Abstract
Granulosa cells (GCs), the largest cell population and primary source of steroid hormones in the ovary, are the important somatic ovarian components. They have critical roles in folliculogenesis by supporting oocyte, facilitating its growth, and providing a microenvironment suitable for follicular development and oocyte maturation, thus having essential functions in maintaining female fertility and in reproductive health in general. Pyroptotic death of GCs and associated inflammation have been implicated in the pathogenesis of several reproductive disorders in females including Premature Ovarian Insufficiency (POI) and Polycystic Ovary Syndrome (PCOS). Here, I reviewed factors, either intrinsic or extrinsic, that induce or inhibit pyroptosis in GCs in various models of these disorders, both in vitro and in vivo, and also covered associated molecular mechanisms. Most of these studied factors influence NLRP3 inflammasome- and GSDMD (Gasdermin D)-mediated pyroptosis in GCs, compared to other inflammasomes and gasdermins (GSDMs). I conclude that a more complete mechanistic understanding of these factors in terms of GC pyroptosis is required to be able to develop novel strategies targeting inflammatory cell death in the ovary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Female subfertility is an increasing reproductive issue worldwide, which is in part due to abnormal ovarian follicular development, affecting one in six couples clinically. The number and the quality of follicles in the ovary is highly related with the fertility status in women [40, 41, 45]. Each follicle consists of an oocyte surrounded by many granulosa cells (GCs). These cells support oocyte, facilitate its growth, and provide a suitable microenvironment for follicular development and oocyte maturation. Although granulosa cell death can be initiated by other programmed cell death pathways such as apoptosis and necroptosis upon certain stimuli [117], here, I focused on pyroptotic death of granulosa cells, factors that induce or limit pyroptosis of these cells, and their implications in reproductive diseases such as Premature Ovarian Insufficiency (POI) and Polycystic Ovary Syndrome (PCOS).
Some transgender men and other gender-diverse people can also experience these disorders; however, since most of the previous work refers to people experiencing them collectively as “women” and does not particularly clarify how these findings might apply to the specific needs of gender-diverse people, I have also used “women” in certain instances, to avoid inappropriate generalisation [7, 9, 36, 50, 59]. More research is urgently needed about the experience of Premature Ovarian Insufficiency (POI) and Polycystic Ovary Syndrome (PCOS) in transgender men and gender-diverse people. Evidence on these disorders in these groups of people is highly scarce, and this research area needs more scientific attention.
Granulosa Cells (GCs)
The development of ovarian follicles capable of ovulating and releasing a mature fertilizable oocyte is a highly complex process that is regulated by both intrafollicular factors and peripheral hormones, including follicle stimulating hormone (FSH). Follicular development can be divided into several stages, including primordial, primary, secondary, and tertiary (antral) follicles [8]. Follicles are structures of critical importance in the ovary, with each follicle consisting of an oocyte surrounded by granulosa cells (GCs). GCs, the largest cell group and primary steroidogenic source in the ovary, are the important somatic cells in the ovary, and play important roles in folliculogenesis by supporting oocyte, facilitating oocyte growth, and providing a suitable microenvironment for follicular development and oocyte maturation, thus having essential functions in maintaining female fertility. GCs primarily secrete multiple growth factors and hormones (essential reproductive hormones such as estrogen and progesterone) that interact with the oocyte during folliculogenesis which is the process of ovarian follicle development from primordial germ cells to fertilizable mature oocytes [82, 153]. The direct communication in addition to the paracrine interactions between the oocyte and GCs provide essential metabolic support for the development and maturation of the oocyte [118]. During ovarian follicular development, oocytes gradually increase in size and progress to maturation accompanied by the proliferation and progressive differentiation of the surrounding somatic cells, including GCs and theca cells (TCs), to support oocyte maturation and ovulation [87]. This proliferation and differentiation of GCs are key events during follicle development, which is regulated by complex communication and interaction among oocytes, GCs, TCs and other somatic cells of the ovary.
There are two major types of GCs: cumulus cells and mural GCs, which surround the oocytes and the antrum (part of an ovarian follicle filled with follicular fluid), respectively. Cumulus cells are involved in providing necessary nutrients to the oocyte and in influencing the development of the oocyte, while mural GCs have endocrine functions and support the growth of the follicle [116]. Estrogens including estradiol and estrone are the key hormones produced by GCs in response to FSH and to the diffusion of androgens from theca cells. Besides, GCs produce different growth factors which interact with the oocyte during its development and impact the process of follicular growth [125, 146]. Therefore, dysfunction of GCs results in abnormal follicular development and ovulation, and ultimately compromises female fertility [80].
Primordial follicles are established perinatally, and they are non-growing (i.e. remain quiescent until activated) and consist of a central primary oocyte surrounded by a single layer of flattened pre-granulosa cells (pre-GCs) [48, 120]. For the females of mammalian species, the pool of primordial follicles provides developing follicles and fertilizable ova resources throughout the reproductive lifespan [2, 148]. In each cycle, only a small number of primordial follicles are selectively activated through a process termed primordial follicle activation. Once activated, primordial follicles undergo rapid growth, resulting in the enlargement of oocytes and proliferation of GCs [84]. During this process, GCs particularly show the most apparent morphological changes, from monolayer flattened to cuboidal in shape. Accordingly, GCs are accompanied by a transition from a relatively transcriptionally inactive state to a more active state [149]. Extensive research have focused on the molecular interactions between oocytes and the surrounding GCs, and this bidirectional signaling has been shown to be required for the proper control of primordial follicle activation, proper oocyte maturation and reproductive health in general [21, 98].
Pyroptosis
Pyroptosis is an inflammatory programmed (i.e. regulated) lytic cell death characterized by the excessive release of proinflammatory cytokines leading to inflammation [143]. Gasdermins are members of a family of pore-forming effector proteins involved in pyroptotic cell death [19]. These proteins (6 members in humans: GSDMA, GSDMB, GSDMC, GSDMD, GSDME and PJVK), except PJVK (Pejvakin, DFNB59), lead to membrane permeabilization and ultimately lytic cell death, upon activation by certain stimuli [11, 19]. Gasdermins contain a cytotoxic N-terminal (NT) domain with an intrinsic pore-forming activity, and a C-terminal domain (CT) which represses pore-forming activity of NT domain in the absence of an activating signal (such as the cleavage by an inflammatory caspase such as caspase-1, -4, -5, -8 or -11) [19, 37, 52, 70, 112]. These two protein domains of gasdermins are connected by a central flexible linker which is cleaved by certain caspases upon induction by certain pathogen-derived or host-derived danger signals (PAMPs and DAMPs, respectively). Following the release of intramolecular inhibition on the NT domain due to proteolytic cleavage of the linker region by caspases, NT pore-forming domain of gasdermin localizes into cell membranes (also to organelle membranes such as mitochondrial outer membrane) to form large oligomeric pores, which results in the disruption of ion homeostasis and in the ultimate induction of cell death [3, 11, 76, 109]. From these pores, certain pro-inflammatory proteins such as IL-18 and IL-1β as well as other small DAMPs (damage-associated molecular patterns) can be released to the extracellular space. In later stages of lytic cell death, NINJ1 (Ninjurin)-mediated plasma membrane rupture enables the release of larger proteins such as LDH and HMGB1 [11, 68, 133].
NLR family pyrin domain containing 3 (NLRP3) is a protein responsible for the activation of the NLRP3 inflammasome, the most well-studied inflammasome complex, resulting in the production of inflammatory cytokines and the subsequent pyroptosis, upon sensing of pathogen-derived or host-derived danger signals [121]. Different caspases are functional in the specific cleavage and activation of certain gasdermins. These inflammatory caspases are activated by various inflammasomes such as NLRP3 inflammasome upon induction by different stimuli [24, 35, 37, 88, 106, 108]. For instance, GSDMD can be cleaved at a certain amino acid position by inflammatory caspases such as caspase-1, -4, -5, and -11 or by caspase-8 depending on cell type, context and stimuli. Similarly, caspase-3 and -8 have been found to be functional in the cleavage of GSDME and GSDMC, respectively, in particular scenarios [55, 101, 131]. It should also be highlighted here that the expression of the NT pore-forming domain of GSDMD or of some other gasdermin family members is sufficient to induce pyroptosis without the need of caspase activation [11, 70, 112].
Since primary ovarian insufficiency (POI) and polycystic ovary syndrome (PCOS) are the most common granulosa cell-related disorders in women, here I focused on pyroptotic cell death of granulosa cells in the context of these two endocrine disorders, as detailed below.
Premature Ovarian Insufficiency (POI)
Premature ovarian insufficiency (POI), also known as premature ovarian failure (POF), is clinically defined as the exhaustion of ovarian reserve in women younger than 40 (i.e. the occurrence of menopause before the age of 40 years), and is a main cause of infertility in women of childbearing age [44]. POI is characterized by menstrual disturbance (amenorrhea (for at least four months) or oligomenorrhea) accompanied by increased gonadotropin levels (e.g. FSH > 25 IU/L) and decreased estradiol (E2) levels [86]. Around 1% of women under 40 suffer from POI, and its clinical etiology manifests with high heterogeneity. Although previous research have suggested that the causes of POI include mutations (certain variants), immune factors, and iatrogenic factors (chemotherapy such as with cisplatin, radiotherapy and ovarian surgery), the pathogenesis of POI is still largely unclear; therefore, no successful standard treatment for this disorder is currently available to the patients [61, 65]. Together with diminished ovarian reserve (DOR), these conditions are likely to lie along a continuum of degrees of decrease in ovarian reserve. Misregulated or abnormal activation of primordial follicles may lead to the exhaustion of the non-renewable pool of primordial follicles, resulting in POI or early menopause [103].
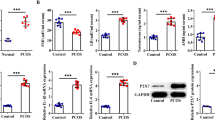
Abnormal inflammation also changes the normal dynamics of ovarian follicles, resulting in poor oocyte quality, anovulation, and related infertility [17, 26] POI is considered to be a pathological chronic inflammatory state that induces the abnormal expression of inflammatory factors of immune cells, granulocytes, and other sources, thus interfering with the normal course of follicular development [58]. NLRP3 inflammasome, caspase-1, and IL-1β expression are increased in GCs of patients with early onset ovarian insufficiency, and the fertility of female mice can be improved by inhibiting the expression of the NLRP3 inflammasome [60]. Besides, NLRP3 inflammasome expression gradually increases with the aging of mouse ovaries, pointing to inflammaging of ovaries [60]. Moreover, apoptosis levels of GCs are substantially higher in POI models, showing that GC death might contribute to the development of POI [92]. Besides apoptosis, pyroptotic death of granulosa cells has also been observed in diverse POI models [27, 28, 75, 137]. For instance, in GCs of POI mice, cyclophosphamide (exposure to which induces POI) promotes an increase in protein levels of NLRP3, ASC, (cleaved) caspase-1, IL-1β and GSDMD, markers of a pyroptotic event [27, 28, 75, 137]. In general, NLRP3 inflammasome-induced pyroptosis is activated during ovarian ageing, resulting in granulosa cell death, follicle depletion and ultimately ovarian dysfunction [60, 79, 137].
Based on the etiology of the disease, the widely used POI animal models can be currently classified as chemotherapy-induced POI models, autoimmune POI models, POI models of mental stress, and galactose-POI models, each with their specific advantages and disadvantages [34]. Among these models, chemotherapy-induced POI animal models are the most commonly used animal models in research [33]. Chemotherapeutic agents, such as cyclophosphamide, tripterygium glycosides, busulfan, cisplatin and doxorubicin (or their combinations), are commonly used to establish animal models of chemotherapy-induced POI due to their irreversible cytotoxicity towards ovaries, including damage to oocytes and follicular depletion [81]. The development of animal models of cyclophosphamide-induced POI is simple, requiring only a single dose of cyclophosphamide [34]. Similarly, a single intraperitoneal injection of combination of cyclophosphamide and busulfan is also used to establish a chemotherapy-induced POI model in mice, as it takes a short time to establish the model with high success rate [123, 136]. Immunization of mice with ZP3 (zona pellucida) glycoprotein is classically used to establish autoimmune POI mice models [34, 46]. This polypeptide induces the production of ZP3 polypeptide antibodies, which bind to ovarian ZP3 to cause an immune response and intervene with the exchange of information between oocytes and surrounding granulosa cells, resulting in ovarian atrophy, anovulation, and other manifestations including human POI [29, 34, 99, 110].
