Abstract
Purpose
Intensive postoperative chemotherapy treatment use in early-onset colon cancer and late-onset colon cancer remains to be defined and their effects on prognosis were unclear. This study aims to investigate whether intensive adjuvant chemotherapy for stage II colon cancer would result in matched survival improvement in young patients (< 50 years) without risk factors and old-aged (70–85 years) patients with risk factors defined by guidelines.
Methods
We extracted eligible patients with pathologically confirmed TNM stage II colon cancer from the Surveillance, Epidemiology, and End Results database between 2004 and 2015. Patients aged < 50 years old without risk factors were defined as non-high-risk early-onset colon cancer (non-HREOCC), and those aged 70 to 85 years with risk factors were defined as high-risk late-onset colon cancer (HRLOCC). Kaplan–Meier (KM) method with log-rank test was performed to calculate the overall survival (OS) and cancer-specific survival (CSS). Multivariate Cox model was used to estimate the association of adjuvant chemotherapy with CSS by adjusting potential confounding factors.
Results
Of 55,366 eligible stage II colon cancer patients, 3341 non-HREOCC patients and 11,722 HRLOCC patients were included. 37.68% and 16.8% of patients received adjuvant chemotherapy among non-HREOCC and HRLOCC patients, respectively. For non-HREOCC patients, there was no significant association between adjuvant chemotherapy and CSS (HR = 1.09, 95%CI0.83–1.44). For HRLOCC patients, adjuvant chemotherapy was associated with a better CSS (HR = 0.88, 95%CI0.79–0.99).
Conclusion
Our findings suggested that potential overuse of adjuvant chemotherapy among non-high-risk young patients with stage II colon cancer did not lead to survival improvement, and caution should be called when using chemotherapy in these patients. However, chemotherapy can be used appropriately for high-risk stage II colon cancer patients aged 70 to 85 years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Colorectal cancer (CRC) is the third most common cause of death from cancer worldwide, and the fourth leading cause of cancer mortality in China [1]. The incidence of CRC has generally declined in several developed countries recently [2], which may be primarily attributed to the widespread implementation of early screening [3]. However, CRC incidence in individuals younger than 50 years keeps dramatically increasing, and so does mortality [2, 4, 5]. In addition, the available evidence shows that the clinical characteristics of younger CRC patients is different from the old ones, presenting more aggressive features, rectum and late-stage CRC in younger patients. Compared with the older CRC patients, better or poorer survival for those patients aged < 50 years have both been reported so far.
Adjuvant chemotherapy is essential for improving the survival of CRC patients, especially for stage III or IV cancer [6, 7]. However, for stage II colon cancer, routine adjuvant chemotherapy is currently not the standard of care. According to the current guideline, whether these patients should receive adjuvant chemotherapy is mainly determined by a series of risk factors, including the lymph node yield during surgery (< 12), poor prognostic features, for example poorly differentiated histology (exclusive of those that are MSI-H), lymphatic or vascular invasion, bowel obstruction and a close, indeterminate, or positive margin [8]. The survival benefit from chemotherapy has been reported for stage II CRC patients with risk factors, but not determined in those aged < 50 years. In addition, studies have shown that compared to old CRC patients, young patients are more likely to receive aggressive postoperative systemic chemotherapy regardless of TNM stage, but the survival benefit is controversial [9,10,11,12] and treatment options for these patients remain unclear [13].
Despite the increased number of younger CRC patients, the majority of the cases are still among the elderly [14]. These old patients tend to not receive adjuvant therapy due to their less tolerance to chemotherapy and less life expectancy [15, 16]. As global life expectancy increases, the proportion of patients aged 70 or older increases and they may become more tolerant than before. However, according to the guideline, adjuvant chemotherapy is only recommended for those stage II colon cancer patients who have risk factors and aged under 70 years of age [8]. Whether adjuvant chemotherapy benefits patients aged 70 years or older with risk factors remains unknown.
