Abstract
Objectives
Acute viral bronchiolitis (AVB) is a major cause of hospitalization for children in developed and developing countries. Nasal high flow (NHF) therapy improves oxygenation and reduces respiratory drive by enhancing carbon dioxide wash-out. However, little is known about the physiological effects of non-invasive helmet continuous positive airway pressure (h-CPAP) and NHF on respiratory work of breathing (WOB) in pediatric patients with AVB. The present study measured esophageal pressure time product over 1 min (PTPes*min−1), as a close surrogate for WOB during standard oxygen therapy (SOT), NHF delivered at incremental flow rates, and h-CPAP in hospitalized patients with AVB.
Methods
This is a physiological randomized crossover study with four 20-min steps: SOT delivered by a Venturi mask; NHF2 set at 2L/kg*min−1PBW; NHF3 set at 3L/kg*min−1PBW; and h-CPAP with PEEP 7 cmH2O. PTPes *min−1, pressure rate product (PRP), respiratory and other physiological parameters were collected towards the end of each step.
Results
Ten hypoxemic children with AVB were enrolled. PTPes*min−1, respiratory rate (RR), PRP, and heart rate (HR) decreased progressively from h-CPAP to NHF3, NHF2, and SOT (p < 0.01). Transcutaneous carbon dioxide tension (tcCO2) was lower during h-CPAP, NHF3, and NHF2 than during SOT (p < 0.001). SpO2:FiO2 was higher during h-CPAP than with all other support (p < 0.01).
Conclusions
In pediatric patients with AVB, h-CPAP was associated with lower WOB, better oxygenation, and lower tcCO2 than with SOT and NHF trials.
Trial registration
Clinicaltrials.gov NCT03689686 Registered 1 August 2018.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute respiratory failure (ARF) due to acute viral bronchiolitis (AVB) is a major cause of hospitalization for children, with admission rates to pediatric intensive care unit (PICU) of 2–6% [1, 2]. AVB in children involves an increase in airway resistance and a decrease in lung compliance due to airway occlusion, alveolar collapse and atelectasis, leading to a rapid shallow breathing pattern [3, 4]. The application of 6–7 cmH2O of continuous positive airway pressure (CPAP) promotes alveolar recruitment, reduces the ventilation-perfusion mismatch and stents the airways, unloading the respiratory muscles, reducing respiratory distress and improving gas exchange [5,6,7].
Non-invasive respiratory support (NRS) with nasal high flow (NHF) was introduced recently in adults and children [8,9,10,11,12,13]. NHF is associated with enhanced carbon dioxide (CO2) wash-out from the upper airways and delivers low end-expiratory positive airway pressure. These effects, combined with optimal airway humidification, reduce the respiratory work of breathing (WOB) and improve gas exchange, potentially lowering the risk of failure of non-invasive approach [12,13,14,15,16,17,18,19]. However, the physiological effects of non-invasive helmet CPAP (h-CPAP) and NHF at different flow rates on respiratory WOB have not been compared in paediatric patients with AVB.
The aim of this physiological randomized crossover study is to compare the effects of standard oxygen therapy (SOT), NHF 2 and 3 L/kg min−1 predicted body weight (PBW) and h-CPAP on the WOB of children with moderate to severe hypoxemic ARF due to AVB.
Methods
The study received approval from the local institutional Ethics Committee, and written informed consent was obtained from the parents or legal guardian of the patients. The study was registered on ClinicalTrial.gov (NCT03689686). Consolidated Standards of Reporting Trials guidelines were followed, and the study was conducted according to the Helsinki 1964 Ethical Declaration Standard, revised in 2008 [20, 21].
Study design
This was a physiological cross-over study that compared four 20-min steps delivered in computer-generated random order:
-
Standard Oxygen Therapy delivered by a non-fitting oxygen Venturi mask (SOT)
-
Nasal high flow delivered at 2 L/kg*min.−1PBW (NHF2)
-
Nasal high flow delivered at 3 L/kg*min.−1PBW (NHF3)
-
CPAP with positive end-expiratory pressure of 7 cmH2O, delivered through a paediatric helmet (h-CPAP) [22, 23].
