Abstract
CAFs (cancer-associated fibroblasts) are highly flexible cells of the cancer microenvironment. They produce the extracellular matrix (ECM) constituents that form the structure of the tumor stroma but are also a source of metabolites, growth factors, chemokines, and exosomes that impact every aspect of the tumor, including its response to treatment. It is believed that exosomal miRNAs facilitate intercellular signaling, which is essential for the development of cancer. The role of miRNAs and CAFs in the tumor microenvironment (TME) and carcinogenesis is reviewed in this paper. The preferred reporting items for systematic reviews and meta-analyses (PRISMA) 2020 guidelines were used to perform a systematic review. Several databases, including Web of Science, Medline, Embase, Cochrane Library, and Scopus, were searched using the following keywords: CAFs, CAF, cancer-associated fibroblasts, stromal fibroblasts, miRNA, exosomal miRNAs, exosome and similar terms. We identified studies investigating exosomal miRNAs and CAFs in the TME and their role in carcinogenesis. A total of 12,572 papers were identified. After removing duplicates (n = 3803), 8774 articles were screened by title and abstract. Of these, 421 were excluded from further analysis. It has been reported that if exosomal miRNAs in CAFs are not functioning correctly, this may influence the secretory phenotype of tip cells and contribute to increased tumor invasiveness, tumor spread, decreased treatment efficacy, and a poorer prognosis. Under their influence, normal fibroblasts (NFs) are transformed into CAFs. Furthermore, they participate in metabolic reprogramming, which allows for fast proliferation of the cancer cell population, adaptation to growing energy demands, and the capacity to avoid immune system identification.
Similar content being viewed by others
Facts
-
CAFs interact with immune cells, cancer vasculature, ECM, and tumor cells in the TME to promote the growth of the tumor by secreting a range of cytokines and chemokines
-
CAFs are dynamic cells that play a crucial role in maintaining tumor structural integrity by producing ECM components and secreting factors that affect tumor growth, invasion, and response to treatment.
-
The exosomal miRNAs can modulate the behavior of NFs, CAFs, and tumor cells, affecting processes such as conversion from NFs to CAFs, tumor invasiveness, and immune evasion.
-
It is well recognized that aberrant expression of exosome-derived miRNAs in CAFs contributes significantly to the growth and spread of cancer.
Open questions
-
Why are CAFs so important in the TME?
-
What are CAFs in the TME?
-
What is the role of CAFs and exosomal miRNAs in cancer progression?
Introduction
MicroRNAs are a class of RNAs that are highly conserved and are approximately 25 nucleotides in length; they bind to the 3′ untranslated region (UTR) or open reading frames (ORFs) [1]. This causes gene silencing after the transcription of target mRNAs. miRNAs regulate gene expression in tumors by utilizing multiple signaling pathways. The miRNAs are important for controlling the interaction between CAFs and tumor cells, which affects how tumors grow and develop. Additionally, they can serve as indicators of cancer and objectives for tumor treatment [2, 3].
New insights have emerged concerning the relationship between normal fibroblasts (NFs), CAFs, and cancer cells through exosomal miRNA secretion. Extracellular vesicles can be classified into three primary forms: extracellular vesicles (EVs) are exosomes (~30–150 nm), ectosomes/microvesicles (100–1000 nm), and oncosomes (1–10 µM) [4]. These types of EVs vary in size and biogenesis. Vesicular trafficking, especially exosome-mediated trafficking, has received attention since each of these three vesicles has a significant function in the biology of cancer [5]. According to a recent study, the exosome secretion route is regulated by the Rab family proteins, such as 27a and 27b [6]. It is believed that exosomes facilitate intercellular transmissions, which are essential for cancer growth [7]. Various cell types in the TME secrete them. They are found in all physiological fluids and are taken up by surrounding cells. Exosomes generated from cancer may operate as intercellular messengers by turning microenvironmental cells into tumor-supportive cells by delivering physiologically active chemicals to these recipient cells [8].
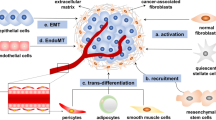
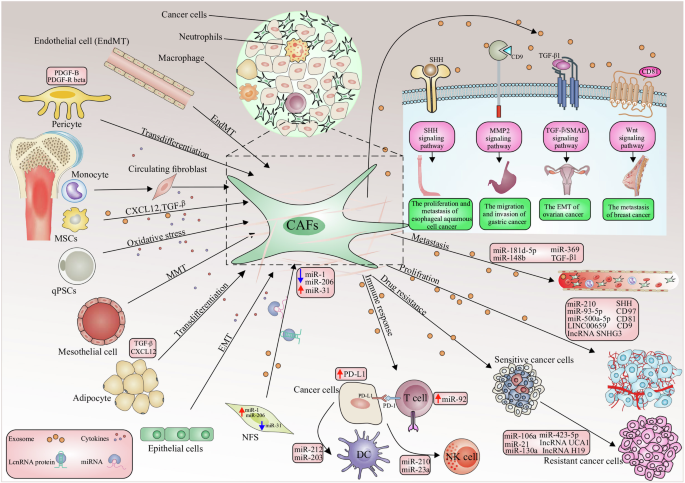
Exosomal miRNAs can originate from tumor cells or stromal cells such as CAFs [9]. The majority of research has focused on exosomal miRNAs produced from cancer cells [10]. Whilst the exosomal miRNAs produced by CAFs are receiving increasing interest, no studies have looked at their abnormal expression patterns in cancer patients. TME includes both the inherent and external variables that influence the development and dissemination of malignancies. It includes the structural, functional, and metabolic aspects of the tumor tissue, as well as the internal environment within the tumor cells. Tumor cells possess the capacity to impact their own survival and growth circumstances through autocrine and paracrine processes, consequently promoting tumor advancement [11]. The TME and the way that cancer cells interact with their microenvironment depend heavily on CAFs [9]. An essential part of CAF production and activation is played by malignant cells [12]. There are several origins of CAFs including 1- bone marrow cells; 2- mesenchymal stem cells (MSCs); 3- ECs (endothelial cells) and pericytes; 4- resident quiescent normal fibroblasts [13] (Fig. 1). Erdogan et al. claim that the direct interactions between tumor cells and other stromal cells, such as immunological and endothelial cells, cause CAFs to establish a certain biological phenotype [14].
The processes of cell conversion to CAFs consist of the following processes: EMT epithelial-to-mesenchymal transition, EndMT endothelial-to-mesenchymal transition, mesothelial-to-mesenchymal transition MMT. Also, CAF-derived exosomes play a role in the following processes, which include: tumor cell proliferation; conversion of drug-sensitive cancer cells into drug-resistant cancer cells; enhancement of the metastatic capacity of cancer cells; an antitumor immune response by regulating the activity of immune cells. CAF-derived exosomes initiate several molecular processes in tumorigenesis, including TGF-β, to promote EMT, the Wnt signaling cascade contributing to the metastasis of breast cancer, the MMP-2 signaling enhancing the migration and invasiveness of gastric cancer cells. The sonic hedgehog (SHH) signaling pathway thus increases the proliferation and metastasis of ESCC cells.
The TME is comprised of endothelial cells, fibroblasts, pericytes, immune cells, stem cells, and bone marrow precursors, as well as the ECM that surrounds them [15]. The homeostatic mechanisms that regulate the production and turnover of the ECM, are disturbed in tumors, resulting in the creation of aberrant blood vessels and accumulations of excess fibrillar collagen with a distinct structure [16]. Disrupted ECM homeostasis results in novel forms of paracrine, cell–cell, and cell–ECM interactions, all of which have significant implications for tumor development, angiogenesis, metastasis, immunosuppression, and treatment resistance [15]. The TME is crucial for the growth of CAF cells [17]. Additionally, there is evidence that miRNAs play a significant role in the TME as well as in tumor cells [18]. The complexity and diversity of the TME are gradually being understood through advancements in science[19].
The relationship between cancer cells and the TME is regulated by extracellular miRNAs (ex-miRNAs) and miRNAs. MicroRNAs have been shown to regulate the expression of genes that are expressed post-transcriptionally and to be involved in a wide range of normal and abnormal cellular processes [20]. Recent progress in the assessment of exosomes has made it possible to use miRNAs as biomarkers or therapeutic tools in clinical practice [21]. Mesenchymal stem cells (MSCs) have the potential to differentiate into CAFs when they interact with miRNAs from tumor exosomes within the TME. CAFs can help with several aspects of tumor development, such as growth, EMT, metabolism, invasion, and metastasis. CAFs have the ability to generate pro-angiogenic factors, which can promote angiogenesis and tumor development [22]. CAFs are proliferative, metabolically active cells that resemble fibroblasts and can be implicated in any stage of the development of cancer. According to earlier research, CAFs and tumor cells communicate with one another through the secretion of several chemokines and cytokines that are part of the ECM [23]. Tumor cells can communicate with neighboring cells by secreting soluble molecules into the extracellular space, according to recent research on small extracellular vesicles (sEVs) [24, 25]. Exosomes contain a great variety of bioactive molecules, including signal peptides, microRNAs, lipids, and DNA [26].
Tumor cells release large amounts of exosomes to interact with the environment across extended distances or to deliver paracrine signals [27]. Therefore, by changing the content of vesicles and transporting chemicals to the tumor site that promote the appearance of oncogenic processes such as proliferation, invasion, proliferation of cancer stem cells, and even treatment resistance, exosomal miRNAs can affect carcinogenesis and cancer progression [28].
