Abstract
The current change in battery technology followed by the almost immediate adoption of lithium as a key resource powering our energy needs in various applications is undeniable. Lithium-ion batteries (LIBs) are at the forefront of the industry and offer excellent performance. The application of LIBs is expected to continue to increase. The adoption of renewable energies has spurred this LIB proliferation and resulted in a dramatic increase in LIB waste. In this review, we address waste LIB collection and segregation approaches, waste LIB treatment approaches, and related economics. We have coined a “green score” concept based on a review of several quantitative analyses from the literature to compare the three mainstream recycling processes: pyrometallurgical, hydrometallurgical, and direct recycling. In addition, we analyze the current trends in policymaking and in government incentive development directed toward promoting LIB waste recycling. Future LIB recycling perspectives are analyzed, and opportunities and threats to LIB recycling are presented.
Similar content being viewed by others
Introduction
Valued at close to 120.5 billion United States dollars (USD) in 2020, the overall battery market has continued to grow1. Lithium-ion batteries (LIBs) have steadily increased in popularity in the battery market over the past two decades, and their market share alone is expected to reach approximately 50 billion USD by 20242. A great amount of funding has been invested in the LIB market, which has led to various technological breakthroughs that have propelled the adoption of this technology3,4. LIBs are arguably the most appealing batteries in the market, offering an array of operational benefits, such as higher energy densities, lower self-discharge capacities, and lighter weights than those of competing technologies5. A key sector that utilizes many LIBs is the electric vehicle (EV) industry, which has experienced considerable growth in market share, with Europe, China, and the United States being the key EV markets6. It is predicted that the EV market will exceed 725 billion USD by 2026, based on an annual compound growth rate of >27%7. A study by Richa et al. predicted that the maximum number of LIBs will be produced for the EV industry by 2040, and the battery mass may exceed 4 million tons8.
LIB market growth and advances in LIB technologies will result in the following: (i) additional resources will be utilized to meet market needs, and (ii) large quantities of LIB waste will be generated. Reserves of key metals used to manufacture LIBs are unevenly distributed and, therefore, have become a matter of geopolitical concern in recent years. An average of 70% of the world’s cobalt (Co) originates from the Democratic Republic of the Congo (DRC), while the other Co-producing countries generate no more than 5% each of the total amount of this resource. Graphite, which is an important component in the production of anodes, is mostly produced in China and Mozambique. These countries generate a combined total of ~70% of the global amount of graphite. Both LIB resource supply and waste production can be addressed through recycling operations, which are aspects of a circular economy, as shown in Fig. 1. The circular economy seeks to foster sustainable consumption by increasing the useful lives of materials and products, reducing waste production through alternate waste treatment approaches that allow materials to benefit the environment and societies, thereby promoting natural ecosystem regeneration9. As an example, the State of Nevada in the United States is spearheading various projects related to the LIB circular economy. A part of the LIB circular economy entails the management of LIBs past their useful life, which, in recent years, has led to increases in research and development (R&D) activities and in the number of organizations that focus on LIB recycling. The goals of this review are to analyze the current LIB recycling trends, recycling methods applied, policies, and incentives for LIB recycling and to provide a summary of the opportunities and threats to the LIB recycling market.
Lithium-ion battery technology
LIBs are categorized based on their composition and are designed to meet specific user needs. Although lithium (Li) is the key component of LIBs, other elements are typically used as building blocks, including Co, nickel (Ni), manganese (Mn), iron (Fe), aluminum (Al), copper (Cu), and graphite. Most LIBs are chiefly composed of a cathode, anode, electrolyte, and separator, with these constituents being housed inside a casing. LIBs operate based on the migration ability of Li+ ions, migrating from the cathode to the anode during charging and in the reverse direction during discharging. The structure and operation principle of a LIB is shown in Fig. 2. Insertion compound anodes and cathodes allow for this migration to occur via an organic liquid electrolyte, such as LiPF6 salt, which is dissolved in an organic solvent mixture. The solvent mixture may comprise ethyl methyl carbonate (EMC), dimethyl carbonate (DMC), ethylene carbonate (EC), and diethyl carbonate (DEC), among other organic solvents10. Graphite has been the primary material utilized for LIB anodes.
