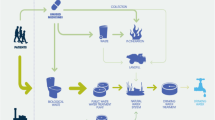
Abstract
China still dominates all other nations as the world's most significant producer and consumer of antibiotics. Antibiotic misuse and overuse have been qualitatively and quantitatively documented in China recently. Antibiotic misuse has alarmed the Chinese population because some antibiotics persist in the environment and adversely affect human health and other non-target organisms. Antibiotic priority setting has been considered the best monitoring tool that is also affordable and time-efficient. Therefore, this article aims to assess the status methods of antibiotic prioritization within ten years ago years (2012–2022) in China and its prevalence and removal by conventional wastewater treatment facilities. Twenty-six priority antibiotics in China may need more attention, according to a recent prioritization assessment. According to other nations, the outcomes of prioritization vary from one nation to another. However, the same antibiotics are frequently reported despite applying various prioritization techniques (method). Their prevalence and frequent detection in China’s environmental media indicate that conventional treatment plants cannot remove them from effluents altogether. Their removal patterns vary from wastewater treatment plant (WWTP) to another and are affected by different factors, including pH, physical–chemical properties of the antibiotic compound, temperature, sludge retention time (SRT), hydraulic retention time (HRT), and the amount of microorganism present.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The treatment and prevention of many bacterial infections frequently involve antibiotics [1]. However, they are considered to be developing contaminants that endanger aquatic habitats and human health [2]. China is the leading manufacturer and buyer of antibiotics worldwide [3,4,5]. A study on antibiotic usage in China assessed the annual production volume at 248,000 tons, of which 160,600 tons were utilized domestically, 88,000 tons were exported, and 600 tons were imported [6, 7]. As a result, antibiotics have been extensively reported as being identified in a variety of environmental media across China over the past ten years due to their misuse and overuse in various contexts, including as preventive and therapeutic drugs for human and animal diseases as well as promoters of animal growth in the aquaculture and livestock industries [8,9,10]. The most often detected antibiotics are sulfonamides (SAs), tetracyclines, quinolones, macrolides, and β-lactams [11].
Since conventional WWTPs cannot altogether remove these drugs from the effluent to be released in surface water, numerous studies indicate that wastewater treatment plant (WWTP) effluent was one of the primary routes for antibiotics to enter the environment [12,13,14,15]. Furthermore, Park (2015) stated that runoff and soil erosion from agricultural lands are significant sources of surface water antibiotics [16]. As a result, different levels of antimicrobial residues have been found in household and farming effluents [17,18,19,20], in food, soil, sediments, and subterranean water [21,22,23].
Antibiotics have both destructive and enriching impacts on the environment. Some antibiotics leak their parent molecules or metabolites into the environment after being ingested by organisms [24]. Long-lasting antimicrobials harm human health by promoting the growth of antibiotic-resistant bacteria (ARB), altering the composition of microbial communities, hastening the detection of antibiotic-resistant mutants, and speeding up the vertical and horizontal spread of antibiotic-resistance genes (ARGs) [25, 26]. According to Yin and Qin, the presence of SAs in the environment has been linked to the spread of genes that cause antibiotic resistance, changes in microbial populations, and other severe issues [27, 28]. Sulfonamides, such as sulfamethoxazole, sulfadiazine, and sulfamethazine, have detrimental consequences on both human health and the environment, according to Zeng et al. Consequently, it is crucial to research how to remove antibiotics from wastewater [8].
Currently, a range of techniques, including better oxidation techniques, adsorption, membrane separation, and others, can be used to extract antibiotics from wastewater [29,30,31,32]. Even though these physicochemical techniques successfully lower antibiotic levels, they cannot be applied in existing wastewater treatment facilities due to several intractable issues. High operational expenses, membrane fouling, and the production of dangerous byproducts during advanced oxidation processes are a few of these problems [33]. Thus, alternative strategies are required [8, 34, 35]. Biological techniques like activated sludge and membranes are still widely used in actual applications. They often remove antibiotic from wastewater [8]. Despite being widely used, these biological methods could not entirely eradicate antibiotic residues from wastewater treatment facilities, which are thought to be the main source of antibiotic residues in aquatic environments. As a result, the widespread use of antibiotics in China’s aquatic and other environmental compartments has been a significant concern over the past ten years.
Given the prevalence of antibiotics in the environment and the potential for adverse health impacts, China is urged to reduce antibiotic consumption promptly [6]. A list of antibiotics with the highest priority is necessary to assist in creating regulations and guide additional environmental surveillance. For the last ten years, China has used antibiotic priority ranking methodologies. Identification of priority antibiotics in China is typically based on criteria such as assessment of environmental risks, effects, and exposure. As a result, 10 different antibiotics were noted as priorities in China in several earlier assessments: Oxytetracycline, trimethoprim, sulfamethoxazole, roxithromycin, erythromycin, ciprofloxacin, clarithromycin, azithromycin, lincomycin, and ketoconazole are some examples of the drugs [5, 36,37,38].
Recently [39], carried out another priority ranking methodology for 105 antibiotics from eight environmental matrices in China. The eight environmental matrices included surface water, sediments, groundwater, animal manure, wastewater from animal farms, wastewater treatment plant effluents, and wastewater treatment plant sludge. Using a ranking method based on national screening data, persistence, bioaccumulation, and toxicity, the top eighteen antibiotics have been identified as the highest priority. These top priority antibiotics consist of four tetracyclines (tetracycline, chlortetracycline, doxycycline, and oxytetracycline), five quinolones (ciprofloxacin, enrofloxacin, norfloxacin, and ofloxacin, fleroxacin), four macrolides (azithromycin, clarithromycin, erythromycin, and roxithromycin), and five sulfonamides (sulfamethoxazole, sulfachlorpyridazine, sulfadiazine, and sulfamethazine and trimethoprim). If we compare the results from the recent research by [39] and other previous studies mentioned above, it is worth mentioning that despite using different ranking methodologies, these studies have a comparable roster of crucial antibiotics. Nonetheless [39], introduced eight more antibiotics to this list. The new antibiotics that have been included are sulfachlorpyridazine, fleroxacin, sulfamethazine, lomefloxacin, sulfamonomethoxine, difloxacin, chloramphenicol, and pefloxacin.
Due to China's huge population density, the enormous amount of antibiotics manufactured and used, and the country’s protracted COVID-19 serious scenario, a methodical strategy is necessary to identify antibiotics that demand extra attention. This review paper aims to examine antibiotics that are classified as priority antibiotics and have been used in China for a past decade (2012–2022), highlighting their occurrence and removal from conventional wastewater treatment plants in China. The intention is to help policymakers and research institutions concentrate on priority antibiotics for better monitoring and economic reasons. To our knowledge, there is no previous review paper with similar content and study objective focusing on China.
2 Occurrence of antibiotics in China
Among all countries, China produces and consumes the most antibiotics [3, 7, 40]. Both veterinary and human antibiotics are widely utilized in China’s feed as growth boosters and medications [37]. As a result, studies on various environmental zones in China, including reservoirs [41], aquaculture farms [42], oceans [43], coastlines [44], lakes [45], upstream and downstream of sewage treatment plants [46], rivers [46], sediments [47] and topsoil of first-tier cities [48], have been conducted about antibiotic emissions and potential risks. Li et al. reported that their concentration levels are mainly ng/L-μg/L in water environments and ng/g-μg/g in sediment and soil environments [49]. For instance, a previous study found 45 antibiotics in samples of Chinese coastal water, with median concentrations of the majority of these antibiotics below 10 ng/L. The concentrations of these antibiotics typically ranged from 0.1 to 100 ng/L [50]. Li et al. reported that the urbanized river Suzhou Creek in Shanghai had antibiotic concentrations of 1936.9 ng/L and 337.3 ng/g in water and sediment samples, respectively [40].
An earlier study found that 85% of the antibiotics released by WWTPs were found in the sludge (in mass: 1780 g/d in sludge, 319 g/d in effluent) [51]. Surface water and sediments in China were discovered to contain priority antibiotics. According to the results, the median antibiotic concentrations in sediments ranged from 0.2 to 46.0 μg/kg, while those in surface water ranged from 0.8 to 48.6 ng/L [11].
The levels of antimicrobial drugs present in animal excrement and animal sewage ranged from roughly 1 μg/kg to 100 mg/kg and 1 ng/L to 10 μg/L, respectively, which confirms that multiple types of antibiotics were found in both the manure and wastewater from animal farms [11]. The previous study by Dong et al. revealed that during 2014 and 2015, antibiotic concentrations in groundwater in China were at ng/L levels; groundwaters near animal farms had greater antibiotic concentrations [52]. Tetracyclines and quinolones had mean concentrations of more than 10 μg/L in groundwater from villages near swine feedlots in Beijing, Hebei, and Tianjin [50]. This data demonstrates that antibiotics are present almost everywhere in China's environment. Thus, there is a need to reference prioritized antibiotics for better monitoring and economic reasons.
3 Prioritization of antibiotics
Several prioritization techniques have recently been developed and validated [5, 38, 53,54,55,56]. Occurrence, exposure potential, and ecological effects (OPT) techniques have been developed to rank 100 pharmaceuticals in China and could be also applied to other chemicals in other countries by updating the corresponding information. Based on predicted environmental concentrations (PEC), applying OPT (occurrence, persistence, bioaccumulation, and toxicity) techniques revealed the 10 pharmaceuticals ranked as priority compounds. This priority ranking was implemented and confirmed after using decision analysis ranking techniques (DART).
Occurrence, toxicity (T), and environmental risk assessment (ERA) were also used as the prioritization method. It has been developed to prioritize pharmaceutical that may pose risks to terrestrial and aquatic systems in Iraq. The prediction has been achieved by taking pharmaceutical usage data and use together with data on physicochemical properties, patient metabolism and wastewater treatment removal. This risk based prioritization technic has also been applied in United Kingdom by utilizing pharmaceutical data in UK and used together with data on physical chemical properties, patient metabolism and wastewater treatment removal in order to estimate the concentrations in the aquatic and terrestrial environment.
The concurrent multi-criteria decision analysis method, which incorporates the factors of exposure, persistence, bioaccumulation, and toxicity (EPBT) together with an environmental risk assessment, have been used to prioritize different pharmaceuticals based on their potential risks in the environment. Optimized Risk quotient based on frequency of measured environmental concentrations (MEC) has also been used as another method to prioritize antibiotics where it revealed different compounds showed a potential environmental risk to aquatic ecosystems.
3.1 Prioritization of antibiotics in China
Sui et al., study marked the beginning of China's history of prioritizing antibiotics for the first time in ten years ago. For the first time in China, 39 pharmaceutical products were ranked using the three criteria: consumption, WWTP removal performance of pharmaceuticals, and potential ecological effects. Among the studied thirty-nine pharmaceutical compounds, twenty-five were antibiotics. According to the results, ecological effects were the most crucial criterion for choosing priority pharmaceuticals [36]. As a result, nine antibiotics: erythromycin, sulfamethoxazole, cefalexin, trimethoprim, lincomycin, ketoconazole, clarithromycin, ciprofloxacin, and clotrimazole, were listed as priority antibiotics among the 25 available antibiotics [36].
Wang et al., used a prioritization scheme to rank 77 veterinary drugs in China's Water and Soil. Three stages of prioritization were used. Seventy-seven veterinary medications were subjected to exposure assessments in Stage I based on usage rates and potential environmental exposure. The ecotoxicity and human health consequences of substances with a high potential to enter the environment were assessed in Stage II, and the results of Stages I and II were used to determine the priority categorization for Stage III. Eight veterinary medications, nevertheless, were left out of Stage II pending for additional research. Thirty-eight compounds out of the 69 veterinary medications studied received high rankings, seven received medium rankings, two received low rankings, and twenty-two received very low rankings. Additionally, antibiotics, endoparasiticides, and aquacultural medicines comprised 57.9%, 28.9%, and 10.5% of the top-ranked compounds. Therefore, among seventy-seven veterinary medicines, thirty-six were antibiotics, where six among them (Amoxicillin, Ciprofloxacin, Florfenicol, Sulfadimidine, Sulfamethoxazole, Tylosin) were ranked high risks [37].
According to three criteria: occurrence (Consumption, Excretion Factor, and WWTP Removal Performance), exposure potential (Persistence and Transportability), and ecological effects (Ecotoxicity and Bioaccumulation), Li et al. performed a ranking system of antibiotics for water bodies in China. Based on the ranking system and the available consumption data, 100 pharmaceuticals were chosen as candidates. China’s wastewater treatment plants (WWTPs) have previously reported finding these drugs in the WWTPs’s effluent. Fourth-two pharmaceuticals out of one hundred examined compounds, were antibiotics). Furthermore, among all therapeutic classes, antibiotics comprised most of the priority pharmaceuticals, suggesting they should be considered based on their behavior in WWTPs. In light of this, nine of the fourth-two antibiotics (erythromycin, penicillin G, azithromycin, amoxicillin, cefalexin, cefotaxime, oxytetracycline, sulfamethoxazole, and clotrimazole) have been designated as priority antibiotics [5].
Prioritizing 100 pharmaceuticals found in China’s water bodies was the focus of a study by Li et al. Several variables, including predicted environmental concentration (PEC), occurrence (O), persistence, bioaccumulation, and toxicity (PBT), were used to determine the ranking. Utilizing the DART method for decision analysis, ten of these compounds were identified as priority substances, of which six were antibiotics. These pharmaceuticals included antibiotics (Azithromycin, Clotrimazole, Erythromycin, Roxithromycin, Trimethoprim, Miconazole), anti-inflammatory (Indometacin, Ibuprofen, Diclofenac), and antilipidemic (Bezafibrate) [38].
Huang et al. examined over 170 studies to gather data describing antibiotics' prevalence and dispersion in China's environment. A total of 110 antibiotics were found, and 28 of those were considered priorities. Except for the atmosphere, these antibiotics were found almost everywhere in China. The investigation focused on various environmental components such as farms with animals, animal waste, wastewater treatment plant (WWTP) discharges, WWTP residual sludge, surface water, sediment, groundwater, and soil. The most common antibiotics across all environmental compartments, according to cluster analysis, were tetracycline, oxytetracycline, chlortetracycline, ofloxacin, enrofloxacin, norfloxacin, and ciprofloxacin. Additionally, water phases frequently contained sulfamethoxazole, sulfadiazine, and sulfamethazine [11].
According to antibiotics’ prevalence (Pv), occurrence (O), persistence, bioaccumulation, and toxicity (PBT) in the environment, Huang et al. [39] developed a novel system for ranking antibiotics. Quantitative structure–activity relationships (QSAR) were used to estimate PBT values, and the Pv and O criteria were quantified and weighted using the decennial national screening datasets, which contained over 15,000 concentration values for 105 potential antibiotics across eight environmental compartments (mentioned above by Huang et al.). PvOPBT was used to identify 26 priority antibiotics, including, five sulfonamides (Sulfamethoxazole, sulfadiazine, sulfamethazine,sulfachloropyridazine,sulfamonomethoxine,fivemacrolides(roxithromycin,erythromycin,azithromycin,clarithromycin,spiramycin);fourtetracycline,tetracyclinedoxycycline, (Chlortetracycline,oxytetracycline);eightquinolones(oflaxacin,norflaxacin,enrofloxacin,ciproflaxaxin,fleroxacin,lomeflaxacin,difloxacin,pefloxacin,) and three antibiotics classified as “others” (trimethoprim, lincomycin, chloramphenicol and ketoconazole). Eighteen antibiotics were listed as priorities in both phases and are suggested as the top priorities deserving of immediate attention, even though there appeared to be a difference between several candidate medicines' PvOPBT ranking scores for the aqueous and solid phases [39].
