Abstract
Background
Methylmalonic acidemia (MMA) is the most common organic acidemia in China, with cblC (cblC-MMA) and mut (mut-MMA) being the predominant subtypes. The present study aimed to investigate the prognostic manifestations and their possible influence in patients with these two subtypes.
Methods
A national multicenter retrospective study of patients with cblC-MMA and mut-MMA between 2004 and 2022 was performed. We compared the clinical features between patients with two subtypes or diagnosed with or without newborn screening (NBS) and further explored the potentially influential factors on the prognosis.
Results
The 1617 enrolled MMA patients included 81.6% cblC-MMA patients and 18.4% mut-MMA patients, with an overall poor prognosis rate of 71.9%. These two subtypes of patients showed great differences in poor prognostic manifestations. The role of NBS in better outcomes was more pronounced in cblC-MMA patients. Predictors of outcomes are “pre-treatment onset”, “NBS”, variants of c.80A > G and c.482G > A and baseline levels of propionylcarnitine and homocysteine for cblC-MMA; “pre-treatment onset”, “responsive to vitB12”, variants of c.914T > C and baseline propionylcarnitine and propionylcarnitine/acetylcarnitine ratio for mut-MMA. Besides, prognostic biochemical indicators have diagnostic value for poor outcomes in mut-MMA.
Conclusions
The study provided potential predictors of the long-term outcome of patients with cblC-MMA and mut-MMA.
Impact
-
Predictors of outcomes are “pre-treatment onset”, “NBS”, MMACHC variants of c.80A > G and c.482G > A and baseline propionylcarnitine and homocysteine for cblC-MMA, “pre-treatment onset”, “responsive to vitB12”, MMUT variants of c.914T > C and baseline propionylcarnitine and propionylcarnitine/acetylcarnitine ratio for mut-MMA.
-
This study with larger sample sizes effectively validated the prediction power and emphasized the importance of NBS in improving the outcomes of both MMA subtypes.
-
The study enhances understanding of the phenotypic and prognostic variations of MMA disease and the predictors will help in the improvement of diagnosis and treatment strategies to achieve a better prognosis for MMA.
Similar content being viewed by others
Introduction
Methylmalonic acidemias (MMA) comprise rare inborn metabolic errors that are mainly inherited in autosomal recessive mode.1,2 The global average incidence of MMA is about 1:100,000 and ranges from 1:3500 to 1:39,000 in China.3,4 MMA is caused by disorders of methylmalonyl-CoA mutase (MCM) or cobalamin metabolism and is characterized by the abnormal accumulation of propionylcarnitine (C3), methylmalonic acid, methylcitrate (MCA) and other metabolites, which often leads to multiorgan damage.5,6 The clinical phenotypes of MMA are variable and atypical, with onset ranging from prenatal to adulthood and clinical symptoms ranging from minor to life-threatening.7,8
According to whether homocysteinemia (Hcy) is combined biochemically, MMA can be divided into combined and isolated types.1 In China, 60%–80% of MMA cases are combined, with the cblC subtype (caused by MMACHC gene mutations, cblC-MMA) predominant (95%) and MMUT gene mutations (mut-MMA) cause 90% of the isolated MMA population.9,10 Both subtypes share some clinical symptoms including feeding difficulties, hypotonia, failure to thrive, acidosis, anemia and cognitive impairments.1, 6,11,12 However, mut-MMA cases are more likely to experience more serious clinical manifestations like acute decompensation and metabolic stroke, chronic kidney disease, and the urine MMA (uMMA) level is higher than that of cblC-MMA children, whereas cblC-MMA patients are more susceptible to hydrocephalus and microangiopathy-related manifestations (i.e., atypical hemolytic uremic syndrome), and retinopathy.13,14,15,16,17,18,19 However, comparative studies of the long-term prognosis between these two subtypes were rarely seen.
The current standard therapy for MMA patients depends on vitamin B12 (VitB12) responsiveness. It has been reported that cblC-MMA are responsive, requiring l-carnitine, betaine, folic acid and intramuscular injections of VitB12, while most mut-MMA patients are VitB12 unresponsive and need a protein-restricted diet and l-carnitine.20,21,22 The tandem mass spectrometry (MS/MS)-based newborn screening (NBS), facilitating early diagnosis and treatment initiation, appeared to have protective effects on the MMA prognosis, especially for the cblC subtype.6, 23, 24 Nevertheless, the prognostic benefit of NBS for the mut-MMA remains controversial.25 In addition, despite improved screening and treatment, some patients still face poor long-term outcomes, including intellectual disabilities, movement disorders, language impairment, ocular complications and even death.6, 23, 26, 27 The long-term outcome for MMA individuals in China remains to be elucidated.
In this national multicenter retrospective study, based on the medical records of a large MMA cohort, we analyzed clinical features and the influence of NBS on two MMA subtypes. Further, we investigated the impact of different factors on disease prognosis.