A phone-call service was available 24/7 for randomization.
End-points
The primary end-point of the study was the difference in esophageal pressure time product per minute (PTPes * min−1), which serves as a surrogate for respiratory WOB.
Inspiratory esophageal pressure swings (ΔPes), pressure rate product (PRP) (i.e., ΔPes * respiratory rate), respiratory physiological parameters, the Modified Woods Clinical Asthma Score (M-WCAS) and the EDIN Score were collected at the end of each step and then compared too. M-WCAS is a combined score used to assess the degree of respiratory distress in children with bronchiolitis. M-WCAS is a combined score used to evaluate the respiratory distress in children with bronchiolitis. It includes indicators such as oxygen saturation, inspiratory breath sounds, expiratory wheezing, use of accessory respiratory muscles, and cerebral function [24]. The EDIN scale is utilized to assess comfort through five behavioral indicators, including facial activity, body movements, quality of sleep, quality of contact with nurses, and consolability [25].
Study population
All consecutive children between the ages of 1 month and 2 years, admitted to the Pediatric Intensive Care Unit (PICU) of the Fondazione Cà Granda, Ospedale Maggiore Policlinico in Milan, Italy, from 1 October 2018 to 30 April 2019, with clinical suspicion of AVB, were screened for eligibility.
The clinical suspicion of AVB was determined based on the following criteria:
-
Presence of infiltrates on chest radiographs
-
Up to three of the following symptoms: body temperature > 38 °C, leucocytosis/leukopenia, purulent secretions, wheezing or abnormal breath sounds.
Nasopharyngeal and/or tracheal secretions were collected by non-bronchoscopic blind technique at admission and AVB infection was detected using an enzyme-linked immunoadsorbent assay [26].
Inclusion criteria were age > 30 days and < 2 years; SpO2:FiO < 264 while receiving additional oxygen; RR > 2SD according to age and/or active contraction of inspiratory muscles and/or paradoxical abdominal motion (2 out of 3 minimum).
Exclusion criteria were as follows: need for immediate intubation; Glasgow Coma Scale < 12; pH < 7.25; impaired cough reflex; upper-airway obstruction; previous facial/gastric surgery; recurrent apneas; hemodynamic instability (need for vasopressor or inotropes); evidence of pneumothorax on lung echo or chest X-ray; contraindications to insertion of an esophageal catheter [22, 23, 27].
Measurements and definitions
The following variables were collected upon admission to PICU admission: age, sex, weight, Pediatric Index of Mortality 2 (PIM2), and pediatric Sequential Organ Failure Assessment (pSOFA) Score [28, 29]. Throughout the study, electrocardiogram traces, transcutaneous measurements of carbon dioxide and oxygen tension, RR, systolic, and diastolic blood pressure were displayed on a multiparametric PICU monitor (DraegerWerk AG and Co., KGaA, Lubeck, Germany).
To measure esophageal pressure, a radio-opaque 6-French (Fr) balloon catheter (CareFusion, San Diego, CA, USA) was inserted through the nose and advanced approximately 15–20 cm until reaching the stomach. The balloon was inflated with the recommended volume of air (0.3–0.5 mL). After confirming positive inspiratory deflection, the catheter was retracted until it reached the lower third of the esophagus, indicated by the appearance of negative inspiratory deflections and cardiac artifacts [15].
Were measured the following variables by offline analysis of tracings collected at the end of each step:
-
A)
Esophageal pressure time product over 1 min (PTPes*min−1). This is a physiological parameter used to quantify WOB in adults and children It is defined as the sum of areas subtended by the esophageal inspiratory pressure curve over a period of 5 min, divided by the number of minutes. This is a simplification of the classic computation of the PTP used in previous studies [14, 15].
-
B)
Inspiratory esophageal pressure swings (ΔPes), equal to the average difference between end-inspiratory and end-expiratory esophageal pressure measured over a 5-min period. ΔPes is used as a measurement of the patient’s inspiratory effort, in line with previous adult studies [14, 15]. By measuring the difference between the highest pressure observed during inspiration and the lowest pressure observed during expiration, ΔPesbr provides valuable information about the magnitude of effort exerted by the patient’s respiratory muscles during each breath.
