Abstract
Purpose
In our previous study, we found that corylifol A (CYA), an active component derived from Psoralea corylifolia L, exhibited ameliorating effects on cancer cachexia. Anamorelin (AML), a ghrelin receptor agonist, is the only approved drug for the clinical treatment of cancer cachexia. Here, we checked the combination of CYA and AML on muscle atrophy and fat lipolysis in cancer cachexia mice to explore the possible clinic therapeutic potency of the combination on cancer cachexia.
Methods
C26 colon tumor-bearing mice, a well-established animal model of cancer cachexia, were used to evaluate the effects of combined therapy involving CYA and AML on cancer cachexia and compared with those of CYA or AML alone. Histological analysis was performed to assess the changes in gastrocnemius (GAS) muscle tissue and epididymal adipose tissue (eWAT) and the possible involved molecular pathways were explored using western blotting and ELISA.
Results
Treatments of CYA, AML, or CYA+AML all significantly ameliorated the body weight decrease, muscle atrophy as well as fat loss in the tumor-bearing mice. Notably, the ameliorating effects of CYA+AML on fat loss were more significant than CYA or AML alone. The eWAT weight of mice in healthy control, C26 model, C26 +CYA, C26 +AML or C26 +CYA +AML group was 0.48 ± 0.07, 0.21 ± 0.10, 0.26 ± 0.10, 0.37 ± 0.10 and 0.48 ± 0.18 g, respectively. CYA+AML could increase food intake, significantly decrease the serum levels of inflammatory cytokines (TNF-α and IL-6) and inhibit the activation (phosphorylation) of hormone-sensitive lipase (HSL) in the eWAT tissues of the C26 tumor-bearing mice. Treatment of CYA along could significantly decrease the serum TNF-α level of the C26 tumor-bearing mice. Treatment of AML alone could increase food intake but could not decrease the serum levels of inflammatory cytokines of the C26 tumor-bearing mice.
Conclusion
These results suggested that the effects of CYA+AML include both the appetite-stimulating effects of AML and the anti-inflammatory effects of CYA. CYA+AML was better than CYA or AML alone in ameliorating cancer cachexia, especially fat loss, of C26 tumor-bearing mice and might be a novel and beneficial therapeutic option for cancer cachexia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cancer cachexia is a multifactorial syndrome affects 50–80% of patients with advanced cancer and contributes to more than 20% of death in cancer patients. It is characterized by un-controlled decrease in body weight resulted from a combination of reduced food intake and increased catabolism, which is induced by inflammation cytokines especially in muscle and adipose tissues [1]. Cancer cachexia is the consequence of a combination of different mechanisms, including alterations in physiological energy balance with an increased resting energy expenditure and reduced energy intake, hormonal and metabolic disturbances in both the periphery and the central nervous system, and a proinflammatory/pro-cachectic environment induced by tumor byproducts and immune-system mediators [2]. The most well established cancer cachexia etiology is that it develops through a series of inflammation-related mechanisms, associated with characteristically high levels of pro-inflammatory cytokines. Both clinical studies and pre-clinical studies confirmed the elevated pro-inflammatory cytokines such as IL-6, TNF-α and immune markers in cancer cachexia patients or model animals [3]. Pro-inflammatory cytokines play the first-rate component in the pathophysiology of cancer cachexia [4]. The inflammatory factors could directly promote the catabolism of adipose and skeletal muscle tissues and lead to fat loss and muscle atrophy [5]. Anorexia in cancer cachexia patients is caused by several factors including dysphagia, nausea, vomiting, gastrointestinal obstruction, decrease in bowel movements and side effects of medications. At the same time, since the paraventricular nucleus and arcuate nucleus express receptors for pro-inflammatory cytokines, the pro-inflammatory cytokines such as IL-1beta, IL-6, TNF-α, and LIF also mimic leptin negative feedback on the hypothalamus and exert a cachexic effect [4]. Therefore, the pro-inflammatory cytokines could directly modulate the protein turnover in skeletal muscle tissues and lipolysis in fat tissues but could also indirectly cause muscle atrophy and fat loss through anorexic stimuli in cancer cachexia [6].
As a multifactorial and multi-organ syndrome, cancer cachexia requires a multi-modal treatment strategy. For inducing appetite and alleviating anorexia, anamorelin (AML), a ghrelin receptor agonist, is a successful example. Ghrelin is a 28-amino acid neuropeptide hormone released from the stomach in response to fasting that stimulates food intake. The secretion of ghrelin from the stomach can be induced by a compensatory mechanism in cachexia and then ghrelin could perform various activities such as increasing fat, preventing muscle atrophy, and reducing muscle breakdown [7]. Ghrelin receptors play a role in releasing growth hormone (GH) from the pituitary gland and increasing appetite through the hypothalamus. GH secretion from the pituitary gland stimulates the liver to release insulin-like growth factor 1 (IGF-1), which enhances muscle protein synthesis [8]. AML was approved by Japan in 2020 for the clinical treating cancer cachexia in patients with non-small cell lung cancer, gastric cancer, pancreatic cancer, and colorectal cancer. Results of clinical studies showed that, by promoting GH secretion via ghrelin receptor activation and increases appetite, AML did improve the body weight loss in patients with cancer cachexia in clinical practice [9]. However, cancer cachexia is mediated and complicated by multiple factors therefore appetite-stimulating agent like AML could not fully attenuate cancer cachexia. Researchers also tried to develop drugs targeting other targets of cancer cachexia such as anti-inflammatory agents like TNF-α blockers infliximab and adalimumab though the clinical trial results were not promising [10].
