Abstract
Background
Pediatric long COVID remains incompletely understood with scant Australian data available. We aimed to assess the impacts of the 2021 Delta variant of SARS-CoV-2 outbreak on symptoms and functioning 12 weeks post-acute infection in a cohort of children and adolescents.
Methods
The parents/carers of 11,864 patients with PCR-confirmed SARS-CoV-2 were invited, via email or text message, to complete an online survey assessing symptoms and functional impairment.
Findings
1731 (17.6%) responded to the survey. 203 (11.7%) reported continued symptoms and/or functional impairment which were flagged for clinical review, all others reported recovery. Of the 169 subsequently clinically reviewed, 63 had already recovered (37.3%) and 17 had exacerbation of pre-existing condition(s) (10.1%); 63 (37.3%) were diagnosed with a Post COVID Condition (PCC). Of these, 21 (12.4%) were considered to have features compatible with the United Kingdom consensus cases definition for Long COVID.
Interpretation
During an outbreak of SARS-CoV-2 an online questionnaire with subsequent clinical review revealed self-reported non-recovery at 12 weeks in a minority of cases, with a spectrum of features. Long COVID comprised only a subset of cases with self-reported non-recovery, and is infrequent in children and adolescents, but still comprises a likely significant burden that warrants attention.
Impact
-
Our study provides the only comprehensive estimate of the frequency and spectrum of post-COVID conditions in children from Australia. The high frequency of self-reported recovery, and low frequency of Long COVID compatible illness adds to the literature from other settings. Risk factors for post-COVID conditions in children are identified and include: age >11 year, and previous medical co-morbidity.
Similar content being viewed by others
Background
Post-COVID-19 conditions including Long COVID remain poorly understood in children and adolescents. Systematic reviews and guidelines have highlighted potential multi-system involvement beyond the period of acute SARS-CoV-2 infection, with more than 200 symptoms reported.1,2,3 However, heterogeneity and biases across various studies have complicated interpretations of prevalence, clinical spectrum, trajectory, and burden of functional and socio-economic impacts.1,2
The pathogenesis of Long COVID is currently considered to be related to all, or any of, the following: immune dysregulation and persistent inflammatory state, autoimmunity, microbiota dysbiosis, endothelial dysfunction and a procoagulant state and dysfunctional neurological signaling, especially of the autonomic nervous system.1,4 In the absence of specific diagnostic tests, Long COVID in adults has been recognized to occur in symptom clusters albeit variably defined in different studies.2,5,6 Pre-morbid factors, such as obesity, diabetes and ethnicity (albeit confounded by socio-economic inequities), are strongly associated with severe acute COVID-19, but predictors of Long COVID including in children and young people have not been well defined.2
Long COVID is likely less frequent in children and adolescents compared with adults, and there is concern that the social and economic – sometimes called indirect effects - of the ‘Pandemic’ on the health and wellbeing of children and adolescents has been substantial and need to be accounted for in determining the burden of long COVID.7,8 Increasingly, Long COVID is being considered a sub-set of those persons with post-acute sequelae of COVID-19 (PASC) that make up a heterogenous group of post-COVID conditions (PCC). Recent publication of a consensus case definition for Long COVID in children (Supplementary Methods: Box 1) is well aligned with the subsequently published WHO case definition for children.9
There have been scant data published on PCC in children in Australia.10 Here, we aimed to determine the prevalence and describe the clinical spectrum of PCC amongst children and adolescents following the 2021 Delta variant of SARS-CoV-2 outbreak in New South Wales (NSW), Australia. NSW is Australia’s most populous state and had the largest Delta variant of concern (VoC) outbreak.