To fill the evidence gap, we used the data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) in 18 registries to investigate the association of adjuvant chemotherapy with overall and CRC-specific survival in stage Ii early-onset colon cancer (< 50 years old) without risk factors and the elder (70 to 85 years old) with risk factors. The objective of this study was to figure out whether it is necessary to use postoperative chemotherapy which was not suggested by guidelines in these two age groups.
2 Method
2.1 Data source and patient selection
Data on colon cancer patients were obtained from the SEER database, which recorded and reported cancer incidence and survival by collecting data from 18 cancer registries.
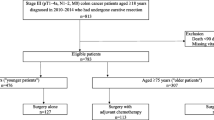
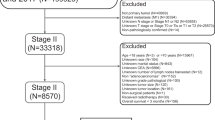
Eligible colon cancer patients between 2004 and 2015 were included according to the following criteria: 1) age at diagnosis ranged from18 to 85 years; 2) underwent surgery with a known number of retrieved lymph nodes; 3) histologically confirmed primary colon cancer; 4) TNM stage II; 5) had an active follow-up. Patients were excluded if they had prior primary tumors or multiple primary colon tumors. Patient demographics, tumor features, treatment regimens, and patient survival of the study cohort were retrospectively retrieved by using the SEER*Stat software. Patients with colon cancer diagnosed at less than 50 years was defined as early-onset colon cancer (EOCC). Late-onset colon cancer (LOCC) is defined as patients with colon cancer diagnosed at 70 to 85 years. Patients of each age category were divided into 4 groups based on the risk factors. Those aged < 50 years old without risk factors were defined as non-high-risk early-onset colon cancer (non-HREOCC), and patients aged 70 to 85 years with risk factors were defined as high-risk late-onset colon cancer (HRLOCC).
Since the SEER data was de-identified, Institutional Review Board approval and informed consents by the study subjects were not required.
2.2 Propensity score matching (PSM)
In this retrospective study, patients were not randomly distributed between the chemotherapy group and the non-chemotherapy group in the elder cohort, which could cause selection bias due to the potential imbalance of baseline covariates. To adjust the potential imbalance between the two groups, we used PSM to create a matched study cohort. We first calculated the propensity scores of every patient by using chemotherapy receipt (received or not received adjuvant chemotherapy) as the dependent variable and using all potential confounding factors as covariates in a multivariable logistic regression model. Second, we matched the chemotherapy group with the non-chemotherapy group utilizing the nearest-neighbor algorithm with a caliper width of 0.02 at a 1:2 ratio. Third, we calculated the standardized mean difference (SMD) of every covariate in unmatched and matched cohorts, and SMD < 0.1 was suggestive of balanced covariates between the two groups.
2.3 Statistical analysis
We first compared the receipt rate of adjuvant chemotherapy by age category using the Chi-square test. The distribution of race, gender, tumor characteristics, and risk factors for adjuvant chemotherapy were compared among chemotherapy and non-chemotherapy groups by Chi-square test or Fisher’s exact test. The primary endpoints were OS and CSS in our study and were assessed using KM plots with log-rank test. Univariate and multivariate Cox models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) to assess the association of chemotherapy with CSS. Age, race, gender, histology type and tumor location were included as covariates for adjustment in the model. For statistical analysis, R software for Windows version 4.2.1 was used. A two-sided P value < 0.05 indicated statistical significance.
3 Results
3.1 Patients characteristics of stage colon cancer in different age groups
Of 55,366 individuals who met the inclusion and exclusion criteria (Fig. 1), 9.7% were early-onset colon patients, 43.7% were 50–69 years old, and 46.6% were diagnosed at an age over 70. Table 1 showed that in stage II colon cancer patients, clinicopathological characteristics varied among different age categories for stage II colon cancer patients. Early-onset patients were more likely to have a left-sided tumor (54.2% vs 49.0% vs 36.3%, p < 0.001). In the meantime, early-onset cancers were more likely to be mucinous adenocarcinoma (10.0% vs 8.48% vs 9.64%, p < 0.001), grade IV(2.17% vs 1.58% vs 1.93%, p < 0.001), and T4 stage (16.8% vs 14.7% vs 13.8%, p < 0.001). As for chemotherapy, young people were more likely to receive adjuvant chemotherapy compared to people 50 years or older, and the chemotherapy receipt rate declined dramatically as age increased (44.6% vs 28.1% vs 9.96%, p < 0.001).
