Abstract
Purpose
Endocrine-disrupting compounds, including bisphenol A (BPA), may promote obesity influencing basal metabolic rate and shifting metabolism towards energy storage. The role of 1,25‑Dihydroxyvitamin D3 (VitD) in counteracting adipogenesis is still a matter of debate. Thus, the current study aims to investigate whether and how VitD exposure during adipogenesis could prevent the pro-adipogenic effect of BPA in two adipocyte models, mouse 3T3-L1 cell line and human adipose-derived mesenchymal stem cells (hAMSC).
Methods
3T3-L1, mouse pre-adipocytes and human adipose-derived mesenchymal stem cells (hAMSC) were treated with VitD (10−7 M) and BPA (10−8 M and 10−9 M), alone or in combination, throughout the differentiation in mature adipocytes. Cellular lipid droplet accumulation was assessed by Oil Red O staining, mRNA and protein expression of key adipogenic markers, transcription factors, and cytokines were investigated by RT-qPCR and WB, respectively. miRNAs involved in the regulation of adipogenic transcription factors were evaluated by RT-qPCR, and highly potent steric-blocking oligonucleotides (miRNA inhibitors) were used to modulate miRNAs expression.
Results
Pre-adipocytes express VitD receptor (VDR) in basal condition, but during the differentiation process VDR expression reduces if not stimulated by the ligand. VitD significantly decreases lipid accumulation, with a consequent reduction in adipogenic marker expression, and counteracts the pro-adipogenic effect of BPA in 3T3-L1 and hAMSC during differentiation. This effect is associated to the increased expression of miR-27a-3p and miR-27b-3p. The blocking of miR-27a-3p and miR-27b-3p through miRNA inhibitors prevents the anti-adipogenic effect of VitD in both cell models.
Conclusions
These results suggest that in cultured 3T3-L1 and hAMSC VitD induces an anti-adipogenic effect and prevents BPA pro-adipogenic effect by triggering at least in part epigenetic mechanisms involving miR-27-3p.

Similar content being viewed by others
Background
Obesity represents a serious public health challenge globally and its prevalence is significantly growing worldwide, mainly in the Western countries [1, 2]. Preventing obesity not only reduces the risk of associated health issues, such as cardiovascular disease, diabetes, metabolic syndrome, cancers and much more but also can lighten the load on the healthcare system. Indeed, according to a 2019 report on the economic burden of obesity from the Organisation for Economic Co-operation and Development (OECD), between 2020 and 2050 countries will spend, on average, 8.4% of their entire annual health budget for treating the consequences of overweight, including obesity [3].
Although the obesity epidemic is strictly linked to sedentary lifestyles combined with excess energy intake, the etiology of obesity is much more complex and multifactorial, including also environmental factors [4].
Increasing evidence from epidemiological, animal and in vitro studies suggest that endocrine-disrupting compounds (EDCs), including bisphenol A (BPA), may have negative health effects by promoting obesity [5,6,7,8,9,10], type 2 diabetes mellitus [11], infertility [12] and cancer [13].
BPA can be found in many common food containers and packaging from which, especially after heating, it can leach into food products by exposing humans to the diet [11].
By binding to nuclear hormonal receptors, mainly but not exclusively estrogen receptors, BPA contributes at the cellular level to increase adipogenesis, the process through which adipose progenitors differentiate into mature adipocytes, influencing the basal metabolic rate and shifting metabolism towards energy storage [10].
Of interest, 25 hydroxyvitamin D3 insufficiency and obesity have concomitantly reached epidemic levels worldwide, with possible bidirectional associations between low 25 hydroxyvitamin D3 and 1,25‑Dihydroxyvitamin D3 (VitD) status and obesity [14,15,16,17]. The role of VitD on adipose biology is still a matter of debate, due to the controversial data obtained mainly in in vitro studies [18,19,20,21], although these latter suggest that VitD exerts catabolic effects in adipocytes, decreasing lipid accumulation. Nevertheless, the molecular mechanisms underlying this inhibition still remain only partially explored.
The current study aims to investigate whether and how VitD exposure during adipogenesis could prevent the pro-adipogenic effect of BPA in adipocyte models: mouse 3T3-L1 cell line and human adipose-derived mesenchymal stem cells (hAMSC).
Materials and methods
Study design
The study design has been summarized in Fig. 1. For this study, the stable 3T3-L1 murine preadipocyte cell line and the limited self-renewal hAMSC human adipose-derived mesenchymal stem cells were used. Because of the rapid senescent of hAMSC the type and the number of experiments with these cells were limited.
Mouse 3T3-L1 and human hAMSC cells were used for the study. VitD and BPA treatment, alone and in combination, was performed during differentiation in white adipocytes in both cell models. VDR protein expression was evaluated by WB in 3T3-L1 pre-adipocytes and in undifferentiated hAMSC and during differentiation in the presence and absence of VitD in both cell models. At the end of the differentiation, gene and protein expression of adipogenic markers were evaluated by RT-qPCR and WB in 3T3-L1 cells and only protein expression by WB in hAMSC with and without treatment. Adipogenesis was also evaluated by staining lipid droplets with Oil Red O dye in both cell models with and without VitD and BPA treatment. Bioinformatic analysis was performed to find conserved miRNAs able to regulate PPARγ in mice and humans. The expression of such miRNAs was investigated in 3T3-L1 treated with VitD and BPA and their role was explored in both cell models by using miRNA inhibitors and by evaluating lipid droplet accumulation using Oil Red O staining and adipogenic marker protein expression by WB.