Polycystic Ovary Syndrome (PCOS)
Polycystic ovary syndrome (PCOS) is a polygenic and polyfactorial reproductive endocrinopathy influenced by a combination of genetic and environmental factors, and it afflicts around 5–10% of women of reproductive age. This disease manifests as a complex interplay of endocrine and metabolic disorders (leading to infertility and recurrent miscarriages, insulin resistance, glucose intolerance, type 2 diabetes mellitus, dyslipidemia, obesity, hirsutism (growth of excessive male-pattern hair in women following puberty), acne and cardiovascular problems, and also psychological disorders such as anxiety and depression) characterized by the thickening of the ovarian capsule and stroma due to increased collagen deposition and fibrous tissue, hyperandrogenism (androgen excess) and a decrease in the production of progesterone and estrogens, anovulation (the absence of ovulation) or oligovulation, disordered gonadotropin secretion, ovarian dysfunction, follicular arrest and polycystic ovary morphology [6, 30, 40, 41, 74, 94, 113]. It is the most common endocrine disorder in women of reproductive age, and the most common cause of anovulatory infertility (accounting for nearly 80% of anovulatory infertility patients) [18]. In women with PCOS, despite the presence of high number of follicles, the amount of high-quality matured oocytes is highly limited, which hugely prevents their intrinsic ability of meiotic maturation and fertilization [32]. This is mostly due to abnormal development of follicles and growth arrest during the early antral phase [39].
Although the cause of PCOS remains largely unknown, previous research suggest that abnormal GC activity is closely associated with the pathogenesis of PCOS, and that apoptosis of GCs are increased in PCOS which induces the premature follicular atresia, i.e. the degeneration and resorption of several follicles and their ovules prior to the maturation and release of one ovule from a healthy follicle [96, 104]. GC death is correlated with unfavorable oocyte quality, ovary dysfunction, and low fertilization rates in PCOS [95, 154]. PCOS patients have also higher levels of low-grade chronic inflammation; for instance, inflammatory cytokines such as IL‐18 are highly expressed in these patients [130, 138]. Although apoptotic cell death does not generally induce inflammation, pyroptotic cell death is highly pro-inflammatory. Indeed, NLRP3 inflammasome activation and subsequent pyroptosis of GCs have a critical role in the pathophysiology of PCOS. Active NLRP3 inflammasome contributes to the development of PCOS, particularly in overweight patients [22]. PCOS process has been recently shown to be accompanied by the pyroptosis of GCs resulting from caspase-1 inflammasome activation [20]. NLRP3 and caspase-1 protein expression (both pro-caspase-1 and cleaved caspase-1) are significantly higher in GCs from patients with PCOS than in GCs from non-PCOS patients [129]. Pyroptosis of GCs is increased in PCOS women [135]. Although the viability of GCs from PCOS mice increases following the inhibition of pyroptosis, blockade of other cell death mechanisms (such as ferroptosis) does not influence GC survival, indicating the relative importance of pyroptotic death of GCs in the context of PCOS compared to other cell death mechanisms [20]. Cleavage of caspase 1 and GSDMD is enhanced in GCs from PCOS mice, and these cells release higher levels of IL-1β and IL-18 compared to GCs from control mice, all pointing to the increased pyroptotic cell death in the case of PCOS. In support, inhibition of caspase-1 activation increases the viability of GCs in PCOS mice, thus rescues the pathogenesis of PCOS [20]. Furthermore, hyperandrogenemia, one of the main pathophysiological changes that take place in patients with PCOS, results in GC pyroptosis in PCOS patients via the activation of NLRP3 inflammasome, in parallel to previous reports [150].
Naturally occurring nonhuman animal models of PCOS are currently unknown. Since hyperandrogenism is a major feature of PCOS, several androgens are used to induce PCOS‐like conditions (symptoms that resemble human PCOS, such as anovulation, cyst-like follicles, elevated LH levels, increased adiposity, and insulin insensitivity) in rodents, including testosterone, 5α‐dihydrotestosterone (DHT), and dehydroepiandrosterone (DHEA) [90, 102, 115]. DHT treatment is the most commonly used approach to establish a PCOS-like phenotype in rodents based on the fact that DHT is a non-aromatizable androgen and can not be converted into estradiol, unlike testosterone; thus, the effect can be fully attributed to DHT [126]. These androgenized animal models are typically established via prenatal or postnatal exposure to these exogenous androgens [42]. Prenatally androgenized animal models are usually developed via exposure to exogenous testosterone or dihydrotestosterone (DHT) during late gestation. Postnatal exposure to androgens is typically performed using DHT or DHEA at the prepubertal or pubertal stages. These exposures ultimately result in hyperandrogenism and PCOS-like phenotypes of the offspring in adulthood [42]. Alterations in the levels of kisspeptin and related molecules have been reported in the hypothalamus of these animal models of PCOS [90]. Besides androgens; estrogens, antiprogestin (progesterone receptor antagonists such as RU486 (mifepristone)), or aromatase inhibitors (for instance, letrozole) are used to generate rodent models of PCOS by subcutaneous injection or implantation. Each of these models have differences in terms of estrous cyclicity, ovarian morphology, gonadotropin and sex steroid profiles, neuropeptide levels in the hypothalamus, and certain metabolic features and adiposity [90]. For instance, prenatal DHT‐treated rats and mice have irregular estrous cycles and PCOS‐like ovarian morphology, increased LH levels with an upregulation of kisspeptin in the hypothalamus [90]. In addition to hormonally-induced mouse models of PCOS; transgenic (estrogen receptor knockout; aromatase knockout), diet-induced or chemically-induced mouse models of PCOS (for instance, by D-galactose, monosodium L-glutamate, bisphenol A or tributyltin) have also been described [31, 42, 90, 114, 126]. Prenatally androgenized rodents exposed to testosterone and letrozole-exposed rodents have been observed to most closely resemble the classic PCOS phenotype, whereas prenatally androgenized mice exposed to DHT better mimic the atypical PCOS patients [42].
Factors that Induce Pyroptotic Cell Death in Ovarian Granulosa Cells
Microplastics
Microplastics (MPs) are defined as plastics smaller than 5 mm in diameter. Polystyrene microplastics (PS MPs) induce the pyroptosis of ovarian granulosa cells via the NLRP3 / caspase-1 signaling pathway in Wistar rats [54]. At certain concentrations, polystyrene microplastics lead to increased protein levels of NLRP3, cleaved caspase-1, and cleaved GSDMD in addition to increased levels of IL-18 and IL-1β in rat granulosa cells, all pointing to the increased levels of pyroptotic cell death. Increases in the levels of these proteins are generally microplastic concentration-dependent. In addition to pyroptosis, apoptotic cell death events are also increased in response to polystyrene microplastics in rat granulosa cells, resulting in lower levels of growing follicles and ovarian reserve due to both programmed cell death pathways [54, 144]. PS MPs-induced NLRP3 inflammasome activation prior to pyroptotic cell death might be due to oxidative stress-induced phosphorylation of NF-κB which then activates NLRP3 inflammasome, based on the findings in this study that PS MPs lead to oxidative stress and disrupt antioxidant capacity in the ovaries of rats, and the previous reports showing that NF-κB is phosphorylated in response to oxidative stress [142], and that phosphorylated-NF-κB activates the NLRP3 inflammasome [147].
Di-(2-Ethylhexyl) Phthalate (DEHP)
Di-(2-ethylhexyl) phthalate (DEHP) is an endocrine-disrupting chemical present in various consumer products including cosmetics, cleaning products, medical devices, textiles, and some other polyvinyl chloride (PVC) products, and this endocrine disruptor has also been observed to be present in the follicular fluid of women [49, 124]. DEHP contributes to ovarian dysfunction by inducing GSDMD-mediated pyroptotic cell death via the SLC39A5 / NF-κB / NLRP3 axis in GCs [119]. Mechanistically, DEHP leads to the overexpression of SLC39A5 which activates NF-κB pathway (higher protein levels of phosphorylated p65 (PP65)/P65 and TNF-α), followed by an increase in the transcript and protein expression levels of NLRP3, an increase in cleaved N-terminal GSDMD (which is able to form gasdermin pores in plasma membrane) protein levels, and also in increases in ratios of mature IL-1β / pro-IL-1β and caspase 1-p12 / pro-caspase 1 in GCs (in KGN cells and primary mouse GCs in vitro), resulting in diminished GC proliferation and impaired ovarian function, with more follicles undergoing atresia following exposure to DEHP. These pyroptotic factors are downregulated following the inhibition of NF-κB, pointing to the involvement of this axis in DEHP-mediated pyroptosis in GCs. DEHP-treated mice have also smaller reproductive organs (uterus and ovaries), fewer healthy follicles, and diminished ovarian reserve, showing that DEHP might negatively influence fertility in mice through increased pyroptotic death of GCs [119].
Hyperandrogen (High Levels of Androgens)
Hyperandrogen induces chronic low-grade inflammation by inflammasome activation in PCOS mice, and leads to the pyroptotic death of ovarian GCs through the activation of the NLRP3 inflammasome [130]. DHT (dihydrotestosterone) treatment for 48 h at a certain concentration significantly increases NLRP3, ASC, activated caspase-1 (p10), GSDMD, GSDMD CT (cleaved GSDMD), IL-1β and IL-18 protein levels in GCs. GCs treated with 0.5 or 2 μM DHT show a rounding up of the cell body, and those treated with 5 μM DHT exhibit the swelling of the cell body and plasma membrane disruption. Besides, DHT mediates the release of LDH from GCs and decreases GC viability in a dose-dependent manner. Hyperandrogen-mediated NLRP3 activation promotes the pyroptosis of GCs, further exacerbating follicular dysfunction by dysregulating the expression of folliculogenesis- and steroidogenesis-related genes in GCs, and also drives ovarian fibrosis [130]. Furthermore, authors suggested that TLR4 pathway may mediate hyperandrogenism-induced chronic low-grade inflammation in PCOS mice, possibly by leading to the activation of NLRP3 inflammasome via the downstream signaling molecule MyD88 and activation of NF-κB [69, 130]. Increased ROS levels might also contribute to inflammasome formation in DHT-treated GCs [130].
Testosterone treatment (10 μM) also increases the mRNA and protein expression of IL-1β and NLRP3, and protein expression of IL-18 in human KGN cells, which also results in certain pyroptotic features including cell swelling and cell body rounding up, and formation of multiple bubble-like protrusions on the plasma membrane, and increased dose-dependent release of LDH [135]. Furthermore, testosterone upregulates the mRNA expression of ASC and caspase-1, and protein expression of caspase-1 and the ratio of GSDMD-NT / GSDMD, pointing that testosterone triggers pyroptosis in KGN cells in vitro [135]. These studies demonstrate that excess androgen might lead to female reproductive disorders such as PCOS by promoting pyroptotic death of GCs, in addition to some other potential mechanisms. Again, here, NF-κB whose expression is increased due to testosterone, and hyperandrogenism-induced endoplasmic reticulum (ER) stress can induce the formation of NLRP3 inflammasomes, ultimately leading to pyroptotic cell death in GCs [25].