3.2 Clinicopathological features of stage II early-onset colon cancer without risk factors
A total of 3341 patients were included in the non-HREOCC group. Among these patients, 2082(66.28%) didn’t receive adjuvant chemotherapy. The clinicopathological features between patients who didn’t receive chemotherapy and those who did are shown in Table 2. Patients who received adjuvant chemotherapy had a slightly younger median age (44 vs 45, p < 0.001). Patients with white race (76.1% vs 71.8%, p = 0.023) or a left-sided tumor (59.8% vs 49.5%, p < 0.001) were more likely to undergo adjuvant chemotherapy. No significant differences were observed for gender, histology type, and differentiation grade between the two groups.
3.3 The association of chemotherapy with survival outcomes in early-onset colon cancer without risk factors
The OS and CSS for patients who received adjuvant chemotherapy and who didn’t receive adjuvant chemotherapy in non-HREOCC cohort are presented in Fig. 2. Patients who received chemotherapy had a 5-year and 10-year OS rate of 93.57% and 91.50%, respectively. For patients who did not receive chemotherapy, the 5-year and 10-year OS rate were 93.73% and 91.07%. No significant differences were observed in both OS and CSS between the two groups.
Comparison of OS and CSS between patients who received adjuvant chemotherapy and who didn’t receive adjuvant chemotherapy in non-HREOCC cohort and matched HRLOCC cohort. a OS between patients who received adjuvant chemotherapy and who didn’t receive adjuvant chemotherapy in non-HREOCC cohort; b CSS between patients who received adjuvant chemotherapy and who didn’t receive adjuvant chemotherapy in non-HREOCC cohort; c OS between patients who received adjuvant chemotherapy and who didn’t receive adjuvant chemotherapy in matched HRLOCC cohort; d CSS between patients who received adjuvant chemotherapy and who didn’t receive adjuvant chemotherapy in matched HRLOCC cohort
In both univariate Cox analysis (Fig. 3a) and multivariate Cox analysis (Fig. 3b), we found that there was no significant association between chemotherapy and CSS in non-HREOCC patients (HR = 1.05, CI95% 0.79–1.38 in univariate analysis; HR = 1.09, CI95% 0.83–1.44 in multivariate analysis). Meanwhile, an older age (HR = 1.031, CI95% 1.004–1.06, p = 0.02), the black race (HR = 2.018, CI95% 1.462–2.79, p < 0.001) and a left-sided tumor (HR = 1.359, CI95% 1.023–1.81, p = 0.03) were independently and negatively related to CSS.
3.4 Propensity score matching and clinicopathological characteristics of late-onset colon cancer with risk factors
Only 16.8% of HRLOCC patients received chemotherapy (1685 in 10,037). The clinicopathological features of HRLOCC patients in chemotherapy and non-chemotherapy groups are presented in Table 3. In the unmatched cohort, patients who received chemotherapy were more likely to be male (49.4% vs 43.9%, p < 0.001) and had a generally younger age (median age 75 vs 78, p < 0.001), a left-sided tumor (50.3% vs 37.0%, p < 0.001), an adequate lymph node yield during surgery (52.4% vs 45.1%, p < 0.001) and a higher T stage (47.7% vs 26.5%, p < 0.001). Patients who received adjuvant chemotherapy had a higher proportion of differentiation grade 2 (59.5% vs 55.2%) and a lower proportion of differentiation grade 3 (30.0% vs 35.0%). No significant difference for the distribution of histology type in the two groups was observed.