Briefly, after assessing the VitD receptor (VDR) expression in pre-adipocytes and in mature white adipocytes, 3T3-L1 and hAMSC cells were treated with BPA and VitD every other day during differentiation. At the end of cell differentiation, gene and protein adipogenic markers expression were evaluated by RT-qPCR, in 3T3-L1, and WB, in both cell lines; lipid accumulation was evaluated by Oil-Red-O staining; miRNAs expression and silencing were estimated by RT-qPCR and by single-strand inhibitors of endogenous miRNAs, respectively, in order to confirm the BPA-induced pro-adipogenic effect and to explore the potential epigenetic protective effect of VitD.
Cell lines, differentiation, and treatment
3T3-L1 mouse fibroblasts were purchased by American Type Culture Collection (ATCC CL-173) and cultured in Dulbecco’s modified Eagle’s medium (DMEM (1X) + GlutaMAX) supplemented with 10% calf serum (CS) and 1 × 105 U/L penicillin and streptomycin (Gibco, Italy) to reach the confluence. Cultures were maintained in humidified atmosphere of 95% air and 5% CO2 at 37 °C. To achieve adipocyte differentiation, 3T3-L1 pre-adipocytes were incubated with differentiation medium (DMEM (1X) + GlutaMAX with 10% fetal bovine serum (FBS) and 1 × 105 U/L penicillin and streptomycin, containing insulin 10−6 M, dexamethasone 10−7 M and 3-isobutyl-1-methylxanthine 10−3 M to starting adipogenesis (day 0). At day 2 of differentiation process, the medium was replaced with the post-differentiation medium (DMEM, 10% FBS and 1 × 105 U/L penicillin and streptomycin, containing only insulin 10−6 M). Then, the post-differentiation medium was changed every 2 days until the mature adipocytes were obtained (day 10).
The primary Human Adipose-derived Mesenchymal Stem Cells (hAMSC) were purchased by PromoCell (hMSC-AT-c; C-12977) and cultured with MesenCult MSC Basal Medium (Stem Cell Technologies; #05401), MesenCult MSC Stimulatory Supplement (Stem Cell Technologies; #05401) and 1 × 105 U/L penicillin and streptomycin (Gibco, Italy) to reach the confluence. Cultures were maintained in a humidified atmosphere of 95% air and 5% CO2 at 37 °C. To achieve adipogenic differentiation, hAMSC were incubated with MesenCult MSC Basal Medium (Stem Cell Technologies; #05413), MesenCult 10X Adipogenic Differentiation Supplement (Stem Cell Technologies; #05414), MesenCult 500X Adipogenic Differentiation Supplement (Stem Cell Technologies; #05415), 1 × 105 L-Glutamine (Gibco, Italy) and 1 × 105 U/L penicillin and streptomycin (Gibco, Italy). According to the protocol, hAMSC were plated in a proliferating medium for 14 days, with a half-medium change on day 7. After 14 days, the medium was replaced with the adipogenic differentiation medium with a medium change every 2 days up to 14 days. Both cell lines were tested and resulted in mycoplasma-free.
For rigorous and reproducible experiments, different batches (3 for 3T3-L1, undergoing passages 1–15, and 2 for hAMSC, undergoing just 2 passages) were used.
Powders of VitD (Selleck Chemicals (UK) and BPA (Sigma-Aldrich) were dissolved in EtOH and DMSO100%, respectively. Aliquots were stored at −80 °C and fresh aliquots were defrosted prior of each new experiment. Serial dilutions were prepared using EtOH and DMSO 40%, respectively, reaching vehicles concentrations of 0.04% in the final volume in each well. VitD was used at a concentration of 10−7 M, a concentration generally used in in vitro studies [22,23,24], and below the cholecalciferol Cmax reached in subjects in condition of VitD deficiency supplemented with different dosing schedules [25]. BPA was used at concentrations of 10−8 M and 10−9 M, concentrations considered biologically relevant [26] according to 0.2 ng BPA/kg bw per day established by EFSA in 2023 [27]. BPA and VitD were inoculated every other day in the medium changed during the 10 or 14 days of differentiation, for 3T3-L1 and hAMSC cells, respectively. BPA and VitD were inoculated as a single and combined treatment, while vehicles (DMSO 40% and EtOH 40%) were added in control wells.
Oil Red O staining
Lipid accumulation was measured using Oil Red O staining at the end of adipocyte differentiation in untreated and treated cells. After incubation, cells were washed three times with PBS 1X and fixed with 4% paraformaldehyde. After 30 min at room temperature, the fixed cells were washed twice with PBS 1X and then stained using Oil Red O, diluted 6/10 in distilled water, for 20 min at room temperature. From this stage on, the cells were no longer exposed to light. Excess Oil Red O was washed with PBS 1X. Stained oil droplets in 3T3-L1 mature adipocytes and differentiated hAMSC were viewed and photographed under the optical microscope Leica DMIL at ×40 magnification. Then, quantification of lipid droplets was performed by dissolving Oil Red O with isopropanol 100% and by measuring the absorbance of the extracts at 490 nm by Victor X4 Multilabel Plate Reader (Perkin Elmer).
Oil Red O staining was performed in 3T3-L1 cells on a 6-well plate in six independent technical experiments and in hAMSC cells on a 24-well plate in one independent technical experiment including four replicates.
