Abstract
Wave intensity and wave speed are measures used to assess the dynamic properties of the arteries and travelling waves within the circulation. Wave intensity and wave speed measured in the carotid artery have the potential to provide hemodynamic and biophysical insights that can advance our understanding of the physiology of cerebral circulation. However, whilst studies have been performed in different patient cohorts exploring different methodological implementations of wave intensity analysis (WIA), to date little work has been done to unify wave measures or provide reference ranges on which to build the field of research and inform clinical practice. This review thus focuses on wave speed and wave intensity in the carotid artery in man with the aim to summarise the current knowledge of the field. From this review, the different methods of measurement and the disparity of the reported values currently hinder efforts to construct reference ranges for a comparator or intervention to be assessed.
Similar content being viewed by others
1 Introduction
Wave intensity is the rate of energy transport in a longitudinal wavefront per unit area. Wave intensity analysis (WIA) provides a quantitative measure of the incremental net energy flux density of travelling waves within the circulation or the power carried by the wave per cross-sectional area. The work done by the wave comprises kinetic energy associated with the velocity of flow and potential energy associated with the expansion of the arterial wall [1]. Wave speed or pulse wave velocity (i.e. the velocity at which the blood pressure pulse propagates in an artery or system of arteries) is often, but by no means invariably, calculated as part of wave intensity analysis. Wave speed is generally evaluated in early systole and is related to the distensibility of the artery as shown by the Moens–Korteweg equation [2, 3], i.e. wave speed is inversely proportional to the square root of distensibility.
So far, little work has focused on the use of WIA for understanding the cerebral circulation, or the impact of the energy transfer from the extracranial to the intracranial vessels, even though WIA has often been measured in the common carotid artery. This is an important area of translational and clinical research. For instance, people with hypertension are more likely to suffer small vessel disease and have poorer cerebral perfusion [4, 5], and increased arterial pressure may heighten the risk of aneurysm formation and rupture at the circle of Willis [6,7,8]. Understanding wave travel could potentially provide a better understanding of such pathology and the analysis is increasingly showing promise in this context. A recent study, for example, found that the forward compression wave intensity in mid-to-late life predicted faster cognitive decline independent of other cardiovascular risk factors as well as markers of carotid structure and stiffness [9]. At present, however, there are very limited direct measurements of the intrinsic mechanical properties of the intracerebral arteries in vivo [10].
Let us consider the setting for this physiological problem. Blood flow to the cerebral circulation arrives through two artery pairs: the left and right internal carotid arteries, which largely supply the anterior circulation of the brain, and the left and right vertebral arteries, responsible mainly for the posterior circulation [11]. The vertebral arteries converge over the brainstem to form the basilar artery. The basilar artery together with the internal carotid arteries form a ring-like anastomosis known as the Circle of Willis. The basic anatomy is shown in Fig. 1, although variants are common [12]. This organisation allows for the re-distribution of blood based on demand, can compensate for resistance such as stenosis, and may therefore represent a collateral system to protect against hypoperfusion. The anastomotic nature of the cerebral arterial anatomy also enables a range of shunt and steal phenomena [13] as illustrated by subclavian steal syndrome [14], reversed Robin Hood syndrome [15], and luxury perfusion syndrome [16, 17].
From a biophysics perspective, the cerebral circulation is unique. Various constraints, which are set out by the Monroe-Kellie doctrine [18], are assumed to hold true for the perfusion of the brain to be maintained within physiological boundaries. Imbalances and breaches of these assumptions are considered to cause marked pathophysiological disturbances, such as Cushing’s triad, ischaemic haemorrhage, or syncope. The first constraint is that the cerebral circulation is encased in the isovolumetric container of the skull. The inlets and outlets (including the cerebrospinal fluid space) are generally through non-compressible bone, which limits the ability for pulsatile movement and hypertrophy at these junctures. Second, the pressure within the skull must be maintained within relatively narrow boundaries to avoid percussion of the brain tissue against the bone [19]. Third, the adaption to the systolic pressure wave must be sufficient to move blood against gravity in the upright position [20], whilst tolerating the orthostatic changes to the supine position and even inversions (such as handstands and other oddities humans master).
In this context, we aim to review current knowledge on wave speed and wave intensity measures in the carotid artery in man. The review will summarise the effect of interventions and any other comparations made within each study but will not aim to compare interventions between reports.
1.1 What is the wave speed measure?
Wave speed (c) is the speed at which a disturbance travels through a medium [21]; in this case, energy through arteries. c is dependent on the viscosity of blood and the mechanical and geometric properties of the vessel [21, 22]. c is an indicator of arterial stiffness (or its inverse distensibility [23], with higher wave speed indicating a stiffer, less distensible vessel.
Whilst aortic wave speed is often measured clinically by femoral-carotid pulse wave velocity, this is a weighted average over the arterial pathlength and does not account for more local variation in arterial vessel mechanics and geometry [24]. Regional, or local, wave speed can be estimated by single-point methods derived from the slope of the loop of two measured variables such as pressure (P) and velocity (U), or flow (Q) and cross-sectional area (A). Specifically, these methods are known as the PU-loop [25], QA-loop [26], ln(D)U-loop [27], and ln(A)U-loop [28] methods. Alternative formulations based on the knowledge of P and U at a single point in the cardiac cycle includes the sum of the squares methods (see [29]). This method has the advantage of being independent of the period of unidirectional waves required for the loop methods but has a larger margin of error when applied to measures taken close to a reflection site.
The linearity between pressure and diameter over the cardiac cycle has been demonstrated using invasive pressure measurements in the carotid artery [30]. However, Kowalski et al. (2017) suggested that the wave reflections may cause an underestimation of c by – 36.9% (± 6.7%) in the carotid artery [31]. Furthermore, it has been shown that reflected waves [32] and the proximity of the measurement to the reflection site [32,33,34] significantly influence the measured wave speed. Using the diameter-velocity loop method, Borlotti et al. [35] showed that proximity to the reflection site affects the measured wave speed so that a positive reflection coefficient leads to an underestimation of wave speed, whilst a negative reflection coefficient leads to an overestimation of wave speed. This is particularly important in the carotid artery given its geometry and anatomy; namely the carotid bifurcation in the neck, the curvature of the internal carotid siphon and the juncture at the Circle of Willis. The degree to which the pressure and flow (or velocity) changes are affected by reflections and the convective contribution is assumed to be negligible in the early part of systole when measurements of c are typically made. The linearity of elasticity of the vessel wall over the physiological pressure range is a necessary assumption, however, there is not sufficient evidence at present to demonstrate the absolute validity of this assumption.