In consideration of the potential effects of confounders on the survival outcomes, we matched 1666 patients in the chemotherapy group with 3202 patients in the non-chemotherapy group using PSM at a ratio of 1:2. Calculation of SMD revealed balanced covariates after matching except for race (SMD = 0.095) (Table 3).
3.5 The association of chemotherapy with survival outcomes in late-onset colon cancer with risk factors
The 5- and 10-year OS rates were 68.4% and 55.7% in the chemotherapy group, and 58.3% and 44.0% in the non-chemotherapy group in unmatched cohort. Multivariate Cox analysis shows that chemotherapy had a positive correlation with CSS (HR = 0.90, CI95% 0.81–0.99, p = 0.04) (Fig. 3c). In the matched cohort, the KM curves show that patients who received chemotherapy had a better OS. But there were no significant differences in CSS between the chemotherapy group and the non-chemotherapy group (Fig. 2c, 2d). Multivariate Cox analysis in the matched cohort also indicates that chemotherapy was positively associated with CSS (HR = 0.88, CI95% 0.79–0.99, p = 0.03) (Fig. 3d). Similar to non-HREOCC cohort, age, race and tumor location had shown strong correlation with survival, as well as tumor grade, T stage and number of lymph node examined (Fig. 3d).
4 Discussion
Young patients usually are given intensive adjuvant chemotherapy treatment, while those aged 70 years or more are recommended no chemotherapy treatment. This study examined whether younger and old-aged patients with stage II colon cancer was overtreated or undertreated. We found adjuvant chemotherapy use did not significantly improve CSS in young stage II colon cancer patients without risk factors after adjusting potential confounding factors, while improved CSS in patients aged 70 to 85 years with risk factors. To some extent, these findings provided evidence for supporting and improving the current clinical guidelines.
In our study, we found that early-onset colorectal patients were nearly 2 times more likely to receive adjuvant chemotherapy after surgery compared to patients aged 50 and older, regardless of tumor stage at diagnosis. Unfortunately, the survival benefit for young patients was not correspondingly improved with this higher use of adjuvant chemotherapy. Manjelievskaia et al. evaluated data from the Central Cancer Registry of the United States Department of Defense and found that chemotherapy might be overused in young (18 to 49 years) with colon cancer but with no matched survival improvement [10]. However, the results were inconsistent with Burnett-Hartman’s study, which indicated that EOCRC patients were associated with more systemic therapy use and tended to have better survival than older patients [12]. The inconsistence findings might be related to an unbalanced distribution of series of factors between the chemotherapy and non-chemotherapy patients. According to the NCCN guideline, adjuvant chemotherapy for stage II colon cancer was only recommended to those with risk factors [e.g. the lymph node yield during surgery less than 12, poorly differentiated histology (exclusive of those that are MSI-H), lymphatic or vascular invasion, bowel obstruction and a close, indeterminate, or positive margin]. In our study, we focused on stage II colon cancer patients aged < 50 years with no risk factors. A high use of adjuvant chemotherapy was also demonstrated among these patients, and no survival benefit from chemotherapy for them was observed in our study, suggesting that an overuse of adjuvant chemotherapy existed in young stage II colon cancer patients with low risk. Furthermore, this finding provided clinical and economical important. Chemotherapy was inevitably related to the adverse effects, such as neurological, sexual or fertility functions, which were very important or young people and might seriously affected their quality of life. In addition, the cost of chemotherapy for colon cancer was quite burden. Therefore, appropriate use of chemotherapy is essential for both individual patient and society.
Clinicopathological features varied between the chemotherapy group and the non-chemotherapy group. People who received adjuvant chemotherapy were younger, more likely to be white people, and more likely to have a left-sided tumor since study has found that stage II left-sided colon cancer exhibited a poorer OS than right-sided colon cancer [17]. It’s interesting that consistent with other studies, White patients were more likely to receive chemotherapy, which may result from different economic level and insurance status [18, 19]. And researchers also found that White patients may achieve better improvement [18] from chemotherapy than Black patients, have an impact on doctors’ treatment strategy.