Non-Esterified Fatty Acids (NEFA)
High producing dairy cows experience a negative energy balance shortly after birth, which is associated with impaired reproductive performance, and which is also accompanied by high plasma concentrations of non-esterified fatty acids (NEFA) up to around 3 weeks post partum [66]. NEFA treatment increases the protein expression of NLRP3 and caspase-1 and the release of IL-1β in a dose-dependent manner in cow GCs, pointing that NEFA may contribute to pyroptotic death of cow GCs, leading to poor reproductive capacity [132]. Besides, NEFA stimulation induces oxidative stress, resulting in increased protein level of TLR4 and in the phosphorylation of NF-κB, and also increases the production of IL-6 and nitric oxide (NO), indicating that NEFA may induce inflammation in these cells. However, these NEFA-mediated effects can be partially reversed when the GCs are pre-treated with N-acetylcysteine (NAC), an antioxidant and radical scavenger. Pre-treatment with NAC limits NEFA-induced increases in NLRP3 and caspase-1 protein levels, and also suppresses the release of IL-1β in GCs. This shows that NEFA can induce pyroptosis and inflammation through NLRP3 inflammasome and oxidative stress-NF-κB pathway, respectively; and NAC can alleviate these conditions to a particular extent [132]. This study also supports other findings detailed above that oxidative stress induced by various factors and TLR4/NF-κB axis is involved in the pyroptotic death of GCs.
High-Fat Diet and Letrozole
Letrozole is an aromatase inhibitor used in the treatment of breast cancer, a hormone-dependent cancer such as ovarian cancer [13, 15, 16]. Letrozole functions by inhibiting the action of aromatase, the enzyme which converts androgens into estrogens by a process called aromatization [12]. Letrozole treatment increases NLRP3 protein levels in GCs of mice compared to control and high-fat diet-fed mice [129]. GCs of both letrozole treated- and high-fat diet-fed mice show increased caspase-1 protein levels compared to GCs of control mice, or compared to GCs of only letrozole treated- or of only high-fat diet-fed mice. The serum concentrations of IL-18 and IL-1β in mice treated with high-fat diet + letrozole are also significantly higher compared to that in only high-fat diet-fed mice. Besides, both letrozole treated- and high-fat diet-fed mice have increased number of cystic follicles and decreased number of corpora lutea compared to control mice [129]. These observations suggest that high-fat diet and treatment with letrozole might result in increased pyroptotic events in GCs of mice, leading to unfavorable reproductive health in female mice. Authors also suggested that NLRP3 may influence follicle development by regulating the expression of proteins involved in the glycosylation of many hormones and growth factors that are indispensable for oogenesis and folliculogenesis [129].
Local Glucose Elevation
Glucose activates NLRP3 inflammasome and pyroptosis in a dose- and time-dependent manner in GCs from patients [140]. It increases NLRP3, ASC, caspase 1, IL-1β mRNA levels, NLRP3, ASC and cleaved IL-1β protein levels, caspase 1 activity, and GSDMD mRNA and NT-GSDMD protein levels, generally in a glucose concentration-dependent manner (from 5 to 35 mM), in GCs, resulting in decreased cell viability at higher concentrations of glucose. Besides, the inhibition of NLRP3 restricts pyroptotic cell death induced by high glucose in GCs, as shown by, for instance, decreased NT-GSDMD protein abundance and lower LDH release [140].
TXNIP facilitates the oligomerization of NLRP3 and stabilizes the inflammasome complex [156]. High glucose treatment stimulates TXNIP mRNA and protein expression in GCs, and knockdown of TXNIP results in reversing the upregulation of NLRP3 inflammasome and certain pyroptosis biomarkers induced by high glucose treatment, pointing that TXNIP participates in high glucose-induced activation of NLRP3 inflammasome and pyroptosis in GCs [140]. Moreover, the activation of NLRP3 inflammasome and pyroptosis impaires estradiol (E2) synthesis in GCs, and decreased E2 synthesis is also observed following high glucose treatment in these cells. In contrast, inhibition of pyroptosis reverses high glucose-induced downregulation of E2 levels in GCs [140]. This points that increased NLRP3 inflammasome activation and pyroptotic cell death induced by higher local glucose may be a potential reason for impaired steroidogenesis and decreased E2 synthesis in human GCs.
Clinically, overweight women have elevated follicular glucose levels which significantly induce NLRP3 inflammasome formation and pyroptotic events in GCs, as shown by, for instance, higher NLRP3, ASC, and cleaved-caspase-1 protein levels, increased caspase-1 activity, higher IL-1β levels in follicular fluid, increased levels of GSDMD mRNA and NT-GSDMD protein, and characteristic pore formation in GC plasma membranes [140]. This shows that high glucose might be a potent inducer of pyroptotic death of GCs, and a specific mediator of obesity-associated reproductive issues in women.
Factors that Limit Pyroptotic Cell Death in Ovarian Granulosa Cells
Plumbagin
Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) is a compound isolated from the root of the plant Plumbago zeylanica L., also known as chitrak [5, 105]. Administration of plumbagin prevents the pyroptosis of GCs and the onset of PCOS, by suppressing WTAP-mediated N6-methylation of ASC mRNA [20]. Normally, the upregulation of WTAP (a key regulator of RNA N6-methylase complex) results in the overactivation of the inflammasome in GCs, since WTAP stabilizes ASC (a NLRP3 inflammasome component) mRNA by mediating its N6-methylation in GCs from the PCOS model. However, plumbagin reverses this inflammasome overactivation by destabilizing ASC mRNA, decreasing its mRNA and protein levels, in GCs isolated from the ovary of mice. It thus decreases the cleavage of both caspase-1 (resulting in the suppression of caspase-1 activation) and GSDMD (resulting in the supression of pyroptosis), and the release of IL-1β, IL-18 and LDH in GCs, which are all normally increased in the case of PCOS. In summary, plumbagin lowers pyroptotic death of granulosa cells, which is normally heightened in the case of PCOS [20].
Quercetin
Cyclophosphamide, a chemotherapeutic drug, damages the ovaries in a dose-dependent manner [91, 92]. Recent studies have demonstrated that cyclophosphamide-induced ovary damage is associated with the facilitation of GC apoptosis [145]. Quercetin, a natural flavonoid widely found in many fruits and plants, including tea, onions, apples, and kale, protects the ovarian reserve from damage due to cyclophosphamide-induced POI by downregulating pyroptosis in mice [27]. More specifically, quercetin reduces the protein levels of NLRP3, GSDMD, caspase-1 and IL-1β in the GCs of cyclophosphamide-induced POI mice, possibly resulting in the inhibition of pyroptosis and in the limitation of cyclophosphamide-induced ovarian damage [27]. Again, in this context, mitochondrial ROS-mediated activation of inflammasomes might be involved in the pyroptosis of GCs, since the expression of oxidative stress products are increased, whereas that of antioxidant products are decreased in this induced POI model, which are reversed after quercetin treatment [27, 51, 141].
HDAC1 (Histone Deacetylase 1)
HDAC1 suppresses the pyroptosis of GCs in PCOS through deacetylation of H3K9ac on the lncRNA H19 promoter to regulate the H19 / miR-29a-3p / NLRP3 axis, as shown in in vitro PCOS cell models (dihydrotestosterone (DHT)-induced HGL5 cells) [26]. HDAC1 expression is lower in patients with PCOS compared to those with non-PCOS at both mRNA and protein level; and its overexpression inhibits PCOS-induced pyroptosis due to decreased protein levels of NLRP3, GSDMD-NT, and cleaved caspase-1, IL- 1β and IL-18, and thus alleviates PCOS phenotype in mouse models (established using dehydroepiandrosterone (DHEA)). Mechanistically, in in vitro PCOS models, HDAC1 inhibits lncRNA H19 transcription through H3K9 deacetylation on H19 promoter, and reduces the binding of H19 to miR-29a-3p to inhibit NLRP3 expression, thus suppressing GC pyroptosis [26]. HDAC1 is also negatively correlated with the levels of certain inflammation markers (IL-6, IL-1β, IL-18, and TNF-α) in the serum of PCOS patients, among which IL-1β and IL-18 are mostly released from cells during pyroptosis, suggesting the association between HDAC1 and pyroptotic cell death in PCOS patients, and anti-pyroptotic function of HDAC1 via H19/miR-29a-3p/NLRP3 axis [23]. This study shows that epigenetic regulators might also be responsible in the regulation of pyroptotic GC death in PCOS.
Leonurine Hydrochloride
Leonurine (4-guanidino-n-butyl syringate), an alkaloid from Leonurus heterophyllus, inhibits the over-activation of NLRP3 inflammasome [77]. Intraperitoneal administration of leonurine hydrochloride prevents cyclophosphamide-induced POI by inhibiting NLRP3 / GSDMD-mediated pyroptosis of GCs and improves cyclophosphamide-induced infertility in mice [28]. Leonurine hydrochloride treatment normalizes the ovarian protein expression of NLRP3, ASC, cleaved caspase-1, and GSDMD, which is increased by cyclophosphamide, and also significantly lowers the levels of serum IL-18 and IL-1β. Furthermore, leonurine hydrochloride treatment increases the weight of reproductive organs, the numbers of primordial follicles, primary follicles, and secondary follicles, reduces the number of atretic follicles, and increases the numbers of live fetuses and implantations (fertility-related indicators) [28]. These observations suggest that leonurine hydrochloride might lead to improved fertility in POI by limiting pyroptosis in GCs, at least in mouse models. Leonurine has been shown to have strong binding ability to PI3K and to suppress IL-1β-induced activation of the PI3K/Akt/NF-κB signaling pathway or to lead to NF-κB inactivation in general [56, 73, 78]. Considering the ability of active NF-κB to activate the NLRP3 inflammasome [147], anti-pyroptotic functionality of leonurine might be due to decreased NF-κB-mediated activation of NLRP3 inflammasome, ultimately resulting in a smaller number of pyroptotic events in GCs.
Bushenhuoluo Decoction (BSHLD)
Bushenhuoluo Decotion (BSHLD) is a traditional empirical formula (of Traditional Chinese medicine (TCM)) which consists of 14 traditional Chinese herbs including Shudihuang, Danggui, Chuanxiong, Baishao, Nvzhenzi, Hanliancao, Tusizi, Chongweizi, Fupenzi, Yinyanghuo, Taoren, Honghua, Lulutong and Huainiuxi [57]. Bushenhuoluo Decoction treatment inhibits NLRP3-mediated GC pyroptosis in ovarian tissues of PCOS rats, thus improving ovarian function, by regulating exosomal miR-30a-5p / SOCS3 (suppressor of cytokine signaling 3) / mTOR / NLRP3 signaling [57]. BSHLD reduces miR-30a-5p expression in serum, serum exosomes and ovarian tissues in PCOS rats, and decreased exosomal miR-30a-5p attenuates LPS-triggered pyroptosis in GCs. Mechanistically, miR-30a-5p directly targets SOCS3 to inactivate mTOR / P70S6K signaling. BSHLD administration significantly reverses IL-1β and IL-18 production in ovarian tissues of PCOS rats, in parallel to decreases in NLRP3, ASC, and caspase 1 mRNA levels and in NLRP3 protein levels, pointing the potential of Chinese herbal medicine BSHLD as an alternative for the treatment of PCOS, by limiting pyroptotic cell death of GCs. In contrast, mTOR agonist rapamycin reverses miR-30a-5p inhibitor (miR-30a-5p antagomir)-mediated decreases in pyroptosis, leads to increased NLRP3 levels and pyroptotic events in GCs. In other words, the inhibitory effect of miR-30a-5p deficiency on GC pyroptosis is attenuated by rapamycin, leading to increased pyroptotic cell death of GCs [57]. This shows that BSHLD and rapamycin might function in reverse directions in the regulation of GC pyroptosis. Here, it should also be noted that miR-30a-5p could drive NF-kB/NLRP3 signaling pathway, and that SOCS family members are capable to regulate various signaling pathways including NF-kB pathway, in some other contexts [93, 134].