When comparing the theoretical local wave speed to that measured experimentally, discrepancies are observed [36, 37]. Namely, based on theoretical grounds from its composition and on experimental studies the relationship between stress and strain in arteries is known to be non-linear [38]. Nonetheless, the assumption of a unique elastic modulus (incremental elastic modulus) may be approximately valid within the pressure range of the cardiac cycle, however, differences in operating pressure may be relevant when comparing measures of c between individuals (e.g. between normotensive and hypertensive individuals) [39]. Extrinsic factors, the tethering of the vessel, and the compressibility of the surrounding tissue have also been suggested to affect c [40].
Therefore, given the special limitations of the cerebral circulation suggested above, and the internal carotid artery in particular, measuring wave speed in this artery experimentally is of particular future interest.
1.2 What is wave intensity analysis?
Unlike wave speed, which is measured in m/s, the units of wave intensity depend on the method of measurement and introduces the variable of local distensibility [41] (e.g. if derived from pressure and velocity, it is commonly reported in W/m2 or mmHg·m·s−3, whereas if derived from diameter and velocity it is commonly reported in m2/s). Despite the variability of units, the measures are essentially equivalent although not interchangeable, and related to each other through the tube law which describes the relationship between the pressure and the area (or diameter) of the artery.
The original method for analysing wave intensity (dI = dPdU) describes the use of pressure and velocity to calculate wave intensity, defined as the maximum value of the composite waves, and wave energy, defined as the integral of the wave intensity over time. Other approaches, such as using a logarithm of the area and velocity, have since been proposed [28, 34]. A large subset of studies report wave intensity using the time-corrected formula, dP/dt × dU/dt. This carries the advantage of being able to compare data acquired with different sampling rates, but with the disadvantage that the resulting units are complex and unintuitive to interpret (mmHg·m·s−3) [42]. An alternative approach where dI is expressed per cardiac cycle and which preserves its interpretability has been proposed [43].
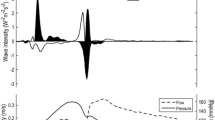
The net wave intensity can be separated into its component forward and backward waves if the wave speed is known [47]. Net wave intensity is a result of the algebraic sum of the forward and backwards travelling waves [44, 45]. Compression waves are associated with a rise in pressure, whilst decompression waves are associated with a fall in pressure. Whilst there are many reflections and re-reflections [46], wave intensity in most large elastic arteries is commonly described in terms of three major wave components. The first is a forward compression wave (FCW) which carries most of the energy over the cardiac cycle and is a measure of the rate of forward wave energy density propagation in early systole due to ventricular ejection. This is followed by a smaller backward compression wave (BCW) which, outside the coronary circulation, represents backward travelling waves (reflections) from various sites of impedance mismatching, such as those due to branching, confluences and changes in cross-sectional areas and/or material properties of the arteries [48]. Finally at the end of systole (protodiastole), a forward decompression wave (also known as the forward expansion wave, FEW) decelerating systolic flow in the arteries is observed. This is caused by the slowing of ventricular contraction which creates a suction effect preceding and contributing to aortic valve closure [49, 50]. It has also been proposed that the end-systolic FEW is caused by the inertial effects of the negative re-reflection of the BCW [49]. Figure 2 illustrates the various wave components.
A strong correlation between the energy of the BCW and FCW has been shown in the common carotid artery of healthy volunteers, suggesting that the forward wave energy generated by the left ventricle is a predictor of the amplitude of the reflected wave [45] Anti-hypertensive treatment, which effectively lowers blood pressure, increases the FCW and decreases the BCW [51]. In a virtual population of 2000 subjects, it was shown that wave intensity analysis may prove useful in the diagnostics and treatment of people with heart failure [52].
Interestingly, in the carotid artery, another forward decompression wave in mid-systole has been reported [42, 49, 53]. This wave is reported to have a variable size. Hughes et al. [53] saw that this augmentation in the carotid artery was associated with a larger second forward decompression wave, suggesting a possible relationship between the additional wave and inertial or re-reflection effects.
2 Methods
This systematic review follows the PRISMA-P guidelines for systematic reviews [54]. The inclusion criteria were original research articles measuring local wave speed or wave intensity in the human carotid artery. Studies reporting any of the measures (i.e. wave speed, wave intensity, forward compression wave energy, forward decompression wave energy, backward compression wave energy) were included regardless of whether they reported only a subset of the measures. Articles were included if they had full-text versions available in English and were published between 1990 and January 1st 2023. Review articles, meta-analyses, studies in non-human species and in silico models were excluded.
The following PubMed searches were run on the 15th of April 2020, repeated on the 1st of April 2021, and on the 12th February 2023 with the following terms: (1.) carotid artery + wave speed, (2.) carotid artery + wave intensity analysis, (3.) internal carotid artery + wave speed, (4.) internal carotid artery + wave intensity analysis.
Data extracted were directly from the articles and checked by the second investigator independently. The data extracted was: (1) Wave speed, (2) Net wave intensity or energy, (3) forward compression wave intensity or energy, (4) forward decompression wave intensity or energy, and (5) backward compression wave intensity or energy.
Risk of bias was not assessed as the bias domains [55] were not considered relevant to the particular type of studies available in the literature. For example, selection, performance and detection bias require the blinding of outcome assessment, which was not a feasible objective. Further, attrition bias, reporting bias and other biases are better assessed against a standard convention of reporting which is not available at the present time for wave speed or wave intensity analysis in the carotid artery. Instead, data are presented with the details of the population studies in each case, as well as the method of measurement.