Corylifol A (CYA) is an isoflavone isolated from the fruit of Psoralea corylifolia L. [11]. CYA has been reported to inhibit inflammatory factors as well as muscle atrophic factor (MAFbx and MuRF1) to ameliorate dexamethasone-induced C2C12 myotubular atrophy and mouse muscle atrophy [12, 13]. In our previous study, we firstly reported that CYA could ameliorate cancer cachexia in C26 tumor-bearing mice in vivo and also could ameliorate myotube atrophy induced by simulated cancer cachexia injuries in vitro [14]. The mechanisms of CYA in ameliorating cancer cachexia include direct inhibiting effects on protein degradation in muscle atrophy and anti-inflammatory effects by targeting the TAOK1/p38-MAPK/FoxO3 pathway [14].
Since single drug intervention might not effective enough to treat cachexia, combined therapy had emerged as an option. Accordingly, active attempts of combination therapy using AML together with other therapies for cancer cachexia were conducted both in clinic [15] and in laboratory research [1]. For example, combination of AML and ActRIIB-Fc was shown to effectively ameliorate cancer cachexia symptoms in a mice model of cancer cachexia [1]. Based on the appetite-inducing effects of AML and the anti-inflammatory and muscle-protecting effects of CYA, we predicted that the combination of AML and CYA might be a promising combination therapy by ameliorating the anorexia, inflammation and muscle atrophy in cancer cachexia at the same time. In the present study, we conducted in vivo experiments using C26 tumor-bearing mice, a well-accepted cancer cachexia mice model, to investigate the anti-cachexia effects of the combination of CYA and AML and compared with that of CYA or AML alone. Possible mechanisms of the combination of CYA and AML in ameliorating cancer cachexia were also explored in the present study. The aim of the present study is to evaluate the possibility of developing CYA into new therapy reagent for cancer cachexia, either as single drug or as a combination therapy together with AML.
2 Materials and methods
2.1 Reagents
CYA, with a purity > 98%, was purchased from Zhenzhun Biotechnology Co. (Shanghai, P.R. China). AML, with a purity of > 99.9%, was purchased from MedChemExpress (NJ, United States). RMPI-1640, Penicillin/streptomycin, and trypsin/EDTA were purchased from Cytiva (Los Angeles, CA, United States). Fetal bovine serum (FBS) was derived from Biological Industries (Kibbutz Beit Haemek, Israel).
2.2 Cell culture
Murine colon adenocarcinoma C26 cells, obtained from Shanghai Institute of Materia Medica, Chinese Academy of Sciences, were maintained in RPMI-1640 medium containing 10% FBS. C26 cells grown in the exponential phase were harvested with trypsin/EDTA for inoculation of mice to establish the cancer cachexia model in vivo.
2.3 Cancer cachexia model in vivo
The establishment of cancer cachexia animal model in vivo by inoculating C26 colon tumor cells into BALB/c mice was conducted similar to previous reports [14, 16, 17]. Male BALB/c mice (6–8 weeks old) were purchased from Shanghai Jihui Laboratory Animal Care Co., Ltd. (Shanghai, China). The care and experimental protocols for this study were in accordance with the Chinese regulations and the Guidelines for the Care and Use of Laboratory Animals drawn up by the National Institutes of Health (USA) and were approved by the Institutional Animal Care and Use Committee of the Shanghai University of Traditional Chinese Medicine (NO. PZSHUTCM2304070004). All animals were acclimatized for a week before beginning the study. Mice were randomly divided into five groups (9 mice in each group): Healthy control group without tumor (Healthy group), C26 tumor-bearing mice group (C26 model group), C26 tumor-bearing mice treated with ip. 20 mg/kg CYA group (CYA group), C26 tumor-bearing mice treated with ig. 30 mg/kg AML group (AML group), and C26 tumor-bearing mice treated with ip. 20 mg/kg CYA and ig. 30 mg/kg AML group (CYA+AML group). C26 tumor cells were inoculated into mice as described in our previous papers [14, 16]. The doses and routes of administration of CYA and AML were selected based on previous studies reporting the efficacy of CYA [14] and AML [1, 18, 19] in ameliorating cancer cachexia.
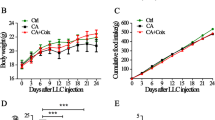
The flow chart illustrating the study design was shown in Fig. 1a. As shown in Fig. 1a, the duration for which the mice received their treatment of CYA, AML or CYA+AML was 18 days and the total study duration was 19 days. Briefly, on Day 0, mice were implanted subcutaneously in the right flank with 200 μL (1.0 × 106) C26 tumour cells. And the mice of group C26 +CYA, C26 +AML or C26 +CYA+AML were given corresponding drugs since the next day after the inoculation of C26 tumor cells till the end of the experiment (Day 18). The healthy control group mice and C26 model group mice received equal volumes of the solvent of AML by gavage (saline) and the solvent of CYA by intraperitoneal injection (2% DMSO + 2% EtOH + 5% Solutol + 91% saline). Body weight and food intake were recorded daily. On day 5 after injection of tumor cells, all mice developed tumors as confirmed by palpation of the injection site. On day 8 after tumor cells injection, the tumors had grown to a measurable size. Tumor measurements were taken using a digital calipers and tumor volumes calculated with the formula: V = x*x*y*0.5, where x represents the width in millimeter and y the length in millimeter. At the end of the experiment, the mice were anaesthetised with 2% isoflurane mixed with oxygen [20,21,22] and blood samples were collected from the retro-orbital region of each mouse. That is, a rigid capillary glass tube is punctured 2–3 mm into the inner corner of the eye between the eyelid and the eyeball, and approximately 0.5–0.6 ml of blood without anticoagulant was collected from each mouse immediately after bleeding. And then, the animals were euthanized by CO2 inhalation. The tumor tissues, gastrocnemius (GAS) muscle tissues, and epididymal adipose tissues (eWAT) of all groups of mice were rapidly dissected and weighed. The tumor-free body weight of each mouse was defined as the value of the body weight minus the weight of tumor tissue. A portion of the GAS tissue and eWAT tissue was then fixed in 4% paraformaldehyde overnight and embedded in paraffin, and the remaining tissue was stored at – 80 ℃ until ready for further analysis.