Li et al.2022 conducted a priority screening-based method on a total of 27 antibiotics from four antibiotic classes (sulfonamides, macrolides, quinolones and tetracycline) in the water and sediment of Suzhou Creek, the most urbanized river in Shanghai, China. The priority-based screening method consisted of four criteria: (1) High detection rate (more than 80%); (2) antibiotics at a high concentration; (3) Significant risk of acute or chronic toxicity to aquatic organisms (RQ > 0.01); and (4) Detection frequency is over 30% in sediment. The results showed that erythromycin, sulfapyridine, clarithromycin, roxithromycin are priority antibiotics in Suzhou creek surface water [40].
3.2 Prioritization of antibiotics in other countries
In Korea, fifty veterinary pharmaceuticals were examined by Kim et al. based on their use, environmental risk, and toxicological hazard. Thirty-one of the fifty veterinary pharmaceuticals were antibiotics. The prioritized list revealed that the top priority class included fourteen antibiotics. These prioritized antibiotics include Ivermectin, oxytetracycline, tylosin, enramycin, fenbendazole, florfenicol, virginiamycin streptomycin, Doxycycline, Apramycin, Ciprofloxacin, Enramycin, spectinomycin, and Amoxicillin. These antibiotics deserve immediate attention in Korean water [57].
In a 2010 study, Kumar and Xagoraraki categorized 100 emerging organic compounds (EOCs) in US stream water, source water, and completed drinking water into different groups. EOCs were categorized as pharmaceuticals, personal care products (PCPs), emerging disruptive chemicals (EDCs), antibiotics, and teratogenics. Emerging Organics compounds (EOCs) chosen for the antibiotics category may also be teratogenic or pharmaceutical substances. Nineteen of the 100 EOCs were antibiotics. Four criteria were used in the study: (1) Occurrence in water; (2) Drinking water utilities' treatment processes; (3) Ecological consequences; and (4) Health implications. Seven antibiotics were ranked as priorities on the list of EOCs in the US as a result, including Demeclocycline, Flumequine, Triclosan, Ciprofloxacin, Norfloxacin, Oxytetracycline, and Sulfathiazole [58].
Ortiz de Garcia etal. [59], conducted a quantitative study structure–activity relationship (QSAR) supported by the Environmental Protection Agency estimation program interface (EPA-EPI) Suite_ interface to evaluate the possible adverse effects of 96 PPCPs and metabolites in Spanish water bodies with insufficient experimental data. Persistence (P), bioaccumulation (B), toxicity (T) (extensive), and occurrence in Spanish aquatic ecosystems (O) (intensive) were among the environmental and toxicological indicators that were investigated in the study. Most compounds' most hazardous qualities were determined by the P index, followed by the T and B indexes. A significant portion of metabolites has concern scores at least as high as their parent compounds. Recently, the European Commission approved the Decision Analysis by Ranking Techniques (DART) tool for presenting three interest rankings through total and partial ranking methods. These rankings include PBT and OPBT. Twelve of the 96 PPCPs were antibiotics. As a result of OPBT rankings of concern, six antibiotics as clarithromycin, levofloxacin, sulfamethoxazole, azithromycin, amoxicillin, ciprofloxacin, were ranked as a priority [59].
Daouk et al. [60], looked into 100 active pharmaceutical ingredients (API) in hospital effluent in Switzerland. These APIs were ranked in order of priority using the OPBT method (Occurrence, Persistence, Bioaccumulation, and Toxicity), where occurrence was defined as consumption, excretion factor, and WWTP removal efficiency. Additionally, an Environmental Risk Assessment (ERA) allowed prioritizing API for 71 compounds based on expected concentrations and data on environmental toxicity found in the literature. The two prioritization strategies were contrasted. ERA identifies antibiotics as potentially harmful to aquatic ecosystems. At the same time, some non-steroidal anti-inflammatory drugs and antiviral medications are highlighted by OPBT prioritization results as being of high concern. 14 of the 100 APIs that were studied were antibiotics. As a result, the findings showed that four antibiotics, including ciprofloxacin, trimethoprim, amoxicillin, and sulfamethoxazole, were frequently ranked as a priority in Table 1. [60]
Menz et al. [61], conducted prioritization research for the entire environmental exposure assessment of 20 veterinary antibiotics in the soil of Germany using a new usage pattern-based exposure screening (UPES) technique. The UPES technique was initially assessed using hypothetical uses of antimicrobial substances in the German meat and poultry industry. The predicted outcome showed that manure concentrations covered seven orders of magnitude from ng/ kg to g/ kg dry weight (dw). Soil concentrations greater than 100 µg/kg dw were estimated to include 14 antibiotic compounds from 10 different antimicrobial classes. As a result, the fourteen veterinary antibiotics with the highest priority in German meat and poultry were identified as amoxicillin, chlortetracycline, enrofloxacin, sulfadiazine, tetracycline, trimethoprim, benzylpenicillin, oxytetracycline, tylosin, lincomycin, neomycin, sulfadimethoxine, and tiamulin [61].
Al-Khazrajy and Boxall [53], studied a risk-based prioritization strategy that was applied to 99 of the most prescribed medications in Baghdad, Mosul, and Basrahn, three cities in Iraq. Based on occurrence (Usage, Excretion Factor, and WWTP Removal Performance), toxicity (T), and environmental risk assessment (ERA) were used as the prioritization method. Active pharmaceutical compounds for antibiotics, antidepressants, and analgesics were shown to be top priorities in surface water, sediment, and the terrestrial environment. Additionally, antibiotics were ranked in order of priority based on how likely they were to kill bacteria, stop their growth, or hasten the spread of antibiotic-resistant genes. Eleven antibiotics were among the top 99 pharmaceuticals prescribed. Ten antibiotics were consequently designated as priority antibiotics, including amoxicillin, azithromycin, ciprofloxacin, erythromycin, clarithromycin, cefalexine, miconazole, tetracycline, metronidazole, and trimethoprim [53].
In Lebanon's water bodies, a priority rank of the eighty-eight most frequently consumed pharmaceuticals was conducted. Twelve of the 88 pharmaceuticals were antibiotics. A simultaneous multi-criteria decision analysis method utilizing the exposure, persistence, bioaccumulation, and toxicity (EPBT) was applied to a subset of 69 pharmaceuticals. An environmental risk assessment (ERA), which determines expected environmental concentrations (PEC) and risk quotients (RQ) at various dilution factors, is used to prioritize a second selection of 84 pharmaceuticals. A priority list of 26 medications from diverse classes is generated to evaluate the two approaches, EPBT prioritization and ERA. Pharmaceuticals for the nervous system, gastrointestinal tract and metabolism account for more than half of those on the priority list (9/26 and 5/26, respectively). The remaining drugs are anti-infectives (4/26), musculoskeletal (3/26), genito-urinary (2/26), respiratory (2/26), and cardiovascular (1/26). As a result, three antibiotics (amoxicillin, azithromycin, and erythromycin) were included as the twenty-six top pharmaceuticals in Lebanon [55].
Guo et al. [54], established a thorough methodology for rating the environmental risks of pharmaceuticals for people, avian and mammalian species, and aquatic and soil organisms in the United Kingdom (UK). The strategy considers potential non-apical effects connected to the therapeutic mode of action and apical ecotoxicological endpoints. One hundred forty-six active pharmaceuticals used in either community or hospital settings in the UK, are used as an example of how the method is implemented. The strategy led to the potential priority identification of sixteen compounds. These substances include associated metabolites and compounds from estrogen, anti-diabetic, antidepressant, inflammatory, antibiotic, and antidepressant. Clarithromycin, azithromycin, ciprofloxacin, and oxytetracycline were the reported priority antibiotics [54].
Zhou et al. [56], prioritized fourth-five pharmaceuticals most frequently examined in European surface waters. Priorities were determined using the RQf values (RQf > 0), where RQf is the optimized risk quotient based on frequency of measured environmental concentrations (MECs) exceeding predicted non-effect concentration (PNEC). Fourth-five analyzed pharmaceutical compounds showed a potential environmental risk to aquatic ecosystems nevertheless, 12 substances were found to pose a significant hazard to aquatic ecosystems, whereas 17 and 7 substances posed a moderate and low risk, respectively. Twelve of the forty-five pharmaceutical compounds analyzed showed a potential environmental risk to aquatic ecosystems. In addition, of the forty-five analyzed pharmaceutical compounds, thirteen were antibiotics. Among the thirteen antibiotics, seven (spiramycin, amoxicillin, ciprofloxacin, ofloxacin, sulfadiazine, clarithromycin, and erythromycin) were prioritized as high and moderate risks [56].
According to the above reviewed reports about prioritization, provide the general insight that results of prioritization show that different countries have different priority pharmaceutical lists, primarily as a result of different patterns of consumption and removal. However, different countries reported similar priority antibiotics despite different prioritization techniques used. Table 1 shows priority antibiotics similarly reported in China and other countries, and they are denoted with “ + ”. It was observed that for the fifteen reports representing 100% of the antibiotic prioritization reports reviewed, seven antibiotics were similarly found at least in 6 reports representing 40% of the reports reviewed. Ciprofloxacin (quinolone class) was the most similarly reported eleven times (73.3%). It was followed by 60% assigned to erythromycin (macrolide class), 53.3% for azithromycin (macrolide class), 53.3% for sulfamethoxazole (sulfonamide class), 46.6% for oxytetracycline (tetracycline class), 40% for trimethoprim (diaminopyrimidine) and 40% for clarithromycin (macrolide class). This implies that these antibiotics are commonly used and detected in many countries and have a high probability of being found in their antibiotic prioritization reports despite what prioritization method is used. In addition, the majority of these antibiotics are found on the model lists of medicines which are often published by the World Health Organization [62].
4 Physical and chemical properties of priority antibiotics
As it has been mentioned in previous paragraphs, antibiotics of priority in China are classified into five classes such as macrolides, quinolones, tetracyclines, sulfonamides, and the ones classified as others (chloramphenicol, ketoconazole, lincomycin and trimethoprim). It was noticed that antibiotics from the same class might exhibit similar behavior, leading to the expectation that their fate under environmental conditions may sound similar [63]. For instance, Carlotte et al. observed that the absorbance maxima of doxycycline and oxytetracycline were 345 and 354 nm respectively, as tetracycline was about 350 nm due to their structural closeness (which differs only in the position and quantity of one OH group) [64]. Additionally, photodegradation had a similar impact on oxytetracycline and doxycycline (41.3 to 43.0%) [63]. For improved research and monitoring, it is crucial to comprehend the physicochemical characteristics of key antibiotics in China.
4.1 Macrolides
Cladinose, desosamine, and a sizable macrocyclic lactone ring that may be attached to one or more deoxy sugars make up most of the chemical compounds known as macrolides (MLs), a class of molecules. They have a low water solubility, are lipophilic, and have mild acids. Additionally, macrolides have a low sorption capacity and a high molecular weight [65, 66]. The molecular makeup of macrolides contains chromophore agents, which enable UV and fluorometric detection of them [67].
4.2 Quinolones
Quinolone antibiotics are characterized as any of a large number of broad-spectrum bactericides with a bicyclic core structure comparable to the substance 4-quinolone [68, 69]. These antibiotics are not affected by acidic or alkaline hydrolysis or elevated temperatures, but they are susceptible to degradation by UV light and are lipid-soluble [70, 71]. Quinolones are poorly soluble in water at pH 6–8 because of their strong polarity and amphoteric properties [67]. They can chelate transition metal ions like copper, lead, zinc, and magnesium, and they are firmly adsorbed by solid substrates rich in excess organic matter and metal oxides (such as Al, Fe hydrous oxides, etc.) [72].
4.3 Tetracyclines
The extensive-spectrum antibiotics known as tetracyclines (TCs) were developed by Benjamin Duggar in 1945 from a soil bacterium of the Streptomyces species. Their designations are based on the four hydrocarbon rings that compose the compound [73]. TCs are light-sensitive amphoteric compounds with a hydroxyl group, a dimethylamino group, and three acid dissociation constants (pKa) that depend on polar functions. These substances show antibacterial action against many microorganisms, including gram-positive and gram-negative bacteria, and are unstable in bases but not in acids [74].
4.4 Sulfonamides
According to Santos and Ramos (2016), p-aminobenzene sulfonamide functional groups are the source of sulfonamides (SAs) [67]. They are composed of a basic amine group (-NH2) joined to an acidic sulfonamide group (-SO2NH-), which displays amphoteric behavior with weakly basic and acidic properties and is prone to salt formation in strongly acidic or basic conditions [71]. The anilinic substituent's nitrogen, which can accept a proton, accounts for the weakly basic properties, whereas the sulfonamide group’s N–H connection, which can release a proton at specific pH levels, accounts for the acidic properties [75]. Table 2 contains a list of their physicochemical characteristics.
5 Analysis of the prevalence of priority antibiotics in the influent and effluent of WWTPs in China and other countries
All antibiotics prioritized by this investigation were almost universal in influent and effluent, according to data from recently analyzed samples, which were collated in Table 3. However, concentrations differed across countries and sewage treatment plants (STPs). Influences are the most concentrated. It was unexpected to learn that the availability of these antibiotics in the influent and effluent is significantly influenced by geographic location. For instance, our research shows China has far higher roxithromycin concentrations in wastewater influent and effluent than in Canada (Table 3). The reason is perhaps the two countries’ demographic, biological, and technological differences. Considering the demographic factors, intriguingly, the population difference between China and other developed nations such as Canada may cause the concentration level's upward trends. This was supported by a report based on a local study of China's diverse population regions. Due to the rapid expansion of the local economy and population, antibiotic concentrations are already high in densely inhabited, economically developed areas, such as Taihu Lake. They are predicted to more than tenfold during the years to come [128]. The abundance of antibiotic-resistant microbial species in the China’s sludge may also be a significant concern because excessive quantities of antibiotics in the environment may cause an increase in the existence of resistant bacteria [76]. The prevalence of associated clinical infections by bacteria has led to a high demand for prioritized antibiotics in countries like China. Another differential factor is WWTPs technology and regulation policies. China, which has the most significant and fastest-growing wastewater sector and water market in the world, faces several issues, including underdeveloped sewage systems and sludge disposal facilities, low treatment process sustainability, questionable effluent discharge standards, and a lack of global thinking on the development of wastewater management in a way that benefits human society, nature, and the environment as a whole [77, 78]. Compared to developed countries like the United States, Spain, and Germany, China's effluents have a higher concentration of prioritized antibiotics, which these considerations may explain. However, as shown in Table 3, developing nations such as Tunisia and Kenya also have a high concentration of prioritized antibiotics compared to China. This may be due to a lack of highly effective wastewater treatment technology and infrastructure, unavailability of expertise, and lack of government wills and regulation policies. It is recommended that China perform more research on wastewater treatment technology to advance the development of wastewater treatment technology and ease the removal of antibiotics from the prioritization list.
6 Removal of priority antibiotics by different biological wastewater treatment technologies
Current research in wastewater treatment technology is focused on removing antibiotics from wastewater. However, conventional urban WWTPs are not designed to remove antibiotics [96], even though they can effectively eliminate conventional organic pollutants and salts. Urban WWTPs are therefore regarded as an important point source for antibiotic pollutants in the environment [97, 98]. Thus, to remove prioritized antibiotics from wastewater, researchers felt it was critical to develop strategies to enhance or support WWTPs. Different biological wastewater treatment technologies were reviewed in the next section. These technologies are classified into two categories (conventional and advanced technologies). At the end of this section, a short discussion on the removal mechanism within China’s WWTPs was provided to illustrate this section.
