Abstract
Agonists targeting α2-adrenergic receptors (ARs) are used to treat diverse conditions, including hypertension, attention-deficit/hyperactivity disorder, pain, panic disorders, opioid and alcohol withdrawal symptoms, and cigarette cravings. These receptors transduce signals through heterotrimeric Gi proteins. Here, we elucidated cryo-EM structures that depict α2A-AR in complex with Gi proteins, along with the endogenous agonist epinephrine or the synthetic agonist dexmedetomidine. Molecular dynamics simulations and functional studies reinforce the results of the structural revelations. Our investigation revealed that epinephrine exhibits different conformations when engaging with α-ARs and β-ARs. Furthermore, α2A-AR and β1-AR (primarily coupled to Gs, with secondary associations to Gi) were compared and found to exhibit different interactions with Gi proteins. Notably, the stability of the epinephrine–α2A-AR–Gi complex is greater than that of the dexmedetomidine–α2A-AR–Gi complex. These findings substantiate and improve our knowledge on the intricate signaling mechanisms orchestrated by ARs and concurrently shed light on the regulation of α-ARs and β-ARs by epinephrine.
Similar content being viewed by others
Introduction
In the sympathetic nervous system, endogenous epinephrine and norepinephrine regulate various physiological functions by activating adrenergic receptors (ARs)1. There are nine distinct AR subtypes in humans: α1A, α1B, α1D, α2A, α2B, α2C, β1, β2, and β32. Three distinct genes that encode α2-AR subtypes have been identified3. Mouse genetic studies with individual α2-AR gene deletions have shown distinct and overlapping physiological functions. While α2A-ARs decrease sympathetic outflow and blood pressure, α2B-ARs increase blood pressure3. Both α2A-AR and α2C-AR are needed to regulate neurotransmitter release3. α2-AR agonists are used to treat hypertension, attention-deficit/hyperactivity disorder, and other diseases4,5,6. Dexmedetomidine is a highly selective α2-AR agonist used for prolonged sedation in hospitalized patients, procedural sedation and general anesthesia and treating emergence delirium7,8,9. The pharmacological blockade of α2A-AR by a clinically used antagonist reduces Alzheimer’s disease-related pathology and improves cognitive deficits in an Alzheimer’s disease transgenic model10. All ARs belong to the G-protein-coupled receptor (GPCR) superfamily11. α2-ARs are Gi-coupled GPCRs that can inhibit adenylyl cyclase and decrease intracellular cAMP levels.
Epinephrine is a hormone and is produced primarily by the chromaffin cells of adrenal glands12. The release of epinephrine is triggered by stress and plays major roles in fight-or-flight responses by increasing blood flow to muscles, heart output, the pupil dilation response, and blood sugar levels by binding to α- and β-ARs2,12. Epinephrine activates the β1-AR–Gs signaling pathway to increase cardiac output, whereas it stimulates the α2A-AR–Gi signaling pathway to decrease blood pressure. Surprisingly, other than the epinephrine–α1A-AR–Gq structure we recently identified, little structural information is available for epinephrine bound to a GPCR–G-protein complex13. There are two structures of epinephrine bound to β1-AR or β2-AR alone with the nanobody 6B9, which stabilizes β-ARs in an active state14,15. Epinephrine binds β1-AR and β2-AR in an identical manner14,15.
GPCRs transduce signals through heterotrimeric G proteins. Although one GPCR is typically coupled to one family of G proteins (Gs, Gi, Gq or G12/13), a GPCR may activate more than one of the four families of G proteins. However, the selective coupling of GPCRs and G proteins is not well understood. While β1-AR is primarily coupled to Gs, it can also couple to Gi16,17,18,19,20. Gi-mediated β1-AR signaling is implicated in decreased receptor responsiveness in myocardial infarction16,17,18,19. On the other hand, α2A-AR is primarily coupled to Gi. Previously, we solved the cryo-EM structure of a β1-AR and Gi complex21. We examined how a single GPCR simultaneously transduces signals through two G proteins by a comparative structural analysis of β1-AR complexed with Gi or Gs21. A structural comparison of the α2A-AR–Gi and β1-AR–Gi complexes should increase our knowledge on the coupling selectivity of GPCR and G-protein.
Here, we used cryo-EM to determine the structures of α2A-AR signaling complexes with Gi and the endogenous agonist epinephrine or the synthetic agonist dexmedetomidine. These results provide structural information on the complexation of epinephrine with a GPCR and a G protein. We identified different conformations of epinephrine in complex with α2A-AR–Gi versus β-AR–6B9 nanobodies. Furthermore, we compared the structures of the α2A-AR–Gi complex and the β1-AR–Gi complex, revealing different coupling modes of Gi to α2A-AR and to β1-AR. Moreover, we performed Gaussian accelerated molecular dynamics (GaMD) simulations to investigate the activation of α2A-AR by agonists and activation of Gi by agonist-bound α2A-AR22,23. GaMD is an enhanced sampling method that works by adding a harmonic boost potential to reduce the system energy barriers22,23. Without the need to set predefined collective variables, GaMD provides unconstrained enhanced sampling and is advantageous for simulating complex biological systems, such as GPCR–G-protein complexes. The harmonic boost potential in GaMD exhibits a Gaussian distribution, which enables us to accurately recover the original biomolecular free energy landscapes by Gaussian approximation, i.e., cumulant expansion to the second order. Thus, through GaMD, unconstrained enhanced sampling and free energy calculations of biomolecules can be simultaneously achieved. Previously, GaMD has been successfully applied to explore the activation and agonist binding of GPCRs24,25,26 and was thus applied in the present study. We proposed a possible sequential order starting from the interaction between activated α2A-AR and the C-terminal half of the α5-helix in Gi and ending with the conformational modification of the GDP/GTP-binding pocket and the opening of the α-helical domain during Gi activation.
Materials and methods
Expression and purification of α2A-AR, Gαi, Gβ1 and Gγ2
An HA signal peptide and a Flag tag were fused to human α2A-AR, followed by the PreScission protease cleavage site, eGFP and an 8xHis tag at the C-terminus. The construct was expressed and purified from Spodoptera frugiperda Sf9 insect cells grown in ESF 921 protein-free medium (Expression Systems), as we previously described21,27. After infection for two days, approximately 2–3 million cells/ml were harvested and flash frozen at −80 °C until use. Thawed cell pellets were sonicated (15 s on/15 s off for 4 min) in buffer containing 25 mM Tris-HCl (pH 7.4), 1 mM EDTA (pH 8.0), 160 μg/ml benzamidine, 1 μg/ml leupeptin, 4 μg/ml aprotinin, 0.5 μM PMSF, 1 μg/ml pepstatin A, 50 μM dexmedetomidine or epinephrine. The membranes were precleared to remove intact cells and debris from the broken cells by centrifugation at 2000 rpm for 10 min. The supernatant was carefully transferred to ultracentrifuge tubes and spun at 40,000 rpm at 6 °C for 1 h. The supernatant was removed, and the pellets were scraped from the bottles. Receptors were extracted using a Dounce homogenizer with the following buffers: 25 mM Tris-HCl (pH 7.4), 0.5 M NaCl, 1% (wt/vol) n-dodecyl-b-d-maltopyranoside (DDM; Anatrace), 0.2% sodium cholate, 0.02% cholesteryl hemisuccinate (CHS), 160 μg/ml benzamidine, 1 μg/ml leupeptin, 4 μg/ml aprotinin, 0.5 μM PMSF, 1 μg/ml pepstatin A, 50 μM dexmedetomidine or epinephrine, and stirring for 1 h at 4 °C. The supernatant was then incubated with HisPur™ Ni-NTA Resin (Thermo Fisher Scientific) for 2 h. The resin was collected by centrifugation, washed four times with 25 mM Tris-HCl (pH 7.4), 0.5 M NaCl, 0.05% DDM, 50 μM dexmedetomidine or epinephrine, and then eluted with 25 mM Tris-HCl (pH 7.4), 0.5 M NaCl, 0.05% DDM, 250 mM imidazole, 50 μM dexmedetomidine or epinephrine. The eluted α2A-AR was concentrated and further purified by size-exclusion chromatography using a Superdex 200 Increase 10/300 column (GE Healthcare) preequilibrated with 20 mM HEPES (pH 7.0), 100 mM NaCl, 0.05% Lauryl Maltose Neopentyl Glycol (LMNG, Anatrace), 50 μM dexmedetomidine or epinephrine, and the peak fractions were pooled and concentrated for complex assembly.
N-terminally His-tagged full-length rat Gαi dominant negative (G203A) proteins were expressed and purified from the E. coli strain BL21 (DE3), as we previously described21,28. The Gαi1(G203A) mutant was used to increase the formation of the α2A-AR–Gi protein complex. The cells were grown in 2 × YT medium at 37 °C until the OD600 reached 0.6. Protein expression was then induced by 100 μM IPTG and continued for 16 h at 16 °C. The cells were harvested by centrifugation, flash-frozen in liquid nitrogen and stored at −80 °C. For protein purification, cell pellets were thawed in lysis buffer containing 50 mM Tris (pH 8.0), 150 mM NaCl, 5 mM β-mercaptoethanol, 10% glycerol, 2 mM MgCl2, 1 mM EDTA, 0.1 mg/ml lysozyme, 10 μM GDP, 0.5 mM PMSF, 1 μg/ml leupeptin, 4 μg/ml aprotinin, and 1 μg/ml pepstatin A and further lysed by sonication (4 s on/4 s off for 2 min). Cell debris was removed by centrifugation at 20,000 × g for 40 min at 4 °C. The supernatant was then collected and incubated with HisPur™ Ni-NTA Resin (Thermo Fisher Scientific) with stirring for 2 h at 4 °C. The resin was then washed four times with lysis buffer containing 25 mM imidazole and eluted with lysis buffer containing 250 mM imidazole. Gαi was concentrated and further purified by size-exclusion chromatography using a Superdex 200 Increase 10/300 column preequilibrated with 50 mM Tris (pH 8.0), 150 mM NaCl, 5 mM β-mercaptoethanol, 10% glycerol, and 10 μM GDP. Purified Gai was concentrated, flash frozen in liquid nitrogen and stored at −80 °C.