3 Results
The search yielded a total of 279 articles, of which 14 were duplicates, resulting in a total of 265 articles that were screened based on abstract content. In total, 59 articles were ultimately included. These contained either wave intensity analysis in the carotid artery, wave speed measurements in the carotid artery, or both. PRISMA flow diagram is shown in Fig. 3.
3.1 Wave speed in the common carotid artery and its branches
Only two studies measured wave speed in the internal carotid artery experimentally [56, 57]. Neumann et al. found wave speeds around 1–1.5 m/s in the internal carotid artery using the lnA-U loop method. Ayadi et al. used two methods of wave speed estimation, i.e. the foot-to-foot method (\(c=\frac{\Delta z}{\Delta t}\), where Delta z represents the distance between two anatomical cross sections, and Delta t is the time interval between the feet of two waves) and a mathematical modification of the foot-to-foot model (summarised as (\(c=\frac{2L}{{t}_{0}}\), where L is the distance between the measurement point and reflection area and t0 is the arrival time of the first reflected wave). Using the traditional foot-to-foot method, the authors reported a similar wave speed of 2.6 ± 0.2 m/s in the young (n = 11, 0 men, 29.4 ± 6.8 years), and 3.3 ± 0.3 m/s in the older group (n = 11, 8 men, 66 ± 14.9 years). However, using the new mathematical model which corrects for the temporal inaccuracy of the foot-to-foot method, the authors reported wave speeds of 5.2 ± 0.5 m/s in the young, and 6.3 ± 0.4 m/s in the older group, suggesting more traditional methods may underestimate wave speed in the internal carotid artery [56].
All other studies report wave speed in the common carotid artery. The measurements in the human carotid artery are summarised in Table 1.
The average wave speed (± standard deviation) reported in healthy volunteers (regardless of method) was 5.58 ± 2.1 m/s, with the median wave speed at 5.38 m/s. The minimum average speed was reported in endurance-trained men (0.8 m/s, age 27 ± 4 years) using the lnDU loop method [58] and the maximum was reported in healthy volunteers (14.2 m/s, 60 ± 9 years) using the PU loop method [59].
Whilst it is known that wave speed increases with age [60], this accounts for 2–3 m/s of the variability and, therefore, the range of values reported in the literature cannot be accounted for by age alone. Interestingly, it was not possible to determine a bias to yield a higher or lower wave speed value based on the method used.
One study reported wave speed in the left and the right common carotid separately, using three different methods of measurement, and found that wave speed was consistently higher in the right compared to the left CCA [61].
Wave speed is consistently reported as marginally higher in men compared to women [45, 62].
3.2 Wave intensity in the carotid artery
Wave intensity in the internal carotid artery was assessed in two studies only [56, 90], while several studies have assessed wave intensity in the common carotid artery. Wave intensity has been assessed during exercise [69], caffeine and tobacco consumption [76], hot water bathing [91], chronic heart failure [59], and in patients with a Fontan circulation [79]. Wave intensity and wave energy measures are summarised in Table 2.
In examining the effect of gender on wave intensity in the common carotid artery, the peak amplitude and the net energy of FCW and BCW were higher in men [45]. However, when normalized for the amplitude of the forward-travelling waves, the difference in BCW was abolished [45]. Rakebrandt et al. (2009) further showed an inverse correlation with age, diastolic blood pressure, local wave speed, beta stiffness index and augmentation index for both the FCW and BCW.
Importantly, Chiesa et al. (2019) reported on a large longitudinal study showing that increased carotid FCW in mid- to late-life was associated with greater 10-year cognitive decline [9]. Higher wave reflection index (a measure relating the magnitude of the BCW to the FCW) and lower FCW has been associated with an increased risk of cardiovascular events [80, 92]. In the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT), it was shown that in the group treated with amlodipine the wave reflection index was significantly attenuated compared to the group treated with atenolol [66]. Manisty et al. also looked at the effect of atorvastin versus placebo and found that whilst there was evidence of reduced augmentation index and greater wave reflection from the body, there was no significant change in wave speed or wave energy [65].
From Table 2 below, it is clear the measures of wave intensity and wave energy cannot easily be compared between methods. However, looking only at the most common method i.e. (dP/dt) × (dU/dt), and removing the outliers reporting a vastly different range of values [73, 78, 79, 83, 87], the mean (± standard deviation) FCW intensity was 9520 ± 2258.7 mmHg m/s3 (median 9308 mmHg m/s3; minimum (reported average) 5750 mmHg m/s3, maximum (reported average) 14,700). The mean BCW energy was 322 ± 1484.5 mmHg m/s2 (median 29 mmHg m/s2; (minimum (reported average) 18 mmHg m/s2, maximum (reported average) 7600 mmHg m/s2). And finally, the mean FEW intensity was 2039 ± 581.6 mmHg m/s3 (median 1900 mmHg m/s3;(minimum (reported average) 1070 mmHg m/s3, maximum (reported average) 3220 mmHg m/s3).
4 Discussion
Wave speed and wave intensity analysis present an opportunity to better our understanding of the physiology of cerebral circulation. At present, a relatively large body of work has been carried out assessing wave speed and wave intensity in the common carotid artery. However, little work has focused on intracranial blood flow or cerebral haemodynamics. When looking at the reports within studies, it is clear that various health conditions and physiological manipulations (such as hot baths, stress tests, exercise and more) are able to modify wave speed and wave intensity suggesting the measures are sensitive to change.
Local wave speed in an artery is intrinsically interesting because of its effect on arterial haemodynamics and because it is a measure of the distensibility of the artery. The separation of the net wave intensity into its forward and backward components requires knowledge of the local wave speed. The disparity in the number of studies that report separated wave intensity results and those that report wave speeds indicates that many studies that measure or estimate wave speeds do not report them. We recommend that any future study that measures local wave speeds should report their results.
With regard to wave speed calculations, a large variability exists between studies and methods. These differences are not obviously explained by method, age, gender or population; however, the current review did not undertake meta-regression to understand the contributions of confounding or modifying factors. Nevertheless, it is clear that, for wave speed to be clinically and scientifically useful for the understanding of the cerebral circulation, a consensus on the reference range for normal wave speed is needed. To obtain this, large-scale studies investigating the contribution and differences in methods as well as the reliability and reproducibility will be necessary.