This review focuses on how exosomal miRNAs influence intercellular interaction and are crucial in the way cancer cells interact with their macro- and microenvironment. Exosomes are a constant carrier of miRNAs. Under different circumstances, miRNAs in the plasma exosome can be maintained. The function of exosomal miRNAs and CAFs in the TME and the development of cancer have been reviewed.
The study search method
Methods
Our study followed the guidelines of preferred reporting items for systematic reviews (PRISMA) 2020 guidelines for systematic reviews [29]. To review exosomal miRNAs and CAFs in the TME and carcinogenesis, we searched: Medline, PubMed, Cochrane Library, Embase, Web of Science, and Scopus. We considered studies up until Jan 2023. Additionally, we searched preprint articles on servers like medRxiv and the Social Science Research Network (SSRN). In this search, the following keywords were used: (([Mesh] “MicroRNAs” MicroRNA OR [Handle/Summary] OR miRNAs [Abstract/Title] OR “MicroRNA” [Abstract/Title] miRNA OR [Handle/Summary] OR [Title/Abstract] “Small Temporal RNA” The terms “Tumor-associated fibroblasts” and “Cancer-Associated Fibroblasts” are interchangeable in Mesh. Fibroblasts linked to OR tumors [Title/Abstract] OR “Fibroblasts associated with cancer” [Mesh] OR Fibroblasts associated with cancer [Title/Abstract] OR Fibroblast [Title/Abstract] OR “Fibroblasts” [Mesh] OR “Stromal fibroblasts “[Mesh] Alternatively stated [Title/Abstract])) AND “tumor-associated”[Mesh]“tumor microenvironment “[Mesh] OR Microenvironment *[Introduction/Title] AND (Cancer)[Handle/Summary] OR Carcinogen* [Abstract/Title] Neoplasm OR *[Handle/Summary] Cancer [Handle/Summary] AND (Exosomes*[Title/Abstract] OR (“Exosomes” [Mesh])). To find articles not found by the automated search, a manual search of reference lists and reviews was conducted for potentially eligible articles. We identified duplicate studies and excluded these. We screened the titles and abstracts of the studies and then reviewed the full texts of the articles in two rounds. Two authors, RN and MM, independently studied the two phases. We resolved any disagreements by consulting with RS, the third author.
Search strategy
Study eligibility criteria: inclusion and exclusion
This review focuses on primary research that examined the relationship between exosomal miRNAs and CAFs in the tumor microenvironment and carcinogenesis. This systematic review was conducted using the following criteria for inclusion: original studies published in either English or Persian that have looked into the role of exosomal miRNAs and CAFs across various types of cancer. Based on the PRISMA- 2020 PICO question formulation checklist, which includes P: Population/Disease/Issue I: Intervention, C: Comparison, O: Outcome, and Text, they will assess the article’s suitability. Our focus was on the TME and carcinogenesis as an outcome, but we also looked at the connection between exosomal miRNAs and CAFs in articles where carcinogenesis was not reported. The current review determined the frequency of exosomal miRNAs and CAFs in cancer patients. Case reports, case series, and studies with less than 6 participants, clinical trials, editorials, commentaries, letters to editors, and narrative reviews were excluded. Additionally, we excluded studies that only looked at exosomal miRNAs and CAFs in specific patient populations, such as those with diabetes or dementia, and studies that involved animals or laboratory settings.
Data extraction
In order to gather articles, eliminate duplicates, and evaluate titles and abstracts, an Endnote library (Version x9) was performed. To begin the process of extracting the data, an expert checklist based on the information gleaned from the articles was created. Only after that was the data extraction carried out. In the studies, information such as the authors’ names, year of publication, country, study design, sample size, age, and gender of patients the study’s specifics, the sample type, the publisher’s nation or area, the detection strategy, and the key findings were collected.
Results
PRISMA flow diagram: study selection process
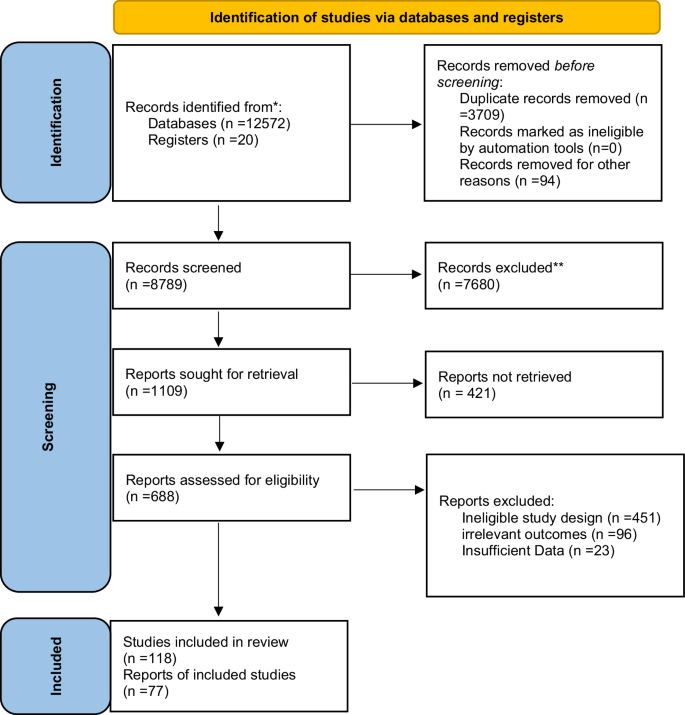
The study selection process is illustrated in the PRISMA Flow Diagram (Fig. 2), outlining the identification, screening, eligibility, and inclusion of studies in this systematic review. 12,572 studies were identified after searching the different databases. 3709 studies were omitted because they were duplicates. The other 8789 articles were checked for eligibility. After evaluating the full texts, 421 studies out of 1109 with titles and abstracts were not included in the analysis (Fig. 2). Finally, 77 studies listed in Table 1 were included to perform a systematic review.
*Consider, if feasible to do so, reporting the number of records identified from each database or register searched (rather than the total number across all databases/registers). **If automation tools were used, indicate how many records were excluded by a human and how many were excluded by automation tools.
Potential role of exosomal miRNAs in the colonization of metastasized CAFs cells
Exosomes and CAFs have important functions in the TME. Exosomes, which are vesicles generated by tumor cells outside of the cells, contain proteins that can interact with immune cells and hinder their capacity to kill tumors, thereby facilitating the evasion of cancer by the immune system. Conversely, CAFs play a crucial role in inhibiting the immune response within the TME, hence promoting tumor invasion and dissemination. Both exosomes and CAFs play a role in creating an immunosuppressive environment in the TME [30].
Because of the distribution of exosomes to neighboring cells, CAFs play a critical role in regulating the formation of malignancies [31]. Drug resistance, angiogenesis, invasion, metastasis, and tumor development are all significantly influenced by exosomal miRNAs. They control mechanisms that affect the microenvironment and cancer immunity. Two examples of the endosomal sorting complex required for transport (ESCRT)-containing proteins that are in charge of exosome formation are Alix (Apoptosis-linked gene 2-interacting protein X) and Tsg101 (Tumor susceptibility gene 101 protein). Proteomic investigations of isolated exosomes from various cell types have found Tsg101, Alix, and ubiquitinated proteins [32].
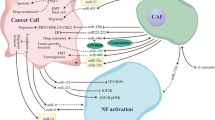
Recent studies have shown that cancer cells generate more exosomes than normal cells, even in the very early phases of carcinogenesis. Analysis of proteomic profiling of blood-circulating extracellular vesicles in breast cancer holds great promise for early detection and diagnosis [33]. Tumor-derived exosomes (TEX) are exosomes generated by tumors that are crucial for managing tumor cells inside the TME. It was discovered that TEXs were significant even in the pre-cancer stage. Furthermore, TEXs with physiologically active miRNAs can mirror the unique features of different types of cancer, impacting tumor growth. Induction of the TEM from melanoma cells [34], transfer of miRNAs supporting 1-integrin-NF-kB signaling from metastatic liver cancer cells [35], improvement of breast cancer cell motility and ECM remodeling pathways [36], and activation of SOCS1/JAK2/STAT3 signaling from melanoma cells [37] (Fig. 1). According to a recent study, the presence of miRNAs in TEXs may help tumors survive by changing NFs into CAFs. In human melanoma cells,miR-21, miRNA-211, miR-222, miR-214, miR-155, and miR-210 may enhance the activation of NFs to undergo CAF transformation [38]. Consequently, there may be a considerable shift in the CAF gene’s expression [39]. It is thought that miR-21 is the primary regulator of oncogenic processes. It enhances cell survival, CAF formation, and activation by regulating TGF-β1 signaling [40]. Healthy hepatic stellate cells were converted into CAFs by isolated exosomes from patients with hepatocellular carcinoma (HCC) via miR-21, which downregulated PTEN (Tumor suppressor gene) and activated the PI3K/AKT signaling pathway [41].
miR-21 has been reported in several types of tumors. The exosomal miR-21-5p released by cancer cells promotes angiogenesis and it is involved in neoplastic processes [42]. An interesting point is related to the promotion of secretion of angiogenic factors through exosomal miR-21 released from cancer cells and its role in neoplastic processes [43]. MiR-21 has been shown to activate NFs and produce the CAF phenotypic markers SMA and S100A4, respectively in studies from colorectal and malignant esophageal cancer cells [39]. The two most common miRNAs, miR-21 and miR-146a, which are recognized as significant regulators of CAF formation, were involved in this process [44, 45]. Furthermore, by stimulating the expression of transforming growth factor-β (TGF-β)/ Smad pathway, which can cause endothelial cells (ECs) to convert into CAFs (EMT), exosomal miR-21-5p induces EMT in gastric cancer (GC) [46] (Fig. 1). Next-generation sequencing technology has shown that the exosomal transfer of miR-21 from CAFs to OVCA (Ovarian cancer) cells inhibited apoptosis and increased resistance to treatment with paclitaxel by reducing the expression of apoptotic peptidase activating factor (APAF1) [47].