6.1 Biological wastewater treatment for removal of priority antibiotics
Prioritized antibiotic removal from wastewater is accomplished through biological removal, which employs organisms including bacteria, microalgae, and fungi. These organisms employed biodegradation, bioaccumulation, and bio-adsorption as removal pathways [99] and sometimes may combine hydrolysis and photolysis for the removal processes [100]. Multiple investigations have underscored the possibility of antibiotics undergoing attenuation and degradation during biological wastewater treatment systems [101, 102]. Nevertheless, the elimination of antibiotics in wastewater treatment plants (WWTPs) exhibits considerable variation, ranging from minimal to almost total, contingent upon their chemical structure and the specific types of biological wastewater treatment systems implemented [103].
6.1.1 Conventional treatment technologies
6.1.1.1 Activated sludge process (ASP)
The activated sludge process (ASP) is the predominant system utilized for biological wastewater treatment. The impact of operating variables, such as solid retention time (SRT), on the biodegradation and adsorption of pollutants during the activated sludge process is widely recognized [104]. Despite its widespread use, activated sludge process is not specifically tailored for antibiotic elimination. Multiple laboratory and field studies have demonstrated that antibiotics are only partially eliminated in conventional wastewater treatment plants (WWTPs), with removal rates varying from zero to over 99% [87, 105].
Therefore, at some extent, activated sludge process may completely or partially remove the antibiotic concentration in the WWTP. However, the activated sludge under consideration in WWTP can be influenced by its properties, operating conditions, the amount microorganisms and the antibiotic chemical structure. Hence the antibiotic may exhibit different removal patterns. For example, in the treatment of some of the priority antibiotics by the activated sludge process, the removal rate of doxycycline was 61% at a starting concentration of 0.18 μg/L; clarithromycin (macrolide class) was 18% at a starting concentration of 0.25 μg/L; erythromycin (macrolide class) was 0% at a starting concentration of 0.06 μg/L; sulfomentoxazole (sulfonamides class) was 60% at a starting concentration of 0.26 μg/L); sulfadimethoxine (sulfonamides class) was 53% at a starting concentration of 3.1 μg/L, and trimethoprim (diaminopyrimidine) was 14% at a starting concentration of 0.1 μg/L. It was reported the observed removal was mainly attributed to the hydraulic retention time ranging between 6 and 16 h [83].
In large-scale application of activated sludge process, the percentage of antibiotic removed from the liquid phase showed considerable variation, ranging from − 1% azithromycin (macrolide class) at a starting concentration 0.13 μg/L to 71%; ciprofloxacin (fluoroquinolones class) at a starting concentration 2.2 μg/L. The removal percentages for other antibiotics were 25% norfloxacin (fluoroquinolones class) at a starting concentration 0.02 μg/L; 52% sulfamethoxazole (sulfonamides class) at a starting concentration 0.44 μg/L), and 66% erythromycin (macrolide class) at a starting concentration 0.045 μg/L) [105]. The primary mechanism for removing fluoroquinolones and macrolides was biosorption, while biodegradation was the primary method for removing sulfonamides [87, 96, 105].
The composition of microbial communities in the activated sludge as well as in sequencing batch reactor (SBR) can also impact the elimination of certain antibiotics. An experiment was conducted where an enriched culture of ammonia-oxidizing microorganisms in a nitrifying activated sludge system removed 98% of sulfamethoxazole from the water phase. The removal of sulfamethoxazole was shown to be linked to the rate of nitrification [106].Therefore this proves that the type of microorganism within the treatment system can influence the rate of antibiotic removal.
Previous studies have reported that low detections of antibiotic concentration in conventional WWTP using ASP in summer than in winter seasons [89, 107]. During the winter season, the reduced rate of biodegradation can be linked to factors such as temperature and other operating parameters of wastewater (WWTP). As a consequence, there is a decrease in the removal of antibiotics. Previous studies in the literature have reported comparable findings with synthetic musks [108]. Similarly, the majority of the investigated antibiotics exhibited greater elimination rates throughout the summer season and might be due to high temperature and dilution in summer season [89, 109].
6.1.1.2 Sequencing batch reactor (SBR)
Recent research has concentrated on investigating the fate and removal of antibiotics in sequencing batch reactors (SBR). These studies have demonstrated significant removal of several antibiotics such as oflaxacin, sulfamethoxazole, trimethoprim, and tetracycline [110,111,112]. The removal rate of 65% was observed for oflaxacin at a starting concentration 15 mg/L; 37% and 80% sulfamethoxazole at a starting concentrations 67 μg/L and 10 mg/L respectively; 21% trimethoprim at a starting concentration 0.5 μg/L; 97% and 68% tetracycline at a starting concentrations 250 μg/L and10 mg/L respectively [110,111,112]. It was reported that either in ASP or SBR, the efficacy of antibiotic was observed to fluctuate according on the physical–chemical characteristics of antibiotics, the particular treatment method used, redox conditions, solid retention time(SRT), hydraulic retention time (HRT), and temperature [89, 109].
A study concerning a lab scale SBR removal of sulfamethoxazole and trimethoprim under redox conditions (aerobic, sequential anoxic/aerobic, and microaerobic with a dissolved oxygen concentration of approximately 0.3 mg/L was conducted. The result revealed the minimal removal of < 10% of trimethoprim at a starting concentration of 500 ng/L) across all redox environments, excluding aerobic conditions where the average rate of removal was 21%. In contrast, the SBR operating under microaerobic conditions exhibited a greater removal of sulfamethoxazole (62% at a starting concentration of 1430 ng/L) compared to the aerobic SBR and sequential anoxic/aerobic SBR (38% and 35% respectively). This enhanced removal was primarily driven by the presence of a unique microenvironment that facilitated both aerobic and anoxic metabolisms, thereby enabling a number of transformation pathways for the more efficient biodegradation of antibiotics [111].
6.1.1.3 Anaerobic digestion (AD)
Anaerobic processes include anaerobic digestion (AD), anaerobic filtration (AF), anaerobic baffled reactor (ABR), and up-flow anaerobic sludge blanket (UASB) [113]. Anaerobic Digestion (AD), particularly the Up Flow Anaerobic Sludge Blanket (UASB) and Expanded Granular Sludge Blanket (EGSB) reactors, has emerged as a significant anaerobic removal technique. Numerous investigations have noted its effectiveness in removing antibiotics from wastewater [114,115,116].
The two most frequently mentioned methods of removing antibiotics from this process are biodegradation and biosorption. Due to the many functional groups on its surface, extracellular polymeric substance (EPS), a material released by microorganisms, is the main component in the biosorption of antibiotics. To draw antibiotics and their metabolites, the microbial membrane and the host cell use hydrophobic partitioning, cation exchange, electrostatic interactions, surface complexation, and electron donor–acceptor interactions [117]. The biodegradation pathway is the main accepted pathway because it involves the enzymatic breakdown of highly harmful antibiotics into intermediate metabolites less harmful than the mother compound.
The most important factors influencing the AD process are high concentration, prolonged antibiotic exposure, and temperature. Tetracycline long-term treatment inhibits anaerobic microbial activity and causes the release of lactate dehydrogenase. At 8 mg/L concentration, tetracycline decreased methane production by 73.28% and disrupted aerobic digestion. EPS polysaccharide content was reduced, and enzyme activity dropped by 66% [118]. It has also been demonstrated that temperature pretreatments and temperature-phased advanced AD increase the elimination efficiency of veterinary antibiotics while enhancing biogas production [119]. Sulfonamides were shown to be removed by anaerobic digestion far more effectively (> 97%) in the summer than in the winter when three groups of antibiotics were compared [120]. Furthermore, the anaerobic digestion (AD) process exhibited greater efficacy in removing antibiotics when carried out under mesophilic (32−37 °C) and thermophilic (50−60 °C) conditions, as contrasted with psychrophilic (< 20 °C) conditions. This can be attributed to the enhanced biochemical reactions and greater rates of metabolic growth of methanogenic bacteria at each of these temperature ranges. For example, a recent study about removal of antibiotics during the anaerobic digestion of pig manure revealed that at temperatures of 52 and 15 °C, a significant reduction of 99% and 20% in erythromycin concentration was observed [121].
In comparison with other sludge treatment methods, anaerobic digestion proved superior to alternative sludge stabilization techniques such as aerobic digestion and anaerobic stabilization ponds in eliminating antibiotics such as trimethoprim, ofloxacin, sulfomethoxazole, ciprofloxacin and norfloxacin from activated sludge. The results of the comparison were in μg/kg dm where “dm” stans for “dry mass” as shown: Aerobic digestion (trimetoprim: 0.66–6.83 μg/kg dm, sulfomethoxazole: < LOD-4.46 μg/kg dm, ofloxacin < LOD-339 μg/kg dm, norfloxacin < LOD-2220, ciprofloxacin: 30.3–2759 μg/kg dm. Anaerobic stabilization pond (trimetoprim: < LOD, sulfomethoxazole: < LOD, ofloxacin < LOD, norfloxacin < LOD, ciprofloxacin: < LOD -36.5 μg/kg dm. Anaerobic digestion (trimetoprim: < LOD, sulfomethoxazole: < LOD, ofloxacin < LOD, norfloxacin < LOD, ciprofloxacin: < LOD -24.4 μg/kgdm [122]. In addition, taking a reference from (Table 4) about the anaerobic digestion reported results from the laboratory and pilot scale, have shown an improved removal of antibiotcs. The observed removal for fluoroquinolones (ofloxacin: 24–85%; ciprofloxacin: 2–82%) and tetracyclines (Oxytetracycline: 50–78%; Chlortetracycline: 76–97%; Tetracycline: 55–85%) was related to biosorption of these antibiotics on the digested sludge and was highlighted as the major removal process Table 4.
6.1.1.4 Constructed wetland treatment systems (CW)
Constructed wetlands have shown promising prospects for enhanced antibiotic treatment and the potential for mitigating antibiotic-resistant bacteria (ARB), their genes, pathogens, and general pollutants in wastewater [123]. Due to the inexpensive construction, operation, and maintenance cost, particularly in developing countries like China, wetland development is a practical wastewater treatment method worldwide. Studies have reported the effective removal of antibiotics by wetland construction methods. Wetland construction demonstrated great removal efficiencies (> 98%) for both sulfamethoxazole (SMX) and tetracycline (TC), two commonly used antibiotics, in a study of their removal performance [124]. Four macrolides, eight sulfonamides, three fluoroquinolones, three tetracyclines, and trimethoprim were among the 19 antibiotics that were studied in two ecological (constructed wetlands and stabilization ponds) and two conventional wastewater treatment processes (activated sludge and micro-power biofilm). The results showed that both the CW and AS demonstrated higher removal efficiencies of target antibiotics in summer than in winter [52].
Nevertheless, the removal pathways include photodegradation, sorption, aerobic/anoxic biodegradation, and phytoremediation. Wetland microbes can change the physicochemical composition of antibiotics, break down antibiotic residues from macromolecules to tiny molecules, and finally to water and carbon dioxide [125]. Wetland plants remove antibiotics in a variety of methods. Antibiotics, for instance, are directly absorbed by plant roots from effluent. Second, vegetation provides oxygen and nutrients for microbial metabolism. Third, vegetation provides places for microbes to attach themselves [126].
The variety and abundance of plants, microbial population diversity, substrate types, as well as CWs operation parameters like hydraulic retention time/hydraulic loading rates, feeding mode, aeration, and influent quality all have a significant impact on antibiotic removal efficiency because constructed wetland (CW) can remove antibiotics from the aquatic environment by combining substrates, plants, animals, and microorganisms. For example, the removal rate of doxycycline (tetracycline class) was 71% at a starting concentration of 0.18 μg/L); clarithromycin (macrolide class) was 31% at a starting concentration of 0.25 μg/L); erythromycin (macrolide class) was 64% at a starting concentration of 0.06 μg/L; sulfamethoxazole (sulfonamides class) was 87% at a starting concentration of 0.26 μg/L; sulfadimethoxine (sulfonamides class) was 99% at a starting concentration of 3.1 μg/L, and trimethoprim (diaminopyrimidine) was 99% at a starting concentration of 0.1 μg/L. The improved removal can be due to the considerably longer hydraulic retention time (HRT) in the CW system, ranging from around 24 to 120 h along with other processes such bioprocess onto soil, uptake by plant and wetland microbial degradation [127].
6.1.2 Advanced biological wastewater treatment technologies
6.1.2.1 Aerobic membrane bioreactor (AeMBR)
An aerobic membrane bioreactor (MBR) represents an innovative wastewater treatment solution that integrates both biological treatment (aerobic digestion) and membrane filtration processes. In the aerobic phase, microorganisms play a crucial role in breaking down organic pollutants within the wastewater. The introduction of microorganisms, including bacteria and fungi, facilitates the consumption and digestion of these pollutants. Nevertheless, this microbial activity necessitates a continuous supply of oxygen.
Following the biological treatment stage, a membrane filtration system is employed to effectively separate the treated water from the remaining suspended solids and microorganisms. This separation is achieved through the utilization of a membrane barrier that selectively permits only clean water to pass through, retaining the solids.
Nowadays, MBR are not only used in removing traditional pollutants (i.e. nitrate, phosphorus, etc.…, but also emerging pollutants (i.e. antibiotics). One of the reason of their use is that they result in decreased loss of slow-growing functional microbes and lead to the formation of more diverse mixed liquors, which can break down a broader variety of organic contaminants [128].
The elimination effectiveness of individual antibiotic varies across various systems and is contingent upon three distinct sets of factors: the physicochemical properties of antibiotic compounds (e.g., hydrophobicity, chemical structure, molecular weight, molecular diameter, and sorption coefficient), membrane attributes (for example, pore size, zeta potential, contact angle, and roughness), and operational parameters (for example, sludge retention time (SRT), pH value, biomass concentration, temperature, and wastewater composition) [129, 130]. A previous study has indicated that an increased SRT can enhance the removal of certain antibiotics. Moreover, it has been observed that hydrophilic compounds are more effectively eliminated by membrane bioreactor (MBR) compared to hydrophobic ones [129]. The variations in removal efficiency under diverse conditions are likely governed by the antibiotic removal mechanisms within the MBR system, encompassing membrane rejection, sorption, biodegradation, air stripping, and photodegradation [131].
Aerobic MBR demonstrates superior performance in antibiotic removal compared to the convetional activated sludge process (ASP) as shown in Table 4.The removal rate of different antibiotics by ASP (ciprofloxacin:71–89%; erythromycin:43–66% amoxillin:100%; sulfamethoxazole 52–70%; trimethoprim 31–33%; tetracycline:67–96%) and by aerobic MBR (ciprofloxacin:29–93%; erythromycin:12–90%; sulfamethoxazole:7–100%; trimethoprim: − 2 to 96%; amoxillin: 86–92%; and tetracycline: 56–97%).The high removal observed in aerobic MBR might due to absolute particle retention facilitated by the membranes and heightened biodegradation achieved through extended SRT and increased biomass concentrations [132].
In different other studies, the same findings about the high performance of AeMBR over ASP for removal of prority antibiotics were reported. For example in aqueous phase the removal rate of trimethoprim by ASP was 33% and 70% by AeMBR at a starting concentration of 0.25 μg/L; oxytetracycline was removed at rate 77% by ASP and 93% by AeMBR at a starting concentration of 30.1 μg/L; tetracycline was removed at rate 67% by ASP and 91% by AeMBR at a starting concentration of 12.3 μg/L; sulfamethazine was removed at rate 77% by ASP and 88% by AeMBR at a starting concentration of 1.8 μg/L; clarithromycin was removed at rate 65% by ASP and 72% by AeMBR at a starting concentration of 1.8 μg/L; ciprofloxacin was removed at rate 86% by ASP and 91% by AeMBR at a starting concentration of 6.4 μg/L [84, 133, 134]. Increased biomass concentration and the presence of smaller sludge flocs, accompanied by a larger surface area, were identified as factors contributing to the heightened sorption of antibiotics within the AeMBR system [135, 136]. Additionally, the extended SRT (Sludge Retention Time) facilitates the enrichment of slow-growing bacteria, such as autotrophic nitrifying bacteria, and the establishment of a more diverse microbial population. This, in turn, promotes improved biodegradation within the AeMBR system [84, 137, 138]. Both sorption and biodegradation play pivotal roles in the comprehensive removal of antibiotics in the AeMBR system, with biodegradation emerging as the predominant pathway for sulfonamides [84, 139, 140].