Methods
Participants
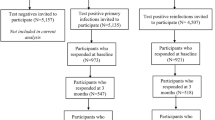
During the Delta VoC outbreak in 2021, the Sydney Children’s Hospitals Network (SCHN) supported the outpatient management of around two thirds of all notified cases of SARS-CoV-2 infection in the Australian state of New South Wales through its ambulatory care service, virtualKIDS-COVID outpatient response team (VK-CORT). Incidence of hospitalization and acute clinical spectrum of disease in children has been previously described.11 A great majority of children had mild or uncomplicated disease and were cared for in the community.11 During the outbreak, SCHN clinicians expressed a need to determine the medium-term outcome of these cases given emerging evidence at the time regarding Long COVID in children. Due to the scale of the outbreak, we devised a digital solution to facilitate outcome assessment using REDCap12 to send a questionnaire to as many cases as possible (Fig. 1).
*Eligible cases were identified in Western, South Western, and South Eastern local health districts via confirmed SARS-CoV-2 infection and referral to the SCHN virtualKIDS‐CORT (as previously described).15 A total of 38 cases of incorrect referrals were identified and removed (reasons included aged over 20 years (n = 20), false positive result (n = 16), incorrect patient matching (n = 2)). A further two children were removed due to social admission to virtualKIDS-CORT and remained test negative. Of responders 63 (3.6%) children responded for themselves; 1691 (96.1%) of responses were from a parent, guardian, or other family member (mother 1364, 81.1%; father 270, 16.1%; grandparent 21, 1.2%; sibling 12, 0.7%; aunt, uncle or other 14, 0.8%); with 5 (0.3%) interviews with one of the researchers.
Outcome measures
We developed a combined 15-minute online questionnaire based on: the International Severe Acute Respiratory and emerging Infection Consortium (ISARIC) Global Tier 1 Ongoing COVID-19 Follow Up Survey including the ISARIC symptom list; the age-specific EuroQOL questionnaire (EQ5D-Y) for health-related quality of life13; selected questions from the World Health Organization disability assessment score (WHO-DAS) for functional impairment14; and, the Kessler K615 as a screen for mental health or Parent and/or Adolescent Strengths and Difficulties Questionnaire (SDQ).16 We added at the beginning of the questionnaire Four screening questions were added to ensure assessment of outcome even where the full questionnaire was not completed (see Table 1).
The questionnaire was distributed to a panel of specialists - the ‘Long COVID clinical panel’ comprising senior clinicians from general pediatrics, infectious diseases, respiratory medicine, adolescent medicine and psychological medicine - within SCHN for review and comment and pre-determined flags for clinical concern were set. These were then set as pre-determined parameters for digital ‘flagging’ of responses in REDCap that would require clinical review (Table 1). Raised SDQ was subsequently removed as a flagging question early in the follow-up process, due to low specificity to a child’s COVID-19 illness, following a case review by a Child and Adolescent Psychiatrist (Supplementary methods).
Procedures
The REDCap based questionnaire was sent to all SCHN VK-CORT patients who tested positive for SARS-CoV-2 between July-November 2021 using a listed parent/carer email address (from 15/11/2021-10/02/2022) and/or mobile number (from 22/12/2021-18/02/2022) 12 weeks after their registration to VK-CORT (see Supplementary Methods for further detail).
Of the cases with responses showing flags for clinical concern beyond SDQ responses, a parent/carer was contacted by a pediatric clinician for a telehealth review of progress and clinical validation of any concerns. These clinical reviews were undertaken by members of a multi-specialty ‘Long COVID clinical panel’ and independently documented in clinical notes. Clinical documentation of these assessments was reviewed by a pediatric infectious diseases physician (PB) and pediatric clinical nurse consultant (EC) together and clinician assessments were pragmatically categorized into five clinical groups: Mental health issues, Pre-existing condition (exacerbated by COVID-19), Single organ symptoms/dysfunction, Persistent symptoms (multi-organ) and Post-viral fatigue. If a parent/carer reported that the symptoms they recorded in the survey had resolved, the child was categorized as ‘recovered’.