Bovine Gβ1 and bovine His6-tagged soluble Gγ2 (C68S) were coexpressed and purified from Sf9 insect cells as we previously described21,27. Sf9 cells were coinfected with 25 ml of each baculovirus when the density of the insect cell culture reached 3 million cells/ml. Forty-eight hours post-infection, the cells were harvested by centrifugation, flash frozen in liquid nitrogen and stored at −80 °C. The cell pellets were thawed in 25 mM Tris (pH 7.5), 150 mM NaCl, 10% glycerol, 2 mM β-mercaptoethanol, 5 mM MgCl2, 10 μM GDP, 0.5 mM PMSF, 160 μg/ml benzamidine, 1 μg/ml leupeptin, 4 μg/ml aprotinin, and 1 μg/ml pepstatin A. The cells were lysed by sonication, and the cell debris was removed by centrifugation at 142,000 × g for 30 min. The supernatants were collected and incubated with HisPur™ Ni-NTA Resin (Thermo Fisher Scientific) with stirring for 2 h at 4 °C. The resin was then washed four times with lysis buffer containing 25 mM imidazole and eluted with lysis buffer containing 250 mM imidazole. The eluted protein was concentrated and further purified using a Superdex 200 Increase 10/300 column preequilibrated with 25 mM Tris (pH 7.5), 150 mM NaCl, and 2 mM β-mercaptoethanol. The purified Gβ1γ2 protein was concentrated, flash frozen in liquid nitrogen and stored at −80 °C.
Protein complex assembly and purification
To assemble the α2A-AR–Gi complex, Gαi and Gβ1γ2 were mixed at a 1:1 molar ratio in the presence of 2 mM MgCl2. The mixture was incubated for 30 min and then mixed with α2A-AR at a 1.8:1 ratio in 500 μl of buffer containing 10 mM HEPES (pH 7), 100 mM NaCl, 0.1 mM TCEP, 0.02% LMNG, 2 mM MgCl2, 0.1 mM TCEP, 0.4 U of Apyrase (Sigma), and 1 mM dexmedetomidine or epinephrine. To remove the GFP tag on α2A-AR, PreScission protease was also added at a ratio of 1:10 (w:w). After an additional overnight incubation at 4 °C, the mixture was centrifuged at 16,000 × g for 10 min to remove any precipitants. The supernatant was then loaded onto a Superdex 200 Increase 10/300 column preequilibrated with 10 mM HEPES (pH 7), 100 mM NaCl, 0.1 mM TCEP, 0.02% LMNG and 25 µM dexmedetomidine or epinephrine. The elution fractions from a single peak containing the pure α2A-AR–Gi complex were concentrated to ~2 mg/ml and used directly for making cryo-EM grids.
Cryo-EM data collection
Next, 3.5 µL of α2A-AR–Gi complex at a concentration of 2 mg/ml was applied to glow-discharged 400 mesh gold Quantifoil R1.2/1.3 holey carbon grids (Quantifoil Micro Tools) and vitrified using a Vitrobot Mark IV (Thermo Fisher Scientific/FEI) at 22 °C and 100% humidity. Micrographs were collected on a 300 kV Titan Krios electron microscope (Thermo Fisher Scientific/FEI) with a Gatan K3 direct electron detector (Gatan, Inc.) in superresolution mode at a nominal 81,000× magnification. For the epinephrine complex, 6454 movies in the defocus range of −0.8 to −1.8 μm were recorded with a total accumulated dose of 50 e-/Å2. For dexmedetomidine, 6766 movies in the defocus range of −0.8 to −2.0 μm were recorded with a total accumulated dose of 51 e-/Å2.
Image processing, 3D reconstruction, modeling, and refinement
Superresolution movies were aligned, Fourier cropped twice, and dose-weighted using MotionCor2 implemented in Relion3.1-beta29,30,31. The effects of the contrast-transfer function were estimated with CTFFIND v4.1.832. For the dexmedetomidine dataset, Relion Laplacian-of-Gaussian picking with minimum and maximum dimensions of 80 Å and 130 Å, respectively, was used to heavily overpick at a rate of approximately 1500 particles per micrograph. The resulting particle stacks of nearly 11 million particles were Fourier cropped twice and processed through 2 rounds of 2D classification in CryoSparc v2.15.0 to remove false-positives, receptors alone, or G-proteins alone33 (Supplementary Fig. 3). A stack of 639,275 intact complex particles was then subjected to 2 rounds of 3D classification in Relion. After 3D classification, a final stack of 188,480 high-resolution particles was selected and further polished by 2 rounds of Bayesian polishing and one round of CTF refinement in Relion. Nonuniform refinement of the polished particles in CryoSparc yielded a map with 3.2 Å resolution using the 0.143 Fourier shell correlation criterion. These consensus stacks were also subjected to G protein-focused refinement in CryoSparc. The G-protein focused refinement map showed significant improvement in the G-protein region compared to the consensus map. A model was built starting from X-ray crystal structures of the antagonist-bound α2A-AR (PDB code 6KUX) and Gi heterotrimer (PDB code 1GG2) in Coot v0.9.1, which, combined with consensus and G-protein focused refinement maps, were used to generate a composite map in Phenix v1.18.234,35. The resulting maps were super-sampled in Coot to 0.8656 Å per pixel with a 320-voxel box. The α2A-AR-Gi model was real-space refined against the composite map in Phenix, and a work/free half-map pair was used to prevent overfitting (Supplementary Fig. 3). For the epinephrine dataset, the processing of the cryo-EM data was similar to that for the dexmedetomidine dataset, except that Relion 2D template-based autopicking was utilized to pick particles instead of Laplacian-of-Gaussian picking (Supplementary Fig. 2), resulting in 7,720,235 particles. The particle stacks were also Fourier cropped twice and processed through 2 rounds of 2D classification in CryoSparc v2.15.0 to remove false-positives, receptors alone, or G-proteins alone (Supplementary Fig. 2). A stack of 1,618,395 intact complex particles was then subjected to 3 rounds of 3D classification in Relion. A final stack of 713,558 high-resolution particles was selected after 3D classification and further polished by 1 round of Bayesian polishing in Relion. Nonuniform refinement of polished final particle stacks in CryoSparc resulted in a map with 2.8 Å resolution using the 0.143 Fourier shell correlation criterion. Since focused refinements of the epinephrine dataset did not further improve map quality, the consensus map was super-sampled in Coot to 0.8560 Å per pixel with 320 voxel boxes and used for model building and refinement. The binding poses of dexmedetomidine and epinephrine were initially determined by densities as well as their geometric restraints and were further supported by MD simulations36. All model statistics were validated using MolProbity37.
Gaussian accelerated molecular dynamics (GaMD)
GaMD is an enhanced sampling method that works by adding a harmonic boost potential to reduce the system energy barriers22,23. When the system potential \(V(\mathop{r}\limits^{ \rightharpoonup })\) is lower than the reference energy E, the modified potential \({V}^{* }(\mathop{r}\limits^{ \rightharpoonup })\) of the system is calculated as follows:
where k is the harmonic force constant. The two adjustable parameters E and k are automatically determined on three enhanced sampling principles. First, for any two arbitrary potential values \({v}_{1}(\mathop{r}\limits^{ \rightharpoonup })\) and \({v}_{2}(\mathop{r}\limits^{ \rightharpoonup })\) found on the original energy surface, if \({V}_{1}(\mathop{r}\limits^{ \rightharpoonup })\, <\, {V}_{2}(\mathop{r}\limits^{ \rightharpoonup })\), \(\Delta V\) should be a monotonic function that does not change the relative order of the biased potential values; i.e., \({V}_{1}^{* }(\mathop{r}\limits^{ \rightharpoonup })\, <\, {V}_{2}^{* }(\mathop{r}\limits^{ \rightharpoonup })\). Second, if \({V}_{1}(\mathop{r}\limits^{ \rightharpoonup })\, <\, {V}_{2}(\mathop{r}\limits^{ \rightharpoonup })\), the potential difference observed on the smoothed energy surface should be smaller than that of the original surface, i.e., \({V}_{2}^{* }(\mathop{r}\limits^{ \rightharpoonup }){\,-\,V}_{1}^{* }(\mathop{r}\limits^{ \rightharpoonup })\, <\, {V}_{2}(\mathop{r}\limits^{ \rightharpoonup }){\,-\,V}_{1}(\mathop{r}\limits^{ \rightharpoonup })\). By combining the first two criteria and using the formulas \({V}^{* }(\mathop{r}\limits^{ \rightharpoonup })\) and \(\Delta V\), we obtain
where \({V}_{\min }\) and \({V}_{\max }\) are the system minimum and maximum potential energies, respectively. To ensure that Eq. 2 is valid, k must satisfy \(k\le 1/\left({V}_{\max }-{V}_{\min }\right)\). Let us define \(k={k}_{0}\cdot 1/\left({V}_{\max }-{V}_{\min }\right)\); then, \(0{ < k}_{0}\le 1\). Third, the standard deviation (SD) of \(\Delta V\) must be small enough (i.e., narrow distribution) to ensure accurate reweighting using cumulant expansion to the second order: \({\sigma }_{\Delta V}=k\left(E-{V}_{{avg}}\right){\sigma }_{V}\le {\sigma }_{0}\), where \({V}_{{avg}}\) and \({\sigma }_{V}\) are the average and SD of ΔV with σ0 as a user-specified upper limit (e.g., \({10k}_{B}T\)) for accurate reweighting. When E is set to the lower bound \(E={V}_{\max }\) according to Eq. 2, \({k}_{0}\) can be calculated as follows:
Alternatively, when the threshold energy E is set to its upper bound E = Vmin + 1/k, k0 is set to:
If \({k}_{0}^{{\prime} {\prime} }\) is calculated between 0 and 1. Otherwise, k0 is calculated using Eq. 3.
