Abstract
Background
Trials assessing the clinical utility of blood-based multi-cancer early detection (MCED) tests are underway. Understanding public attitudes towards MCED screening is essential if these tests are to be used. We aimed to quantify MCED screening intention and potential barriers and facilitators to uptake.
Methods
Adults aged 50–77 (n = 958) completed an online survey. The primary outcome was intention to have MCED screening if offered. Psychological variables including barriers and facilitators were assessed. We used logistic regressions to explore associations between socio-demographics and psychological factors and intention.
Results
93.8% of participants said they would ‘definitely’ or ‘probably’ have MCED screening if offered. Intention was significantly associated with previous screening participation and general cancer attitudes but not with socio-demographic factors. Participants were more likely to be intenders if they had higher health motivation, and perceived greater benefits of blood tests. Participants were less likely to be intenders if they perceived greater disadvantages of blood tests, more practical barriers, were more worried about the outcome and more concerned about a positive result.
Conclusions and implications
MCED screening intention was high. The lack of socio-demographic variation suggests equitable interest in this type of screening; however, future research should consider how intention translates to uptake.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Earlier stage of cancer diagnosis is generally associated with improved survival [1], as it often increases the chance of successful treatment and outcomes [2]. The NHS Long Term plan set the ambition that 75% of cancers should be diagnosed at stage 1 or 2 by 2028 [3]. Cancer screening aims to identify cancer and pre-cancerous conditions in asymptomatic individuals, but at present only around 6% of cancers are diagnosed through screening in England [4]. Although England currently has three single cancer screening programmes and targeted lung health checks [5, 6], many other cancers have no effective screening test, including aggressive cancers such as pancreatic and stomach cancer. Multi-cancer early detection (MCED) blood tests show potential for detecting cancers before symptoms appear. Blood-based MCED tests look for the presence of circulating tumour DNA or other biomarkers in a standard blood sample and can predict the location of the cancer, in order to direct diagnostic follow-up [7]. A number of MCED tests are in development [8] and trials are currently underway to establish the clinical utility of these tests in asymptomatic individuals, with the hope that MCED tests could be used for population screening in the future [9,10,11].
The UK National Screening Committee recognises that any new screening programme should be acceptable to the target population and the public [12], since acceptability has implications for engagement, uptake and the overall success of a screening programme [13]. One qualitative study has suggested that the introduction of a population-based MCED screening programme will be appealing due to the simplicity and familiarity of the blood test procedure. However, the potential for positive results and follow-up testing to cause anxiety was an area of concern for participants, and personal beliefs about the benefits of diagnosing cancer early influenced desire for the test [14]. The proportion of people who would want MCED screening, and the barriers and facilitators to this kind of test, have not yet been quantified. It is essential that public attitudes to MCED screening are better understood prior to any future implementation [15].
Many studies investigating the acceptability of screening programmes use intention to participate as a proxy for acceptability [16,17,18,19]. Intention has been shown to be a good predictor of future screening uptake, and to be indicative of programme acceptability [20, 21], though there is a gap between intention and behaviour [22]. Quantifying intention can support estimates of cost-effectiveness of a screening programme and plans for resource allocation. There are well-established inequalities in uptake of cancer screening programmes [23], so, research identifying health inequalities should be embedded in the evaluation and introduction of a new screening programme [12]. Understanding the barriers and facilitators to screening participation and whether these might be more/less prevalent among people from lower socio-economic or ethnic minority backgrounds is also important. This can help identify content for information materials to ensure they address questions or concerns that people might face in response to screening invitations and inform proactive interventions to promote equitable uptake.
To our knowledge, no other studies to date have explored intention to participate in population-based MCED blood test screening. Our objectives were: (1) to estimate the proportion of people in a population-representative sample with positive intentions to have an MCED blood test for screening, (2) to quantify the prevalence of barriers and facilitators to having an MCED blood test, (3) to explore socio-demographic predictors of barrier and facilitator endorsement and (4) to explore socio-demographic and psychological predictors of intention to have an MCED blood test.
Methods
This study is reported following the CROSS reporting guidelines [24] and a copy of the checklist is available in Supplementary material A.
Design
We ran a cross-sectional online population-based survey of men and women aged 50–77 years in England. The sample was representative of the English population within this age-group in terms of age, sex, ethnicity, social grade and region. Ethical approval for this study was granted by King’s College London Research Ethics committee (LRM-22/23-36139, 06/06/2023). Informed consent was obtained. The study protocol is available at: https://osf.io/zm4v6/.