6.1.2.2 Anaerobic membrane bioreactor (AnMBR)
Since they can produce more biomethane for renewable energy than conventional wastewater energy recovery systems and do not require energy-intensive oxygen delivery, anaerobic membrane bioreactors (AnMBR) are crucial to plans for sustainable wastewater treatment and resource recovery [141, 142]. The use of conventional anaerobic processes for pharmaceutical wastewater treatment is typically limited by many challenges, including a lengthy start-up period, a slow growth rate of anaerobic microbes, and poor biomass retention. However, in addition to manufacturing useful products and managing effluent methane emissions, AnMBR offers functionalities that help overcome the restrictions mentioned earlier [142].
Minimal research has looked at removing antibiotics from wastewater in AnMBR systems despite AnMBR's significant potential for doing so. Another study of AnMBR incorporating powdered activated carbon (PAC) reported that with 0.5–1.5 mg CIP/L in the feed, 50–76% of the ciprofloxacin in an AnMBR was removed [143]. Trimethoprim and sulfamethoxazole were removed at a rate of 35–98 and 85–100% respectively (Table 4). According to another previous report it was denoted that the primary method of removing antibiotics like sulfomethoxazole and trimetoprim in AnMBR, was through biodegradation, which was found to be highly efficient and correlated with the hydrophobic properties and specific molecular characteristics of the antibiotics, such as the presence of electron withdrawing groups and electron donating groups, as well as the inclusion of nitrogen and sulfur in the molecular structure [112]. Sulfomethoxazole can be easily eliminated in the AnMBR through reductive processes, which are facilitated by the presence of strong electron-withdrawing groups (sulfonyl) in its molecular structure. The exceptional biodegradability of trimethoprim in anaerobic environments can be attributed to the inclusion of substituted pyrimidine in its molecular composition [112].
The extended solid retention time (SRT exceeding 200 days) in an Anaerobic Membrane Bioreactor (AnMBR) led to improved elimination of antibiotics (refer to Table 4). However, a reduction in Hydraulic Retention Time (HRT) from 35 to 24 h resulted in a notable decline in antibiotics removal. This decrease is attributed to the inhibitory effects stemming from the elevated loading rate of antibiotics, which adversely impacted microbial activities [144].
AnMBR system may be a viable treatment for removing priority antibiotics from wastewater. However, further research on functionality improvement is required along with more full scale-AnMBR studies on the removal of priority antibiotics.
6.1.2.3 Moving-bed biofilm reactor (MBBR)
A moving bed biofilm reactor (MBBR) is a biological method for treating drinking water, stormwater, and wastewater. This method uses specially-made plastic carriers in an aerated tank to produce the perfect conditions for bacteria to multiply and develop biofilms. Antibiotics dissipation, nitrification, denitrification, and phosphorus removal are a few other services that the moving bed biofilm reactor (MBBR) technology can offer [145]. Since antibiotics are present in wastewater at concentrations (ng/L to µg/L) and are too low to enable biomass development, antibiotics are micropollutants occasionally recognized as non-growth substrates (secondary substrates). The biological conversion of prioritized antibiotics in moving bed biofilm reactor (MBBR) is primarily the result of co-metabolic mechanisms, whereby the removal of non-growth substrates (antibiotics) necessitates the presence of primary substrates like COD and nutrients to support the biomass growth of the bacteria that are converting the antibiotics [145]. For example, in bioreactor tests, adding sucrose (growth substrate) increased the breakdown of sulfomethoxazole (SMX), a non-growth substrate. It is possible to hasten the decomposition of SMX by adding electron acceptors (sodium hydrogen carbonate, sodium sulfate, and sodium nitrate) [146]. Additionally, three main pathways for the breakdown of antibiotics in the moving bed biofilm reactor (MBBR) have been identified: co-metabolism, organic matter as an electron acceptor, and antibiotics as a growth substrate [147]. The biological removal mechanism must be understood to optimize the prioritized antibiotic removal processes in MBBR. For instance, when the influent NO3-N concentration was 100 mg/L in moving bed biofilm reactor (MBBR), SMX degradation peaked at 73.31%. Nevertheless, the reduction in SMX was only 17.2% and 32.74%, respectively, when the influent NO3-N concentration was 5 and 10 mg/L [148]. This shows that denitrification improves some types of prioritized (SMX) antibiotic removal, as denitrifying bacteria typically utilize antibiotics as a co-substance to co-metabolize nitrate and antibiotics [106, 149]; thus, when designing MBBR for antibiotic removal, greater emphasis should be placed on improving the denitrifying bacteria community to achieve a high-efficiency result.
Moving-bed biofilm reactors (MBBRs) are a successful conventional wastewater treatment system for removing antibiotics in a priority manner and enhancing water output quality from wastewater treatment processes. Chlortetracycline (CTC), tetracycline (TC), and oxytetracycline (OTC) removal efficiencies of an anaerobic/aerobic MBBR reactor were 52.03, 41.79, and 38.42%, respectively, when mixed tetracycline antibiotics (50 g/L) were added to the system [150]. Furthermore, the concentration level of a standalone antibiotic may affect the ability of the MBBR removal capacity. For example, tetracycline decreased steadily as the concentration of tetracyline rose. Upon increasing the tetracycline concentration from 150 to 200 μg L−1, there was a decrease in the antibiotic removal rate from 37.65 to 33.19%. Nevertheless, reducing the tetracyline concentration from 10 to 2.8 μg L−1 resulted in a high removal rate of 72.06%. The rapid breakdown of tetracycline at low concentrations was caused by the backflow of sludge, which extended the duration of exposure of tetracycline to microorganisms [150]. The biodegradation of the most persistent antibiotics in a study of moving bed biofilm reactor (MBBR) with carriers increased for ciprofloxacin, with degradation efficiencies of 63.14 ± 2.70% [151].
A previous study was conducted to investigate the removal of the macrolides (erythromycin and clindamycin) in a Moving Bed Biofilm Reactor (MBBR).The compounds erythromycin and clindamycin were removed to a lesser extent during the continuous flow experiment, with erythromycin showing a removal rate of less than 20% and clindamycin showing a removal rate close to 90%.The rationale for this effective elimination was that the biofilm in this system harbors more efficient microorganisms capable of degradation compared to those typically found in activated sludge (of a membrane bioreactor) [152].
The most significant advantages of activated sludge and biofilm technology are combined in moving-bed biofilm reactors (MBBRs). Moving bed biofilm reactor (MBBR) have strong anti-shock load capability, great treatment efficacy, and low-temperature functionality [150]. It is both ecologically friendly and economical.
6.1.2.4 Biological aerated filter system
The biological aerated filter system (BAF) uses the aerobic process to treat wastewater by combining oxidation and filtration through biological interaction. The BAF is made up of three phases: a solid phase that acts as a substrate for microbial growth; a liquid phase into which the solid material is dissolved; and a gas phase that is created when air is added to the reactor [153]. It has been utilized to treat a range of wastes, including pharmaceuticals, due to its incredible effectiveness and low cost [154], and industrial, municipal, and swine flush water [155]. The use of biological aerated filter systems in wastewater treatment is gaining importance because of the high antibiotic removal rate. For instance, in a study on removing antibiotics from piggery effluent by a biological aerated filter system, the BAF system showed excellent removal rates for the nine specific antibiotics (82.1–100%) [155]. In another previous study about removing antibiotics from swine wastewater, the use of BAF revealed the ability to significantly remove the concentration of different classes of antibiotics. BAFs achieved a 49% removal of sulfachloropyridazine (sulfonamides) at a stating concentration of 131 μg/L, a 51% removal of sulfamethoxazole (sulfonamides) at a stating concentration of 0.095 μg/L, and a 20% elimination of ofloxacin (fluoroquinolones) at a stating concentration of 0.024 μg/L in the aqueous phase [155]. BAF system demonstrated improved elimination of ciprofloxacin (fluoroquinolones) at a concentration of up to 1 mg/L, with an overall effectiveness for removal of 95% [156].For example, the efficiency of removing ciprofloxacin decreased considerably from an initial value of 95% to 79% when the hydraulic retention time was reduced from 10 to 5 h. This decrease was attributed to the occurrence of short-circuiting in the liquid flow, which resulted in reduced contact time between antibiotics and microbial biomass [156].
The significant predictors of BAF efficiency are hydraulic retention time and the amount of biomass retained in the inert granular media. The BAF system's ability to remove antibiotics is generally enhanced by high biomass concentration and higher HRT.
6.1.2.5 Microalgae-bacteria symbiosis-based bioreactor technology
Due to its exceptional capacity to remove contaminants and high biomass yield, microalgae-bacteria symbiosis for wastewater treatment is thriving. This is in part because of the robust adaptability of microalgae to a variety of contaminants, including chemical oxygen demand (COD), total ammonia nitrogen (TAN), total phosphorus (TP), and antibiotics based on their distinctive defense mechanism towards exogenous pollutants [157]. Additionally, it has been demonstrated that microalgae can effectively remove several antibiotics from wastewater, giving them a competitive alternative to biological wastewater treatment [158,159,160].
Microalgae need CO2 for their metabolic processes, and this is where bacterial symbiosis is crucial. In the microalgae-bacteria consortium, CO2 released by bacteria could support microalgae cell growth while degrading antibiotics, whereas O2 produced by microalgae may be used as an electron acceptor for organics degradation by bacteria, resulting in an increased wastewater treatment efficiency [161, 162]. It has generally been shown that selected microalga-bacteria symbiosis is very effective in treating wastewater with a wide range of antibiotic concentrations. For instance, at concentrations between 0.1 and 200 mg/L, it was found that the microalga-bacteria consortium successfully removed more than 95% of oxytetracycline and ofloxacin [157]. When chlortetracycline was tested utilizing a microalga-bacteria consortium at concentrations greater than 10 mg/L, its biodegradation efficiency was 48.52% [163]. The maximum sulfamethoxazole removal efficiency was reported to be 99.0 ± 0.2% in a microalga-bacteria consortium photobioreactor at an initial concentration of 500 μg/L for 7 days [161].
The microalgae-bacteria consortia respond to these antibiotics and other hazardous pollutants in various ways, such as physical adsorption, covalent bonding, bio-accumulation, and biodegradation. Enzymatic activities tend to rise during biodegradation, which encourages the biotransformation of the antibiotics. As seen in the co-metabolism of sulfamethoxazole, the transformation route includes oxidation, hydroxylation, formylation, side chain degradation, and pterin-related conjugation [160]. The superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and malondialdehyde (MDA) concentrations are antioxidant enzymes released for biodegradation. The effectiveness of the microalgae-bacteria system, physiological reactions, and indicators of antibiotic toxicity have all been monitored using these enzyme concentrations [164].
Nutrient concentrations affect antibiotic removal because they affect the cellular biological activity and thus significantly influence the microalgae-bacteria consortium metabolism of antibiotics. To maintain an adequate growth rate, the microalga-bacteria consortium needs specific amounts of essential nutrients like carbon, nitrogen, phosphorus, and sulfur. A lack of or an excess of these nutrients will have an impact on growth, which will have an impact on how efficiently antibiotics degrade [165, 166]. Since microalgae need light energy to thrive, light intensity and wavelength are essential for microalgae-bacteria functionality. As a result, when removing priority antibiotics that use microalgae-bacteria symbiosis, light is a crucial consideration. In addition to the previously mentioned role of light energy, several antibiotics, such as fluoroquinolones and tetracyclines, and sulfonamides, are better broken down in the presence of energy [70, 167]. Other factors are temperature, pH, and microbial growth inhibitors.
6.1.3 Discussion of the removal mechanism within China’s WWTPs
The removal of prioritized antibiotics was primarily facilitated by WWTPs, as previously stated. Although, in China, little research has been reported on WWTPs removal of prioritized antibiotics. In WWTPs, conventional contaminants are often removed from effluents using physical, chemical, and biological techniques that don't harm the environment. However, the removal efficiency of antibiotics in WWTPs in China is significantly influenced by climate, population, sludge retention time (SRT), hydraulic retention time (HRT), antibiotic nature, nitrification, and other factors [80, 86].
Taking into account how different treatment methods affect the effectiveness of removing priority antibiotics, research on 12 WWTPs in various Chinese cities showed higher removal efficiencies (70—91%) of total pharmaceuticals, primarily norfloxacin, Erythromycin-H2O, clarithromycin, acetaminophen, and sulfamethoxazole. Sequencing batch reactor (SBR), anaerobic/anoxic/oxic (AAO), and membrane bioreactor (MBR) processes were used by aforementioned WWTPs [183]. The removal efficiency of sulfamethoxazole, erythromycin, and norfloxacin were 44%, 24%, and 82%, respectively, when compared to WWTPs using a combination system of anaerobic/anoxic/oxic (A2/O); activated sludge process (ASP) and cyclic activated sludge technology (CAST) [81]. More extended treatment periods in these two systems and less washout of slow-growing biomass were related to the former's more effective removal performances [183]. Research of 12 WWTPs in Dalian, China, used the anoxic/oxic treatment system (A/O), cyclic activated sludge technology (CAST), biological filtration oxygenated reactor (BFOR), as well as ASP and AAO as treatment methods. Different removal efficiencies of the same antibiotics were recorded from different WWTPs. The researcher ascribed the discrepancy to several WWTP-specific factors, including operating parameters (HRT, SRT, temperature), treatment techniques (ASP, CAST), and other aspects [184]. In another study, WWTPs with screen, grit chamber, anaerobic/anoxic/oxic (A2/O), sedimentation, and UV disinfection treatment components showed improved tetracycline 80%, norfloxacin 80%, and sulfamethoxazole (50–80%) removal efficiency [185]. It recommended that the WWTPs system targeting the removal of the three main groups of antibiotics (fluoroquinolones, sulfonamides, and macrolides), which account for the majority of the priority antibiotics in China, may find it advantageous to employ the first WWTPs component design that incorporated sequencing batch reactor (SBR), the combination is anaerobic/anoxic/oxic (AAO), and membrane bioreactor (MBR) processes.
When location is considered as a factor in removing priority antibiotics in WWTPs in China, it has been shown that population density affects the concentration of antibiotics in the influents of WWTPs. This has a direct effect on how efficiently antibiotics are removed in WWTPs. For example, WWTP in Shanghai had a higher cumulative concentration level of total priority antibiotics in the influent (9988.08 ng/L), attributed to its high population density [183]. Climatic considerations such as rainfall and temperature at WWTPs' locations are crucial for removing priority antibiotics in China. For instance, a study of the removal performance of 29 pharmaceuticals in 5 distinct WWTPs in China showed that the removal efficiency in these WWTPs varied significantly from 37.4% to 64.4%. Shanghai, Guangzhou, and Jinan WWTPs demonstrated better removal efficiency than Beijing and Chengdu WWTPs. In addition, the comparatively high temperature may have also contributed to the extremely high total removal efficiency of the WWTPs in Shanghai and Guangzhou [183]. Regional variations in antibiotics concentrations and compositions accounted for the discrepancies in removal efficiency. Significantly, more total antibiotics were detected in the effluents of North Shaanxi WWTPs than effluents of other regions of the 51 Shaanxi province WWTPs studied, with tetracycline and macrolides predominating; this correlates with the removal efficiency of the antibiotics [186].