-
C)
Pressure rate product (PRP), defined as the mean ΔPes * RR. PRP is a physiological parameter used to assess a patient's inspiratory effort over a 1-min period. Previous pediatric studies have utilized PRP as a reliable indicator of inspiratory effort, which makes it a valuable parameter in assessing and comparing the effects of different NRS strategies in children ARF or AVB [17,18,19].
Study protocol
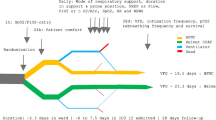
Patients were kept in semi-recumbent position, under sedation with low-dose dexmedetomidine (0.5 mcg/kg/h) delivered through all study steps, according to local PICU protocol [30]. FiO2 was set to target a peripheral saturation of 90–95% during the first step, then kept constant throughout the study. FiO2 during NHF was also measured using a dedicated system (AIRVO™2; Fisher & Paykel Healthcare, Auckland, New Zealand). NHF2 and NHF3 were delivered using specific paediatric nasal prongs (Fisher & Paykel Healthcare) and h-CPAP was delivered by a free-flow gas circuit, as described in previous paediatric studies [22, 23] (Fig. 1).
The choice of interface plays a crucial role in determining the success or failure of NRS. The use of a helmet for delivering CPAP in adults and children has gained extensive experience [5,6,7, 22, 23]. The helmet offers advantages such as more consistent airway pressurization, lower leakages compared to oronasal masks, and faster resolution of pediatric ARF compared to NHF2 [5,6,7, 31, 32].
The pediatric helmet utilized in the study (Castar Starmed, Mirandola, Italy) is designed with a collar diameter of 27 cm and a volume of 6 L. It is crafted from transparent, latex-free polyvinyl chloride and securely attached to a soft collar that conforms to the child's neck. The helmet system is connected to a diaper using two braces. One port of the helmet connects to a fresh gas source, while the other is linked to an underwater positive end-expiratory pressure valve. To ensure safety, an overpressure device is set at 20 cmH2O on the inspiratory line. High fresh gas flow of 40 L/min is employed to prevent CO2 rebreathing. In case of emergencies, the helmet can be quickly and easily removed. An anti-asphyxia valve is integrated to prevent CO2 rebreathing if a circuit disconnection or interruption in the gas supply occurs. This valve can be effortlessly detached to facilitate nursing and suctioning procedures. Tracheal and oral suction can be performed through an opening on the helmet's surface. The inspiratory line allows for the measurement and display of pressure, FIO2, and temperature using the Sensor OPT system (Starmed, Mirandola, Italy).
Each patient received the pre-planned steps (SOT; NHF2; NHF3; h-CPAP) in random order. The first 15 min of each step were considered as a washout period to minimize the carry-over effect. During washout periods, data were monitored but not considered for study measurements. Esophageal pressure traces were sampled at 100 Hz for 5 min towards the end of each trial and analyzed offline (ICU Kleistek, Bari, Italy). Two senior physicians not involved in the study analyzed all traces offline. Each file was assigned a classification based on an order number, with no indication of the type of respiratory support displayed on the screen. Physiological parameters were collected in the same last 5 min of each trial. A PICU senior physician not involved in the study was always present for monitoring and treatment of potential adverse events. For safety reasons, the protocol included the following termination criteria: SaO2 < 90% despite FiO2 > 0.6; tcCO2 > 10% compared to baseline; need for intubation; hemodynamic instability.
Sample size and statistical analysis
No previous data have been published comparing PTPes*min−1 values during spontaneous breathing (SB) and NHF at different flow rate in paediatric population. Therefore, we calculated the sample size according to previous published studies reporting mean value of PTPes*min−1 during SB (216 ± 100 cmH2O sec min−1) and NHF (154 ± 84 cmH2O sec min−1) in adults with ARF [14].