When exosomal miR-21-5p causes the peritoneal cavity to undergo mesothelial–mesenchymal transition (MMT), lung cancer is more likely to spread [48]. Mesothelial cells have been identified as a source of CAFs in peritoneal carcinomatosis (PC) [49]. Therefore, the exosomal interaction between tumor cells, CAFs, and tumor-associated macrophages (TAMs) is a major factor in the growth of malignancies [50]. While miR-10b, miR-6819-5p, miR-6737-5p, and miR-1249-5p have been shown to have indirect oncogenic activity via controlling fibroblast function [51,52,53]. CAFs release exosomal miR-1228, which has the ability to enhance the invasion and migration of osteosarcoma by targeting suppressor of cancer cell invasion (SCAI). Exosomal collagen type VI alpha 1 (COL6A1), transforms healthy fibroblasts into CAFs, facilitating the invasion and migration of osteosarcoma (OS) cells via the TGF-β/COL6A1 signaling pathway [54, 55]. In the context of OS, studies have shown that OS cell-derived exosomal components, such as COL6A1 and leukemia inhibitory factor receptor antisense RNA1 (LIFR-AS1), can induce the transformation of NFs into CAFs. Once activated, these CAFs can enhance the migration and invasion of OS cells through the secretion of proinflammatory cytokines and interaction with microRNAs. Additionally, the interaction between CAF-secreted exosomes and OS cells can promote malignancy grade and contribute to the development of OS. Moreover, CAFs contribute to immune evasion and tumor progression by modulating the immune microenvironment, promoting the differentiation of myofibroblasts, and enhancing angiogenesis [55]. Furthermore, CAF-derived exosomes have been implicated in promoting angiogenesis within the TME, which is a crucial process for tumor growth. These exosomes can deliver pro-angiogenic factors and signaling molecules to endothelial cells, stimulating the formation of new blood vessels to support the growing tumor. Additionally, CAF-derived exosomes have been associated with immunosuppressive effects, influencing the immune response within the TME and facilitating immune escape by cancer cells [54].
To what extent can the presence of CAFs affect the development of cancer?
Numerous cell processes, including tumor development, EMT, metabolism, metastasis, and invasion can all be accelerated by CAFs [56]. The EMT plays an important role in both carcinogenesis and embryogenesis. EMT is mostly responsible for the invasion of single cells, which modifies the morphology and adhesion characteristics of cancer cells and represses epithelial markers in favor of mesenchymal signatures. The expression of genes associated with EMT may be stimulated by the interaction between β-catenin accumulation and T-cell factor/lymphoid enhancer factor (TCF/LEF), a crucial EMT initiator [57]. A study has demonstrated that the high heterogeneity of CAFs allows them to exhibit two functionalities in disease: while CAFs can promote angiogenesis, inflammation, immunosuppression, and metastasis, which ultimately lead to the growth of tumors, the antitumor characteristics of fibroblasts contribute to processes such as ECM production, the growth and differentiation of epithelial cells, and the response to tissue damage [58]. CAFs can also produce a variety of growth factors and proinflammatory cytokines, such as TGF-β, vascular endothelial growth factor(VEGF), interleukin-6 (IL-6), and CXC chemokine ligand 12 (CXCL12), to support angiogenesis and attract immunosuppressive cells into the TME to aid in immune evasion [59]. Furthermore, CAFs can contribute to cancer development indirectly (via soluble factors and miRNA) as well as directly (through intercellular communication). Exosomes generated by CAFs control the TME and mediate the growth and dissemination of cancer cells [56]. Numerous bioactive substances, including DNA, lipids, signal peptides, and miRNAs, are carried by exosomes. Among these molecules are exosomal miRNAs, which are important regulators of the TME. It is well recognized that aberrant expression of exosome-derived miRNAs in CAFs contributes significantly to the growth and spread of cancer [60] for example, miR-9 and miR-200s induce NFs in TME to transform into CAFs and promote tumor metastasis [36, 61].
CAFs interact with immune cells, cancer vasculature, ECM, and tumor cells in the TME to promote the growth of the tumor by secreting a range of cytokines and chemokines. Proliferative signaling is aided by a number of CAF-derived substances that help cancer cells evade growth suppressors and withstand cell death. Exosomes from the CRC cell lines HT-29 and SW480, perhaps via miRNA let-7d, inhibited the migration of CC-type chemokine receptor 2 (CCR2) monocytes (THP-1 cells) and the release of chemokine (C-C motif) ligand 7 (CCL7) from CAFs in vitro [62]. According to Fullár et al., membrane-type 1 matrix metalloproteinase (MT1-MMP), which is generated by tumor cells, activates inactive matrix metalloproteinase-2 (MMP-2) produced by fibroblasts. The fine-tuning of cancer cell invasion is dependent on the activated MMP-2 [63]. In oral squamous cell carcinoma (OSCC) is further increased by the miR-34a-5p/AXL axis. The AKT/GSK3β/β-catenin signaling pathway facilitates this process. This may trigger EMT, which raises the metastasis of cancer cells [31]. MiR-451 is a tumor suppressor that is regulated in various types of tumors. In contrast, CAFs use small molecules called exosomal miR-451 to signal and help cancer cells move and grow [64]. Furthermore, it has been shown that the miRNA levels in CAFs and cancer cells may vary differ from those in exosomes [9]. Tumor stroma has been demonstrated to have a large distribution of CAFs. Myofibroblasts and fibroblasts combine to become CAFs [65]. It is well known that these CAFs stimulate angiogenesis, which contributes to tumor development and, consequently, to the growth and spread of cancer cells [66] for example miR-526b and miR-655 promote angiogenesis and lymphangiogenesis in TME [67].
Recent studies suggest that unregulated epigenetic control of gene expression and metabolic adaptive response may be the cause of the aberrant activation of CAFs [68]. Regular fibroblasts (NFs) are not like activated CAFs; instead, they change cell surface markers. During carcinogenesis, CAFs’ pro- and antitumorigenic activities are probably dynamic. For example, in order to enhance the CAF phenotype in breast cancer, cancer cells might interact with fibroblasts to stimulate Notch signaling [69]. However, since squamous cell carcinoma may enhance CAF phenotypes when Notch signaling is absent, this method probably isn’t relevant in all situations [70]. There is a transfer of gastric cancer (GC)-derived exosomal miR-27a to fibroblasts. This transfection leads to a decrease in cysteine and glycine rich protein 2 (CSRP2) expression, an increase in α-SMA expression, and the separation of fibroblasts into CAFs [71]. Pancreatic adenocarcinoma (PAAD) remains one of the most common and lethal tumors. Comparing the tissues of PAAD to the corresponding normal tissues, it was shown that the expression of ACTA2, FAP (fibroblast activation protein), PDGFRα/β (platelet-derived growth factor receptor-α/β), and S100A4 (which is widely used as a marker to detect CAFs) was significantly overexpressed [72]. Numerous inflammatory modulators can activate the CAFs [73]. Interleukin-1 (IL-1) acts via NF-kB and IL-6, and the transcription factor STAT (signal transducer and activator of transcription) [74]. Crosstalk and positive feedback mechanisms such as JAK (Janus kinase)-STAT signaling, the contractile cytoskeleton, and modifications in histone acetylation are the root causes of the further increase in CAF activation [74]. Furthermore, physical alterations occur in the ECM, and CAF is activated [75]. Therefore, tumor cells are the primary source of CAFs, which accelerates the development of tumors.
The Importance of exosomal miRNAs secreted from CAFs in the spread of cancer and the CAFs
The importance of the microenvironment for the development, maintenance, and spreading of the tumor mass has been mainly shown [76]. The majority of cell types, including cancer cells, release exosomes, which are nanoparticles with a diameter of 30–150 nm that facilitate communication between surrounding cells and tumors [75].
Given that miRNAs are crucial regulators of tumor functionalities, it is becoming more and clearer that alterations driven by cancer govern these miRNA-based networks. A member of the α-arrestin protein family is thioredoxin-interacting protein (TXNIP). It is regarded as a thioredoxin-activity endogenous inhibitor. It has been established that TXNIP had a role in the development of colorectal cancer (CRC). Key components in the formation of CRC are TXNIP and miR-135b-5p from CAFs. CAF exosomes may regulate the production of miR-135b-5p to affect CRC cell proliferation by blocking TXNIP [18].