RNA isolation and quantitative real‑time PCR
Total RNA was extracted from differentiated 3T3-L1 cells following the same protocol previously reported [28], with the only exception regarding the use of the QIAzol reagent (Qiagen) in the current study. cDNA was generated from 1 μg RNA retro-transcribed using the RT2 First Strand Kit (Qiagen) as previously described [28]. The cDNA obtained was used for quantification of mRNA levels of all investigated genes or the main markers involved in adipogenesis (PPARγ; C/EBPα; Adiponectin; Leptin; and LPL). Cyclophilin A was used as an internal control for normalization. The primers used (Invitrogen; Thermo Fisher Scientific, Inc.) were as follows: peroxisome proliferator-activated receptor-γ (PPARγ) forward 5’-CAAGAATACCAAAGTGCGATCAA-3’ and reverse 5’-GAGCTGGGTCTTTTCAGAATAATAAG-3’; CCAAT/enhancer-binding protein-α (C/EBP-α) forward 5’-GACCATTAGCCTTGTGTGTTAC-3’ and reverse 5’-TGGATCGATTGTGCTTCAAGTT-3’; Adiponectin forward 5’-TGCCGAAGATGACGTTACTA-3’ and reverse 5’-TCTCACCCTTAGGACCAAGA-3’; Leptin forward 5’-CTGGCAGTCTATCAACAGGTC-3’ and reverse 5’-TCCACCTCTGTGGAGTAGAG-3’; LPL forward 5’-GCTCTCAGATGCCCTACAAA-3’ and reverse 5’-GATGTCCACCTCCGTGTAAA-3’; Cyclophilin A forward 5’-GCAGACAAAGTTCCAAAGACAG-3’and reverse 5’CACCCTGGCACATGAATCC-3’.
cDNAs were amplified in Power Up SYBR Green Master mix (Applied Biosystems by Thermo Fisher Scientific) using the StepOne Plus Real-Time PCR System (Applied Biosystems, Life Technologies) running as previously reported [28]. The relative expression of target genes was calculated using the comparative threshold method, 2−ΔCt correction of target and reference gene transcripts [29]. PCR was performed in 3T3-L1 cells in six independent technical experiments.
Protein extraction and western blot
Protein expression of VDR was evaluated in both cell lines during the proliferation/differentiation period at different time points. Proteins were extracted from 3T3-L1 and hAMSC cells: (1) grown with proliferation medium for 24 h; (2) grown with differentiation medium for 48 h; (3) after 4, 6, and 10 days of post-differentiation medium for 3T3-L1 and after 4, 6 and 14 days of differentiation medium for hAMSC; (4) after 4, 6 and 10 days of post-differentiation medium plus VitD 10−7 M for 3T3-L1 and after 4, 6 and 14 days of differentiation medium plus VitD 10−7 M for hAMSC. Protein expression of the main adipogenic markers was evaluated on pellets collected by treated cells at the end of 10 and 14 days of differentiation, for 3T3-L1 and hAMSC, respectively.
3T3-L1 and hAMSC cells were plated at a density of 3 × 105 in 60 mm dishes and treated with BPA and VitD. Then the cells were washed two times with PBS 1X. Then, lysis buffer (1% NP-40, 10% Glycerol, 137 mM NaCl, 20 mM Tris pH7.6, 20 mM NaF, 2 μg/mL aprotinin, 2 μg/mL leupeptin, 2 μg/mL pepstatin, 200 μM Na3VO4, 1 mM PMSF) was added and cells were stored at 4 °C for 30 min. The homogenate was centrifuged for 15 min, at 12,000 × g and 4 °C and the supernatant was collected and stored at −80 °C until use. Protein concentrations were measured using Pierce BCA Protein Assay (Thermo Scientific) and 40 μg of protein was subjected to SDS-PAGE. The loaded gel was then electroblotted onto a nitrocellulose membrane for 1 h and half in a TransBlot Amersham membrane. After a blocking treatment for 1 h with 5% of milk, the membrane was incubated with primary antibodies at 4 °C overnight and secondary antibodies at room temperature for 1 h. The specific primary antibodies were as follows: VDR (1:1000; #sc-13133, Santa Cruz), peroxisome proliferator-activated receptor-γ (PPARγ; 1:1000; #2443, Cell Signaling); CCAAT/enhancer-binding protein-α (C/EBP-α; 1:1000; #2295, Cell Signaling); Adiponectin (1:1000; #2789, Cell Signaling); Leptin (1:500; #PA1-051; Invitrogen); Lipoprotein Lipase (1:1000; # MA5-18055, Invitrogen); β-actin (1:10,000; #A5441, Sigma-Aldrich). The secondary antibodies goat anti-mouse IgG, HRP Conjugate and donkey anti-rabbit IgG, HRP Conjugate (1:2000; #GtxMu-003-DHRPX and #RbxGt-003-DHRPX, respectively) were purchased from ImmunoReagents.
The proteins were detected using the ECL chemiluminescent HRP substrate (Millipore) and imaged by ImageQuant™ LAS 4000 mini luminescent image analyzer (GE Healthcare).
WB was performed in 3T3-L1 cells in five independent technical experiments to assess adipogenic markers expression and in three independent technical experiments to assess VDR expression and adipogenic markers after VitD and miR-27 inhibitors treatment.
WB was performed in hAMSC cells in two independent technical experiments to assess adipogenic markers expression and in three independent technical experiments to assess VDR expression and adipogenic markers with and without VitD and miR-27 inhibitors treatment.