Data collation and analysis
Duplicates in combined datasets were identified, assessed, and reconciled as outlined in supplementary methods. We defined complete recovery as recovery on all four screening questions without any subsequent questionnaire variables flagging for concern. We defined those who reported non-recovery on screening questions or any subsequent flagging variables as having possible post-COVID condition. These cases were eligible for clinician contact and review. We report the proportion of reported non-recovery on the four screening questions and across multiple other variables. Amongst flagged cases reporting non-recovery, we describe the clinician assessed spectrum of disease amongst those with self-reported non-recovery. We defined clinician assessed non-recovery with symptoms not better explained by an alternative diagnosis as a Post-COVID condition (PCC). This was considered the primary outcome of the study. We considered two clinical sub-groups – post-viral fatigue and persistent symptoms (multiorgan) - to be potentially consistent with the UK consensus case definition for ‘Long COVID’ if a reviewing clinician validated the persistence and functional impact of symptoms (Supplementary Methods).
Descriptive analyses were completed using IBM SPSS Statistics (Version 25) and Microsoft Excel (2013). For nominal data frequency (n) and percentages are provided, 95% confidence intervals (CI) around proportions were calculated across a binomial distribution. For continuous data either mean and standard deviation, or median and inter-quartile range (IQR) are provided. We compared proportions of persistent symptoms comparing older and younger children (cut off 11 years).17 We calculated relative risks of non-recovery at clinician review compared with self-reported and clinician assessed recovery by demographic and clinical variables.
The study received Human Research Ethics Committee (HREC) approval with waiver of consent to contact Sydney Local health District (RPA Zone) inset HREC ID 2021/ETH11819 & X21-0370.
Results
Of 11,864 eligible children and families, 9819 (82.8%) had contact details (mobile phone number or email address) extractable from electronic medical records (2045 had blank or nonsense records). Of these, 1825 (18.6%) opened the URL to the REDCap questionnaire and 1731 (17.6%) answered one or more of the four screening questions (Fig. 1). We compared those eligible cases who responded with non-responders. The epidemiologic curve of responders and non-responders were broadly overlapping (Supplementary Fig. 1) and there was also broad similarity between the groups in terms of demographic variables and acute disease severity with some exceptions (Supplementary Table 1). The responder cohort showed a higher prevalence of fever and cough at assessment of acute infection, was slightly younger and was less likely to have a postcode of residence from more socioeconomically disadvantaged areas.
Of the responders (n = 1731), 1528 (88.3%) reported complete recovery. Of the 443 that showed responses that flagged for concern, upon review around half (n = 240, 54.2%) of these were for elevated scores on the SDQ only. The remaining 203 cases (11.7%) of responses were considered as possible post-COVID condition, see Fig. 1. The responses for pre-determined ‘flagging’ questions from the questionnaire are shown in Table 1. Persistence of COVID-19 associated symptoms was reported in 6.1% (106/1731) of participants, non-recovery to baseline health in 6.0% (104/1728), inability to return to usual (baseline) activities in 3.0% (52/1730) and a need for additional help to recover from COVID-19 in 1.7% (30/1730) of responses. Of those who reported persistent symptoms and went on to complete the full ISARIC symptom check list (n = 64), a wide spectrum of symptoms were reported with individual symptom frequencies in Fig. 2 and Supplementary Table 2, and their frequencies by system are shown in Supplementary Fig. 2. Age-related differences were evident when comparing children above and below an age cut-off at 11 years.17 All symptom groups were more frequent in older children, with loss of taste or smell, fatigue, headache, dizziness, breathlessness, abdominal pain, and nausea significantly more frequent (Fig. 2 and Supplementary Fig. 2).
Measures of the overall effect of disease showed 0.5% (8/1576) of responses rating the child’s health as ‘bad’ or worse on the WHO-DAS, 2.4% (33/1380) of responses indicating the child had missed 10 or more days of school/preschool or daycare in the preceding 30 days, and 5.1% (79/1559) of responses indicating they had ‘visited a doctor/health center because of COVID-19 health consequences’. In terms of the self-reported “Total Health Score” (maximum score possible = 100) the mean score from responders was 90.7 (IQR 89–100) prior to acute COVID-19 and 87.8 (IQR 82–100) on the day of questionnaire completion (Supplementary Table 3).