Combined treatment of corylifol A (CYA) and anamorelin (AML) ameliorated weight loss of C26 tumor-bearing mice without affecting tumor growth (a) Schematic diagram of the experimental protocol. b Time-dependent change in body weight of mice in different groups. (c) Tumor-free body weight of mice in different groups at the end of the experiment. d Accumulative food intake of mice in different groups. e Tumor volume of mice. f Photo and weight of the isolated tumor tissues from C26 tumor-bearing mice. Values are expressed as mean ± SD (n = 9). Statistical analysis of one-way ANOVA/Tukey's post hoc test was performed. *p < 0.05, **p < 0.01 compared with C26 model group; ####p < 0.0001 compared with healthy control group
2.4 Hematoxylin–eosin staining
The paraffin-embedded GAS or eWAT tissues were sectioned at 10 μm and stained with Hematoxylin–eosin (H&E) using the standard procedure. The H&E stained tissue sections were observed under a bright field microscope and the cross-sectional area of muscle myofibers or adipocytes were quantified using Image J software as described in our previous reports [14, 16].
2.5 Western blot analysis
Western blot analysis was performed as described in our previous reports [23, 24]. Briefly, eWAT tissues were homogenized in RIPA buffer with a phosphatase protease inhibitor. Equal amounts of protein samples were separated on 7.5% SDS–PAGE gels and transferred to polyvinylidene fluoride membranes. Membranes were blocked with 5% skimmed milk for 1 h at room temperature and then the membrane strips containing the target proteins were cut based on their molecular weight before antibody hybridization. After that, the membrane strips were incubated with corresponding primary antibodies at 4 °C overnight. Subsequently, the membrane strips were incubated with secondary HRP-conjugated antibody at room temperature for 2 h. Antibody-antigen interactions were observed using the ECL kit (Meilunbio, Shanghai, China) and membrane chemiluminescence was detected using the ChemiDocTM MP Imaging System (BioRad, California, USA). Quantification of band intensity was conducted using Image J. The primary antibodies used were as follows: rabbit anti-HSL (Hormone sensitive lipase) polyclonal antibody (1:1000, Cell Signaling Technology), rabbit anti-p-HSL polyclonal antibody (1:1000, Cell Signaling Technology), and mouse anti-β-actin monoclonal antibody (1:1000, Santa Cruz Biotechnology). The secondary antibodies used were HRP-conjugated goat anti-mouse secondary antibody (1:5000, ZEN BIO) and HRP-conjugated goat anti-rabbit secondary antibody (1:5000, ZEN BIO).
2.6 Measurement of inflammatory cytokines and glycerol in mice serum
After the blood samples were collected and allowed to stand at 37 ℃ for more than 30 min, the serum samples were obtained by centrifugation of the blood samples at 4000 rpm for 10 min. The isolated serum samples were then stored at − 80 ℃ for further use. The Mouse IL-6 High Sensitivity ELISA Kit (#EK206/3–96, Multi Sciences, Hangzhou, China), the Mouse TNF-α High Sensitivity ELISA Kit (#EK282HS-96, Multi Sciences, Hangzhou, China), and the Glycerol Assay Kit (#F005-1-1, Nanjing Jiancheng Bioengineering Institute, Nanjing, China) were used to analyze the IL-6, TNF-α and glycerol levels in the serum samples, respectively.
2.7 Statistical analysis
All values are presented as the mean ± SD. Differences between groups were tested for statistical significance using one-way ANOVA with Tukey’s post hoc multiple comparisons using Prism 8.0.2 software, and p < 0.05 was considered significant.
3 Results
3.1 CYA and AML combination treatment ameliorated cancer cachexia symptoms of C26 tumor-bearing mice
The time-dependent change of the mice body weight in each group of mice was shown in Fig. 1b. As shown in Fig. 1b, body weight of healthy control mice continued to increase during the experimental period, while the body weight of C26 model mice increased slowly and even began to decrease from Day 12 after tumor inoculation. At the end of the experiment, the body weight of C26 model mice was significantly lower than that of the healthy control mice. While, the body weight of mice in the CYA, AML or CYA+AML treatment groups were higher than that of the C26 model group. Figure 1c showed the tumor-free body weight of each group of mice at the end of the experiment. The tumor-free body weight of C26 model mice (19.76 ± 1.28 g) was significantly lower than that of healthy control mice (27.87 ± 0.96 g). The tumor-free body weight of mice in C26 +CYA, C26 +AML, C26 +CYA +AML group (21.30 ± 1.63 g, 21.77 ± 1.49 g, and 22.13 ± 1.31 g, respectively) at the end of the experiment were all significantly higher than that of C26 model mice. As shown in Fig. 1d, AML treatment increased the accumulative food intake from the beginning of the experiment. The food intake of AML-treated tumor-bearing mice was even higher than that of the healthy control mice until Day 14 after tumor inoculation, the time point when the cancer cachexia symptoms of tumor-bearing were obvious. The results clearly showed the appetite-inducing effects of AML. CYA+AML treatment also increased the food intake of mice. The time-dependent changes in the tumor volume of each group of mice were shown in Fig. 1e. The photo of tumor tissues and the values of tumor weight at the end of the experiment were shown in Fig. 1f. As shown in Fig. 1e and f, there is no significant difference in the tumor volume or tumor weight among different groups. Together, these results showed that the treatments of CYA, AML, CYA+AML ameliorated weight loss of C26 tumor-bearing mice without affecting tumor growth.