Chemical, physical properties, and the influent concentrations of prioritized antibiotics are other factors that played essential roles for their removal in different WWTPs [187, 188]. Research findings indicate that higher initial influent concentrations of antibiotics correlate with decreased removal efficiency in WWTPs [186]. However, the removal is subjected to the adopted treatment processes. For example, granular activated carbon (GAC) adsorption in advanced WWTPs removed erythromycin and carbamazepine, which were resistant to biological treatment, by an average of 74% and 88%, respectively [189].
The physicochemical properties, such as hydrophobicity, influence antibiotics sorption and removal efficiency in WWTPs.When the antibiotics are significantly hydrophobic (log Kow > 3.2), removal efficiency, particularly through absorption, is enhanced [187]. Notably, 51 wastewater treatment plants in Shaanxi province had higher detectable concentrations of prioritized antibiotics like ofloxacin (OFL), roxithromycin (RXM), norfloxacin (NOR), and tetracycline (TCN) in the effluent. One possible explanation for this could be that the antibiotics physiochemical properties made them recalcitrant, allowing them to withstand the removal processes of the WWTPs [86]. Although the research stated that different treatment processes of WWTPs play major roles in removing the targeted antibiotics, OFL, RXM, NOR, and TCN were dominant in the effluents. They are categorized as potential risk compounds to the province [86]. Most antibiotics, especially those in the aminoglycoside family, are polar, and hydroxylation increases their polarity and hydrophilicity, making them more biodegradable in WWTPs.
7 Proposed induced antibiotic resistance genes (ARGs) of priority antibiotics in China
According to most prioritization results, higher antibiotic usage amounts are generally associated with a higher potential for concentration in the aquatic environment, which poses a high risk to humans, animals, and water bodies [39]. For example, one of the significant risks that may be brought on by using priority antibiotics in water bodies is developing resistance to antibiotics bacteria. The excessive and improper use of antibiotics has reduced their efficacy against human and animal pathogens, thereby hastening the emergence of antibiotic-resistant bacteria (ARB) and antibiotic-resistant genes (ARGs), which has diminished the therapeutic value of antibiotics [3]. According to earlier research, antibiotics that are adsorbed on soils and sediments and those that are dissolved in water exhibit effective biological activity, such as exerting pressure on bacteria to produce antibiotic resistance [190].
As shown in a report from the World Health Organization (WHO), antimicrobial resistance is an urgent global public health issue that needs to be handled immediately [191]. The issue is particularly severe in China. Due to its widespread use of sub-therapeutic antibiotic doses in agriculture, strong incentives for overprescribing, and antibiotic prescribing practices [192]. For example, it has been approximated that nearly 75% of patients suffering from seasonal flu are given prescriptions for antibiotics, while 80% of patients admitted to hospitals receive antibiotic prescriptions. This percentage is significantly higher than the maximum limit of 30% recommended by the World Health Organization [193]. The primary reason for this over-prescription could be that hospitals generate a significant portion of their revenue from the sale of drugs [194]. In China, antibiotic-resistant bacteria's (ARB) emergence has become a global concern, with other countries reporting infections caused by such bacteria [3, 195]. Moreover, China has also reported cases of multidrug-resistant (MDR) bacteria, commonly known as "superbugs," that can resist several types of antibiotics.
Sulfonamides, tetracyclines, macrolides, quinolones, and antibiotics categorized as others (chloramphenicol, trimethoprim, ketoconazole, and lincomycin) are the five classes into which priority antibiotics in China are divided. Because the corresponding antibiotics are commonly used and persistent in the environment, tetracycline (tet) and sulfonamide (sul) ARGs are frequently found. ARGs have been found in several Chinese environmental matrices, such as WWTPs and sludge, surface water, sediment, and livestock farm waste.
7.1 Antibiotic resistance genes (ARGs) in WWTPs
According to Guo et al., one of the hotspots of ARGs is wastewater treatment plants (WWTPs) [196]. WWTPs are anticipated to have a high level of ARG diversity and abundance because they provide an environment conducive to ARB growth and ARG spread [197]. Tetracycline and sulfonamide resistance genes are most frequently detected and reported in different environmental matrices. Tetracycline genes which are recognized as the most frequently occurring tet genes in China's WWTPs consist of (tet M, tet O, tet Q, and tet W) which are four genes for ribosomal protection proteins; (tet A, tet C, and tet G) which are the three efflux pump genes and (tetX) which are one gene for enzyme modification [198, 199].
sulI and sul II are sulfonamide-resistance genes often detected in WWTPs throughout China. The antibiotic-resistant genes (ARGs) concentrations in WWTPs varied substantially across different WWTPs, even after normalization by sample volumes. Studies have shown that the concentration of tet genes ranged from 102 to 1010 copies/mL [199]. The WWTP in Zhejiang province, Eastern China, had the highest level (1011.17 copies/mL) of tet genes in raw wastewater [200]. Besides, antibiotic resistance genes from other antibiotic classes, such as quinolones and macrolide, have been detected in WWTPs. For example, ARGs like quinolone (qnr) and macrolide resistance gene (erm) have been quantified in sludge and effluent from northern China’s WWTPs [201, 202].
According to the abundance of sulfonamides and tetracyclines resistance genes, it has been reported that the abundance of sul was generally higher than that of tet. This is because sulI is often linked to class I integrons [3]. By comparing the abundance of ARGs in WWTPs, it was reported that WWTPs from pharmaceutical industries had higher ARGs than that municipal and rural WWTPs. The composition of ARGs may remain the same, but the proportion of their distributions in wastewater may change after treatment. Most ARGs may be transferred into the sludge, even if their relative abundance has decreased during treatment [203]. A previous study has shown that the abundance of ARGs was 1.0 –14.1 times greater in the effluent and sludge than in the influent, indicating that the proliferation of ARGs occurs during the treatment process [204].
7.2 Antibiotic resistance genes (ARGs) in surface water
As a result of its significance in maintaining environmental standards and promoting human health, research on antibiotic resistance in Escherichia coli present in surface water has gained considerable attention. For example, Hu et al. (2008) screened the Wenyu River Basin in Beijing, China, for E. coli resistant to one or more antibiotics, revealing a mean frequency of 48.7 ± 8.7% of 388 isolates in the wet season and 47 ± 6% of 236 isolates in the dry season. The most commonly observed drugs with high resistance rates were sulfonamides, tetracyclines, and ampicillin [205]. Culture-dependent approaches and qualitative PCR methods were used in previous studies to identify resistant bacteria and their respective ARGs for the five antibiotic classes (tetracycline, sulfonamide, fluoroquinolone, and chloramphenicol) in the surface water of China. The measured result by qPCR shows that tet and sul were the most prevalent resistance genes in the study area [3].
Two sulfonamide ARGs (sulI and sul II) were quantified in the 20 samples from the Beijing River in South China. SulI was present at higher concentrations than Sul II (p < 0.05), with mean values of (1.41 ± 1.12) × 10−2 and (1.58 ± 1.71) × 10−3 copies of 16S rDNA, respectively [206].
In the Huangpu River, Jiang et al. [207] discovered eight genes for tetracycline resistance (tet A, tet B, tet C, tet G, tet M, tet O, tet W, and tet X) and two genes for sulfonamide resistance (sul1 and sul2), with tet B having the lowest average absolute abundance of 3.7 × 101 copies/mL and tet X having the highest of 1.6 × 105 [207]. Shen et al. [208] conducted a similar study in the Huangpu River and found that sul genes were more prevalent than tet genes A [208].
Niu et al. [209] quantified nine ARGs in the Bohai Bay coastal zone near Tianjin City, with sulfonamide ARGs having the highest abundances of 105–103 copies/16S copies, followed the tetracycline ARGs (10−5–10−4 copies/16S copies) and the macrolide ARGs(10−6–10−5 copies/16S copies [209]. Xu et al. [210] found that sul1 and sul2 were the most common and dominant genes in a study on antibiotics and antibiotic resistance genes in Qingcaosha Reservoir in the Yangtze River Delta, China, with tet A and sul1 having the highest relative abundances (> 102 copies/16S copies). The total relative abundance of ARGs was higher in the site closest to the inflow than in other sites [210].
7.3 Antibiotic resistance genes (ARGs) in sediment
Sediments are considered a relatively significant reservoir of ARGs in most earlier studies [198]. Sediment samples may have higher ARG concentrations than water samples. For example, Luo et al. showed that the concentration levels of sul I and sul II in sediments were 120 to 2000 times greater than in water samples collected from China's Haihe River [211]. Similar findings were made in samples from the Northern Yellow Sea, where the concentrations of sulI and sul II in the sediment were 103 times higher than those in the water, indicating that the sediment was a significant repository for some ARGs [212].
The presence of ARGs in the sediments of surface water and estuaries was examined to reflect the effects of anthropogenic activities. The regions of some developed cities and coastal areas in China were the main focus of the study by Zhao et al., on the incidence of ARGs. Zhao et al. studied the presence of ARGs in surface water sediments and estuaries in developed cities and coastal areas in China. They found that six ARGs had an abundance of roughly 105–101 copies/16S copies in sediments, with Sul1 and Sul2 present in all nine provinces ((Liaoning, Hebei, Shandong, Tianjin, Shanghai, Zhejiang, Fujian, Guangdong, and in Guangxi) out of the six genes examined. The exception was Hebei Province, which had a low relative abundance (< 10−8 copies/16S copies) [203]. Guangdong Province and Shanghai had a higher relative abundance of sul genes than other provinces, which may partly reflect the impact of human activities on the occurrence of ARGs.In comparison to other provinces, Shandong Province and Shanghai had a higher relative abundance of tet genes [203]. According to the report by Ben et al., it was noticed that swine feedlots were nearby when Shandong Province sediments were sampled, which may have contributed to the relatively high abundance of ARGs that was found [213].
7.4 Antibiotic resistance genes (ARGs) in livestock animal farm waste
Pigs, hens, and cows are the primary animals raised for food production in China and are also the animals most frequently studied for their manure content. Most studies on antibiotic-resistant bacteria in these animals rely on culture-dependent assays. For instance, Yang et al. found that many E. coli isolates from pig and hen farms in Beijing and Heibei Province, China, 2000 were resistant to several categories of antibiotics. The resistant isolates showed resistance to trimethoprim-sulfamethoxazole (76%), streptomycin (77%), ampicillin (79%), sulfamethoxazole (84%), tetracycline (98%), among other drugs. Levofloxacin resistance was 64%, ciprofloxacin resistance was 79%, and difloxacin resistance was 95%. Similar results were obtained for E. coli isolates from cow, pig, and hen farms in Eastern China's Shandong Province, with 52% of hen isolates, 25% of pig isolates, and 30% of cow isolates showing resistance to 12, 10, and 1 antimicrobial agent, respectively [214].
Numerous reports have been about tetracycline and sulfonamide resistance genes in livestock manures. Eight ARGs (sulI, sul II, sul III, and sul A) were quantified in manure samples from representative swine, poultry, and cattle feedlots in Shanghai, China [215]. All ARGs tested were found in the collected samples except for tet B. Sulfonamide, and tetracycline resistance genes were relatively common, with relative abundances of 10−5 to 10−2 and10−6 to 10−3, respectively. Except for sul II, overall results have shown that antibiotic-resistance genes from sulfonamide were more prevalent than antibiotic resistance from tetracycline, and only a sluggishly positive relationship between ARGs and the respective antibiotics was found [215].
7.5 Antibiotic resistance genes (ARGs) in in soils
Antibiotics are commonly released into agricultural soils in China through manure or compost as fertilizer. Many studies [215,216,217,218,219,220,221,222] have reported a significant increase in the diversity and abundance of antibiotic resistance genes (ARGs) in soils that have been treated with livestock manure over long periods. ARGs for sulfonamide and tetracycline resistance are commonly found in soils that have been amended with manure or compost [83, 215, 219, 220, 222,223,224]. The sul and tet resistance gene levels in agricultural soils in China range from 10−6–10−2 to 10−8–10−2 gene copies/16S rRNA gene copies. ARGs are most prevalent in Northeast China because the region has long-term wastewater irrigation [222].
7.6 Antibiotic resistance genes (ARGs) and other environmental matrices in China
Besides the aforementioned environmental compartments, antibiotic-resistance genes have been reported in another environmental compartments of China, including sludge [225,226,227], and landfill refuse and leachates [228,229,230,231,232]; groundwater [233] and even drinking water [234,235,236].
Numerous studies have found a positive relationship between ARGs and paired antibiotics. For example, water and sediment samples from the Pearl River Estuary showed a positive relationship (correlation) between the total abundance of tet genes and the total concentration of antibiotics [237]. Furthermore, a municipal wastewater treatment plant study found that the abundance of the sul1 gene was related to the level of sulfonamides [81]. Another study investigating Antibiotic resistance genes (ARGs) in municipal wastewater discovered a positive relationship between two tet genes (tet B and tet W) and tetracycline. At the same time, a negative correlation was observed between qnr C and enrofloxacin [238]. In manure-amended soils, the relationships between ARGs and antibiotics were investigated [229]. Only three genes (sul2, sul3, and tet M) out of nine correlated with the concentrations of specific antibiotics in 13 mariculture environments. On the other hand, Tet W abundance in water showed a positive correlation with total sulfonamide concentrations [239]. The discussion above means that the ARGs might also be correlated with other antibiotic classes and have a positive or negative relationship with the paired antibiotics [208, 240, 241].
8 Rationale of prioritization of antibiotics in China
Antibiotics are widely produced and used in China for both people and animals. In China, therapeutic drugs for humans and animals and feed additives (growth promoters) are widely used. They may cause environmental and human health issues due to their existence in the environment. As a result, previous studies have documented numerous instances of the detection of various antibiotic classes in various environmental compartments of China, including coastal waters [44, 242] surface water and sediment [49, 143, 242,243,244,245]. Effluent and sludge of STPs [3, 11], animal wastewater and manure [246,247,248], Soil [197, 249], Groundwater [11, 250, 251] and many others [11]. Therefore, to manage the environment in China, it is crucial to list the antibiotics considered of relative priority [37].
Different prioritization results in this review showed that countries have different priority antibiotic lists, primarily because of different consumption and removal patterns. Most prioritization results ranking systems revealed that higher antibiotic usage amounts are likely to result in higher concentrations of antibiotics in aquatic environments, which would pose a greater risk to aquatic environments [5].
Since the 2009 health system reform, China has given antibiotic control considerable attention by increasing national antimicrobial stewardship and establishing the National Essential Medicines System. The use of antibiotics at tertiary institutions was successfully reduced by a national antimicrobial stewardship program. Still, the drug policy change did not address the current problems with antibiotic misuse in primary care and rural settings [10]. As a result, the overuse of antibiotics will continue to increase the concentration of antibiotics detected in China's various aquatic environments, putting both human health and the aquatic environment at risk.
Precision equipment, skilled analysis technologies, and effective pre-treatment are needed to identify antibiotics in the environment. Antibiotic simultaneous quantification is also exceedingly expensive, time-consuming, and challenging. Therefore, the priority monitoring antibiotics must be filtered out of the antibiotics detected in the environment. Therefore, it's crucial to sort the antibiotics in the environment and eliminate those requiring closer monitoring [5, 40].
Prioritizing antibiotic use also helps to create a focus on ongoing antibiotic management and monitoring. It also provides a critical framework for evaluating ecological risks and managing antibiotic contamination in aquatic ecosystems.
