Abstract
Background
Neuroendocrine carcinomas (NECs) of the esophagus are extremely rare and poorly understood. This study aimed to delineate the clinicopathological and immunohistochemical features of esophageal NECs using a retrospective survey.
Methods
Patients with histologically proven esophageal neuroendocrine carcinomas (NECs) were recruited from Zhongshan Hospital, Fudan University, China, between 2006 and 2016. Clinical, endoscopic, pathological, and immunohistochemical data were collected retrospectively.
Results
Of 43 patients with NEC, older male patients were predominant. In total, 93.0% of the tumor masses were located in the middle or lower esophagus. Twenty-nine (67.4%) and 22 (51.2%) cases showed ulceration and esophageal stenosis, respectively. The average index of Ki-67 staining was 67.1% ± 21.4% with positive immunostaining for CD56 (88.4%), chromogranin A (51.2%), and synaptophysin (72.1%). Small cell carcinomas accounted for 95.3% of cases, and 16.3% of patients had mixed components with adenocarcinoma or squamous cell carcinoma.
Conclusion
Esophageal NECs are rare and mainly affect men in their sixties or seventies. They show a similar endoscopic appearance to squamous cell carcinoma or adenocarcinoma and present with a proliferative mass with stenosis or ulcers. Therefore, esophageal NECs are difficult to diagnose by endoscopy. Esophageal NECs can be mixed with other subtypes of neoplasms, such as adenocarcinoma and squamous cell carcinoma. In addition to chromogranin A, synaptophysin, and CD56, NSE, S100, and CKpan might be candidate biomarkers to diagnose esophageal NECs. Overall, we provide new insights into the biology of esophageal NECs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophageal NECs are extremely rare with a reported incidence between 0.4% and 2% among all malignancies [1]. In accordance with the WHO classification, NECs are poorly differentiated with a Ki-67 index of > 20% [2, 3]. The WHO definition for NECs includes positive neuroendocrine markers such as chromogranin A, synaptophysin, and CD56 [4]. NECs are subtyped into small- and large-cell neoplasms [3, 5], the former of which are much more frequent. In addition to neuroendocrine cells, NECs can also be mixed with non-neuroendocrine components, such as adenocarcinoma or SCC, which account for ≥ 30% of the tumor mass. In the WHO classification, NECs are termed mixed neuroendocrine-non-neuroendocrine neoplasms (MiNENs), which are well or poorly differentiated with a variable Ki-67 index [2, 3]. The most recent WHO classification of NENs in the digestive system is shown in Supplementary Fig. 1 [2, 3, 6].
Macroscopically, esophageal NECs have relatively prominent features, including submucosal growth, and are usually covered by normal epithelium with or without ulcerous lesions in the center. The endoscopic appearance of esophageal SCC correlates with the tumor size and depth of infiltration. Occasionally, it can also resemble submucosal tumors similar to esophageal NECs, although this is rare. Esophageal SCC primarily presents as ulcerative, polypoid, infiltrative, fungating, stenosing, or circumferential infiltrative, and is generally distinguishable from esophageal NECs.
Because of the rarity of esophageal NECs, there is a paucity of studies regarding the clinicopathological and immunohistochemical features of esophageal NECs. Moreover, because of the high frequency of venous, lymphatic, and perineural invasions, the tumor is often in an advanced stage at diagnosis. Additionally, no guideline or consensus has been established for the treatment of esophageal NECs, which leads to a poor prognosis. Therefore, studies with large sample sizes are urgently needed. The present study aimed to collect data from a series of patients with a diagnosis of neuroendocrine carcinoma of the esophagus, which was confirmed by pathologists with expertise in neuroendocrine neoplasms, to inform clinicians on the clinicopathological characteristics and biological behavior of this poorly understood disease.
Methods
Patients
Sixty-two eligible subjects were initially enrolled, and 19 patients who were diagnosed with adenocarcinoma or SCC mixed with neuroendocrine components accounting for < 30% of the tumor mass were excluded. Eventually, we performed a retrospective study of 43 patients with esophageal NECs at Zhongshan Hospital, Fudan University, from 2006 to 2016. The patients were examined for neuroendocrine markers (chromogranin A, synaptophysin, and CD56) and underwent gastroscopy, computed tomography, endoscopic ultrasonography (EUS), or somatostatin receptor imaging (SRI) preoperatively. Tumors positive for one of these pathological markers were diagnosed as NECs. The inclusion criteria were cases with a final pathological diagnosis of esophageal NEC, regardless of pure NECs or MiNENs. Patients with MiNENs fulfilled the 2019 WHO diagnostic criteria stating that each component accounts for ≥ 30% of the tumor mass. The exclusion criteria were patients with a history of neuroendocrine carcinoma in other organs. Demographic characteristics of the eligible patients and morphological and immunohistochemical data of the corresponding tumor samples were collected from medical records. This study was approved by the Medical Ethics Committee of Zhongshan Hospital, Fudan University (B-2018–222). Informed consent was obtained from all patients.