Methods
Patients
A total of 1617 MMA patients who underwent genetic analysis were diagnosed and treated at multiple Chinese hospitals from January 2004 to December 2022. Among them, 1319 cases were caused by the MMACHC gene variations and 298 cases were caused by the MMUT gene variations. All of these patients were followed up at least once and included in our study. This study has been in accordance with the Helsinki Declaration and was approved by the Ethics Committee of Xinhua Hospital, Shanghai Jiaotong University School of Medicine (Approval ID: XHEC-D-2023-142).
Biochemical examination
Dried blood spots (DBSs) were collected and the levels of amino acids, free carnitine, and acylcarnitines were analyzed using API4000/API4500 MS/MS (Applied Biosystems, Foster City, CA). Urinary organic acids including methylmalonic acid (uMMA) and methylcitric acid (uMCA) were measured by QP2010/QP2020 gas chromatography–mass spectrometry (GC–MS, Shimadzu Limited, Kyoto, Japan). Plasma Hcy was detected using a fluorescence polarization immunoassay. Furthermore, routine blood and urine tests were performed to assess liver, renal and cardiac function. In the study, the baseline levels of characteristic metabolites are from the test results at the time of diagnosis; the levels after treatment are from the best test result during the follow-up.
Genetic testing
Genomic DNA was extracted from peripheral blood using Qiagen Blood DNA Mini Kits (Qiagen, Hilden, Germany). Sanger sequencing or next-generation sequencing were performed and variations were identified using reference sequences from Genbank (MMACHC: NM_015506; MMUT: NC_000006.12). For the variants that were not recorded in the Human Gene Mutation Database, the ClinVar Database (https://www.ncbi.nlm.nih.gov/clinvar/) or previous literature, the pathogenicity analysis of these variants was performed according to the American College of Medical Genetics and Genomics and the Association for Molecular Pathology (ACMG-AMP) guidelines.28 Patients with other variant genes associated with MMA were excluded from this study.
Treatment
Totally 1418 patients underwent a VitB12 loading test as previously reported.9 A reduction of over 50% in the blood C3/acetylcarnitine (C2) ratio or uMMA level following VitB12 treatment was regarded as complete responsiveness.22 A decrease of 50%–30% was considered partially responsive.29 Other cases were considered VitB12 unresponsive. In this study, 1295 patients (1189 with cblC-MMA and 106 with mut-MMA) who were completely or partially responsive to VitB12 treatment were regarded as VitB12 responsive.
The cblC-MMA and VitB12 complete responsive mut-MMA patients received treatment with hydroxocobalamin (1–20 mg, once every 1–20 days based on age, weight, and condition, intramuscular injection), levocarnitine (50–100 mg/kg/day, oral), betaine (50–100 mg/day, oral) and folinic acid (5–15 mg/day, oral). VitB12 partially responsive mut-MMA patients were treated with hydroxocobalamin (1 mg, once every 2–7 days), levocarnitine, and a protein-restricted diet limiting isoleucine, valine, threonine and methionine. Unresponsive cases were restricted to natural proteins, supplemented with a specially formulated nutritional powder and received levocarnitine. Blood amino acid and carnitine profiles, especially branched-chain and essential amino acids, were monitored to guide diet and drug therapy.30,31,32 Additionally, four mut-MMA patients underwent liver transplants.
NBS
DBSs were taken 72–120 h after birth and were immediately sent for MS/MS examination.33 Newborns with positive results (C3/C2 > 0.2 and/or C3 > 4.0 μmol/L) in the initial screening were recalled and re-examined. The still-positive individuals were referred for confirmatory tests including GC–MS (uMMA and uMCA), genetic testing and examination of their Hcy level. Patients who underwent NBS (including those who developed symptoms during the screening process) were enrolled in the NBS group, and all patients were treated immediately after diagnosis. Patients who refused to receive NBS, developed disease before NBS, or were confirmed by NBS but refused treatment were included in the non-NBS group.
Follow-up and prognostic evaluation
MMA patients were followed monthly initially, later extending to 3 months when stable. Follow-up methods included telephone surveys and in-person patient visits. During telephone surveys, parents supplied information on their children’s height, weight, motor, speech and cognitive development during telephone surveys. During follow-up visits, outpatients were evaluated by experienced pediatric clinicians.
The prognosis was evaluated according to the biochemical results and development status, which were categorized as poor or good. A good prognosis means no onset or significant abnormalities at the last visit. Poor outcomes included neurologic disorders, ocular complications, renal diseases, anemia, death and other abnormalities not individually counted (heart lesions, etc.).30 “Ocular complications” manifest as fixate inability, strabismus, nystagmus, and various ocular abnormalities such as maculopathy, retinopathy and optic atrophy. “Nephropathy” was primarily diagnosed through urine routine, renal function tests and renal ultrasound. “Anemia” mainly relied on routine blood tests. The “neurologic disorders” were based on the basic motor and language function assessed as previously described,23, 34 neuropsychological tests and cranial magnetic resonance imaging (MRI). The developmental quotient and intelligence quotient determined by neuropsychological tests followed a reported protocol.9 MRI abnormalities included hydrocephalus (ventriculomegaly, severe cerebral parenchyma atrophy and subdural effusion), periventricular white matter changes, corpus callosal thinning, myelination delay and basal ganglionic abnormalities.