9 Conclusion
The results of the prioritization studies showed that different countries have different priority pharmaceutical lists, primarily as a result of different patterns of consumption and removal. According to most prioritization results ranking systems, the more pharmaceuticals are used, the more the indication of a higher potential for concentration in the aquatic environment and a higher risk to the aquatic environment. The development of antibiotic-resistance genes and bacteria is one of the main risks of prolonged antibiotic exposure in aquatic environments. This issue has been identified as a global public health emergency that requires rapid attention.
The removal of antibiotics in order of priority by wastewater treatment facilities varies between WWTPs. Several variables, including pH, temperature, the physical and chemical characteristics of the antibiotic, HRT, SRT, the number of microorganisms present and the removal technology used, primarily impact it. Priority antibiotics in China could be placed at the forefront of the emerging pollutants to be monitored in the aquatic environment of China to reduce and get rid of human risks and the impacts of these priority antibiotics on the non-target organisms. This awareness could be also followed and practiced by the rest of the world in advance by in order to be proactive and get rid of antibiotic pollution in their respective aquatic environment as this been reported that monitoring antibiotics according to a priority list is cost-effective and not time-consuming.
Data availability
Not applicable.
References
Yang S-F, et al. Fate of sulfonamide antibiotics in contact with activated sludge: sorption and biodegradation. Water Res. 2012;46(4):1301–8.
Yu Z, et al. Removal and degradation mechanisms of sulfonamide antibiotics in a new integrated aerobic submerged membrane bioreactor system. Bioresour Technol. 2018;268:599–607.
Qiao M, et al. Review of antibiotic resistance in China and its environment. Environ Int. 2018;110:160–72.
Xu Z, et al. Spatiotemporal heterogeneity of antibiotic pollution and ecological risk assessment in Taihu Lake Basin, China. Sci Total Environ. 2018;643:12–20.
Li Y, et al. Ranking and prioritizing pharmaceuticals in the aquatic environment of China. Sci Total Environ. 2019;658:333–42.
Ying G-G, et al. China must reduce its antibiotic use. Environ Sci Technol. 2017;51(3):1072–3.
Zhang Q-Q, et al. Comprehensive evaluation of antibiotics emission and fate in the river basins of china: source analysis, multimedia modeling, and linkage to bacterial resistance. Environ Sci Technol. 2015;49(11):6772–82.
Zeng L, et al. Bibliometric analysis of microbial sulfonamide degradation: development, hotspots and trend directions. Chemosphere. 2022;293: 133598.
Yang Y, et al. Antibiotics and antibiotic resistance genes in global lakes: a review and meta-analysis. Environ Int. 2018;116:60–73.
He P, et al. Rational use of antibiotics in the context of China’s health system reform. BMJ. 2019;365: l4016.
Huang F, et al. Recognition of typical antibiotic residues in environmental media related to groundwater in China (2009–2019). J Hazard Mater. 2020;399: 122813.
Li B, Zhang T. Mass flows and removal of antibiotics in two municipal wastewater treatment plants. Chemosphere. 2011;83(9):1284–9.
Wang H, et al. Ecotoxicological effects, environmental fate and risks of pharmaceutical and personal care products in the water environment: a review. Sci Total Environ. 2021;788: 147819.
Zhao B, et al. Occurrence and fate of ten sulfonamide antibiotics in typical wastewater treatment plants in the City of Jinan of Northeastern China. Desalin Water Treat. 2020. https://doi.org/10.5004/dwt.2020.26346.
Cui J, et al. Occurrence, ecotoxicological risks of sulfonamides and their acetylated metabolites in the typical wastewater treatment plants and receiving rivers at the Pearl River Delta. Sci Total Environ. 2020;709: 136192.
Park JY. Transport and fate of veterinary sulfonamide antibiotics in soil. 2015. https://epub.uni-bayreuth.de/2143/. Accessed 19 Oct 2023.
García-Galán MJ, Díaz-Cruz S, Barceló D. Multiresidue trace analysis of sulfonamide antibiotics and their metabolites in soils and sewage sludge by pressurized liquid extraction followed by liquid chromatography–electrospray-quadrupole linear ion trap mass spectrometry. J Chromatogr A. 2013;1275:32–40.
Li W, et al. Occurrence and removal of antibiotics in a municipal wastewater reclamation plant in Beijing, China. Chemosphere. 2013;92(4):435–44.
Liu C, et al. Simultaneous adsorption of Cd2+ and BPA on amphoteric surfactant activated montmorillonite. Chemosphere. 2016;144:1026–32.
Östman M, Fick J, Tysklind M. Detailed mass flows and removal efficiencies for biocides and antibiotics in Swedish sewage treatment plants. Sci Total Environ. 2018;640–641:327–36.
K’Oreje KO, et al. Occurrence, fate and removal of pharmaceuticals, personal care products and pesticides in wastewater stabilization ponds and receiving rivers in the Nzoia Basin, Kenya. Sci Total Environ. 2018;637–638:336–48.
Ondarza PM, et al. Pharmaceuticals, illicit drugs and their metabolites in fish from Argentina: implications for protected areas influenced by urbanization. Sci Total Environ. 2019;649:1029–37.
Williams M, et al. Emerging contaminants in a river receiving untreated wastewater from an Indian urban centre. Sci Total Environ. 2019;647:1256–65.
Guo J, Selby K, Boxall AB. Assessment of the risks of mixtures of major use veterinary antibiotics in European surface waters. Environ Sci Technol. 2016;50(15):8282–9.
Fridman O, et al. Optimization of lag time underlies antibiotic tolerance in evolved bacterial populations. Nature. 2014;513(7518):418–21.
Zhang A-N, et al. An omics-based framework for assessing the health risk of antimicrobial resistance genes. Nat Commun. 2021;12(1):4765.
Yin R, et al. In situ photoreduction of structural Fe(III) in a metal–organic framework for peroxydisulfate activation and efficient removal of antibiotics in real wastewater. J Hazard Mater. 2020;388: 121996.
Qin K, et al. A review of ARGs in WWTPs: sources, stressors and elimination. Chin Chem Lett. 2020;31(10):2603–13.
Bai C-X, Shen F, Qi X-H. Preparation of porous carbon directly from hydrothermal carbonization of fructose and phloroglucinol for adsorption of tetracycline. Chin Chem Lett. 2017;28(5):960–2.
Du J, et al. Hydroxyl radical dominated degradation of aquatic sulfamethoxazole by Fe0/bisulfite/O2: Kinetics, mechanisms, and pathways. Water Res. 2018;138:323–32.
Li S-Z, Li X-Y, Wang D-Z. Membrane (RO-UF) filtration for antibiotic wastewater treatment and recovery of antibiotics. Sep Purif Technol. 2004;34(1):109–14.
Si Q-S, et al. Carbon quantum dots-based semiconductor preparation methods, applications and mechanisms in environmental contamination. Chin Chem Lett. 2020;31(10):2556–66.
Luo Y, et al. Removal and fate of micropollutants in a sponge-based moving bed bioreactor. Bioresour Technol. 2014;159:311–9.
Song H-L, et al. Degradation of sulfamethoxazole in low-C/N ratio wastewater by a novel membrane bioelectrochemical reactor. Bioresour Technol. 2020;305: 123029.
Zhang L, et al. Quantifying the contribution rates of sulfonamide antibiotics removal mechanisms in constructed wetlands using multivariate statistical analysis. Environ Pollut. 2022;292: 118463.
Sui Q, et al. Identification of priority pharmaceuticals in the water environment of China. Chemosphere. 2012;89(3):280–6.
Wang N, et al. Prioritization of veterinary medicines in China’s environment. Hum Ecol Risk Assess Int J. 2014;20(5):1313–28.
Li Y, et al. Prioritization of pharmaceuticals in water environment in China based on environmental criteria and risk analysis of top-priority pharmaceuticals. J Environ Manage. 2020;253: 109732.
Huang F, et al. Prioritization of antibiotic contaminants in China based on decennial national screening data and their persistence, bioaccumulation and toxicity. Sci Total Environ. 2022;806: 150636.
Li X, et al. Occurrence, comparison and priority identification of antibiotics in surface water and sediment in urbanized river: a case study of Suzhou creek in Shanghai. Sustainability. 2022;14:8757.
Chen Y, et al. Occurrence and risk assessment of antibiotics in multifunctional reservoirs in Dongguan, China. Environ Sci Pollut Res. 2020;27(12):13565–74.
Han QF, et al. Distribution, combined pollution and risk assessment of antibiotics in typical marine aquaculture farms surrounding the Yellow Sea, North China. Environ Int. 2020;138: 105551.
Zhang R, et al. Occurrence and risks of antibiotics in the coastal aquatic environment of the Yellow Sea, North China. Sci Total Environ. 2013;450–451:197–204.
Lu J, et al. Occurrence, distribution, and ecological-health risks of selected antibiotics in coastal waters along the coastline of China. Sci Total Environ. 2018;644:1469–76.
Li W, et al. Occurrence of antibiotics in water, sediments, aquatic plants, and animals from Baiyangdian Lake in North China. Chemosphere. 2012;89(11):1307–15.
Wu M-H, et al. Occurrence, fate and interrelation of selected antibiotics in sewage treatment plants and their receiving surface water. Ecotoxicol Environ Saf. 2016;132:132–9.
Xu J, et al. Distribution, sources and composition of antibiotics in sediment, overlying water and pore water from Taihu Lake, China. Sci Total Environ. 2014;497–498:267–73.
Gao L, et al. Occurrence and distribution of antibiotics in urban soil in Beijing and Shanghai, China. Environ Sci Pollut Res Int. 2015;22(15):11360–71.
Li S, et al. A duodecennial national synthesis of antibiotics in China’s major rivers and seas (2005–2016). Sci Total Environ. 2018;615:906–17.
Li S, et al. Antibiotics in water and sediments of rivers and coastal area of Zhuhai City, Pearl River estuary, South China. Sci Total Environ. 2018;636:1009–19.
Wang Y, et al. Monitoring, mass balance and fate of pharmaceuticals and personal care products in seven wastewater treatment plants in Xiamen City, China. J Hazard Mater. 2018;354:81–90.
Dong W, et al. Review: Micro-organic contaminants in groundwater in China. Hydrog J. 2018;26(5):1351–69.
Al-Khazrajy OS, Boxall AB. Risk-based prioritization of pharmaceuticals in the natural environment in Iraq. Environ Sci Pollut Res Int. 2016;23(15):15712–26.
Guo J, et al. Toxicological and ecotoxicological risk-based prioritization of pharmaceuticals in the natural environment. Environ Toxicol Chem. 2016;35(6):1550–9.
Mansour F, et al. Environmental risk analysis and prioritization of pharmaceuticals in a developing world context. Sci Total Environ. 2016;557–558:31–43.
Zhou S, et al. Optimization of screening-level risk assessment and priority selection of emerging pollutants: the case of pharmaceuticals in European surface waters. Environ Int. 2019;128:1–10.
Kim Y, et al. Prioritizing veterinary pharmaceuticals for aquatic environment in Korea. Environ Toxicol Pharmacol. 2008;26(2):167–76.
Kumar A, Xagoraraki I. Pharmaceuticals, personal care products and endocrine-disrupting chemicals in U.S. surface and finished drinking waters: a proposed ranking system. Sci Total Environ. 2010;408(23):5972–89.
Ortiz de García S, et al. Ranking of concern, based on environmental indexes, for pharmaceutical and personal care products: an application to the Spanish case. J Environ Manage. 2013;129:384–97.
Daouk S, et al. Prioritization methodology for the monitoring of active pharmaceutical ingredients in hospital effluents. J Environ Manage. 2015;160:324–32.
Menz J, Schneider M, Kümmerer K. Usage pattern-based exposure screening as a simple tool for the regional priority-setting in environmental risk assessment of veterinary antibiotics: a case study of north-western Germany. Chemosphere. 2015;127:42–8.
Organization, W.H., WHO model list of essential medicines, 20th list (March 2017, amended August 2017). 2017.
Chabilan A, et al. Mesocosm experiment to determine the contribution of adsorption, biodegradation, hydrolysis and photodegradation in the attenuation of antibiotics at the water sediment interface. Sci Total Environ. 2023;866: 161385.
Carlotti B, Fuoco D, Elisei F. Fast and ultrafast spectroscopic investigation of tetracycline derivatives in organic and aqueous media. Phys Chem Chem Phys. 2010;12(48):15580–91.
Carvalho IT, Santos L. Antibiotics in the aquatic environments: a review of the European scenario. Environ Int. 2016;94:736–57.
Etebu E, Arikekpar I. Antibiotics: classification and mechanisms of action with emphasis on molecular perspectives. Int J Appl Microbiol Biotechnol Res. 2016;4:90–101.
Santos L, Ramos F. Analytical strategies for the detection and quantification of antibiotic residues in aquaculture fishes: a review. Trends Food Sci Technol. 2016;52:16–30.
Pham TDM, Ziora ZM, Blaskovich MAT. Quinolone antibiotics. Medchemcomm. 2019;10(10):1719–39.
Andriole VT. Use of quinolones in treatment of prostatitis and lower urinary tract infections. Eur J Clin Microbiol Infect Dis. 1991;10(4):342–50.
Kümmerer K. Antibiotics in the aquatic environment: a review–part I. Chemosphere. 2009;75(4):417–34.
Thiele-Bruhn S. Pharmaceutical antibiotic compounds in soils: a review. J Plant Nutr Soil Sci. 2003;166:145–67.
Belal F, Al-Majed AA, Al-Obaid AM. Methods of analysis of 4-quinolone antibacterials. Talanta. 1999;50(4):765–86.
Sánchez AR, Rogers RS 3rd, Sheridan PJ. Tetracycline and other tetracycline-derivative staining of the teeth and oral cavity. Int J Dermatol. 2004;43(10):709–15.
Díaz-Cruz MS, Barceló D. Chapter 2.1 Analysis of antibiotics in aqueous samples. In: Petrović M, Barceló D, editors. Comprehensive analytical chemistry. Amsterdam: Elsevier; 2007. p. 61–93.
Hoff R, Pizzolato TM, Diaz-Cruz MS. Trends in sulfonamides and their by-products analysis in environmental samples using mass spectrometry techniques. Trends Environ Anal Chem. 2016;9:24–36.
Zhu Y-G, et al. Continental-scale pollution of estuaries with antibiotic resistance genes. Nat Microbiol. 2017;2:16270. https://doi.org/10.1038/nmicrobiol.2016.270.
Qu J, et al. Municipal wastewater treatment in China: development history and future perspectives. Front Environ Sci Eng. 2019;13(6):88.
Huang J, et al. Characterizing the river water quality in China: recent progress and on-going challenges. Water Res. 2021;201: 117309.
Zhang H, et al. Fate of antibiotics during wastewater treatment and antibiotic distribution in the effluent-receiving waters of the Yellow Sea, Northern China. Mar Pollut Bull. 2013;73(1):282–90.
Guerra P, et al. Occurrence and fate of antibiotic, analgesic/anti-inflammatory, and antifungal compounds in five wastewater treatment processes. Sci Total Environ. 2014;473–474:235–43.
Gao L, et al. Occurrence of antibiotics in eight sewage treatment plants in Beijing, China. Chemosphere. 2012;86(6):665–71.
Tahrani L, et al. Identification and risk assessment of human and veterinary antibiotics in the wastewater treatment plants and the adjacent sea in Tunisia. Water Sci Technol. 2017;76(11):3000–21.
Zhang H, et al. Occurrence, seasonal variation and removal efficiency of antibiotics and their metabolites in wastewater treatment plants, Jiulongjiang River Basin, South China. Environ Sci Process Impacts. 2015;17(1):225–34.
