Abstract
Gynecologic cancer is a critical concern in the field of women’s health, and traditional treatment methods have demonstrated limited efficacy for certain advanced and recurrent gynecologic cancers. In recent years, antibody–drug conjugate (ADC) therapy, as an emerging targeted approach, has gained increasing attention as a research hotspot. This review aims to elucidate the structure and mechanism of ADC drugs and explore their application and clinical research progress in gynecologic cancers such as ovarian cancer, cervical cancer, and endometrial cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Gynecologic cancer poses a significant threat to women’s health, with its global prevalence on the rise. While traditional treatments like surgery, radiation, and chemotherapy have shown considerable progress, their effectiveness in addressing recurrent or advanced gynecological cancers remains constrained. Therefore, there is an urgent demand for innovative therapeutic approaches.
Recently, antibody–drug conjugates (ADCs) have garnered significant attention as an emerging strategy for anti-tumor therapy. An ADC comprises a monoclonal antibody that targets tumor-specific or tumor-associated antigens, along with multiple molecules of cytotoxic drugs connected by linkers [1]. ADC drugs offer a dual advantage: firstly, the monoclonal antibody provides robust targeting capabilities, enabling precise localization to tumor cells. Secondly, the release of the cytotoxic drug within cancer cells leads to a highly localized and potent cytotoxic effect. In comparison to conventional chemotherapeutic drugs, ADCs demonstrate superior performance in minimizing damage to normal cells.
The utilization of ADCs in the realm of gynecological oncology displays promising potential. Studies on gynecological malignancies such as ovarian, cervical, and endometrial cancers indicate that ADCs offer notable advantages in the therapeutic management of recurrent or metastatic cancers. This review aims to delineate the current applications of ADC drugs in gynecological cancers and provide an overview of the current state of clinical research in this area.
2 Structure and mechanism of action of ADCs
The concept of ADC originated from the “magic bullet” theory proposed by Paul Ehrlich, widely recognized as the “Father of Immunology” [2]. ADCs can be divided into three main components: (1) a highly specific antibody targeting cancer-associated antigens; (2) a cytotoxic payload capable of inducing cell death upon internalization into target cells; (3) a linker designed to stabilize the cytotoxic drugs during transportation and facilitate their release within tumor cells [3, 4] (Fig. 1).
2.1 Antibody
The crucial component of ADC is the antibody, which should possess the following properties: Firstly, targeting, allowing the delivery of the toxic drug into the target cells through the antibody [5]. The second factor is binding affinity, where a strong affinity between the antibody and antigen facilitates the efficient endocytosis of ADCs [6]. Additionally, the antibody should exhibit low immunogenicity and minimal cross-reactivity [7].
Early ADCs utilized mouse antibodies. To overcome the limitations of mouse antibodies, including strong immunogenicity, low affinity for binding to human antigens, and short half-life, human-mouse chimeric antibodies were introduced [8]. However, treatment with chimeric antibodies can still elicit an immune response, with anti-drug antibodies primarily targeting the murine Fab region of the antibody [9]. Subsequently, humanized antibodies were developed [10], and out of the 15 ADCs that have received marketing approval so far, 13 employ humanized antibodies. It is worth noting that research has started on creating ADCs utilizing completely human antibodies [11].
2.2 Cytotoxic payloads
The antitumor effect of ADCs is significantly dependent on cytotoxic drugs, which are required to have the following properties: (1) high cytotoxicity to kill tumor cells; (2) modifiability to enable successful coupling to the antibody part [12]; (3) high stability; (4) strong hydrophobicity and membrane permeability to facilitate easier passage through biomembranes and entry into cancer cells [13].
The most widely used cytotoxic drugs currently can be divided into two categories: microtubule inhibitors and DNA-damaging drugs. Microtubule inhibitors act by inhibiting microtubule proteins, disrupting microtubules, and hindering the G2/M phase of the cell cycle, leading to cell death. Notably, microtubule inhibitors specifically target actively growing cells [14]. While the latter doesn’t depend on the cell cycle. In addition to this, RNA polymerase inhibitors and other drugs are currently used by next-generation ADCs as cytotoxic molecules [15].
2.3 Linkers
The effective fusion of the cytotoxic molecule and the antibody is dependent on the linkers in ADCs. To guarantee the security of ADC, it needs to maintain a steady state during transportation to avoid the premature release of toxic molecules. However, to achieve therapeutic benefits, it is essential for ADCs to effectively release toxic molecules into tumor tissues or cells when required [16].
Linkers can be classified into two categories according to the mechanism of action: cleavable and non-cleavable. The former type can be cleaved to release toxic molecules according to the difference between the tumor microenvironment and the normal physiological environment, and can be further subdivided into acid-sensitive, protease-sensitive and glutathione-sensitive, etc. [17]. Some cleavable linkers also have the ability to transport drugs extracellularly, causing a bystander effect that kills nearby tumor cells that don’t express the target antigen [18]. The latter mechanism of action is that non-cleavable linkers and toxin molecules are released together, after the lysosomal degradation of the antibody component of the ADC [19].
2.4 Mechanism of action of ADC
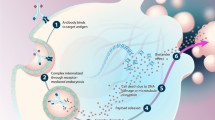
Firstly, the ADC-antigen complex is internalized into the cell through receptor-mediated endocytosis after the antibody recognizes and attaches to its target [20]. A portion of the drugs exits the cell again after binding to neonatal Fc receptors (FcRns) in endosomes. Extracellular physiological pH 7.4 facilitates the release of the ADC from FcRn [21], and the recycling mechanism serves as a buffer to avoid normal cell death in case of misdelivery. Then, ADCs remained in early endosomes are translocated into late endosomes. Finally, toxin molecules are released from endosomes (cleavable linkers) or after fusion with lysosomes (non-cleavable linkers) through acidic conditions or hydrolytic enzymes [22, 23]. These toxin molecules further induce apoptosis, thereby killing the tumor cells (Fig. 2).
3 ADCs in gynecologic cancer
Chemotherapy and radiotherapy exhibit limited efficacy in recurrent or metastatic gynecological malignancies, with an objective response rate (ORR) of only 20–30% [24,25,26]. In recent years, the emergence of targeted therapy has brought a ray of hope for these patients. ADC, as a novel form of targeted therapy, has drawn increasing interest, and many basic and clinical studies on ADCs for gynecological tumors are actively underway both domestically and internationally (Table 1).
3.1 ADCs targeting FRα
Alpha-folate receptor (FRα) transports folate via receptor-mediated endocytosis. The distribution of FRα in normal tissues is constrained, and its minimal expression can be seen in a few polarized epithelia [27]. Whereas in epithelial ovarian cancer (EOC), more than 80% of tumors exhibit high FRα expression, and it has been suggested that its high expression may be a predictor of poor efficacy of chemotherapy [28]. Additionally, it has a high level of expression in endometrioid carcinoma [29]. Thus, this difference in expression levels makes targeted therapy against FRα a viable option.
3.1.1 IMGN853
IMGN853(also known as Mirvetuximab soravtansine) consists of a FRα-binding antibody combined with a tubulin-disrupting drug, maytansinoid DM4 [30]. In preclinical studies, IMGN853 showed excellent anti-tumor activity in FRα-positive tumors such as the EOC, uterine-serous-carcinoma (USC) and their xenograft models [29, 30].