Statistical analysis
SPSS 26.0 (SPSS Inc., Chicago, IL) and GraphPad Prism Software 7.0 (Dotmatics, Boston, MA) were used for statistical analyses. Analyses using the Shapiro–Wilk method indicated a non-normal distribution for continuous variables, so the values are expressed as the interquartile range (IQR) and the differences between two groups were analyzed by a two-tailed unpaired or paired Student’s t-test with a Mann–Whitney U test. The differences between three or more groups were analyzed using Kruskal–Wallis one-way ANOVA and the post hoc Tukey–Kramer test. The comparison of rates used a two-tailed Fisher’s exact test with or without the Benjamini–Hochberg correction. A p value of <0.05 was considered statistically significant.
Results
Demographics and clinical features of enrolled patients
A total of 1617 MMA patients (900 males and 717 females) were enrolled in this study, which included 1319 (81.6%) cblC-MMA cases and 298 (18.4%) mut-MMA cases. Demographic, clinical and biochemical data are presented in Tables 1, 2 and Fig. S1. Among them, 1330 patients experienced onset, from minutes after birth to 32.3 years, and there are 21 cases in which the onset of symptoms was unknown. A total of 1586 patients provided information on whether they participated in the extended NBS program. Of the 670 patients who were diagnosed through NBS, 252 were asymptomatic and 206 experienced onsets before treatment (Fig. S1). Of the 916 patients who were not screened, 899 (98.11%) were diagnosed symptomatically, and 20 (21.8%) were confirmed via sibling diagnosis, including 13 asymptomatic patients (Fig. S1). Among the 1418 patients with treatment information, 1295 (91.3%) responded to VitB12 treatment, 942 of the 1220 patients (77.2%) had developed disease prior to treatment and 198 patients lost treatment onset information (Table 1, Fig. S1). All patients were followed up at least once, of whom 455 (28.1%) had normal development, while 1162 (71.9%) had poor outcomes, of which 135 (11.6%) were deceased. The median follow-up period was 24.1 months (IQR = 11.3–46.8 months). The overall levels of characteristic metabolites (blood C3, C3/C2 ratio, Hcy, uMMA and uMCA) before and after treatment are presented in Table 2, which shows that all indicators have improved after treatment.
Comparison of clinical features in cblC-MMA and mut-MMA patients
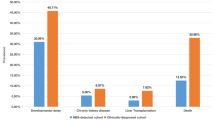
The clinical features of patients with two major MMA subtypes, cblC-MMA and mut-MMA, were first compared (Tables 1, 2). The median age of disease onset in the mut-MMA group was significantly younger than it was in the cblC-MMA group. As for the treatment, the VitB12-responsive rate was 100% in the cblC-MMA group, while it was only 46.3% in the mut-MMA group. For the prognosis, the two groups had a comparable proportion of good outcomes but a variable distribution of poor outcome manifestations. In patients with poor prognosis, the rate of death and anemia in the mut-MMA group was much higher, while the proportions of ocular complications and neurologic disorders including language impairment and hydrocephalus were statistically higher in the cblC-MMA group (Table 1). Biometabolically, the cblC-MMA group had lower blood C3, C3/C2 ratio, uMMA and uMCA before and after treatment compared to the mut-MMA group (Table 2). The above results show that mut-MMA has worse clinical symptoms and a worse prognosis than cblC-MMA.
The influence of NBS on the clinical features of cblC-MMA and mut-MMA patients
Given that the clinical characteristics of individuals with cblC-MMA and mut-MMA differ, the effect of NBS on both groups was further evaluated (Tables 2, 3). The NBS group demonstrated a younger age at onset and lower proportions of pre-treatment onset and onset of symptoms when compared to the non-NBS group for both MMA subtypes (Table 3). Besides, NBS shortened the onset-to-treatment interval and decreased the early onset rate in cblC-MMA patients rather than mut-MMA patients. For prognosis, NBS reduced the proportions of death and anemia among cblC-MMA patients with poor prognosis and improved MRI results among mut-MMA patients (Table 3). In terms of characteristic metabolites, NBS significantly decreased levels of all markers after treatment in both subtypes, with mut-MMA NBS cases also showing lower pre-treatment levels (Table 2). The above indicates that NBS could improve the overall prognosis of MMA patients.