Bioinformatic miRNAs prediction
Using the algorithm Target Scan, a computational analysis was performed to find miRNA families broadly conserved among the vertebrates and targeting PPARγ both in humans and mice. The analysis revealed miR-27-3p as a modulator of the target PPARγ, thus with a potential role in adipogenesis.
miRNAs isolation and quantitative real‑time PCR
For miRNA analysis, total RNA was extracted using a miRNeasy Mini Kit (#217004; Qiagen). After treatment with BPA 10−8 and 10−9 M and VitD 10−7 M, cells were lysed in QIAzol Lysis Reagent. miRNAs isolation and RT-qPCR have been performed according to the previously used protocol [30]. After washings with buffers and centrifugations for several minutes, miRNeasy Mini spin columns were used to inhibit RNases and to remove cellular DNA and proteins from the samples. Obtained RNA samples were eluted in 30 μL of RNAse-free water. Then the total RNA was used to generate cDNAs via reverse transcription with TaqMan® MicroRNA Reverse Transcription Kit (#4366596, Applied Biosystem). cDNAs derived from the reverse-transcription reaction were pre-amplified using TaqMan® PreAmp Master Mix (#4488593, Applied Biosystem) and specific pre-formulated TaqMan® MicroRNA Assay 20X (TaqMan® probe and primer set) miR-27a-3p and miR-27b-3p (#4427975, assay ID:00408 and assay ID:00409, respectively).
Once samples were ready, RT-qPCR was performed to detect the mRNA expression levels of miR-27a-3p and miR-27b-3p using 2 µl of cDNA that was added to 10 µl of PCR mix prepared by adding 0.5 µl of TaqMan® MicroRNA assay 20X, 5 µl PCR master mix (TaqMan® Fast Advanced Master mix, #4444964, Applied Biosystem), and 4.5 µl of sterile water. The miR-27a-3p and miR-27b-3p levels were normalized to U6 small nuclear RNA (#4427975, assay ID: 001973). PCR was performed in 3T3-L1 cells in ten independent technical experiments.
Statistical analysis
The GraphPad Prism 9 software was used to perform statistical analysis. The experiments were expressed as the means and standard errors of the mean (S.E.M.). For statistical comparison, analysis of variance (ANOVA) or Student’s t tests with post hoc testing, Tukey’s multiple comparison test and Mann–Whitney test two-tailed for parametric and nonparametric data, were used. A p value < 0.05 with a 95% confidence interval (CI) was considered significant.
Results
VDR is expressed in 3T3-L1 and hAMSC cells
The analysis of protein VDR revealed that its expression was higher in undifferentiated 3T3-L1 and hAMSC and during the early stages (48 h) of adipogenesis but then its expression decreased in the further 4, 6 and 10/14 days of differentiation in both cell models (Fig. 2). Interestingly, in both cell models the presence of the natural ligand VitD maintained VDR expression through the differentiation period particularly in 3T3-L1 model (Fig. 2A) and at least day 4 of adipogenesis to further decline until the end of differentiation even in the presence of VitD in hAMSC model (Fig. 2B).
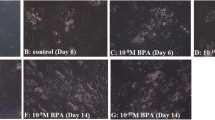
Pictures of Petri dishes and images captured at ×40 in translucence with and without Oil Red O staining with and without VitD treatment in 3T3-L1 (A) and hAMSC (B). Blots of VDR, PPARγ and Adiponectin in 3T3-L1 (A) and hAMSC (B) with and without VitD treatment. VDR protein expression in 3T3-L1 and hAMSC in proliferation, differentiation, and post-differentiation medium with and without VitD.
VitD reduces lipid accumulation and counterbalances BPA adipogenic effect in 3T3-L1 and hAMSC cells
In 3T3-L1, Red Oil O staining quantification evidenced a significant anti-adipogenic outcome (∼54%, p = 0.0022) of VitD and a significant pro-adipogenic outcome of BPA (∼29%, p = 0.047 for BPA 10−8 M and ∼42%, p = 0.047 for BPA 10−9 M) throughout the differentiation of 3T3-L1 cells compared to untreated cells. Interestingly, when cells were treated with both VitD and BPA during differentiation, VitD counterbalanced BPA pro-adipogenic effect by reducing lipid accumulation by ∼72%, p = 0.0043 (BPA 10−8 M vs. VitD + BPA 10−8 M) and of ∼76%, p = 0.0022 (BPA 10−9 M vs. VitD + BPA 10−9 M), and the co-treatment resulted in a lower but significant reduction of lipid accumulation (42.5%, p = 0.0022 (control vs. VitD + BPA 10−8 M); 34.9%, p = 0.0022 (control vs. VitD + BPA 10−9 M) compared with untreated cells (Fig. 3A).
Percentage of Red Oil O staining in A 3T3-L1 and B hAMSC treated with VitD and BPA alone and in combination during differentiation in mature adipocytes. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. C Percentage of PPARγ, C/EBPα, leptin, adiponectin, and LPL gene expression in 3T3-L1 white adipocytes. Data normalized on cyclophilin. D Percentage of PPARγ, C/EBPα, leptin, adiponectin, and LPL protein expression in 3T3-L1 white adipocytes. Data normalized on actin. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. E Protein expression of PPARγ, C/EBPα, leptin, adiponectin, and LPL in hAMSC.
In hAMSC, Red Oil O staining quantification evidenced a significant anti-adipogenic outcome (∼27%, p < 0.0001) of VitD and a significant pro-adipogenic outcome of the only one tested BPA concentration (∼8%, p = 0.0007 for BPA 10−9 M) throughout the differentiation of hAMSC cells compared to untreated cells. Interestingly, as also observed in 3T3-L1, when hAMSC cells were treated with both VitD and BPA during differentiation, VitD counterbalanced BPA pro-adipogenic effect by reducing lipid accumulation of ∼12%, p = 0.0003 (BPA 10−9 M vs. VitD + BPA 10−9 M) (Fig. 3B).