A Phase I study enrolled 44 individuals who have solid tumours, including ovarian (23), endometrial (11), cervical (1), renal (5) and non-squamous cell lung (4) cancers. The most common adverse events (AEs) reported were fatigue (43%), blurred vision and keratopathy (29%), and treatment-related serious adverse events (TRSAEs) were grade 3 hypophosphatemia and grade 3 punctate keratitis. Among the 43 evaluable patients, 2 EOC patients had partial response (PR) with an ORR of 4.7%. CA125 response was seen in 4 EOC and 1 endometrial carcinoma, while twenty-two individuals showed stable disease (SD) [31].
In the Phase III monotherapy study FORWARD I (NCT02631876), 336 patients, including those with platinum-resistant and FRα-positive advanced EOC, were randomized in a 2:1 ratio to receive either IMGN853 or traditional chemotherapy. The ORRs for IMGN853 and chemotherapy were 22% and 12%, respectively, and the results for progression-free survival (PFS) and overall survival (OS) were similar. Although the primary endpoint was not met, IMGN853 demonstrated better clinical activity and fewer side effects compared to chemotherapy [32].
The FDA decided to approve IMGN853 for the therapy of recurrent ovarian carcinoma with high FRα expression as well as platinum resistance according to the outcome of the trial SORAYA (NCT04296890). Among the 106 patients, roughly 32% of the participants presented an objective response and the median duration of response (DoR) was nearly 7 months. TRAEs caused 32% of patients to delay dosing, 19% of patients to reduce their doses, and 7% of patients to stop taking this formulation [33].
MIRASOL (NCT04209855) was a phase 3 clinical study which compared IMGN853 with traditional chemotherapy. The study included 453 patients, of whom 14% had been given first-line treatment, 39% had received second-line treatment, and 47% had received third-line treatment [34]. According to the latest data from the MIRASOL trial, comparing to the chemotherapy group, OS was extended from 12.75 months to 16.46 months, representing a 33% reduction in mortality risk. PFS increased from 3.98 months to 5.62 months, and the risk of disease progression or mortality decreased by 35%. The ORR improved from 15.9% to 42.3%, with a safety profile superior to that of chemotherapy. Based on these data, IMGN853 is expected to progress from accelerated to full approval.
In addition, GLORIOSA (NCT05445778), a Phase III randomized trial, is being conducted to evaluate the effectiveness of bevacizumab ± IMGN853 as maintenance therapy in patients with high FRα and platinum-sensitive ovarian carcinoma who have received second-line chemotherapy with platinum + bevacizumab.
3.1.2 STRO-002
The drug includes a FRα-targeting antibody SP8166, a Hemiasterlin derivative and a cleavable linker [35]. Its payload can lead to cell death by inhibiting microtubule proteins or triggering immunogenic responses. In mice models of ovarian and endometrial cancer, the formulation effectively targeted and inhibited tumor cells, indicating that it may be a potentia therapeutic alternative for both tumor types [35].
A Phase I dose-escalation trial of STRO-002 (NCT03748186) included 39 platinum- resistant patients (FRα expression levels were not required). The results showed that the ORR was nearly 31%,and the median PFS was over 7 months [36]. Regarding side effects, STRO-002 behaves differently from many other drugs, because no ocular toxicity was observed [37].
In light of these findings, a new phase II/III trial named REFRaME has been initiated. In addition, STRO-002-GM2 (NCT05200364), a Phase I multicenter dose-escalation study, is also underway to explore STRO-002 + bevacizumab in patients with refractory or relapsed advanced ovarian cancer after standard treatment.
However, it’s important to note that in comparison, STRO-002 did not demonstrate superior efficacy and safety when compared to IMGN853. Specifically, the occurrence of neutropenia, affecting approximately 70% of patients, raises safety concerns for STRO-002 compared to IMGN853.
3.1.3 MORAb-202
This formulation is composed of an FRα-binding antibody, farletuzumab, coupled to the anticancer drug eribulin via a cleavable linker [38]. 22 patients with solid tumors (including ovarian cancer) expressing high levels of FRα, were enrolled in a phase I study (NCT03386942). A total of 95% of patients reported mild to moderate TRAEs, with leukopenia and neutropenia occurring in 10 (45%) of these patients. Additionally, severe serum alanine aminotransferase and gamma-glutamyltransferase elevations occurred in one patient. Regarding of effectiveness, 18 patients (81%) had positive response, including 1 complete response (CR) (4.5%), 9 PRs (40.5%) and 8 SD (36%) [39]. According to the results, a Phase I/II trial (NCT04300556) was also initiated to thoroughly investigate the effectiveness and security of this formulation among cancer patients, including endometrial cancer and ovarian cancer.
In terms of ORR statistics for the three medications when administered alone, MORAb-202 demonstrated the best performance. Additionally, it exhibited a lower incidence of AEs, suggesting that MORAb-202 holds promising potential for future research and development.
3.2 ADCs targeting HER-2
Human epidermal growth factor receptor 2 (HER-2) is a transmembrane protein with tyrosine kinase activity encoded by the HER-2/neu oncogene, capable of promoting cell proliferation and tumorigenesis [40]. It is frequently overexpressed across multiple kinds of cancers and related to poor prognosis, resistance to radiotherapy, hormone treatment, and chemotherapy, as well as an increased likelihood of metastasis and recurrence [41]. According to whole-exome sequencing and immunohistochemical data, HER-2 is highly expressed in more than 35% of patients with USC, highlighting HER-2 as a promising target in gynecological oncology therapy [42].
3.2.1 T-DM1
T-DM1(Ado-trastuzumab emtansine) that is composed of trastuzumab coupled to DM1, a sulfur-containing derivative of emtansine, via a non-cleavable linker, has been approved by the FDA for the therapy of breast cancer with high expression level of HER-2 [43]. A phase II clinical study (NCT02465060) that included 38 people who have HER-2 amplified ovarian or endometrial cancer, resulted in stable disease in 8/10 of 36 evaluable individuals, and the median DoR is 4.6 months [44]. In another trial (NCT02675829), which included 58 tumor patients (including ovarian and endometrial cancer), an ORR of 22% for endometrial cancers (4/18, 2 CR), and an ORR of 17% for ovarian cancers (1/6) were detected. In addition to this, ongoing studies are exploring the use of T-DM1 in combination with other drugs for recurrent endometrial and ovarian carcinoma [45].
3.2.2 BDC-1001
BDC-1001 consists of a trastuzumab analogue as the targeting component, coupled through a non-cleavable linker to a dual agonist of the Toll-like receptor (TLR) 7/8, which is crucial for innate immunity, acting as a link between specific and non-specific immune responses.
Data from a phase I/II study (NCT04278144) suggested that the most frequent TRAEs are weakness, infusion-related responses, nausea and gastrointestinal reaction. Approximately 42.1% patients experienced grade 3 or higher AEs, and 33.3% patients showed SAEs. Regarding efficacy aspects, only 13 patients (32.5%) had disease control among the 40 evaluable people, including 1 PR and 12 cases of SD [46, 47].
3.2.3 DS-8201a
DS-8201a (Trastuzumab Deruxtecan, trade name Enhertu®) is composed of an anti-HER-2 monoclonal antibody coupled via a protease-cleavable linker to Deruxtecan, a topoisomerase I inhibitor. FDA and EMA have approved it for the therapy of metastatic breast cancer that over expresses the HER-2 receptor [48, 49].