Gene variants identified in cblC-MMA and mut-MMA patients
All patients involved in this study were ascertained by genetic analysis. Among 1319 cblC-MMA patients, 244 (18.5%) carried homozygous variants, 1051 (79.7%) carried compound heterozygous variants and 24 (1.8%) carried mono-heterozygous variants (Table S1). Totally 105 MMACHC variants were identified, including 35 missense, 21 nonsense, 6 splicing, 27 frameshift, 4 exon deletions, 12 small deletions and 28 variants were novel (Table S1). The five most frequent variants were c.609G > A (43.4%), c.658_660delAAG (10.0%), c.80A > G (8.0%), c.482G > A (6.1%) and c.567dupT (5.8%, Fig. 1 and Table S1), and the allele of c.609G > A was found in all top 5 most common combinations, another allele included c.609G > A (15.2%), c.658_660delAAG (9.9%), c.80A > G (8.7%), c.567dupT (5.2%) and c.217C > T (4.0%). Of the 298 mut-MMA patients, 16 (5.4%) carried homozygous variants, 276 (92.6%) carried compound heterozygous variants and 6 (2.0%) carried mono-heterozygous variants (Table S2). There were 159 variants identified in the MMUT gene, including 35 missense, 22 nonsense, 13 splicing, 19 frameshift, 2 exon deletions and 3 small deletions (Table S4). The c.729_730insTT (11.9%), c.1106G > A (6.0%), c.323G > A (6.0%), c.914T > C (4.8%) and c.1663G > A (4.1%) compromised the five most common variants (Fig. 1 and Table S2). The homozygous c.729_730insTT (2.0%), c.729_730insTT with c.1663G > A (2.0%), c.1106G > A (1.7%), c.323G > A (1.3%) and c.1106G > A with c.1663G > A (1.3%) were the top 5 common allele combinations.
Factors affecting variable outcomes
Both cblC-MMA and mut-MMA patients were categorized into two groups based on whether they had poor outcomes and detailed abnormalities such as anemia, ocular complications, nephropathy, neurologic disorders (language impairment, motor disturbance, abnormal neuropsychological test results and abnormal MRI results) and death. Factors including “NBS”, “Pre-treatment onset”, “Responsive to VitB12 treatment”, biochemical metabolites at baseline and the top 5 most common variants in the MMACHC or MMUT genes. The differences in the incidence of these factors between poor outcome and control groups in patients with two subtypes are summarized in Tables S3–S6, and based on these, multivariate logistic regression was performed to explore factors independently associated with various prognostic phenotypes.
For cblC-MMA patients (Fig. 2), multivariate analysis revealed that “pre-treatment onset” (OR = 8.62–26.7) and “NBS” (OR = 0.03–0.42) were independent predictors of nearly all analyzed poor prognostic manifestations except death and anemia. A higher Hcy level was independently associated with a higher risk of anemia (OR = 1.01), and a higher baseline level of C3 could independently increase the risk of having an overall poor prognosis (OR = 1.11) and neurologic disorders (OR = 1.14), especially abnormal results of MRI (OR = 1.11) and neuropsychological tests (OR = 1.18). Moreover, the MMACHC hotspot variant of c.482G > A was a negative predictor of almost all poor prognostic phenotypes except anemia (OR = 0.06–0.22) and the variant of c.80A > G could independently decrease the risk of ocular complications (OR = 0.09), language impairment (OR = 0.35) and motor disturbance (OR = 0.45).
For mut-MMA patients (Fig. 3), “pre-treatment onset” (OR = 8.54–37.25) and “responsive to VitB12 treatment” (OR = 0.19–0.25) were independent predictors of overall poor prognosis and neurologic disorders (especially language impairment and motor disturbance). Higher levels of baseline C3 and C3/C2 independently predicted a higher risk of death (OR = 1.05) and anemia (OR = 99.3), respectively. Moreover, the MMUT hotspot variant of c.914T > C (OR = 14.2) was a significant predictor of death.
Metabolite levels after treatment and their diagnostic value for prognostic manifestations
Levels of prognostic metabolite indicators can assess the extent of recovery after treatment. Generally, patients with poor prognosis had significantly higher levels of prognostic metabolite markers when compared to patients without corresponding symptoms, and the mut-MMA group had higher marker levels than the cblC-MMA group (Table S4). However, for some poor outcomes like death, anemia and ocular complications, there was little difference in indicator levels between the groups with and without poor phenotypes for both MMA subtypes. Moreover, ROC curves illustrated that levels of all four characteristic metabolites after treatment had moderate diagnostic value for various poor outcomes in mut-MMA patients, such as C3 for overall poor prognosis (area under the curve [AUC] = 0.763, Fig. 4a); C3/C2 and C3 for abnormal neuropsychological test results (AUC = 0.722 and 0.737, Fig. 4b); uMMA, C3 and C3/C2 for motor disturbance (AUC = 0.714, 0.766 and 0.772, Fig. 4c); and uMCA, uMMA, C3 and C3/C2 for anemia (AUC = 0.726, 0.704, 0.750 and 0.768, Fig. 4d), neurologic disorders (AUC value = 0.718, 0.755, 0.780 and 0.779, Fig. 4e) and abnormal MRI results (AUC value = 0.772, 0.815, 0.827 and 0.829, Fig. 4f). However, none of the prognostic metabolic indicators has diagnostic value for poor prognosis in cblC-MMA patients (AUC < 0.7).
Discussion
The long-term prognosis of MMA patients varies from normal to life-threatening and is influenced by multiple factors. In this national multicenter retrospective study with one of the largest Chinese MMA cohorts, we provided rich and reliable information on prognostic manifestations and their possible influence of the two major subtypes in combined and isolated MMA, cblC and mut, in mainland China.