Tran NH, et al. Occurrence and removal of multiple classes of antibiotics and antimicrobial agents in biological wastewater treatment processes. Water Res. 2016;104:461–72.
Zhou L-J, et al. Occurrence and fate of eleven classes of antibiotics in two typical wastewater treatment plants in South China. Sci Total Environ. 2013;452–453:365–76.
Sun Q, et al. Seasonal and spatial variations of PPCP occurrence, removal and mass loading in three wastewater treatment plants located in different urbanization areas in Xiamen, China. Environ Poll. 2016;208:371–81.
Hu J, et al. Occurrence and fate of antibiotics in a wastewater treatment plant and their biological effects on receiving waters in Guizhou. Proc Saf Environ Prot. 2018;113:483–90.
Verlicchi P, et al. Hospital effluent: Investigation of the concentrations and distribution of pharmaceuticals and environmental risk assessment. Sci Total Environ. 2012;430:109–18.
Mohapatra S, et al. Occurrence and fate of pharmaceuticals in WWTPs in India and comparison with a similar study in the United States. Chemosphere. 2016;159:526–35.
Rodriguez-Mozaz S, et al. Occurrence of antibiotics and antibiotic resistance genes in hospital and urban wastewaters and their impact on the receiving river. Water Res. 2015;69:234–42.
Shao B, et al. Determination of 76 pharmaceutical drugs by liquid chromatography–tandem mass spectrometry in slaughterhouse wastewater. J Chromatogr A. 2009;1216(47):8312–8.
Rossmann J, et al. Simultaneous determination of most prescribed antibiotics in multiple urban wastewater by SPE-LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;969:162–70.
Jia A, et al. Occurrence and fate of quinolone and fluoroquinolone antibiotics in a municipal sewage treatment plant. Water Res. 2012;46(2):387–94.
Chang X, et al. Determination of antibiotics in sewage from hospitals, nursery and slaughter house, wastewater treatment plant and source water in Chongqing region of Three Gorge Reservoir in China. Environ Pollut. 2010;158(5):1444–50.
K’Oreje KO, et al. Occurrence patterns of pharmaceutical residues in wastewater, surface water and groundwater of Nairobi and Kisumu city, Kenya. Chemosphere. 2016;149:238–44.
Batt AL, Kim S, Aga DS. Comparison of the occurrence of antibiotics in four full-scale wastewater treatment plants with varying designs and operations. Chemosphere. 2007;68(3):428–35.
Valdés ME, et al. Distribution of antibiotics in water, sediments and biofilm in an urban river (Córdoba, Argentina, LA). Environ Pollut. 2021;269: 116133.
Wang Y, et al. Distribution, sources, and potential risks of antibiotic resistance genes in wastewater treatment plant: a review. Environ Pollut. 2022;310: 119870.
Xiong J-Q, Kurade MB, Jeon B-H. Biodegradation of levofloxacin by an acclimated freshwater microalga, Chlorella vulgaris. Chem Eng J. 2017;313:1251–7.
Abramović BF, et al. Experimental and computational study of hydrolysis and photolysis of antibiotic ceftriaxone: degradation kinetics, pathways, and toxicity. Sci Total Environ. 2021;768: 144991.
Ben W, et al. Occurrence, removal and risk of organic micropollutants in wastewater treatment plants across China: comparison of wastewater treatment processes. Water Res. 2018;130:38–46.
Zhou X, et al. Partitioning of fluoroquinolones on wastewater sludge. Clean: Soil, Air, Water. 2013;41(8):820–7.
Lin AY-C, Tsai Y-T. Occurrence of pharmaceuticals in Taiwan’s surface waters: impact of waste streams from hospitals and pharmaceutical production facilities. Sci Total Environ. 2009;407(12):3793–802.
Kim S, et al. Removal of antibiotics in wastewater: effect of hydraulic and solid retention times on the fate of tetracycline in the activated sludge process. Environ Sci Technol. 2005;39(15):5816–23.
Verlicchi P, et al. Removal of selected pharmaceuticals from domestic wastewater in an activated sludge system followed by a horizontal subsurface flow bed: analysis of their respective contributions. Sci Total Environ. 2013;454–455:411–25.
Kassotaki E, et al. Enhanced sulfamethoxazole degradation through ammonia oxidizing bacteria co-metabolism and fate of transformation products. Water Res. 2016;94:111–9.
Deng W, et al. Occurrence and risk assessment of antibiotics in river water in Hong Kong. Ecotoxicol Environ Saf. 2016;125:121–7.
Lv Y, et al. Seasonal occurrence and behavior of synthetic musks (SMs) during wastewater treatment process in Shanghai, China. Sci Total Environ. 2010;408(19):4170–6.
Dong H, et al. Occurrence and removal of antibiotics in ecological and conventional wastewater treatment processes: a field study. J Environ Manage. 2016;178:11–9.
Huang M, et al. Removal performance and changes in the microbial communities of SBRs under aerobic and anoxic conditions with trace tetracycline pressure. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2014;49(8):940–7.
Stadler LB, et al. Effect of redox conditions on pharmaceutical loss during biological wastewater treatment using sequencing batch reactors. J Hazard Mater. 2015;282:106–15.
Oberoi AS, et al. Insights into the fate and removal of antibiotics in engineered biological treatment systems: a critical review. Environ Sci Technol. 2019;53(13):7234–64.
de Ilurdoz MS, Sadhwani JJ, Reboso JV. Antibiotic removal processes from water and wastewater for the protection of the aquatic environment: a review. J Water Proc Eng. 2022;45: 102474.
Wu Q, et al. Effects of antibiotics on anaerobic digestion of sewage sludge: performance of anaerobic digestion and structure of the microbial community. Sci Total Environ. 2022;845: 157384.
Zhang M, et al. Variations of antibiotic resistome in swine wastewater during full-scale anaerobic digestion treatment. Environ Int. 2021;155: 106694.
Zhou H, et al. Zero-valent iron enhanced in-situ advanced anaerobic digestion for the removal of antibiotics and antibiotic resistance genes in sewage sludge. Sci Total Environ. 2021;754: 142077.
Cao D-Q, et al. Role of extracellular polymeric substance in adsorption of quinolone antibiotics by microbial cells in excess sludge. Chem Eng J. 2019;370:684–94.
Liu Y, et al. Inhibition of tetracycline on anaerobic digestion of swine wastewater. Bioresour Technol. 2021;334: 125253.
Gurmessa B, et al. Manure anaerobic digestion effects and the role of pre- and post-treatments on veterinary antibiotics and antibiotic resistance genes removal efficiency. Sci Total Environ. 2020;721: 137532.
Liu H, et al. Removal of tetracyclines, sulfonamides, and quinolones by industrial-scale composting and anaerobic digestion processes. Environ Sci Pollut Res. 2018;25(36):35835–44.
Feng L, et al. Removal of antibiotics during the anaerobic digestion of pig manure. Sci Total Environ. 2017;603–604:219–25.
Martín J, et al. Pharmaceutically active compounds in sludge stabilization treatments: anaerobic and aerobic digestion, wastewater stabilization ponds and composting. Sci Total Environ. 2015;503–504:97–104.
Hazra M, Durso LM. Performance efficiency of conventional treatment plants and constructed wetlands towards reduction of antibiotic resistance. Antibiotics (Basel). 2022;11(1):114.
Song H-L, et al. Vertical up-flow constructed wetlands exhibited efficient antibiotic removal but induced antibiotic resistance genes in effluent. Chemosphere. 2018;203:434–41.
Zheng S, et al. Current progress in natural degradation and enhanced removal techniques of antibiotics in the environment: a review. Int J Environ Res Public Health. 2022;19(17):10919.
Chen M, et al. Collision of emerging and traditional methods for antibiotics removal: taking constructed wetlands and nanotechnology as an example. NanoImpact. 2019;15: 100175.
Hijosa-Valsero M, et al. Removal of antibiotics from urban wastewater by constructed wetland optimization. Chemosphere. 2011;83(5):713–9.
Zhao W, et al. Efficient elimination of sulfonamides by an anaerobic/anoxic/oxic-membrane bioreactor process: performance and influence of redox condition. Sci Total Environ. 2018;633:668–76.
Kırıl Mert B, et al. Efficient removal approach of micropollutants in wastewater using membrane bioreactor. In: Yonar T, editor., et al., Wastewater and water quality. London: InTech; 2018.
Li C, Cabassud C, Guigui C. Evaluation of membrane bioreactor on removal of pharmaceutical micropollutants: a review. Desalin Water Treat. 2015;55(4):845–58.
Besha AT, et al. Removal of emerging micropollutants by activated sludge process and membrane bioreactors and the effects of micropollutants on membrane fouling: a review. J Environ Chem Eng. 2017;5(3):2395–414.
Zhang M, et al. Introduction to aerobic membrane bioreactors: current status and recent developments. Curr Dev Biotechnol Bioeng. 2020. https://doi.org/10.1016/B978-0-12-819809-4.00001-2.
Radjenović J, Petrović M, Barceló D. Fate and distribution of pharmaceuticals in wastewater and sewage sludge of the conventional activated sludge (CAS) and advanced membrane bioreactor (MBR) treatment. Water Res. 2009;43(3):831–41.
García Galán MJ, Díaz-Cruz MS, Barceló D. Removal of sulfonamide antibiotics upon conventional activated sludge and advanced membrane bioreactor treatment. Anal Bioanal Chem. 2012;404(5):1505–15.
Sipma J, et al. Comparison of removal of pharmaceuticals in MBR and activated sludge systems. Desalination. 2010;250(2):653–9.
Fernandez-Fontaina E, et al. Biodegradation kinetic constants and sorption coefficients of micropollutants in membrane bioreactors. Biodegradation. 2013;24(2):165–77.
Wang L, et al. Behavior of tetracycline and macrolide antibiotics in activated sludge process and their subsequent removal during sludge reduction by ozone. Chemosphere. 2018;206:184–91.
Park J, et al. Removal of pharmaceuticals and personal care products by ammonia oxidizing bacteria acclimated in a membrane bioreactor: Contributions of cometabolism and endogenous respiration. Sci Total Environ. 2017;605–606:18–25.
Wen Q, et al. Insight into effects of antibiotics on reactor performance and evolutions of antibiotic resistance genes and microbial community in a membrane reactor. Chemosphere. 2018;197:420–9.
Shi B-J, et al. Application of membrane bioreactor for sulfamethazine-contained wastewater treatment. Chemosphere. 2018;193:840–6.
Pretel R, et al. Navigating environmental, economic, and technological trade-offs in the design and operation of submerged anaerobic membrane bioreactors (AnMBRs). Water Res. 2015;87:531–41.
Smith AL, et al. Navigating wastewater energy recovery strategies: a life cycle comparison of anaerobic membrane bioreactor and conventional treatment systems with anaerobic digestion. Environ Sci Technol. 2014;48(10):5972–81.
Qiu W, et al. Occurrence of antibiotics in the main rivers of Shenzhen, China: association with antibiotic resistance genes and microbial community. Sci Total Environ. 2019;653:334–41.
Huang B, et al. Treatment of pharmaceutical wastewater containing β-lactams antibiotics by a pilot-scale anaerobic membrane bioreactor (AnMBR). Chem Eng J. 2018;341:238–47.
Torresi E, et al. Impact of external carbon dose on the removal of micropollutants using methanol and ethanol in post-denitrifying moving bed biofilm reactors. Water Res. 2017;108:95–105.
Yang C-W, Tsai L-L, Chang B-V. Anaerobic degradation of sulfamethoxazole in mangrove sediments. Sci Total Environ. 2018;643:1446–55.
Zhou Q, et al. Enhanced strategies for antibiotic removal from swine wastewater in anaerobic digestion. Trends Biotechnol. 2021;39(1):8–11.
Chen Y, et al. Sulfamethoxazole removal from mariculture wastewater in moving bed biofilm reactor and insight into the changes of antibiotic and resistance genes. Chemosphere. 2022;298: 134327.
Bílková Z, Malá J, Hrich K. Fate and behaviour of veterinary sulphonamides under denitrifying conditions. Sci Total Environ. 2019;695: 133824.
Chen HY, Liu YD, Dong B. Biodegradation of tetracycline antibiotics in A/O moving-bed biofilm reactor systems. Bioprocess Biosyst Eng. 2018;41(1):47–56.
Carneiro RB, et al. Influence of organic loading rate on ciprofloxacin and sulfamethoxazole biodegradation in anaerobic fixed bed biofilm reactors. J Environ Manage. 2020;273: 111170.
Casas ME, et al. Biodegradation of pharmaceuticals in hospital wastewater by staged Moving Bed Biofilm Reactors (MBBR). Water Res. 2015;83:293–302.
Ibrahim Faskol AS, Racoviţeanu G. Technology review of the biological aerated filter systems BAFs for removal of the nitrogen in wastewater. IOP Conf Ser Earth Environ Sci. 2021;664(1): 012106.
Priya VS, Philip L. Treatment of volatile organic compounds in pharmaceutical wastewater using submerged aerated biological filter. Chem Eng J. 2015;266:309–19.
Chen J, et al. Removal of antibiotics from piggery wastewater by biological aerated filter system: treatment efficiency and biodegradation kinetics. Bioresour Technol. 2017;238:70–7.
Arya V, Philip L, Murty Bhallamudi S. Performance of suspended and attached growth bioreactors for the removal of cationic and anionic pharmaceuticals. Chem Eng J. 2016;284:1295–307.
Zhang J, et al. Removal of oxytetracycline and ofloxacin in wastewater by microalgae-bacteria symbiosis for bioenergy production. Bioresour Technol. 2022;363: 127891.
Chu Y, et al. Biotransformation of sulfamethoxazole by microalgae: removal efficiency, pathways, and mechanisms. Water Res. 2022;221: 118834.
Peng J, et al. Removal of levofloxacin by an oleaginous microalgae Chromochloris zofingiensis in the heterotrophic mode of cultivation: removal performance and mechanism. J Hazard Mater. 2022;425: 128036.
Xiong Q, et al. Co-metabolism of sulfamethoxazole by a freshwater microalga Chlorella pyrenoidosa. Water Res. 2020;175: 115656.
Xie B, et al. Biological sulfamethoxazole degradation along with anaerobically digested centrate treatment by immobilized microalgal-bacterial consortium: performance, mechanism and shifts in bacterial and microalgal communities. Chem Eng J. 2020;388: 124217.
Zhang B, et al. Microalgal-bacterial consortia: from interspecies interactions to biotechnological applications. Renew Sustain Energy Rev. 2020;118: 109563.
Wang Y, et al. Enhanced biodegradation of chlortetracycline via a microalgae-bacteria consortium. Bioresour Technol. 2022;343: 126149.
Li J, et al. Effect of tetracycline on the growth and nutrient removal capacity of Chlamydomonas reinhardtii in simulated effluent from wastewater treatment plants. Bioresour Technol. 2016;218:1163–9.
Ashraf N, Ahmad F, Lu Y. Synergy between microalgae and microbiome in polluted waters. Trends Microbiol. 2023;31(1):9–21.
Moldes Plaza D. Factors influencing the bioremoval of copper and zinc from wastewater using microalgae, bacteria, and their consortia. 2020.
Wang C-Y, et al. A new Eu-MOF for ratiometrically fluorescent detection toward quinolone antibiotics and selective detection toward tetracycline antibiotics. Chin Chem Lett. 2022;33(3):1353–7.
Lakshminarasimman N, et al. Biotransformation and sorption of trace organic compounds in biological nutrient removal treatment systems. Sci Total Environ. 2018;640–641:62–72.