This drug exhibits a strong bystander impact due to the high membrane permeability of its payload in comparison to T-DM1. In a preclinical investigation, T-DM1 did not induce cell death in HER2-negative MDA-MB-468 cells or HER2-positive KPL-4 cells, while DS-8201a demonstrated efficacy against both cell types [50].
DS-8201a demonstrated an ORR of around 26% and a PFS of almost 1 year in a phase I research (NCT02564900) which recruited some cancer patients who develop metastasis at a late stage (involving endometrial carcinoma) [51]. However, these data were calculated for the full range of tumor types selected, and separate data specifically for endometrial cancer were not reported.
34 patients with uterine carcinosarcoma that are HER2-positive were enrolled in the STATICE phase II trial.22 of them had HER2 expression of 2 + or 3 + , and 10 had 1 + expression. Among those with 2 + or 3 + expression, the ORR was 55% (12/22), 10 had SD, none progressed, and the disease control rate (DCR) was 100%. Among HER2 1 + expressers, the ORR was 70% (7/10), with 3 cases of SD, none progressing, and DCR of 100% [52].
In addition to this, some studies are now being conducted to assess if the formulation is effective alone in treating endometrial and cervical carcinoma (NCT04482309) and together with Olaparib or Ceralasertib in treating endometrial carcinoma (NCT04585958, NCT04704661).
3.2.4 SYD985
SYD985 (Trastuzumab duocarmazine) is composed of trastuzumab coupled to duocarmazine via a cleavable linker [53]. This agent has shown excellent anti-cancer effectiveness in rat models of uterine plasma cancer and ovarian carcinoma [54, 55].
SYD985 was evaluated in a phase I/II trial (NCT02277717) involving a variety of malignancies that highly expresses HER-2. Regarding efficacy aspects, among the evaluable participants, 25 people (57%) showed shrinkage of the tumor, with a median PFS of 3–4 months in endometrial carcinoma patients [56].Except above, the effectiveness of SYD985 ± niraparib for those who have advanced or metastatic endometrial carcinoma are being researched in several further clinical trials.
Compared with T-DM1, SYD985 has been found to be significantly more cytotoxic to epithelial ovarian cancer cell lines both in vitro and in vivo. This difference in cytotoxicity may be associated with the presence of different payloads. In other words, the duocarmycin payload in SYD985 appears to be more toxic than the maytansin payload in T-DM1.
3.2.5 A166
A166 consists of trastuzumab coupled through a cleavable linker to a novel toxin molecule Duo-5 [57]. A phase I/II clinical trial (NCT03602079) is exploring the effects of A166 in those who have cancers with high HER-2 expression (including cervical cancer), with the early outcomes suggesting that approximately 60% of patients achieve SD and PR. However, these data are based on an overall calculation, and individual results for each cancer type have not yet been reported [58].
3.2.6 DB1303
DB1303 consists of a trastuzumab biosimilar coupled to a topoisomerase I inhibitor via a cleavable linker, and it has received Fast Track Designation from the FDA. It has demonstrated potential anticancer efficacy and safety in tumor models expressing HER-2, whether highly or poorly. A Phase I/II trial (NCT05150691) is underway to investigate the effectiveness of the drug in those who have advanced or unresectable, recurrent, or metastatic endometrial cancer.
3.2.7 DHES0815A
DHES0815A consists of a monoclonal antibody targeting HER-2, MHES0488A, bond to a novel DNA monoalkylation reagent, PBD-MA, by a stable disulfide. Notably, its binding site to the target antigen is different from that of other antibodies. Results from a preclinical study evaluating the effectiveness of this drug on USC and xenograft tumors showed that it exhibits notable antitumor cytotoxicity. In addition, DHES0815A was not significantly cytotoxic in USC lacking HER2 expression, which is in stark contrast to DS-8201a.Therefore, for HER2 -overexpressing USC or other cancer patients in whom trastuzumab and conventional chemotherapy have been ineffective, the use of DHES0815A, either alone or in combination with other HER-2 targeted drugs may be a new therapeutic choice [59].
3.3 ADCs targeting Mesothelin
Mesothelin is a glycoprotein that attaches to cell membranes via the GPI. Mesothelin exists in the upper vagina, uterus, and fallopian tubes under physiological conditions [60]. However, when malignant transformation occurs, mesothelin expression increases significantly, especially in pancreatic, mesothelioma, lung, ovarian, endometrial and cervical cancers [61]. It has been demonstrated that over 70% of ovarian cancers have elevated Mesothelin expression, which makes it a viable target for cancer treatment [62].
3.3.1 Anetumab ravtansine
Anetumab Ravtansine (BAY94-9343) consists of an anti-mesothelin monoclonal antibody coupled to DM-4 via a disulfide-containing linker [63]. Apart from its ability to kill mesothelin-positive tumor cells in vivo, it has also been demonstrated to have a bystander effect on nearby mesothelin-negative tumor cells [63].
A phase I clinical study (NCT01439152) enrolled 148 participants with highly expressed mesothelin cancers, including 21 ovarian cancers. In terms of side effects, some patients treated with the drug experienced TRAEs similar to other ADCs, such as fatigue and gastrointestinal reactions. Regarding the efficacy, the DCR was 57%, including 5% with PR and 52% with SD. The median DoR more than 2 months and the median PFS nearly 3 months [64].
Anetumab ravtansine together with pegylated liposomal doxorubicin in a phase Ib research for patients who have platinum-resistant ovarian cancer, fallopian tube cancer or primary peritoneal cancer, a DCR of 86% was observed in 21 evaluable patients. Among them, there were 11 cases of PR and 7 SD.Additionally, 6 patients had a DoR of more than 250 days [65].
3.3.2 DMOT4039A
DMOT4039A consists of an anti-mesothelin monoclonal antibody linked to an anti-microtubule drug MMAE through a dipeptide [66]. A phase I study (NCT01469793) included some patients with advanced pancreatic cancer or platinum-resistant ovarian carcinoma. According to their findings, among 31 ovarian cancer patients who highly express mesothelin, 3 of 10 patients were in PR, and 3 others showed CA125 response, with a median PFS of nearly 5 months. However, 20% of patients developed microtubule-inhibitor-specific toxicity resulting in peripheral neuropathy (grades 1–3) [67].
3.3.3 BMS-986148
BMS-986148 consists of a completely human IgG1 anti-mesothelin antibody coupled to a cytotoxic peptide, Tubulysin [68].The effectiveness and security of this drug in individuals with high MESOLINE expression, including ovarian carcinoma, were evaluated in a phase II study (NCT02341625). All treatment cohorts experienced hepatic TRAEs, with increased levels of aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase being the most frequent. Additionally, one patient with ovarian cancer died due to pneumonia associated with this ADC. Regarding efficacy, 9% of ovarian cancer patients achieved objective response, and the DCR was 13%, including 2 PR and 11 SD [69].
3.3.4 RC88
RC88 is composed of an anti-mesothelin antibody coupled through a cleavable linker to MMAE [70]. A multicenter, single-arm, Phase 2 study (NCT06173037) is evaluating the efficacy, safety, and pharmacokinetics of RC88 monotherapy in platinum-resistant recurrent epithelial ovarian, fallopian tube, and primary peritoneal cancer.
3.4 ADCs targeting NaPi2B
NaPi2B is a transmembrane protein which involves in the absorption of inorganic phosphate and the preservation of phosphate homeostasis [71]. It has been shown that NaPi2B is highly expressed in 2/3 of patients with high-grade plasmacytoid ovarian cancer, but not in normal tissues [72].