Wave speed in the extracranial common carotid, as opposed to the intracranial internal carotid, has been extensively reported. It is interesting that whilst the mean wave speed is in line with the systemic circulation at approximately 5 m/s, the range is wide and appears biologically improbable, at least at the lower end (minimum 0.8 m/s and maximum 14 m/s). In the intracranial internal carotid artery, traditional wave speed estimation suggested a greatly reduced speed at 2-3 m/s. This must be interpreted with caution given the limited number of observations but could suggest that the internal carotid, and by extension the intracranial circulation, behaves differently to the systemic pressure/velocity wave associations, or that corrections must be applied to the measures to account for the limited resolution in this space. There is evidence that intracranial arteries undergo cyclic distension but the distensibility appears much reduced compared with extracranial arteries [10]. On that basis, the wave speed is likely to be higher albeit not infinite.
A similar limitation exists for wave intensity. Indeed, the axial distension wave as assumed for the analysis of wave intensity may be absent or much reduced under the isovolumetric constraints of the cranial cavity. Nonetheless, with the limited number of observations in the internal carotid artery and no studies directly comparing the dynamics between the internal and the external segment of the carotid artery, we are limited to the interpretation of each in isolation.
Here, a further complication comes from the inconsistent reporting both in terms of the measurement and calculations presented, that is, whether the peak amplitude or the area under the curve is reported, but also in terms of the variation in units of measurements, making it challenging to directly compare between studies. A consensus on a standardisation of units, e.g. to be reported as Standard International units for future studies would be useful. Whilst the conversion between units is relatively trivial, comparing results of different measures and different methodologies is not. In addition, wave intensity varies across the cardiac cycle. However, the temporal variation is commonly ignored as only the peak systolic intensity or the total energy across the cardiac cycle is usually reported.
From a methodological perspective, it is worth mentioning that the estimate of velocity using pulsed Dopper use peak velocity since this is what the US device typically provides, Other devices (e.g. ALOKA) which use Colour Doppler calculate average velocity. This could introduce substantial differences in estimates of both wave speed and WI.
Despite a substantial body of research spanning 30 years, both on the theoretical side but also on clinical applications relating specifically to the carotid artery and clinically important scenarios such as hypertension, the use of wave intensity analysis is still not widespread in clinical practice. There are examples of ultrasound machines that can yield wave intensity values as part of the cardiovascular analytics [108] but consensus on units and prognostic value of wave intensity indices, in addition to reference ranges, is required to establish this indicator as a useful clinical variable.
It is interesting to note that, despite the clear implications of wave speed and energy on cerebral haemodynamics and the potential development of therapeutic markers of cerebrovascular health, no study has, to our knowledge, assessed the direct impact of wave energy or wave speed on the cerebral circulation by means of functional or perfusion imaging. It should of course be noted that a limitation of the measurements in the common carotid is the bifurcation of this artery into the external and internal carotid artery of which only the internal carotid branch is relevant for the cerebral circulation. Also, an investigation of the impact of wave energy and wave speed on the carotid sinus baroreceptor, a homeostatic sensor of blood pressure, has not been performed. Importantly, the internal carotid arteries supply the anterior circulation of the cerebrum and as such the posterior circulation as supplied by the vertebral arteries are neglected, largely due to the limitations of the current imaging resolution and the high risk of invasive measurements in these arteries.
Overall, despite over thirty years of research and – to some degree – implementation of the analysis, some points remain to be resolved, for carotid analysis and wave analysis in the vasculature in general:
-
Consistency around nomenclature would be beneficial: this includes terms such as ‘wave’ (which often needs to be defined due to its many meanings) and terminology such as decompression vs. expansion (for the protodiastolic wave); the former also relates to the theoretical importance of framing the analysis based on successive wave fronts vs. sinusoidal waves, potentially posing theoretical complications, such as discussing the arrival time of a sinusoidal wave which theoretically has no beginning or end. Usually, this is overcome by utilising phase differences, however, this approach is not universally applied at present.
-
Consistency with respect to the use of FCW, BCW and FEW from wave separation as opposed to net wave intensity.
-
Consistency around symbols: as different formulations can be adopted, particularly (P, U) formulation vs. (A, U) or (D, U) formulations, clarity around symbols would be helpful.
-
Consistency around the formulation itself, with consensus about the use of differences or differentials, i.e. dI = dPdU vs. dI/dt = dP/dt dU/dt, which in turn would raise the questions of I = dPdU vs. dI = dPdU.
-
Systematic identification of wave intensity peaks could be beneficial particularly when considering clinical implementation, analogous to ECG peaks identification.
4.1 Outlook
Wave intensity and the measurement of wave speed are accepted techniques in the field of vascular physiology and arterial research. Here, we propose that wave speed and wave intensity may be useful non-invasive measures to better our understanding of cerebral haemodynamic mechanisms. We suggest further work is needed to understand the reported variability of the measures across studies and to evaluate relevant reference values for wave speed and the wave components in the healthy human artery.
Availability of data and material
Included in the manuscript.
References
Caro CG, Pedley TJ, Schroter RC, Seed WA. The mechanics of the circulation. Oxford University Press; 1978.
Moens I. Die Pulscurve. Leiden EJ Brill; 1978. Available online https://archive.org/details/diepulscurve00iseb/page/4/mode/2up. Accessed 08 Aug 2024.
Korteweg DJ. Ueber die Fortpflanzungsgeschwindigkeit des Schalles in elastischen Röhren. Annalen der Physik und Chemie. 1878;241(12):525–42.
Warnert EAH, Rodrigues JCL, Burchell AE, Neumann S, Ratcliffe LEK, Manghat NE, et al. Is high blood pressure self-protection for the brain? Circ Res. 2016;119(12):e140–e151.
Mok V, Kim JS. Prevention and management of cerebral small vessel disease. J Stroke. 2015;17(2):111.
Tutino VM, Mandelbaum M, Takahashi A, Pope LC, Siddiqui A, Kolega J, et al. Hypertension and estrogen deficiency augment aneurysmal remodeling in the rabbit circle of willis in response to carotid ligation. Anat Rec. 2015;298(11):1903–10.