Intercalation cathodes that store migrated Li+ ions via insertion may be composed of transition metal oxides, metal chalcogenides (such as NbSe3 and TiS3), or polyanions (XO43−, X = Si, P, S, W, Mo, As)11. In particular, metal oxide cathodes are relatively mature components of the market because they are at the forefront of advances in LIB technologies, and they will be the focus of this review. Metal oxide cathodes can be divided into three classes: i) spinel, ii) layered oxides, and iii) polyanion oxides12. These materials can include Li[Ni1-x-yCoxMny]O2 (NMC), LiFePO4 (LFP), LiMnO2 (LMO), LiCoO2 (LCO) and Li[Ni1-x-yCoxAly]O2 (NCA), and their compositions, performance characteristics, and applications are given in Fig. 3. As seen from the provided list of LIB cathode chemistries, the cathode contains most of the economic elements. Therefore, it is appealing to conduct investigations into material recovery from the cathode material.
Different metal oxide cathode compositions: Li[Ni1-x-yCoxMny]O2 (NMC), LiFePO4 (LFP), LiMnO2 (LMO), LiCoO2 (LCO) and Li[Ni1-x-yCoxAly]O2 (NCA). Their performance characteristics are given in terms of the gravimetric energy density (GED) and volumetric energy density (VED), in addition to examples of LIB chemical applications105,140,141,142.
Supply of lithium-ion battery materials
Resource supply for LIBs is vital to their commercial success; therefore, complex geopolitical structures must be considered with regard to the supply chain. Of the most common elements used in LIBs, Co, and Li are predicted to pose the greatest supply risks13. To reduce the sensitivities of supply fluctuations, several techniques may be employed, such as expanding supply sources, increasing recycling abilities, and establishing international communication among agencies14. In this review, the elements that are essential for LIB manufacturing are categorized as critical or noncritical. Table 1 provides a list of these key elements and their designation. The critical elements are designated as such because they are the primary focus of this review article, unlike the noncritical elements. According to the 2023 Mineral Commodity Summaries15, the production of noncritical elements in 2021 is as follows:
-
a.
China, India, and Russia produced the greatest amounts of smelter Al, with 2022 production estimates showing an increase in output.
-
b.
Chile, Peru and China had the greatest Cu mine production, with estimated figures showing a decrease in production across the board in 2022.
-
c.
Phosphate rock was chiefly produced by China, Morocco, and the United States.
-
d.
Australia, Brazil, and China were the largest producers of usable iron ore, while China, India, and Japan were the largest producers of pig iron.
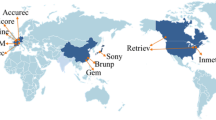
The top producing countries for each of the highlighted LIB critical elements (including graphite) are given in Fig. 4, with Australia and China clearly standing out as top producers of several highly desirable materials.
Lifetime, waste collection, and treatment options for LIBs
The collection of LIBs at the end-of-life (EoL) is a vital aspect of recycling endeavors. Pristine batteries are considered to have reached their EoL when they are only able to retain 80% of their original rated capacity16. The remaining capacity of the battery can be determined using the state of health (SOH), which is evaluated using the battery-rated capacity (Crat) and the maximum battery current operating capacity (Ccur) in Eq. 117:
Therefore, a LIB reaches its EoL upon reaching 0.8 SOH. Other important parameters usually used when describing battery health and capacity include the state of charge (SOC) and depth of discharge (DOD). The SOC at any point in time is the ratio of the battery level of charge at that moment to the maximum capacity of the battery18. The DOD may be defined as the ratio of the discharge capacity of the battery in the fully charged state to the nominal capacity of the battery19. The determination of SOH may be accomplished through direct measurement, modeling, or the application of data-driven approaches20,21,22.