Guo X, et al. Sulfamethoxazole and COD increase abundance of sulfonamide resistance genes and change bacterial community structures within sequencing batch reactors. Chemosphere. 2017;175:21–7.
Zhang Y, et al. Characterization of microbial community and antibiotic resistance genes in activated sludge under tetracycline and sulfamethoxazole selection pressure. Sci Total Environ. 2016;571:479–86.
Zheng W, et al. Removal of veterinary antibiotics from anaerobically digested swine wastewater using an intermittently aerated sequencing batch reactor. J Environ Sci. 2018;65:8–17.
Yin F, et al. Antibiotic degradation and microbial community structures during acidification and methanogenesis of swine manure containing chlortetracycline or oxytetracycline. Bioresour Technol. 2018;250:247–55.
Akyol Ç, et al. The fate of oxytetracycline in two-phase and single-phase anaerobic cattle manure digesters and its effects on microbial communities. J Chem Technol Biotechnol. 2016;91(3):806–14.
Huang X, et al. Performance and bacterial community dynamics of vertical flow constructed wetlands during the treatment of antibiotics-enriched swine wastewater. Chem Eng J. 2017;316:727–35.
Choi Y-J, Kim L-H, Zoh K-D. Removal characteristics and mechanism of antibiotics using constructed wetlands. Ecol Eng. 2016;91:85–92.
Ávila C, et al. Attenuation of emerging organic contaminants in a hybrid constructed wetland system under different hydraulic loading rates and their associated toxicological effects in wastewater. Sci Total Environ. 2014;470–471:1272–80.
Senta I, et al. Removal of antimicrobials using advanced wastewater treatment. J Hazard Mater. 2011;192(1):319–28.
Bourgin M, et al. Evaluation of a full-scale wastewater treatment plant upgraded with ozonation and biological post-treatments: abatement of micropollutants, formation of transformation products and oxidation by-products. Water Res. 2018;129:486–98.
Monsalvo VM, et al. Removal of trace organics by anaerobic membrane bioreactors. Water Res. 2014;49:103–12.
Wijekoon KC, et al. Development of a predictive framework to assess the removal of trace organic chemicals by anaerobic membrane bioreactor. Bioresour Technol. 2015;189:391–8.
Polesel F, et al. Removal of pharmaceuticals in pre-denitrifying MBBR—influence of organic substrate availability in single- and three-stage configurations. Water Res. 2017;123:408–19.
Torresi E, et al. Biofilm thickness influences biodiversity in nitrifying MBBRs: implications on micropollutant removal. Environ Sci Technol. 2016;50(17):9279–88.
Liu H-Q, et al. Spatial distribution and removal performance of pharmaceuticals in municipal wastewater treatment plants in China. Sci Total Environ. 2017;586:1162–9.
Zhang X, et al. Occurrence, removal, and risk assessment of antibiotics in 12 wastewater treatment plants from Dalian, China. Environ Sci Pollut Res Int. 2017;24(19):16478–87.
Ashfaq M, et al. Occurrence, fate, and mass balance of different classes of pharmaceuticals and personal care products in an anaerobic-anoxic-oxic wastewater treatment plant in Xiamen, China. Water Res. 2017;123:655–67.
Alegbeleye O, et al. Efficient removal of antibiotics from water resources is a public health priority: a critical assessment of the efficacy of some remediation strategies for antibiotics in water. Environ Sci Pollut Res Int. 2022;29(38):56948–7020.
Gu Y, et al. Fate of pharmaceuticals during membrane bioreactor treatment: status and perspectives. Bioresour Technol. 2018;268:733–48.
Krzeminski P, et al. Performance of secondary wastewater treatment methods for the removal of contaminants of emerging concern implicated in crop uptake and antibiotic resistance spread: a review. Sci Total Environ. 2019;648:1052–81.
Yang X, et al. Occurrence and removal of pharmaceuticals and personal care products (PPCPs) in an advanced wastewater reclamation plant. Water Res. 2011;45(16):5218–28.
Zhang Q, et al. Occurrences of three classes of antibiotics in a Natural River Basin: association with antibiotic-resistant Escherichia coli. Environ Sci Technol. 2014;48(24):14317–25.
WHO, Global Action Plan on Antimicrobial Resistance. Witte W. Selective pressure by antibiotic use in livestock. Int J Antimicrob Resist Agents. 2015;16:19–24.
Yezli S, Li H. Antibiotic resistance amongst healthcare-associated pathogens in China. Int J Antimicrob Agents. 2012;40(5):389–97.
Li Y. China’s misuse of antibiotics should be curbed. BMJ. 2014;348: g1083.
Currie J, Lin W, Meng J. Addressing antibiotic abuse in China: an experimental audit study. J Dev Econ. 2014;110:39–51.
Wang M. Antimicrobial resistance in China: challenges and actions. Clin Infect Dis. 2018;67(2):S127–S127.
Guo J, et al. Metagenomic analysis reveals wastewater treatment plants as hotspots of antibiotic resistance genes and mobile genetic elements. Water Res. 2017;123:468–78.
Zhao F, et al. Distribution, dynamics and determinants of antibiotics in soils in a peri-urban area of Yangtze River Delta, Eastern China. Chemosphere. 2018;211:261–70.
Chen B, et al. Metagenomic profiles of antibiotic resistance genes (ARGs) between human impacted estuary and deep ocean sediments. Environ Sci Technol. 2013;47(22):12753–60.
Wen Q, et al. Monitoring and evaluation of antibiotic resistance genes in four municipal wastewater treatment plants in Harbin, Northeast China. Environ Poll. 2016;212:34–40.
Li J, et al. Occurrence and removal of antibiotics and the corresponding resistance genes in wastewater treatment plants: effluents’ influence to downstream water environment. Environ Sci Pollut Res Int. 2016;23(7):6826–35.
Luo Y, et al. Proliferation of multidrug-resistant New Delhi Metallo-β-lactamase genes in municipal wastewater treatment plants in Northern China. Environ Sci Technol Lett. 2013;1:26.
Mao D, et al. Prevalence and proliferation of antibiotic resistance genes in two municipal wastewater treatment plants. Water Res. 2015;85:458–66.
Zhao W, Wang B, Yu G. Antibiotic resistance genes in China: occurrence, risk, and correlation among different parameters. Environ Sci Pollut Res Int. 2018;25(22):21467–82.
Zhai W, et al. Fate and removal of various antibiotic resistance genes in typical pharmaceutical wastewater treatment systems. Environ Sci Pollut Res. 2016;23(12):12030–8.
Hu J, et al. Phenotyping and genotyping of antibiotic-resistant Escherichia coli isolated from a natural river basin. Environ Sci Technol. 2008;42(9):3415–20.
Ling Z, et al. A preliminary investigation on the occurrence and distribution of antibiotic resistance genes in the Beijiang River, South China. J Environ Sci. 2013;25(8):1656–61.
Jiang L, et al. Prevalence of antibiotic resistance genes and their relationship with antibiotics in the Huangpu river and the drinking water sources, Shanghai, China. Sci Total Environ. 2013;458–460:267–72.
Shen Q, et al. Preliminary studies on the pollution levels of antibiotic and antibiotic resistance genes in Huangpu River, China. Ecol Environ Sci. 2012;21:1717–23.
Niu Z-G, Zhang K, Zhang Y. Occurrence and distribution of antibiotic resistance genes in the coastal area of the Bohai Bay, China. Mar Poll Bull. 2016;107(1):245–50.
Xu T, et al. Characteristics of antibiotics and antibiotic resistance genes in Qingcaosha Reservoir in Yangtze River Delta, China. Environ Sci Eur. 2020;32(1):82.
Luo Y, et al. Trends in antibiotic resistance genes occurrence in the Haihe River, China. Environ Sci Technol. 2010;44(19):7220–5.
Na G, et al. Sulfonamide antibiotics in the Northern Yellow Sea are related to resistant bacteria: implications for antibiotic resistance genes. Mar Pollut Bull. 2014;84(1):70–5.
Ben W, et al. Dissemination of antibiotic resistance genes and their potential removal by on-farm treatment processes in nine swine feedlots in Shandong Province, China. Chemosphere. 2017;167:262–8.
Yang H, et al. Characterization of multiple-antimicrobial-resistant Escherichia coli isolates from diseased chickens and swine in China. J Clin Microbiol. 2004;42(8):3483–9.
Ji X, et al. Antibiotic resistance gene abundances associated with antibiotics and heavy metals in animal manures and agricultural soils adjacent to feedlots in Shanghai, China. J Hazard Mater. 2012;235–236:178–85.
Chen Q, et al. Long-term field application of sewage sludge increases the abundance of antibiotic resistance genes in soil. Environ Int. 2016;92–93:1–10.
Cheng W, et al. Abundance and persistence of antibiotic resistance genes in livestock farms: a comprehensive investigation in eastern China. Environ Int. 2013;61:1–7.
Mu Q, et al. Occurrence of sulfonamide-, tetracycline-, plasmid-mediated quinolone- and macrolide-resistance genes in livestock feedlots in Northern China. Environ Sci Pollut Res Int. 2015;22(9):6932–40.
Wang N, et al. Sulfonamide-resistant bacteria and their resistance genes in soils fertilized with manures from Jiangsu Province, Southeastern China. PLoS ONE. 2014;9: e112626.
Wu N, et al. Abundance and diversity of tetracycline resistance genes in soils adjacent to representative swine feedlots in China. Environ Sci Technol. 2010;44(18):6933–9.
Zhang S, et al. Characterization of antibiotics and antibiotic resistance genes on an ecological farm system. J Chem. 2015;2015:1–8.
Zhou X, et al. Use of commercial organic fertilizer increases the abundance of antibiotic resistance genes and antibiotics in soil. Environ Sci Pollut Res Int. 2017;24(1):701–10.
Cheng W, et al. Behavior of antibiotics and antibiotic resistance genes in eco-agricultural system: a case study. J Hazard Mater. 2016;304:18–25.
Peng S, et al. Long-term application of fresh and composted manure increase tetracycline resistance in the arable soil of eastern China. Sci Total Environ. 2015;506–507:279–86.
Yang Y, et al. Quantification and characterization of β-lactam resistance genes in 15 sewage treatment plants from East Asia and North America. Appl Microbiol Biotechnol. 2012;95(5):1351–8.
Zhang T, et al. Tetracycline resistance genes and tetracycline resistant lactose-fermenting Enterobacteriaceae in activated sludge of sewage treatment plants. Environ Sci Technol. 2009;43(10):3455–60.
Zhang XX, Zhang T. Occurrence, abundance, and diversity of tetracycline resistance genes in 15 sewage treatment plants across China and other global locations. Environ Sci Technol. 2011;45(7):2598–604.
Song L, et al. Sulfamethoxazole, tetracycline and oxytetracycline and related antibiotic resistance genes in a large-scale landfill, China. Sci Total Environ. 2016;551–552:9–15.
Wang Y, et al. Occurrence and prevalence of antibiotic resistance in landfill leachate. Environ Sci Pollut Res Int. 2015;22(16):12525–33.
Wu S, et al. Preparation of ceramic filler from reusing sewage sludge and application in biological aerated filter for soy protein secondary wastewater treatment. J Hazard Mater. 2015;283:608–16.
Yu Z, et al. Co-occurrence of mobile genetic elements and antibiotic resistance genes in municipal solid waste landfill leachates: a preliminary insight into the role of landfill age. Water Res. 2016;106:583–92.
Zhang XH, et al. Occurrence of antibiotic resistance genes in landfill leachate treatment plant and its effluent-receiving soil and surface water. Environ Pollut. 2016;218:1255–61.
Chen Q-L, et al. An underappreciated hotspot of antibiotic resistance: the groundwater near the municipal solid waste landfill. Sci Total Environ. 2017;609:966–73.
Guo X, et al. Prevalence of sulfonamide and tetracycline resistance genes in drinking water treatment plants in the Yangtze river Delta, China. Sci Total Environ. 2014;493:626–31.
Xu L, et al. High-throughput profiling of antibiotic resistance genes in drinking water treatment plants and distribution systems. Environ Pollut. 2016;213:119–26.
Xi C, et al. Prevalence of antibiotic resistance in drinking water treatment and distribution systems. Appl Environ Microbiol. 2009;75(17):5714–8.
Chen B, et al. Differentiating anthropogenic impacts on ARGs in the Pearl River Estuary by using suitable gene indicators. Water Res. 2013;47(8):2811–20.
Xu J, et al. Occurrence of antibiotics and antibiotic resistance genes in a sewage treatment plant and its effluent-receiving river. Chemosphere. 2015;119:1379–85.
Chen CQ, et al. Persistence and risk of antibiotic residues and antibiotic resistance genes in major mariculture sites in Southeast China. Sci Total Environ. 2017;580:1175–84.
McKinney CW, et al. tet and sul antibiotic resistance genes in livestock lagoons of various operation type, configuration, and antibiotic occurrence. Environ Sci Technol. 2010;44(16):6102–9.
Su H-C, et al. Contamination profiles of antibiotic resistance genes in the sediments at a catchment scale. Sci Total Environ. 2014;490:708–14.
Li F, et al. Identification of the priority antibiotics based on their detection frequency, concentration, and ecological risk in urbanized coastal water. Sci Total Environ. 2020;747: 141275.
Li S, et al. Antibiotics in water and sediments of Danjiangkou Reservoir, China: spatiotemporal distribution and indicator screening. Environ Pollut. 2019;246:435–42.
Sharma BM, et al. Health and ecological risk assessment of emerging contaminants (pharmaceuticals, personal care products, and artificial sweeteners) in surface and groundwater (drinking water) in the Ganges River Basin, India. Sci Total Environ. 2019;646:1459–67.
Xie H, et al. Pharmaceuticals and personal care products in water, sediments, aquatic organisms, and fish feeds in the Pearl River Delta: occurrence, distribution, potential sources, and health risk assessment. Sci Total Environ. 2019;659:230–9.
Qian M, et al. Occurrence of trace elements and antibiotics in manure-based fertilizers from the Zhejiang Province of China. Sci Total Environ. 2016;559:174–81.
Liu X, et al. Occurrence of typical antibiotics and source analysis based on PCA-MLR model in the East Dongting Lake, China. Ecotoxicol Environ Saf. 2018;163:145–52.
Wang R, et al. Screening and quantitation of residual antibiotics in two different swine wastewater treatment systems during warm and cold seasons. Sci Total Environ. 2019;660:1542–54.
Wei R, et al. Occurrence of seventeen veterinary antibiotics and resistant bacterias in manure-fertilized vegetable farm soil in four provinces of China. Chemosphere. 2019;215:234–40.
Ma L, et al. Impacts of irrigation water sources and geochemical conditions on vertical distribution of pharmaceutical and personal care products (PPCPs) in the vadose zone soils. Sci Total Environ. 2018;626:1148–56.
Yang Y-Y, et al. Pharmaceuticals and personal care products (PPCPs) and artificial sweeteners (ASs) in surface and ground waters and their application as indication of wastewater contamination. Sci Total Environ. 2018;616–617:816–23.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (Grant No. 52091542) and the Shanghai Science and Technology Commission (Grant No. 20XD1430600).
Author information
Authors and Affiliations
Contributions
To the study conception and design: JPB and EI and HY-Material preparation, data collection, and analysis were performed: JPB-The writing first draft, and preparation of original manuscript JPB. All authors: JPB, EI, YH, AMW, GDSQJr., PU, RW and JZ commented on previous versions of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bavumiragira, J.P., Eheneden, I., Yin, H. et al. Insight on prioritization of antibiotics in China, their occurrence, and removal by different wastewater treatment technologies. Discov Environ 2, 28 (2024). https://doi.org/10.1007/s44274-024-00047-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44274-024-00047-z