Unlike other solid tumors, gynecological malignancies have seldom been observed to include exosomes; in particular, endometrial cancer (EC) exosomal miRNAs are thought to play a significant role as a mediator in this two-way communication between cells [77]. However, exosomal miRNAs act as a bridge for information exchange between TAMs, CAFs, and EC cells. They are crucial for the growth of tumor cells, the EMT transition, and ultimately the development of TMEs. Exosomes generated by CAF stimulate the proliferation of EC. Exosomes produced from EC-CAFs may include downregulated miRNAs with tumor suppressor characteristics. Comparatively to NFs, EC- CAFs contained and released substantially less miR-148b in exosomes. DNA methyltransferase 1 (DNMT1) is suppressed when miR-148b translocates to EC cells. In the event that this suppression is not achieved, EMT will rise and EC will get stronger. Accordingly, the downregulation of miR-148b in EC-CAF-derived exosomes contributes to both EC cell invasion and metastasis. Consequently, treating EC with miR-148b overexpression may be therapeutic [78]. Similarly, miR-320a expression in EC cells, EC- CAFs-derived exosomes, and EC-CAFs will decline. MiR-320a suppresses the HIF1α/VEGFA axis, which inhibits the proliferation of EC cells [79]. Poor prognostic indicators such as HIF1α and VEGFA are indicative of EC [63]. Additionally, there is a link between the rise in EC cell radiation sensitivity and the decline in HIF1α/VEGFA levels [80]. Exosomes that overexpress miR-320a may also be employed to treat EC sufferers. Studies on serum exosomal miRNAs and miRNAs produced from CAFs in solid tumors [81, 82] as well as supraglottic laryngeal squamous cell carcinomas (SLSCC) have also been conducted [83, 84]. In patients with SLSCC, exosomal miRNAs generated from CAFs had aberrant expression. Together with their target genes (CCND1, CDKN1B, CDK6, PTEN, and FOS), miR-16-5p, miR-29a-3p, miR-34c-5p, miR-32-5p, and miR-490-5p may form a carcinogenic TME and function as biomarkers for SLSCC therapy [10].
There is evidence that in hepatocellular carcinomas (HCCs), exosomes produced from CAF may control the TME. The wnt/β-catenin signaling pathway is decreased by CAF-derived exosomes containing miR-20a-5p oncogene. It causes the inhibition of actin-binding 1 (LIMA1) in HCC and the inhibition of tumor suppressor LIM domain [85]. GSK3β was effective in β-catenin degradation in prostate cancer (PC). The reason for the growth of PC cells and metastasis through the regulation of GSK3β/β-catenin signaling was actually miR-1290 exosomally secreted CAFs [86]. Exosomes have a crucial function in facilitating the spread of metastatic castration-resistant prostate cancer (CRPC) by transporting proteins such as APOE, LRG1, and ITIH that are directly implicated in tumor growth and the formation of secondary tumors in the bones. The diagnostic relevance of these exosomal proteins in differentiating between CRPC and PC patients is important, and targeting them could be a feasible strategy for CRPC therapy. Moreover, exosomes discharged by PC cells exhibiting diverse androgen response characteristics can impact the formation of new blood vessels in CRPC, with miR-27a-3p being recognized as a crucial mediator in this mechanism. Gaining a comprehensive understanding of how extracellular vesicles contribute to the spread of metastatic CRPC is essential for identifying and developing novel treatment targets for advanced CRPC [87].
The reverse transcriptase telomerase, which lengthens telomeres, is one of the characteristics that set cancer cells apart. This is due to the fact that whereas most somatic cells lack the enzyme, the majority of malignant cells possess this enzyme. Through the upregulation of two CAF markers, α-smooth muscle actin (SMA) and vimentin, exosomal telomerase may aid in the conversion of NFs into CAFs. Telomerase activity modifies the miRNA transcriptomes in these fibroblasts. The proliferative phenotype that these cells adopt after eating exosomal human telomerase reverse transcriptase (hTERT) may be influenced by one of the most well-known miRNAs, miR-342, which is thought to be expressed ectopically as a survival benefit [88]. Moreover, exosomes contain a significant amount of circular RNAs (circRNAs), which have a crucial function in various physiopathological processes including the migration and growth of tumor cells, vascularization in tissues, and the development of tissues and organs. Tumor cells communicate with one another by exchanging exosomal circRNAs, which are signaling molecules, to enhance the growth and progression of tumors. Exosomes have a regulatory impact on tumor formation and change of the tumor microenvironment by carrying circRNAs to tumor cells or other cells in the microenvironment [89]. Exosomal circRNAs play crucial roles in the TME by modulating cancer hallmarks, including angiogenesis, EMT, invasion, and metastasis. They can also regulate anticancer immunity and enhance chemoresistance [89, 90]. Exosomal circRNAs can be used to monitor tumor prognosis and predict postoperative recurrence. For instance, in bladder urethral epithelial carcinoma (UCB), circPRMT5 is overexpressed and positively correlated with low survival in patients. Similarly, a high expression of exosomal circPDE8A is a risk factor for patients with pancreatic ductal adenocarcinoma (PADC), and lower levels of exo-FECR1 are associated with longer disease remissions in small cell lung cancer (SCLC) patients [90]. In bladder cancer, the expression of CircNFIX in serum exosomes is significantly increased in patients with tumor recurrence compared to those with primary tumors [90]. As research on exosomal circRNAs progresses, exosomal circRNAs are anticipated to serve as biomarkers for early detection of bladder cancers and as targeted therapy tools [89]. Exosomal circRNAs have been shown to regulate melanoma progression by modulating the TME. For example, exosomal circRPS5 can inhibit melanoma cell proliferation and invasion [91]. Recent studies have shown that exosomal circRNAs play critical roles in conferring chemotherapy resistance in diverse cancers. Exosomal circRNAs can act as miRNA sponges, interact with RNA-binding proteins, and even encode proteins, thereby modulating the expression of genes involved in drug resistance pathways [92]. In these cancers (lung cancer, gynecological cancers, glioma, breast cancer, prostate cancer, multiple myeloma, and oral squamous cell carcinoma), specific exosomal circRNAs were found to be upregulated and associated with increased proliferation, migration, invasion, and apoptosis resistance in chemotherapy-resistant cancer cells. Mechanistic investigations revealed that these exosomal circRNAs typically regulate chemoresistance by sponging miRNAs and altering the expression of target genes [92].
The role of CAFs in the resistance to anticancer drugs
Exosomal miRNAs originating from cancer can promote CAF differentiation, and exosomal miRNAs released by CAFs in the TME are essential in the development of therapeutic resistance. Tumor-derived exosomes may have a major impact on the differentiation of CAFs, which increases tumor development, invasive, pro-angiogenic, and drug-resistant phenotypes [34, 93]. Furthermore, Cancer’s response to chemotherapy is controlled by CFAs. Premetastatic lung fibroblasts were similarly stimulated by exosomal miR-1247-3p from HCC cells, which resulted in the overexpression of IL1B, IL-6, and IL-8 as well as resistance to sorafenib therapy [35]. Similarly, exosomal miR-1247-3p from HCC cells triggered CAF in fibroblasts of a lung premetastatic niche. Proinflammatory genes such as IL1B, IL-6, and IL-8 were upregulated as a result, and sorafenib therapy led to the development of therapeutic resistance [35]. Sarfenib is regarded as a targeted therapy for the treatment of advanced HCC, despite its modest effect in terms of overall survival because of drug resistance [94]. In terms of clinical prognosis, sorafenib is unsuccessful due to inflammatory interleukins such as IL-6 [94].