The BPA-dependent adipogenic effect was accompanied by alterations in the expression of adipogenic markers counteracted by VitD in 3T3-L1 and hAMSC cells
To characterize the effect of VitD, RT-qPCR, in 3T3-L1 cells, and WB, in both cell lines, were carried out to evaluate the expression of the main adipogenic markers, including the early transcription factors, such as PPARγ, C/EBPα, leptin, adiponectin, and LPL at the end of the differentiation process, during which 3T3-L1 and hAMSC cells were chronically treated with VitD and BPA. In 3T3-L1, the expression of most adipogenic markers was significantly induced by BPA, at both concentrations tested, and inhibited by VitD at gene and protein levels as shown in Fig. 3C, D. Interestingly, when co-treated, the expression of adipogenic markers remains downregulated, demonstrating a superimposable effect of VitD on the pro-adipogenic effect of BPA.
In hAMSC, although the observed effects of BPA and VitD were more contained than that observed in 3T3-L1, BPA 10−9 M stimulated while VitD reduced the protein expression of adipogenic markers as shown in Fig. 3E. Particularly, adiponectin and leptin were strongly reduced by VitD while PPARγ was only partially lowered. Nevertheless, even in this cell model, VitD treatment during differentiation counterbalanced the BPA pro-adipogenic effect as demonstrated by the reduced expression of adipogenic markers compared to control and BPA-treated cells.
A VitD-dependent epigenetic mechanism underpinning the regulation of adipogenesis in 3T3-L1 and hAMSC cells involves miR-27a-3p and miR-27b-3p
To further address the mechanisms supporting the anti-adipogenic effect of VitD, a potential epigenetic mechanism orchestrated by VitD was investigated in both 3T3-L1 and hAMSC cells. Bioinformatic analysis performed with Target Scan predicted PPARγ as a target of miR-27a-3p and miR-27b-3p, in both mice and humans (Fig. 4A), thus RT-qPCR was performed to evaluate both miRNA expression regulation by VitD compared with BPA in 3T3-L1 cells during adipogenesis. As shown in Fig. 4B, in 3T3-L1 the expression of miR-27a-3p and miR-27b-3p was significantly up-regulated by VitD compared to untreated cells (64.7%, p = 0.001 and 74.3%, p < 0.0001 respectively). Accordingly, to the pro-adipogenic effect of BPA, both tested concentrations significantly down-regulated miR-27a-3p (BPA 10−9 M 38.6%, p < 0.0001; BPA 10−8 M 35.6%, p = 0.013) and miR-27b-3p (BPA 10−9 M 31.1%, p < 0.0001; BPA 10−8 M 34.6%, p < 0.0001) compared to untreated cells.
A miR-27a-3p and miR-27b-3p predicted seed match in 3’UTR of PPARγ mRNA in mice and humans. B Percentage of miR-27a-3p and miR-27b-3p expression in 3T3-L1 white adipocytes treated with VitD and BPA during differentiation. *p < 0.05; **p < 0.01; ****p < 0.0001. C Percentage of Red Oil O staining in 3T3-L1 and hAMSC treated with VitD and miR-27a-3p and miR-27b-3p inhibitors during differentiation in mature adipocytes. *p < 0.05, ***p < 0.001, ****p < 0.0001. D Regulation of protein expression of adipogenic markers by VitD with and without miR-27a-3p and miR-27b-3p inhibitors in 3T3-L1 and hAMSC differentiated in white adipocytes.
Interestingly, in both cell models, the treatment with VitD was not further able to inhibit adipogenesis in the presence of miR-27a-3p and miR-27b-3p inhibitors, as demonstrated by lipid droplet accumulation (Fig. 4C). Indeed, in 3T3-L1 VitD significantly reduced lipid accumulation compared to untreated cells (57.3%, p < 0.0001) whereas, in the presence of miR-27a-3p and miR-27b-3p inhibitors, the anti-adipogenic effect of VitD was reverted (+51.0%, p = 0.003 and +75.7%, p < 0.0001 of lipid accumulation compared to VitD, respectively) (Fig. 4C). Accordingly, in hAMSC VitD significantly reduced lipid accumulation compared to untreated cells (32.9%, p < 0.0001) whereas, in the presence of miR-27a-3p and miR-27b-3p inhibitors, the anti-adipogenic effect of VitD was reverted (30.1%, p < 0.0001 and 30.4%, p < 0.0001 of lipid accumulation compared to VitD, respectively) (Fig. 4C).
In 3T3-L1 and hAMSC, the analysis of protein adipogenic markers demonstrated that the VitD treatment during adipogenesis reduced PPARγ, C/EBPα, adiponectin and leptin expression. Conversely, the effect triggered by VitD was reverted when miR-27a-3p and miR-27b-3p were blocked by inhibitors (Fig. 4D). These findings demonstrated that both miR-27a-3p and miR-27b-3p are effectors of VitD-dependent anti-adipogenic action.
Discussion
The results of the present study provide a demonstration of a novel VitD-dependent epigenetic mechanism, involving miR-27-3p, underpinning the control of adipogenesis and pro-adipogenic activity of BPA in 3T3-L1 and hAMSC models.