According to the clinical guidelines, adjuvant chemotherapy were currently not recommended to patients aged 70 years or more because of the limited evidence. Previous study has demonstrated that compared to patients under 70, older patients with stage II or III colon cancer gained equally prolonged DFS from single-agent fluopyrimidine [20], leading us to rethink the survival benefits of chemotherapy for elderly patients. In this study, we focused on old-aged stage II colon cancer patients with risk factors for adjuvant chemotherapy. Since patients aged more than 85 years tended not to receive chemotherapy, we only included stage II colon cancer patients aged 70 to 85 years and 16.8% of these patients received adjuvant chemotherapy. Patients received adjuvant chemotherapy were more likely to be male, relatively younger, more likely to have a left-sided tumor, an adequate lymph node yield during surgery, and a higher T stage. Interestingly, an adequate lymph node yield was associated with chemotherapy receipt, while it was a protective factor in multivariate Cox analysis and it is the insufficient lymph node detection that makes up the risk factors for adjuvant chemotherapy [21]. One reasonable explanation is that relatively young and fit patients are more tolerant to both surgery and chemotherapy, and therefore, may be treated more aggressively with excessive resection extent and extra adjuvant chemotherapy.
In our study, patients who received chemotherapy exhibited a better OS and similar CSS compared to patients who didn’t receive chemotherapy. One possible reason for the better OS was that patients who received chemotherapy were relatively in better health, maybe with fewer chronic diseases, such as hypertension and diabetes mellitus, which were associated with loss of years of life. For future studies, it’s necessary to include information of previous medical history as confounding factors to analyse the correlation between chemotherapy and survival.
Cox analysis in both unmatched and matched cohort indicated that chemotherapy had a positive effect on CSS for HRLOCC patients. This finding implies that for high risk stage II colon cancer patients aged 70–85, adjuvant chemotherapy can still prolong their survival. The results consistent with Kim’s study conducted in Korean population [22]. He found that adjuvant chemotherapy an independent prognostic factor for better survival in both the low-risk and high-risk patients over 70 years old. Liu et al. conducted a study in 80-year-old or older CRC patients based on the SEER database and came to the conclusion that chemotherapy could offer survival benefit for very old patients diagnosed with CRC [23]. However, there were some previous studies showing the opposite results. One study, which included patients underwent surgery of stage II primary colon cancer at Seoul National University Hospital from 2002 to 2015, pointed out that adjuvant chemotherapy does not appear to yield survival benefits in elderly patients (aged 70 to 93) with stage II colon cancer [24]. However, in real-life clinical practice, adjuvant chemotherapy is rarely given to patients over 80 years. Moreover, patients older than 90 tend to have more competing issues that might affect their life expectancy and the decline of organ functions makes them more vulnerable to the adverse effects of chemotherapy. Therefore, we consider it more reasonable to limit patient age under 85 years old and the results may be more accurate. In a systematic review, Meyers reported that receiving an oxaliplatin plus fluoropyrimidino regimen showed no benefit for patients older than 70 years in clinical trial MOSAIC, NSABP C-07 and XELOXA [25], which indicated that the choice of chemotherapy regimen was also essential for improving survival. Elderly patients with stage II disease should be encouraged to participate in randomized trials to further investigate the best treatment strategy for them.
To our knowledge, this was the first study to assess the survival benefit of adjuvant chemotherapy for non-high-risk young and old-aged patients with stage II colon cancer who are in controversial regarding the use of chemotherapy. Moreover, the large sample size, detailed follow-up information, and use of PSM also made the results more robust. However, several limitations should be noted. First, since the data was extracted from the SEER database, the study population restricted to patients from US. Further studies were warranted to verify this finding in Asian or Chinese population. Second, although we adjusted several covariates, other potential confounding factors related to CRC prognosis might exist, such as microsatellite instability (MSI) status and symptoms. Third, this was a retrospective study and recalling bias might be inevitable.