Data collection was carried out in June 2023 by YouGov. Participants were identified through YouGov’s online research panel. The UK panel includes 2.7 million people recruited via the YouGov website and through social media advertising. Participants receive points for taking part in surveys which can then be exchanged for shopping vouchers.
Participants
Eligible participants were aged 50–77 years, and currently living in England. This age range matches participants in the ongoing trial of an MCED test (the NHS-Galleri trial; NCT05611632 [11]) and is likely to reflect the population who would be offered MCED blood test screening if it were rolled out at population level. Participants who had taken part in the NHS-Galleri trial and/or had received a cancer diagnosis in the previous three years were not eligible to participate. Potential participants were identified by YouGov from their panel.
Our target sample size was n = 1000. This was designed to allow us to estimate intention to have an MCED blood test with good precision (±3%, with 95% confidence, assuming a prevalence of 60–70% for positive intentions).
Materials and measures
A survey was created by the authors (behavioural scientists) in collaboration with patient and public involvement (PPI) representatives. A detailed description of all survey items is available at: https://osf.io/ka3t7. The survey was only available in English. Participants were shown introductory information about MCED screening followed by additional information about diagnostic work-up following a positive result, including an estimated false positive rate of 0.5% (1 in 200) and a positive predictive value of 50% (1 in 2 with a positive result having cancer); see Box 1. After the introductory information participants answered three knowledge-based attention check questions before completing questions assessing intention, barriers and facilitators. After the additional information about follow-up diagnostic testing, they answered more questions. All questions were mandatory to avoid missing data, except for questions relating to personal details such as health and gender identity, which participants could skip. Participants were excluded from any analyses for which their data were missing.
The full survey is available here: https://osf.io/68wvb. The primary outcome was intention to have MCED screening if offered, measured on a five-point scale with a single item: ‘If you were offered a blood test that looks for a range of cancers would you have it?’ (response options ordered as follows: definitely not; probably not; yes probably; yes definitely and don’t know). Participants who responded probably or definitely not were offered a free-text box to say why. We asked this question twice, at the beginning of the survey after the introductory information (initial intention) and then again after additional information had been provided (considered intention).
The survey included items assessing perceived barriers and facilitators to MCED screening. These items were developed to cover a range of constructs from several theories e.g. the Health Belief Model [25], previous research in the cancer screening context (e.g. [26, 27]) and a qualitative study exploring attitudes to MCED blood tests [14]. It also contained items assessing cancer-related attitudes: attitudes to screening [28], cancer fatalism [29], cancer risk perceptions [30, 31] and attitudes to overdiagnosis [28]. Detail about the source of each item is in the protocol (https://osf.io/ka3t7).
We adopted YouGov’s standard socio-demographic questions for age, ethnicity, social grade (based on occupation of the highest household earner), and region, with additional items from the UK census included to assess sex, marital status, education and self-reported disability. We also assessed previous screening behaviour (items adapted from the Cancer Awareness Measure [26]), and global self-rated health [32]. The survey items were a combination of validated and unvalidated measures and were assessed for face validity via PPI feedback.
Procedure
Participants were sent an email including a unique re-direct link taking them to the survey. This allowed YouGov to link responses to socio-demographic data and avoided multiple submissions. Participants who clicked the link were shown a participant information page and asked to indicate consent to take part.
When participants completed the survey, they were thanked for their participation and offered sources of support. Participants received 150 YouGov points (approximately £1.50) upon completion of the survey.
Analysis
All datasets were anonymous. The data were analysed using the Complex Samples functionality in IBM SPSS Statistics 29 and followed a pre-written analysis plan (available here: https://osf.io/zm4v6/). Deviations from this plan were recorded in a separate document (https://osf.io/zm4v6/). Weights were provided by YouGov to account for outstanding variation between the recruited sample and the wider population with respect to age, sex, social grade, region and ethnicity so that the sample was representative of English adults aged 50–77 years [33]. All analyses were weighted so that the sample was population representative. Participants were excluded prior to analyses if they completed the survey too quickly (in less than 5 min), too slowly (in more than 3 standard deviations above the mean time taken) or if they answered all three attention check questions incorrectly.
Frequencies and proportions (with 95% confidence intervals) of individuals giving each response option for the two intention items (initial and considered) were reported. For further analysis exploring intention, a single binary variable was created using considered intention and classifying participants as ‘intenders’ or ‘non-intenders/don’t knows’. We used logistic regression to assess whether intention (using the binary outcome) was associated with socio-demographic characteristics.