Considering an α-error = 0.05 and power = 80%, the study needed 15 patients to detect a 30% reduction in the primary end-point between SOT and NHF3 (MedCalc V19.1.7. software; Lt-Ostend, Belgium). Interim statistical analysis was pre-planned for 8–10–12 patients to detect excessive benefits from one treatment.
Data distribution was assessed using Shapiro–Wilk analysis. Due to the distribution of the data, a non-parametric analysis of variance (ANOVA) using the Friedman test was conducted, followed by post-hoc Bonferroni correction. Significance was considered p < 0.05. Outcome variables are presented as median and interquartile range (IQR), specifically the 1st to 3rd quartiles (MedCalc V19.1.7. software; Lt-Ostend, Belgium).
Results
The study was concluded with 10 enrolled patients because the interim statistical analysis indicated that the primary endpoint had already been achieved with a high level of significance. Therefore, it was deemed unethical to enrol additional children who would require invasive procedures such as the placement of an esophageal catheter.
Study flowchart is depicted in Fig. 2.
All enrolled patients completed the study without interruptions. No major adverse events such as hemodynamic instability, cardiac arrest, or hypercapnic coma were reported during study.
Baseline patients’ characteristics are summarized in Tables 1 and 2.
All children were enrolled early after admission and the study protocol started within a median time of 3 h from PICU admission. Enrolled children received a median period of NRS of 4[4.25–5] days with a median PICU LOS of 4[3–4]) days. No patient died during PICU and hospital stay. All patients survived at 3- and 6-months follow-up.
Primary and secondary end-point per protocol analysis is reported in Table 3 and Fig. 3.
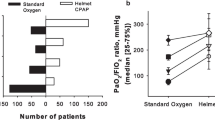
Primary and secondary end points. Depicts the effects of the different trials on the respiratory drive and patient’s effort over the study period. A, B Esophageal PTP and ΔPesBr are not affected by increasing flow rates during NHF 2 and 3 L/kg. On the other side, h-CPAP reduces the respiratory effort indexes compared to both NHF trials and standard oxygen mask. C, D Although a trend to a reduction in pressure rate product (ΔPesBr * RR) and respiratory rate and during NHF trials compared to oxygen mask was found, only h-CPAP was associated to a reduction of all respiratory effort parameters. NHF nasal high flow nasal; h-CPAP helmet continuous positive airway pressure. Within patient variability was analyzed with Friedman test with post hoc Bonferroni correction. Significance was taken at p < 0.05. §p < 0.001 h-CPAP versus NHF 3 L/kg, NHF 2 L/kg and oxygen mask
PTPes*min−1 decreased during h-CPAP (179[97–376]cmH2O*sec*min−1) compared to NHF3 (500[164–600]cmH2O*sec*min−1), NHF2 (508[216–672]cmH2O*sec*min−1), and SOT (535[228–701] cmH2O*sec*min−1) (p < 0.001). There were no differences between PTPes *min−1 values for SOT, NHF2, and NHF3.
ΔPesbr decreased during h-CPAP (10[5–13]cmH2O) compared to NHF3(15[5.5–25]cmH2O), NHF2 (17[4–26.5]cmH2O) and SOT (17[8.5–30.5] cmH2O) (p < 0.01) without differences between NHF3, NHF2, and SOT.
Pressure rate product (PRP) decreased during h-CPAP (410[207–61]) vs NHF3 (792[241–1100]), NHF2 (617[228–1100]) and SOT (812[399–1500]) (p < 0.01) without differences between NHF3, NHF2 and SOT.
MWCAS score was decreased during h-CPAP (3[2.7–4]) vs NHF3 (4.5[3–5]), NHF2 (5[3–5]), and SOT (6[5, 6]) (p < 0.01) and during NHF3 vs NHF2 and SOT (p < 0.01).
Physiological parameters across the study are reported in Table 3 and in Fig. 4. FiO2 was kept constant across the study and no variations > 5% were observed across the trials between FIO2 values displayed on AIRVO2 and values displayed by an external oximeter on inspiratory circuit limb.