Bushen Cuyun Recipe
Bushen Cuyun Recipe (BCR) is an ancient Chinese herbal decoction and a product marketed in China (with tradename of Yueling Yin) with proposed positive effects on female infertility [64]. It comprises of 10 different herbs [64]. Bushen Cuyun Recipe protects GCs against pyroptosis in the case of diminished ovarian reserve induced by cyclophosphamide in rats, by reversing the elevated protein levels of GSDMD and caspase-1 in GCs, suggesting that the inhibition of pyroptosis by BCR could ameliorate ovarian function in the case of diminished ovarian reserve. This treatment relieves the reduction in the numbers of growing follicles and corpus lutea from last ovulation, potentially leading to improved fertility in rats [64]. Authors showed in another study by performing molecular docking analyses that the main active components of BCR (kaempferol, quercetin, and hesperetin) can stably bind to GSDMD, resulting in decreased levels of available GSDMD [63]. Therefore, it can be speculated that BCR or its certain components more directly limit pyroptotic death of GCs by possibly binding to its main executioner proteins (i.e. gasdermins) and preventing its oligomerization to be able to form membrane pores.
α-Ketoglutarate
α-Ketoglutarate is a key intermediate in the tricarboxylic acid (TCA) cycle, also known as Krebs cycle, a series of chemical reactions used to generate energy by the oxidation of acetylcoenzyme A (CoA) derived from carbohydrates, fatty acids and proteins in aerobic organisms. α-Ketoglutarate treatment reduces the expression of inflammatory factors in the ovaries and reduces systemic inflammatory cytokines, thus inhibiting the level of intracellular inflammation [62, 78, 111, 151]. Long-term use of α-ketoglutarate delays the decline of fertility in mice and improves the number and quality of follicles and oocytes [151]. α-Ketoglutarate improves ovarian reserve function in POI by inhibiting NLRP3-mediated pyroptosis of GCs [75]. It decreases mRNA levels of NLRP3, caspase-1, GSDMD and IL-18, and protein levels of NLRP3, cleaved-caspase 1, GSDMD, IL-1B and IL-18, which are normally increased in cyclophosphamide-induced POI models compared to controls, in rat GCs [75]. In vitro, in KGN cells (ovarian granulosa cell tumor cell line) treated with LPS and nigericin (microbial toxin derived from the gram-positive bacteria Streptomyces hygroscopicus, used to trigger the NLRP3 inflammasome) to mimic pyroptosis, α-ketoglutarate (2 mM) limits pyroptotic cell death (as quantified by decreased LDH release from cells), decreases the released IL-18 and IL-1β protein levels, and lowers inflammatory and pyroptosis factors such as NLRP3, GSDMD, caspase-1, IL-18 and IL-1β at both mRNA and protein levels, showing that α-ketoglutarate could delay or even reverse cyclophosphamide-induced POI by inhibiting NLRP3-mediated pyroptosis in granulosa cells [75]. Authors also reported that α-ketoglutarate intervention elevates lactate levels in POI rats, thereby restoring the expression of certain glycolytic enzymes. Since the disruption of glycolytic flux is a signal for inflammasome signaling and pyroptosis mostly due to decreased NADH levels and induction of mitochondrial ROS production [107], the restoration of glycolysis or downstream TCA cycle metabolism with the supplemantation of specific metabolites such as α-ketoglutarate might limit inflammasome activation and downstream pyroptotic cell death in GCs.
Human Bone Marrow Derived-Mesenchymal Stem Cells (hBMSCs)
Autoimmune-related disorders are responsible for approximately 4%-30% of POI cases [43]. In vitro, co-culture of hBMSCs (human bone marrow derived-mesenchymal stem cells) with autoimmune-damaged GCs (modeled by IFN-γ-induced human GC line KGN) reduces the occurrence of pyroptosis in GCs in autoimmune POI [83]. In more detail, hBMSC cell co-culture reduces the level of LDH released (a pyroptotic cell death marker, as a marker of cell membrane breakdown) from KGN cells, and also decreases NLRP3 and caspase-1 mRNA levels, and caspase-1 protein levels, suggesting that hBMSCs might repair IFN-γ-induced autoimmune damage in KGN cells occurred due to pyroptotic cell death [83]. hBMSCs co-culture treatment does not change NLRP3 and GSDMD protein levels; however, it decreases caspase-1 protein levels in KGN cells. Therefore, it can be suggested that hBMSCs co-culture treatment limits pyroptotic cell death of GCs mostly by decreasing the processing and activation of GSDMD (free NT domain of GSDMD formed following caspase cleavage is able to form pores), rather than regulating its protein levels. In vivo, hBMSCs improve estrous cycle disorders in autoimmune POI mice, increases serum estradiol levels, decreases serum FSH levels, improves ovarian function and morphology, increases the number of primordial, primary and mature follicles, and decreases the number of atretic follicles [83]. Some of these in vivo effects in mice might be attributed, at least to a certain extent, to decreased pyroptotic events in GCs following hBMSC treatment.
Exosomes derived from bone marrow mesenchymal stem cells also suppress NLRP3/Caspase1/GSDMD pyroptosis axis in vitro (KGN cells exposed to IFN-gamma) and in vivo (BALB/c mouse model of autoimmune POI, induced by zona pellucida glycoprotein 3 (ZP3)), resulting in the restoration of ovarian function (normalization of the irregular oestrous cycles, rescue of the follicular loss, and increased the pregnancy rate and number of offspring) in autoimmune POI [137]. Exosomes derived from these mesenchymal stem cells downregulate the expression of the NLRP3, caspase 1 and GSDMD, decrease the level of LDH released, and also inhibit activation of the NF-κB pathway in ovarian granulosa cells, indicating that exosomal treatment promotes the function of ovarian granulosa cells by suppressing the NF-κB pathway [137]. Furthermore, the rising levels of IL-1β in the serum of the POI group (compared to controls) significantly decrease after this exosomal treatment in mice [137].
He’s Yang Chao Recipe
He’s Yang Chao Recipe (HSYC) is a traditional Chinese medicine originated from ‘Cong Rong Tu Si Wan’, a classic and famous formula recorded in the traditional Chinese medicinal book Ji-Yin-Zong-Lu in the period of Ming Dynasty. This recipe primarily contains eight herbs [85]. Treatment with He’s Yang Chao recipe significantly reduces the mRNA and protein expression of NLRP3, caspase-1, GSDMD, IL-18, and IL-1B in GCs of cyclophosphamide-induced POI mouse models [85]. This points that He’s Yang Chao recipe protects GCs in mice with POI potentially by the contribution from the inhibition of NLRP3 inflammasome activation [85]. The mechanism by which He’s Yang Chao recipe limits pyroptotic death of GC in mice remains to be studied. Besides, specific molecules of this recipe which are particularly functional in the regulation of pyroptosis in GCs should be identified in future studies.
Metformin
Cisplatin is a DNA-damaging chemotherapy drug used in the first-line standard treatment of ovarian cancer, in addition to the treatment of some other solid malignant tumors [14]. However, it can adversely affect ovarian health and fertility (for instance, by reducing ovarian primordial follicles, decreasing ovarian reserve and diminishing the number of sinusoidal follicles) in premenopausal cancer patients; thus, the ovarian endocrine and reproductive functions of female patients with cancer should be safeguarded against cisplatin-induced damage. Metformin is a biguanide antihyperglycemic agent and first-line pharmacotherapy used in the management of type II diabetes [127]. It is also used off-label for the treatment of insulin resistance in PCOS [4]. Besides, metformin extends average lifespan in certain model organisms [10]. Metformin mitigates cisplatin-induced ovarian damage by the inhibition of GC pyroptosis via ROS/TXNIP/NLRP3 signaling pathway [128]. The number of GC pyroptosis events significantly decreases in the cisplatin + metformin compared to only cisplatin group, in parallel to decreases in the protein levels of caspase-1, GSDMD, IL-1β, and in mRNA and protein levels of TXNIP and NLRP3 [129]. Treatment with a TXNIP inhibitor results in the loss of metformin-induced decreases in cisplatin-induced pyroptotic cell death in GCs. In summary, metformin can, to a certain extent, inhibit GC pyroptosis due to cisplatin, potentially by the inhibition of the TXNIP / NLRP3 / caspase-1 / GSDMD axis in mice [128]. Metformin also downregulates cellular levels of ROS which is known to induce NLPR3 inflammasome activation, and to augment S-palmitoylation of GSDMD which is required for it to be able to form pores [1, 38].
Metformin also inhibits human KGN cell pyroptosis via the miR-670-3p/NOX2/ROS pathway [155]. It decreases NLRP3 and ASC mRNA levels, and NLRP3, cleaved caspase-1, ASC and GSDMD-NT protein levels, released LDH levels and caspase-1 activity in LPS-treated human KGN cells. Some of these metformin-mediated decreases in pyroptotic factors are further decreased with additional treatment with N-acetyl-L-cysteine (NAC), a ROS scavenger. Mechanistically, metformin upregulates miR-670-3p which inhibits the NOX2-ROS axis by targeting NOX2 in KGN cells, leading to the repression of GC pyroptosis in vitro [155].
Genipin and Tauro-ursodeoxycholic Acid (TUDCA)
Genipin (GP), an inflammation inhibitor which was initially isolated from the Chinese medicinal plant Gardenia jasminoides [97], suppresses testosterone-induced inflammation in human GCs (KGN cells) [135]. Genipin decreases mRNA levels of IL-1β, NLRP3, ASC and caspase-1, and protein levels of IL-18, IL-1β, NLRP3, caspase-1 and the ratio of GSDMD-NT/GSDMD, and also reduces released LDH levels, showing that the suppression of inflammation attenuates androgen-induced pyroptosis in GCs in vitro [135]. Similar reduction in the mRNA and protein levels of pyroptosis-related factors and in LDH release are observed following the treatment of KGN cells with TUDCA (a bile acid and an inhibitor of ER stress), resulting in the suppression of testosterone-induced inflammation in these cells [135]. This points that ER stress might mediate testosterone-induced pyroptosis and inflammation in KGN cells. ER stress is known to promote NLRP3 inflammasome activation through multiple mechanisms including the unfolded protein response (UPR), calcium or lipid metabolism, and ROS generation [25, 71, 89, 122]. The limiting effect of ER stress inhibition on pyroptotic death of GCs might be mainly due to decreased activation of NLRP3 inflammasome, possibly resulting in decreased caspase-1 activation and pro-IL-1β, pro-IL-18 and GSDM processing.
Cysteine-rich 61 (CYR61)
Rats with premature ovarian failure have lower levels of Cysteine-rich 61 (CYR61; also known as CCN1) in their GCs [139]. This protein limits cyclophosphamide-induced inhibition of proliferation in rat GCs by suppressing NLRP3 / caspase 1-mediated pyroptotic cell death [139]. Overexpression of CYR61 decreases NLRP3, caspase 1, GSDMD and IL-1β protein levels, whereas its silencing results in increased expression of these proteins in cyclophosphamide-induced GCs [139]. CYR61 overexpression leads to higher viability of cyclophosphamide-treated rat GCs, whereas CYR61 silencing results in decreased viability of these cells [139]. These observations suggest that CYR61 is involved in the suppression of pyroptotic death of GCs induced by cyclophosphamide, leading to increased GC viability. CYR61 also inhibits cyclophosphamide-induced senescence of rat ovarian granulosa cells (as shown by downregulation of senescence markers p53 and p21, and less SA-β-gal positivity), and mechanistically this may be related to the regulation of caspase-1/NLRP3-induced pyroptosis. This can be supported by previous research demonstrating a potential association between cellular senescence and pyroptosis [47, 53, 67, 72, 152, 157].
Cyproterone Acetate
Cyproterone acetate (CYA) is a progestin-based drug frequently used to reduce androgen in PCOS patients. Cyproterone acetate treatment decreases the expression of pyroptosis-related proteins in mice GCs. More specifically, cyproterone acetate leads to decreases in the protein levels of NLRP3, GSDMD, GSDMD-NT, caspase 1 and cleaved-caspase 1 in GCs from mice with hyperandrogen-induced PCOS, in addition to decreases in IL-18 and IL-1β concentration in serum [150]. Mechanistically, in vitro, cyproterone acetate ameliorates the pyroptosis of KGN cells by decreasing the activation of the IRE1 signaling pathway [150]. Activated IRE1 increases TXNIP mRNA stability, and in turn, elevated TXNIP protein activates the NLRP3 inflammasome [71, 100, 122]. This IRE1-TXNIP axis-regulated NLRP3 inflammasome activation has been also shown to be manipulated in the case of other pyroptosis-inducer or -inhibitor factors in granulosa cells as detailed above.