3.2 CYA and AML combination treatment reduced the muscle atrophy of C26 tumor-bearing mice
As shown in Fig. 2a (photo of the GAS muscle tissues) and Fig. 2b (weight of the GAS muscle tissues), the GAS weight of C26 model mice (0.10 ± 0.01 g) was significantly lower than that of healthy control mice (0.15 ± 0.01 g). The GAS weight of mice in C26 +CYA, C26 +AML, C26 +CYA+AML group was 0.12 ± 0.01 g, 0.12 ± 0.01 g, and 0.12 ± 0.01 g, respectively. All three treatment groups, CYA, AML and CYA + AML significantly attenuated the decrease in GAS weight induced by tumor load. While, there was no significant difference among the three treatment groups in the ameliorating effects on muscle atrophy. Similarly, as showed in Fig. 2c and d, skeletal muscle cross-sectional area was reduced in the C26 model group of mice compared to the healthy group, suggesting muscle atrophy while all treatments could ameliorate the decrease in the myofiber cross-sectional area. These results suggested that CYA alone or AML alone could significantly ameliorate the muscle atrophy in cancer cachexia thus CYA+AML also could efficiently ameliorate the muscle atrophy but its effects were not better than that of CYA alone or AML alone.
Combined treatment of corylifol A (CYA) and anamorelin (AML) ameliorated muscle atrophy in C26 tumor-bearing mice (a) Photo of gastrocnemius (GAS) muscle tissues of mice in different groups. b Weight of GAS muscle tissues of mice in different groups. Values are expressed as mean ± SD (n = 9). c Representative figures of GAS tissue sections stained with H&E. Scale bar, 200 μm. d Quantification results of the myofiber cross-sectional area of GAS tissues. Statistical analysis of one-way ANOVA/Tukey's post hoc test was performed. *p < 0.05, **p < 0.01 compared with C26 model group; ####p < 0.0001 compared with healthy control group
3.3 CYA and AML combination treatment reduced the fat loss in C26 tumor-bearing mice with better effects than that of CYA alone or AML alone
As shown in Fig. 3, significant decrease in eWAT weight (Fig. 3a and b) and decrease in adipocyte cross-sectional area of eWAT tissues (Fig. 3c and d) were observed in C26 tumor model group. The eWAT weight of mice in C26 model group (0.21 ± 0.10 g) was significantly lower than that of healthy control mice (0.48 ± 0.07 g). The eWAT weight of mice in C26 +CYA, C26 +AML, C26 +CYA+AML group was 0.26 ± 0.10, 0.37 ± 0.10 and 0.48 ± 0.18 g, respectively. CYA slightly ameliorated the decrease in eWAT weight and adipocyte cross-sectional area of eWAT, but the effects were not significant. Treatment with AML or CYA+AML significantly ameliorated the decrease in eWAT weight. Notably, the ameliorating effects of CYA+AML on fat loss were significantly stronger than that of CYA alone or AML alone. Similar results were observed in the results of analyzing the adipocytes cross-sectional area of eWAT tissues induced by C26 tumor burden (Fig. 3c and d).
Combined treatment of corylifol A (CYA) and anamorelin (AML) ameliorated the fat loss in C26 tumor-bearing mice (a) Photo of epididymal adipose tissues (eWAT) of mice in different groups. b Weight of eWAT of mice in different groups. Values are expressed as mean ± SD (n = 9). c Representative figures of eWAT tissue sections stained with H&E. Scale bar, 200 μm. d Quantification results of the adipocyte cross-sectional area of eWAT tissues. Statistical analysis of one-way ANOVA/Tukey's post hoc test was performed. *p < 0.05, ***p < 0.001 compared with C26 model group; ###p < 0.001 compared with healthy control group. $$p < 0.01 compared with CYA group
As shown in Fig. 4a and b, the expression and the activation (phosphorylation) of hormone-sensitive lipase (HSL) increased in C26 model mice compared with that of healthy control mice. The Western blotting assay images showed in Fig. 4a were representative images. The original images of the Western blotting assay, together with results of other replicate experiments, were provided as Supplementary materials of the manuscript. CYA, AML or CYA+AML could significantly reduce the activation of HSL in fat tissues of C26 tumor-bearing mice. These results suggested that CYA+AML combination therapy was an efficient combination therapy in ameliorating fat loss in cancer cachexia.
Combined treatment of corylifol A (CYA) and anamorelin (AML) inhibited the activation of Hormone sensitive lipase (HSL) in the eWAT of C26 tumor-bearing mice. a Representative western blotting of phosphorylated HSL and total HSL in the eWAT of mice. b Results of quantification analysis of the ratio values of phosphorylated HSL (p-HSL) to total HSL in (a). Values were expressed as mean ± SD (n = 3). Statistical analysis of one-way ANOVA/Tukey's post hoc test was performed. *p < 0.05, **p < 0.01 compared with C26 model group; ##p < 0.01 compared with healthy control group
3.4 CYA and AML combination treatment decreased serum levels of inflammatory cytokines and glycerol
Results of analysis of the serum levels of TNF-α in each group of mice were shown in Fig. 5a. As shown in Fig. 5a, the serum level of TNF-α in C26 model mice (35.74 ± 18.66 pg/mL) was significantly higher than that of healthy control mice (13.26 ± 4.89 pg/mL). The serum TNF-α level of mice in C26 +CYA, C26 +AML, C26 +CYA+AML group was 9.32 ± 8.00, 28.01 ± 18.63, and 11.37 ± 6.42 pg/mL, respectively. CYA or CYA+AML treatment could significantly inhibit the increase of serum TNF-α in C26 tumor-bearing mice, but AML treatment did not. Results of analysis of the serum levels of IL-6 in each group of mice were shown in Fig. 5b. As shown in Fig. 5b, the serum level of IL-6 in C26 model mice (1061.81 ± 533.87 pg/mL) was significantly higher than that of healthy control mice (9.89 ± 2.48 pg/mL). The serum IL-6 level of mice in C26 +CYA, C26 +AML, C26 +CYA+AML group was 826.45 ± 141.87, 1086.21 ± 387.43, 382.71 ± 213.01 pg/mL, respectively. CYA or AML treatment alone could not ameliorate the elevation of IL-6 in the serum of C26 tumor bearing mice. Only CYA+AML could significantly inhibit the increase of serum IL-6 in C26 tumor bearing mice. These results suggested that CYA+AML combination treatment could significantly inhibit the increase of inflammatory cytokines such as TNF-α and IL-6 in C26 tumor-bearing mice. The anti-inflammatory effects of CYA+AML might be mainly resulted from the effects of CYA. As shown in Fig. 5c, CYA, CYA+AML but not AML could significantly inhibit the increase of glycerol in C26 tumor-bearing mice. These results suggested that, the ameliorating effects of CYA on fat loss in cancer cachexia might be based on inhibiting lipolysis induced by inflammatory cytokines while the ameliorating effects of AML on fat loss might be mainly based on increasing food intake. CYA+AML combination treatment could both inhibit lipolysis induced by inflammatory cytokines and increase food intake therefore exhibit considerable ameliorating effects, better than both CYA alone or AML alone, on fat loss in cancer cachexia.