3.4.1 Upifitamab rilsodotin
Upifitamab rilsodotin (UpRi) is composed of a NaPi2B-targeting antibody coupled to an AF-HPA payload via a cleavable linker. It is worth mentioning that this formulation has payloads of more than 10 molecules thanks to dolaflexin technology [73].
UPLIFT (NCT03319628) is a phase II trial in platinum-resistant patients (including ovarian and fallopian tube cancers) who had been given 1–4 lines of prior therapy. According to the results, fatigue, nausea, vomiting, and fever were the most frequent TRAEs. Among 26 ovarian cancer patients who have high expression of NaPi2B, 39% had an objective response and 81% had disease control [74].
In addition, two clinical trials are ongoing. A Phase III research called UPNEXT (NCT05329545) is comparing the maintenance treatment of UpRi to placebo in recurring plasmacytoid ovarian cancer patients who are platinum-sensitive and have over expression of NaPi2B. First-line maintenance therapies for ovarian carcinoma include bevacizumab and PARP inhibitors, however there is no established therapy for individuals who recur after receiving these drugs. It is worth mentioning that the outcomes of UPNEXT trial promise to provide promising options. Another Phase I trial, UPGRADE-A (NCT04907968), is investigating UpRi in together with carboplatin for the therapy of patients who have metastatic or recurring ovarian carcinoma and is expected to provide an alternative platinum-based multidrug combination therapy.
3.4.2 Lifastuzumab vedotin
Lifastuzumab vedotin (DNIB0600A) consists of a humanised anti-NaPi2B monoclonal antibody coupled to an anti-mitotic cytotoxic payload MMAE. In a Phase Ia trial (NCT01363947) involving individuals with platinum-resistant ovarian cancer and other cancers, the safety and anti-cancer effectiveness of this agent were evaluated. Among the observed TRAEs, fatigue was reported in 52% of patients, nausea in 38%, decreased appetite in 33%, and peripheral neuropathy in 29%, with 16% discontinuing the study due to AEs. Regarding efficacy, although this drug’s activity in the lung cancer cohort was not particularly robust, 46% of patients in the ovarian cancer cohort achieved PR, suggesting that this formulation may be particularly effective for ovarian cancer [75].
In another study (NCT01995188), lifastuzumab vedotin in combination with bevacizumab and carboplatin displayed promising antitumor ability in ovarian cancer patients who are resistant to platinum. Approximately 60% of patients achieved CR or PR,with a median PFS of around 10 months. However, more than 80% of patients had TRAEs, emphasizing the necessity for further studies to confirm the therapeutic value of this multi-drug combination therapy [76].
3.5 ADCs targeting TF
Tissue factor (TF), also called coagulation factor III, serves as the primary activator of the exogenous coagulation pathway [77]. It has been demonstrated to promote tumour development by enhancing cell proliferation, angiogenesis, and epithelial-mesenchymal transition [78]. Notably, over 90% of cervical tumors exhibit elevated TF expression [79], making it a powerful target for the cancer therapy.
3.5.1 Tisotumab vedotin
This ADC consists of a humanized anti-TF IgG1 antibody coupled to MMAE via a valine-citrulline linker [80]. The FDA’s decision to grant accelerated approval for the drug in the treatment of recurring or metastatic cervical carcinoma with disease progression during or after traditional chemotherapy was in accordance with the outcomes of InnovaTV 204 (NCT03438396). In this trial, the ORR was 24%, with CR accounting for 7%, PR accounting for 17%, and a median DoR of 8.3 months [81]. It is noteworthy that ocular side effects happened in 53% of patients, so the FDA issued a warning about ocular toxicity at the same time as the approval of it.
Innova TV 205 is a global, randomized, multi-cohort Phase I/II study assessing the effectiveness of tisotumab vedotin in together with bevacizumab, pabolizumab or carboplatin. The ORR was 55% in 33 patients treated with TV + carboplatin and 35% in 35 patients treated with TV + pabrolizumab, which suggests potential for new combination therapies [82]. In addition to this, a phase III trial, Innova TV 301, evaluating the effectiveness of this ADC in those with recurring or metastatic cervical carcinoma whose disease has progressed after prior 1–2-line therapy in comparison to traditional chemotherapy is ongoing.
3.6 ADCs targeting TROP-2
Trophoblast cell surface antigen-2 (TROP-2) is a glycoprotein with high expression levels observed in many kinds of epithelial neoplasm, correlating with tumor aggressiveness, metastasis and poor prognosis [83, 84]. Unlike normal cells, tumor cells exhibit elevated levels of TROP-2, making it a favorable target for research.
3.6.1 Sacituzumab govitecan
Sacituzumab govitecan (IMMU-132) is an ADC targeting Trop-2 [85]. It was accepted for the therapy of metastatic breast cancer in 2020 and is being assessing its efficacy in endometrial carcinoma.
In a Phase II clinical study (NCT01631552), TRAEs occurred in 97.6% participants, with nearly 63% experiencing nausea, 58% neutropenia, and 56% gastrointestinal reactions. In terms of efficacy, 18 patients with endometrial carcinoma showed better response to the ADC compared with traditional chemotherapy, with an ORR of 22% and a median PFS of 3.2 months [86].
Notably, a case report showed that in a case of recurrent and extensively drug-resistant USC, a reduction in tumor volume of approximately 66% was detected, and such a high level of clinical remission suggests that the drug has greater therapeutic promise in endometrial cancer [87]. In addition, another Phase II study (NCT04251416) evaluating the effectiveness of this formulation in recurring endometrial carcinoma is underway.
3.6.2 SKB264
SKB264 consists of a humanized anti-TROP2 antibody coupled via 2-methylsulfonylpyrimidine to belotecan derivative, KL610023, a topoisomerase I inhibitor. It has been shown that SKB264 performs better than IMMU-132 in terms of targeting, anti-tumor activity and half-life, thus the drug has great research significance [88]. The first clinical study (NCT04152499) of SKB264 in those with unresectable or metastatic cancers, including ovarian cancer, is currently recruiting, and future data may better resolve questions about the drug’s efficacy and safety.
3.6.3 PF-06664178
PF-06664178 is also an ADC targeting Trop-2. A phase I dose-escalation study (NCT02122146) was designed to assess the effectiveness and security of this drug in cancer patients, including those with ovarian or cervical cancer. However, further studies of this formulation were halted due to extreme sensitivity observed in skin and mucosal cells. This sensitivity resulted in severe rash, mucosal inflammation, and neutropenia even at very low doses, hindering the anti-tumor activity of the drug [89].
3.7 ADCs targeting MUC16
MUC16, also known as CA125, is the largest type I transmembrane mucin and serves as an important serological marker for screening and surveillance of ovarian cancer. The high expression level of MUC16 can be found in many cancers, like ovarian cancer, cervical cancer and breast cancer. Additionally, research have revealed a correlation between the elevated level of its expression and tumor progression, as well as poor prognosis [90]. The distinct expression pattern in ovarian cancer tissues and normal ovarian epithelium makes it a good choice for targeted therapy in ovarian cancer.
3.7.1 Sofituzumab vedotin
Sofituzumab vedotin (DMUC5754A) consists of anti-MUC16 antibody coupled to MMAE through a cleavable linker. Results from a clinical trial (NCT01335958) involving ovarian and pancreatic carcinomas showed that peripheral neuropathy, fatigue, and gastrointestinal problems occurred at all dose levels. In terms of efficacy, the ORR was only 11%, including 1 CR and 6 PRs, which may be related to the level of MUC16 expression because objective response was observed only in patients with high MUC16 expression [91].