The Chinese MMA population exhibits distinct characteristics, mainly due to the different subtype distribution and genetic variation spectrum compared to studies from America and Europe. In contrast to many Western countries where MMA is predominantly of the isolated type, the combined type accounts for 60%–80% of cases in China.35, 36 Furthermore, the variation hotspots of MMACHC and MMUT also differ greatly between China and other countries. For instance, the most prevalent MMACHC variants were c.609G > A in China,37 while c.271dupA and c.331C > T occurred predominantly in European and American populations and c.394C > T was frequent in patients from the Middle East and India.38, 39 The c.729_730insTT in the MMUT gene had the highest frequency in our study, which contrasts with variants like c.322C > T in Spanish, c.349G > T in Japanese and c.655A > T in French.40 It is noteworthy that the incidence of MMA in China exceeds the global average. A detailed analysis of the prognosis of MMA patients in China will help to make more specific recommendations for the management of our patients.
The MMA prognosis depends largely on the subtypes. Despite similar poor prognosis rates of around 70%, mut-MMA patients have a markedly higher mortality rate and higher levels of all four MMA biochemical indicators after treatment when compared to cblC-MMA patients. These findings indicated a worse outcome in the mut-MMA group, which may be attributed to the more frequent episodes of acute metabolic decompensation, earlier onset and lower VitB12-responsive rate of mut-MMA patients (Table 1).41,42,43 Furthermore, the “pre-treatment onset” and unresponsiveness to VitB12 treatment were also independently associated with poor prognosis and neurologic disorders in mut-MMA patients, as evidenced by our multivariate analysis and previous studies.44 Moreover, elevated baseline levels of C3 and C3/C2 were predicted as independent positive predictors of death and anemia in mut-MMA patients, respectively. In MMA, a significant quantity of propionyl-CoA is unable to be converted to succinyl-CoA to enter the TCA cycle due to enzyme defects and needs to be combined with carnitine to form C3 for excretion. Therefore, elevated C3 can be an indicator of abnormal propionate catabolism. C3/C2 were considered a more specific metabolic indicator than C3. Consequently, abnormal propionate metabolism was highly correlated with death and anemia observed in mut patients, which requires strict monitoring and control. Finally, the c.914T > C variant in the MMUT gene, specific to the Chinese population, was identified as an independent risk factor for mortality, emphasizing the importance of rigorous management in affected patients.
For cblC-MMA prognosis, a higher incidence of language impairment, ocular complications and hydrocephalus among survivors with poor outcomes was observed compared to those with mut-MMA, with the latter two having been previously reported.45, 46 This underscores the importance of ophthalmological, MRI, and language assessments during follow-up for cblC-MMA patients. With regard to the factors affecting the prognostic manifestations, our findings showed that higher baseline C3 levels increased the risk of poor prognosis and neurologic disorders, suggesting that the severe suppression of the propionate metabolic pathway is prone to irreversible nerve damage. The baseline Hcy levels independently predicted anemia. This discovery is supported by previous studies demonstrating the prevalence of megaloblastic anemia in patients with the cblC type, which is associated with hyperhomocysteine.47, 48 In addition, variant hotspots c.80A > G and c.482G > A in the MMACHC gene, mainly found in patients with late-onset and mild phenotypes, were identified to be strongly related to a better prognosis.49, 50
Similar to the findings of mut-MMA, pre-treatment onset predicted most manifestations of poor outcomes for cblC-MMA, highlighting the irreversible damage of MMA onset and underscoring the urgency of timely diagnosis and treatment for prognostic recovery. NBS holds potential for early detection and intervention in rare, treatable illnesses and its function in improving the prognosis of patients with cblC-MMA was confirmed by prior studies and our multivariate analysis.23, 25 In contrast, no poor outcomes were identified to be independently associated with NBS in mut-MMA, possibly due to the fact that more mut-MMA patients develop symptoms before or during the process of NBS, a more severe condition with limited therapeutic effect, as shown in our study (Table 1). Nevertheless, NBS can facilitate early treatment and significantly reduce the symptom onset in mut patients, and the lower metabolic indicator levels before treatment in the NBS group of mut-MMA patients indicated that the early diagnosis due to NBS can effectively reduce the occurrence of damaging or even fatal acute metabolic derangement at initial presentation. Therefore, the importance of NBS for the diagnosis, treatment and prognosis of mut-MMA cannot be ignored, and the screening window needs to be as early as possible. Notably, in contrast to studies based on Chinese populations, the benefit of NBS on MMA outcomes is less evident in some Western countries.51, 52 This discrepancy may be attributed to the fact that Western countries have a greater proportion of patients with cobalamin-nonresponsive MMA and a different spectrum of genetic variants.35 In conclusion, NBS for MMA is necessary for China, which has a large proportion of a cobalamin-responsive MMA population.