Direct measurements can be carried out using the ampere-hour counting method or electrochemical impedance spectroscopy (EIS). The ampere-hour counting method involves fully charging and discharging the LIB with a small constant current at ambient temperature23. The charge transferred during the complete charge‒discharge cycle is accurately measured, and Eq. 1 can be applied for SOH computation23. Measuring the SOH using EIS involves first using an electrical signal that is sent to the system and then recording the impedance-dependent response24. For example, the ohmic resistance has been noted to increase linearly with the age of the battery; thus, using a defined diagnostic map between the available capacity and the ohmic resistance allows for the determination of SOH25. Apart from the SOH, EIS can be used to determine the internal temperature and SOC of a battery, which can be used to infer its health17. The modeling approach for estimating the SOH involves electrical and electrochemical models and nonlinear partial differential equations to correlate the battery SOH with measured signals, such as the current, voltage, and temperature26,27. Data-driven approaches, such as support vectors and fuzzy logic, can leverage machine learning to calculate battery degradation indicators using collected data during battery operation28,29. By applying different approaches or a combination thereof, the LIB EoL can be determined. Although LIBs may be considered to have reached their EoL upon reaching a SOH of 80%, LIBs may be utilized for different purposes at their EOLs, such as stationary energy storage.
The collection and reuse of EoL LIBs is an evolving area of recycling with the potential to grow drastically. This field can ensure reliable management and supply of critical materials in-house. It is important to switch from the casual discarding of spent and EoL LIBs to establishing robust LIB collection strategies for waste LIB. The poor to nonexistent LIB collection rates across different geographic regions, especially those associated with domestic consumer electronics, impede the expansion of LIB waste treatment efforts. It has been reported that the highest waste LIB collection rates are in Asian countries at ~70%30. This high rate is apparent mainly because LIB manufacturing has been concentrated in Asia, thereby promoting targeted LIB waste treatment efforts. The European Union (EU), Australia, and the Americas are receiving some traction, but they still face challenges related to the volumes necessary for profitability31.
The collection, transport, and storage of LIBs must be appropriate, as LIBs are classified as highly combustible hazardous materials. Flammable electrolytes (in the form of liquid electrolytes) in LIBs pose an ignition risk, which may lead to thermal runaway. In the case of waste and spent batteries, improper discarding of the LIBs can lead to (i) LIB puncturing during waste processing by crushing; (ii) subjecting the LIBs to dangerously high temperatures; or (iii) short-circuiting, which may result from residual charges in the discarded LIBs. To treat LIB waste, refurbishing, repurposing, and recycling are potential pathways that have been explored32,33. Figure 5 shows the possible pathways that can be explored for the treatment of waste LIBs.
The refurbishing and repurposing of LIBs chiefly target batteries that can still be applied for purposes requiring a relatively low LIB capacity, although they have reached their EoLs as described earlier. These batteries can be directly reused for the same application as before LIB refurbishment or for cascade use for a different, less demanding application33. With respect to LIB recycling, several approaches have been developed that generally fall under two categories: direct and indirect recycling. Direct LIB recycling involves the direct regeneration of the cathode material by relithiation, while indirect LIB recycling completely breaks down the electrode material to recover the separate constituents as compounds (usually salts or alloys). Of the two recycling approaches mentioned, indirect recycling is more prevalent with full-scale commercial applications than direct recycling, which is still at the experimental level. Further details regarding these LIB waste treatment pathways are discussed in the preceding sections.
Refurbishing and repurposing
Refurbishment of LIBs for direct reuse in EVs has been considered for tackling LIB waste. This process may be applicable for LIBs that have not necessarily reached their EoLs but are obtained from EVs that require significant automotive repairs or that have suffered damage from collision while the LIBs remain intact8. Furthermore, even upon reaching the defined EoL, EV LIBs may be reused for less demanding EV commutes34. A preferable strategy for handling LIBs at the EoL is refurbishing and repurposing the LIBs to meet different energy storage needs35. A good example of LIBs that fit this criterion is EV LIBs that have experienced capacity fade, thereby degrading the driving range capability. Although these LIBs are not appropriate for EVs, they still retain ~80% of their original capacities from the beginning of life (BOL) and may be utilized until they retain only 50–60%, as depicted in Fig. 634,36. These LIBs have been the subject of second-life applications, such as in EV charging infrastructure and in industrial and renewable energy storage systems37,38,39,40. The refurbishment and repurposing process steps include initial testing and screening, sorting, regrouping, and new system management integration41. Some of the technical challenges associated with refurbishing include safety issues in treatment and a lack of homogeneity in terms of capacity and cell type, which increase with increasing cell age37.