5 Conclusion
For stage II colon cancer patients, people under 50 years old were more likely to receive adjuvant chemotherapy. The additional use of postoperative chemotherapy did not result in matched survival improvement in non-HREOCC cohort. However, regarding to HRLOCC patients, adjuvant chemotherapy can prolong patients’ survival. Elderly patients should not be excluded from receiving adjuvant chemotherapy on age alone.
Availability of data and materials
The datasets generated during and/or analysed during the current study are available in the SEER repository, https://seer.cancer.gov/data-software/.
Abbreviations
- non-HREOCC:
-
Non-high-risk early-onset colon cancer
- HRLOCC:
-
High-risk late-onset colon cancer
- KM:
-
Kaplan–Meier
- OS:
-
Overall survival
- CSS:
-
Cancer-specific survival
- CRC:
-
Colorectal cancer
- SEER:
-
The National Cancer Institute’s Surveillance, Epidemiology, and End Results
- EOCC:
-
Early-onset colon cancer
- LOCC:
-
Late-onset colon cancer
- PSM:
-
Propensity score matching
- SMD:
-
Standardized mean difference
- HR:
-
Hazard ratio
- CI:
-
Confidence intervals
References
Zheng R, Zhang S, Zeng H, et al. Cancer incidence and mortality in China, 2016. Journal of the National Cancer Center. 2022;2(1):1–9.https://doi.org/10.1016/j.jncc.2022.02.002.
Sinicrope FA. Increasing Incidence of Early-Onset Colorectal Cancer. N Engl J Med. 2022;386(16):1547–58. https://doi.org/10.1056/NEJMra2200869.
Cardoso R, Guo F, Heisser T, et al. Colorectal cancer incidence, mortality, and stage distribution in European countries in the colorectal cancer screening era: an international population-based study. Lancet Oncol. 2021;22(7):1002–13. https://doi.org/10.1016/s1470-2045(21)00199-6.
Sung JJY, Chiu HM, Jung KW, et al. Increasing Trend in Young-Onset Colorectal Cancer in Asia: More Cancers in Men and More Rectal Cancers. Am J Gastroenterol. 2019;114(2):322–329.https://doi.org/10.14309/ajg.0000000000000133.
Lui RN, Tsoi KK, Ho JM, et al. Global Increasing Incidence of Young-Onset Colorectal Cancer Across 5 Continents: A Joinpoint Regression Analysis of 1,922,167 Cases. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2019.https://doi.org/10.1158/1055-9965.EPI-18-1111.
André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350(23):2343–51. https://doi.org/10.1056/NEJMoa032709.
Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med. 2005;352(5):476–87. https://doi.org/10.1056/NEJMra040958.
Benson AB, Venook AP, Al-Hawary MM, et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network : JNCCN. 2021;19(3):329–359.https://doi.org/10.6004/jnccn.2021.0012.
Cercek A, Chatila WK, Yaeger R, et al. A Comprehensive Comparison of Early-Onset and Average-Onset Colorectal Cancers. J Natl Cancer Inst. 2021;113(12):1683–92. https://doi.org/10.1093/jnci/djab124.
Manjelievskaia J, Brown D, McGlynn KA, Anderson W, Shriver CD, Zhu K. Chemotherapy Use and Survival Among Young and Middle-Aged Patients With Colon Cancer. JAMA Surg. 2017;152(5):452–9. https://doi.org/10.1001/jamasurg.2016.5050.
Patel SG, Ahnen DJ. Colorectal Cancer in the Young. Curr Gastroenterol Rep. 2018;20(4):15. https://doi.org/10.1007/s11894-018-0618-9.
Burnett-Hartman AN, Powers JD, Chubak J, et al. Treatment patterns and survival differ between early-onset and late-onset colorectal cancer patients: the patient outcomes to advance learning network. Cancer causes & control : CCC. 2019;30(7):747–55. https://doi.org/10.1007/s10552-019-01181-3.