The frequency and proportion of individuals responding ‘agree’/‘strongly agree’ or ‘quite a bit’/‘a great deal’ to each barrier/facilitator item is reported (full breakdown of responses available in Supplementary Tables 1 and 2). Following Exploratory Factor Analysis (EFA, see supplementary material), the 21 items assessing barriers and facilitators were reduced to five scales assessing: Health Motivation (4 items; Cronbach’s alpha = 0.85); Benefits of blood tests (4 items; Cronbach’s alpha = 0.87); Disadvantages of blood tests (4 items, Cronbach’s Alpha = 0.80); Practical barriers (3 items, Cronbach’s alpha = 0.71) and Fear of outcome (3 items, Cronbach’s alpha = 0.82). The six items assessing concern about a positive result formed an additional scale (Cronbach’s alpha = 0.90). Scales were created by summing the items and adjusting for a minimum score of zero. Three items did not fit in the scales and were analysed individually. We used fully adjusted ANCOVAs to assess whether age, sex, social grade, ethnicity or employment status were independently associated with barriers/facilitators scores. For the three single item barriers, we ran logistic regressions to examine demographic associations. We used a Bonferroni correction to adjust for multiple comparisons (45 in total), resulting in a p-value of <0.001 being considered statistically significant.
We used logistic regression to assess whether intention (using the binary outcome) was associated with any of the psychological variables including: perceived risk of cancer, cancer worry, fatalism, attitudes to screening/overdiagnosis and the six scales or three individual items assessing barriers/facilitators. We used content analysis to analyse the free text responses recorded by participants who responded ‘probably not’ or ‘definitely not’ to the intention item. Two authors worked together to develop a coding frame and independently coded each response. Discrepancies were discussed and resolved.
Public and patient involvement (PPI)
A PPI panel consisting of five participants was involved in the development of the participant information page, consent form, and survey. The panel included two women and three men aged 50–70 years and included people with and without personal cancer experience. Development of the research questions was carried out before recruitment of the PPI panel. Our PPI panel had substantial influence on the information that was presented and the need to split this into different sections. Discussion also included in-depth debate about the best wording for items and their response options. The discussions provided an indication of the face validity of newly created items, and ensured they were presented in a logical and user-friendly way.
Results
Sample
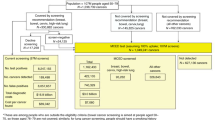
Of the 1250 people who clicked the survey link, 1052 were eligible and consented (representing an 84.2% completion rate based on survey completes/survey started). The survey was fully completed by 1002 participants but 44 were excluded prior to analysis (n = 10 for answering all three attention check items incorrectly; n = 34 for completing the survey too quickly/slowly). Data from 958 participants was included in the analysis. Sample characteristics are presented in Table 1.
Intention to have MCED screening
Initially, most participants said they would ‘probably’ (N = 299; 31.3%) or ‘definitely’ (n = 595; 62.2%) have MCED screening (initial intention) with a slight increase in intention strength after reading additional information, (n = 257; 26.9% and n = 641; 66.9% respectively). All 36 people who said they would probably or definitely not have the test after the initial intention question recorded a free-text response. Participants gave a range of different reasons for their response. The most frequent were: ‘test causing anxiety’ (n = 12), ‘not wanting to know about cancer’ (n = 7), ‘low perceived need for the test’ (n = 7) and ‘wanting to focus on living life’ (n = 5) (see Supplementary Table 3 for all responses).
Using the binary outcome (intenders versus non-intenders/don’t know), we did not find significant associations between intention to have MCED screening and any of the socio-demographic characteristics we assessed (age, sex, social grade, ethnicity, education, employment and marital status) or with self-rated health (see Table 2). Of the 860 participants who were eligible for any of the three screening programmes, those who reported never attending any screening or only attending some screening were less likely to be intenders than those who had attended all the screening they were eligible for (n = 5/7; 71.2% who had never attended and n = 172/203; 84.7% who had attended some compared with n = 629/650; 96.6% who had attended all). Those who reported ‘a bad experience’ of screening in the past were less likely to be intenders (83.0%) than those who had not (94.4%)
Barriers and facilitators to MCED screening
The percentage of participants who endorsed each barrier and facilitator to MCED screening is presented in Table 3. The most frequently endorsed barriers were: being frightened about what the test might find (45.0%), needing to know more about how the test works (39.6%) and the potential for MCED screening to cause worry about cancer (32.0%). The least frequently endorsed barrier to MCED screening was being ‘too busy’ (1.2%). The most frequently endorsed facilitators to MCED screening related to blood tests being safe (92.7%), quick (92.9%) and familiar (86.7%).