The capacities of pristine LIBs decrease from 100% at BOL to approximately 80% of their original capacity at the end-of-life, thereby necessitating refurbishment. Depending on the application during the second life, the refurbished LIBs may be utilized until they retain 50–60% of their original BOL capacity, leading to refurbished-end-of-life.
Direct recycling
Direct recycling is a low-cost LIB recycling approach that has been explored with other conventional methods. Relithiation is used in direct LIB recycling, which allows for the retention of constituent materials to the greatest extent. The active materials are not broken down into their constituents. This preservation allows recyclers to bypass the costly components of the remanufacturing process, which utilizes recycled cathode constituent metals. Direct recycling includes electrochemical, mechanical, cathode-to-cathode, and cathode-healingTM (trademarked by Hulico LLC) methodologies42,43. These methodologies share the common feature of reactivating the cathode material without the need to decompose it to its constituent elements or compounds. Figure 7 provides a schematic example of a direct recycling process.
Indirect recycling
Pyrometallurgical treatment
Pyrometallurgical approaches, which utilize elevated temperatures to thermally treat EoL LIBs, can be divided into (i) thermal pretreatment and (ii) extractive pyrometallurgy processes. Pretreatment is used to prepare cathode materials by removing the binder, recovering the electrolyte, and/or removing carbon. Conversely, extractive metallurgy is used to isolate and recoup constituent metals from LIBs, producing gases, slag, metal alloy mixtures, and salts.
Thermal pretreatment may be conducted on EoL LIBs for the thermochemical conversion of some constituents. This process may be achieved through incineration and pyrolysis. Incineration involves the use of elevated temperatures in an oxygen or air atmosphere and is used to burn off carbon and binder; thermal decomposition begins at ~350 °C44,45. Since the binder has adhesive characteristics, it may result in cathode powder retention on the Al foil and affect the metal recovery efficiency. Carbon removal helps to improve the Li leaching efficiency when Li leaching is conducted downstream, as the presence of carbon allows the absorption of Li ions45. Pyrolysis chiefly facilitates the thermal decomposition of organic compounds into products that can be used downstream as chemical feedstock or fuel, and when coupled with condensation, pyrolysis can enable electrolyte recovery46. For example, Zachmann et al. utilized a low-temperature process in which the recovery of electrolyte solvents (EMC, DMC, and EC) was optimized at ~130 °C. The off-gas produced includes toxic hydrogen fluoride gas (HFg) and phosphoryl fluoride (POF3) from the decomposition of LiPF6 salt. When the gas is bubbled off through deionized water, which serves as a gas wash, HFg forms hydrofluoric acid (HF), and POF3 forms phosphoric acid (H3PO4)47. The increased treatment temperature correlates directly with the increases in volume and concentration of off-gas48. Furthermore, pyrolysis crispens the Al foil, facilitating separation from the cathode material49.
Extractive pyrometallurgy processes include roasting and smelting. These processes are less vulnerable to constituent chemistries, such as reduced vulnerability to organic impurities in the black mass (BM), than hydrometallurgical processes.