3.7.2 DMUC4064A
DMUC4064A is another ADC designed to target MUC16. A phase I study (NCT02146313) involving patients with platinum-resistant ovarian carcinoma reported AEs like DMUC5754A. The difference was that ocular AEs, such as blurred vision, dry eyes, keratitis, and ocular pain and photophobia, were found in 40% of the participants, suggesting that the toxicity was agent related. In terms of efficacy, objective responses were observed in more than 45% of participants with MUC16 IHC scores of 2 + or higher [92]. However, further clinical studies of this drug have been discontinued due to non-safety related issues.
3.8 ADCs targeting PTK7
Protein tyrosine kinase 7 (PTK7) is an important transmembrane receptor protein which plays an important role in cell adhesion and migration [93]. Numerous cancers, including ovarian carcinoma, exhibit elevated levels of PTK7, which are associated with tumorigenesis, metastasis, and prognosis. Despite the absence of tyrosine kinase activity in PTK7, making it impractical to explore inhibitors, it holds potential as a therapeutic target.
3.8.1 PF-06647020
PF-06647020 (Cofetuzumab Pelidotin) consists of an antibody targeting PTK7 and a microtubule inhibitor [94]. The outcomes of a clinical study (NCT02222922) that enrolled 63 individuals with platinum-resistant ovarian cancer showed that patients experienced AEs such as gastrointestinal reactions and fatigue during the administration of this formulation. The ORR was approximately 27%, and it is important to emphasize that PTK7 expression levels were generally higher in patients who achieved objective responses, suggesting that the low response rate correlates with the low expression of this target [95].
3.9 ADCs targeting CD116
CD166 is a cell-surface glycoprotein and a member of the immunoglobulin receptor subfamily, playing a crucial role in cell adhesion and migration. Elevated expression of CD166 is observed in various types of cancers, correlating with increased tumor aggressiveness and poor prognosis [96]. For endometrial cancer in the early stage, CD116 has been suggested as a trustworthy biomarker [97], and therapeutic approaches targeting ALCAM are under investigation.
3.9.1 Praluzatamab ravtansine
Praluzatamab ravtansine (CX-2009) is composed an antibody targeting CD166 and DM4 coupled by a cleavable linker. A phase I/II trial (NCT03149549) was conducted in participants with various types of advanced cancer, including 22 patients with ovarian cancer. However, only 2 (9%) patients achieved PR [98]. The limited success might be attributed to the small sample size, highlighting the necessity for a larger cohort to further investigate the efficacy of this ADC in gynecologic cancers.
3.9.2 ADCs targeting AXL
AXL is a member of the receptor tyrosine kinase TAM family, and its high level of expression can be found in many types of cancers, like breast and ovarian cancer. Its overexpression is associated with the promotion of cancer cell proliferation, invasion, and metastasis, contributing to a poor prognosis [99]. The response to paclitaxel and carboplatin can be improved by inhibiting AXL [100]. Therefore, targeting AXL may be a promising strategy for the therapy of chemoresistant gynecologic cancers.
3.9.3 Enapotamab vedotin
Enapotamab vedotin (EnaV) consists of a monopoly antibody targeting to AXL, coupled to MMAE through a cleavable linker [101]. In a phase II trial (NCT0298887) of this ADC, only 2 (8%) ovarian carcinoma patients with platinum-resistant achieved an objective response. However, it’s important to note that this outcome might be influenced by the small sample size. In terms of side effects, the most common AEs of grade 3 and above were gastrointestinal reactions, including nausea, vomiting, constipation, colitis, and diarrhea and bloating.
3.9.4 ADCs targeting EFNA-4
Ephrin receptor A4 (EFNA-4) is a tyrosine kinase receptor that is involved in signaling pathways regulating both embryogenesis and adult tissue homeostasis [102]. EFNA-4 and other ephrin family members are highly expressed in tumors like triple-negative breast, ovarian and colorectal cancers compared to normal tissues [103, 104], which makes it a potential therapeutic target.
3.9.5 PF-06647263
PF-06647263 is composed of an anti-EFNA-4 monoclonal antibody coupled through a cleavable linker to calicheamicin, a DNA damaging agent. A preclinical study reported that it shows good anticancer activity in ovarian and triple-negative breast cancer [103]. However, the results of a phase I clinical study (NCT02078752) in patients who are resistant to traditional therapy, including ovarian cancer, showed that only 10% of patients demonstrated slight tumor shrinkage. Additionally, no relativity between tumor remission and EFNA4 expression levels was observed [105]. This result suggests that EFNA-4 does not have the desired effect of being selected as a target.
3.9.6 ADCs targeting DPEP3
Dipeptidase3 (DPEP3) is a glycosylphosphatidylinositol-anchored metallopeptidase that plays an important role in the hydrolysis of many dipeptides. It has been shown to be expressed at high levels in ovarian cancer and may be a potential target [106].
3.9.7 Tamrintamab pamozirine
Tamrintamab pamozirin (SC-003) consists of a monoclonal antibody targeting DPEP3 coupled via a cleavable linker to a pyrrolobenzodiazepine dimer, SC-DR002. 74 individuals with platinum-resistant ovarian carcinoma were enrolled in a phase I study (NCT02539719). The result was that only 3 out of 58 evaluable patients had a PR, with a 4% ORR [107]. The drug was not studied further due to safety concerns and low efficacy.
4 Limitations and future directions of ADCs
It is essential to acknowledge that although this new class of drugs shows much potential in anti-cancer therapy, there are certain limitations. For example, as with other cancer treatments, tumor cells can develop resistance to ADC drugs, which can reduce efficacy. Insufficient target specificity may cause ADC drugs to bind to normal cells, increasing toxicity. Additionally, the instability of the linker can lead to premature release or degradation of the toxin.
At the same time, scientists and pharmaceutical developers are constantly exploring innovative approaches to overcome these limitations for the future development of ADC drugs. For example, further in-depth research to find more suitable antibody targets to enhance the selectivity and therapeutic effectiveness of ADCs. Combining with other treatments, such as chemotherapy, immunotherapy or radiotherapy, may help to improve effectiveness and reduce the probability of drug resistance. Furthermore, efforts are being made to design more stable linkers to ensure that ADC drugs release toxins in the body at a more precise time and location.
5 Conclusion
Overall, ADC drugs, as a novel targeted therapeutic approach, have shown great potential in the field of gynecological cancer and other cancers. Despite current limitations, ongoing advancements in science and technology, coupled with a deeper understanding of the mechanisms of ADC drugs, are expected to contribute to continuous improvement and development of this therapeutic strategy. It is anticipated that these efforts will result in the delivery of more effective therapeutic options for patients in the future.
Availability of data and materials
Not applicable.