In addition to the factors mentioned above, significantly elevated baseline uMMA and uMCA levels, higher proportions of patients with the MMACHC variants c.609G > A, c.80A > G or c.658_660delAAG, or with the MMUT variant c.323G > A and a lower percentage of patients with the MMUT variant c.1663G > A were found in some poor outcome groups compared to those without corresponding manifestations (Table 2 and Tables S4–S6). The elevated MMA level has been linked to increased risk for mortality and basal ganglia stroke,53, 54 and MCA could contribute to brain damage through impairing glutamate metabolism and mitochondria.55 Moreover, the genotype–phenotype associations have also been reported for some of the above variants: irreversible brain disorders and poor prognosis were more common in cblC patients with the MMACHC c.609G > A homozygous variant; the MMACHC c.80A > G variant was prevalent among Chinese cblC patients with prominent renal complications; and the c.1663G > A variant in MMUT is associated with better outcomes in mut-MMA.7, 56, 57 However, the multivariate analysis in our study identified no strong association between these elements and any poor prognostic manifestations. We must recognize that the exclusions in multivariate analysis due to missing information might introduce bias. The associations of MMA prognosis with these elements still need to be further explored based on an intact and large patient database.
Biometabolic indicators can reflect an individual’s response to a therapeutic intervention and the prognosis of a disease.58 In line with this, significant differences in the characteristic metabolite levels after treatment were found between the groups with and without corresponding prognostic symptoms, especially for neurologic disorders (Table 2 and Table S4). The distinctions were more pronounced for mut-MMA, evident in the diagnostic value of the four indicators for prognostic symptoms (Fig. 4), which are meaningful for prognostic surveillance and management. However, for cblC-MMA patients and symptoms like death, ocular complications and anemia, the differences and diagnostic value were inconclusive. The onset of symptoms in these cases may involve a combination of metabolites or other factors, which need further research.
Conclusions
The study outlines the long-term prognosis of a large MMA cohort. The comparative study of the clinical features between two major MMA subtypes enhances understanding of the phenotypic and prognostic variations of this disease. Our analysis provided strong evidence of the clinical effectiveness and long-term benefits of MS/MS-based NBS for MMA. It is crucial to expand NBS coverage and optimize the screening window. There are limitations to incomplete data collection, which affected the conduct and accuracy of some statistical analysis, and the prognostic manifestations involve other systems such as blood and viscera that need further attention. Nonetheless, the identified influential factors will refine diagnosis and treatment strategies, improving the MMA prognosis.
Data availability
Data will be made available on request.
References
Zhou, W., Li, H., Wang, C., Wang, X. & Gu, M. Newborn screening for methylmalonic acidemia in a Chinese population: molecular genetic confirmation and genotype phenotype correlations. Front. Genet. 9, 726 (2018).
Forny, P. et al. Integrated multi-omics reveals anaplerotic rewiring in methylmalonyl-CoA mutase deficiency. Nat. Metab. 5, 80–95 (2023).
Zhou, X., Cui, Y. & Han, J. Methylmalonic acidemia: current status and research priorities. Intractable Rare Dis. Res. 7, 73–78 (2018).
Zhao, Z. et al. Newborn screening for inherited metabolic diseases using tandem mass spectrometry in China: outcome and cost-utility analysis. J. Med. Screen. 29, 12–20 (2022).
Head, P. E. et al. Aberrant methylmalonylation underlies methylmalonic acidemia and is attenuated by an engineered sirtuin. Sci. Transl. Med. 14, eabn4772 (2022).
Wang, C. et al. Phenotypic and genotypic analysis of children with methylmalonic academia: a single-center study in China and a recent literature review. Clin. Chim. Acta Int. J. Clin. Chem. 522, 14–22 (2021).
He, R. et al. Variable phenotypes and outcomes associated with the MMACHC c.609G > A homologous mutation: long term follow-up in a large cohort of cases. Orphanet J. Rare Dis. 15, 200 (2020).
Head, P. E., Meier, J. L. & Venditti, C. P. New insights into the pathophysiology of methylmalonic acidemia. J. Inherit. Metab. Dis. 46, 436–449 (2023).
Yu, Y. et al. Different mutations in the MMUT gene are associated with the effect of vitamin B12 in a cohort of 266 Chinese patients with mut-type methylmalonic acidemia: a retrospective study. Mol. Genet. Genom. Med. 9, e1822 (2021).
Kang, L. et al. A study on a cohort of 301 Chinese patients with isolated methylmalonic acidemia. J. Inherit. Metab. Dis. 43, 409–423 (2020).
Sun, S., Jin, H., Rong, Y., Song, W. & Li, Q. Methylmalonic acid levels in serum, exosomes, and urine and its association with cblC type methylmalonic acidemia-induced cognitive impairment. Front. Neurol. 13, 1090958 (2022).
Gupta, N. et al. Clinical and molecular spectrum of patients with methylmalonic acidemia. Indian J. Pediatr. 91, 675–681 (2024).
Hörster, F. et al. Delineating the clinical spectrum of isolated methylmalonic acidurias: cblA and Mut. J. Inherit. Metab. Dis. 44, 193–214 (2021).
Esser, A. J. et al. Versatile enzymology and heterogeneous phenotypes in cobalamin complementation type C disease. iScience 25, 104981 (2022).