Clinical review
Of the 203 responses showing flags for clinical concern, 169 (83%) were able to be contacted by a pediatric clinician for telehealth review (34 unable to be contacted). Of these, 156/169 (92%) had sufficient clinical documentation of a pediatric assessment to support pragmatic clinical case categorization (Fig. 1 and Table 2). 63 children and adolescents showed complete recovery at clinical review despite having prior self-reported non-recovery; with a median time from questionnaire completion to clinical review being 23.2 days (IQR 15.4–47.4). We identified 63 children with evidence of a Post-COVID condition (PCC). Amongst these were children with single-organ persistent symptoms, most prominently upper or lower respiratory tract symptoms not impacting on daily function, multi-organ persistent symptoms or predominantly fatigue. There were 21 children (1.2% of responder cohort, 95% CI 0.8–1.8) with persistent symptoms and confirmed to be impacting on daily function (most often ability to attend school regularly) and so considered compatible with the UK consensus definition of Long COVID.
We compared demographics and acute illness variables between responders who self-reported recovery, and responders with self-reported concern for non-recovery stratified by the outcome of clinical review (Table 3 and Supplementary Table 4). Those children with clinician confirmed PCC were more likely to be older (relative risk 4.25 (RR), 95% CI 2.1–4.4; 12–15 years compared with <5 years) and more likely to have medical co-morbidity (RR 2.1, 95% CI 1.3–3.4). Children with PCC were also more frequently female and considered more unwell/higher risk acutely than those who self-reported recovery although this was not statistically significant.
Discussion
In this study we assessed the medium-term outcome of acute COVID-19 in children and adolescents with laboratory confirmed SARS-CoV-2 infection in metropolitan Sydney following a discrete COVID outbreak with the Delta VoC in 2021. Several key findings have emerged. A great majority of respondents showed self-reported complete recovery at 12 weeks following infection. Amongst those who reported persistent symptoms, a wide spectrum was evident, and we conclude that Post COVID conditions (PCC) in children are both identifiable and heterogeneous. Those with features compatible with the UK consensus definition for Long COVID made up only a small subset of children and adolescents with persistent symptoms. Other subsets include those with a worsening of prior medical or developmental/behavioral co-morbidities, and those with emergent and persistent respiratory symptoms (albeit mild and without significant impact on daily function).
Reported prevalence estimates of PCC in children and adolescents have varied considerably with variability dependent on definitions used, location, presence or absence of controls groups.7,8 One systematic review of symptom prevalence in controlled follow-up studies (>4 weeks following COVID-19) showed significant risk differences (difference in symptom prevalence between cases of SARS-CoV-2 infection and controls without known SARS-CoV-2 infection) of 2–8% for individual symptoms including loss of smell, headaches, cognitive difficulties, sore throat and sore eyes; fatigue risk difference was 7% but not statistically significant.18 In the two included studies examining combinations of symptoms, the risk differences for 3 or more symptoms were 5% and 14%. Another, more recent, systematic review showed of 6 studies reported the prevalence difference was less than 4%.11 In these reviews, several studies reported symptom prevalence >4 weeks from infection, and only a minority in those only >12 weeks from infection. A large, national study from Denmark, not included in these prior reviews, reported a significantly higher prevalence of at least one symptom persisting more than 2 months in children and adolescents following confirmed SARS-CoV-2 infection when compared with controls. The difference was greatest in the youngest children; 0–3 years 12.8% difference, 4–11 years 4.4%, and 12–14 years 4.7%.19 The impact of vaccination on Long COVID prevalence and the effect newer variants have not been evaluated in children. We note emerging evidence that vaccination reduces prevalence, that is also potentially reduced following Omicron VoC infection.20
The accuracy of our prevalence estimates of PCC is limited by the response rate to our questionnaire and the absence of a non-infected control group in our study. However, our estimate for any persistent symptoms of concern of 6%, symptoms impacting on function of 3% and Long COVID (UK consensus definition compatible) of 1.2% are relatively low compared to other uncontrolled studies in the published literature and align well with those estimates from controlled studies.7,18,19 Further, our responder cohort showed broad similarity across measured demographic variables with our non-responder cohort. Where differences were evident in medical comorbidity and socioeconomic status, the current literature suggests these factors are likely to be associated with an increased risk of PCC.2,18 We therefore consider it likely that any bias introduced by these differences would result in an overestimation of PCC prevalence rather than underestimation.