Diverse subtypes of CAFs coexist in pancreatic cancer tissues, where they have the dual ability to accelerate and impede the disease’s progression. Here, cancer cells with p53 mutations that cause GOF (gain of function) give rise to the prevailing population known as CAFs [95]. As a result, it gives p53-null and GOF cancer cells a metastatic environment [95]. GOF mutant p53 cells or their CAFs have the ability to rewire CAFs that were trained by null p53 cancer cells. One important component of this pro-metastatic milieu is perlecan (Heparin sulfate proteoglycan 2, or HSPG2). These dominating CAFs impede the capacity of cancer cells to respond to chemotherapy. Because decreasing perlecan in the stroma in conjunction with chemotherapy boosts mice survival, perlecan is a possible target for anti-stromal treatments in pancreatic cancer. CAFs have the ability to release exosomes carrying miR-20a, which can speed up the development and chemoresistance of non-small-cell lung cancer (NSCLC) [96]. CAF-derived exosomes, especially those that are CD9-positive, inhibit the growth of malignant melanoma cells. The five-year disease-free survival rate was considerably greater in patients who had CD9-positive CAF-made exosomes compared to patients who lacked CD9 exosomes [97]. Tumors with high levels of periostin-positive (POSTN) CAFs significantly decreased the overall survival of the patients [98]. POSTN+CAF showed minimal expression of smooth muscle actin and high rates of protein synthesis and proliferation when they were found in peri-/pre-tumoral regions. They were associated with highly malignant tumors and macrophage infiltrates. The recruitment of dendritic cells and immune-related markers was associated with CAFs that were podoplanin-positive. Specific TME traits were associated with the simultaneous presence of POSTN+CAF and podoplanin-positive CAFs, as measured by stromal abundance and immune cell infiltrates. Although the published myofibroblastic CAF (myCAF)/iCAF categorization was unrelated to POSTN+CAF, podoplanin-positive CAFs showed a fraction that resembled the inflammatory CAF (iCAF). According to these findings, a POSTN+CAF is an early, activated CAF that is associated with aggressive malignancies, whereas a podoplanin-positive CAF is connected to an immune-related phenotype. Together, these two categories establish specific TME and influence patient prognosis; they might be useful for future patient stratification. Targeting or reprogramming “bad” CAF populations (e.g., POSTN) may lead to the development of a novel treatment strategy for pancreatic ductal adenocarcinoma (PDAC) [98]. The platinum-based medication cisplatin creates adducts in the DNA of cancer cells to exert its anticancer effects. These adducts drive cancer cells to undergo apoptosis, or cell death, by inducing the DNA damage response. Cisplatin resistance is a major issue for people with bladder cancer (BC). It has been demonstrated that the delivery of bioactive compounds by exosomes derived from CAFs (CAF-Exo) can enhance chemotherapy resistance in a range of human tumors. Exosomal LINC00355, generated from CAFs, enhances BC cell resistance to cisplatin by modulating the miR-34b-5p/ABCB1 axis [99]. A recent study found that CAF-Exo miR-98-5p downregulates CDKN1A, a crucial regulator of cell cycle arrest, increasing OVCA cell proliferation and fostering a cisplatin-resistant phenotype [100]. Interestingly, several exosomal miRNAs from CAF have been shown to enhance cisplatin resistance. For example, in head and neck cancer cells, CAF-Exo miR-196a reduced the expression of CDKN1B (Cyclin Dependent Kinase Inhibitor 1B), a gene critical for the change from the G1 to the S phases of the cell cycle [101]. Furthermore, miR-522, which was produced from CAF exosomes, conferred cisplatin resistance to gastric cancer cells [102]. In the pancreatic TME, CAF-Exo miR-106b was found to directly reduce tumor protein 53-induced nuclear protein 1 (TP53INP1) expression, which in turn increased gemcitabine resistance in pancreatic cancer cells [12]. In patients with PAAD receiving gemcitabine therapy, exosomal miR-146a, which is released by CAF, has also been shown to target Snail pathways and accelerate the establishment of the gemcitabine-resistant phenotype [103]. Nano-drug delivery systems (nano-DDSs) can be designed to respond to TME stimuli, enhancing drug retention, accumulation, penetration, and tumor cell uptake [104]. Exosomes have emerged as a promising drug delivery platform for cancer therapies, including melanoma treatment [91, 105]. Exosomes can be engineered to enhance their tumor-targeting capabilities and drug delivery efficiency. Strategies include surface decoration and loading exosomes with therapeutic cargoes [91, 105]. Engineered exosomes can deliver drugs to tumor sites and temporally release therapeutic molecules, improving on the limitations of conventional molecular-targeted drugs [91]. Moreover, nanomaterials used in tumor immunotherapy are into two main categories: organic and inorganic nanomaterials, organic nanomaterials include polymers such as polylactic–co-glycolic acid (PLGA), polyethyleneimine (PEI), polyethylene glycol (PEG), polycaprolactone (PCL), and polyglutamic acid (γ-PGA), as well as cell membrane-derived structures like tumor cell membranes, macrophage membranes, platelet membranes, and erythrocyte membranes. These organic nanomaterials exhibit advantageous properties like biocompatibility but may face challenges such as instability and rapid metabolism. In contrast, inorganic nanomaterials like graphene, black phosphorus, and silicon demonstrate enhanced stability and drug delivery capabilities but potentially pose greater toxicity concerns [106]. Lili Cheng et al. designed and created a therapeutic nanovesicle known as hGLV. Cheng et al. developed a hybrid therapeutic nanovesicle, named hGLV, by combining genetically modified exosomes with drug-containing thermosensitive liposomes. The overexpression of CD47 in hGLV was seen to modify the process of tumor cell phagocytosis by macrophages through the inhibition of CD47 signaling [107] Nanoparticle-enhanced radiotherapy is significant in triggering robust cancer immunotherapy because it allows for the combination of antitumor drugs to kill tumors while also enhancing the body’s immunity. This multi-treatment effect is achieved through the use of multifunctional nanomaterials, which can overcome the limitations of traditional immunotherapy strategies such as low bioavailability, low response rates, and severe side effects. By utilizing nanotechnology, researchers have been able to break through the bottleneck problem of antitumor immunotherapy, offering new possibilities for more effective cancer treatment [108].
Discussion
The CAFs within the TME interact with cancer cells in ways that are essential to the growth and dissemination of disease. It is now recognized that within the TME, CAFs, and myofibroblasts are extremely diverse cells with unique gene expression patterns and a variety of biological roles that often contradict one another [72] (Fig. 1). Multiple CAF subpopulations may be seen inside a single tumor. CAFs partly mediate their actions by altering the ECM and secreting soluble components and EVs. EVs, of which exosomes are a subclass, are microscopic sacs that contain a range of biomolecules, such as proteins, nucleic acids, and lipids. Exosomes transport a payload of lipids, proteins, and nucleic acids that resemble the biological makeup of the cells from which they were derived. The tumor’s malignant nature is regulated by its surroundings. The TME is composed of biological components such as tumor cells, CAFs, endothelium, and immunological cells, as well as non-cellular components such as exosomes and cytokines, which all play important roles [109]. CAFs are stromal cells within the TME that have been found to promote tumor growth, invasion, and metastasis. They play a significant role in creating a supportive environment for cancer cells by secreting various factors that aid in tumor progression. In particular, CAF-derived exosomes have been implicated in mediating communication between CAFs and cancer cells, thereby promoting tumor progression and therapy resistance. These exosomes may contain specific bioactive molecules that contribute to the crosstalk within the TME, ultimately impacting tumor growth and response to therapy [92]. CAF secretes proinflammatory cytokines such as IL-1 and IL-8, both of which have pro-tumor effects. According to studies, tumor-derived exosome miRNA-1247-3p can activate tumor-associated fibroblasts, causing them to produce cytokines such as IL-6 and IL-8, which promotes lung metastasis of liver cancer [35]. Numerous investigations have revealed that exosome-associated miRNA-9 and miRNA-200s enhance metastasis and the transformation of NFs into CAFs [36]. Multiple vascular supports are required for cancer progression and can provide nourishment and growth factors to tumors. Exosomes released in low oxygen conditions augment the stem cell characteristics in Ewing’s sarcoma by delivering a concentrated amount of miR-210 that suppresses the apoptotic pathway, resulting in cell viability and promoting the creation of cellular spheres. In the microenvironment of bone sarcomas, EVs have a vital function in facilitating communication between different cells. Additionally, they can be used as biomarkers to aid in the diagnosis and prognosis of these conditions. An analysis is conducted on the role of EVs in facilitating communication between various cells inside the microenvironment of bone sarcomas. This research yields novel insights that can aid in the identification of therapeutic targets and diagnostic analysis [110].
Previous studies have demonstrated that exosomal miRNAs released by tumor cells have a significant influence on vasculature remodeling via IL-8-activated VCAM-1. Exosome-associated miRNA-526b and miRNA-655 also increase lymphangiogenesis and angiogenesis [111], whereas miRNA-340-5p and miRNA-561 promote an immunosuppressive TME [112, 113]. Exosomes produced from CAFs have been demonstrated to greatly promote OVCA tumor growth. CAFs directly transported exosomes into OVCA cells to boost intracellular circIFNGR2 levels. Exosomal circIFNGR2 activation inhibited cell proliferation, metastasis, and EMT. Mechanistically, elevated circIFNGR2 activated the miR-378/ST5 axis, preventing tumor cells from evolving into malignant cells [109]. The methods by which CAFs influence PC tumorigenesis remain unknown. When CAFs-Exo was compared to NFs-Exo, the level of miR-1290 was considerably greater. CAFs dramatically improved PC cell motility, invasion, stemness, and metastasis by transmitting exosomal miR-1290 [114]. The lack of precise CAF markers substantially limits depletion techniques. Nonetheless, CAF depletion was the first technique to be shown as a supplement to immunotherapies. Reducing FAzP (fibroblast activation protein)+ CAFs enhanced the anticancer vaccine’s efficacy, according to seminal research by Kraman et al. [115]. Recent research has identified CAF-mediated CXCL12 expression as a defining characteristic of the “CAF-S1” immunosuppressive subtype of breast cancer myofibroblastic CAF (myCAF) [116]. Pre-clinical studies have also shown that the clinically approved inhibitor AMD3100 is effective in blocking the interaction between CXCL12 and its cognate receptor CXCR4 [117, 118]. The complex and intricate signaling network that involves TGF-β, PI3Ks/AKT/mTOR (Mammalian target of rapamycin), MAPK (Mitogen-activated protein kinase), Wnt, Janus kinase/signal transducers and activators of transcription, EGFR (Epidermal growth factor receptor), Hippo, and nuclear factor kappa-light-chain-enhancer of activated B cells, among other signaling pathways, makes CAFs vulnerable to crosstalk with cancer cells. Exosomes carrying miRNA are released by CAFs, healthy fibroblasts, and cancer cells that form a network of cell-to-cell communication in the TME. These CAF signals reveal distinct characteristics as the disease advances and may be the target of anticancer therapy.
Conclusion
CAFs are essential elements of the TME and play an important role in the interaction between cancer cells and their environment. An essential function of cancer cells is to mediate the development and activation of CAFs. Furthermore, the development of cancer is dependent on both CAF activation and malignant cancer cells. Oncological behaviors are caused by crosstalk between cancer cells and CAFs. Within the TME, NFs, cancer cells, and CAFs all release miRNA-containing exosomes that facilitate cell-to-cell communication. Consequently, CAFs require a focused treatment that improves anticancer therapy in both in vivo and in vitro investigations. MiRNAs are involved in cancer cells as well as the tumor microenvironment. According to the body of data, the relationship between tumor cells and the TME may be impacted by the dysregulation of miRNAs and ex-miRNAs. Thus, it is anticipated that removing CAFs will make it easier to cure cancer and prevent cancer cells from metastasizing and developing resistance. However, a major barrier to more readily treating cancer with therapeutic techniques is the absence of precise markers for CAFs.