System setup and simulation analysis
The structures of epinephrine–α2A-AR–Gi and dexmedetomidine–α2A-AR–Gi cryo-EM were used for setting up simulation systems to explore the stability of the complex. The missing residues in the ECL2 of α2A-AR (PLISEKKGGGGGPQPA, residue number 168–184) were modeled by SWISS-MODEL38. To explore the G-protein activation mechanism, another two models with the α-helical domain were built as follows: Gαi in the dexmedetomidine–α2A-AR–Gi cryo-EM structure was substituted with the GDP-bound conformation of Gαi (residues 11-354 of PDB 1GG2) with or without GDP. The missing residues in 1GG2 (349–354) were added. All four simulation systems were prepared with the CHARMM-GUI web server using a membrane protein input generator39. The receptor was inserted into a palmitoyl-oleoyl-phosphatidyl-choline (POPC) bilayer. All chain termini were capped with neutral patches (acetyl and methylamide). All the disulfide bonds in the complexes that were resolved in the cryo-EM structures were maintained in the simulations. The systems were solvated in 0.15 M NaCl solution at 310 K. The CHARMM36m parameter set was used for the receptor, lipids and GDP40,41,42. The CGenFF 2.4.0 parameters were used for epinephrine and dexmedetomidine43. Force field parameters with high penalties were optimized with FFParm44. GaMD simulations of these systems followed a protocol similar to that used in previous studies of GPCRs24,26. For each of the complex systems, initial energy minimization, thermalization, and 20 ns cMD equilibration were performed using NAMD2.1245. A cutoff distance of 12 Å was used for the van der Waals and short-range electrostatic interactions, and the long-range electrostatic interactions were computed with the particle‒mesh Ewald summation method46. A 2-fs integration time step was used for all MD simulations, and a multiple-time-stepping algorithm was used with bonded and short-range nonbonded interactions computed every time step and long-range electrostatic interactions every two timesteps. The SHAKE algorithm was applied to all hydrogen-containing bonds. The NAMD simulation started with equilibration of the lipid tails. With all other atoms fixed, the lipid tails were energy minimized for 1000 steps using the conjugate gradient algorithm and melted with a constant number, volume, and temperature (NVT) run for 0.5 ns at 310 K. The four systems were further equilibrated using a constant number, pressure, and temperature (NPT) run at 1 atm and 310 K for 10 ns with 5 kcal/(mol. Å2) harmonic position restraints applied to the protein and ligand atoms. Final equilibration of each system was performed using an NPT run at 1 atm pressure and 310 K for 0.5 ns with all atoms unrestrained. After energy minimization and system equilibration were complete, conventional MD simulations were performed on each system for 20 ns at 1 atm pressure and 310 K with a constant ratio constraint applied on the lipid bilayer in the X-Y plane.
With the NAMD output structures, the system topology and CHARMM36m force field files, the ParmEd tool in the AMBER package was used to convert the simulation files into the AMBER format47. The GaMD module implemented in the GPU version of AMBER20 was then applied to perform the simulations22,47. GaMD simulations included an 8-ns short cMD simulation used to collect the potential statistics for calculating GaMD acceleration parameters and a 48-ns equilibration after the boost potential was added. Finally, three independent 500-ns GaMD simulations with randomized initial atomic velocities were performed for the epinephrine–α2A-AR–Gi and dexmedetomidine–α2A-AR–Gi complexes without the α-helical domain. More replicas and longer simulations are needed to capture the G protein activation process because this process is slow. Therefore, four independent 3000-ns GaMD production simulations with randomized initial atomic velocities were performed for the intact G-protein-bound dexmedetomidine–α2A complex with or without GDP. The average and SD of the system potential energies were calculated every 800,000 steps (1.6 ns). All GaMD simulations were run at the “dual-boost” level by setting the reference energy to the lower bound. One boost potential was applied to the dihedral energetic term, and the other was applied to the total potential energetic term. The upper limit of the boost potential SD, σ0, was set to 6.0 kcal/mol for the dihedral and total potential energetic terms. Similar temperature and pressure parameters were used as in the NAMD simulations.
CPPTRAJ was used to analyze the GaMD simulations48. Snapshots of all three GaMD production simulations (1500 ns in total) were combined for structural clustering to identify the representative agonist binding conformations. The hierarchical agglomerative algorithm in CPPTRAJ was applied for clustering. The cutoff was set to 2.0 Å for the RMSD of the agonist and surrounding receptor residues (residues within 4 Å of agonist) to form a cluster. Interactions between epinephrine and the receptor were measured using the distance between the CG atom of D128 and the N1/O3 atoms of epinephrine, the OH atom of Y431 and the N1 atom of epinephrine, the OG atom of S215 and the O1 atom of epinephrine, and the OG atom of S219 and the O2 atom of epinephrine. Interactions between dexmedetomidine and the receptor were measured using the distance between the O atom of F427 and the N2 atom of dexmedetomidine, OG in the side chain of Y431 and the N2 atom of dexmedetomidine, and the O atom from the backbone of Y431 and the N2 atom of dexmedetomidine. The distance between the NZ atoms of K46 in the P-loop and the CG atom of D200 in β3 of the Switch II region in Gαi was used to measure the salt bridge formed between K46 and D200. The degree of domain separation (Ras-like domain and α-helical domain) was measured by the distance between residue E276 in the Ras-like domain and residue A138 in the α-helical domain. The C-terminal α5−helix RMSD was measured relative to the α5-helix RMSD in the cryo-EM structure of α2A-AR–Gi. The time courses of these reaction coordinates obtained from the GaMD simulation are plotted in Figs. 2, 3, 6, 7, and Supplementary Fig. 11.
cAMP measurement
Gα-depleted HEK293 cells (in which the genes for all Gα subunits expressed in HEK cells were mutated by using the CRISPR/Cas system)49 were cotransfected with α2A-AR and Gαi1 or β1-AR and Gαi1 for the studies presented in Supplementary Fig. 9. CHO cells were transfected with wild-type or mutant α2A-AR plasmids for the studies presented in Supplementary Fig. 8. The cells were starved from serum overnight, washed twice with Hank’s balanced salt solution containing 25 mM HEPES-NaOH (pH 7.4) and 0.1% BSA, and incubated in buffer containing 0.5 mM IMBX (Sigma) for 20 min at room temperature27. For the Gi-mediated cAMP inhibition assay, the cells were first stimulated with 20 μM forskolin for 20 min at 37 °C. Dexmedetomidine was added to cells cotransfected with α2A-AR and Gαi1. Isoproterenol was used to treat cells cotransfected with β1-AR and Gαi1. To terminate the reaction, the medium was aspirated and the solution was immediately treated with 0.1 M HCl for 10 min at room temperature. The cells were harvested by centrifugation, and the supernatant was collected for determination of cAMP concentration in triplicate with a Direct Cyclic AMP Enzyme Immunoassay kit (Enzo Life Sciences). The cAMP assays were repeated three times, and the data are presented as the means ± SDs of three independent experiments. The analysis was performed using the log(agonist) vs. response function of Prism 9 (GraphPad)27,50.
Quantification and statistical analysis
In Supplementary Figs. 8 and 9, the cAMP assays were repeated three times, and the data are presented as the means ± SDs of three independent experiments. The analysis was performed using the log(agonist) vs. response function of Prism 9 (GraphPad), as indicated in the figure legends. The cryo-EM data collection and refinement statistics are listed in Supplementary Table 1.
Results
Cryo-EM structures of the signaling complexes of epinephrine–α2A-AR–Gi and dexmedetomidine–α2A-AR–Gi
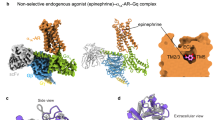
To investigate the mechanism underlying the molecular recognition of epinephrine by an α-AR, we solved the structures of human α2A-AR bound to epinephrine (an endogenous agonist) or dexmedetomidine (a synthetic agonist) and in complex with its cognate signaling Gi heterotrimer (Gαi(G203A)Gβ1Gγ2). We used Gαi1(G203A) to increase the formation of the ligand–α2A-AR–Gi protein complex without using any stabilizing nanobodies (Supplementary Fig. 1)51. We solved a 2.8 Å cryo-EM structure of the epinephrine-bound α2A-AR and Gi signaling complex (Fig. 1a, Supplementary Fig. 2, and Supplementary Table 1) and a 3.2 Å cryo-EM structure of α2A-AR–Gi in complex with dexmedetomidine (Fig. 1b, Supplementary Fig. 3, and Supplementary Table 1). Overall, the structures of the α2A-AR–Gi complex are similar in the presence of epinephrine and dexmedetomidine, with a root-mean-square deviation (RMSD) of 1.1 Å over 865 Cα atoms (Fig. 1 and Supplementary Fig. 4). However, there are local conformational differences, especially in the ligand-binding pockets (Figs. 2, 3). While some of the interacting residues are common to both ligands, epinephrine and dexmedetomidine each form different interactions in the orthosteric ligand-binding pocket (Supplementary Figs. 5, 6 and 7).
a The density map, model and ligand-binding pocket of epinephrine–α2A-AR–Gi are shown. b The density map, the model and the ligand-binding pocket of dexmedetomidine–α2A-AR–Gi are shown. Epinephrine-bound α2A-AR is colored in pink. Dexmedetomidine-bound α2A-AR is colored in purple. Gαi1 in green. Gβ in orange. Gγ in blue. Epinephrine and dexmedetomidine are colored in orange and yellow, respectively. Oxygen and nitrogen atoms are depicted in red and blue, respectively.