In the last decade, the obesogen hypothesis proposed that environmental chemicals, including EDCs, termed obesogens, promote obesity by inducing molecular cellular mechanisms in adipocytes in which commitment, differentiation and size are altered [31]. Several pieces of research, including preclinical studies on human and murine cell models, demonstrated that BPA, a lipophilic compound with estrogen mimetic activity, is an obesogen able to induce adipogenesis [32], and high BPA circulating levels are associated with obesity and obesity-related comorbidities, like type 2 diabetes, as reported in clinical studies on humans [11, 33]. Particularly, several studies demonstrated that BPA induces adipogenesis at low (micromolar and nanomolar) concentrations in 3T3-L1 [5, 7, 34,35,36,37], human primary cultures adipocytes [37, 38] and hAMSC [8, 39] and the results of the current study confirm these findings. Indeed, BPA at both tested concentrations (10−8 M and 10−9 M) increased lipid accumulation and adipogenic markers expression in 3T3-L1 and hAMSC models treated during differentiation.
The secosteroid VitD plays multiple physiological roles and is therefore known as a pleiotropic hormone. VitD and 25(OH)vitD deficiency are a frequent and common feature of people with obesity and, despite this negative association has been reported by several studies [40], a causal relationship between obesity onset and low circulating VitD levels has not been completely clarified yet. Several hypotheses for low VitD levels observed in people with obesity have been proposed: dilution of VitD in a larger body volume of distribution, VitD strongly entangled in fatty tissues, VitD reduced absorption, and nutritional regimens with low VitD. Although only partially explored, VitD orchestrates cellular mechanisms underpinning the inhibition of adipogenesis, by regulating transcription factors and adipogenic marker expression and increasing the expression of lipolytic enzymes and genes involved in β-oxidation [18, 19, 21, 41, 42]. Previous data demonstrated that in 3T3-L1 VDR is expressed in pre-adipocytes, but also the unbounded VDR is expressed early in differentiation in 3T3-L1 cells and may play a role in some aspect of the process. Contrarily, VDR nuclear expression is stable in the presence of VitD, which in turn negatively regulates adipogenesis [18]. This observation, confirmed by the results of the current study both in 3T3-L1 and in hAMSC, allowed us to postulate a protective role of VitD during adipogenesis, mainly during the first phase of adipogenesis, in the presence of pro-adipogenic factors, such as BPA. These findings could deserve further consideration due to the growing use of BPA substitutes in BPA-free products, whose obesogen effects are not sufficiently investigated [32]. A recent study investigated the role of VitD plus BPA, both inoculated during the differentiation period at the same concentrations (VitD 10−10 M plus BPA 10−10 M and VitD 10−8 M plus BPA 10−8 M) on adipogenesis in hAMSC cells [43]. The Authors explored lipid droplet accumulation and gene expression of adipogenic markers during differentiation (6 days) and at the end of the differentiation process (14 days), observing increased levels of PPARγ and C/EBPα gene expression and increased lipid droplet only when hAMSC were treated with VitD 10−8 M plus BPA 10−8 M at 6 days of differentiation. Surprisingly, although the Authors reported a significant increase in relative lipid vacuole staining after 14 days of differentiation, they did not report a concomitant rise in adipogenic markers gene expression at both concentrations tested [43]. Contrary to the results of the of Salehpour’s study, the findings of the current study demonstrated that VitD inhibits adipogenesis in mouse 3T3-L1 and human hAMSC cell models and antagonizes BPA-adipogenic effect. The discrepancies between the results could be addressed to: (1) a different VitD concentration used and (2) probably, to the different drug inoculation, which in the current study is every other day, although this difference cannot be deduced by the description of methods of Salehpour’s study.
Epigenetics has emerged as a key regulator of various physiological and pathological processes, including body weight regulation and obesity. Among the different epigenetic mechanisms, miRNAs play a crucial role in adipogenesis, by binding to specific sequences of mRNA targets involved in adipocytes differentiation process [44]. miR-27a-3p and miR-27b-3p were previously described as anti-adipogenic miRNAs acting on the regulation of PPARγ and C/EBPα expression both in mice and human fat cell models [45,46,47]. By examining the promoter regions of miR-27a-3p and miR-27b-3p, some Authors reported three putative VDR binding sites in the promoter of miR-27a-3p, and one VDR binding site in the promoter of miR-27b-3p through, which VitD enhanced miR-27a-3p and miR-27b-3p transcripts expression in human oral keratinocytes [48]. Nevertheless, the hypothesis that VitD could modulate adipogenesis through epigenetic mechanisms, particularly through miR-27a-3p and miR-27b-3p, has never been explored yet. The results of the current study demonstrated that expression of both miRNAs is up-regulated by VitD in adipocytes, and that both miRNAs are necessary for the VitD anti-adipogenic action in both 3T3-L1 and hAMSC cell models during differentiation. These findings demonstrate a novel epigenetic modulation of adipocyte differentiation by VitD, involving miR-27, during the early steps of the process.
The possible translational research questions that arise from our findings suggest the promising role of early VitD supplementation in clinical practice to prevent uncontrolled adipogenesis, mainly induced by exposure to BPA.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
World Obesity Federation. World Obesity Atlas 2023. World Obesity Federation; London, 2023.
Boutari C, Mantzoros CS. A 2022 update on the epidemiology of obesity and a call to action: as its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism. 2022;133:155217.
OECD (2019), The Heavy Burden of Obesity: The Economics of Prevention, OECD Health Policy Studies, OECD Publishing, Paris, https://doi.org/10.1787/67450d67-en.
Panuganti KK, Nguyen M, Kshirsagar RK. Obesity. Treasure Island (FL): StatPearls Publishing; 2023.
Ariemma F, D’Esposito V, Liguoro D, Oriente F, Cabaro S, Liotti A, et al. Low-dose bisphenol-A impairs adipogenesis and generates dysfunctional 3T3-L1 adipocytes. PLoS ONE. 2016;11:e0150762.