Backes D, Rinkel GJE, Laban KG, Algra A, Vergouwen MDI. Patient- and aneurysm-specific risk factors for intracranial aneurysm growth. Stroke. 2016;47(4):951–7.
Hainsworth AH, Markus HS, Schneider JA. Cerebral small vessel disease, hypertension, and vascular contributions to cognitive impairment and dementia. Hypertension. 2024;81(1):75–86.
Chiesa ST, Masi S, Shipley MJ, Ellins EA, Fraser AG, Hughes AD, et al. Carotid artery wave intensity in mid- to late-life predicts cognitive decline: the Whitehall II study. Eur Heart J. 2019;40(28):2300–9.
Warnert EAH, Verbree J, Wise RG, van Osch MJP. Using high-field magnetic resonance imaging to estimate distensibility of the middle cerebral artery. Neurodegener Dis. 2016;16(5–6):407–10.
Roloff EvL, Tomiak-Baquero AM, Kasparov S, Paton JFR. Parasympathetic innervation of vertebrobasilar arteries: is this a potential clinical target? J Physiol. 2016;594(22):6463–85.
Jones JD, Castanho P, Bazira P, Sanders K. Anatomical variations of the circle of Willis and their prevalence, with a focus on the posterior communicating artery: a literature review and meta-analysis. Clin Anat. 2021;34(7):978–90.
Mosmans PCM, Jonkman EJ. The significance of the collateral vascular system of the brain in shunt and steal syndromes. Clin Neurol Neurosurg. 1980;82(3):145–56.
North RR, Fields WS, Debakey ME, Crawfors ES. Brachial-basilar insufficiency syndrome. Neurology (Minneap). 1962;12:810.
Alexandrov AV, Sharma VK, Lao AY, Tsivgoulis G, Malkoff MD, Alexandrov AW. Reversed robin hood syndrome in acute ischemic stroke patients. Stroke. 2007;38(11):3045–8.
Feindel W, Perot P. Red cerebral veins. J Neurosurg. 1965;22(4):315–25.
Waltz AG. Red venous blood: occurrence and significance in ischemic and nonischemic cerebral cortex. J Neurosurg. 1969;31(2):141–8.
Wilson MH. Monro-Kellie 2.0: the dynamic vascular and venous pathophysiological components of intracranial pressure. J Cereb Blood Flow Metab. 2016;36(8):1338–50.
Kasprowicz M, Lalou DA, Czosnyka M, Garnett M, Czosnyka Z. Intracranial pressure, its components and cerebrospinal fluid pressure-volume compensation. Acta Neurol Scand. 2016;134(3):168–80.
Seymour RS, Hargens AR, Pedley TJ. The heart works against gravity. Am J Physiol-Regul Integr Comp Physiol. 1993;265(4):R715–20.
Schmitz G, Lighthill J. Waves in fluids. Cambridge-London-New York-Melbourne, Cambridge University Press 1978. XV, 504 S., £ 17.50 A. ZAMM - Zeitschrift für Angewandte Mathematik und Mechanik. 1979;59(11):671–671.
Bramwell JC, Hill AV. The velocity of the pulse wave in man. Proc R Soc B Biol Sci. 1922;93(652):298–306.
Blacher J, Asmar R, Djane S, London GM, Safar ME. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension. 1999;33(5):1111–7.
Borlotti A, Khir AW, Rietzschel ER, De Buyzere ML, Vermeersch S, Segers P. Noninvasive determination of local pulse wave velocity and wave intensity: changes with age and gender in the carotid and femoral arteries of healthy human. J Appl Physiol. 2012;113(5):727–35.
Khir AW, O’Brien A, Gibbs JSR, Parker KH. Determination of wave speed and wave separation in the arteries. J Biomech. 2001;34(9):1145–55.
Rabben SI, Stergiopulos N, Hellevik LR, Smiseth OA, Slørdahl S, Urheim S, et al. An ultrasound-based method for determining pulse wave velocity in superficial arteries. J Biomech. 2004;37(10):1615–22.
Feng J, Khir AW. Determination of wave speed and wave separation in the arteries using diameter and velocity. J Biomech. 2010;43(3):455–62.
Biglino G, Steeden JA, Baker C, Schievano S, Taylor AM, Parker KH, et al. A non-invasive clinical application of wave intensity analysis based on ultrahigh temporal resolution phase-contrast cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2012;14(1):57.
Aguado-Sierra J, Parker KH, Davies JE, Francis D, Hughes AD, Mayet J. Arterial pulse wave velocity in coronary arteries. In: 2006 International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE; 2006. p. 867–70.
Sugawara M, Niki K, Furuhata H, Ohnishi S, Suzuki S. Relationship between the pressure and diameter of the carotid artery in humans. Heart Vessels. 2000;15(1):49–51.
Kowalski R, Beare R, Willemet M, Alastruey J, Smolich JJ, Cheung MMH, et al. Robust and practical non-invasive estimation of local arterial wave speed and mean blood velocity waveforms. Physiol Meas. 2017;38(11):2081–99.
Segers P, Swillens A, Taelman L, Vierendeels J. Wave reflection leads to over- and underestimation of local wave speed by the PU- and QA-loop methods: theoretical basis and solution to the problem. Physiol Meas. 2014;35(5):847–61.
Swillens A, Taelman L, Degroote J, Vierendeels J, Segers P. Comparison of non-invasive methods for measurement of local pulse wave velocity using FSI-simulations and in vivo data. Ann Biomed Eng. 2013;41(7):1567–78.
Li Y, Borlotti A, Parker KH, Khir AW. Variation of wave speed determined by the PU-loop with proximity to a reflection site. In: 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE; 2011. p. 199–202.
Borlotti A, Li Y, Parker KH, Khir AW. Experimental evaluation of local wave speed in the presence of reflected waves. J Biomech 2014;47(1):87–95.
Parker KH. Arterial tube laws and wave speeds. 2021. arxiv:2106.10061
Hermeling E, Vermeersch S, Rietzschel E, De Buyzere M, Gillebert T, van de Laar R, et al. The change in pulse wave velocity over the cardiac cycle is independently associated with left ventricular mass index in middle-aged healthy subjects. J Hypertens. 2011;29: e38.