Distinct conformations of epinephrine binding to α2A-AR and β-ARs
Epinephrine, a chiral endogenous full agonist for all ARs, displays different binding conformations when interacting with α2A-AR and β-ARs (Fig. 2a and Supplementary Fig. 6). The unique conformations of epinephrine within these receptor complexes shed light on the structural and functional implications of these variations. In the structure of the epinephrine–α2A-AR–Gi complex, the β-carbon hydroxyl group is oriented toward the extracellular side (Fig. 2a and Supplementary Fig. 6). Notably, the N-methyl group is directed toward the transmembrane domain (TM) 7 residue F4277.38 (superscript denotes Ballesteros-Weinstein numbering)52 (Fig. 2a). Moreover, the meta-hydroxyl group of epinephrine interacts with S2155.43, and the para-hydroxyl group interacts with S2155.43, S2195.46, and T1333.37 (Fig. 2a). Additionally, the amino group interacts with D1283.32 and Y4317.42 (Fig. 2a), forming a comprehensive interaction network.
a Schematic diagram of the epinephrine-binding pocket of α2A-AR from the cryo-EM structure. Hydrogen bonds are depicted as dashed black lines. b–f Time courses of the interactions between epinephrine and specific residues of α2A-AR were calculated from the GaMD simulations: the distance between the CG atom of D128 and the N1 atom of epinephrine (b), the CG atom of D128 and the O3 atoms of epinephrine (c), the OH atom of Y431 and the N1 atom of epinephrine (d), the OG atom of S215 and the O1 atom of epinephrine (e), the OG atom of S219 and the O2 atom of epinephrine (f), and the phenyl group of F427 and the methyl group of epinephrine (g). h Functional studies of the epinephrine-interacting residues of α2A-AR. The effects of wild-type and mutant α2A-AR on the cAMP inhibition signaling initiated by epinephrine were studied. The data are shown as the means ± SDs of three independent experiments.
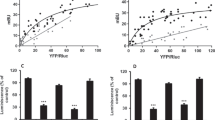
To validate the conformation of epinephrine within the cryo-EM structure of the epinephrine–α2A-AR–Gi complex, GaMD simulations were conducted. These simulations indicated that epinephrine forms consistent interactions with adjacent receptor residues, which is consistent with the cryo-EM structure. Although slight fluctuations were observed across multiple GaMD simulations (Fig. 2b–g), the overall conformation of epinephrine remained stable and aligned with that observed in the cryo-EM structure. Hydrogen bonds were formed between epinephrine and D1283.32, with average distances of 3.32 ± 0.15 Å and 3.63 ± 0.63 Å (Fig. 2b, c). Additionally, the hydrogen bonds formed between epinephrine and the receptor residues Y4317.42, S2155.43 and S2195.46 were measured at 4.20 ± 0.15 Å, 3.74 ± 0.88 Å and 3.45 ± 0.79 Å, respectively (Fig. 2d–f). A strong CH3-Π interaction between epinephrine and F4277.38 formed at a distance of 4.10 ± 0.65 Å (Fig. 2g). This reaffirms the reliability of the cryo-EM structure of the epinephrine–α2A-AR–Gi complex. To substantiate the insights garnered from cryo-EM and GaMD simulations, functional studies were carried out by mutating the residues that interacted with epinephrine on α2A-AR. The data extracted from these studies provide compelling support for the pivotal roles played by these residues in α2A-AR signaling (Fig. 2h and Supplementary Fig. 8).
Although structural data concerning epinephrine in complex with β-ARs and G-proteins remain scarce, we leveraged available information from epinephrine-bound β1-AR and β2-AR structures stabilized by nanobody 6B9 (PDB: 7BTS and 4LDO, respectively)14,15. The conformations of epinephrine within these β-AR complexes are identical. However, the conformation of epinephrine could differ in β-AR-G-protein complexes compared with β-AR-nanobody 6B9 complexes. The differing orientations of the β-carbon hydroxyl and N-methyl groups of epinephrine when complexed with α2A-AR and β-ARs stand out. Notably, this disparity arises due to the rotation around the chemical bond that links the catechol ring and β-carbon of epinephrine (Supplementary Fig. 6a). The critical hydrogen bond interactions of N3637.38 (in β1-AR) and N3127.38 (in β2-AR) with the β-carbon hydroxyl and N-methyl groups of epinephrine underscore their functional relevance14,15. Correspondingly, F4277.38 in α2A-AR occupies a similar position, participating in hydrophobic interactions with the N-methyl group of epinephrine (Fig. 2a). These structural findings align well with previous functional investigations, revealing the distinct roles of specific residues in subtype-specific ligand recognition53,54. Taken together, the multifaceted structural findings obtained by examining the interactions of epinephrine with α2A-AR and β-ARs enhance our knowledge of receptor‒ligand dynamics and functional outcomes. N3127.38 of β2-AR was shown to be essential for epinephrine binding and signaling54. Moreover, the N3127.38 mutation in F abolished the binding and signaling of β2-AR by epinephrine54. Furthermore, F4277.38 at the equivalent position was shown to be critical for α2A-AR function53. The F4277.38 mutation to N in α2A-AR increased the binding of β-AR antagonists while decreasing the binding of epinephrine to this mutant α2A-AR53. Researchers have functionally demonstrated that N (conserved in all β-ARs) or F (conserved in all α-ARs) at this position of TM7 are the main subtype determinant (α-ARs versus β-ARs)55. The structural studies of β-ARs and our current study of α2A-AR in complex with epinephrine provide the structural basis for subtype-specific ligand recognition. These insights further knowledge on the intricate ligand recognition mechanisms that are intrinsic to different AR subtypes.
Interaction of dexmedetomidine and α2-ARs
Dexmedetomidine is a selective agonist for α2-AR56,57,58. The conformation of dexmedetomidine shows flexibility in the complexes of α2A-AR–Gi, α2B-AR–Gi–the antibody fragment scFv16 (which binds to the interface between Gαi and Gβ and was used to stabilize the entire complex) (PDB: 6K42), and α2A-AR–Go–scFv16 (PDB: 7EJA)59,60(Supplementary Fig. 7). While the two methyl groups on the phenyl ring are in similar positions, the imidazole rings rotate and are in different positions in the different structural complexes (Supplementary Fig. 7). In the complex of dexmedetomidine–α2A-AR-Gi, the conserved and critical S2155.43 and S2195.46 residues (which interact with the hydroxyl groups on the catechol ring of epinephrine) are located near the two methyl groups on the phenyl ring of dexmedetomidine; however, the residues do not interact with these groups directly (Fig. 3a). Researchers have shown that S2155.43 and S2195.46, as well as their corresponding Ser residues in all other ARs, are critical for the agonist-induced activation of ARs61. Even though the imidazole group shows greater flexibility, the most interactions occur with F4277.38 and Y4317.42 (Fig. 3a). Furthermore, we employed GaMD simulations to verify the conformation of dexmedetomidine in the complex of dexmedetomidine–α2A-AR-Gi. Interactions between dexmedetomidine and residues F4277.38 and Y4317.42 in α2A-AR were similar in the GaMD simulations and in our cryo-EM structure (Fig. 3b–h). Hydrogen bonds were formed between dexmedetomidine and the receptor residues F4277.38 and Y4317.42 (which are both the backbone and the side chain), with average distances of 3.08 ± 0.25 Å, 4.06 ± 0.32 Å and 4.19 ± 0.37 Å, respectively (Fig. 3b–d). A salt-bridge interaction was observed between dexmedetomidine and D1283.32, with the CZ atom of D128 (negative charge center) and the N atom of dexmedetomidine (positive charge center) maintaining an average distance of 5.47 ± 0.45 Å (Fig. 3e). Hydrophobic interactions between dexmedetomidine and F4277.38 occurred, and the phenyl group of F4277.38 interacted with the phenyl group, 2-methyl group, and 3-methyl group of dexmedetomidine at distances of 6.43 ± 0.62 Å, 6.85 ± 0.66 Å, and 5.12 ± 0.50 Å, respectively (Fig. 3f–h). Moreover, we mutated the dexmedetomidine-interacting residues on α2A-AR, and functional studies supported their roles in dexmedetomidine-initiated α2A-AR signaling (Fig. 3i and Supplementary Fig. 8).
a Schematic diagram of the dexmedetomidine binding pocket of α2A-AR from the cryo-EM structure. Hydrogen bonds are depicted as dashed black lines. b–d Time courses of the interactions between dexmedetomidine and specific residues of α2A-AR were calculated from the GaMD simulations: the distance between the O atom of F427 and the N2 atom of dexmedetomidine (b), OG in the side chain of Y431 and the N2 atom of dexmedetomidine (c), the O atom in the backbone of Y431 and the N2 atom of dexmedetomidine (d), the CZ atom of D128 and the nitrogen atom of dexmedetomidine (e), the phenyl group of F406 and the phenyl group of dexmedetomidine (f), the phenyl group of F406 and the 2-methyl group of dexmedetomidine (g), and the phenyl group of F406 and the 3-methyl group of dexmedetomidine (h). i Functional studies of the dexmedetomidine-interacting residues of α2A-AR. The effects of wild-type and mutant α2A-AR on the cAMP inhibition signaling initiated by dexmedetomidine were examined. The data are shown as the means ± SDs of three independent experiments.
Activation of α2A-AR by epinephrine and dexmedetomidine
We investigated the mechanism by which α2A-ARs are activated by epinephrine and dexmedetomidine. We compared the structures of α2A-AR in its inactive and active states. The inactive state of α2A-AR was captured by binding with the antagonist RS 79948 (PDB: 6KUX) or the partial agonist RES (PDB: 6KUY)62. Compared with these inactive state X-ray crystal structures, the active state α2A-AR in the complexes of epinephrine–α2A-AR–Gi and dexmedetomidine–α2A-AR–Gi shows characteristic conformational changes for class A GPCR activation (Fig. 4a).
a–c Differential images of the inactive state of α2A-AR (gray; PDB 6KUX) and the active state of α2A-AR in complex with Gi (this work). d The movements of TM6 and TM7 during α2A-AR activation are shown. e Conformational changes in the CWxP motif during α2A-AR activation are shown. f Conformational changes in the PIF motif during α2A-AR activation are shown. g Y441 within the NPxxY motif packs against L139 and I142 in the active state of α2A-AR. h The ionic lock between R146 (within the DRY motif) and E384 located at the cytoplasmic end of TM6 is disrupted in the active state of α2A-AR. The ionic bonding between R146 and E384 is indicated by a dashed black line.