A summary of pyroptosis-inducer and -inhibitor factors in granulosa cells in diverse models systems of POI and PCOS is given in Fig. 1 and Table 1.
Pyroptosis in ovarian granulosa cells (GCs), pyroptosis-inducers and -inhibitors in these cells, in various models of Premature Ovarian Insufficiency (POI) and Polycystic Ovary Syndrome (PCOS). Both pyroptosis-inducer and -inhibitor factors mostly influence NLPR3-, caspase 1- and GSDMD-mediated pyroptosis in GCs in vitro and in vivo models of POI and PCOS. In the presence of a pyroptosis inducer (such as microplastics), caspase 1, following its activation by NLRP3 inflammasome, cleaves GSDMD between its N-terminal and C-terminal domains (NT and CT, respectively), and then free GSDMD-NT domains oligomerize to form pores in the plasma membrane leading to the pore-mediated release of certain pro-inflammatory molecules including IL-18 and LDH, and ultimately leading to lytic pro-inflammatory cell death
Conclusion
Pyroptosis of ovarian granulosa cells is involved in the pathogenesis of multiple reproductive disorders including Premature Ovarian Insufficiency (POI) and Polycystic Ovary Syndrome (PCOS). Several factors, either endogenous / intrinsic or exogenous / extrinsic, have been identified so far, which have been shown to induce or inhibit / limit pyroptotic death of GCs, ultimately influencing fertility in females. Microplastics, di-(2-ethylhexyl) phthalate (DEHP), hyperandrogen (high levels of androgens), non-esterified fatty acids (NEFA), high-fat diet and letrozole, local glucose elevation have been reported to induce pyroptotic cell death in GCs, mostly mediated by NLRP3 inflammasome and GSDMD, in different in vitro and in vivo models. However, more research has focused on factors that inhibit or limit pyroptosis in GCs. Plumbagin, quercetin, HDAC1 (histone deacetylase 1), leonurine hydrochloride, certain Chinese traditional medicine recipes (Bushenhuoluo decoction (BSHLD), Bushen Cuyun recipe and He’s Yang Chao recipe), α-ketoglutarate, human bone marrow derived-mesenchymal stem cells (hBMSCs), metformin, genipin, tauro-ursodeoxycholic acid (TUDCA), cysteine-rich 61 (CYR61) and cyproterone acetate have been found to suppress pyroptotic death of GCs in different model systems of POI and PCOS. Similar to pyroptosis-inducers in GCs, these factors almost all the time influence NLPR3 inflammasome- and GSDMD-mediated pyroptotic cell death in GCs, decreasing pro-inflammatory death of these cells.
Combined, a better and more complete mechanistic understanding of pyroptosis-inducer and-inhibitor factors in granulosa cells is required to develop novel and more effective strategies against abnormal ovarian follicular development and associated reproductive disorders in women.
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Abais JM, Xia M, Zhang Y, Boini KM, Li PL. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid Redox Signal. 2015;22(13):1111–29. https://doi.org/10.1089/ars.2014.5994.
Adhikari D, Liu K. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr Rev. 2009;30:438–64.
Aglietti RA, Estevez A, Gupta A, Ramirez MG, Liu PS, Kayagaki N, Ciferri C, Dixit VM, Dueber EC. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc Natl Acad Sci U S A. 2016;113(28):7858–63. https://doi.org/10.1073/pnas.1607769113.
Attia GM, Almouteri MM, Alnakhli FT. Role of Metformin in Polycystic Ovary Syndrome (PCOS)-Related Infertility. Cureus. 2023;15(8):e44493. https://doi.org/10.7759/cureus.44493.
Aziz MH, Dreckschmidt NE, Verma AK. Plumbagin, a medicinal plant-derived naphthoquinone, is a novel inhibitor of the growth and invasion of hormone-refractory prostate cancer. Cancer Res. 2008;68(21):9024–32. https://doi.org/10.1158/0008-5472.CAN-08-2494.
Azziz R, Adashi EY. Stein and Leventhal: 80 years on. Am J Obstet Gynecol. 2016;214:247 e241-247 e211. https://doi.org/10.1016/j.Ajog.2015.12.013.
Baba T, Endo T, Honnma H, Kitajima Y, Hayashi T, Ikeda H, Masumori N, Kamiya H, Moriwaka O, Saito T. Association between polycystic ovary syndrome and female-to-male transsexuality. Hum Reprod. 2007;22:1011–6. https://doi.org/10.1093/humrep/del474.
Baerwald AR, Adams GP, Pierson RA. Ovarian antral folliculogenesis during the human menstrual cycle: A review. Hum Reprod Update. 2012;18:73–91.
Bailie E, Maidarti M, Jack S, Hawthorn R, Watson N, Telfer E, Anderson RA. The ovaries of transgender men indicate effects of high dose testosterone on the primordial and early growing follicle pool. Reprod Fertil. 2023;4(2):e220102. https://doi.org/10.1530/RAF-22-0102.
Berkel C, Cacan E. A collective analysis of lifespan-extending compounds in diverse model organisms, and of species whose lifespan can be extended the most by the application of compounds. Biogerontology. 2021;22(6):639–53. https://doi.org/10.1007/s10522-021-09941-y.
Berkel C, Cacan E. Differential Expression and Copy Number Variation of Gasdermin (GSDM) Family Members, Pore-Forming Proteins in Pyroptosis, in Normal and Malignant Serous Ovarian Tissue. Inflammation. 2021;44(6):2203–16. https://doi.org/10.1007/s10753-021-01493-0.
Berkel C, Cacan E. Estrogen- and estrogen receptor (ER)-mediated cisplatin chemoresistance in cancer. Life Sci. 2021;286:120029. https://doi.org/10.1016/j.lfs.2021.120029.
Berkel C, Cacan E. Lower expression of NINJ1 (Ninjurin 1), a mediator of plasma membrane rupture, is associated with advanced disease and worse prognosis in serous ovarian cancer. Immunol Res. 2023;71(1):15–28. https://doi.org/10.1007/s12026-022-09323-7.
Berkel C, Kucuk B, Usta M, Yılmaz E, Cacan E. The Effect of Olaparib and Bortezomib Combination Treatment on Ovarian Cancer Cell Lines. Eur J Biol Aralık. 2020;79(2):115–23.
Berkel C. Estrogen receptor- and progesterone receptor-positive breast tumors have higher mRNA levels of NR3C1 and ZBTB16, with implications in prognosis for luminal A subtype. Hum Cell. 2024;37:376–9. https://doi.org/10.1007/s13577-023-01014-1.
Berkel C. KIF18A as a potential biomarker to distinguish different breast cancer subtypes based on receptor status. Genome Instab Dis. 2024. https://doi.org/10.1007/s42764-024-00126-8.
Boots C, Jungheim C. Semin Reprod Med. 2015;33:270.
Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841–55.
Broz P, Pelegrín P, Shao F. The gasdermins, a protein family executing cell death and inflammation. Nat Rev Immunol. 2020;20(3):143–57. https://doi.org/10.1038/s41577-019-0228-2.
Cai Z, He S, Liu R, Zhou L, Zhao L. Plumbagin rescues the granulosa cell’s pyroptosis by reducing WTAP-mediated N6-methylation in polycystic ovary syndrome. J Ovarian Res. 2022;15(1):126. https://doi.org/10.1186/s13048-022-01058-1.
Cavalcante AY, Gouveia BB, Barberino RS, Lins TL, Santos LP, Goncalves RJ, et al. Kit ligand promotes the transition from primordial to primary follicles after in vitro culture of ovine ovarian tissue. Zygote. 2016;24:578–82.
Chamanara S, Hozouri V, Irandoost E. Inhibition of NLRP3 inflammasome-A potential mechanistic therapeutic for treatment of polycystic ovary syndrome? J Biochem Mol Toxicol. 2024;38(1):e23592. https://doi.org/10.1002/jbt.23592.
Chen J, Zhu Z, Xu S, Li J, Huang L, Tan W, Zhang Y, Zhao Y. HDAC1 participates in polycystic ovary syndrome through histone modification by regulating H19/miR-29a-3p/NLRP3-mediated granulosa cell pyroptosis. Mol Cell Endocrinol. 2023;573:111950. https://doi.org/10.1016/j.mce.2023.111950.
Chen KW, Demarco B, Heilig R, Shkarina K, Boettcher A, Farady CJ, Pelczar P, Broz P. Extrinsic and intrinsic apoptosis activate pannexin-1 to drive NLRP3 inflammasome assembly. EMBO J. 2019;38(10):e101638.
Chen X, Guo X, Ge Q, Zhao Y, Mu H, Zhang J. ER Stress Activates the NLRP3 Inflammasome: A Novel Mechanism of Atherosclerosis. Oxid Med Cell Longev. 2019;2019:3462530. https://doi.org/10.1155/2019/3462530.
Chen Y, Miao C, Zhao Y, Yang L, Wang R, Shen D, Ren N, Zhang Q. Mol Hum Reprod. 2023;29:gaad035.
Chen Y, Zhao Y, Miao C, Yang L, Wang R, Chen B, Zhang Q. Quercetin alleviates cyclophosphamide-induced premature ovarian insufficiency in mice by reducing mitochondrial oxidative stress and pyroptosis in granulosa cells. J Ovarian Res. 2022;15(1):138. https://doi.org/10.1186/s13048-022-01080-3.
Chi YN, Hai DM, Ma L, Cui YH, Hu HT, Liu N, Juan-Du, Lan XB, Yu JQ, Yang JM. Protective effects of leonurine hydrochloride on pyroptosis in premature ovarian insufficiency via regulating NLRP3/GSDMD pathway. Int Immunopharmacol. 2023;114:109520. https://doi.org/10.1016/j.intimp.2022.109520.
Chon SJ, Umair Z, Yoon MS. Premature ovarian insufficiency: past, present, and future. Front Cell Dev Biol. 2021;9:672890. https://doi.org/10.3389/fcell.2021.672890.
Christ JP, Cedars MI. Current guidelines for diagnosing PCOS. Diagnostics (Basel). 2023;13(6):55. https://doi.org/10.3390/diagnostics13061113.
Corrie L, Gulati M, Singh SK, Kapoor B, Khursheed R, Awasthi A, Vishwas S, Chellappan DK, Gupta G, Jha NK, Anand K, Dua K. Recent updates on animal models for understanding the etiopathogenesis of polycystic ovarian syndrome. Life Sci. 2021;280:119753. https://doi.org/10.1016/j.lfs.2021.119753.
Da Broi MG, et al. Influence of follicular fluid and cumulus cells on oocyte quality: clinical implications. J Assist Reprod Genet. 2018;35:735–51. https://doi.org/10.1007/s10815-018-1143-3.
Dai F, Liu H, He J, Wu J, Yuan C, Wang R, Yuan M, Yang D, Deng Z, Wang L, Wang Y, Yang X, Wang H, Hu W, Cheng Y. Model construction and drug therapy of primary ovarian insufficiency by ultrasound-guided injection. Stem Cell Res Ther. 2024;15(1):49. https://doi.org/10.1186/s13287-024-03646-y.
Dai F, Wang R, Deng Z, Yang D, Wang L, Wu M, Hu W, Cheng Y. Comparison of the different animal modeling and therapy methods of premature ovarian failure in animal model. Stem Cell Res Ther. 2023;14(1):135. https://doi.org/10.1186/s13287-023-03333-4.