Abbreviations
- ADC:
-
Antibody–drug conjugate
- AEs:
-
Adverse events
- ALCAM:
-
Activated leukocyte cell adhesion molecule
- CR:
-
Complete response
- DCR:
-
Disease Control Rate
- DoR:
-
Duration of response
- DPEP3:
-
Dipeptidase3
- EFNA-4:
-
Ephrin receptor A4
- EnaV:
-
Enapotamab vedotin
- EOC:
-
Epithelial ovarian cancer
- FcRns:
-
Neonatal Fc receptors
- FRα:
-
Alpha-folate receptor
- HER-2:
-
Human epidermal growth factor receptor 2
- NaPi2B:
-
Sodium-dependent phosphate transport protein 2B
- ORR:
-
Objective response rate
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- PR:
-
Partial response
- PTK7:
-
Protein tyrosine kinase 7
- SD:
-
Stable disease
- TF:
-
Tissue factor
- TLR:
-
Toll-like receptor
- TROP-2:
-
Trophoblast cell surface antigen-2
- TRSAEs:
-
Treatment-related serious adverse events
- TV:
-
Tisotumab vedotin
- UpRi:
-
Upifitamab rilsodotin
- USC:
-
Uterine-serous-carcinoma
References
Heh E, et al. Peptide drug conjugates and their role in cancer therapy. Int J Mol Sci. 2023;24(1):829.
Strebhardt K, Ullrich A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat Rev Cancer. 2008;8(6):473–80.
Ritchie M, Tchistiakova L, Scott N. Implications of receptor-mediated endocytosis and intracellular trafficking dynamics in the development of antibody drug conjugates. MAbs. 2013;5(1):13–21.
Tarcsa E, et al. Antibody-drug conjugates as targeted therapies: are we there yet? A critical review of the current clinical landscape. Drug Discov Today Technol. 2020;37:13–22.
Peters C, Brown S. Antibody-drug conjugates as novel anti-cancer chemotherapeutics. Biosci Rep. 2015;35(4):e00225.
Egan PC, Reagan JL. The return of gemtuzumab ozogamicin: a humanized anti-CD33 monoclonal antibody-drug conjugate for the treatment of newly diagnosed acute myeloid leukemia. Onco Targets Ther. 2018;11:8265–72.
Hughes B. Antibody-drug conjugates for cancer: poised to deliver? Nat Rev Drug Discov. 2010;9(9):665–7.
Abdollahpour-Alitappeh M, et al. Antibody-drug conjugates (ADCs) for cancer therapy: strategies, challenges, and successes. J Cell Physiol. 2019;234(5):5628–42.
Carter PJ. Potent antibody therapeutics by design. Nat Rev Immunol. 2006;6(5):343–57.
Waldmann H. Human monoclonal antibodies: the benefits of humanization. Methods Molec Biology (Clifton, NJ). 2019;1904:1.
Hellmann I, et al. Novel antibody drug conjugates targeting tumor-associated receptor tyrosine kinase ROR2 by functional screening of fully human antibody libraries using Transpo-mAb display on progenitor B cells. Front Immunol. 2018;9: 2490.
Walsh SJ, et al. Site-selective modification strategies in antibody-drug conjugates. Chem Soc Rev. 2021;50(2):1305–53.
Birrer MJ, et al. Antibody-drug conjugate-based therapeutics: state of the science. J Natl Cancer Inst. 2019;111(6):538–49.
Chen H, et al. Tubulin inhibitor-based antibody-drug conjugates for cancer therapy. Molecules. 2017;22(8):1281.
Pahl A, Lutz C, Hechler T. Amanitins and their development as a payload for antibody-drug conjugates. Drug Discov Today Technol. 2018;30:85–9.
Tolcher AW. Antibody drug conjugates: the dos and don’ts in clinical development. Pharmacol Ther. 2022;240: 108235.
Bargh JD, et al. Cleavable linkers in antibody-drug conjugates. Chem Soc Rev. 2019;48(16):4361–74.
Burton JK, Bottino D, Secomb TW. A systems pharmacology model for drug delivery to solid tumors by antibody-drug conjugates: implications for bystander effects. AAPS J. 2019;22(1):12.
Ducry L, Stump B. Antibody-drug conjugates: linking cytotoxic payloads to monoclonal antibodies. Bioconjug Chem. 2010;21(1):5–13.
Jaracz S, et al. Recent advances in tumor-targeting anticancer drug conjugates. Bioorg Med Chem. 2005;13(17):5043–54.
Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7(9):715–25.
Chalouni C, Doll S. Fate of antibody-drug conjugates in cancer cells. J Exp Clinical Cancer Res. 2018;37(1):20.
Pander G, et al. Antibody-drug conjugates: what drives their progress? Drug Discovery Today. 2022;27(10): 103311.
Baert T, et al. The systemic treatment of recurrent ovarian cancer revisited. Ann Oncol. 2021;32(6):710–25.
Marshall C, et al. Overview of systemic treatment in recurrent and advanced cervical cancer: a primer for radiologists. Abdom Radiol (NY). 2019;44(4):1506–19.
Rütten H, et al. Recurrent endometrial cancer: local and systemic treatment options. Cancers. 2021;13(24):6275.
Salazar MDA, Ratnam M. The folate receptor: what does it promise in tissue-targeted therapeutics? Cancer Metastasis Rev. 2007;26(1):141–52.
Vergote IB, Marth C, Coleman RL. Role of the folate receptor in ovarian cancer treatment: evidence, mechanism, and clinical implications. Cancer Metastasis Rev. 2015;34(1):41–52.
Altwerger G, et al. In vitro and in vivo activity of IMGN853, an antibody-drug conjugate targeting folate receptor alpha linked to DM4, in biologically aggressive endometrial cancers. Mol Cancer Ther. 2018;17(5):1003–11.
Ab O, et al. IMGN853, a folate receptor-α (FRα)-targeting antibody-drug conjugate, exhibits potent targeted antitumor activity against FRα-expressing tumors. Mol Cancer Ther. 2015;14(7):1605–13.
Moore KN, et al. Phase 1 dose-escalation study of mirvetuximab soravtansine (IMGN853), a folate receptor α-targeting antibody-drug conjugate, in patients with solid tumors. Cancer. 2017;123(16):3080–7.
Moore KN, et al. Phase III, randomized trial of mirvetuximab soravtansine versus chemotherapy in patients with platinum-resistant ovarian cancer: primary analysis of FORWARD I. Ann Oncol. 2021;32(6):757–65.
Matulonis UA, et al. Efficacy and safety of mirvetuximab soravtansine in patients with platinum-resistant ovarian cancer with high folate receptor alpha expression: results from the SORAYA study. J Clin Oncol. 2023;41(13):2436–45.
Moore KN, Van Gorp T, Wang J, et al. MIRASOL (GOG 3045/ENGOT OV-55): a randomized, open-label, phase III study of mirvetuximab soravtansine versus investigator’s choice of chemotherapy in advanced high-grade epithelial ovarian, primary peritoneal, or fallopian tube cancers with high folate-alpha(FRa) expression. Gynecol Oncol. 2020;38(15_suppl):TPS6103–TPS6103.
Abrahams C, et al. Abstract NT-090: preclinical activity and safety oF STRO-002, a noveL ADC targeting folate receptor alpha for ovarian and endometrial cancer. Clin Cancer Res. 2019;25(22_Supplement):NT-090-NT-090.
Naumann RW, et al. Phase 1 dose-escalation study of STRO-002, an antifolate receptor alpha (FRα) antibody drug conjugate (ADC), in patients with advanced, progressive platinum-resistant/refractory epithelial ovarian cancer (EOC). J Clin Oncol. 2021;39(15_suppl):5550–5550.
Eaton JS, et al. Ocular adverse events associated with antibody-drug conjugates in human clinical trials. J Ocul Pharmacol Ther. 2015;31(10):589–604.