The overall root-mean-square deviation between the structures of α2A-AR in the active and inactive states is 2.7 Å for 257 Cα atoms. The largest structural changes upon activation occurred on the cytoplasmic side of α2A-AR (Fig. 4b, c), with an outward rotation of TM6 by ~16 Å (measured at the Cα of E3846.30) and an inward ~5 Å movement of TM7 (measured at the Cα of Y4417.53) (Fig. 4d). In addition to these TM conformational changes, the side chains of certain residues are rearranged as part of the α2A-AR activation process. Immediately below the orthosteric ligand pocket, the rotameric change in W4026.48 (within the CWxP motif) indicates that TM6 in class A GPCRs opens for G-protein engagement63 (Fig. 4e). The layer below the CWxP motif is the PIF motif (P2235.50, I1363.40, and F3986.44) (Fig. 4f). Closer to the G protein-interacting site below the PIF motif, the highly conserved NPxxY motif at the cytoplasmic end of TM7 is another key microswitch of GPCR activation63 (Fig. 4g). TM7 rotates around the NPxxY motif. This shifts Y4417.53 toward the position occupied by TM6 in the inactive structure (Fig. 4g). Among the G protein-interacting residues, the rearrangement of side chains in the highly conserved D(E)/RY motif in TM3 is critical for GPCR activation63 (Fig. 4h). In inactive α2A-AR, the ionic-lock salt bridge is preserved between the side chains of R1463.50 and D1453.49, but it is broken in the active state α2A-AR structure (Fig. 4h). Additionally, R1463.50 forms a salt bridge with E3846.30 in the inactive state of α2A-AR, but this interaction is disrupted in the active state of α2A-AR (Fig. 4h). In the active state structure, the C-terminal end of the α5-helix of Gαi occupies the space originally occupied by E3846.30 in the inactive state (Fig. 4h). The new position of E3846.30 in the active state is ~16 Å outward (Fig. 4h). The R1463.50 side chain forms a new packing interaction with C351 in the α5-helix of Gαi (Fig. 4h). Therefore, α2A-ARs undergo conformational changes that propagate from the orthosteric ligand-binding site to the Gi-interacting site during activation. Similar conformational changes are observed in the epinephrine–α2A-AR–Gi and dexmedetomidine–α2A-AR–Gi structures.
Molecular recognition of Gi by α2A-AR and β1-AR
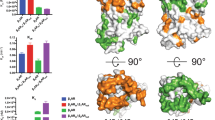
To determine the molecular mechanism by which α2A-AR activates Gi, we investigated how α2A-AR recognizes Gi during the activation process. As revealed by the cryo-EM structures, α2A-AR mainly recognizes Gαi (with fewer interactions with Gβγ subunits) (Fig. 5a). On α2A-AR, the interacting elements include TM3, TM5, TM6, and ICL2 (Fig. 5b). On Gαi, the C-terminal α5-helix contributes to the majority of interactions with α2A-AR (Fig. 5c). α2A-AR is a Gi-coupled receptor, while β1-AR couples primarily to Gs and secondarily to Gi16,21,62,64. We recently solved the cryo-EM structure of the β1-AR and Gi complex21. The α2A-AR–Gi complex and β1-AR–Gi complex were compared, revealing that the interactions between the receptors and Gi are different (Fig. 5d, e). To functionally support these structural data, receptor-interacting residues on Gi were mutated, and their effects on the signaling initiated by α2A-AR and β1-AR were examined (Supplementary Fig. 9a–d). Gα-depleted HEK293 cells (in which the genes for all Gα subunits expressed in HEK cells were mutated by using the CRISPR/Cas system) were cotransfected with α2A-AR or β1-AR and mutated Gαi149 (Supplementary Fig. 9). These functional data support the specific interactions revealed by the structures. K345 (located in the C-terminal α5-helix) of Gi interacts with both α2A-AR and β1-AR (Fig. 5d), and the K345A mutation blocked α2A-AR- and β1-AR-initiated Gi-mediated cAMP inhibition (Supplementary Fig. 9a, b). K345, as well as nearby I344, L348, and F354 on the α5-helix, seem to form interactions with Gi-coupled GPCRs in general (within packing distance with TM5 and TM6); Ala mutations in these residues individually reduce Gi interactions with the receptors65,66. In the α2A-AR–Gi complex, K345 of Gi is within the packing distance of R2415.68 of α2A-AR (Fig. 5d). In the β1-AR–Gi complex, K345 of Gi could form interactions with Q2375.51 of β1-AR (Fig. 5d). Furthermore, L194 (in the loop between β2 and β3) and F336 (in the C-terminal α5-helix) of Gαi form hydrophobic interactions with F14734.51 in ICL2 of β1-AR (Fig. 5e). In α2A-AR, the equivalent residue is I15434.51 (Fig. 5e). I154 in α2A-AR and F14734.51 in β1-AR have different interactions due to side chain differences (Fig. 5e). Compared to I15434.51, α2A-AR, F14734.51 in β1-AR seems to insert deeper to form more contacts with F336 (Fig. 5e), and the I15434.51 in α2A-AR mainly contacts L194 (Fig. 5e). Functional studies revealed that the F336A mutation diminished β1-AR-initiated Gi-mediated cAMP inhibition but had a smaller effect on α2A-AR-initiated Gi-mediated cAMP inhibition (Supplementary Fig. 9d). On the other hand, L194A impaired α2A-AR-initiated and Gi-mediated cAMP inhibition but had a smaller effect on β1-AR-initiated and Gi-mediated cAMP inhibition (Supplementary Fig. 9c). Overall, Gi forms fewer interactions with β1-AR than with α2A-AR. Together, the structural and functional data demonstrate that α2A-AR (a primarily Gi-coupled GPCR) and β1-AR (a GPCR secondarily coupled to Gi) interact with and signal through Gi differently.
Mechanism of Gi activation by α2A-AR
To understand the α2A-AR-catalyzed activation of Gi, we compared the GDP/GTP-binding pocket in Gαi in the complex with α2A-AR and in the inactive, GDP-bound Gi heterotrimer (Gαi1(G203A)Gβ1Gγ2 trimer, PDB: 1GG2)67 (Fig. 6 and Supplementary Figs. 10, 11). Our structure reveals that during activation, the α-helical domain opens by ~77o, which is the principal change. This change results in an ~38 Å displacement of its mass center relative to the Ras-like GTPase domain, which anchors the heterotrimer to α2A-AR (Supplementary Fig. 10b). Critically, the maximum rotation is limited by the position of Gβγ, with Gβγ acting as a buttress to prevent further rotation (Supplementary Fig. 10c–e).
a Diagram showing the GDP-binding pocket of Gαi. Ionic and hydrogen bonds are depicted as dashed black lines. b E43 interacts with R178 in the inactive GDP-bound Gαi. c K46 interacts with D200 in nucleotide-free Gαi. Ionic and hydrogen bonds are depicted as dashed black lines. d, e The distance between K46 and D200 of Gαi calculated from the GaMD simulations when Gi is bound to GDP (d) or is nucleotide free (e). f, g The distance between the Ras-like domain and the α-helical domain of Gαi calculated from the GaMD simulations when Gi is bound to GDP (f) or is nucleotide free (g). (h, i) The distance between K192 and D341 of Gαi calculated from the GaMD simulations when Gi is bound to GDP (h) or is nucleotide free (i). j, k The distance between Q52 and A326 of Gαi calculated from the GaMD simulations when Gi is bound to GDP (j) or is nucleotide free (k). l, m The distance between E43 and R178 of Gαi calculated from the GaMD simulations when Gi is bound with GDP (l) or is nucleotide-free (m). Four independent 3000 ns GaMD simulations are shown for each condition.
In complex with α2A-AR, Gαi is nucleotide free and represents an intermediate state in the GDP/GTP exchange reaction (Supplementary Fig. 11a). In the inactive state of Gαi, the β-phosphate of GDP is coordinated by the sidechains of K46 and S44 in the P-loop and by the main chains of E43, S44, K46 and S47 in the P-loop (Fig. 6a). These structural comparisons revealed that the Switch I region (Linker 2) of Gαi moves away from the GDP/GTP-binding pocket as the α-helical domain rotates away from the Ras-like domain of Gαi (Supplementary Fig. 10). The ionic interaction between E43 on the P-loop and R178 on Switch I was disrupted (Fig. 6b). This E43-R178 interaction might serve as a gate to prevent GDP release (Fig. 6b). In the α2A-AR–Gi complex structure, the side chain of K46 interacts with the side chain of D200 on β3 of the Switch II region and the main chain of G40 (Fig. 6c). Moreover, the interaction network between the N-terminal region of Gαi1 and C-terminal region of Gαi1 in Gαi1Gβ1Gγ2 was disrupted in the α2A-AR–Gi complex (Supplementary Fig. 11c). In the Gi trimer, the sidechain of D341 in the α5-helix forms an ionic interaction with the sidechain of K192 in the β2-β3 loop (Supplementary Fig. 11c). In the α2A-AR–Gi complex, the rotation and translation of the α5-helix move D341 away and disrupt this interaction (Supplementary Fig. 11c). Furthermore, the sidechain of Q52 in the α1-helix forms a hydrogen bond with the backbone carbonyl of A326 in the β6-α5 loop (the TCAT motif) and interacts with the sidechain of T329 in the α5-helix in the Gi trimer (Supplementary Fig. 11f). In the α2A-AR–Gi complex, this contact network is disrupted, leading to movements of the α1-helix, the P-loop and the TCAT motif (Supplementary Fig. 11f). Since these regions form the GDP binding pocket, the combined disruption of these interactions should lead to GDP release and Gi activation.