Cohen IC, Cohenour ER, Harnett KG, Schuh SM. BPA, BPAF and TMBPF alter adipogenesis and fat accumulation in human mesenchymal stem cells, with implications for obesity. Int J Mol Sci. 2021;22:5363
Ahmed S, Atlas E. Bisphenol S- and bisphenol A-induced adipogenesis of murine preadipocytes occurs through direct peroxisome proliferator-activated receptor gamma activation. Int J Obes. 2016;40:1566–73.
Salehpour A, Shidfar F, Hedayati M, Neshatbini Tehrani A, Farshad AA, Mohammadi S. Bisphenol A enhances adipogenic signaling pathways in human mesenchymal stem cells. Genes Environ. 2020;42:13.
Hoepner LA. Bisphenol A: a narrative review of prenatal exposure effects on adipogenesis and childhood obesity via peroxisome proliferator-activated receptor gamma. Environ Res. 2019;173:54–68.
Biemann R, Bluher M, Isermann B. Exposure to endocrine-disrupting compounds such as phthalates and bisphenol A is associated with an increased risk for obesity. Best Pr Res Clin Endocrinol Metab. 2021;35:101546.
Provvisiero DP, Pivonello C, Muscogiuri G, Negri M, de Angelis C, Simeoli C. et al. Influence of bisphenol A on type 2 diabetes mellitus. Int J Environ Res Public Health. 2016;13:989
Pivonello C, Muscogiuri G, Nardone A, Garifalos F, Provvisiero DP, Verde N, et al. Bisphenol A: an emerging threat to female fertility. Reprod Biol Endocrinol. 2020;18:22.
Khan NG, Correia J, Adiga D, Rai PS, Dsouza HS, Chakrabarty S, et al. A comprehensive review on the carcinogenic potential of bisphenol A: clues and evidence. Environ Sci Pollut Res Int. 2021;28:19643–63.
Savastano S, Barrea L, Savanelli MC, Nappi F, Di Somma C, Orio F, et al. Low vitamin D status and obesity: role of nutritionist. Rev Endocr Metab Disord. 2017;18:215–25.
Parikh SJ, Edelman M, Uwaifo GI, Freedman RJ, Semega-Janneh M, Reynolds J, et al. The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. J Clin Endocrinol Metab. 2004;89:1196–9.
Konradsen S, Ag H, Lindberg F, Hexeberg S, Jorde R. Serum 1,25-dihydroxy vitamin D is inversely associated with body mass index. Eur J Nutr. 2008;47:87–91.
Bennour I, Haroun N, Sicard F, Mounien L, Landrier JF. Vitamin D and obesity/adiposity-a brief overview of recent studies. Nutrients. 2022;14:2049
Blumberg JM, Tzameli I, Astapova I, Lam FS, Flier JS, Hollenberg AN. Complex role of the vitamin D receptor and its ligand in adipogenesis in 3T3-L1 cells. J Biol Chem. 2006;281:11205–13.
Kong J, Li YC. Molecular mechanism of 1,25-dihydroxyvitamin D3 inhibition of adipogenesis in 3T3-L1 cells. Am J Physiol Endocrinol Metab. 2006;290:E916–24.
Narvaez CJ, Simmons KM, Brunton J, Salinero A, Chittur SV, Welsh JE. Induction of STEAP4 correlates with 1,25-dihydroxyvitamin D3 stimulation of adipogenesis in mesenchymal progenitor cells derived from human adipose tissue. J Cell Physiol. 2013;228:2024–36.
Salehpour A, Hedayati M, Shidfar F, Neshatbini Tehrani A, Farshad AA, Mohammadi S. 1,25-Dihydroxyvitamin D3 modulates adipogenesis of human adipose-derived mesenchymal stem cells dose-dependently. Nutr Metab. 2021;18:29.
Vaughan-Shaw PG, Blackmur JP, Grimes G, Ooi LY, Ochocka-Fox AM, Dunbar K, et al. Vitamin D treatment induces in vitro and ex vivo transcriptomic changes indicating anti-tumor effects. FASEB J. 2022;36:e22082.
Yamaguchi M, Weitzmann MN. High dose 1,25(OH)2D3 inhibits osteoblast mineralization in vitro. Int J Mol Med. 2012;29:934–8.
Provvisiero DP, Negri M, de Angelis C, Di Gennaro G, Patalano R, Simeoli C, et al. Vitamin D reverts resistance to the mTOR inhibitor everolimus in hepatocellular carcinoma through the activation of a miR-375/oncogenes circuit. Sci Rep. 2019;9:11695.
Fassio A, Adami G, Rossini M, Giollo A, Caimmi C, Bixio R. Pharmacokinetics of oral cholecalciferol in healthy subjects with vitamin D deficiency: a randomized open-label study. Nutrients. 2020;12:1553
Ahmed F, Sarsenbayeva A, Katsogiannos P, Aguer C, Pereira MJ. The effects of bisphenol A and bisphenol S on adipokine expression and glucose metabolism in human adipose tissue. Toxicology. 2020;445:152600.
EFSA Panel on Food Contact Materials, Enzymes and Processing Aids, Lambre C, Barat Baviera JM, Bolognesi C, Chesson A, Cocconcelli PS, et al. Re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J. 2023;21:e06857.
Negri M, Pivonello C, Simeoli C, Di Gennaro G, Venneri MA, Sciarra F, et al. Cortisol circadian rhythm and insulin resistance in muscle: effect of dosing and timing of hydrocortisone exposure on insulin sensitivity in synchronized muscle cells. Neuroendocrinology. 2021;111:1005–28.