Roasting can be subdivided into carbothermic and salt-assisted roasting, while smelting involves high temperatures above the material melting point, resulting in metal reduction and the generation of immiscible molten liquid phases50. Cathode materials obtained from the thermal pretreatment of EoL LIBs may be used as feedstocks for roasting operations. During carbothermic roasting, the cathode material is heated in the presence of a carbon-based reducing agent, such as coke or charcoal, to produce a mixture of alloys, impure metals, or oxides requiring further refining, in addition to carbon residue51. The sequence of steps that occur is the decomposition of the cathode material, which is followed by the oxidation of carbon and the reduction in the level of decomposed metal oxides. The graphite present in the spent anode has been investigated as an agent for carbothermic roasting to reduce the high valence state of cathode metal elements to prepare for hydrometallurgical processing52. Furthermore, the incorporation of microwave technology in carbothermic roasting has led to the emergence of microwave-assisted reduction, which is conducted in a microwave furnace53. This process takes advantage of the carbon present in the cathode material to absorb microwave energy, which leads to the formation of lattice cracks and to interparticle dissociation through the destruction of the crystal structures of the cathode material, and it effectively increases the material temperature54,55. This phenomenon reduces the reaction time relative to conventional heating through the material bed55. In salt-assisted reduction roasting, chlorination (using NaCl, NH4Cl, HCl(g), or Cl2(g)), sulfation (using Na2SO4, NH4SO4, MgSO4, SO2, or H2SO4) or nitration (using HNO3) may be utilized to convert the constituent metal elements into water-soluble compounds56,57,58.
Smelting is used to isolate and recoup constituent metals from LIBs, producing slag, metal alloy mixtures, and off-gases. The oxygen potential of the contained metals is a key factor, as metals with low oxygen potential require less effort for recovery by smelting than metals with higher oxygen potential, affecting the efficiency and selectivity of recovery. It is because of the oxygen potential that smelting operations fail to effectively recover Li (with a high oxygen potential) as part of the matte (the metal alloy with values); however, the Li enters the slag59. The slag mostly consists of unwanted impurities that preferentially separate into the slag due to reactions with the added fluxing agents, which assist in reducing the operating temperature51. Slag often undergoes hydrometallurgical treatment for complete metal extraction. The metal alloy mixture product contains transition metals, such as Ni, Co, and Mn, that are specific to the feedstock LIB chemistry. The oxygen potential contributes to the state of the metal alloy, making it difficult to separate metals with similar oxygen potentials, such as Ni and Co, and thereby affecting the process selectivity. A novel smelting approach utilizes multiple induction coils layered through a graphite bed. By melting materials at several different layers and temperatures, it is possible to recover Li and transition metals (Co, Ni, Mn)59. Figure 8 shows the sequence of steps that may be conducted in the pyrometallurgical treatment of a mixed feedstock of spent LIBs.
To make the process sustainable, alternative energy sources for alleviating the high energy draw associated with pyrometallurgy while presenting relatively green solutions are a subject of investigation. Solar energy has recently been explored for LIB recycling in the form of concentrated solar power (CSP). CSP technologies use a series of optics, such as mirrors and lenses, to concentrate solar irradiation over a large area onto a small receiving surface60. The concentrated energy can then be directly used to provide industrial heat, converted to electricity, or stored as heat in thermal energy storage systems that use molten salts. The industrial application of CSP in ceramic material processing and extractive metallurgy has allowed for the investigation of the application of CSP in the carbothermic reduction of LIB waste61,62.
Hydrometallurgical treatment
During hydrometallurgical processing, the LIBs are initially discharged to prevent short-circuiting. This phenomenon may be accomplished through various methods, such as connecting a load to the battery or applying a solution that may be made from alkaline bicarbonates, ammonium bicarbonate, and hydrogen phosphates63. When using aqueous solutions for discharge, care must be taken because some solutions damage the battery terminals via corrosion64. Selection is highly dependent on the desired state of the discharged battery. Some nonconventional methods for discharging batteries before hydrometallurgical treatment include the use of an external short circuit or resistor, but care must be taken because heat may accumulate65,66. After battery discharge, the LIBs are disassembled, and the metal casing and any wrapping material are selectively removed to expose the electrode material, which can then be subjected to leaching operations. Material leaching occurs via material dissolution in a solvent (lixiviant). As LIB active materials are bound with a binder, such as polyvinylidene fluoride (PVDF), to the Al and Cu current collectors, organic solvents, such as N-methyl-2-pyrrolidone (NMP), N-dimethylacetamide (DMAC), or N,N-dimethylformamide (DMF), may be employed to break down the binder67.