Reesink KD, Spronck B. Constitutive interpretation of arterial stiffness in clinical studies: a methodological review. Am J Physiol-Heart Circ Physiol. 2019;316(3):H693-709.
Spronck B, Avolio AP, Tan I, Butlin M, Reesink KD, Delhaas T. Arterial stiffness index beta and cardio-ankle vascular index inherently depend on blood pressure but can be readily corrected. J Hypertens. 2017;35(1):98–104.
Hodis S, Zamir M. Pulse wave velocity as a diagnostic Index: The pitfalls of tethering versus stiffening of the arterial wall. J Biomech. 2011;44(7):1367–73.
Crowe LA, Ariff B, Keegan J, Mohiaddin RH, Yang GZ, Hughes AD, et al. Comparison between three-dimensional volume-selective turbo spin-echo imaging and two-dimensional ultrasound for assessing carotid artery structure and function. J Magn Reson Imaging. 2005;21(3):282–9.
Zambanini A, Cunningham SL, Parker KH, Khir AW, McG. Thom SA, Hughes AD. Wave-energy patterns in carotid, brachial, and radial arteries: a noninvasive approach using wave-intensity analysis. Am J Physiol-Heart Circ Physiol. 2005;289(1):H270–6.
Su J, Hilberg O, Howard L, Simonsen U, Hughes AD. A review of wave mechanics in the pulmonary artery with an emphasis on wave intensity analysis. Acta Physiol. 2016;218(4):239–49.
Parker KH, Jones CJH. Forward and backward running waves in the arteries: analysis using the method of characteristics. J Biomech Eng. 1990;112(3):322–6.
Rakebrandt F, Palombo C, Swampillai J, Schön F, Donald A, Kozàkovà M, et al. Arterial wave intensity and ventricular-arterial coupling by vascular ultrasound: rationale and methods for the automated analysis of forwards and backwards running waves. Ultrasound Med Biol. 2009;35(2):266–77.
Davies JE, Alastruey J, Francis DP, Hadjiloizou N, Whinnett ZI, Manisty CH, et al. Attenuation of wave reflection by wave entrapment creates a “Horizon Effect” in the human aorta. Hypertension. 2012;60(3):778–85.
Parker KH. An introduction to wave intensity analysis. Med Biol Eng Comput [Internet]. 2009;47(2):175–88. https://doi.org/10.1007/s11517-009-0439-y.
Avolio AP, Van Bortel LM, Boutouyrie P, Cockcroft JR, McEniery CM, Protogerou AD, et al. Role of pulse pressure amplification in arterial hypertension. Hypertension. 2009;54(2):375–83.
Mynard JP, Kondiboyina A, Kowalski R, Cheung MMH, Smolich JJ. Measurement, analysis and interpretation of pressure/flow waves in blood vessels. Front Physiol. 2020;27:11.
Parker KH, Jones CJH, Dawson JR, Gibson DG. What stops the flow of blood from the heart? Heart Vessels. 1988;4(4):241–5.
Fujimoto S, Mizuno R, Saito Y, Nakamura S. Clinical application of wave intensity for the treatment of essential hypertension. Heart Vessels. 2004;19(1):19–22.
Reavette RM, Sherwin SJ, Tang MX, Weinberg PD. Wave intensity analysis combined with machine learning can detect impaired stroke volume in simulations of heart failure. Front Bioeng Biotechnol. 2021;9:737055.
Hughes AD, Park C, Davies J, Francis D, McG. Thom SA, Mayet J, et al. Limitations of augmentation index in the assessment of wave reflection in normotensive healthy individuals. Bauer WR, editor. PLoS ONE. 2013;8(3): e59371.
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349(jan02 1):g7647–g7647.
Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(Oct 18):d5928–d5928.
Neumann S, Sophocleous F, Kobetic MD, Hart EC, Nightingale AK, Parker KH, et al. Wave intensity analysis in the internal carotid artery of hypertensive subjects using phase-contrast MR angiography and preliminary assessment of the effect of vessel morphology on wave dynamics. Physiol Meas. 2018;39(10): 104003.
Ayadi A, Sahtout W, Baledent O. A novel non-invasive method for estimating the local wave speed at a single site in the internal carotid artery. Biomed Eng/Biomedizinische Technik. 2020;65(5):557–566.
Pomella N, Wilhelm EN, Kolyva C, González-Alonso J, Rakobowchuk M, Khir AW. Noninvasive assessment of the common carotid artery hemodynamics with increasing exercise work rate using wave intensity analysis. Am J Physiol-Heart Circ Physiol. 2018;315(2):H233–41.
Curtis SL, Zambanini A, Mayet J, McG Thom SA, Foale R, Parker KH, et al. Reduced systolic wave generation and increased peripheral wave reflection in chronic heart failure. Am J Physiol-Heart Circ Physiol. 2007;293(1):H557–62.
Lucini D, Palombo C, Malacarne M, Pagani M. Relationship between carotid artery mechanics and the spontaneous baroreflex. J Hypertens. 2012;30(9):1809–16.
Aguado-Sierra J, Davies JE, Hadjiloizou N, Francis D, Mayet J, Hughes AD, et al. Reservoir-wave separation and wave intensity analysis applied to carotid arteries: A hybrid 1D model to understand haemodynamics. In: 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE; 2008. p. 1381–4.
Borlotti A, Vermeersch S, Rietzschel E, Segers P, Khir AW. A comparison between local wave speed in the carotid and femoral arteries in healthy humans: Application of a new method. In: 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology. IEEE; 2010. p. 2857–60.
Di Lascio N, Gemignani V, Bianchini E, Bruno RM, Ghiadoni L, Faita F. Effects of carotid pressure waveforms on the results of wave separation, wave intensity and reservoir pressure analysis. Physiol Meas. 2018;39(11): 114003.
Rietzschel ER, De Buyzere ML, Bekaert S, Segers P, De Bacquer D, Cooman L, et al. Rationale, design, methods and baseline characteristics of the Asklepios Study. Eur J Cardiovasc Prev Rehabil. 2007;14(2):179–91.