Physiological parameters. Depicts the effects of the different trials on physiological parameters over the study period. A, B Oxygenation increased progressively by increasing NHF rates whereas transcutaneous carbon dioxide tension was significatively reduced compared to oxygen mask. H-CPAP was associated to a significative increase in oxygenation compared to all study trials. Effects of h-CPAP on transcutaneous carbon dioxide tension was similar if compared with NHF 2 and 3 L/kg. C, D Similar effects were reported for m-WCAS score, showing that h-CPAP and NHF 3L/kg reduced the respiratory distress and heart rate compared to NHF 2 L/kg and oxygen mask. NHF Nasal High Flow oxygen therapy; h-CPAP helmet continuous positive airway pressure; m-WCAS Modified Wood’s Clinical Asthma Score. Within patient variability was analyzed with Friedman test with post hoc Bonferroni correction. Significance was taken at p < 0.05. §p < 0.001 h-CPAP versus NHF 3 L/kg, 2 L/kg and oxygen mask; *p < 0.01 NHF 2 and 3 L/kg versus oxygen mask
SpO2:FiO2 showed a progressive significative increase during h-CPAP (326[320–326]) vs NHF3 (264[248–350]), NHF2 (270[250–300]), and SOT (200[160–215]) (p < 0.01). Moreover, we observed an increase in oxygenation even during NHF3 vs NHF2 and SOT (p < 0.01).
TCCO2decreased progressively during h-CPAP (33[31–42] mmHg), NHF3(33[31–45] mmHg), NHF2(36[35–51] mmHg) compared to SOT (46[34–58] mmHg) (p < 0.001).
Respiratory rate decreased during h-CPAP (40[35–45] breath*min−1) compared both to NHF3 (45[40–51] breath*min−1), NHF2 (45[37–55] breath*min−1), and to SOT (55[37–60] breath*min−1) (p < 0.01).
Heart rate decreased during h-CPAP (104[91–118] beats min−1) compared to NHF3 (130[120–150] bpm), NHF2 (130[120–150] beats min−1) and SOT (140[113–136] beats min−1) (p < 0.001). No difference in MAP were found across the study. Comfort improved during all NRS trials compared to SOT.
Discussion
The main findings of the study are as follows: (a) h-CPAP demonstrated a reduction in WOB, PTPes*min−1, PRP, and RR, along with improved gas exchange compared to NHF and SOT; (b) NHF2 and NHF3 showed enhanced gas exchange compared to SOT without affecting WOB; (c) increasing the flow rate from 2 to 3 L/min*kg−1PBW did not provide any additional benefits in terms of reducing WOB or improving gas exchange.
Furthermore, there were no differences in WOB between NHF2 and NHF3, supporting the notion that the lower flow rate can be considered the standard clinical setting. Notably, NHF2 may represent the upper limit of flow rate beyond which a lack of clinical response in terms of RR and HR indicates persistently elevated WOB and the need to escalate to CPAP [19, 31,32,33,34,35].
While previous studies have discussed the effects of NHF and CPAP in pediatric ARF, most of them have been observational analyses or quality improvement studies, with limited physiological investigations to determine the factors contributing to the success or failure of each method. Findings from our study underscore the importance of measuring the effects of different NRS techniques on WOB in order to effectively manage respiratory assistance and prevent potential Self-Induced Lung Injury (SILI) since the early stages of ARF. Although the normal level of WOB generated in pediatric ARF is not well-known, evidence from adult studies suggests that excessive WOB, accompanied by significant swings in intrapleural pressure, can lead to diaphragmatic fatigue and SILI if not promptly detected and treated since the early phases of ARF.
Currently, esophageal pressure measurement is considered the gold standard for assessing WOB in adults and children. The pressure time product (PTPes*min−1) correlates well with WOB in pediatric populations and enables monitoring the effects of various interventions on respiratory muscle unloading.
The present study was designed to investigate the effects of different NRS systems on WOB and explore the feasibility of translating these findings into clinical practice, where esophageal pressure monitoring is still considered experimental and individual respiratory effort is primarily assessed using clinical scores.
The findings from our study align with pediatric studies, highlighting the optimal flow rate for NHF as 2 L/min*kg−1PBW. Additionally, h-CPAP was found to provide more effective support for escalating care after NHF failure, and its physiological benefits on respiratory mechanics are associated with improved clinical outcomes [16,17,18,19, 36].