Combined treatment of corylifol A (CYA) and anamorelin (AML) inhibited the increase of serum levels of inflammatory cytokines and glycerol induced by tumor load in C26 tumor-bearing mice (a) Level of TNF-α in serum of mice in different groups. b Level of IL-6 in serum of mice in different groups. c Level of glycerol in serum of mice in different groups. Values were expressed as mean ± SD (n = 6). One-way ANOVA test was performed followed by Tukey’s post hoc test. *p < 0.05, **p < 0.01 compared with C26 model group; #p < 0.05, ##p < 0.01, ####p < 0.0001 compared with healthy control group; $$p < 0.01 compared with CYA group; &&p < 0.01 compared with AML group
4 Discussion
Currently, there is a lack of effective and specifically targeted drugs for the clinical treatment of cancer cachexia. Results of the present study showed that the combination of CYA and AML exhibited ameliorating effects on cancer cachexia in C26 tumor-bearing mice, a well-accepted and widely used animal model of cancer cachexia. CYA+AML could significantly ameliorate the body weight decrease, muscle atrophy and fat loss in cancer cachexia. The results suggested the possibility of developing combination therapy strategy for cancer cachexia based on the appetite-inducing effects of AML and the anti-inflammatory effects of CYA. Up to now, AML is the only drug approved for the treatment of cancer cachexia while it was only approved by Japan but had not been approved by the FDA [25]. AML is a ghrelin receptor agonist which could ameliorate cancer cachexia mainly by inducing appetite [26]. The ghrelin is a peptide hormone produced by endocrine cells in the stomach that activates ghrelin receptors in the hypothalamus to participate in the release of growth hormone from the pituitary gland and increase appetite. At the same time, ghrelin also could inhibit TNF-α–induced pro-inflammatory cytokine production in vitro and inhibits cytokine release in a rat model of endotoxic shock [27]. Growth hormone released by the pituitary gland could stimulate the liver to secrete insulin-like growth factor 1 (IGF-1), which promotes protein synthesis [8]. Therefore, by activating ghrelin receptors, AML could promote food intake, inhibit inflammatory responses, stimulate release of growth hormone, and thus improve weight loss in patients with cancer cachexia [28]. While, AML still could not fully ameliorate the cancer cachexia symptoms. Use of AML in combination with other pharmacologic interventions in the treatment of cancer cachexia was expected to have better therapeutic outcomes.
CYA is one of the active components of the traditional Chinese medicine Psoralea corylifolia L. It had been shown that CYA could ameliorate dexamethasone-induced muscle atrophy in mice [13] and C2C12 myotubular atrophy [12] by inhibiting the expression of muscle-specific ubiquitin-E3 ligases (MAFbx and MuRF1) and muscle growth inhibitors. And CYA also down-regulated the phosphorylation of NF-κB and inhibitd the expression of inflammatory cytokines (IL-6 and TNF-α) and improved mitochondrial function to alleviate muscle atrophy in diabetic mice [13]. Furthermore, it was found in our previous report that CYA exhibited ameliorating effects on cancer cachexia by inhibiting TAOK1/p38-MAPK/FoxO3 pathway both in vivo and in vitro [14]. The success of the combination of CYA and AML in ameliorating cancer cachexia of C26 tumor-bearing mice in the present study supports further study of using the combination of CYA and AML in the treatment of cancer cachexia.