We looked at whether socio-demographic characteristics (age, sex, social grade, ethnicity and employment status) were associated with any of the six composite scales (Supplementary Tables 6 and 7). Scores for the ‘practical barriers’ were significantly higher in individuals from lower social grades (C1, C2 and D/E) compared to those from the highest social grade (A/B; p < 0.001). Older age groups had lower ‘fear of outcome’ scores compared with the youngest group (p < 0.001). Participants from ethnic minority backgrounds were also more likely to say that they would need more information than participants from white ethnic backgrounds (p < 0.001).
Psychological predictors of intention
General beliefs about cancer risk, fatalism and general attitudes to cancer screening were significantly associated with intention to have MCED screening (see Tables 2 and 4). Participants were less likely to be intenders if they felt their risk of cancer was ‘above average’ or ‘below average’ (82.2%) versus ‘same as average’ (95.4%), and if they worried about cancer ‘often’ or ‘very often’ (88.4%) versus ‘never’/‘rarely’/‘sometimes’ (94.4%). Participants who believed cancer is predetermined were also less likely to be intenders (86.4%) than those who did not hold this fatalistic belief (95.7%).
Participants were more likely to be intenders if they believed: cancer screening is always a good idea, that finding cancer early means that treatment saves lives, or that finding cancer means a person has less treatment (p < 0.001). Views on overdiagnosis were also associated with intention, with participants more likely to be intenders if: they said they would want to be tested for cancer, even if nothing could be done; they said they would still have screening, even if it was for a slow-growing cancer that would not cause them harm in their lifetime; they said they would have the recommended treatment for early-stage cancer (p < 0.001).
Using the six barrier and facilitator scales we looked at the association between barriers and facilitators and intention. In unadjusted analyses, all scales were significantly associated with intention to have MCED screening (p < 0.001) (Table 5). Higher ‘Health motivation’ and ‘Benefits of blood tests’ scores were associated with increased odds of being an intender. Conversely, higher scores on ‘Disadvantages of blood tests’, ‘Fear of outcome’, ‘Practical barriers’ and ‘Concerns about a ‘positive’ result’ were associated with decreased odds of being an intender. Participants who said that they would need more information (F(1957) = 16.15 p < 0.001), that they wouldn’t trust the results (F(1957) = 30.79 p < 0.001), or that they had more important things to worry about (F(1957) = 24.83 p < 0.001) were less likely to be intenders. After adjusting for all the barriers and facilitators (scales and items), three scales remained significant predictors of intention to have MCED screening: health motivation (F(1957) = 64.37 p < 0.001), practical barriers (F(1957) = 6.19 p = 0.013) and concerns after results (F(1957) = 16.66 p < 0.001) (Supplementary Tables 4 and 5).
Discussion
In this population-based survey in England, most people said they would have MCED screening and intention remained high after receiving additional information about diagnostic work-up following a positive result and the potential for false-positive results. Intention to have MCED screening was not associated with socio-demographic characteristics but did vary according to previous screening behaviours and attitudes to cancer and screening in general. General health motivation, anticipated practical barriers and concerns about what would happen following a positive result, were the strongest predictors of intention to have MCED screening. The need for additional information, fear of the outcome and concern about what would happen after results were endorsed by a sizeable minority (i.e. over a third of participants).
This is the first study to quantify intention to have MCED screening in a sample representative of the English population following provision of brief information. The findings add to evidence from a 2021 report investigating public priorities in cancer research which reported that 70% of participants would want a single blood test for multiple cancers, but they were not given any further information about blood tests for cancer screening [34]. Since intention is a strong predictor of uptake [20], the high intention demonstrated in our study, following brief information, suggests MCED screening uptake could be high if rolled out across England. Nevertheless, it is important to consider that intention does not always translate into action in cancer screening contexts [22]. For example, in colorectal cancer screening 80% of people who said they would probably or definitely have screening subsequently attended flexi-sigmoidoscopy [21]. Future research is needed to assess how intention translates to action in the context of MCED screening and factors influencing this. The finding that intention did not vary by socio-economic and demographic characteristics suggests motivation to have MCED screening may be equally high across socio-demographic groups. Nevertheless, as outlined in widely used behaviour change frameworks (e.g. COM-B [35]), capability (whether someone has the knowledge, skills and abilities to engage in particular behaviour) and opportunity (external factors influencing whether a behaviour can be executed) are also important determinants of health behaviour.