References
Javanmard SH, Vaseghi G, Ghasemi A, Rafiee L, Ferns GA, Esfahani HN, et al. Therapeutic inhibition of microRNA-21 (miR-21) using locked-nucleic acid (LNA)-anti-miR and its effects on the biological behaviors of melanoma cancer cells in preclinical studies. Cancer cell Int. 2020;20:384. https://doi.org/10.1186/s12935-020-01394-6
Zhang T, Zhang P, Li H-XJ. CAFs-derived exosomal miRNA-130a confers cisplatin resistance of NSCLC cells through PUM2-dependent packaging. Int J Nanomed. 2021:561–77.
Yang S-S, Ma S, Dou H, Liu F, Zhang S-Y, Jiang C. et al. Breast cancer-derived exosomes regulate cell invasion and metastasis in breast cancer via miR-146a to activate cancer associated fibroblasts in tumor microenvironment. Exp Cell Res. 2020;391:111983
Kumar A, Kumar P, Sharma M, Kim S, Singh S, Kridel SJ, et al. Role of extracellular vesicles secretion in paclitaxel resistance of prostate cancer cells. Cancer Drug Resist. 2022;5:612–24. https://doi.org/10.20517/cdr.2022.26
Lu Y, Eguchi T, Sogawa C, Taha EA, Tran MT, Nara T, et al. Exosome-based molecular transfer activity of macrophage-like cells involves viability of oral carcinoma cells: size exclusion chromatography and concentration filter method. Cells. 2021;10:1328.
Jaé N, McEwan DG, Manavski Y, Boon RA, Dimmeler S. Rab7a and Rab27b control secretion of endothelial microRNA through extracellular vesicles. FEBS Lett. 2015;589:3182–8.
Albino D, Falcione M, Uboldi V, Temilola DO, Sandrini G, Merulla J, et al. Circulating extracellular vesicles release oncogenic miR-424 in experimental models and patients with aggressive prostate cancer. Commun Biol. 2021;4:119. https://doi.org/10.1038/s42003-020-01642-5
Maacha S, Bhat AA, Jimenez L, Raza A, Haris M, Uddin S, et al. Extracellular vesicles-mediated intercellular communication: roles in the tumor microenvironment and anti-cancer drug resistance. Mol Cancer. 2019;18:55. https://doi.org/10.1186/s12943-019-0965-7
Yang F, Ning Z, Ma L, Liu W, Shao C, Shu Y, et al. Exosomal miRNAs and miRNA dysregulation in cancer-associated fibroblasts. Mol Cancer. 2017;16:148. https://doi.org/10.1186/s12943-017-0718-4
Wu C, Wang M, Huang Q, Guo Y, Gong H, Hu C, et al. Aberrant expression profiles and bioinformatic analysis of CAF‐derived exosomal miRNAs from three moderately differentiated supraglottic LSCC patients. J Clin Lab Anal. 2022;36:e24108.
Xu M, Li S. The opportunities and challenges of using PD-1/PD-L1 inhibitors for leukemia treatment. Cancer Lett. 2024;593:216969. https://doi.org/10.1016/j.canlet.2024.216969
Fang Y, Zhou W, Rong Y, Kuang T, Xu X, Wu W, et al. Exosomal miRNA-106b from cancer-associated fibroblast promotes gemcitabine resistance in pancreatic cancer. Exp Cell. Res. 2019;383:111543.
Akkız H. Emerging role of cancer-associated fibroblasts in progression and treatment of hepatocellular carcinoma. Int J Mol Sci. 2023;24:3941.
Erdogan B, Ao M, White LM, Means AL, Brewer BM, Yang L, et al. Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. J Cell Biol. 2017;216:3799–816. https://doi.org/10.1083/jcb.201704053
Li Y, Gu L. Establishment and characterization of HXWMF-1: the first mouse fibroblastic tumor cell line derived from leukemia-associated fibroblasts. Cancer Cell Int. 2021;21:177. https://doi.org/10.1186/s12935-021-01870-7
Sun B. The mechanics of fibrillar collagen extracellular matrix. Cell Rep Phys Sci. 2021;2. https://doi.org/10.1016/j.xcrp.2021.100515
Mao X, Xu J, Wang W, Liang C, Hua J, Liu J, et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer. 2021;20:131. https://doi.org/10.1186/s12943-021-01428-1
Yin H, Yu S, Xie Y, Dai X, Dong M, Sheng C, et al. Cancer-associated fibroblasts-derived exosomes upregulate microRNA-135b-5p to promote colorectal cancer cell growth and angiogenesis by inhibiting thioredoxin-interacting protein. Cell Signal. 2021;84:110029.
Kang Y, Li S. Nanomaterials: Breaking through the bottleneck of tumor immunotherapy. Int J Biol Macromol. 2023;230:123159. https://doi.org/10.1016/j.ijbiomac.2023.123159
Wu H-J, Hao M, Yeo SK, Guan J-L. FAK signaling in cancer-associated fibroblasts promotes breast cancer cell migration and metastasis by exosomal miRNAs-mediated intercellular communication. Oncogene. 2020;39:2539–49.
Wang J-W, Wu X-F, Gu X-J, Jiang X-H. Exosomal miR-1228 from cancer-associated fibroblasts promotes cell migration and invasion of osteosarcoma by directly targeting SCAI. Oncol Res. 2019;27:979.
You X, Wu J, Zhao X, Jiang X, Tao W, Chen Z, et al. Fibroblastic galectin-1-fostered invasion and metastasis are mediated by TGF-β1-induced epithelial-mesenchymal transition in gastric cancer. Aging. 2021;13:18464.
Ene–Obong A, Clear AJ, Watt J, Wang J, Fatah R, Riches JC, et al. Activated pancreatic stellate cells sequester CD8 + T cells to reduce their infiltration of the juxtatumoral compartment of pancreatic ductal adenocarcinoma. Gastroenterology 2013;145:1121–32.
de la Fuente A, Alonso-Alconada L, Costa C, Cueva J, Garcia-Caballero T, Lopez-Lopez R, et al. M-Trap: exosome-based capture of tumor cells as a new technology in peritoneal metastasis. J Natl Cancer Inst. 2015;107. https://doi.org/10.1093/jnci/djv184
Jeon B-H, Jang C, Han J, Kataru RP, Piao L, Jung K, et al. Profound but dysfunctional lymphangiogenesis via vascular endothelial growth factor ligands from CD11b+ macrophages in advanced ovarian cancer. Cancer Res. 2008;68:1100–9.
Nedaeinia R, Manian M, Jazayeri M, Ranjbar M, Salehi R, Sharifi M. et al. Circulating exosomes and exosomal microRNAs as biomarkers in gastrointestinal cancer. Cancer Gene Ther. 2017;24:48–56.
Whiteside TL. Tumor-derived exosomes and their role in cancer progression. Adv Clin Chem.2016;74:103–41.
Saravanan PB, Kalivarathan J, Khan F, Shah R, Levy MF, Kanak MA. Exosomes in transplantation: role in allograft rejection, diagnostic biomarker, and therapeutic potential. Life Sci. 2023;324:121722. https://doi.org/10.1016/j.lfs.2023.121722
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71/J
Zhu X, Li S. Nanomaterials in tumor immunotherapy: new strategies and challenges. Mol Cancer. 2023;22:94. https://doi.org/10.1186/s12943-023-01797-9
Li Y-Y, Tao Y-W, Gao S, Li P, Zheng J-M, Zhang S-E, et al. Cancer-associated fibroblasts contribute to oral cancer cells proliferation and metastasis via exosome-mediated paracrine miR-34a-5p. EBioMedicine 2018;36:209–20.
Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P. et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126:5553–65. https://doi.org/10.1242/jcs.128868.
Rontogianni S, Synadaki E, Li B, Liefaard MC, Lips EH, Wesseling J, et al. Proteomic profiling of extracellular vesicles allows for human breast cancer subtyping. Commun Biol. 2019;2:325. https://doi.org/10.1038/s42003-019-0570-8
Yeon JH, Jeong HE, Seo H, Cho S, Kim K, Na D, et al. Cancer-derived exosomes trigger endothelial to mesenchymal transition followed by the induction of cancer-associated fibroblasts. Acta Biomater. 2018;76:146–53.
Fang T, Lv H, Lv G, Li T, Wang C, Han Q, et al. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat Commun. 2018;9:191.
Baroni S, Romero-Cordoba S, Plantamura I, Dugo M, D’ippolito E, Cataldo A, et al. Exosome-mediated delivery of miR-9 induces cancer-associated fibroblast-like properties in human breast fibroblasts. Cell Death Dis. 2016;7:e2312–e.
Zhou X, Yan T, Huang C, Xu Z, Wang L, Jiang E, et al. Melanoma cell-secreted exosomal miR-155-5p induce proangiogenic switch of cancer-associated fibroblasts via SOCS1/JAK2/STAT3 signaling pathway. J Exp Clin Cancer Res. 2018;37:1–15.
Jorge NA, Cruz JG, Pretti MAM, Bonamino MH, Possik PA, Boroni M. Poor clinical outcome in metastatic melanoma is associated with a microRNA-modulated immunosuppressive tumor microenvironment. J Transl Med. 2020;18:1–17.
Shu SL, Yang Y, Allen CL, Maguire O, Minderman H, Sen A, et al. Metabolic reprogramming of stromal fibroblasts by melanoma exosome microRNA favours a pre-metastatic microenvironment. Sci Rep. 2018;8:12905.
Li Q, Zhang D, Wang Y, Sun P, Hou X, Larner J, et al. MiR-21/Smad 7 signaling determines TGF-β1-induced CAF formation. Sci Rep. 2013;3:1–9.