Pivonello C, Negri M, De Martino MC, Napolitano M, de Angelis C, Provvisiero DP, et al. The dual targeting of insulin and insulin-like growth factor 1 receptor enhances the mTOR inhibitor-mediated antitumor efficacy in hepatocellular carcinoma. Oncotarget. 2016;7:9718–31.
Pivonello C, Patalano R, Simeoli C, Monto T, Negri M, Amatrudo F. Circulating myomiRNAs as biomarkers in patients with Cushingas syndrome. J Endocrinol Invest. 2023;47:655–669.
Heindel JJ, Blumberg B. Environmental obesogens: mechanisms and controversies. Annu Rev Pharm Toxicol. 2019;59:89–106.
Varghese SV, Hall JM. Bisphenol A substitutes and obesity: a review of the epidemiology and pathophysiology. Front Endocrinol. 2023;14:1155694.
Lin MH, Lee CY, Chuang YS, Shih CL. Exposure to bisphenol A associated with multiple health-related outcomes in humans: an umbrella review of systematic reviews with meta-analyses. Environ Res. 2023;237:116900.
Pomatto V, Cottone E, Cocci P, Mozzicafreddo M, Mosconi G, Nelson ER, et al. Plasticizers used in food-contact materials affect adipogenesis in 3T3-L1 cells. J Steroid Biochem Mol Biol. 2018;178:322–32.
Masuno H, Iwanami J, Kidani T, Sakayama K, Honda K. Bisphenol a accelerates terminal differentiation of 3T3-L1 cells into adipocytes through the phosphatidylinositol 3-kinase pathway. Toxicol Sci. 2005;84:319–27.
Longo M, Zatterale F, Naderi J, Nigro C, Oriente F, Formisano P. Low-dose bisphenol-A promotes epigenetic changes at Ppargamma promoter in adipose precursor cells. Nutrients. 2020;12:3498
Valentino R, D’Esposito V, Passaretti F, Liotti A, Cabaro S, Longo M, et al. Bisphenol-A impairs insulin action and up-regulates inflammatory pathways in human subcutaneous adipocytes and 3T3-L1 cells. PLoS ONE. 2013;8:e82099.
Desai M, Ferrini MG, Jellyman JK, Han G, Ross MG. In vivo and in vitro bisphenol A exposure effects on adiposity. J Dev Orig Health Dis. 2018;9:678–87.
Ohlstein JF, Strong AL, McLachlan JA, Gimble JM, Burow ME, Bunnell BA. Bisphenol A enhances adipogenic differentiation of human adipose stromal/stem cells. J Mol Endocrinol. 2014;53:345–53.
Migliaccio S, Di Nisio A, Mele C, Scappaticcio L, Savastano S, Colao A, et al. Obesity and hypovitaminosis D: causality or casualty? Int J Obes Suppl. 2019;9:20–31.
Ji S, Doumit ME, Hill RA. Regulation of adipogenesis and key adipogenic gene expression by 1, 25-dihydroxyvitamin D in 3T3-L1 cells. PLoS ONE. 2015;10:e0126142.
Chang E, Kim Y. Vitamin D decreases adipocyte lipid storage and increases NAD-SIRT1 pathway in 3T3-L1 adipocytes. Nutrition. 2016;32:702–8.
Salehpour A, Shidfar F, Hedayati M, Farshad AA, Tehrani AN, Mohammadi S. Molecular mechanisms of vitamin D plus bisphenol A effects on adipogenesis in human adipose-derived mesenchymal stem cells. Diabetol Metab Syndr. 2021;13:41.
Wu FY, Yin RX. Recent progress in epigenetics of obesity. Diabetol Metab Syndr. 2022;14:171.
Lin Q, Gao Z, Alarcon RM, Ye J, Yun Z. A role of miR-27 in the regulation of adipogenesis. FEBS J. 2009;276:2348–58.
Karbiener M, Fischer C, Nowitsch S, Opriessnig P, Papak C, Ailhaud G, et al. microRNA miR-27b impairs human adipocyte differentiation and targets PPARgamma. Biochem Biophys Res Commun. 2009;390:247–51.
Wu H, Pula T, Tews D, Amri EZ, Debatin KM, Wabitsch M. et al. microRNA-27a-3p but not -5p is a crucial mediator of human adipogenesis. Cells. 2021;10:3205
Ge X, Yuan L, Wei J, Nguyen T, Tang C, Liao W, et al. Vitamin D/VDR signaling induces miR-27a/b expression in oral lichen planus. Sci Rep. 2020;10:301.
Funding
The study was funded by the Ministry of Education, University and Research Grants PRIN 20203AMKTW (to SS).
Author information
Authors and Affiliations
Contributions
CP conceived the study, designed the experiments, performed data analysis and interpretation, and drafted the manuscript; DPP, MN and FA performed the experiments, data analysis and prepared figures; RoPa, TM, CdeA, CG, GP, GdA performed the literature search and contributed to data analysis; AC revised the manuscript improving the scientific content; RP and SS critically reviewed and revised the manuscript for intellectual content, by relevantly improving the scientific content and the formal style.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Provvisiero, D.P., Negri, M., Amatrudo, F. et al. 1,25‑Dihydroxyvitamin D3 mitigates the adipogenesis induced by bisphenol A in 3T3-L1 and hAMSC through miR-27-3p regulation. Int J Obes (2024). https://doi.org/10.1038/s41366-024-01629-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41366-024-01629-w
- Springer Nature Limited