Clinical data collection
Clinical data, such as patient age and sex, were extracted from the electronic medical records. On the basis of endoscopic findings, esophageal tumor locations were divided into upper (16–25 cm from incisor teeth), middle (25–32 cm from incisor teeth), and lower (32–40 cm from incisor teeth). Additionally, the mucosal surface condition (smooth, rough, and ulcerative) and the degree of esophageal stenosis (none, mild, and severe) were noted.
Pathology and immunohistochemistry
Pathological samples obtained by either tissue biopsy or surgical resection from primary tumor sites were reviewed by pathologists with expertise in neuroendocrine neoplasms. All cases in our study were NECs, esophageal squamous carcinoma, or esophageal adenocarcinoma with neuroendocrine differentiation categorized as MiNENs. The results of immunohistochemical staining were extracted from the pathology reports and are presented as either positive or negative. Chromogranin A, synaptophysin, CD56, and other markers were assessed.
Statistical analysis
Statistical analysis was performed using Statistical Package for Social Sciences (SPSS) version 26.0.0.2 software. Categorical data are expressed as percentages. Continuous data are expressed as the mean or median ± standard deviation (SD) and ranges as appropriate. Associations between pathological characteristics and endoscopic findings were investigated by applying the independent-samples t-test and chi-squared test (Fisher’s exact test). P < 0.05 was considered statistically significant.
Results
Clinical characteristics
Of the 43 NEC patients, the mean age at diagnosis was 72.3 ± 8.6 years (range: 49–88 years). Male patients were predominant, comprising 72.1% of cases (N = 31).
Endoscopic findings
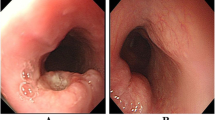
The endoscopic findings are summarized in Table 1. The majority were centered in the middle and lower esophagus with proportions of 37.2% and 55.8%, respectively. The mucosal surface of 29 (67.4%) patients exhibited ulceration. The maximum diameter of the lesions was 27.3 ± 18.6 mm. The muscularis propria was the most commonly involved layer (n = 28, 65.1%), followed by the mucosa (n = 7, 16.3%) and submucosa (n = 8, 18.6%). Additionally, three cases (7.0%) had severe stenosis. Therefore, gastroscopy was difficult to pass through the lesions, and 19 patients (44.2%) had mild stenosis. Among the 29 patients with ulceration, 20 (69.0%) patients developed esophageal stenosis (P = 0.001). Therefore, stenosis of the esophageal lumen might be closely related to ulceration. On the basis of the above endoscopic findings, esophageal NECs had a similar endoscopic appearance to SCC and adenocarcinoma, and presented as a proliferative mass with stenosis or an ulcer (Fig. 1). Therefore, they were difficult to distinguish by endoscopy.
Pathology and immunohistochemistry
The WHO definition for NECs includes positive neuroendocrine markers such as chromogranin A, synaptophysin, and CD56. Furthermore, a Ki-67 index of ≥ 20% is necessary to diagnose NECs. Tumors with < 20% Ki-67 positivity were diagnosed as neuroendocrine tumors. In accordance with the WHO recommendation, we examined the three neuroendocrine markers, chromogranin A, synaptophysin, and CD56, to screen NECs of the esophagus, and tumors positive for one of these markers were diagnosed as NEC. Among the patients with NECs, 38 (88.4%), 22 (51.2%), and 31 (72.1%) showed positive immunostaining for CD56, chromogranin A, and synaptophysin, respectively, and one of the three neuroendocrine markers was positive. Ki-67 staining in all cases was > 30%. The average index of Ki-67 staining was 67.1% ± 21.4%, ranging from 30 to 100%. Additionally, some NECs were positive for NSE or S100, which are neural markers.
Among these patients, 36 (83.7%) were pathologically diagnosed with pure esophageal NEC. Seven (16.3%) patients had mixed components with either adenocarcinoma (N = 2) or SCC (N = 5). To characterize these tumors more precisely, we analyzed other epithelial markers, including CKpan, p63, and HCK, which are essential for carcinogenesis of the esophagus, and examined the cell origin. As a result, 27 (62.8%), 13 (30.2%), and seven (16.3%) cases were positive for CKpan, p63, and HCK, respectively.