There are few studies to help determine the risk factors for and overall severity of PCC in children and adolescents. Older age, female sex, and medical comorbidity have been associated with increased risk of persistent symptoms in two systematic reviews.2,18 Our study adds support to older age and medical co-morbidity being risk factors for PCC, and female sex was over-represented in children clinically considered to have Long COVID.
Few studies have quantified impact of persistent symptoms post COVID in children on functioning or measures of HRQoL. In a Danish study,19 differences in health-related quality of life (PedsQL) scores were seen in younger children with worse physical symptom scores in confirmed SARS-CoV-2 infected children than in uninfected controls. In a population based Norwegian study,21 children (aged <16 years) following SARS-CoV-2 infection showed a relative increased use of primary health services but not specialist outpatient or hospital services compared with uninfected controls for 3 months. The effect was largest and lasted longest (to 6 months) in children aged 1–5 years. In our study, the relative difference between children reporting persistent symptoms and those in whom it was reported to impact on function or result in seeking additional health assessment adds support to the view that a considerable proportion of PCC in children are mild. In the children clinically reviewed, a majority had already recovered or reported single-organ symptoms which were not impacting on daily activities including return to school. The data from Magnusson et al.21 also suggest a natural history of PCC of recovery over a 6 month period, although the longitudinal course of PCC needs further study. Recently published results from a longitudinal analysis of symptom prevalence from the UK CLoCK study showed that a majority of measured symptoms reduced in prevalence between 3 and 6 months post COVID suggesting a trajectory to recovery of PCC is likely across this time period.22
Our data suggest that Long COVID is a debilitating, multi-system illness, that is experienced by only a minority of children with persistent symptoms 12 weeks following acute COVID-19, determined by a survey/questionnaire approach. We identified a relatively greater number of children with single symptom, mainly respiratory, upper and lower tract, persistent problems including loss of taste/smell. We acknowledge that some would consider these cases also to be Long COVID, and we support addressing the needs of these children beyond the consideration of Long COVID specifically as determined in our study. We have also shown evidence of COVID-19 exacerbating the symptoms of pre-existing medical, developmental-behavioral and mental health conditions in children. We cannot determine whether these effects are direct or indirect, although the impression of the clinicians participating in this work was that it was more likely to have arisen indirectly from the disruption of the COVID-19 pandemic more broadly or the disruptions to the child/family from the isolation requirements of the acute illness. This additional disease burden may be attributed directly to COVID-19 from the perspective of patients/families and the wider community.
Although children on clinical review who met the clinical definition of Long COVID only represent a small proportion of our responder cohort, this proportion potentially constitutes a large number of children where population wide infection occurs. Serosurvey data from Australia showed that the only ~2% of the child population showed serological evidence of infection following the Delta VoC wave23; however a recent survey performed after 7 months of Omicron VoC transmission showed 60–80% of children had been infected (see: 2022 serosurvey summary report at https://ncirs.org.au/reports). Although we do not know whether PCC following Omicron VoCs are similar in frequency to Delta in our setting, the absolute number of infections indicates a need for scalable person-centered interventions is now more pressing. Health services need to develop pathways to support screening of children for non-recovery and provide better guidance for primary care services to support detection, initial case management and improved pathways to escalation of care and thereby provided tailored treatment strategies to deliver the right care at the right time. At present, Australia lacks a comprehensive approach to collect data on PCC across the lifespan. Without such data evaluation of the prevalence and severity of PCC with current and future variants in the Australian context will be hampered, as well as a more comprehensive evaluation of long-term outcomes and the burden on the health system. Also at present, the management of PCC, including Long COVID, is empiric by analogy with other causes of post-viral fatigue states. Without better case identification and tracking in Australia, implementation of interventional studies will not be possible.