Previous screening behaviour significantly predicted intention to have MCED screening, consistent with previous research in other screening contexts [19, 36]. MCED screening does hold potential to engage people whose previous non-attendance/participation is driven by aversion to more invasive procedures, and intention to have an MCED test was still high in those who had never or inconsistently attended (71% and 85% respectively). This suggests uptake may be high even in those who do not participate in other screening programmes. However, individuals who had never or inconsistently attended screening were still less likely to intend to have MCED screening. Non-intenders were also less likely to support screening in general and scored significantly lower on the ‘health motivation’ scale. This suggests that for some, cancer screening is not in line with their values, and if this is the case it makes sense that previous behaviour would continue to predict intention to some degree. This finding has also been observed in qualitative work [14]. An individual’s informed choice not to have MCED screening should be respected. Nevertheless, efforts should be made to ensure that never or infrequent screening participation is a choice, and not the result of specific, modifiable barriers to screening such as lower knowledge or fatalistic attitudes that could warrant intervention.
The benefits of blood tests as a procedure were highly endorsed with few perceiving disadvantages. This supports qualitative findings suggesting that the primary test procedure will be appealing to most people [14]. Individuals who were non-intenders were more likely to be concerned about a positive result, the need for follow-up testing and the potential for this to cause anxiety. If MCED screening is rolled out in the future, it will be important to manage these concerns and detailed information materials should be developed to give reassurance regarding follow-up tests, since it seems these could put individuals off MCED screening at the point of invitation. Interestingly, providing participants with estimated detail about the positive predictive value of an MCED test (i.e. ‘2 out of 200 people will have a cancer marker found in their blood. 1 of these would have cancer diagnosed after further tests and 1 would not’) did not decrease intention, in fact it was stronger after this information was provided. Research exploring attitudes to mammography suggests that people are extremely tolerant of false-positive results [37]; however, further work is needed to explore public tolerance of false-positive results in MCED testing, for finding cancer overall and for different types of cancer. We did not present information about false negative results (of which there is much lower public tolerance), since it is not possible to make an estimate about this at the moment.
Another frequently cited barrier was the need for additional information. This finding is not surprising since MCED screening is still relatively unheard of, and participants received limited information within the survey. If implemented, participants will likely be given comprehensive informational resources at the time of invitation to support informed choice, as seen in other national screening programmes [38,39,40]. Materials outlining the benefits and harms of screening, and explaining procedural elements, are highly valued by potential participants, as has been reported elsewhere [41,42,43]. At present there is limited information available about MCED tests [44]. Care and thorough testing should be undertaken to ensure that sufficiently detailed and accessible materials answering relevant questions are available.
Whilst motivation did not vary by demographic characteristics, some of the barriers and enablers were more prominent in particular sub-groups. Average scores on the ‘fear of the outcome’ scale were higher for younger than older participants. This is in line with some evidence suggesting that cancer worry decreases with age [45], although this relationship has been refuted in other studies [46]. This suggests that interventions designed to reassure people about the treatability of cancer and emphasise good survival outcomes could be particularly important for younger participants. Practical barrier scores were higher in those from lower occupational social grades. Other studies have found that practical barriers to screening such as appointment times, lacking spare time [47] and transport issues [48] are more prevalent in individuals from more deprived backgrounds. If MCED screening is implemented, flexible appointments should be offered in accessible locations and booking processes should be simplified to reduce inequity.
Strengths and limitations
The use of a sample that was population-representative with respect to age, sex, ethnicity, social grade and region should allow the results to be generalised beyond the research setting and could help inform understanding of potential uptake if MCED screening is implemented in England. There are nevertheless some limitations. The survey was set up so that the proportion of people from ethnic minority backgrounds represented the proportion in the English population. However, this meant there were relatively small numbers of participants from each ethnic minority background. Consequently, we were not able to consider variation within broader ethnic minority groups. For example, there is evidence that within South Asian minority groups, uptake of existing screening is lowest among participants from Bangladeshi backgrounds [49]. In addition, since the survey was text-based and in English, those who were unable to read English would not have been able to take part. A deeper understanding of attitudes to MCED screening in different ethnic minority communities and non-English speakers will be important.