Cheng X, et al. MORAb-202, an antibody-drug conjugate utilizing humanized anti-human FRα farletuzumab and the microtubule-targeting agent eribulin, has potent antitumor activity. Mol Cancer Ther. 2018;17(12):2665–75.
Shimizu T, et al. First-in-human phase 1 study of MORAb-202, an antibody-drug conjugate comprising farletuzumab linked to eribulin mesylate, in patients with folate receptor-α-positive advanced solid tumors. Clin Cancer Res. 2021;27(14):3905–15.
Gutierrez C, Schiff R. HER2: biology, detection, and clinical implications. Arch Pathol Lab Med. 2011;135(1):55–62.
Connell CM, Doherty GJ. Activating HER2 mutations as emerging targets in multiple solid cancers. ESMO Open. 2017;2(5): e000279.
Zhao S, et al. Landscape of somatic single-nucleotide and copy-number mutations in uterine serous carcinoma. Proc Natl Acad Sci USA. 2013;110(8):2916–21.
Verma S, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–91.
Jhaveri KL, et al. Ado-trastuzumab emtansine (T-DM1) in patients with HER2-amplified tumors excluding breast and gastric/gastroesophageal junction (GEJ) adenocarcinomas: results from the NCI-MATCH trial (EAY131) subprotocol Q. Ann Oncol. 2019;30(11):1821–30.
Li BT, et al. A multi-histology basket trial of ado-trastuzumab emtansine in patients with HER2 amplified cancers. J Clin Oncol. 2018;36(15_suppl):2502–2502.
Dumbrava EI, et al. Abstract OT-03–02: Phase 1/2 study of a novel HER2 targeting TLR7/8 immune-stimulating antibody conjugate (ISAC), BDC-1001, as a single agent and in combination with an immune checkpoint inhibitor in patients with advanced HER2-expressing solid tumors. Cancer Res. 2021;81(4_Supplement):OT-03-02-OT-03-02.
Sharma M, et al. Preliminary results from a phase 1/2 study of BDC-1001, a novel HER2 targeting TLR7/8 immune-stimulating antibody conjugate (ISAC), in patients (pts) with advanced HER2-expressing solid tumors. J Clin Oncol. 2021;39(15_suppl):2549–2549.
Andrikopoulou A, et al. Trastuzumab deruxtecan (DS-8201a): the latest research and advances in breast cancer. Clin Breast Cancer. 2021;21(3):e212–9.
Keam SJ. Trastuzumab deruxtecan: first approval. Drugs. 2020;80(5):501–8.
Beck A, et al. Strategies and challenges for the next generation of antibody-drug conjugates. Nat Rev Drug Discovery. 2017;16(5):315–37.
Tsurutani J, et al. Targeting HER2 with trastuzumab deruxtecan: a dose-expansion, phase i study in multiple advanced solid tumors. Cancer Discov. 2020;10(5):688–701.
Hasegawa K, et al. 813P efficacy and safety of trastuzumab deruxtecan in HER2-expressing uterine carcinosarcoma (STATICE trial, NCCH1615): a multicenter, phase II clinical trial. Ann Oncol. 2021;32:S767.
Nadal-Serrano M, et al. The second generation antibody-drug conjugate SYD985 overcomes resistances to T-DM1. Cancers. 2020;12(3):670.
Black J, et al. SYD985, a novel duocarmycin-based HER2-targeting antibody-drug conjugate, shows antitumor activity in uterine serous carcinoma with HER2/Neu expression. Mol Cancer Ther. 2016;15(8):1900–9.
Menderes G, et al. SYD985, a novel duocarmycin-based HER2-targeting antibody-drug conjugate, shows promising antitumor activity in epithelial ovarian carcinoma with HER2/Neu expression. Gynecol Oncol. 2017;146(1):179–86.
Banerji U, et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019;20(8):1124–35.
Rinnerthaler G, Gampenrieder SP, Greil R. HER2 directed antibody-drug-conjugates beyond T-DM1 in breast cancer. Int J Mol Sci. 2019;20(5):1115.
Liu Y, et al. A first in-human study of A166 in patients with locally advanced/metastatic solid tumors which are HER2-positive or HER2-amplified who did not respond or stopped responding to approved therapies. J Clin Oncol. 2020;38(15_suppl):1049–1049.
Tymon-Rosario J, et al. DHES0815A, a novel antibody-drug conjugate targeting HER2/neu, is highly active against uterine serous carcinomas in vitro and in vivo. Gynecol Oncol. 2021;163(2):334–41.
Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci USA. 1996;93(1):136–40.
Argani P, et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE). Clin Cancer Res. 2001;7(12):3862–8.
Hassan R, et al. Localization of mesothelin in epithelial ovarian cancer. Appl Immunohistochem Mol Morphol. 2005;13(3):243–7.
Golfier S, et al. Anetumab ravtansine: a novel mesothelin-targeting antibody-drug conjugate cures tumors with heterogeneous target expression favored by bystander effect. Mol Cancer Ther. 2014;13(6):1537–48.
Hassan R, et al. First-in-human, multicenter, phase I dose-escalation and expansion study of anti-mesothelin antibody-drug conjugate anetumab ravtansine in advanced or metastatic solid tumors. J Clin Oncol. 2020;38(16):1824–35.
Santin A, et al. 372 Safety and activity of the anti-mesothelin antibody–drug conjugate anetumab ravtansine in combination with pegylated-liposomal doxorubicin in platinum-resistant ovarian, fallopian tube or primary peritoneal cancer. Int J Gynecol Cancer. 2020;30(Suppl 3):A154–A154.
Scales SJ, et al. An antimesothelin-monomethyl auristatin e conjugate with potent antitumor activity in ovarian, pancreatic, and mesothelioma models. Mol Cancer Ther. 2014;13(11):2630–40.
Weekes CD, et al. Phase I study of DMOT4039A, an antibody-drug conjugate targeting mesothelin, in patients with unresectable pancreatic or platinum-resistant ovarian cancer. Mol Cancer Ther. 2016;15(3):439–47.
Clarke J, et al. Abstract B057: BMS-986148, an anti-mesothelin antibody-drug conjugate (ADC), alone or in combination with nivolumab demonstrates clinical activity in patients with select advanced solid tumors. Molecular Cancer Therapeutics. 2019;18(12_Supplement):B057–B057.
Rottey S, et al. Phase I/IIa trial of BMS-986148, an anti-mesothelin antibody-drug conjugate, alone or in combination with nivolumab in patients with advanced solid tumors. Clin Cancer Res. 2022;28(1):95.
Jiang J, et al. Preclinical safety profile of RC88-ADC: a novel mesothelin-targeted antibody conjugated with Monomethyl auristatin E. Drug Chem Toxicol. 2023;46(1):24–34.
Xu H, et al. Molecular cloning, functional characterization, tissue distribution, and chromosomal localization of a human, small intestinal sodium-phosphate (Na+-Pi) transporter (SLC34A2). Genomics. 1999;62(2):281–4.
Levan K, et al. Immunohistochemical evaluation of epithelial ovarian carcinomas identifies three different expression patterns of the MX35 antigen, NaPi2b. BMC Cancer. 2017;17(1):303.
Yurkovetskiy AV, et al. Dolaflexin: a novel antibody-drug conjugate platform featuring high drug loading and a controlled bystander effect. Mol Cancer Ther. 2021;20(5):885–95.
Richardson DL, et al. A phase 1 study of XMT-1536 in patients with solid tumors likely to express NaPi2b: a summary of dose escalation. Gynecol Oncol. 2020;159:52.