Kneuertz PJ, Chang GJ, Hu CY, et al. Overtreatment of young adults with colon cancer: more intense treatments with unmatched survival gains. JAMA Surg. 2015;150(5):402–9. https://doi.org/10.1001/jamasurg.2014.3572.
Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA: a cancer journal for clinicians. 2020;70(3):145–164.https://doi.org/10.3322/caac.21601.
Abrams TA, Brightly R, Mao J, et al. Patterns of adjuvant chemotherapy use in a population-based cohort of patients with resected stage II or III colon cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(24):3255–62. https://doi.org/10.1200/jco.2011.35.0058.
Potosky AL, Harlan LC, Kaplan RS, Johnson KA, Lynch CF. Age, sex, and racial differences in the use of standard adjuvant therapy for colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20(5):1192–202. https://doi.org/10.1200/jco.2002.20.5.1192.
Meguid RA, Slidell MB, Wolfgang CL, Chang DC, Ahuja N. Is there a difference in survival between right- versus left-sided colon cancers? Ann Surg Oncol. 2008;15(9):2388–94. https://doi.org/10.1245/s10434-008-0015-y.
Mitsakos AT, Irish W, Parikh AA, Snyder RA. The association of health insurance and race with treatment and survival in patients with metastatic colorectal cancer. Plos One. 2022;17(2):e0263818.https://doi.org/10.1371/journal.pone.0263818.
Knisely AT, Michaels AD, Mehaffey JH, et al. Race is associated with completion of neoadjuvant chemotherapy for breast cancer. Surgery. 2018;164(2):195–200. https://doi.org/10.1016/j.surg.2018.03.011.
Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345(15):1091–7. https://doi.org/10.1056/NEJMoa010957.
Benson AB 3rd, Schrag D, Somerfield MR, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22(16):3408–19. https://doi.org/10.1200/jco.2004.05.063.
Kim MK, Won DD, Park SM, et al. Effect of Adjuvant Chemotherapy on Stage II Colon Cancer: Analysis of Korean National Data. Cancer Res Treat. 2018;50(4):1149–63. https://doi.org/10.4143/crt.2017.194.
Liu W, Zhang M, Wu J, Tang R, Hu L. Oncologic Outcome and Efficacy of Chemotherapy in Colorectal Cancer Patients Aged 80 Years or Older. Frontiers in medicine. 2020;7:525421.https://doi.org/10.3389/fmed.2020.525421.
Lee KY, Park JW, Lee KY, et al. Adjuvant chemotherapy does not provide survival benefits to elderly patients with stage II colon cancer. Sci Rep. 2019;9(1):11846. https://doi.org/10.1038/s41598-019-48197-y.
Meyers BM, Cosby R, Quereshy F, Jonker D. Adjuvant Chemotherapy for Stage II and III Colon Cancer Following Complete Resection: A Cancer Care Ontario Systematic Review. Clin Oncol (R Coll Radiol). 2017;29(7):459–65. https://doi.org/10.1016/j.clon.2017.03.001.
Acknowledgements
Not applicable.
Funding
The Fundamental Research Funds for the Central Universities (No. 226–2022-00009); the Project of the regional diagnosis and treatment center of the Health Planning Committee (No. JBZX-201903).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Conception of the work: KFD, QX, LJW; Methodology: TJ, WL, YSZ, CQL; Investigation, TJ, YSZ, WL; Writing – Original Draft: TJ; Writing – Review & Editing: TJ, YSZ, LJW; Funding Acquisition: KFD; Supervision: KFD. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Consent for publication bas been obtained from all authors of this manuscript.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jin, T., Zhu, Y., Lu, W. et al. Postoperative chemotherapy use and survival in non-high-risk young and high-risk old-aged patients with stage II colon cancer. Holist Integ Oncol 2, 6 (2023). https://doi.org/10.1007/s44178-023-00027-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44178-023-00027-y