Manisty C, Mayet J, Tapp RJ, Sever PS, Poulter N, McG. Thom SA, et al. Atorvastatin treatment is associated with less augmentation of the carotid pressure waveform in hypertension. Hypertension. 2009;54(5):1009–13.
Manisty CH, Zambanini A, Parker KH, Davies JE, Francis DP, Mayet J, et al. Differences in the magnitude of wave reflection account for differential effects of amlodipine- versus atenolol-based regimens on central blood pressure. Hypertension. 2009;54(4):724–30.
Zambanini A, Khir AW, Byrd SM, Parker KH, Thom SAM, Hughes AD. Wave intensity analysis: a novel non-invasive method for determining arterial wave transmission. In: Computers in Cardiology. IEEE, 2002; p. 717–20.
Kowalski R, Lee MGY, Doyle LW, Cheong JLY, Smolich JJ, D’Udekem Y, et al. Reduced aortic distensibility is associated with higher aorto‐carotid wave transmission and central aortic systolic pressure in young adults after coarctation repair. J Am Heart Assoc. 2019;8(7):e011411.
Pomella N, Wilhelm EN, Kolyva C, González-Alonso J, Rakobowchuk M, Khir AW. Common carotid artery diameter, blood flow velocity and wave intensity responses at rest and during exercise in young healthy humans: a reproducibility study. Ultrasound Med Biol. 2017;43(5):943–57.
Rowland EM, Riemer K, Lichtenstein K, Tang MX, Weinberg PD. Non-invasive assessment by B-mode ultrasound of arterial pulse wave intensity and its reduction during ventricular dysfunction. Ultrasound Med Biol. 2023;49(2):473–88.
Giudici A, Palombo C, Morizzo C, Kozakova M, Cruickshank JK, Wilkinson IB, et al. Transfer‐function‐free technique for the noninvasive determination of the human arterial pressure waveform. Physiol Rep. 2021;9(18):e15040.
Kozakova M, Morizzo C, Goncalves I, Natali A, Nilsson J, Palombo C. Cardiovascular organ damage in type 2 diabetes mellitus: the role of lipids and inflammation. Cardiovasc Diabetol. 2019;18(1):61.
Babcock MC, Lefferts WK, Hughes WE, Fitzgerald KL, Leyer BK, Redmond JG, et al. Acute effect of high-intensity cycling exercise on carotid artery hemodynamic pulsatility. Eur J Appl Physiol. 2015;115(5):1037–45.
Palombo C, Malshi E, Morizzo C, Rakebrandt F, Corretti V, Santini F, et al. Arterial wave reflection during antihypertensive therapy with barnidipine: a 6-month, open-label study using an integrated cardiovascular ultrasound approach in patients with newly diagnosed hypertension. Clin Ther. 2009;31(12):2873–85.
Heffernan KS, Lefferts WK, Atallah-Yunes NH, Glasgow AC, Gump BrooksB. Racial differences in left ventricular mass and wave reflection intensity in children. Front Pediatr. 2020;8:132.
Swampillai J, Rakebrandt F, Morris K, Jones CJH, Fraser AG. Acute effects of caffeine and tobacco on arterial function and wave travel. Eur J Clin Invest. 2006;36(12):844–9.
Liu J, Cao TS, Duan YY, Yang YL, Yuan LJ. Effects of cold pressor-induced sympathetic stimulation on the mechanical properties of common carotid and femoral arteries in healthy males. Heart Vessels. 2011;26(2):214–21.
Babcock MC, Lefferts WK, Heffernan KS. Relation between exercise central haemodynamic response and resting cardiac structure and function in young healthy men. Clin Physiol Funct Imaging. 2017;37(4):372–8.
Saiki H, Kurishima C, Masutani S, Senzaki H. Cerebral circulation in patients with fontan circulation: assessment by carotid arterial wave intensity and stiffness. Ann Thorac Surg. 2014;97(4):1394–9.
Vriz O, Pellegrinet M, Zito C, di Bello V, Bettio M, Carerj S, et al. One-point carotid wave intensity predicts cardiac mortality in patients with congestive heart failure and reduced ejection fraction. Int J Cardiovasc Imaging. 2015;31(7):1369–78.
Vriz O, Zito C, di Bello V, La Carrubba S, Driussi C, Carerj S, et al. Non-invasive one-point carotid wave intensity in a large group of healthy subjects. Heart Vessels. 2016;31(3):360–9.
Vriz O, Favretto S, Jaroch J, Wojciech R, Bossone E, Driussi C, et al. Left ventricular function assessed by one-point carotid wave intensity in newly diagnosed untreated hypertensive patients. J Ultrasound Med. 2017;36(1):25–35.
Lefferts WK, DeBlois JP, Augustine JA, Keller AP, Heffernan KS. Age, sex, and the vascular contributors to cerebral pulsatility and pulsatile damping. J Appl Physiol. 2020;129(5):1092–101.
Heffernan KS, Lefferts WK, Augustine JA. Hemodynamic correlates of late systolic flow velocity augmentation in the carotid artery. Int J Hypertens. 2013;2013:1–7.
Palombo C, Kozakova M, Morizzo C, Gnesi L, Barsotti M, Spontoni P, et al. Circulating endothelial progenitor cells and large artery structure and function in young subjects with uncomplicated Type 1 Diabetes. Cardiovasc Diabetol. 2011;10(1):88.
Zhang Z, Luo R, Tan B, Qian J, Duan Y, Wang N, et al. Carotid artery stiffness evaluated early by wave intensity in normal left ventricular function in post-radiotherapy patients with nasopharyngeal carcinoma. J Med Ultrason. 2018;45(2):301–6.
Lefferts WK, Augustine JA, Heffernan KS. Effect of acute resistance exercise on carotid artery stiffness and cerebral blood flow pulsatility. Front Physiol. 2014;19:5.
Kozakova M, Morizzo C, Bianchi C, Di Filippi M, Miccoli R, Paterni M, et al. Glucose-related arterial stiffness and carotid artery remodeling: a study in normal subjects and type 2 diabetes patients. J Clin Endocrinol Metab. 2014;99(11):E2362–6.