Data collection was completed online. There is some evidence that this type of data collection is equivalent to home-based interviewer led data collection [50], but as with all research methods there are likely to be some participation biases, for example those with low digital literacy are unlikely to participate. It is possible that there are broader inequalities in the opportunity to participate. For example, our research likely under-represents the least literate in the population. The small drop-out between engaging with the invitation (which did not describe what the survey was about) and survey completion suggests that interest in the topic area itself did not bias participation (i.e. self-selection bias). However, those on the YouGov panel may be people who are more interested in research participation broadly. It is likely that alternative methodologies (e.g. community-based face-to-face surveys, using multilingual interviewers) will be required to reach some groups and provide a more complete picture of attitudes to blood-based MCED tests across all sections of the society.
Whilst it was essential to provide participants with information about MCEDs within the study, it is still uncertain how MCED screening would be offered and what the performance characteristics of any test might be. If MCED screening is offered in a context that is very different from what was described to participants, intentions and attitudes may differ. As the potential for MCED screening develops, future work will be needed to ensure our understanding of acceptability accurately reflects the provision on offer. For most of our analyses we combined those who would not have a blood-based MCED tests and those who were unsure. These groups are likely quite different and further work to understand why people (i) actively do not want the test or (ii) are not sure is needed.
Since this was the first study in this context, we developed many of the items for the questionnaire rather than using validated scales. The items drew largely on our qualitative work as well as the existing literature in the screening field. The six scales we developed could be further validated and used in future work. This would allow for comparison of the literature as more evidence becomes available. However, since the questionnaire was developed in a UK context, the items likely exclude domains that will warrant exploration in different populations e.g. the relevance of insurance pay-outs in the US healthcare system. Engagement with PPI prior to development of the research questions would have strengthened the study.
Conclusion
This is the first study to quantify intention to have MCED blood test screening in a population-based sample. The findings suggest that intentions to take up MCED screening in England are high. While motivation to have MCED screening appears to be equitable across socio-demographic groups, inequity in uptake will likely be driven by access and opportunity which will require further consideration if a programme is implemented. Further work is needed to explore attitudes to blood-based MCED tests in different countries with different healthcare contexts. Supporting individuals with clear and accessible information will be imperative prior to an offer of an MCED test and measures should be put in place to reduce concern surrounding MCED screening results.
Data availability
The dataset and syntax will be made available upon reasonable request.
References
NHS Digital. Cancer Survival in England, cancers diagnosed 2015 to 2019, followed up to 2020 2022 [Available from: https://digital.nhs.uk/data-and-information/publications/statistical/cancer-survival-in-england/cancers-diagnosed-2015-to-2019-followed-up-to-2020/cancer-survival-by-stage]. Accessed April 2024.
World Health Organisation. Promoting cancer early diagnosis 2024 [Available from: https://www.who.int/activities/promoting-cancer-early-diagnosis] Accessed April 2024.
NHS. The NHS Long Term Plan. 2019. [Available from: https://www.longtermplan.nhs.uk/publication/nhs-long-term-plan/] Accessed August 2024.
NHS Digital. Routes to Diagnosis, 2018 2022 [Available from: https://digital.nhs.uk/data-and-information/publications/statistical/routes-to-diagnosis/2018]. Accessed April 2024.
NHS. NHS Screening 2024 [Available from: https://www.nhs.uk/conditions/nhs-screening/]. Accessed April 2024.
NHS. Lung health checks [Available from: https://www.nhs.uk/conditions/lung-health-checks]. Accessed April 2024.
Klein EA, Richards D, Cohn A, Tummala M, Lapham R, Cosgrove D, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncol. 2021;32:1167–77.
Hackshaw A, Clarke CA, Hartman AR. New genomic technologies for multi-cancer early detection: rethinking the scope of cancer screening. Cancer Cell. 2022;40:109–13.
Lennon AM, Buchanan AH, Kinde I, Warren A, Honushefsky A, Cohain AT, et al. Feasibility of blood testing combined with PET-CT to screen for cancer and guide intervention. Science. 2020;369:eabb9601.
Liu MC, Oxnard GR, Klein EA, Swanton C, Seiden MV. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann Oncol. 2020;31:745–59.
Neal RD, Johnson P, Clarke CA, Hamilton SA, Zhang N, Kumar H, et al. Cell-free DNA-based multi-cancer early detection test in an asymptomatic screening population (NHS-Galleri): design of a pragmatic, prospective randomised controlled trial. Cancers. 2022;14:4818.
UK National Screening Committee. Criteria for a population screening programme. 2022. [Available from: https://www.gov.uk/government/publications/evidence-review-criteria-national-screening-programmes/criteria-for-appraising-the-viability-effectiveness-and-appropriateness-of-a-screening-programme] Accessed August 2024.