The most interesting finding in the present study is the efficiency of CYA+AML in ameliorating fat loss in cancer cachexia mice. CYA+AML treatment exhibited a significant alleviation on the weight loss of eWAT. CYA+AML almost totally inhibited the fat loss thus the eWAT weight in CYA+AML-treated mice was similar to that of the healthy control mice. CYA+AML was more effective than CYA alone or AML alone in ameliorating fat loss. It can also be observed from the HE stained sections of eWAT tissues that, the adipocyte cross-sectional area of mice in the model group was reduced compared to the healthy group, suggesting degradation of adipose tissues. CYA, AML, and CYA+AML could all alleviated the decrease in the adipocyte cross-sectional area, with the best alleviation observed in CYA+AML. The promising ameliorating effects of CYA+AML on fat loss might be based on its significant inhibiting effects on the increase of serum pro-inflammatory cytokines and lipolysis in cancer cachexia. Among the pro-inflammatory cytokines that contribute to the development of cancer cachexia, the inflammatory cytokines IL-6 and TNF-α play important roles and contribute to weight loss, skeletal muscle atrophy, and depletion of adipose tissue [29]. IL-6 accelerates the rate of lipolysis in adipocytes [30], activates the STAT3 signaling pathway and induces protein degradation in muscle cells [31]. TNF-α inhibits lipid synthesis in adipocytes [32], induces activation of NF-κB in muscle cells and causes muscle atrophy [33]. Therefore, inhibition of CYA+AML on the increase of serum IL-6 and TNF-α in C26 tumor-bearing mice could decrease lipolysis and thus ameliorate fat loss. HSL (Hormone sensitive lipase) is the rate-limiting enzyme of lipolysis, and it could increase the activity of the lipolytic pathway so that lead to the decrease of adipose tissue and the increase of circulating free fatty acids and glycerol [34]. Increased adipose tissue catabolism in cancer cachexia would result in higher blood glycerol levels [35]. As shown in the results of analyzing the levels of inflammatory cytokines in mice serum, AML alone exhibited no influence on the levels of serum TNF-α or IL-6. CYA could only significantly inhibit the decrease of TNF-α but not IL-6. Notably, CYA+AML could significantly inhibit the increase of both serum TNF-α and IL-6 in cancer cachexia mice. Results of analyzing HSL activation in fat tissues and the serum level of glycerol also clearly showed that CYA+AML significantly inhibited the lipolysis in C26 tumor-bearing mice. CYA+AML could significantly inhibit the activation of HSL in eWAT and also decrease the serum level of glycerol in C26 tumor-bearing mice. The appetite-inducing effects of CYA+AML might also contribute to its ameliorating effects on fat loss in cancer cachexia. As showed in the results of checking the food intake of each group of mice, CYA+AML, similar to AML alone, could increase the food intake of C26 tumor-bearing mice. Both increase in food intake and decrease in lipolysis might be involved in the mechanisms of CYA+AML to ameliorate fat loss in cancer cachexia.
To our surprise, though CYA+AML did significantly ameliorate muscle atrophy in C26 tumor-bearing mice, its effects in ameliorating muscle atrophy did not exhibit to be better than CYA alone or AML alone. There might be two reasons for the no-better effects of CYA+AML in ameliorating muscle atrophy, compared with CYA alone or AML alone. Firstly, since CYA or AML already had significant ameliorating effects on muscle atrophy, it might be difficult for CYA+AML to further ameliorate muscle atrophy in cancer cachexia mice. Secondly, the experiment period of only 18 days might be not long enough to show the combined effects of CYA and AML in ameliorating muscle atrophy. In clinic, weight loss of cancer cachexia patients was mainly resulted from muscle atrophy since the skeletal muscle accounts for ~ 40% of total body weight. While, in animal model of cancer cachexia, fat loss (decrease in weight of fat tissues) develops quickly and thus appears to be more considerable than muscle atrophy in the short time period. In different models of cancer cachexia, adipose tissue loss was identified before a loss of skeletal muscle mass [34]. Therefore, it is possible that CYA+AML combination therapy might exhibit better efficacy in ameliorating muscle atrophy than CYA alone or AML alone in clinic.
In summary, results of the present study showed the efficacy of the combination of CYA and AML in ameliorating cancer cachexia. While, more experiments and further study are necessary to confirm the possibility of using the drugs in cancer cachexia therapy. Firstly, the effective doses range and the best administration route of the drugs needs to be determined. The doses of CYA and AML used in the present study were only one dose which was selected based on previous reports [1, 14, 18, 19]. Efficacy of more doses and different administration routes should be checked. Secondly, other kinds of cancer cachexia animal models need to be used to check the efficacy of the drugs. Especially, cancer cachexia animal models with relatively slow development of tumor growth and long experiment period might be more suitable to check the efficacy of the drugs in ameliorating muscle atrophy. Thirdly, more strategies of combination therapy for cancer cachexia might be tried. For example, muscle atrophy in cancer cachexia was resulted from both the decrease in protein synthesis and the increase in protein degradation, it might be reasonable to use CYA+AML together with other treatment with inducing effects on protein synthesis such as amino acids supplementation to further ameliorate the cancer cachexia.
5 Conclusion
In conclusion, combination therapy of CYA and AML could exhibit both the effects of CYA and the effects of AML in alleviating cancer cachexia symptoms thus might be a novel and beneficial therapeutic option for cancer cachexia. The possible mechanisms of CYA+AML in alleviating cancer cachexia were shown in Fig. 6. As shown in Fig. 6, in the combination of CYA and AML, AML alleviates muscle and fat loss in cachexia by promoting appetite in mice while CYA decreases the levels of inflammatory cytokines and also has direct protecting effects on muscle cells. Therefore, combination therapy of CYA and AML could ameliorate both fat loss and muscle atrophy thus efficiently alleviate cancer cachexia.
Illustration of the possible mechanisms by which combination therapy of corylifol A (CYA) and anamorelin (AML) alleviates cancer cachexia. In the combination of CYA and AML, AML alleviates muscle and fat loss in cachexia by promoting appetite in mice while CYA decreases the levels of inflammatory cytokines and also has direct protecting effects on muscle cells. Therefore, combination therapy of CYA and AML could ameliorate both fat loss and muscle atrophy thus alleviate cancer cachexia
To be noted, as a preclinical study, the results of this research need to face many challenges before being translated into clinical trials. These challenges include determining appropriate drug dosage and administration routes, identifying suitable clinical patients, clarifying possible side effects and addressing potential implementation issues. More pharmacology studies, such as pharmacokinetic studies of the combination of CYA and AML, which can help in the determination of dosing regimens and the prediction of associated side effects, are necessary to check the druggability of the combination. Further in-depth studies clarifying the mechanisms of CYA+AML in ameliorating cancer cachexia are also necessary for possible translation of CYA and AML combination therapy into clinical practice for the effective management of cancer cachexia.
Data availability
The data that support the findings of this study are available from the corresponding author, Xuan Liu, on reasonable request.
Code availability
Not applicable.
References
Queiroz AL, Dantas E, Ramsamooj S, Murthy A, Ahmed M, Zunica ERM, et al. Blocking ActRIIB and restoring appetite reverses cachexia and improves survival in mice with lung cancer. Nat Commun. 2022;13:4633. https://doi.org/10.1038/s41467-022-32135-0.
Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nat Rev Dis Prim. 2018;4:17105. https://doi.org/10.1038/nrdp.2017.105.
Zhang L, Bonomi PD. Immune system disorder and cancer-associated cachexia. Cancers. 2024. https://doi.org/10.3390/cancers16091709.
Malla J, Zahra A, Venugopal S, Selvamani TY, Shoukrie SI, Selvaraj R, et al. What role do inflammatory cytokines play in cancer cachexia? Cureus. 2022;14: e26798. https://doi.org/10.7759/cureus.26798.
Talbert EE, Guttridge DC. Emerging signaling mediators in the anorexia-cachexia syndrome of cancer. Trends in cancer. 2022;8:397–403. https://doi.org/10.1016/j.trecan.2022.01.004.
Mangano GD, Fouani M, D’Amico D, Di Felice V, Barone R. Cancer-related cachexia: the vicious circle between inflammatory cytokines, skeletal muscle, lipid metabolism and the possible role of physical training. Int J Mol Sci. 2022. https://doi.org/10.3390/ijms23063004.
Setiawan T, Sari IN, Wijaya YT, Julianto NM, Muhammad JA, Lee H, et al. Cancer cachexia: molecular mechanisms and treatment strategies. J Hematol Oncol. 2023;16:54. https://doi.org/10.1186/s13045-023-01454-0.
Nishie K, Sato S, Hanaoka M. Anamorelin for cancer cachexia. Drugs Today. 2022;58:97–104. https://doi.org/10.1358/dot.2022.58.3.3381585.
Wakabayashi H, Arai H, Inui A. Anamorelin in Japanese patients with cancer cachexia: an update. Curr Opin Support Palliat Care. 2023;17:162–7. https://doi.org/10.1097/spc.0000000000000658.
da Fonseca GWP, Sato R, de Nazaré Nunes Alves MJ, von Haehling S. Current advancements in pharmacotherapy for cancer cachexia. Expert Opin Pharmacother. 2023;24:629–39. https://doi.org/10.1080/14656566.2023.2194489.
Gao Q, Xu Z, Zhao G, Wang H, Weng Z, Pei K, et al. Simultaneous quantification of 5 main components of Psoralea corylifolia L. in rats’ plasma by utilizing ultra high pressure liquid chromatography tandem mass spectrometry. J Chromatogr B, Anal Technol Biomed Life Sci. 2016;1011:128–35. https://doi.org/10.1016/j.jchromb.2015.12.044.
Han Y, Lee H, Li H, Ryu JH. Corylifol A from Psoralea corylifolia L. Enhances myogenesis and alleviates muscle atrophy. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21051571.
Yeon MH, Seo E, Lee JH, Jun HS. Bavachin and corylifol A improve muscle atrophy by enhancing mitochondria quality control in type 2 diabetic mice. Antioxidants. 2023. https://doi.org/10.3390/antiox12010137.
Zhang R, Shen Q, Wang Y, Deng X, Fan J, Gu X, et al. Corylifol A ameliorates muscle atrophy by inhibiting TAOK1/p38-MAPK/FoxO3 pathway in cancer cachexia. J Cachexia Sarcopenia Muscle. 2023;14:2098–113. https://doi.org/10.1002/jcsm.13288.
Oshima Y, Matsuura H, Sakurai Y, Hirai K, Tani E, Yoshimoto N, et al. A case of squamous cell lung cancer treated with anamorelin in combination with a multidisciplinary collaborative approach for treating cancer cachexia. Respir Med Case Rep. 2022;36:101609. https://doi.org/10.1016/j.rmcr.2022.101609.
Fan M, Gu X, Zhang W, Shen Q, Zhang R, Fang Q, et al. Atractylenolide I ameliorates cancer cachexia through inhibiting biogenesis of IL-6 and tumour-derived extracellular vesicles. J Cachexia Sarcopenia Muscle. 2022;13:2724–39. https://doi.org/10.1002/jcsm.13079.
Chrysostomou SE, Eder S, Pototschnig I, Mayer AL, Derler M, Mussbacher M, et al. R-ketorolac ameliorates cancer-associated cachexia and prolongs survival of tumour-bearing mice. J Cachexia Sarcopenia Muscle. 2024;15:562–74. https://doi.org/10.1002/jcsm.13422.
Hanada K, Fukasawa K, Hinata H, Imai S, Takayama K, Hirai H, et al. Combination therapy with anamorelin and a myostatin inhibitor is advantageous for cancer cachexia in a mouse model. Cancer Sci. 2022;113:3547–57. https://doi.org/10.1111/cas.15491.
Kudamatsu H, Kawashiri T, Mine K, Mori K, Inoue M, Ishida H, et al. Ameliorating effects of cystine and theanine in a cancer cachexia mouse model. J Pharmacol Sci. 2023;152:163–6. https://doi.org/10.1016/j.jphs.2023.04.008.
Rosa-Caldwell ME, Lim S, Haynie WA, Brown JL, Deaver JW, Morena Da Silva F, et al. Female mice may have exacerbated catabolic signalling response compared to male mice during development and progression of disuse atrophy. J Cachexia, Sarcopenia Muscle. 2021;12:717–30. https://doi.org/10.1002/jcsm.12693.
Rosa-Caldwell ME, Lim S, Haynie WS, Jansen LT, Westervelt LC, Amos MG, et al. Altering aspects of mitochondrial quality to improve musculoskeletal outcomes in disuse atrophy. J Appl Physiol. 2020;129:1290–303. https://doi.org/10.1152/japplphysiol.00407.2020.
van de Worp W, Theys J, González AS, van der Heyden B, Verhaegen F, Hauser D, et al. A novel orthotopic mouse model replicates human lung cancer cachexia. J Cachexia Sarcopenia Muscle. 2023;14:1410–23. https://doi.org/10.1002/jcsm.13222.