Demarco B, Grayczyk JP, Bjanes E, Le Roy D, Tonnus W, Assenmacher CA, Radaelli E, Fettrelet T, Mack V, Linkermann A, Roger T, Brodsky IE, Chen KW, Broz P. Caspase-8-dependent gasdermin D cleavage promotes antimicrobial defense but confers susceptibility to TNF-induced lethality. Sci Adv. 2020;6(47):eabc3465. https://doi.org/10.1126/sciadv.abc3465.
De Roo C, Lierman S, Tilleman K, Peynshaert K, Braeckmans K, Caanen M, Lambalk CB, Weyers S, Tsjoen G, Cornelissen R, et al. Ovarian tissue cryopreservation in female-to-male transgender people: insights into ovarian histology and physiology after prolonged androgen treatment. Reprod Biomed Online. 2017;34:557–66. https://doi.org/10.1016/j.rbmo.2017.03.008.
Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, Sun H, Wang DC, Shao F. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535(7610):111–6. https://doi.org/10.1038/nature18590.
Du G, Healy LB, David L, Walker C, El-Baba TJ, Lutomski CA, Goh B, Gu B, Pi X, Devant P, Fontana P, Dong Y, Ma X, Miao R, Balasubramanian A, Puthenveetil R, Banerjee A, Luo HR, Kagan JC, Oh SF, Robinson CV, Lieberman J, Wu H. ROS-dependent S-palmitoylation activates cleaved and intact gasdermin D. Nature. 2024;. https://doi.org/10.1038/s41586-024-07373-5.
Du J, Ruan X, Jin F, Li Y, Cheng J, Gu M, Mueck AO. J Ovarian Res. 2021;14(1):36.
Dumesic DA, Meldrum DR, Katz-Jaffe MG, Krisher RL, Schoolcraft WB. Oocyte environment: follicular fluid and cumulus cells are critical for oocyte health. Fertil Steril. 2015;103:303–16.
Dumesic DA, et al. Scientific Statement on the Diagnostic Criteria, Epidemiology, Pathophysiology, and Molecular Genetics of Polycystic Ovary Syndrome. Endocr Rev. 2015;36:487–525.
Dunn AJ. Animal models in polycystic ovarian syndrome. Clin Obstet Gynecol. 2021;64(1):126–33. https://doi.org/10.1097/GRF.0000000000000580.
Ebrahimi M, Akbari AF. The role of autoimmunity in premature ovarian failure. Iran J Reprod Med. 2015;13(8):461–72.
ESHRE Guideline Group on Female Fertility Preservation, Anderson RA, Amant F, Braat D, D’Angelo A, Chuva de Sousa Lopes SM, Demeestere I, Dwek S, Frith L, Lambertini M, Maslin C, Moura-Ramos M, Nogueira D, Rodriguez-Wallberg K, Vermeulen N. ESHRE guideline: female fertility preservation. Hum Reprod Open. 2020;2020(4):hoaa052. https://doi.org/10.1093/hropen/hoaa052.
Farquhar CM, Bhattacharya S, Repping S, Mastenbroek S, Kamath MS, Marjoribanks J, et al. Female subfertility. Nat Rev Dis Primers. 2019;5:1–21.
Feng G, Lin M, Zhou X, Zhang L, Li J, Ouyang J. Efficacy of Bushenjianpi prescription on autoimmune premature ovarian failure in mice. J Tradit Chin Med. 2017;37(5):667–74.
Fernández-Duran I, Quintanilla A, Tarrats N, Birch J, Hari P, Millar FR, Lagnado AB, Smer-Barreto V, Muir M, Brunton VG, Passos JF, Acosta JC. Cytoplasmic innate immune sensing by the caspase-4 non-canonical inflammasome promotes cellular senescence. Cell Death Differ. 2022;29(6):1267–82. https://doi.org/10.1038/s41418-021-00917-6.
Fortune JE, Yang MY, Muruvi W. The earliest stages of follicular development: follicle formation and activation. Soc Reprod Fertil Suppl. 2010;67:203–16. https://doi.org/10.7313/upo9781907284991.018.
Fu X, et al. Di-(2-ethylhexyl) phthalate exposure induces female reproductive toxicity and alters the intestinal microbiota community structure and fecal metabolite profile in mice. Environ Toxicol. 2021;36:1226–42. https://doi.org/10.1002/tox.23121.
Grynberg M, Fanchin R, Dubost G, Colau JC, Brémont-Weil C, Frydman R, Ayoubi JM. Histology of genital tract and breast tissue after long-term testosterone administration in a female-to-male transsexual population. Reprod Biomed Online. 2010;20:553–8. https://doi.org/10.1016/j.rbmo.2009.12.021.
Harijith A, Ebenezer DL, Natarajan V. Reactive oxygen species at the crossroads of inflammasome and inflammation. Front Physiol. 2014;5:352. https://doi.org/10.3389/fphys.2014.00352.
He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang ZH, Zhong CQ, Han J. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015;25(12):1285–98. https://doi.org/10.1038/cr.2015.139.
Hom LM, Sun S, Campbell J, Liu P, Culbert S, Murphy IM, Schafer ZT. A role for fibroblast-derived SASP factors in the activation of pyroptotic cell death in mammary epithelial cells. bioRxiv [Preprint]. 2023;2023.02.21.529458. https://doi.org/10.1101/2023.02.21.529458. Update in: J Biol Chem. 2023 Jun 13;:104922.
Hou J, Lei Z, Cui L, Hou Y, Yang L, An R, Wang Q, Li S, Zhang H, Zhang L. Polystyrene microplastics lead to pyroptosis and apoptosis of ovarian granulosa cells via NLRP3/Caspase-1 signaling pathway in rats. Ecotoxicol Environ Saf. 2021;212:112012. https://doi.org/10.1016/j.ecoenv.2021.112012.
Hou J, Zhao R, Xia W, Chang CW, You Y, Hsu JM, Nie L, Chen Y, Wang YC, Liu C, Wang WJ, Wu Y, Ke B, Hsu JL, Huang K, Ye Z, Yang Y, Xia X, Li Y, Li CW, Shao B, Tainer JA, Hung MC. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat Cell Biol. 2020;10:1264–75. https://doi.org/10.1038/s41556-020-0575-z.
Hu ZC, Gong LF, Li XB, Fu X, Xuan JW, Feng ZH, Ni WF. Inhibition of PI3K/Akt/NF-κB signaling with leonurine for ameliorating the progression of osteoarthritis: In vitro and in vivo studies. J Cell Physiol. 2019;234(5):6940–50. https://doi.org/10.1002/jcp.27437.
Huang Q, Li Y, Chen Z, Ou H, Tan Y, Lin H. Bushenhuoluo Decoction improves polycystic ovary syndrome by regulating exosomal miR-30a-5p/ SOCS3/mTOR/NLRP3 signaling-mediated autophagy and pyroptosis. J Ovarian Res. 2024;17(1):29. https://doi.org/10.1186/s13048-024-01355-x.
Huang Y, Hu C, Ye H, Luo R, Fu X, Li X, Huang J, Chen W, Zheng Y. J Immunol Res. 2019;1:8069898.
Ikeda K, Baba T, Noguchi H, Nagasawa K, Endo T, Kiya T, Saito T. Excessive androgen exposure in female-to-male transsexual persons of reproductive age induces hyperplasia of the ovarian cortex and stroma but not polycystic ovary morphology. Hum Reprod. 2013;28:453–61. https://doi.org/10.1093/humrep/des385.
Navarro-Pando JM, Alcocer-G´omez E, Castej´on-Vega B, Navarro-Villar´an E, Cond´es-Herv´as M, Mundi-Roldan M, Muntan´e J, P´erez-Pulido AJ, Bullon P, Wang C, Hoffman HM, Sanz A, Mbalaviele G, Ryffel B, Cordero MD. Inhibition of the NLRP3 inflammasome prevents ovarian aging. Sci Adv. 2021;7(1). https://doi.org/10.1126/sciadv.abc7409.
Jaillard S, Bell K, Akloul L, Walton K, McElreavy K, Stocker WA, Beaumont M, Harrisson C, Jääskeläinen T, Palvimo JJ, Robevska G, Launay E, Satié AP, Listyasari N, Bendavid C, Sreenivasan R, Duros S, van den Bergen J, Henry C, Domin-Bernhard M, Cornevin L, Dejucq-Rainsford N, Belaud-Rotureau MA, Odent S, Ayers KL, Ravel C, Tucker EJ, Sinclair AH. New insights into the genetic basis of premature ovarian insufficiency: Novel causative variants and candidate genes revealed by genomic sequencing. Maturitas. 2020;141:9–19. https://doi.org/10.1016/j.maturitas.2020.06.004.
Jia Y, Yin C, Ke W, Liu J, Guo B, Wang X, Zhao P, Hu S, Zhang C, Li X, Liu R, Zheng X, Wang Y, Wang G, Pan H, Hu W, Song Z. Sci Total Environ. 2023;878:163069.
Jiang M, Huang L, Wang Y, Wang Y, Kang Q, Chen C, Hu Y, Li J, Wang T. Yueliang Yin Ameliorates Endometrial Receptivity in Mice with Embryo Implantation Failure by Reducing Pyroptosis and Activating BDNF/TrkB Pathway. Mol Nutr Food Res. 2023;67(23):e2300339. https://doi.org/10.1002/mnfr.202300339.
Jiang M, Wang W, Zhang J, Wang C, Bi Y, Li P, Yang S, Li J, Xu YT, Wang T. Protective effects and possible mechanisms of actions of Bushen Cuyun recipe on diminished ovarian reserve induced by cyclophosphamide in rats. Front Pharmacol. 2020;11:546. https://doi.org/10.3389/fphar.2020.00546.
Jiao X, Zhang H, Ke H, Zhang J, Cheng L, Liu Y, et al. Premature Ovarian Insufficiency: Phenotypic Characterization within Different Etiologies. J Clin Endocrinol Metab. 2017;102:2281–90.
Jorritsma R, César ML, Hermans JT, Kruitwagen CL, Vos PL, Kruip TA. Effects of non-esterified fatty acids on bovine granulosa cells and developmental potential of oocytes in vitro. Anim Reprod Sci. 2004;81(3–4):225–35. https://doi.org/10.1016/j.anireprosci.2003.10.005.
Joshi CS, Salazar AM, Wang C, Ligon MM, Chappidi RR, Fashemi BE, Felder PA, Mora A, Grimm SL, Coarfa C, Mysorekar IU. D-Mannose reduces cellular senescence and NLRP3/GasderminD/IL-1β-driven pyroptotic uroepithelial cell shedding in the murine bladder. Dev Cell. 2024;59(1):33-47.e5. https://doi.org/10.1016/j.devcel.2023.11.017.
Kayagaki N, Kornfeld OS, Lee BL, Stowe IB, O’Rourke K, Li Q, Sandoval W, Yan D, Kang J, Xu M, Zhang J, Lee WP, McKenzie BS, Ulas G, Payandeh J, Roose-Girma M, Modrusan Z, Reja R, Sagolla M, Webster JD, Cho V, Andrews TD, Morris LX, Miosge LA, Goodnow CC, Bertram EM, Dixit VM. NINJ1 mediates plasma membrane rupture during lytic cell death. Nature. 2021;591(7848):131–6. https://doi.org/10.1038/s41586-021-03218-7.
Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637–50. https://doi.org/10.1016/j.immuni.2011.05.006.
Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu J, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang Y, Bertram EM, Goodnow CC, Dixit VM. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526(7575):666–71. https://doi.org/10.1038/nature15541.
Lerner AG, Upton JP, Praveen PV, Ghosh R, Nakagawa Y, Igbaria A, Shen S, Nguyen V, Backes BJ, Heiman M, Heintz N, Greengard P, Hui S, Tang Q, Trusina A, Oakes SA, Papa FR. IRE1α induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab. 2012;16(2):250–64. https://doi.org/10.1016/j.cmet.2012.07.007.