Dennison RA, Boscott RA, Thomas R, Griffin SJ, Harrison H, John SD, et al. A community jury study exploring the public acceptability of using risk stratification to determine eligibility for cancer screening. Health Expect. 2022;25:1789–806.
Schmeising-Barnes N, Waller J, Marlow LAV. Attitudes to multi-cancer early detection (MCED) blood tests for population-based screening: a qualitative study in Great Britain. Soc Sci Med. 2024;347:116762.
Marlow LAV, Schmeising-Barnes N, Brain K, Duncombe S, Robb KA, Round T, et al. Multi-cancer early detection tests for cancer screening: a behavioural science perspective. Lancet Oncol. 2022;23:837–9.
Quaife SL, Vrinten C, Ruparel M, Janes SM, Beeken RJ, Waller J, et al. Smokers’ interest in a lung cancer screening programme: a national survey in England. BMC Cancer. 2018;18:497.
Wagner CV, Verstraete W, Hirst Y, Nicholson BD, Stoffel ST, Laszlo H. Public preferences for using quantitative faecal immunochemical test versus colonoscopy as diagnostic test for colorectal cancer: evidence from an online survey. BJGP Open. 2020;4:bjgpopen20X101007.
Al-Ani A, Hammouri M, Sultan H, Al-Huneidy L, Mansour A, Al-Hussaini M. Factors affecting cervical screening using the health belief model during the last decade: a systematic review and meta-analysis. Psychooncology. 2024;33:e6275.
Ivanova A, Kvalem IL. Psychological predictors of intention and avoidance of attending organized mammography screening in Norway: applying the Extended Parallel Process Model. BMC Women’s Health. 2021;21:67.
Huang J, Wang J, Pang TW, Chan MK, Leung S, Chen X, et al. Does theory of planned behaviour play a role in predicting uptake of colorectal cancer screening? A cross-sectional study in Hong Kong. BMJ Open. 2020;10:e037619.
von Wagner C, Bonello B, Stoffel ST, Skrobanski H, Kerrison R, McGregor LM. Predictors of intention translation in flexible sigmoidoscopy screening for colorectal cancer. Health Psychol. 2019;38:1083–95.
Sheeran P, Orbell S. Using implementation intentions to increase attendance for cervical cancer screening. Health Psychol. 2000;19:283.
von Wagner C, Good A, Whitaker KL, Wardle J. Psychosocial determinants of socioeconomic inequalities in cancer screening participation: a conceptual framework. Epidemiol Rev. 2011;33:135–47.
Sharma A, Minh Duc NT, Luu Lam Thang T, Nam NH, Ng SJ, Abbas KS, et al. A consensus-based checklist for reporting of survey studies (CROSS). J Gen Intern Med. 2021;36:3179–87.
Rosenstock IM. The health belief model and preventive health behavior. Health Educ Monogr. 1974;2:354–86.
Cancer Research UK. The Cancer Awareness Measures (CAM) 2023 [Available from: https://www.cancerresearchuk.org/health-professional/awareness-and-prevention/the-cancer-awareness-measures-cam.
Waller J, Bartoszek M, Marlow L, Wardle J. Barriers to cervical cancer screening attendance in England: a population-based survey. J Med Screen. 2009;16:199–204.
Waller J, Osborne K, Wardle J. Enthusiasm for cancer screening in Great Britain: a general population survey. Br J Cancer. 2015;112:562–6.
Powe BD. Fatalism among elderly African Americans: effects on colorectal cancer screening. Cancer Nurs. 1995;18:385–92.
Ferrer RA, Klein WMP, Persoskie A, Avishai-Yitshak A, Sheeran P. The tripartite model of risk perception (TRIRISK): distinguishing deliberative, affective, and experiential components of perceived risk. Ann Behav Med. 2016;50:653–63.
Riedinger C, Campbell J, Klein WMP, Ferrer RA, Usher-Smith JA. Analysis of the components of cancer risk perception and links with intention and behaviour: a UK-based study. PLOS ONE. 2022;17:e0262197.
Mossey JM, Shapiro E. Self-rated health: a predictor of mortality among the elderly. Am J Public Health. 1982;72:800–8.
YouGov. Methodology 2024 [Available from: https://yougov.co.uk/about/panel-methodology.
David Taylor MH, Mark Emberton. British public attitudes towards cancer research and treatment in 2021. UCL School of Pharmacy: University College London; 2021.
Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci. 2011;6:42.