Miao C, Lv Y, Zhang W, Chai X, Feng L, Fang Y, et al. Pyrrolidine dithiocarbamate (PDTC) attenuates cancer cachexia by affecting muscle atrophy and fat lipolysis. Front Pharmacol. 2017;8:915. https://doi.org/10.3389/fphar.2017.00915.
Shen Q, Kuang JX, Miao CX, Zhang WL, Li YW, Zhang XW, et al. Alantolactone ameliorates cancer cachexia-associated muscle atrophy mainly by inhibiting the STAT3 signaling pathway. Phytomedicine. 2021;95:153858. https://doi.org/10.1016/j.phymed.2021.153858.
Wakabayashi H, Arai H, Inui A. The regulatory approval of anamorelin for treatment of cachexia in patients with non-small cell lung cancer, gastric cancer, pancreatic cancer, and colorectal cancer in Japan: facts and numbers. J Cachexia Sarcopenia Muscle. 2021;12:14–6. https://doi.org/10.1002/jcsm.12675.
Takayama K, Takiguchi T, Komura N, Naito T. Efficacy and safety of anamorelin in patients with cancer cachexia: post-hoc subgroup analyses of a placebo-controlled study. Cancer Med. 2023;12:2918–28. https://doi.org/10.1002/cam4.5206.
Li WG, Gavrila D, Liu X, Wang L, Gunnlaugsson S, Stoll LL, et al. Ghrelin inhibits proinflammatory responses and nuclear factor-kappaB activation in human endothelial cells. Circulation. 2004;109:2221–6. https://doi.org/10.1161/01.Cir.0000127956.43874.F2.
Bai Y, Hu Y, Zhao Y, Yu X, Xu J, Hua Z, et al. Anamorelin for cancer anorexia-cachexia syndrome: a systematic review and meta-analysis. Support Care Cancer. 2017;25:1651–9. https://doi.org/10.1007/s00520-016-3560-0.
Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012;16:153–66. https://doi.org/10.1016/j.cmet.2012.06.011.
Tsoli M, Swarbrick MM, Robertson GR. Lipolytic and thermogenic depletion of adipose tissue in cancer cachexia. Semin Cell Dev Biol. 2016;54:68–81. https://doi.org/10.1016/j.semcdb.2015.10.039.
Barton BE. IL-6-like cytokines and cancer cachexia: consequences of chronic inflammation. Immunol Res. 2001;23:41–58. https://doi.org/10.1385/ir:23:1:41.
Cawthorn WP, Heyd F, Hegyi K, Sethi JK. Tumour necrosis factor-alpha inhibits adipogenesis via a beta-catenin/TCF4(TCF7L2)-dependent pathway. Cell Death Differ. 2007;14:1361–73. https://doi.org/10.1038/sj.cdd.4402127.
Patel HJ, Patel BM. TNF-α and cancer cachexia: molecular insights and clinical implications. Life Sci. 2017;170:56–63. https://doi.org/10.1016/j.lfs.2016.11.033.
Joshi M, Patel BM. The burning furnace: alteration in lipid metabolism in cancer-associated cachexia. Mol Cell Biochem. 2022;477:1709–23. https://doi.org/10.1007/s11010-022-04398-0.
Fang R, Yan L, Liao Z. Abnormal lipid metabolism in cancer-associated cachexia and potential therapy strategy. Front Oncol. 2023;13:1123567. https://doi.org/10.3389/fonc.2023.1123567.
Chiappalupi S, Sorci G, Vukasinovic A, Salvadori L, Sagheddu R, Coletti D, et al. Targeting RAGE prevents muscle wasting and prolongs survival in cancer cachexia. J Cachexia Sarcopenia Muscle. 2020;11:929–46. https://doi.org/10.1002/jcsm.12561.
Funding
This study was supported by the National Nature Science Foundation of China (No. 82373317, 82374085), the Natural Science Foundation of Shanghai (No. 23ZR1460500, 21ZR1464400), the Key Fields Excellent Ph.D Student Education Program of Shanghai University of TCM (No. GJ2023031).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Supervised the project and designed the experiment: XWZ, XDG and XL. Performed the experiments: NL and RQZ. Material preparation, data collection and analysis were performed by XD, XFG, JXW and QLX. The first draft of the manuscript was written by NL and RQZ and then all authors were involved in the revision of the manuscript. All the authors reviewed the manuscript and agreed to publish it.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The present study has been approved by the Institutional Animal Care and Use Committee of the Shanghai University of Traditional Chinese Medicine (NO. PZSHUTCM2304070004).
Tumor size/burden statement
The tumor size/burden in the present study was not exceeded the maximal tumor size/burden permitted by the ethics committee, the Institutional Animal Care and Use Committee of the Shanghai University of Traditional Chinese Medicine. Generally, the maximal tumor size/burden permitted by the Institutional Animal Care and Use Committee of the Shanghai University of Traditional Chinese Medicine is that, the tumor volume in mice should not exceed 2000 mm3 or the weight of the tumor should not be more than 10% of the body weight of the mice. While, since cancer cachexia is a syndrome observed in cancer patients in the late stages of the disease, larger tumor size/burden was necessary for studies establishing animal models of cancer cachexia. For example, more than 8000 mm3 tumor volume was observed in previous studies [36]. Therefore, no specific limitation of tumor size/burden is required for cancer cachexia-related studies.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, N., Zhang, R., Deng, X. et al. Combination therapy of corylifol A and anamorelin attenuates cachexia by decreasing pro-inflammatory cytokines, inhibiting lipolysis and increasing food intake in a murine colon cancer model. Discov Med 1, 49 (2024). https://doi.org/10.1007/s44337-024-00016-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44337-024-00016-8