Berraondo, P., Martini, P. G. V., Avila, M. A. & Fontanellas, A. Messenger RNA therapy for rare genetic metabolic diseases. Gut 68, 1323–1330 (2019).
Sloan, J. L., Carrillo, N., Adams, D. & Venditti, C. P. in Genereviews® (eds Adam, M. P. et al.) (University of Washington, Seattle Copyright© 1993–2023).
Manoli, I., Sloan, J. L. & Venditti, C. P. in Genereviews® (eds Adam, M. P. et al.) (University of Washington, Seattle Copyright© 1993–2023).
Dao, M. et al. Long-term renal outcome in methylmalonic acidemia in adolescents and adults. Orphanet J. Rare Dis. 16, 220 (2021).
Beck, B. B., van Spronsen, F., Diepstra, A., Berger, R. M. & Kömhoff, M. Renal thrombotic microangiopathy in patients with cblC defect: review of an under-recognized entity. Pediatr. Nephrol. 32, 733–741 (2017).
Forny, P. et al. Guidelines for the diagnosis and management of methylmalonic acidaemia and propionic acidaemia: first revision. J. Inherit. Metab. Dis. 44, 566–592 (2021).
Huemer, M. et al. Guidelines for diagnosis and management of the cobalamin-related remethylation disorders cblC, cblD, cblE, cblF, cblG, cblJ and MTHFR deficiency. J. Inherit. Metab. Dis. 40, 21–48 (2017).
Baumgartner, M. R. et al. Proposed guidelines for the diagnosis and management of methylmalonic and propionic acidemia. Orphanet J. Rare Dis. 9, 130 (2014).
Ling, S. et al. The follow-up of Chinese patients in cblC type methylmalonic acidemia identified through expanded newborn screening. Front. Genet. 13, 805599 (2022).
Ahrens-Nicklas, R. C. et al. Efficacy of early treatment in patients with cobalamin C disease identified by newborn screening: a 16-year experience. Genet. Med. Off. J. Am. Coll. Med. Genet. 19, 926–935 (2017).
Mütze, U. et al. How longitudinal observational studies can guide screening strategy for rare diseases. J. Inherit. Metab. Dis. 45, 889–901 (2022).
Gizicki, R. et al. Long-term visual outcome of methylmalonic aciduria and homocystinuria, cobalamin C type. Ophthalmology 121, 381–386 (2014).
Nizon, M. et al. Long-term neurological outcome of a cohort of 80 patients with classical organic acidurias. Orphanet J. Rare Dis. 8, 148 (2013).
Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. Off. J. Am. Coll. Med. Genet. 17, 405–424 (2015).
Yang, Y. L. & Han, L. S. Expert consensus on dietary therapy and nutrition management of isolated methylmalonic aciduria. Chin. J. Pract. Pediatr. 33, 481–486 (2018).
Liang, L. et al. Evaluation of the clinical, biochemical, genotype and prognosis of mut-type methylmalonic acidemia in 365 Chinese cases. J. Med. Genet. 61, 8–17 (2023).
Fraser, J. L. & Venditti, C. P. Methylmalonic and propionic acidemias: clinical management update. Curr. Opin. Pediatr. 28, 682–693 (2016).
Haijes, H. A., van Hasselt, P. M., Jans, J. J. M. & Verhoeven-Duif, N. M. Pathophysiology of propionic and methylmalonic acidemias. Part 2: treatment strategies. J. Inherit. Metab. Dis. 42, 745–761 (2019).
Mak, C. M., Lee, H. C., Chan, A. Y. & Lam, C. W. Inborn errors of metabolism and expanded newborn screening: review and update. Crit. Rev. Clin. Lab. Sci. 50, 142–162 (2013).
Huishu, E. et al. Evaluation of the clinical, biochemical, neurological, and genetic presentations of glutaric aciduria type 1 in patients from China. Front. Genet. 12, 702374 (2021).
Almási, T. et al. Systematic literature review and meta-analysis on the epidemiology of methylmalonic acidemia (MMA) with a focus on MMA caused by methylmalonyl-CoA mutase (mut) deficiency. Orphanet J. Rare Dis. 14, 84 (2019).
Liu, Y. et al. [Heterogeneous phenotypes, genotypes, treatment and prevention of 1 003 patients with methylmalonic acidemia in the mainland of China]. Zhonghua er ke za zhi Chin. J. Pediatr. 56, 414–420 (2018).
Zhang, R. et al. Spectrum analysis of inborn errors of metabolism for expanded newborn screening in a northwestern Chinese population. Sci. Rep. 11, 2699 (2021).
Fischer, S. et al. Clinical presentation and outcome in a series of 88 patients with the cblC defect. J. Inherit. Metab. Dis. 37, 831–840 (2014).
Kaur, R., Attri, S. V., Saini, A. G. & Sankhyan, N. A high frequency and geographical distribution of MMACHC R132* mutation in children with cobalamin C defect. Amino Acids 53, 253–264 (2021).