Zhou Y, Ren H, Dai B, Li J, Shang L, Huang J, et al. Hepatocellular carcinoma-derived exosomal miRNA-21 contributes to tumor progression by converting hepatocyte stellate cells to cancer-associated fibroblasts. J Exp Clin Cancer Res. 2018;37:1–18.
He Q, Ye A, Ye W, Liao X, Qin G, Xu Y, et al. Cancer-secreted exosomal miR-21-5p induces angiogenesis and vascular permeability by targeting KRIT1. Cell Death Dis. 2021;12:576. https://doi.org/10.1038/s41419-021-03803-8
Wu D, Qin H, Wang Z, Yu M, Liu Z, Peng H, et al. Bone mesenchymal stem cell-derived sEV-encapsulated thermosensitive hydrogels accelerate osteogenesis and angiogenesis by release of exosomal miR-21. Front Bioeng Biotechnol. 2022;9:829136.
Kumarswamy R, Volkmann I, Jazbutyte V, Dangwal S, Park D-H, Thum T. Transforming growth factor-β–induced endothelial-to-mesenchymal transition is partly mediated by microRNA-21. Arterioscler Thromb Vasc Biol. 2012;32:361–9.
Hsieh J-Y, Huang T-S, Cheng S-M, Lin W-S, Tsai T-N, Lee OK, et al. miR-146a-5p circuitry uncouples cell proliferation and migration, but not differentiation, in human mesenchymal stem cells. Nucleic Acids Res. 2013;41:9753–63.
Li Q, Li B, Li Q, Wei S, He Z, Huang X, et al. Exosomal miR-21-5p derived from gastric cancer promotes peritoneal metastasis via mesothelial-to-mesenchymal transition. Cell Death Dis. 2018;9:854.
Au Yeung CL, Co N-N, Tsuruga T, Yeung T-L, Kwan S-Y, Leung CS, et al. Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat Commun. 2016;7:11150.
Watabe S, Kikuchi Y, Morita S, Komura D, Numakura S, Kumagai‐Togashi A, et al. Clinicopathological significance of microRNA‐21 in extracellular vesicles of pleural lavage fluid of lung adenocarcinoma and its functions inducing the mesothelial to mesenchymal transition. Cancer. Medicine. 2020;9:2879–90.
Lee J, Hong BS, Ryu HS, Lee H-B, Lee M, Park IA, et al. Transition into inflammatory cancer-associated adipocytes in breast cancer microenvironment requires microRNA regulatory mechanism. PLoS ONE. 2017;12:e0174126.
Li M, He L, Zhu J. Targeting tumor-associated macrophages for cancer treatment. Cell Biosci. 2022;12:85. https://doi.org/10.1186/s13578-022-00823-5.
Yoshii S, Hayashi Y, Iijima H, Inoue T, Kimura K, Sakatani A, et al. Exosomal microRNAs derived from colon cancer cells promote tumor progression by suppressing fibroblast TP 53 expression. Cancer Sci. 2019;110:2396–407.
Li H, Li F. Exosomes from BM-MSCs increase the population of CSCs via transfer of miR-142-3p. Br J Cancer. 2018;119:744–55.
Dai G, Yao X, Zhang Y, Gu J, Geng Y, Xue F, et al. Colorectal cancer cell–derived exosomes containing miR-10b regulate fibroblast cells via the PI3K/Akt pathway. Bull Cancer. 2018;105:336–49.
Yue J, Chen ZS, Xu XX, Li S. Functions and therapeutic potentials of exosomes in osteosarcoma. Acta Mater Med. 2022;1:552–62. https://doi.org/10.15212/amm-2022-0024
Gao X, Gao B, Li S. Extracellular vesicles: a new diagnostic biomarker and targeted drug in osteosarcoma. 2022;13. https://doi.org/10.3389/fimmu.2022.1002742
Hu J, Wang W, Lan X, Zeng Z, Liang Y, Yan Y, et al. CAFs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial-mesenchymal transition in colorectal cancer. Mol Cancer. 2019;18:1–15.
Medici D, Hay ED, Olsen BR. Snail and Slug promote epithelial-mesenchymal transition through β-catenin–T-cell factor-4-dependent expression of transforming growth factor-β3. Mol Biol Cell. 2008;19:4875–87.
Monteran L, Erez N. The dark side of fibroblasts: cancer-associated fibroblasts as mediators of immunosuppression in the tumor microenvironment. Front Immunol. 2019;10:1835. https://doi.org/10.3389/fimmu.2019.01835
Ahmadzadeh M, Rosenberg SA. TGF-β1 attenuates the acquisition and expression of effector function by tumor antigen-specific human memory CD8 T cells. J Immunol. 2005;174:5215–23.
Minciacchi VR, Spinelli C, Reis-Sobreiro M, Cavallini L, You S, Zandian M, et al. MYC mediates large oncosome-induced fibroblast reprogramming in prostate cancer. Cancer Res. 2017;77:2306–17.
Tang X, Hou Y, Yang G, Wang X, Tang S, Du Y. et al. Stromal miR-200s contribute to breast cancer cell invasion through CAF activation and ECM remodeling. Cell Death Differ. 2016;23:132–45.
Noh GT, Kwon J, Kim J, Park M, Choi D-W, Cho K-A, et al. Verification of the role of exosomal microRNA in colorectal tumorigenesis using human colorectal cancer cell lines. PLoS ONE. 2020;15:e0242057.
Fullár A, Kovalszky I, Bitsche M, Romani A, Schartinger VH, Sprinzl GM, et al. Tumor cell and carcinoma-associated fibroblast interaction regulates matrix metalloproteinases and their inhibitors in oral squamous cell carcinoma. Exp Cell Res. 2012;318:1517–27.
Khazaei S, Nouraee N, Moradi A, Mowla SJC, Oncology T. A novel signaling role for miR-451 in esophageal tumor microenvironment and its contribution to tumor progression. ClinTransl Oncol. 2017;19:633–40.
Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–48.
Erez N, Truitt M, Olson P, Hanahan D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-κB-dependent manner. Cancer Cell. 2010;17:135–47.
Hunter S, Nault B, Ugwuagbo K, Maiti S, Majumder M. Mir526b and Mir655 promote tumour associated angiogenesis and lymphangiogenesis in breast cancer. Cancers. 2019;11. https://doi.org/10.3390/cancers11070938
Lee YT, Tan YJ, Falasca M, Oon CE. Cancer-associated fibroblasts: epigenetic regulation and therapeutic intervention in breast cancer. Cancers. 2020;12. https://doi.org/10.3390/cancers12102949
Strell C, Paulsson J, Jin S-B, Tobin NP, Mezheyeuski A, Roswall P. et al. Impact of epithelial-stromal interactions on peritumoral fibroblasts in ductal carcinoma in situ. J Natl Cancer Inst. 2019;111:983–95.
Procopio M-G, Laszlo C, Al Labban D, Kim DE, Bordignon P, Jo S-H, et al. Combined CSL and p53 downregulation promotes cancer-associated fibroblast activation. Nat Cell Biol. 2015;17:1193–204.
Wang J, Guan X, Zhang Y, Ge S, Zhang L, Li H. et al. Exosomal miR-27a derived from gastric cancer cells regulates the transformation of fibroblasts into cancer-associated fibroblasts. Cell Physiol Biochem. 2018;49:869–83.
Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther. 2006;5:1640–6.
Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM. et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer. 2020;20:174–86.
Albrengues J, Bertero T, Grasset E, Bonan S, Maiel M, Bourget I, et al. Epigenetic switch drives the conversion of fibroblasts into proinvasive cancer-associated fibroblasts. Nat Commun. 2015;6:10204.
Calvo F, Ranftl R, Hooper S, Farrugia AJ, Moeendarbary E, Bruckbauer A, et al. Cdc42EP3/BORG2 and septin network enables mechano-transduction and the emergence of cancer-associated fibroblasts. Cell Rep. 2015;13:2699–714.
Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, Chaudhry SI. et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Cell report. 2013;15:637–46.
Sykaras AG, Christofidis K, Politi E, Theocharis S. Exosomes on endometrial cancer: a biomarkers treasure trove?. Cancer. 2022;14:1733
Li BL, Lu W, Qu JJ, Ye L, Du GQ, Wan XP. Loss of exosomal miR-148b from cancer-associated fibroblasts promotes endometrial cancer cell invasion and cancer metastasis. J Cell Physiol. 2019;234:2943–53. https://doi.org/10.1002/jcp.27111
Zhang N, Wang Y, Liu H, Shen W. Extracellular vesicle encapsulated microRNA-320a inhibits endometrial cancer by suppression of the HIF1α/VEGFA axis. Exp Cell Res. 2020;394:112113.
Miyasaka A, Oda K, Ikeda Y, Sone K, Fukuda T, Inaba K, et al. PI3K/mTOR pathway inhibition overcomes radioresistance via suppression of the HIF1-α/VEGF pathway in endometrial cancer. Gynecol Oncol. 2015;138:174–80. https://doi.org/10.1016/j.ygyno.2015.04.015
Shan G, Zhou X, Gu J, Zhou D, Cheng W, Wu H, et al. Downregulated exosomal microRNA-148b-3p in cancer associated fibroblasts enhance chemosensitivity of bladder cancer cells by downregulating the Wnt/β-catenin pathway and upregulating PTEN. Cell Oncol. 2021;44:45–59.
Liu Y, Yang Y, Du J, Lin D, Li F. MiR‐3613‐3p from carcinoma‐associated fibroblasts exosomes promoted breast cancer cell proliferation and metastasis by regulating SOCS2 expression. IUBMB Life. 2020;72:1705–14.