The histological findings were most commonly small cell carcinomas. The proportions of small and large cell types were 95.3% and 4.7%, respectively. Additionally, lymphatic metastasis had occurred in 22 (51.2%) cases. The pathological features of patients with esophageal NECs are presented in Table 2.
Clinical outcomes
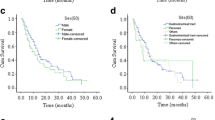
In our study, 25 (58.1%) patients underwent surgical treatment, whereas 18 (41.9%) patients underwent endoscopic resection. Eleven (25.6%) patients experienced postoperative recurrence. Among them, seven experienced liver metastasis, two had lung metastasis, and two had brain metastasis. The relapsed patients all underwent chemotherapy, but eventually died. The overall survival (OS) rate was 62.8% (n = 27). We further compared the OS rate between surgical (17/25, 68%) and endoscopic (10/18, 55.6%) resection methods and found no statistically significant difference in OS between the two groups (P = 0.405).
Discussion
The incidence of esophageal NECs is relatively low, ranging between 0.4% and 2% among all esophageal malignancies [1]. Moreover, as doctors’ awareness increases, more NECs are being diagnosed. However, most studies have been case reports. Data summarizing the characteristics of NECs are scarce.
Esophageal NECs have positive immunostaining for chromogranin A, synaptophysin, and CD56 with > 20% Ki-67 positivity [4]. Similar to other esophageal malignancies, patients with NECs are mostly older men. The major locations of esophageal NECs are the distal esophagus and gastroesophageal junction [7]. Indeed, we found that upper (8.6%) esophageal sites were uncommon. Stenosis of the esophageal lumen was closely related to ulceration. Moreover, Lee et al. found that 65.4% of patients had polypoid lesions with a smooth but discolored surface [8]. Dysphagia is the most common symptom, followed by weight loss due to stenosis of the esophageal lumen [9].
Considering the difficulty in differentiating NECs from other esophageal malignancies by endoscopy, pathological examination and immunohistochemistry are particularly important for definitive diagnosis. Consistent with other studies, among the NEC patients, our results showed that chromogranin A, synaptophysin, and CD56 should be widely used for diagnosis. Some patients were also positive for NSE or S100. The Ki-67 index of all patients was ≥ 30% (67.1% ± 21.4%). Squamous components are often mixed with NECs [1]. We found that 16.3% of patients had mixed components with either adenocarcinoma or SCC. Therefore, some NECs were positive for epithelial markers, such as CKpan, p63, and HCK. p53 loss has been found in poorly differentiated NECs. However, our hospital did not detect p53 in the tissue biopsies or surgical specimens. Consistent with previous reports, we found that esophageal NECs were common small cell types. These cells are derived from local multipotent gastrointestinal stem cells instead of migrating from the neural crest [10]. We believe that, in addition to chromogranin A, synaptophysin, and CD56, NSE, S100, and CKpan might be candidate biomarkers to diagnose NECs.
NECs are aggressive and their prognosis is poor [11]. Because of insufficient data about the clinicopathological and immunohistochemical characteristics of esophageal NECs, a standard treatment has not been established. Surgery, chemotherapy, and radiotherapy are recommended for small- and large-cell NECs [12, 13]. Endoscopic resection is suggested for tumors that are well differentiated, less than 1 cm, and without lymph node metastasis or lymphovascular invasion [8]. For early diagnosis and optimal therapy of esophageal NECs, studies with large sample sizes are urgently needed.
There are several limitations in our study. The retrospective study design made it challenging to analyze all clinicopathological and immunohistochemical features of esophageal NECs because of small patient numbers and missing data. Moreover, the immunohistochemical profile of each case was not consistent because the pathological reports were not standardized. However, a prospective trial cannot be performed because of the rarity of this disease. Regardless, further prospective studies are needed to confirm our results. We analyzed chromogranin A by immunohistochemistry, but not blood chromogranin A. However, we analyzed other neuroendocrine markers to compensate for this inadequacy. Furthermore, inaccuracies in the diagnosis of patients with biopsy samples as the only diagnostic material may exist because verifying the 30% threshold of each component may be challenging based on the limited amount of tissue. Nevertheless, biopsy samples were reviewed by expert pathologists.
Our study provides further understanding of esophageal NECs, which may improve the accuracy of clinical diagnosis. Thus, our study provides a new perspective on esophageal NECs.
Conclusion
We analyzed all eligible patients from 2006 to 2016 and provided detailed information on clinical, pathological, and immunohistochemical features. A larger study might be necessary to clarify the molecular mechanisms underlying esophageal NECs. We look forward to more accurate identification and optimal therapeutic modalities for this rare disease.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available, but are available from the corresponding author upon reasonable request.