Nowadays, the timing of escalation of NRS from NHD to CPAP is still widely debated.
In the current pediatric literature, the need to escalate from NHF to CPAP has been associated with persistent tachypnea, tachycardia, and increased oxygen requirement during NHF treatment [33]. Data from this study suggest that a lack of response in terms of RR and HR during NHF2 may indicate persistently increased WOB, potentially leading to muscle exhaustion or the need for intubation. Although measuring WOB remains challenging in clinical practice, it can be hypothesized that persistently elevated RR and HR might reflect sustained WOB and predict the need for escalation from NHF to CPAP, consistent with findings from the PARIS study [33].
This study has several strengths: the patient population was homogeneous in terms of the severity of ARF, and the study endpoints were clearly established a priori, minimizing subjective decisions on the main outcomes. WOB was measured using a gold standard method applicable in the pediatric population.
The study also has some limitations. Firstly, it was a short-term physiological study that included a small sample of children. Although this physiological study was able to address our main objective, the power of the study may have been insufficient to show small differences between conditions or to conduct subgroup analyses. Furthermore, our population was selected based on age, sex, cause of ARF, and pSOFA score, indicating only respiratory involvement. Therefore, the generalizability of these results deserves further evaluation and cannot be extended to ARF due to other etiologies or in the presence of multiple organ failures. Secondly, the study was not blinded to treatment as it was impossible to conceal the interface from healthcare providers. However, the analysis of esophageal pressure tracings was performed blindly on an unidentified database. Thirdly, CO2 tension was measured using a transcutaneous gas analyzer, and oxygenation was determined only using the SpO2:FiO2 ratio to avoid the need for arterial cannulation [37].
In conclusion, the results of this study suggest that:
-
a)
h-CPAP offers the greatest physiological benefits, including reduced WOB, improved gas exchange, and increased patient comfort, making it the preferred option for escalating respiratory support when signs of respiratory distress persist under NHF.
-
b)
NHF2 remains the optimal flow rate for improving gas exchange in successfully treated patients.
-
c)
NHF2 may serve as the upper limit of flow rate beyond which a lack of clinical response could predict treatment failure and necessitate escalation to h-CPAP.
-
d)
Improvements in simple physiological parameters such as RR and HR are correlated with lower WOB and can guide the optimization of NRS, even in the absence of other invasive effort monitoring techniques.
Additional clinical studies are required to develop new WOB monitoring systems reliable during NRS for each pediatric age group.
Availability of data and materials
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available on account of privacy or ethical restrictions.
References
Balfour Lynn RE, Marsh G, Gorayi D et al (2014) Non-invasive ventilation for children with acute respiratory failure in the developing world: literature review and implementation example. Paediatr Respir Rev 15:181–187
Murthy S, Kissoon N (2014) Management of severe viral infections in the paediatric intensive care unit. J Paediatr Intensive Care 3:205–216
Ganu SS, Gautam A, Wilkins B et al (2012) Increase in use of non-invasive ventilation for infants with severe bronchiolitis is associated with decline in intubation rates over a decade. Intensive Care Med 38:1177–1183
Essouri S, Laurent M, Chevret L et al (2014) Improved clinical and economic outcomes in severe bronchiolitis with pre-emptive nCPAP ventilatory strategy. Intensive Care Med 40:84–91