Gregory TA, Wilson C, Duncan A, Turnbull D, Cole SR, Young G. Demographic, social cognitive and social ecological predictors of intention and participation in screening for colorectal cancer. BMC Public Health. 2011;11:38.
Schwartz LM, Woloshin S, Sox HC, Fischhoff B, Welch HG. US women’s attitudes to false-positive mammography results and detection of ductal carcinoma in situ: cross-sectional survey. West J Med. 2000;173:307–12. https://doi.org/10.1136/ewjm.173.5.307.
NHS England. Cervical screening: leaflet for women considering screening. Last updated 2024 [Available from: https://www.gov.uk/government/publications/cervical-screening-description-in-brief] Accessed August 2024.
NHS England. Breast screening: information leaflets, last updated 2022 [Available from: https://www.gov.uk/government/collections/breast-screening-information-leaflets] Accessed August 2024.
Public Health England. Bowel cancer screening: information leaflets. Last updated 2022 [Available from: https://www.gov.uk/government/collections/bowel-cancer-screening-information-leaflets] Accessed August 2024.
Crothers K, Kross EK, Reisch LM, Shahrir S, Slatore C, Zeliadt SB, et al. Patients’ attitudes regarding lung cancer screening and decision aids. a survey and focus group study. Ann Am Thorac Soc. 2016;13:1992–2001.
Dieng M, Trevena L, Turner RM, Wadolowski M, McCaffery K. What Australian women want and when they want it: cervical screening testing preferences, decision-making styles and information needs. Health Expect. 2013;16:177–88.
Hoover DS, Pappadis MR, Housten AJ, Krishnan S, Weller SC, Giordano SH, et al. Preferences for communicating about breast cancer screening among racially/ethnically diverse older women. Health Commun. 2019;34:702–6.
Greene MP, Vassy JL. Helping patients understand multi-cancer early detection tests: a scoping review. Per Med. 2024;21:131–37.
Hidalgo JL, Sotos JR, Herráez MJ, Rosa MC, López JL, Ortiz MP. Factors Associated with cancer worry among people aged 50 or older, Spain, 2012-2014. Prev Chronic Dis. 2015;12:E226.
Vrinten C, van Jaarsveld CHM, Waller J, von Wagner C, Wardle J. The structure and demographic correlates of cancer fear. BMC Cancer. 2014;14:597.
Logan L, McIlfatrick S. Exploring women’s knowledge, experiences and perceptions of cervical cancer screening in an area of social deprivation. Eur J Cancer Care. 2011;20:720–7.
Peters K. Politics and patriarchy: barriers to health screening for socially disadvantaged women. Contemp Nurse 2012;42:190–7.
Marlow LA, Wardle J, Waller J. Understanding cervical screening non-attendance among ethnic minority women in England. Br J Cancer. 2015;113:833–9.
Connor K, Hudson B, Power E. Awareness of the signs, symptoms, and risk factors of cancer and the barriers to seeking help in the UK: comparison of survey data collected online and face-to-face. JMIR Cancer. 2020;6:e14539.
Acknowledgements
We would like to thank our patient and public involvement representatives including: Sue Duncombe, Tim Ward, Rashmi Kumar and Julian Ashford for their insight throughout the design of this study.
Funding
This work was supported by GRAIL Bio UK Ltd. King’s College London sponsored this study. The funder played no role in the design, conduct, analysis or interpretation of the findings but did have the opportunity to review a draft of the manuscript prior to submission.
Author information
Authors and Affiliations
Contributions
Ninian Schmeising-Barnes: data curation, formal analysis, investigation, methodology, project administration, visualisation, writing—original draft, writing—review and editing. Jo Waller: conceptualisation, funding acquisition, investigation, methodology, supervision, writing—review and editing, visualisation. Laura A.V. Marlow: conceptualisation, data curation, formal analysis, investigation, methodology, project administration, resources, supervision, visualisation, writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
JW reports research income from GRAIL Bio UK Ltd, which funds 20% of her salary and the full salaries of LAVM and NSB through a contract with King’s College London/Queen Mary University of London.
Ethics approval and consent to participate
Ethics approval was granted by King’s College London, June 2023 (LRM-22/23-36139). Consent was assessed with a single question presented to participants after they had read the information page. This study was performed in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schmeising-Barnes, N., Waller, J. & Marlow, L.A.V. Intention to have blood-based multi-cancer early detection (MCED) screening: a cross-sectional population-based survey in England. Br J Cancer (2024). https://doi.org/10.1038/s41416-024-02822-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41416-024-02822-4
- Springer Nature Limited