Zhao Q, Zheng X, Guo H, Xue X, Zhang Y, Niu M, et al. Serum exosomal miR-941 as a promising oncogenic biomarker for laryngeal squamous cell carcinoma. J Cancer. 2020;11:5329.
Wang J, Zhou Y, Lu J, Sun Y, Xiao H, Liu M, et al. Combined detection of serum exosomal miR-21 and HOTAIR as diagnostic and prognostic biomarkers for laryngeal squamous cell carcinoma. Med Oncol. 2014;31:1–8.
Qi Y, Wang H, Zhang Q, Liu Z, Wang T, Wu Z, et al. CAF-released exosomal miR-20a-5p facilitates HCC progression via the LIMA1-mediated β-catenin pathway. Cells. 2022;11:3857.
Wang S, Du P, Cao Y, Ma J, Yang X, Yu Z. et al.Cancer associated fibroblasts secreted exosomal miR-1290 contributes to prostate cancer cell growth and metastasis via targeting GSK3β. Cell Death Discov. 2022;8:371
Li S, Kang Y, Zeng Y. Targeting tumor and bone microenvironment: Novel therapeutic opportunities for castration-resistant prostate cancer patients with bone metastasis. Biochim Biophys Acta Rev Cancer. 2024;1879:189033. https://doi.org/10.1016/j.bbcan.2023.189033
Likonen D, Pinchasi M, Beery E, Sarsor Z, Signorini LF, Gervits A, et al. Exosomal telomerase transcripts reprogram the microRNA transcriptome profile of fibroblasts and partially contribute to CAF formation. Sci Rep. 2022;12:16415.
Liu Q, Li S. Exosomal circRNAs: novel biomarkers and therapeutic targets for urinary tumors. Cancer Lett. 2024;588:216759. https://doi.org/10.1016/j.canlet.2024.216759
Xu Y, Kong S, Qin S, Shen X, Ju S. Exosomal circRNAs: sorting mechanisms, roles and clinical applications in tumors. Front Cell Dev Biol. 2020;8:581558. https://doi.org/10.3389/fcell.2020.581558
Xu M, Li S. Nano-drug delivery system targeting tumor microenvironment: a prospective strategy for melanoma treatment. Cancer Lett. 2023;574:216397. https://doi.org/10.1016/j.canlet.2023.216397
Guo X, Gao C, Yang DH, Li S. Exosomal circular RNAs: a chief culprit in cancer chemotherapy resistance. Drug Resist Update. 2023;67:100937. https://doi.org/10.1016/j.drup.2023.100937
Ringuette Goulet C, Bernard G, Tremblay S, Chabaud S, Bolduc S, Pouliot F. Exosomes induce fibroblast differentiation into cancer-associated fibroblasts through TGFβ signaling. Mol Cancer Res. 2018;16:1196–204.
Lai S-C, Su Y-T, Chi C-C, Kuo Y-C, Lee K-F, Wu Y-C, et al. DNMT3b/OCT4 expression confers sorafenib resistance and poor prognosis of hepatocellular carcinoma through IL-6/STAT3 regulation. J Exp Clin Cancer Res. 2019;38:1–18.
Vennin C, Mélénec P, Rouet R, Nobis M, Cazet AS, Murphy KJ, et al. CAF hierarchy driven by pancreatic cancer cell p53-status creates a pro-metastatic and chemoresistant environment via perlecan. Nat Commun. 2019;10:3637.
Shi L, Zhu W, Huang Y, Zhuo L, Wang S, Chen S, et al. Cancer‐associated fibroblast‐derived exosomal microRNA‐20a suppresses the PTEN/PI3K‐AKT pathway to promote the progression and chemoresistance of non‐small cell lung cancer. Clin Transl Med. 2022;12:e989.
Fujii N, Yashiro M, Hatano T, Fujikawa H, Motomura H. CD9-positive exosomes derived from cancer-associated fibroblasts might inhibit the proliferation of malignant melanoma cells. Anticancer Res. 2023;43:25–33. https://doi.org/10.21873/anticanres.16130
Neuzillet C, Nicolle R, Raffenne J, Tijeras-Raballand A, Brunel A, Astorgues-Xerri L, et al. Periostin- and podoplanin-positive cancer-associated fibroblast subtypes cooperate to shape the inflamed tumor microenvironment in aggressive pancreatic adenocarcinoma. J Pathol. 2022;258:408–25. https://doi.org/10.1002/path.6011
Luo G, Zhang Y, Wu Z, Zhang L, Liang C, Chen X. Exosomal LINC00355 derived from cancer-associated fibroblasts promotes bladder cancer cell resistance to cisplatin by regulating miR-34b-5p/ABCB1 axis. Acta Biochim Biophys Sin. 2021;53:558–66.
Guo H, Ha C, Dong H, Yang Z, Ma Y, Ding Y. Cancer-associated fibroblast-derived exosomal microRNA-98-5p promotes cisplatin resistance in ovarian cancer by targeting CDKN1A. Cancer Cell Int. 2019;19:1–15.
Qin X, Guo H, Wang X, Zhu X, Yan M, Wang X, et al. Exosomal miR-196a derived from cancer-associated fibroblasts confers cisplatin resistance in head and neck cancer through targeting CDKN1B and ING5. Genome Biol. 2019;20:1–21.
Zhang H, Deng T, Liu R, Ning T, Yang H, Liu D, et al. CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol Cancer. 2020;19:1–17.
Richards KE, Zeleniak AE, Fishel ML, Wu J, Littlepage LE, Hill R. Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene. 2017;36:1770–8.
Zhu H, Zhang P, Shi J, Kou D, Bai X. Exosome-delivered circRPS5 inhibits the progression of melanoma via regulating the miR-151a/NPTX1 axis. PLoS ONE. 2023;18:e0287347. https://doi.org/10.1371/journal.pone.0287347
Zhang M, Hu S, Liu L, Dang P, Liu Y, Sun Z, et al. Engineered exosomes from different sources for cancer-targeted therapy. Signal Transduct Target Ther. 2023;8:124 https://doi.org/10.1038/s41392-023-01382-y
Chen Z, Yue Z, Yang K, Shen C, Cheng Z, Zhou X, et al. Four ounces can move a thousand pounds: the enormous value of nanomaterials in tumor immunotherapy. 2023;12:2300882. https://doi.org/10.1002/adhm.202300882.
Cheng L, Zhang X, Tang J, Lv Q, Liu J. Gene-engineered exosomes-thermosensitive liposomes hybrid nanovesicles by the blockade of CD47 signal for combined photothermal therapy and cancer immunotherapy. Biomater. 2021;275:120964.
Chen Z, Yue Z, Yang K, Li S. Nanomaterials: small particles show huge possibilities for cancer immunotherapy. J Banobiotechnol. 2022;20:484. https://doi.org/10.1186/s12951-022-01692-3
Chen X, Ren X, E J, Zhou Y, Bian R Exosome-transmitted circ IFNGR2 Modulates Ovarian Cancer Metastasis via miR-378/ST5 Axis. Molecular and cellular biology. 2023:1-21.
Li S. The basic characteristics of extracellular vesicles and their potential application in bone sarcomas. J Nanobiotechnol. 2021;19:277. https://doi.org/10.1186/s12951-021-01028-7
Hunter S, Nault B, Ugwuagbo KC, Maiti S, Majumder M. Mir526b and Mir655 promote tumour associated angiogenesis and lymphangiogenesis in breast cancer. Cancers. 2019;11:938.
Chen E-B, Zhou Z-J, Xiao K, Zhu G-Q, Yang Y, Wang B, et al. The miR-561-5p/CX3CL1 signaling axis regulates pulmonary metastasis in hepatocellular carcinoma involving CX3CR1+ natural killer cells infiltration. Theranostics. 2019;9:4779.
Liu Y, Li X, Zhang Y, Wang H, Rong X, Peng J, et al. An miR-340-5p-macrophage feedback loop modulates the progression and tumor microenvironment of glioblastoma multiforme. Oncogene. 2019;38:7399–415.
Wang S, Du P, Cao Y, Ma J, Yang X, Yu Z, et al. Cancer associated fibroblasts secreted exosomal miR-1290 contributes to prostate cancer cell growth and metastasis via targeting GSK3&beta. Cell Death Discov. 2022;8:371.
Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein–α. Science. 2010;330:827–30.
Costa A, Kieffer Y, Scholer-Dahirel A, Pelon F, Bourachot B, Cardon M, et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell. 2018;33:463–79.e10.
Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti–PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci USA. 2013;110:20212–7.
Chen IX, Chauhan VP, Posada J, Ng MR, Wu MW, Adstamongkonkul P, et al. Blocking CXCR4 alleviates desmoplasia, increases T-lymphocyte infiltration, and improves immunotherapy in metastatic breast cancer. Proc Natl Acad Sci USA. 2019;116:4558–66.
Funding
This work was supported by the Vice Chancellery for Research, Isfahan University of Medical Science, Isfahan, Iran (No. 140033).
Author information
Authors and Affiliations
Contributions
RN and MM designed research. RS, MR, MG, SHJ, SN, and HN were involved in the search strategy and writing original draft preparation. RN wrote the paper. GAF revised the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nedaeinia, R., Najafgholian, S., Salehi, R. et al. The role of cancer-associated fibroblasts and exosomal miRNAs-mediated intercellular communication in the tumor microenvironment and the biology of carcinogenesis: a systematic review. Cell Death Discov. 10, 380 (2024). https://doi.org/10.1038/s41420-024-02146-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41420-024-02146-5
- Springer Nature Limited