Cambonie G, Milési C, Jaber S et al (2008) Nasal continuous positive airway pressure decreases respiratory muscles overload in young infants with severe acute viral bronchiolitis. Intensive Care Med 34:1865–1872
Milési C, Matecki S, Jaber S et al (2013) 6 cmH2O continuous positive airway pressure versus conventional oxygen therapy in severe viral bronchiolitis: a randomized trial. Pediatr Pulmonol 48:45–51
Essouri S, Durand P, Chevret L et al (2011) Optimal level of nasal continuous positive airway pressure in severe viral bronchiolitis. Intensive Care Med 37:2002–2007
Papazian L, Corley A, Hess D, Fraser JF, Frat JP et al (2016) Use of high-flow nasal cannula oxygenation in ICU adults: a narrative review. Intensive Care Med 42:1336–1349
Nishimura M (2016) High-flow nasal cannula oxygen therapy in adults: physiological benefits, indication, clinical benefits, and adverse effects. Respir Care 61:529–541
Maggiore SM, Idone FA, Vaschetto R, Festa R et al (2014) Nasal high-flow versus Venturi mask oxygen therapy after extubation. Effects on oxygenation, comfort, and clinical outcome. Am J Respir Crit Care Med 190:282–288
Frat JP, Thille AW, Mercat A, Girault C et al (2015) High-flow oxygen through nasal cannula: FLORALI Study Group; REVA Network. New Eng J Med 372:2185–2196
Lee JH, Rehder KJ, Williford L, Cheifetz IM, Turner DA (2013) Use of high flow nasal cannula in critically ill infants, children, and adults: a critical review of the literature. Intensive Care Med 39:247–257
Nielsen KR, Ellington EL, Gray AJ et al (2017) Effect of high-flow nasal cannula on expiratory pressure and ventilation in infant, pediatric, and adult models. Respir Care. https://doi.org/10.4187/respcare.05728
Mauri T, Turrini C, Eronia N, Grasselli G et al (2016) Physiologic effects of high-flow nasal cannula in acute hypoxemic respiratory failure. Am J Respir Crit Care Med 195:1207–1215
Mauri T, Alban L, Turrini C, Cambiaghi B et al (2017) Optimum support by high-flow nasal cannula in acute hypoxemic respiratory failure: effects of increasing flow rates. Intensive Care Med 43:1453–1463
Shetty SM, Hickey A, Rafferty GF, Peacock JL et al. (2016) Work of breathing during CPAP and heated humidified high-flow nasal cannula. Arch Dis Child Fetal Neonatal 0: F1–F4
Rubin S, Guman A, Deakers T, Khemani R, Ross P, Newth CJ (2014) Effort of breathing in children receiving high flow nasal cannulas. Pediatr Critic Care Med 15:1–6
Pham TM, O’Malley L, Mayfield S, Martin S, Schibler A (2015) The effect of high flow nasal cannula therapy on the work of breathing in infants with bronchiolitis. Pediatr Pulmonol 50:713–720
Weiler T, Kamerkar A, Hotz J, Ross PA, Newth CJ, Khemani RG (2017) The relationship between High Flow Nasal Cannula Flow Rate and Effort of Breathing in Children. J Pediatr 189:66–71
Begg C, Cho M, Eastwood S et al (1996) Improving the quality of reporting of randomized controlled trials The CONSORT statements. JAMA 276:637–639
Puri KS, Suresh KR, Goga NJ et al (2008) Declaration of Helsinki, 2008: Implications for stakeholders in research. J Postgrad Med 55:131–134
Chidini G, Piastra M, Marchesi T, De Luca D et al (2015) Continuous positive airway pressure with helmet versus mask in infants with bronchiolitis: an RCT. Pediatrics. https://doi.org/10.1542/peds.2014-1142
Chidini G, Calderini E, Pelosi P (2010) Treatment of acute hypoxemic respiratory failure with continuous positive airway pressure delivered by a new paediatric helmet in comparison with a standard full-face mask: a prospective pilot study. Pediatr Crit Care Med 11:502–508
Wood DW, Downes JJ, Lecks HI (1972) A clinical scoring system for the diagnosis of respiratory failure. Preliminary report on childhood status asthmaticus. Am J Dis Child 123:122
Debillon T, Zupan V, Ravault N et al (2001) Development and initial validation of the EDIN scale, a new tool for assessing prolonged pain in preterm infants. Arch Dis Child Fetal Neonatal 85:F36–F41
Puri KS, Suresh KR, Gogtay NJ, Thatte UM (2009) Declaration of Helsinki: implications for stakeholders in research. Ethics Forum. J Postgrad Med. 55:131–34.