Abbreviations
- CgA:
-
Chromogranin A
- EUS:
-
Endoscopic ultrasonography
- GEJ:
-
Gastroesophageal junction
- GINECs:
-
Gastrointestinal neuroendocrine carcinomas
- HCK:
-
High molecular weight cytokeratin
- MiNENs:
-
Mixed neuroendocrine-nonneuroendocrine neoplasms
- NECs:
-
Neuroendocrine carcinomas
- NENs:
-
Neuroendocrine neoplasms
- NETs:
-
Neuroendocrine tumors
- P value:
-
Probability value
- SCC:
-
Squamous-cell carcinoma
- SD:
-
Standard deviation
- SPSS:
-
Statistical Package for Social Sciences
- SRI:
-
Somatostatin receptor imaging
- SYN:
-
Synaptophysin
References
Huang Q, et al. Primary high-grade neuroendocrine carcinoma of the esophagus: a clinicopathologic and immunohistochemical study of 42 resection cases. Am J Surg Pathol. 2013;37(4):467–83.
Nagtegaal ID, et al. The 2019 WHO classification of tumors of the digestive system. Histopathology. 2020;76(2):182–8.
Assarzadegan N, Montgomery E. What is New in the 2019 World Health Organization (WHO) Classification of Tumors of the Digestive System: Review of Selected Updates on Neuroendocrine Neoplasms, Appendiceal Tumors, and Molecular Testing. Arch Pathol Lab Med. 2021;145:664–77.
Giannetta E, et al. A rare rarity: neuroendocrine tumor of the esophagus. Crit Rev Oncol Hematol. 2019;137:92–107.
Klöppel G. Classification and pathology of gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer. 2011;18(Suppl 1):S1-16.
Rindi G, et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol. 2018;31(12):1770–86.
Nayal B, et al. Primary small cell carcinoma of the esophagus - an eight year retrospective study. J Clin Diagn Res. 2015;9(5):Ec04-6.
Lee CG, et al. The clinical features and treatment modality of esophageal neuroendocrine tumors: a multicenter study in Korea. BMC Cancer. 2014;14:569.
Wu IC, Chu YY, Wang YK, et al. Clinicopathological features and outcome of esophageal neuroendocrine tumor: A retrospective multicenter survey by the digestive endoscopy society of Taiwan. J Formos Med Assoc. 2021;120:508–14.
Cives M, Strosberg JR. Gastroenteropancreatic neuroendocrine tumors. CA Cancer J Clin. 2018;68(6):471–87.
Tustumi F, et al. Primary neuroendocrine neoplasm of the esophagus - report of 14 cases from a single institute and review of the literature. Arq Gastroenterol. 2017;54(1):4–10.
Hou X, et al. Multidisciplinary modalities achieve encouraging long-term survival in resectable limited-disease esophageal small cell carcinoma. PLoS One. 2013;8(7):e69259.
Tomiyama T, et al. Esophageal large-cell neuroendocrine carcinoma with inconsistent response to treatment in the primary and metastatic lesions. Case Rep Gastroenterol. 2018;12(2):234–9.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Zi-Han Geng (Conceptualization: Equal; Data curation: Equal; Formal analysis: Equal; Investigation: Equal; Methodology: Equal; Software: Equal; Validation: Equal; Visualization: Equal; Writing - original draft: Lead; Writing - review & editing: Lead). Ya-Lan Liu (Conceptualization: Equal; Software: Equal; Writing - original draft: Equal; Writing - review & editing: Equal). Chen Xu (Conceptualization: Equal). Yan Zhu (Conceptualization: Equal). Ping-Hong Zhou (Conceptualization: Equal; Supervision: Equal). Ming-Yan Cai (Conceptualization: Equal; Supervision: Equal).
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Medical Ethics Committee of Zhongshan Hospital, Fudan University (B-2018-222). Informed consent was obtained from all patients.
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Figure 1.
Most current World Health Organization classification of NENs in the digestive system. NENs, Neuroendocrine neoplasms; NETs, Neuroendocrine tumors; NECs, Neuroendocrine carcinomas; MiNENs, Mixed neuroendocrine-non-neuroendocrine neoplasms; SCC, Squamous cell carcinoma.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Geng, ZH., Liu, YL., Xu, C. et al. Neuroendocrine carcinomas of the esophagus: clinicopathological and immunohistochemical features of 43 cases. CCB 2, 5 (2023). https://doi.org/10.1007/s44272-023-00004-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44272-023-00004-6