Leaching in acidic or alkaline solutions is used to extract the constituent metals from the LIB active materials that have been separated from the current collectors. Figure 9 shows a general summary of the steps associated with LIB recycling hydrometallurgical operations. Ammonia (NH3) and its sulfate or sulfite derivatives may be used as alkaline leaching solutions, while organic (such as citric, ascorbic, and oxalic acids) and inorganic (HCl, H2SO4, and H3PO4) acids may be used as acidic leaching solutions68. The inclusion of hydrogen peroxide in an acidic media leaching solution has been shown to increase the recovery rates of Co and Li in solution. This rate increase arises due to the role of peroxide as a reductant; this reductant creates more Co(II) species than Co(III) species69. The Co(II) species have better leaching characteristics compared to the Co (III) species. The kinetics of the leaching process can be explained using the empirical logarithmic law model of the rate, which depends on the surface layer diffusion of the solvent and is supplemented by X-ray powder diffraction and scanning electron microscopy analysis70. Despite the reputation of ammonia as a less effective leaching agent than other materials, its tendency to favor complexes with Cu and Co shows promise for its leaching abilities. By using sodium sulfite as a reductant, ammonia leaching can exceed selectivity values of 98% for Li, Ni, and Co71. Methods that have been employed to improve the effectiveness of the leaching process include the addition of a calcination step (removal of impurities) and fusion with potassium hydrogen sulfate72. The addition of ultrasonic technology to the existing process results in distinctive cavitation of the material surface, which aids in the recovery of precious metals while remaining less environmentally impactful than other methods, such as highly acidic solutions73.
Bioleaching is a novel LIB metal recovery approach that has been investigated74. In a study by Bahaloo-Horeh & Mousavi, bacteria of the family Aspergillus niger were used to produce organic acids for the leaching process. Organic acid production was initially optimized by altering the inoculum size, initial pH, and sucrose concentration. When producing the acids, leaching was conducted, and the highest metal recoveries of Li (100%), Cu (100%), Mn (77%), and Al (75%) were achieved at 2% w/v, while the highest recoveries of Co (64%) and Ni (54%) are obtained at 1% w/v75. Jagen Roy et al. investigated metal recovery from NMC batteries through the application of Acidithiobacillus ferrooxidans, which is an autotrophic bacterium. The spent LIBs were discharged, mechanically shredded, and finely crushed before leaching. When bacteria are used, biogenic H2SO4 and Fe3+ are produced, and they act as biolixiviants. Recoveries of 92% Mn, 90% Ni, 89% Li, and 82% Co from the spent LIBs after 72 h of leaching were achieved74. While bioleaching processes can recover high amounts of metal, they often exhibit poor kinetics and subsequently low throughput rates59.
In summary, the leaching process results in a mixture of metal ion species in solution and a solid residue consisting of insoluble components. With the target metals in solution, the separation and recovery of these metals are the next steps in the recycling process. These steps have led to the development of three main techniques: (i) precipitation, (ii) solvent extraction, and (iii) electrochemical deposition.
Precipitation is a chemical process in which a chemical agent is used to forcibly convert dissolved species into insoluble compounds. After leaching the LIB cathode powders, remaining solid residues are filtered, and various chemical agents are used to systematically precipitate the various metals to recover them as metal salts, thereby affecting separation76. A myriad of chemicals have been investigated for the precipitation step, including carbonates and hydroxides77,78. Often, coprecipitation occurs, such that the precipitated material contains several metal ions with a somewhat uniform distribution. This ability to produce a mixed and homogenous material can aid in the resynthesis of the cathode material while avoiding potentially complicated separation steps79. The following are some representative precipitation studies performed during the recycling of materials from LIBs.