Niederman MS, Mandell LA, Anzueto A et al (2001) American Thoracic Society Guidelines for the management of adults with community acquired pneumonia: diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med 16:1730–1817
Slater A, Shann F, Pearson G (2003) Paediatric Index of Mortality (PIM) Study Group: PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med 29:278–285
Matics TJ, Sanchez-Pinto LN (2017) Adaptation and validation of a pediatric sequential organ failure assessment score and evaluation of the sepsis-3 definitions in critically ill children. JAMA Pediatr 171:e172352
Venn RM, Hell J, Grounds RM (2000) Respiratory effects of dexmedetomidine in the surgical patient requiring intensive care. Crit Care 4:302–308
Vitaliti G, Vitaliti MG, Finocchiaro MC, Di Stefano VA et al (2017) Randomized comparison of helmet CPAP versus high-flow nasal cannula oxygen in pediatric respiratory distress. Respir Care 62:1036–1042
Lin J, Zhang Y, Xiong L, Liu S, Gong C, Dai J (2019) High-flow nasal cannula therapy for children with bronchiolitis: a systematic review and meta-analysis. Arch Dise Child 0: 1–13
Franklin D, Babl FE, Schlapbach LJ, Oakley E et al (2018) A randomized trial of high-flow therapy in infants with bronchiolitis. N Engl J Med 378:1121–1131
Milési C, Essouri S, Pouyau R, Liet J-M et al (2017) High flow nasal cannula [HFNC] versus nasal continuous positive airway pressure [nCPAP] for the initial respiratory management of acute viral bronchiolitis in young infants: a multicentre randomized controlled trial [TRAMONTANE study]. Intensive Care Med 43:209–216
Kepreotes E, Whitehead B, Attia J, Oldmeadow C, Collison A, Searles A et al (2017) High-flow warm humidified oxygen versus standard low-flow nasal cannula oxygen for moderate bronchiolitis [HFWHO RCT]: an open, phase 4, randomised controlled trial. Lancet 389:930–939
Milesi C, Pierre AF, Deho A, Liets JM et al (2018) Multicenter randomized controlled trial of a 3-L/kg/min versus 2-L/kg/min high-flow nasal cannula flow rate in young infants with severe viral bronchiolitis [TRAMONTANE 2]. Intensive Care Med 44:1870–1878
Mayordomo-Colunga J, Pons M, López Y et al (2013) Predicting noninvasive ventilation failure in children from the SpO2/FiO2 (SF) ratio. Intensive Care Med 39:1095–1110
Code availability
Not applicable.
Funding
The study was supported by the Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan, Italy.
Author information
Authors and Affiliations
Contributions
Giovanna Chidini: conceptualization—equal, data curation—equal, formal analysis—equal, supervision—equal, writing—original draft-Equal, and writing—review and editing—equal. Giorgio Conti: conceptualization—equal, formal analysis—equal, methodology—equal, writing—original draft—equal, and writing—review and editing-equal. Tommaso Mauri: conceptualization-equal, formal analysis—equal, methodology—equal, writing—original draft—equal, and writing—review and editing—equal. Stefano Scalia Catenacci: software—equal, supervision—equal, writing—original draft—equal, and writing—review and editing—equal. Tiziana Marchesi: software—equal, supervision-supporting, validation-supporting, writing—original draft—supporting, and writing—review and editing-supporting. Giada Dona: data curation—equal, software—supporting, supervision—supporting, writing—original draft—equal, and writing—review and editing—equal. Maria Adele Figini: data curation—equal, software—supporting, validation—equal, writing—original draft—equal, and writing—review and editing—equal. Giovanni Babini: investigation—equal, methodology—supporting, software—supporting, supervision—supporting, and writing—review and editing—equal. Edoardo Calderini: conceptualization—supporting, investigation—supporting, methodology—equal, and writing—review and editing—supporting. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chidini, G., Mauri, T., Conti, G. et al. Physiological effects of standard oxygen therapy, high-flow nasal cannula, and helmet CPAP in acute bronchiolitis: a randomized cross-over study. Intensive Care Med. Paediatr. Neonatal 1, 13 (2023). https://doi.org/10.1007/s44253-023-00013-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44253-023-00013-2