To functionally test the roles of these identified interacting residues, mutations of these residues were generated. These mutated and wild-type Gi constructs were then expressed in Gα-depleted (by CRISPR) HEK293 cells together with α2A–AR49 (Supplementary Fig. 9e). Forskolin was used to increase cellular cAMP levels, and subsequent epinephrine-induced activation of α2A–AR reduced cAMP levels (Supplementary Fig. 9e). These mutations decreased the activation and thus signaling of α2A-AR to inhibit cAMP (Supplementary Fig. 9e).
We performed GaMD simulations to examine the G protein activation process26 (Fig. 6d, e). We started by examining the complex of dexmedetomidine–α2A-AR–Gi and replaced nucleotide-free Gi with inactive GDP-bound Gi, mimicking the starting point of the G protein activation reaction pathway. However, no GDP release was observed from this complex during the 3000 ns GaMD simulations, reflecting the slow kinetics of GDP dissociation (Supplementary Fig. 11b). We then carried out further GaMD simulations by directly removing GDP from the Gi protein. In the absence of GDP, K46 and D200 formed an ionic interaction with a distance of ~3.5 Å between their charge centers during most of the simulation time period (Fig. 6e). In contrast, when GDP was bound to Gi, the salt bridge between K46 and D200 fluctuated between ~3.5 Å and ~6 Å (Fig. 6d). The conformation with an ~6 Å distance revealed that K46 and the β-phosphate of GDP interact (Fig. 6a). Therefore, the GaMD simulations showed that D200 forms a stable salt bridge with K46 in the P-loop in an intermediate step during G protein activation. In contrast, the β-phosphate of GDP competes with this Lys in the inactive state of G proteins.
Additionally, we investigated the conformational changes propagating from the GPCR contact site (the C-terminal α5-helix of Gi that interacts with α2A-AR) to the GDP/GTP binding pocket of Gi. In our GaMD simulations, the α-helical domain moved away from the Ras-like domain after ~1400 ns in two of the four independent 3000 ns GaMD simulations, as measured by the distance between the Cα atoms of residues A138 (in the α-helical domain) and E276 (in the Ras-like domain) (Fig. 6g). On the other hand, in the presence of GDP, this interdomain separation was not observed (Fig. 6f). We then inspected the interactions between the α5-helix and structural elements linked to the GDP/GTP-binding pocket and examined the temporal correlation between the interaction changes and α-helical domain separation. One correlated change is that the C-terminal α5-helix adopts a conformation similar to that in the α2A-AR–Gi complex (Supplementary Fig. 11e). In this conformation, the α5-helix forms tight interactions with α2A-AR. After the second correlated conformational change, the ionic interaction between K192 on the β2-β3 loop and D341 on the α5-helix is disrupted (from ~3 Å in Fig. 6h to ~12.5 Å in Fig. 6i and Supplementary Fig. 11c). After the third correlated conformational change, the interaction between Q52 in the α1-helix and A326 in the β6-α5 loop at the end of the α5-helix is disturbed (Fig. 6j,k, Supplementary Fig. 11f). The α1-helix is immediately downstream of the P-loop and is directly involved in GDP binding (Fig. 6a). Therefore, both the changes in the K192 and D341 interaction, as well as the Q52 and A326 interaction, propagate the tight binding of the C-terminal α5-helix to α2A-AR across ~35 Å to the P-loop (the GDP/GTP-binding pocket). Concomitantly, the ionic interaction between E43 on the P-loop and R178 on Switch I is disrupted, leading to α-helical domain separation (Fig. 6b, l, m). Hence, α2A-AR interacts strongly with the C-terminal half of the α5-helix, leading to the disruption of interactions between K192 on the β2-β3 loop and D341 on the α5-helix, as well as between Q52 on the α1-helix and A326 on the β6-α5 loop. These structural changes lead to modification of the GDP/GTP-binding pocket.
The epinephrine-bound complex is more stable than the dexmedetomidine-bound complex
Finally, we analyzed the structural flexibility of different agonist-bound α2A-AR–Gi complexes (Fig. 7). In GaMD simulations of the epinephrine–α2A-AR–Gi and dexmedetomidine–α2A-AR–Gi complexes, the α2A-ARs underwent small fluctuations, except for ICL1 and the extracellular loop 2 (ECL2) (Fig. 7a, b). On the other hand, Gi proteins exhibited greater flexibility than α2A-ARs, especially in the C-terminal α5-helix, Switch III, and the αN helix in Gαi and the N-termini of Gβγ (Fig. 7a, b). Compared with epinephrine, dexmedetomidine increased the fluctuations in the α2A-AR and Gi interface and in α2A-AR, especially in ECL2 and the orthosteric ligand-binding site (Fig. 7c). Thus, when the compound is bound with dexmedetomidine, the interaction between α2A-AR and Gi is less stable. On the other hand, dexmedetomidine binding to α2A-AR led to lower flexibility in the Gi than that observed with epinephrine (Fig. 7c).
a–c GaMD simulations showing the changes in the flexibility of the ligand–α2A-AR–Gi complexes. The root-mean-square fluctuations (RMSFs) of the epinephrine–α2A-AR–Gi complex (a) and the dexmedetomidine–α2A-AR–Gi complex (b) are shown. c Changes in the RMSF of α2A-AR and Gi in the presence of the dexmedetomidine–α2A-AR–Gi complex compared with the presence of the epinephrine–α2A-AR–Gi complex. The dashed box indicates the α2A-AR–Gi-interacting regions. d, e Comparison of the agonist flexibilities of the two complexes. The time courses of the root-mean-square deviation (RMSD) of the two agonists in the complexes are shown. f, g Stable hydrogen bond formed between Y4317.42 and D1283.32 in the epinephrine–α2A-AR–Gi complex. The time courses of the distance between residues Y4317.42 and D1283.32 in the epinephrine–α2A-AR–Gi (f) and dexmedetomidine-α2A-AR–Gi (g) complexes are shown. h–k Complex dynamic and 2D free energy profiles. Time courses of the distance between the intracellular ends of TM3 and TM6 (measured as the distance in Å between R146 and T388) in the epinephrine–α2A–AR–Gi (h) and dexmedetomidine–α2A–AR–Gi (i) complexes. 2D free energy profiles of the agonist RMSD relative to cryo-EM conformations (Å) and R146 and T388 distances (Å) calculated from GaMD simulations in the epinephrine–α2A–AR–Gi (j) and dexmedetomidine–α2A–AR–Gi (k) complexes are shown. The time courses of the distance between the DRY motif in α2A-AR and the last five C-terminal residues in Gi in the epinephrine–α2A-AR–Gi complex (l) and in the dexmedetomidine–α2A-AR–Gi complex (m) are shown.
Furthermore, in the α2A-AR–Gi complexes, the fluctuations were less pronounced in the presence of epinephrine than in the presence of dexmedetomidine (Fig. 7c, d, e). More stable interactions were observed for epinephrine with receptor residues in the epinephrine–α2A-AR–Gi complex. Epinephrine formed a salt bridge with D1283.32 and six hydrogen bonds with residues Y4317.42, C1323.36, S2155.43, and S2195.46 (Fig. 2). In contrast, dexmedetomidine formed only three hydrogen bonds with the α2A-AR residues Y4317.42, C1323.36, and F4277.38 (Fig. 3). Moreover, differences in the residue interactions were observed at the orthosteric ligand-binding site of α2A-AR in GaMD simulations of the epinephrine–α2A-AR–Gi and dexmedetomidine–α2A-AR–Gi complexes (Fig. 7f–i). In the epinephrine–α2A-AR–Gi complex, the hydrogen bond between residues Y4317.42 and D1283.32 was stable, while large fluctuations were observed in the dexmedetomidine–α2A-AR–Gi complex (Fig. 7f, g). The ligand RMSD and the TM3-TM6 distance (measured by the distance between R1463.50 and T3886.34) were used to calculate the 2D free energy profiles of the simulation systems (Fig. 7h–k). The low-energy state of the epinephrine–α2A-AR–Gi complex was sampled with the ligand RMSD and the TM3–TM6 distance centered at (1.2 Å, 15.7 Å) (Fig. 7j), which was consistent with the cryo-EM structure. The dexmedetomidine-α2A-AR–Gi complex sampled a larger conformational space and two low-energy wells with a ligand RMSD and a TM3-TM6 distance centered at (1.9 Å, 15.0 Å) and (3.8 Å, 17.2 Å) (Fig. 7k). Additionally, the interaction between α2A-AR and Gi is less stable when bound with dexmedetomidine than with epinephrine, as measured by the distance between the DRY motif on TM3 of α2A-AR and the last 5 C-terminal residues of Gi (Fig. 7l and m). Together, these data suggest that the dexmedetomidine–α2A-AR–Gi complex is less stable than the epinephrine–α2A-AR–Gi complex and provide the structural basis for ligand efficacy.
Discussion
Here, we investigated the mechanisms underlying the molecular recognition of epinephrine by ARs. Surprisingly, we found that epinephrine adopts two different conformations when bound to α2A-AR and β-ARs. These two different epinephrine conformations are isomers that result from the rotation around the chemical bond that links the catechol ring and β-carbon of epinephrine. Furthermore, when bound to α2A-AR, the β-carbon hydroxyl group of epinephrine faces the extracellular side, and the N-methyl group points to TM7. When bound to β1-AR, the β-carbon hydroxyl group faces the intracellular side, and the N-methyl group points toward TM3. Recently, we observed that epinephrine exhibits the same conformation in complex with α1A-AR and α2A-AR13. This new information can be utilized to perform structural modifications and produce α-AR and β-AR selective agonists.