Hasegawa H, Hongo K, Kanai H. Measurement of regional pulse wave velocity using very high frame rate ultrasound. J Med Ultrason. 2013;40(2):91–8.
Zhang X, Liu J, Cheng Z, Wu B, Xie J, Zhang L, et al. Personalized <scp>0D‐1D</scp> multiscale hemodynamic modeling and wave dynamics analysis of cerebral circulation for an elderly patient with dementia. Int J Numer Methods Biomed Eng. 2021;37(9):e3510.
Hathano S, Nobuoka S, Aono J, Nagashima J, Tokuoka S, Ozawa Y, et al. Influence of hot water bathing on reflection pressure wave. J Jpn Soc Balneol Climatol Phys Med. 2002;65(2):83–8.
Manisty C, Mayet J, Tapp RJ, Parker KH, Sever P, Poulter NH, et al. Wave reflection predicts cardiovascular events in hypertensive individuals independent of blood pressure and other cardiovascular risk factors. J Am Coll Cardiol. 2010;56(1):24–30.
Aghilinejad A, Amlani F, Liu J, Pahlevan NM. Accuracy and applicability of non-invasive evaluation of aortic wave intensity using only pressure waveforms in humans. Physiol Meas. 2021;42(10): 105003.
Nogami Y, Seo Y, Yamamoto M, Ishizu T, Aonuma K. Wave intensity as a useful modality for assessing ventilation–perfusion imbalance in subclinical patients with hypertension. Heart Vessels. 2018;33(8):931–8.
Zhang Y, Liu M, Wang M, Zhang L, Lv Q, Xie M, et al. Wave intensity analysis of carotid artery: a noninvasive technique for assessing hemodynamic changes of hyperthyroid patients. J Huazhong Univ Sci Technol [Medical Sciences]. 2010;30(5):672–7.
Liu J, Yuan LJ, Zhang ZM, Duan YY, Xue JH, Yang YL, et al. Effects of acute cold exposure on carotid and femoral wave intensity indexes: evidence for reflection coefficient as a measure of distal vascular resistance. J Appl Physiol. 2011;110(3):738–45.
Niki K, Sugawara M, Chang D, Harada A, Okada T, Tanaka R. Effects of sublingual nitroglycerin on working conditions of the heart and arterial system: analysis using wave intensity. J Med Ultrason. 2005;32(4):145–52.
Sugawara M, Niki K, Ohte N, Okada T, Harada A. Clinical usefulness of wave intensity analysis. Med Biol Eng Compu. 2009;47(2):197–206.
Lefferts WK, DeBlois JP, Receno CN, Barreira TV, Brutsaert TD, Carhart RL, et al. Effects of acute aerobic exercise on arterial stiffness and cerebrovascular pulsatility in adults with and without hypertension. J Hypertens. 2018;36(8):1743–52.
Niki K, Sugawara M, Chang D, Harada A, Okada T, Sakai R, et al. A new noninvasive measurement system for wave intensity: evaluation of carotid arterial wave intensity and reproducibility. Heart Vessels. 2002;17(1):12–21.
Niki K, Sugawara M, Kayanuma H, Takamisawa I, Watanabe H, Mahara K, et al. Associations of increased arterial stiffness with left ventricular ejection performance and right ventricular systolic pressure in mitral regurgitation before and after surgery: wave intensity analysis. IJC Heart Vasc. 2017;16:7–13.
Smith DL, DeBlois JP, Wharton M, Fehling PC, Ranadive SM. Effect of moderate exercise-induced heat stress on carotid wave intensity. Eur J Appl Physiol. 2015;115(10):2223–30.
Takaya Y, Taniguchi M, Sugawara M, Nobusada S, Kusano K, Akagi T, et al. Evaluation of exercise capacity using wave intensity in chronic heart failure with normal ejection fraction. Heart Vessels. 2013;28(2):179–87.
Bleasdale RA, Mumford CE, Campbell RI, Fraser AG, Jones CJH, Frenneaux MP. Wave intensity analysis from the common carotid artery: a new noninvasive index of cerebral vasomotor tone. Heart Vessels. 2003;18(4):202–6.
Du GQ, Li HR, Xue JY, Chen S, Du P, Wu Y, et al. Wave intensity analysis can identify eccentric cardiac hypertrophy in hypertensive patients with varied left ventricular configurations. J Ultrasound Med. 2015;34(11):2019–27.
Li Y, Guo L. Clinical value of carotid wave intensity analysis for differentiating nonobstructive hypertrophic cardiomyopathy from left ventricular hypertrophy secondary to systemic hypertension. J Clin Ultrasound. 2013;41(3):151–7.
Tanaka M, Sugawara M, Ogasawara Y, Suminoe I, Izumi T, Niki K, et al. Noninvasive evaluation of left ventricular force−frequency relationships by measuring carotid arterial wave intensity during exercise stress. J Med Ultrason. 2015;42(1):65–70.
HITACHI. Products. Wave Intensity - The new Index of Cardiovascular Circulation Dynamics. [cited 2020 Jun 10]. Available from: http://www.hitachi-medical-systems.eu/products/ultrasound/technologies/cardiovascular-technologies/wave-intensity.html. Accessed 03 Aug 2023.
Acknowledgements
This work was inspired by the collaborative efforts on wave intensity analysis in the internal carotid artery.
Funding
None to declare.
Author information
Authors and Affiliations
Contributions
SN has contributed to the conception and design of the work; the acquisition, analysis, and interpretation of data and have drafted the work, edited revisions of the manuscript and have approved the submitted version. GB has contributed to the conception and design of the work; the acquisition, analysis, and interpretation of data and have drafted the work, edited revisions of the manuscript and have approved the submitted version. KP has contributed to the conception and interpretation of data, provided revisions of the manuscript and have approved the submitted version. AH has contributed to the conception and interpretation of data, provided revisions of the manuscript and have approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
None to declare.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Neumann, S., Parker, K.H., Hughes, A.D. et al. A Systematic Review of Wave Speed and Wave Intensity Measures in the Human Carotid Arteries. Artery Res 30, 12 (2024). https://doi.org/10.1007/s44200-024-00058-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44200-024-00058-4