Our study has several strengths: all cases of SARS-CoV-2 infection were laboratory confirmed by PCR testing in a context of very high-test frequency at a population level. Furthermore, prior to the Delta VoC outbreak infection rates in children generally in New South Wales had been extremely low with COVID-19 well controlled, potentially allowing for better assessment of the direct effect of COVID-19 itself on post-COVID symptoms, rather than indirect pandemic effects. The follow-up questionnaire included explicit evaluation of responses relative to the child’s acute SARS-CoV-2 illness and all cases had been managed during their acute infection through a structured ambulatory care model.11 Our study sought responses from all aged children; the inclusion of children aged <5 years has been identified as a gap in the already limited literature on PCC in children.24,25 In addition to a standardized symptom list, our questionnaire collected data on unmet healthcare need, HRQoL and school attendance. An additional strength was the clinician review and validation of responses amongst >80% of cases who were flagged for symptoms of concern, and the application of the UK consensus case definition for long COVID in children.
Despite these strengths, our study has several limitations. Firstly, we did not have a control group of either SARS-CoV-2 test-negative children or children with another respiratory virus. This is a well-recognized issue in the field, but again we point to the relatively low prevalence of persistent symptoms identified in our responder cohort that aligns with risk differences reported in controlled studies. Secondly, our survey response rate was <20%. Again, this is a recognized weakness of other questionnaire-based studies in the field - the 3-month response rate in the UK CLoCK study was 13.4%, but we point to the broad similarity of our responder cohort with the measured characteristics of the non-responder cohort. Thirdly, amongst respondents, beyond the initial screening questions, the full questionnaire was completion was variable. Fourthly, the questionnaire was only available in English that may have impacted on the capacity for children and families from culturally and linguistically diverse backgrounds to respond. Lastly, the clinical review was undertaken via telehealth in almost all cases and clinical categorization was pragmatic and post-hoc.
In conclusion, we have described a low estimated prevalence, but broad spectrum and severity of post-COVID conditions at >12 weeks following predominantly mild SARS-CoV-2 infection in children aged <16 years.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available under human research ethics committee guidance but are available from the corresponding author on reasonable request.
References
Nalbandian, A. et al. Post-acute COVID-19 syndrome. Nat. Med. 27, 601–615 (2021).
Michelen, M. et al. Characterising long COVID: a living systematic review. BMJ Glob. 6, 09 (2021).
Lopez-Leon, S. et al. Long-COVID in children and adolescents: a systematic review and meta-analyses. Sci 12, 9950 (2022).
Davis, H. E., McCorkell, L., Vogel, J. M. & Topol, E. J. Long COVID: major findings, mechanisms and recommendations. Nat. Rev. Microbiol 21, 133–146 (2023).
Kenny, G. et al. Identification of Distinct Long COVID Clinical Phenotypes Through Cluster Analysis of Self-Reported Symptoms. Open Forum Infect. Dis. 9, ofac060 (2022).
O’Mahoney, L. L. et al. The prevalence and long-term health effects of Long Covid among hospitalised and non-hospitalised populations: A systematic review and meta-analysis. EClinicalMedicine 55, 101762 (2023).
Zimmermann, P., Pittet, L. F. & Curtis, N. How Common is Long COVID in Children and Adolescents? Pediatr. Infect. Dis. J. 40, e482–e487 (2021).
Zimmermann, P., Pittet, L. F. & Curtis, N. The Challenge of Studying Long COVID: An Updated Review. Pediatr. Infect. Dis. J. 41, 424–426 (2022).
Stephenson, T. et al. Long COVID (post-COVID-19 condition) in children: a modified Delphi process. Arch. Dis. Child 107, 674–680 (2022).
Howard-Jones, A. R. et al. COVID-19 in children. II: Pathogenesis, disease spectrum and management. J. Paediatr. Child Health 58, 46–53 (2022).