Li J, Wang X, Yao Z, Yuan F, Liu H, Sun Z, Yuan Z, Luo G, Yao X, Cui H, Tu B, Sun Z, Fan C. NLRP3-dependent crosstalk between pyroptotic macrophage and senescent cell orchestrates trauma-induced heterotopic ossification during aberrant wound healing. Adv Sci (Weinh). 2023;10(19):e2207383. https://doi.org/10.1002/advs.202207383.
Li N, Xu Q, Liu Q, Pan D, Jiang Y, Liu M, Liu M, Xu H, Lin C. Leonurine attenuates fibroblast-like synoviocyte-mediated synovial inflammation and joint destruction in rheumatoid arthritis. Rheumatology (Oxford). 2017;56(8):1417–27. https://doi.org/10.1093/rheumatology/kex142.
Li R, et al. Prevalence of polycystic ovary syndrome in women in China: a large community-based study. Hum Reprod. 2013;28:2562–9. https://doi.org/10.1093/humrep/det262.
Liu K, Wu Y, Yang W, Li T, Wang Z, Xiao S, Peng Z, Li M, Xiong W, Li M, Chen X, Zhang S, Lei X. α-Ketoglutarate improves ovarian reserve function in primary ovarian insufficiency by inhibiting NLRP3-mediated pyroptosis of granulosa cells. Mol Nutr Food Res. 2024;5:e2300784. https://doi.org/10.1002/mnfr.202300784.
Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, Lieberman J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535(7610):153–8. https://doi.org/10.1038/nature18629.
Liu Y, Duan C, Chen H, Wang C, Liu X, Qiu M, Tang H, Zhang F, Zhou X, Yang J. Inhibition of COX-2/mPGES-1 and 5-LOX in macrophages by leonurine ameliorates monosodium urate crystal-induced inflammation. Toxicol Appl Pharmacol. 2018;351:1–11. https://doi.org/10.1016/j.taap.2018.05.010.
Liu Z, Gan LU, Zhang T, Ren Q, Sun C. J Pineal Res. 2018;64:e12455.
Lliberos C, Liew SH, Mansell A, Hutt KJ. The Inflammasome contributes to depletion of the ovarian reserve during aging in mice. Front Cell Dev Biol. 2021;8:628473. https://doi.org/10.3389/fcell.2020.628473.
Lo BK, Sheikh S, Williams SA. In vitro and in vivo mouse follicle development in ovaries and reaggregated ovaries. Reproduction. 2019;157:135–48.
Lv SJ, Hou SH, Gan L, Sun J. Establishment and Mechanism Study of a Primary Ovarian Insufficiency Mouse Model Using Lipopolysaccharide. Anal Cell Pathol (Amst). 2021;2021:1781532. https://doi.org/10.1155/2021/1781532.
M’Baye M, Hua G, Khan HA, Yang L. RNAi-mediated knockdown of INHBB increases apoptosis and inhibits steroidogenesis in mouse granulosa cells. J Reprod Dev. 2015;61:391–7. https://doi.org/10.1262/jrd.2014-158.
Ma WQ, Zhuo AP, Xiao YL, Gao M, Yang YT, Tang LC, Wu YH, Tian D, Fu XF. Human bone marrow derived-mesenchymal stem cells treatment for autoimmune premature ovarian insufficiency. Stem Cell Rev Rep. 2024;20(2):538–53. https://doi.org/10.1007/s12015-023-10629-8.
Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 2002;296:2178–80.
Miao C, Zhao Y, Chen Y, Wang R, Ren N, Chen B, Dong P, Zhang Q. Investigation of He’s Yang Chao recipe against oxidative stress-related mitophagy and pyroptosis to improve ovarian function. Front Endocrinol (Lausanne). 2023;14:1077315. https://doi.org/10.3389/fendo.2023.1077315.
Nelson LM. Primary ovarian insufficiency. N Engl J Med. 2009;360:606–14.
Orisaka M, Tajima K, Tsang BK, Kotsuji F. Oocyte-granulosa-theca cell interactions during preantral follicular development. J Ovarian Res. 2009;2:9.
Orning P, Weng D, Starheim K, Ratner D, Best Z, Lee B, Brooks A, Xia S, Wu H, Kelliher MA, Berger SB, Gough PJ, Bertin J, Proulx MM, Goguen JD, Kayagaki N, Fitzgerald KA, Lien E. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science. 2018;362(6418):1064–9. https://doi.org/10.1126/science.aau2818.
Oslowski CM, Hara T, O’Sullivan-Murphy B, Kanekura K, Lu S, Hara M, Ishigaki S, Zhu LJ, Hayashi E, Hui ST, Greiner D, Kaufman RJ, Bortell R, Urano F. Thioredoxin-interacting protein mediates ER stress-induced β cell death through initiation of the inflammasome. Cell Metab. 2012;16(2):265–73. https://doi.org/10.1016/j.cmet.2012.07.005.
Osuka S, Nakanishi N, Murase T, Nakamura T, Goto M, Iwase A, Kikkawa F. Animal models of polycystic ovary syndrome: A review of hormone-induced rodent models focused on hypothalamus-pituitary-ovary axis and neuropeptides. Reprod Med Biol. 2018;18(2):151–60. https://doi.org/10.1002/rmb2.12262.
Overbeek A, van den Berg MH, van Leeuwen FE, Kaspers GJ, Lambalk CB, van Dulmen-den BE. Chemotherapy-related late adverse effects on ovarian function in female survivors of childhood and young adult cancer: A systematic review. Cancer Treat Rev. 2017;53:10–24.
Park HS, Chugh RM, El Andaloussi A, Hobeika E, Esfandyari S, Elsharoud A, et al. Human BM-MSC secretome enhances human granulosa cell proliferation and steroidogenesis and restores ovarian function in primary ovarian insufficiency mouse model. Sci Rep. 2021;11:4525.
Park SH, Kim KE, Hwang HY, Kim TY. Regulatory effect of SOCS on NF-kappaB activity in murine monocytes/macrophages. DNA Cell Biol. 2003;22(2):131–9. https://doi.org/10.1089/104454903321515931.
Patel S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J Steroid Biochem Mol Biol. 2018;182:27–36. https://doi.org/10.1016/j.jsbmb.2018.04.008.
Peng SL, Wu QF, Xie Q, Tan J, Shu KY. PATL2 regulated the apoptosis of ovarian granulosa cells in patients with PCOS. Gynecol Endocrinol. 2021;37:629–34.
Qiu X, Wei Y, Liu C, Ding C, Zhao S. Hyperandrogen enhances apoptosis of human ovarian granulosa cells via up-regulation and demethylation of PDCD4. Gynecol Endocrinol. 2020;36:333–7. https://doi.org/10.1080/09513590.2019.1653844.
Rajanbabu V, Galam L, Fukumoto J, Enciso J, Tadikonda P, Lane TN, Bandyopadhyay S, Parthasarathy PT, Cho Y, Cho SH, Lee YC, Lockey RF, Kolliputi N. Genipin suppresses NLRP3 inflammasome activation through uncoupling protein-2. Cell Immunol. 2015;297(1):40–5. https://doi.org/10.1016/j.cellimm.2015.06.002.
Reddy P, Zheng W, Liu K. Mechanisms maintaining the dormancy and survival of mammalian primordial follicles. Trends Endocrinol Metab. 2010;21:96–103.
Rhim SH, Millar SE, Robey F, Luo AM, Lou YH, Yule T, Allen P, Dean J, Tung KS. Autoimmune disease of the ovary induced by a ZP3 peptide from the mouse zona pellucida. J Clin Invest. 1992;89(1):28–35. https://doi.org/10.1172/JCI115572.
Robblee MM, Kim CC, Porter Abate J, Valdearcos M, Sandlund KL, Shenoy MK, Volmer R, Iwawaki T, Koliwad SK. Saturated Fatty Acids Engage an IRE1α-Dependent Pathway to Activate the NLRP3 Inflammasome in Myeloid Cells. Cell Rep. 2016;14(11):2611–23. https://doi.org/10.1016/j.celrep.2016.02.053.
Rogers C, Fernandes-Alnemri T, Mayes L, Alnemri D, Cingolani G, Alnemri ES. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun. 2017;3(8):14128. https://doi.org/10.1038/ncomms14128.
Ryu Y, Kim SW, Kim YY, Ku SY. Animal Models for Human Polycystic Ovary Syndrome (PCOS) Focused on the Use of Indirect Hormonal Perturbations: A Review of the Literature. Int J Mol Sci. 2019;20(11):2720. https://doi.org/10.3390/ijms20112720.
Saatcioglu HD, Cuevas I, Castrillon DH. Control of oocyte reawakening by kit. PLoS Genet. 2016;12:e1006215.
Salehi R, et al. Ovarian mitochondrial dynamics and cell fate regulation in an androgen-induced rat model of polycystic ovarian syndrome. Sci Rep. 2020;10:1021. https://doi.org/10.1038/s41598-020-57672-w.
Sandur SK, Ichikawa H, Sethi G, et al. Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) suppresses NF-κB activation and NF-κB-regulated gene products through modulation of p65 and IκBα kinase activation, leading to potentiation of apoptosis induced by cytokine and chemotherapeutic agents. J Biol Chem. 2006;281:17023–33.
Sanjo H, Nakayama J, Yoshizawa T, Fehling HJ, Akira S, Taki S. Cutting edge: TAK1 safeguards macrophages against proinflammatory cell death. J Immunol. 2019;203(4):783–8. https://doi.org/10.4049/jimmunol.
Sanman LE, Qian Y, Eisele NA, Ng TM, van der Linden WA, Monack DM, Weerapana E, Bogyo M. Disruption of glycolytic flux is a signal for inflammasome signaling and pyroptotic cell death. Elife. 2016;24(5):e13663. https://doi.org/10.7554/eLife.13663.
Sarhan J, Liu BC, Muendlein HI, Li P, Nilson R, Tang AY, Rongvaux A, Bunnell SC, Shao F, Green DR, Poltorak A. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc Natl Acad Sci U S A. 2018;115(46):E10888–97. https://doi.org/10.1073/pnas.1809548115.
Sborgi L, Rühl S, Mulvihill E, Pipercevic J, Heilig R, Stahlberg H, Farady CJ, Müller DJ, Broz P, Hiller S. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016;35(16):1766–78. https://doi.org/10.15252/embj.201694696.
Setiady YY, Samy ET, Tung KS. Maternal autoantibody triggers de novo T cell-mediated neonatal autoimmune disease. J Immunol. 2003;170(9):4656–64. https://doi.org/10.4049/jimmunol.170.9.4656.
Shahmirzadi AA, Edgar D, Liao CY, Hsu YM, Lucanic M, Wiley CD, Gan G, Kim DE, Kasler C, Kuehnemann HG, Kaplowitz B, Bhaumik D, Riley RR, Kennedy BK, Lithgow GJ. Cell Metab. 2020;32:447.e6.
Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660–5. https://doi.org/10.1038/nature15514.
Shrivastava S, Conigliaro RL. Polycystic ovarian syndrome. Med Clin N Am. 2023;107(2):227–34. https://doi.org/10.1016/j.mcna.2022.10.004.
Stener-Victorin E. Update on animal models of polycystic ovary syndrome. Endocrinology. 2022;163(12):bqac164. https://doi.org/10.1210/endocr/bqac164.
Stener-Victorin E, Padmanabhan V, Walters KA, Campbell RE, Benrick A, Giacobini P, Dumesic DA, Abbott DH. Animal models to understand the etiology and pathophysiology of polycystic ovary syndrome. Endocr Rev. 2020;41(4):bnaa010. https://doi.org/10.1210/endrev/bnaa010.
Strauss JF, Williams CJ. in Yen & Jaffe's Reproductive Endocrinology (Seventh Edition) (eds Jerome F. Strauss & Robert L. Barbieri) 157–191.e158 (W.B. Saunders, 2014).
Stringer JM, Alesi LR, Winship AL, Hutt KJ. Beyond apoptosis: evidence of other regulated cell death pathways in the ovary throughout development and life. Hum Reprod Update. 2023;29(4):434–56. https://doi.org/10.1093/humupd/dmad005.