Previous functional studies have extensively elucidated that the ligand-binding residues identified within our structures, namely, D1283.32, V1293.33, C1323.36, T1333.37, S2155.43, S2195.46, W4026.48, F4056.51, F4066.52, Y4096.55, F4277.38, and Y4317.42, play pivotal roles in orchestrating the intricate interactions between α2A-AR and its ligands (Fig. 2a, and Supplementary Figs. 6, 12)53,54,60,62,68. Although epinephrine and dexmedetomidine occupy a common binding site, their engagement with α2A-AR diverges significantly. Through dedicated functional analyses, we investigated select ligand-binding residues (namely, D1283.32, T1333.37, S2155.43, S2195.46, F4056.51, F4066.52, Y4096.55, and Y4317.42) to determine their distinctive responses to epinephrine and dexmedetomidine. While different ligands engage similar residues within the orthosteric ligand-binding pockets of ARs, the nuances of these interactions subtly deviate among the ligands. The roles of the corresponding residues in accommodating distinct ligands within ARs are remarkably diverse. Moreover, recent advancements in structure-based docking and optimization have unveiled novel α2A-AR agonists, which serve as nonopioid analgesics and are characterized by attenuated side effects69. Compared to canonical agonists such as dexmedetomidine and norepinephrine, these innovative agonists, which are endowed with novel chemotypes, forge more potent interactions with F4277.3869. These findings underscore the pivotal significance of F4277.38 in conferring subtype-specific discernment of ligands.
Our data also revealed that α2A-AR (a primarily Gi-coupled GPCR) and β1-AR (a GPCR primarily coupled to Gs and secondarily coupled to Gi) interact differently with Gi. These results contribute to our knowledge on the coupling specificity among GPCRs and G proteins, which is not fully understood. Previously, we solved and compared the cryo-EM structures of β1-AR with Gs or with Gi and revealed that β1-AR recognizes Gs versus Gi through three-dimensional interactions21,27. The overall β1-AR–G-protein complex structure (not the α5-helix structure alone) dictates β1-AR–G-protein interaction modes, and the different modes of interaction between β1-AR–Gs and β1-AR–Gi contribute to the differences in activation selectivity and efficiency21. Furthermore, we conducted a structural comparison between our α2A-AR–Gi complex and an α2A-AR–Go complex (Supplementary Fig. 13). Upon superimposing the α2A-ARs, we observed variations in the orientations of Gi and Go (Supplementary Fig. 13a). Notably, the most prominent distinction was observed in the N-termini position within the G-proteins (Supplementary Fig. 13a). This difference could be attributed to the incorporation of scFv16 in the α2A-Go complex. Additionally, we identified disparities in the interactions between α2A-AR and G proteins. For instance, K345 on Gi was closely packed against R2415.68 of α2A-AR (Supplementary Fig. 13b, c), while in Go, the corresponding residue was A345 (Supplementary Fig. 13b, d). Notably, A345 of Go did not interact with any residues on α2A-AR (Supplementary Fig. 13b, d). These findings collectively highlight the distinct interactions between α2A-AR and Gi versus Go.
In summary, we investigated the interactions between epinephrine and the α and β families of ARs and revealed new information on the structural characteristics of the interactions. The data show that epinephrine adopts different conformations to interact with these families of ARs. We also provide structural information on the binding of epinephrine to a GPCR-G protein complex. We further elucidated the selectivity of GPCR-G-protein coupling by investigating the coupling modes of Gi to α2A-AR and β1-AR. GaMD simulations were used to propose a possible sequential order for the activation of Gi by α2A-AR. These findings advance our knowledge of the mechanisms underlying GPCR and G protein activation.
Data availability
The cryo-EM reconstructions of the epinephrine–α2A-AR–Gi complex and the Dexmedetomidine–α2A-AR–Gi complex have been deposited in the Election Microscopy Data Bank (EMDB) under ID codes EMD-45425 and EMD-45426, respectively. The corresponding atomic models have been deposited in the Protein Data Bank (PDB) under ID codes 9CBL and 9CBM, respectively.
References
von Euler, U. S. Adrenergic neurotransmitter functions. Science 173, 202–206, https://doi.org/10.1126/science.173.3993.202 (1971).
Philipp, M. & Hein, L. Adrenergic receptor knockout mice: distinct functions of 9 receptor subtypes. Pharm. Ther. 101, 65–74, https://doi.org/10.1016/j.pharmthera.2003.10.004 (2004).
Brede, M., Philipp, M., Knaus, A., Muthig, V. & Hein, L. alpha2-adrenergic receptor subtypes—novel functions uncovered in gene-targeted mouse models. Biol. Cell 96, 343–348, https://doi.org/10.1016/j.biolcel.2004.03.006 (2004).
Giovannitti, J. A. Jr, Thoms, S. M. & Crawford, J. J. Alpha-2 adrenergic receptor agonists: a review of current clinical applications. Anesth. Prog. 62, 31–39, https://doi.org/10.2344/0003-3006-62.1.31 (2015).
Keating, G. M. Dexmedetomidine: a review of its use for sedation in the intensive care setting. Drugs 75, 1119–1130, https://doi.org/10.1007/s40265-015-0419-5 (2015).
Pichot, C., Ghignone, M. & Quintin, L. Dexmedetomidine and clonidine: from second- to first-line sedative agents in the critical care setting? J. Intensive Care Med. 27, 219–237, https://doi.org/10.1177/0885066610396815 (2012).
Weerink, M. A. S. et al. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin. Pharmacokinet. 56, 893–913, https://doi.org/10.1007/s40262-017-0507-7 (2017).
Nguyen, V., Tiemann, D., Park, E. & Salehi, A. Alpha-2 agonists. Anesthesiol. Clin. 35, 233–245, https://doi.org/10.1016/j.anclin.2017.01.009 (2017).
Preskorn, S. H. et al. Effect of sublingual dexmedetomidine vs placebo on acute agitation associated with bipolar disorder: a randomized clinical trial. JAMA 327, 727–736, https://doi.org/10.1001/jama.2022.0799 (2022).
Scullion, G. A., Kendall, D. A., Marsden, C. A., Sunter, D. & Pardon, M. C. Chronic treatment with the alpha2-adrenoceptor antagonist fluparoxan prevents age-related deficits in spatial working memory in APPxPS1 transgenic mice without altering beta-amyloid plaque load or astrocytosis. Neuropharmacology 60, 223–234, https://doi.org/10.1016/j.neuropharm.2010.09.002 (2011).
Kobilka, B. K. Structural insights into adrenergic receptor function and pharmacology. Trends Pharmacol. Sci. 32, 213–218, https://doi.org/10.1016/j.tips.2011.02.005 (2011).
Dalal, R. & Grujic, D. Epinephrine in StatPearls, https://www.ncbi.nlm.nih.gov/books/NBK482160/ (2023).
Su, M. et al. Structural basis of agonist specificity of alpha(1A)-adrenergic receptor. Nat. Commun. 14, 4819, https://doi.org/10.1038/s41467-023-40524-2 (2023).
Xu, X. et al. Binding pathway determines norepinephrine selectivity for the human beta1AR over beta2AR. Cell Res. 31, 569–579, https://doi.org/10.1038/s41422-020-00424-2 (2021).
Ring, A. M. et al. Adrenaline-activated structure of beta2-adrenoceptor stabilized by an engineered nanobody. Nature 502, 575–579, https://doi.org/10.1038/nature12572 (2013).
Kompa, A. R., Gu, X. H., Evans, B. A. & Summers, R. J. Desensitization of cardiac beta-adrenoceptor signaling with heart failure produced by myocardial infarction in the rat. Evidence for the role of Gi but not Gs or phosphorylating proteins. J. Mol. Cell Cardiol. 31, 1185–1201, https://doi.org/10.1006/jmcc.1999.0951S0022-2828(99)90951-3 (1999).
Martin, N. P., Whalen, E. J., Zamah, M. A., Pierce, K. L. & Lefkowitz, R. J. PKA-mediated phosphorylation of the beta1-adrenergic receptor promotes Gs/Gi switching. Cell Signal 16, 1397–1403, https://doi.org/10.1016/j.cellsig.2004.05.002 (2004).
Belevych, A. E., Juranek, I. & Harvey, R. D. Protein kinase C regulates functional coupling of beta1-adrenergic receptors to Gi/o-mediated responses in cardiac myocytes. FASEB J. 18, 367–369, https://doi.org/10.1096/fj.03-0647fje (2004).
Wang, J. et al. Galphai is required for carvedilol-induced beta1 adrenergic receptor beta-arrestin biased signaling. Nat. Commun. 8, 1706, https://doi.org/10.1038/s41467-017-01855-z (2017).
Dwivedi, H., Baidya, M. & Shukla, A. K. GPCR signaling: the interplay of galphai and beta-arrestin. Curr. Biol. 28, R324–R327, https://doi.org/10.1016/j.cub.2018.02.027 (2018).
Alegre, K. O. et al. Structural basis and mechanism of activation of two different families of G proteins by the same GPCR. Nat. Struct. Mol. Biol. 28, 936–944, https://doi.org/10.1038/s41594-021-00679-2 (2021).
Miao, Y., Feher, V. A. & McCammon, J. A. Gaussian accelerated molecular dynamics: unconstrained enhanced sampling and free energy calculation. J. Chem. Theory Comput. 11, 3584–3595, https://doi.org/10.1021/acs.jctc.5b00436 (2015).
Wang, J. N. et al. Gaussian accelerated molecular dynamics: principles and applications. Wires Comput Mol. Sci. 11, e1521, https://doi.org/10.1002/wcms.1521 (2021).
Miao, Y. & McCammon, J. A. Graded activation and free energy landscapes of a muscarinic G-protein–coupled receptor. Proc. Natl. Acad. Sci. 113, 12162–12167, https://doi.org/10.1073/pnas.1614538113 (2016).
Su, M. et al. Structures of beta1-adrenergic receptor in complex with Gs and ligands of different efficacies. Nat. Commun. 13, 4095, https://doi.org/10.1038/s41467-022-31823-1 (2022).
Draper-Joyce, C. J. et al. Positive allosteric mechanisms of adenosine A1 receptor-mediated analgesia. Nature 597, 571–576, https://doi.org/10.1038/s41586-021-03897-2 (2021).