Williams, P. et al. COVID-19 in New South Wales children during 2021: severity and clinical spectrum. Med J. Aust. 217, 303–310 (2022).
Harris, P. A. et al. Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 42, 377–381 (2009).
Wille, N. et al. Development of the EQ-5D-Y: a child-friendly version of the EQ-5D. Qual. Life Res. 19, 875–886 (2010).
Ustun, T. B. et al. Developing the World Health Organization Disability Assessment Schedule 2.0. Bull. World Health Organ 88, 815–823 (2010).
Mewton, L. et al. The psychometric properties of the Kessler Psychological Distress Scale (K6) in a general population sample of adolescents. Psychol. Assess. 28, 1232–1242 (2016).
Goodman, R. The Strengths and Difficulties Questionnaire: a research note. J. Child Psychol. Psychiatry 38, 581–586 (1997).
Stephenson, T. et al. Long COVID and the mental and physical health of children and young people: national matched cohort study protocol (the CLoCk study). BMJ Open 11, e052838 (2021).
Behnood, S. A. et al. Persistent symptoms following SARS-CoV-2 infection amongst children and young people: A meta-analysis of controlled and uncontrolled studies. J. Infect. 84, 158–170 (2022).
Kikkenborg Berg, S. et al. Long COVID symptoms in SARS-CoV-2-positive adolescents and matched controls (LongCOVIDKidsDK): a national, cross-sectional study. Lancet Child Adolesc. Health 6, 240–248 (2022).
Antonelli, M. et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. Lancet Infect. Dis. 22, 43–55 (2022).
Magnusson, K. et al. Healthcare use in 700 000 children and adolescents for six months after covid-19: before and after register based cohort study. BMJ 376, e066809 (2022).
Pinto Pereira, S. M. et al. Natural course of health and well-being in non-hospitalised children and young people after testing for SARS-CoV-2: a prospective follow-up study over 12 months. Lancet Reg. Health Eur. 25, 100554 (2023).
Koirala, A., Gidding, H. F., Vette, K., Macartney, K. & Group, P. S. The seroprevalence of SARS-CoV-2-specific antibodies in children, Australia, November 2020 - March 2021. Med. J. Aust. 217, 43–45 (2022).
Zimmermann, P., Pittet, L. F. & Curtis, N. Long covid in children and adolescents. BMJ 376, o143 (2022).
Franco, J. V. A. et al. Long-Term Health Symptoms and Sequelae Following SARS-CoV-2 Infection: An Evidence Map. Int. J. Environ. Res. Public Health 19, 11 (2022).
Acknowledgements
We acknowledge the many clinicians (nursing, medical and allied health) who contributed to the acute care of children with SARS-CoV-2 infection under the auspices of the SCHN virtualKIDS COVID‐19 outpatient response team. New South Wales Health COVID-19 Emergency Response Priority Research Funding, 2021.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
P.N.B., R.D., and A.B. conceptualized and designed the study. P.N.B., R.B., E.C., J.B., and A.B. carried out the data collection and extraction. P.N.B., S.A., T.B., J.D.-P., K.K., C.L., B.Mc., B.M., M.P., J.N., H.S., and R.D. conducted clinical review of cases. P.N.B. and E.C. clinically categorized cases. P.N.B., R.B., and A.B. conducted initial analyses. P.N.B. and A.B. interpreted the data. P.N.B., R.B., and A.B. drafted the initial manuscript. All authors contributed to review of the manuscript. P.N.B., R.B., and A.B. contributed to manuscript revision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
The study received Human Research Ethics Committee (HREC) approval with waiver of consent to contact Sydney Local health District (RPA Zone) inset HREC ID 2021/ETH11819 & X21-0370.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Britton, P.N., Burrell, R., Chapman, E. et al. Post COVID-19 conditions in an Australian pediatric cohort, 3 months following a Delta outbreak. Pediatr Res (2024). https://doi.org/10.1038/s41390-024-03492-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-024-03492-x
- Springer Nature America, Inc.