Gerber DE, et al. Phase Ia study of anti-NaPi2b antibody-drug conjugate lifastuzumab vedotin DNIB0600A in patients with non-small cell lung cancer and platinum-resistant ovarian cancer. Clin Cancer Res. 2020;26(2):364–72.
Moore KN, et al. Phase 1b study of anti-NaPi2b antibody-drug conjugate lifastuzumab vedotin (DNIB0600A) in patients with platinum-sensitive recurrent ovarian cancer. Gynecol Oncol. 2020;158(3):631–9.
Bogdanov VY, et al. Alternatively spliced human tissue factor: a circulating, soluble, thrombogenic protein. Nat Med. 2003;9(4):458–62.
Magnus N, et al. Tissue factor expression provokes escape from tumor dormancy and leads to genomic alterations. Proc Natl Acad Sci USA. 2014;111(9):3544–9.
Zhao X, et al. Expression of tissue factor in human cervical carcinoma tissue. Exp Ther Med. 2018;16(5):4075–81.
Breij ECW, et al. An antibody-drug conjugate that targets tissue factor exhibits potent therapeutic activity against a broad range of solid tumors. Can Res. 2014;74(4):1214–26.
Coleman RL, et al. Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021;22(5):609–19.
Song X, et al. Tisotumab vedotin for the treatment of cervical carcinoma. Drugs of Today (Barc). 2022;58(5):213–22.
Bignotti E, et al. Trop-2 overexpression as an independent marker for poor overall survival in ovarian carcinoma patients. Eur J Cancer. 2010;46(5):944–53.
Trerotola M, et al. Upregulation of Trop-2 quantitatively stimulates human cancer growth. Oncogene. 2013;32(2):222–33.
Cardillo TM, et al. Sacituzumab govitecan (IMMU-132), an anti-Trop-2/SN-38 antibody-drug conjugate: characterization and efficacy in pancreatic, gastric, and other cancers. Bioconjug Chem. 2015;26(5):919–31.
Bardia A, et al. Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Anna Oncol. 2021;32(6):746–56.
Han C, et al. Sacituzumab govitecan (IMMU-132) in treatment-resistant uterine serous carcinoma: a case report. Gynecol Oncol Rep. 2018;25:37–40.
Cheng Y, et al. Preclinical profiles of SKB264, a novel anti-TROP2 antibody conjugated to topoisomerase inhibitor, demonstrated promising antitumor efficacy compared to IMMU-132. Front Oncol. 2022;12: 951589.
King GT, et al. A phase 1, dose-escalation study of PF-06664178, an anti-Trop-2/Aur0101 antibody-drug conjugate in patients with advanced or metastatic solid tumors. Invest New Drugs. 2018;36(5):836–47.
Shen H, et al. MUC16 facilitates cervical cancer progression via JAK2/STAT3 phosphorylation-mediated cyclooxygenase-2 expression. Genes Genomics. 2020;42(2):127–33.
Liu JF, et al. Phase I study of safety and pharmacokinetics of the anti-MUC16 antibody-drug conjugate DMUC5754A in patients with platinum-resistant ovarian cancer or unresectable pancreatic cancer. Ann Oncol. 2016;27(11):2124–30.
Liu J, et al. An open-label phase I dose-escalation study of the safety and pharmacokinetics of DMUC4064A in patients with platinum-resistant ovarian cancer. Gynecol Oncol. 2021;163(3):473–80.
Bin-Nun N, et al. PTK7 modulates Wnt signaling activity via LRP6. Development (Cambridge, England). 2014;141(2):410–21.
Sachdev JC, et al. PF-06647020 (PF-7020), an antibody-drug conjugate (ADC) targeting protein tyrosine kinase 7 (PTK7), in patients (pts) with advanced solid tumors: results of a phase I dose escalation and expansion study. J Clin Oncol. 2018;36(15_suppl):5565–5565.
Maitland ML, et al. First-in-human study of PF-06647020 (Cofetuzumab Pelidotin), an antibody-drug conjugate targeting protein tyrosine kinase 7, in advanced solid tumors. Clin Cancer Res. 2021;27(16):4511–20.
Weidle UH, et al. ALCAM/CD166: cancer-related issues. Cancer Genomics Proteomics. 2010;7(5):231–43.
Devis L, et al. Activated leukocyte cell adhesion molecule (ALCAM) is a marker of recurrence and promotes cell migration, invasion, and metastasis in early-stage endometrioid endometrial cancer. J Pathol. 2017;241(4):475–87.
Boni V, et al. CX-2009, a CD166-directed probody drug conjugate (PDC): results from the first-in-human study in patients (Pts) with advanced cancer including breast cancer (BC). J Clin Oncol. 2020;38(15_suppl):526–526.
Zhu C, Wei Y, Wei X. AXL receptor tyrosine kinase as a promising anti-cancer approach: functions, molecular mechanisms and clinical applications. Mol Cancer. 2019;18(1):153.
Quinn JM, et al. Therapeutic inhibition of the receptor tyrosine kinase AXL improves sensitivity to platinum and taxane in ovarian cancer. Mol Cancer Ther. 2019;18(2):389–98.
Boshuizen J, et al. Cooperative targeting of melanoma heterogeneity with an AXL antibody-drug conjugate and BRAF/MEK inhibitors. Nat Med. 2018;24(2):203–12.
Pasquale EB. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat Rev Cancer. 2010;10(3):165–80.
Damelin M, et al. Anti-EFNA4 calicheamicin conjugates effectively target triple-negative breast and ovarian tumor-initiating cells to result in sustained tumor regressions. Clin Cancer Res. 2015;21(18):4165–73.
Kumar SR, et al. The receptor tyrosine kinase EphB4 is overexpressed in ovarian cancer, provides survival signals and predicts poor outcome. Br J Cancer. 2007;96(7):1083–91.
Garrido-Laguna I, et al. First-in-human, phase I study of PF-06647263, an anti-EFNA4 calicheamicin antibody-drug conjugate, in patients with advanced solid tumors. Int J Cancer. 2019;145(7):1798–808.
Hayashi K, et al. Structure of human DPEP3 in complex with the SC-003 antibody Fab fragment reveals basis for lack of dipeptidase activity. J Struct Biol. 2020;211(1): 107512.
Hamilton E, et al. Tamrintamab pamozirine (SC-003) in patients with platinum-resistant/refractory ovarian cancer: findings of a phase 1 study. Gynecol Oncol. 2020;158(3):640–5.
Acknowledgements
Not applicable.
Funding
This work was funded by Guangdong Basic and Applied Basic Research Foundation (2022A1515012432), Beijing Xisike Clinical Oncology Research Foundation (Y-Young2022–0145), Sun Yat-sen Clinical Research Cultivating Program (SYS-C-202001), and Beijing Kanghua Foundation for the Development of Traditional Chinese and Western Medicine (KH-2021-LLZX-049).
Author information
Authors and Affiliations
Contributions
DDX (1) substantial contributions to collection and organizing of the published articles; (2) drafting the article; XWP, ZSC and ZQL (1) contributions to revising the article; HWL (1) contributions to conception and design; (2) making important changes to the paper; and (3) final approval of the version to be published. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, D., Chen, ZS., Peng, X. et al. Research progress of antibody–drug conjugates in gynecologic cancer. Holist Integ Oncol 3, 42 (2024). https://doi.org/10.1007/s44178-024-00114-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44178-024-00114-8