Su Y-Q, Sugiura K, Eppig JJ. Mouse oocyte control of granulosa cell development and function: Paracrine regulation of cumulus cell metabolism. Semin Reprod Med. 2009;27:32–42. https://doi.org/10.1055/s-0028-1108008.
Sun J, Gan L, Lv S, Wang T, Dai C, Sun J. Exposure to Di-(2-Ethylhexyl) phthalate drives ovarian dysfunction by inducing granulosa cell pyroptosis via the SLC39A5/NF-κB/NLRP3 axis. Ecotoxicol Environ Saf. 2023;252:114625. https://doi.org/10.1016/j.ecoenv.2023.114625.
Suzuki H, Kanai-Azuma M, Kanai Y. From sex determination to initial folliculogenesis in mammalian ovaries: morphogenetic waves along the anteroposterior and dorsoventral axes. Sex Dev. 2015;9:190–204.
Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19:477–89.
Talty A, Deegan S, Ljujic M, Mnich K, Naicker SD, Quandt D, Zeng Q, Patterson JB, Gorman AM, Griffin MD, Samali A, Logue SE. Inhibition of IRE1α RNase activity reduces NLRP3 inflammasome assembly and processing of pro-IL1β. Cell Death Dis. 2019;10(9):622. https://doi.org/10.1038/s41419-019-1847-z. (Erratum In:Cell Death Dis. 2020 Jan 6;11(1):12).
Tang D, Feng X, Ling L, Zhang W, Luo Y, Wang Y, Xiong Z. Experimental study for the establishment of a chemotherapy-induced ovarian insufficiency model in rats by using cyclophosphamide combined with busulfan. Regul Toxicol Pharmacol. 2021;122:104915. https://doi.org/10.1016/j.yrtph.2021.104915.
Trnka B, Polan M, Zigmont VA. Exposure to Di-2-ethylhexyl phthalate (DEHP) and infertility in women, NHANES 2013–2016. Reprod Toxicol. 2021;103:46–50. https://doi.org/10.1016/j.reprotox.2021.05.010.
Tu J, Cheung AH-H, Chan CL-K, Chan W-Y. The role of microRNAs in ovarian granulosa cells in health and disease. Front Endocrinol. 2019. https://doi.org/10.3389/fendo.2019.00174.
van Houten EL, Visser JA. Mouse models to study polycystic ovary syndrome: a possible link between metabolism and ovarian function? Reprod Biol. 2014;14(1):32–43. https://doi.org/10.1016/j.repbio.2013.09.007.
Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond). 2012;122(6):253–70. https://doi.org/10.1042/CS20110386.
Wang B, Li J, Zhang Q, Li Y, Ren W, He D. Metformin mitigates cisplatin-induced ovarian damage through inhibiting the pyroptosis of granulosa cells via ROS/TXNIP/NLRP3 signaling pathway. Aging (Albany NY). 2024;16. https://doi.org/10.18632/aging.205659.
Wang B, Shi M, Yu C, Pan H, Shen H, Du Y, Zhang Y, Liu B, Xi D, Sheng J, Huang H, Ding G. NLRP3 inflammasome-dependent pathway is involved in the pathogenesis of polycystic ovary syndrome. Reprod Sci. 2024;31(4):1017–27. https://doi.org/10.1007/s43032-023-01348-z.
Wang D, Weng Y, Zhang Y, Wang R, Wang T, Zhou J, Shen S, Wang H, Wang Y. Exposure to hyperandrogen drives ovarian dysfunction and fibrosis by activating the NLRP3 inflammasome in mice. Sci Total Environ. 2020a;745:141049. https://doi.org/10.1016/j.scitotenv.2020.141049.
Wang Y, Gao W, Shi X, Ding J, Liu W, He H, Wang K, Shao F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547(7661):99–103. https://doi.org/10.1038/nature22393.
Wang Y, Li C, Ali I, Li L, Wang G. N-acetylcysteine modulates non-esterified fatty acid-induced pyroptosis and inflammation in granulosa cells. Mol Immunol. 2020b;127:157–63. https://doi.org/10.1016/j.molimm.2020.09.011.
Wang Y, Shao F. NINJ1, rupturing swollen membranes for cataclysmic cell lysis. Mol Cell. 2021;81(7):1370–1. https://doi.org/10.1016/j.molcel.2021.03.005.
Wu YX, Xu RY, Jiang L, Chen XY, Xiao XJ. MicroRNA-30a-5p Promotes Chronic Heart Failure in Rats by Targeting Sirtuin-1 to Activate the Nuclear Factor-κB/NOD-Like Receptor 3 Signaling Pathway. Cardiovasc Drugs Ther. 2023;37(6):1065–76. https://doi.org/10.1007/s10557-021-07304-w.
Xiang Y, Wang H, Ding H, Xu T, Liu X, Huang Z, Wu H, Ge H. Hyperandrogenism drives ovarian inflammation and pyroptosis: A possible pathogenesis of PCOS follicular dysplasia. Int Immunopharmacol. 2023;125(Pt A):111141. https://doi.org/10.1016/j.intimp.2023.111141.
Xiao GY, Cheng CC, Chiang YS, Cheng WT, Liu IH, Wu SC. Exosomal miR-10a derived from amniotic fluid stem cells preserves ovarian follicles after chemotherapy. Sci Rep. 2016;6:23120. https://doi.org/10.1038/srep23120.
Xie J, Yang Y, Zhuo A, Gao M, Tang L, Xiao Y, Zhu H, Fu X. Exosomes derived from mesenchymal stem cells attenuate NLRP3-related pyroptosis in autoimmune premature ovarian insufficiency via the NF-κB pathway. Reprod Biomed Online. 2024;48(6):103814. https://doi.org/10.1016/j.rbmo.2024.103814.
Xiong YL, Liang XY, Yang X, Li Y, Wei LN. Low-grade chronic inflammation in the peripheral blood and ovaries of women with polycystic ovarian syndrome. Eur J Obstet Gynecol Reprod Biol. 2011;159:148–50. https://doi.org/10.1016/j.Ejogrb.2011.07.012.
Xu H, Bao X, Yang J, Kong H, Li Y, Sun Z. Cysteine-rich 61 (CYR61) alleviates cyclophosphamide-induced proliferation inhibition in ovarian granulosa cells via suppressing NLRP3/caspase1-mediated pyroptosis. Hum Exp Toxicol. 2023;42:9603271231152832. https://doi.org/10.1177/09603271231152831.
Xu R, Zhao H, Qi J, Yao G, He Y, Lu Y, Zhu Q, Wang Y, Ding Y, Zhu Z, Li X, Vankelecom H, Sun Y. Local glucose elevation activates pyroptosis via NLRP3 inflammasome in ovarian granulosa cells of overweight patients. FASEB J. 2023;37(3):e22807. https://doi.org/10.1096/fj.202201796RR.
Xu X, Zhang L, Ye X, Hao Q, Zhang T, Cui G, Yu M. Nrf2/ARE pathway inhibits ROS-induced NLRP3 inflammasome activation in BV2 cells after cerebral ischemia reperfusion. Inflamm Res. 2018;67(1):57–65. https://doi.org/10.1007/s00011-017-1095-6.
Yang Y, Wang Y, Kong Y, Zhang X, Zhang H, Gang Y, Bai L. Carnosine Prevents Type 2 Diabetes-Induced Osteoarthritis Through the ROS/NF-κB Pathway. Front Pharmacol. 2018;9:598. https://doi.org/10.3389/fphar.2018.00598.
Yu SY, Li XL. Pyroptosis and inflammasomes in obstetrical and gynecological diseases. Gynecol Endocrinol. 2021;37:385–91.
Yuan Y, Qin Y, Wang M, Xu W, Chen Y, Zheng L, Chen W, Luo T. Microplastics from agricultural plastic mulch films: A mini-review of their impacts on the animal reproductive system. Ecotoxicol Environ Saf. 2022;244:114030. https://doi.org/10.1016/j.ecoenv.2022.114030.
Yuksel A, Bildik G, Senbabaoglu F, Akin N, Arvas M, Unal F, et al. The magnitude of gonadotoxicity of chemotherapy drugs on ovarian follicles and granulosa cells varies depending upon the category of the drugs and the type of granulosa cells. Hum Reprod. 2015;30:2926–35.
Zanjirband M, Hodayi R, Safaeinejad Z, Nasr-Esfahani MH, Ghaedi-Heydari R. Evaluation of the p53 pathway in polycystic ovarian syndrome pathogenesis and apoptosis enhancement in human granulosa cells through transcriptome data analysis. Sci Rep. 2023;13(1):11648. https://doi.org/10.1038/s41598-023-38340-1.
Zeng J, Chen Y, Ding R, Feng L, Fu Z, Yang S, Deng X, Xie Z, Zheng S. Isoliquiritigenin alleviates early brain injury after experimental intracerebral hemorrhage via suppressing ROS- and/or NF-κB-mediated NLRP3 inflammasome activation by promoting Nrf2 antioxidant pathway. J Neuroinflammation. 2017;14(1):119. https://doi.org/10.1186/s12974-017-0895-5.
Zhang H, Liu K. Cellular and molecular regulation of the activation of mammalian primordial follicles: somatic cells initiate follicle activation in adulthood. Hum Reprod Update. 2015;21:779–86.
Zhang H, Risal S, Gorre N, Busayavalasa K, Li X, Shen Y, et al. Somatic cells initiate primordial follicle activation and govern the development of dormant oocytes in mice. Curr Biol. 2014;24:2501–8.
Zhang Y, Xie X, Ma Y, Du C, Jiao Y, Xia G, Xu J, Yang Y. Cyproterone Acetate Mediates IRE1α Signaling Pathway to Alleviate Pyroptosis of Ovarian Granulosa Cells Induced by Hyperandrogen. Biology (Basel). 2022;11(12):1761. https://doi.org/10.3390/biology11121761.
Zhang Z, He C, Gao YU, Zhang L, Song Y, Zhu T, Zhu K, Lv D, Wang J, Tian X, Ma T, Ji P, Cui W, Liu G. Aging Cell. 2021;20:e13291.
Zhao P, Yue Z, Nie L, Zhao Z, Wang Q, Chen J, Wang Q. Hyperglycaemia-associated macrophage pyroptosis accelerates periodontal inflamm-aging. J Clin Periodontol. 2021;48(10):1379–92. https://doi.org/10.1111/jcpe.13517.
Zhao R, Jiang Y, Zhao S, Zhao H. Multiomics analysis reveals molecular abnormalities in granulosa cells of women with polycystic ovary syndrome. Front Genet. 2021;12:648701. https://doi.org/10.3389/fgene.2021.648701.
Zheng Q, Li Y, Zhang D, Cui X, Dai K, Yang Y, Liu S, Tan J, Yan Q. ANP promotes proliferation and inhibits apoptosis of ovarian granulosa cells by NPRA/ PGRMC1/EGFR complex and improves ovary functions of PCOS rats. Cell Death Dis. 2017;8:e3145.
Zhou LH, Zou H, Hao JY, Huang Y, Zhang JN, Xu XH, Li J. Metformin inhibits ovarian granular cell pyroptosis through the miR-670–3p/NOX2/ROS pathway. Aging (Albany NY). 2023;15(10):4429–43. https://doi.org/10.18632/aging.204745.
Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–40. https://doi.org/10.1038/ni.1831.
Zhou R, Xie X, Qin Z, Li X, Liu J, Li H, Zheng Q, Luo Y. Cytosolic dsDNA is a novel senescence marker associated with pyroptosis activation. Tissue Cell. 2021;72:101554. https://doi.org/10.1016/j.tice.2021.101554.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). No funding has been received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflicts of Interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Berkel, C. Inducers and Inhibitors of Pyroptotic Death of Granulosa Cells in Models of Premature Ovarian Insufficiency and Polycystic Ovary Syndrome. Reprod. Sci. (2024). https://doi.org/10.1007/s43032-024-01643-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43032-024-01643-3