Su, M. et al. Structural basis of the activation of heterotrimeric Gs-protein by isoproterenol-bound beta1-adrenergic receptor. Mol. Cell 80, 59–71 e54, https://doi.org/10.1016/j.molcel.2020.08.001 (2020).
Liu, S. et al. Differential activation mechanisms of lipid GPCRs by lysophosphatidic acid and sphingosine 1-phosphate. Nat. Commun. 13, 731, https://doi.org/10.1038/s41467-022-28417-2 (2022).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332, https://doi.org/10.1038/nmeth.4193 (2017).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. Elife 7, https://doi.org/10.7554/eLife.42166 (2018).
Zivanov, J., Nakane, T. & Scheres, S. H. W. A Bayesian approach to beam-induced motion correction in cryo-EM single-particle analysis. IUCrJ 6, 5–17, https://doi.org/10.1107/S205225251801463X (2019).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221, https://doi.org/10.1016/j.jsb.2015.08.008 (2015).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296, https://doi.org/10.1038/nmeth.4169 (2017).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D. Biol. Crystallogr. 60, 2126–2132, https://doi.org/10.1107/S0907444904019158 (2004).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D. Biol. Crystallogr. 66, 213–221, S0907444909052925 [pii] https://doi.org/10.1107/S0907444909052925 (2010).
Friesner, R. A. et al. Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 49, 6177–6196, https://doi.org/10.1021/jm051256o (2006).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D. Biol. Crystallogr. 66, 12–21, https://doi.org/10.1107/S0907444909042073 (2010).
Waterhouse, A. et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 46, W296–W303, https://doi.org/10.1093/nar/gky427 (2018).
Lee, J. et al. CHARMM-GUI input generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM simulations using the CHARMM36 additive force field. J. Chem. Theory Comput. 12, 405–413, https://doi.org/10.1021/acs.jctc.5b00935 (2016).
Vanommeslaeghe, K. & MacKerell, A. D. CHARMM additive and polarizable force fields for biophysics and computer-aided drug design. Biochim. Biophys. Acta (BBA) - Gen. Subj. 1850, 861–871, https://doi.org/10.1016/j.bbagen.2014.08.004 (2015).
Huang, J. et al. CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nat. Methods 14, 71, https://doi.org/10.1038/nmeth.4067 (2017).
Klauda, J. B. et al. Update of the CHARMM all-atom additive force field for lipids: validation on six lipid types. J. Phys. Chem. B 114, 7830–7843, https://doi.org/10.1021/jp101759q (2010).
Vanommeslaeghe, K., Raman, E. P. & MacKerell, A. D. Automation of the CHARMM general force field (CGenFF) II: assignment of bonded parameters and partial atomic charges. J. Chem. Inf. Model. 52, 3155–3168, https://doi.org/10.1021/ci3003649 (2012).
Kumar, A., Yoluk, O. & MacKerell, A. D. Jr. FFParam: standalone package for CHARMM additive and drude polarizable force field parametrization of small molecules. J. Comput. Chem. 41, 958–970, https://doi.org/10.1002/jcc.26138 (2020).
Phillips, J. C. et al. Scalable molecular dynamics with NAMD. J. Comput. Chem. 26, 1781–1802, https://doi.org/10.1002/jcc.20289 (2005).
Darden, T., York, D. & Pedersen, L. Particle mesh Ewald: An N⋅ log (N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089, https://doi.org/10.1063/1.464397 (1993).
Case, D. A. et al. Amber 2020, University of California, San Francisco (2020).
Roe, D. R. & Cheatham, T. E. PTRAJ and CPPTRAJ: software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 9, 3084–3095, https://doi.org/10.1021/ct400341p (2013).
Hisano, Y. et al. Lysolipid receptor cross-talk regulates lymphatic endothelial junctions in lymph nodes. J. Exp. Med. 216, 1582–1598, https://doi.org/10.1084/jem.20181895 (2019).
Huang, J., Sun, Y., Zhang, J. J. & Huang, X. Y. Pivotal role of extended linker 2 in the activation of Galpha by G protein-coupled receptor. J. Biol. Chem. 290, 272–283, https://doi.org/10.1074/jbc.M114.608661 (2015).
Manglik, A., Kobilka, B. K. & Steyaert, J. Nanobodies to study G Protein-coupled receptor structure and function. Annu Rev. Pharm. Toxicol. 57, 19–37, https://doi.org/10.1146/annurev-pharmtox-010716-104710 (2017).
Ballesteros, J. A. W. H. Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci. 25, 366–428 (1995).
Suryanarayana, S., Daunt, D. A., Von Zastrow, M. & Kobilka, B. K. A point mutation in the seventh hydrophobic domain of the alpha 2 adrenergic receptor increases its affinity for a family of beta receptor antagonists. J. Biol. Chem. 266, 15488–15492 (1991).
Suryanarayana, S. & Kobilka, B. K. Amino acid substitutions at position 312 in the seventh hydrophobic segment of the beta 2-adrenergic receptor modify ligand-binding specificity. Mol. Pharm. 44, 111–114 (1993).
Wu, Y., Zeng, L. & Zhao, S. Ligands of adrenergic receptors: a structural point of view. Biomolecules 11, https://doi.org/10.3390/biom11070936 (2021).
Jasper, J. R. et al. Ligand efficacy and potency at recombinant alpha2 adrenergic receptors: agonist-mediated [35 S]GTPgammaS binding. Biochem. Pharmacol. 55, 1035–1043, https://doi.org/10.1016/s0006-2952(97)00631-x (1998).
Peltonen, J. M., Pihlavisto, M. & Scheinin, M. Subtype-specific stimulation of [35 S]GTPgammaS binding by recombinant alpha2-adrenoceptors. Eur. J. Pharm. 355, 275–279, https://doi.org/10.1016/s0014-2999(98)00518-4 (1998).
Tan, C. M., Wilson, M. H., MacMillan, L. B., Kobilka, B. K. & Limbird, L. E. Heterozygous alpha 2A-adrenergic receptor mice unveil unique therapeutic benefits of partial agonists. Proc. Natl Acad. Sci. USA 99, 12471–12476, https://doi.org/10.1073/pnas.122368499 (2002).
Yuan, D. et al. Activation of the alpha2B adrenoceptor by the sedative sympatholytic dexmedetomidine. Nat. Chem. Biol. 16, 507–512, https://doi.org/10.1038/s41589-020-0492-2 (2020).
Xu, J. et al. Structural insights into ligand recognition, activation, and signaling of the alpha2A adrenergic receptor. Sci. Adv. 8, eabj5347, https://doi.org/10.1126/sciadv.abj5347 (2022).
Liapakis, G. et al. The forgotten serine. A critical role for Ser-2035.42 in ligand binding to and activation of the beta 2-adrenergic receptor. J. Biol. Chem. 275, 37779–37788, https://doi.org/10.1074/jbc.M002092200 (2000).
Qu, L. et al. Structural basis of the diversity of adrenergic receptors. Cell Rep. 29, 2929–2935.e2924, https://doi.org/10.1016/j.celrep.2019.10.088 (2019).
Zhou, Q. et al. Common activation mechanism of class A GPCRs. Elife 8, https://doi.org/10.7554/eLife.50279 (2019).
Asano, T., Katada, T., Gilman, A. G. & Ross, E. M. Activation of the inhibitory GTP-binding protein of adenylate cyclase, Gi, by beta-adrenergic receptors in reconstituted phospholipid vesicles. J. Biol. Chem. 259, 9351–9354 (1984).
Sun, D. et al. Probing Galphai1 protein activation at single-amino acid resolution. Nat. Struct. Mol. Biol. 22, 686–694, https://doi.org/10.1038/nsmb.3070 (2015).
Kang, Y. et al. Cryo-EM structure of human rhodopsin bound to an inhibitory G protein. Nature 558, 553–558, https://doi.org/10.1038/s41586-018-0215-y (2018).
Wall, M. A. et al. The structure of the G protein heterotrimer Gi alpha 1 beta 1 gamma 2. Cell 83, 1047–1058 (1995).
Wang, C. D., Buck, M. A. & Fraser, C. M. Site-directed mutagenesis of alpha 2A-adrenergic receptors: identification of amino acids involved in ligand binding and receptor activation by agonists. Mol. Pharm. 40, 168–179 (1991).
Fink, E. A. et al. Structure-based discovery of nonopioid analgesics acting through the alpha(2 A)-adrenergic receptor. Science 377, eabn7065, https://doi.org/10.1126/science.abn7065 (2022).
Acknowledgements
We thank members of our research groups for helpful discussion and comments on the manuscript and Li-Hua Yu for technical support. We thank Dr. A. Inoue (Tohoku University, Japan) for the Gα-depleted HEK293 cells. We thank the Laboratory for BioMolecular Structure (LBMS) staff for help with cryo-EM data acquisition. This work was supported by NIH grants GM138676 (X.Y.H.) and GM132572 (Y.M.). This work used supercomputing resources with the TG-MCB180049 allocation award through the Extreme Science and Engineering Discovery Environment (XSEDE), the M2874 project through the National Energy Research Scientific Computing Center (NERSC), and the Research Computing Cluster at the University of Kansas. LBMS is supported by the DOE Office of Biological and Environmental Research (KP1607011).
Author information
Authors and Affiliations
Contributions
J.L. expressed and purified α2A-AR, Gαi, Gβ1γ2, β1-AR, and the protein complexes and performed cAMP assays. M.S. generated the cryo-EM grids and performed the cryo-EM screening, data collection, EM density map determination, and model building. J.W. and H.N.D. performed GaMD simulations under the supervision of Y.M. X.Y.H. supervised the project, interpreted the data, and wrote the manuscript. All the authors contributed to the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lou, JS., Su, M., Wang, J. et al. Distinct binding conformations of epinephrine with α- and β-adrenergic receptors. Exp Mol Med (2024). https://doi.org/10.1038/s12276-024-01296-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s12276-024-01296-x
- Springer Nature Limited