Acquaviva, C. et al. Molecular basis of methylmalonyl-CoA mutase apoenzyme defect in 40 European patients affected by Mut° and Mut- forms of methylmalonic acidemia: identification of 29 novel mutations in the Mut gene. Hum. Mutat. 25, 167–176 (2005).
Jiang, Y. Z. et al. Factors influencing in-hospital death for pediatric patients with isolated methylmalonic acidemia: a nationwide inpatient database analysis. Orphanet J. Rare Dis. 15, 154 (2020).
Heringer, J. et al. Impact of age at onset and newborn screening on outcome in organic acidurias. J. Inherit. Metab. Dis. 39, 341–353 (2016).
Couce, M. L. et al. Evaluation and long-term follow-up of infants with inborn errors of metabolism identified in an expanded screening programme. Mol. Genet. Metab. 104, 470–475 (2011).
Waisbren, S. E. Review of neuropsychological outcomes in isolated methylmalonic acidemia: recommendations for assessing impact of treatments. Metab. Brain Dis. 37, 1317–1335 (2022).
Weisfeld-Adams, J. D., McCourt, E. A., Diaz, G. A. & Oliver, S. C. Ocular disease in the cobalamin C defect: a review of the literature and a suggested framework for clinical surveillance. Mol. Genet. Metab. 114, 537–546 (2015).
He, R. et al. Analysis of 70 patients with hydrocephalus due to cobalamin C deficiency. Neurology 95, e3129–e3137 (2020).
Zaric, B. L. et al. Homocysteine and hyperhomocysteinaemia. Curr. Med. Chem. 26, 2948–2961 (2019).
Green, R. & Datta Mitra, A. Megaloblastic anemias: nutritional and other causes. Med. Clin. N. Am. 101, 297–317 (2017).
Ding, S. et al. Late-onset cblC defect: clinical, biochemical and molecular analysis. Orphanet J. Rare Dis. 18, 306 (2023).
Wu, S. N. et al. Variable phenotypes and outcomes associated with the MMACHC c.482G > A mutation: follow-up in a large cblC disease cohort. World J. Pediatr. 20, 848–858 (2024).
Reischl-Hajiabadi, A. T. et al. Outcomes after newborn screening for propionic and methylmalonic acidemia and homocystinurias. J. Inherit. Metab. Dis. 47, 674–689 (2024).
Haijes, H. A. et al. Retrospective evaluation of the Dutch pre-newborn screening cohort for propionic acidemia and isolated methylmalonic acidemia: what to aim, expect, and evaluate from newborn screening? J. Inherit. Metab. Dis. 43, 424–437 (2020).
Riphagen, I. J. et al. Methylmalonic acid, vitamin B12, renal function, and risk of all-cause mortality in the general population: results from the prospective Lifelines-Minuthe study. BMC Med. 18, 380 (2020).
Dionisi-Vici, C., Deodato, F., Röschinger, W., Rhead, W. & Wilcken, B. ‘Classical’ organic acidurias, propionic aciduria, methylmalonic aciduria and isovaleric aciduria: long-term outcome and effects of expanded newborn screening using tandem mass spectrometry. J. Inherit. Metab. Dis. 29, 383–389 (2006).
Amaral, A. U., Cecatto, C., Castilho, R. F. & Wajner, M. 2-Methylcitric acid impairs glutamate metabolism and induces permeability transition in brain mitochondria. J. Neurochem. 137, 62–75 (2016).
Liang, L. et al. A rare mutation c.1663G > A (p.A555t) in the MMUT gene associated with mild clinical and biochemical phenotypes of methylmalonic acidemia in 30 Chinese patients. Orphanet J. Rare Dis. 16, 22 (2021).
Liu, X. et al. Prominent renal complications associated with MMACHC pathogenic variant c.80A > G in Chinese children with cobalamin C deficiency. Front. Pediatr. 10, 1057594 (2022).
Longo, N., Sass, J. O., Jurecka, A. & Vockley, J. Biomarkers for drug development in propionic and methylmalonic acidemias. J. Inherit. Metab. Dis. 45, 132–143 (2022).
Funding
This work was supported by the Shanghai Sailing Program (No. 23YF1435000) and the Scientific Research Project Plan of the Shanghai Municipal Health Commission (No. 202140346).
Author information
Authors and Affiliations
Contributions
L.H., L.L. and L.H. (Lianshu Han) were in charge of the idea, project design and concept of the paper. T.C., S.L. and S.D. did laboratory work like MS/MS, GC/MS, PCR and DNA sequencing. L.L., W.Q., H.Z., X.G., K.Z. and L.H. (Lianshu Han) collected the clinical data. L.H. and S.L. did data analysis and wrote the draft. W.Q., H.Z., X.G. and L.H. (Lianshu Han) edited and revised the manuscript. All authors read and approved the manuscript and believe that the manuscript represents honest work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hao, L., Ling, S., Ding, S. et al. Long-term follow-up of Chinese patients with methylmalonic acidemia of the cblC and mut subtypes. Pediatr Res (2024). https://doi.org/10.1038/s41390-024-03581-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-024-03581-x
- Springer Nature America, Inc.