Sa et al. used NaOH and NH3.H2O for coprecipitation to produce Ni1/3Mn1/3Co1/3(OH)2 after NMC LIB leaching. A total of 1 M NH3.H2O was initially heated to 60 °C, after which 5 M NH3 was added. H2O and 2 M of leached MSO4 (M = Co, Ni, Mn) were added at rates of 10 mL/hr and 30 mL/hr, respectively, for two hours, while 5 M NaOH was added automatically throughout the precipitation process for pH control. Care was taken in adjusting the Ni:Mn:Co to close to 1:1:1 to obtain a high-quality precursor for NMC cathode production after coprecipitation. Mn2+ oxidation was particularly monitored during coprecipitation. Coprecipitation was conducted in a nitrogen (N2) atmosphere to prevent the formation of Mn3+, which could lead to multiphase formation and affect coprecipitation. The coprecipitation process was completed after 24 h, producing Ni1/3Mn1/3Co1/3(OH)2; this material was then physically mixed with Li2CO3 and heated for 12 h in air at 900 °C to synthesize the LiNi1/3Mn1/3Co1/3O2 cathode material79.
Dhiman & Gupta applied a mixture of precipitation and solvent extraction after leaching. Concentrated NaOH was added to the product leach solution, and the resulting mixture was heated for 2 h at 95 °C, causing Fe to precipitate as iron hydroxide [Fe(OH)2], which was filtered. Moreover, the resulting solution was treated with ammonium persulfate [(NH4)2S2O8] to precipitate Mn as manganese oxide (MnO2), which was filtered. A NaOH solution was added to precipitate any Cu and Al, which were filtered as Cu(OH)2 and Al(OH)3, respectively. Then, solvent extraction was applied using a phosphonium ionic liquid reagent to selectively extract Co from the remaining solution containing Li, Ni, and Co. Co was recovered from the organic material used for extraction via one of two approaches. The first approach involved the direct precipitation of Co from the loaded organic material using 0.04 M oxalic acid, which was followed by calcining the resulting cobalt oxalate (CoC2O4) at 600 °C to obtain cobalt oxide (Co3O4). The second approach involved stripping the organic reagent using 0.05 M HCl solution to recover the cobalt in solution. The raffinate produced from the solvent extraction process was treated with ammonia (NH3) and dimethylglyoxime to precipitate Ni as a dimethylglyoxalate complex. The mixture remaining after Ni precipitation was filtered and treated with NaOH for pH adjustment, after which a saturated Na2CO3 solution was added at 100 °C to precipitate the Li2CO380.
Solvent extraction is a process by which various ions/compounds are transferred between two immiscible phases based on the selectivity of the extracting phase for the target ion/compound. Highly selective reagents are used to separate ions/compounds; therefore, this process is proven to be very effective. Some reagents that have been used for LIBs include 2-ethylhexyl phosphonic acid (PC88A), trioctylamine (TOA), di-2-ethylhexyl phosphoric acid (D2EHPA), benzoyltrifluoroacetone (HBTA), trioctylphosphine oxide (TOPO) and bis(2,4,4-trimethylpentyl)phosphoric acid (Cyanex 272), as shown in Table 2. Since solvent extraction processes are chiefly focused on the separation of ions/compounds, they are usually paired with another process to recover the metal species, and for LIBs, precipitation is used to obtain metal salts as the product80. Solvent extraction may be used with precipitation to extract impurities before precipitation.
Electrochemical deposition utilizes the differences in electrode potentials to recover and separate metals. An applied potential causes the metallic ions in the solution to obtain electrons, resulting in metal deposition on the cathode. With respect to LIBs, Al, and Fe recovery via electrochemical deposition has not been of primary concern because of the economics involved and the relative ease of precipitating Al and Fe80. Li has a strong negative potential, which complicates its electrochemical deposition81. Mn electrodeposition is strongly affected by the purity of the electrolyte, and it requires the use of additives such as selenite or selenate, which improve the deposition quality; conversely, the addition of ammonia increases the current efficiency82. In contrast, Co and Ni have ideal standard reduction potentials for electrodeposition, but their electrodeposition from a mixture consisting of both species can be challenging because their potentials are comparable, which affects selectivity and often results in the formation of a Co-Ni alloy83. The following are examples of electrodeposition studies that have been conducted.
To improve the selectivity during Co and Ni electrodeposition, Kim et al. utilized an interfacial design using functionalized electrodes and speciation control using concentrated chloride to recover Co and Ni from NMC cathode powder83. An anionic cobalt chloride complex (CoCl42−) formed, while Ni was in the cationic form [Ni(H2O)5Cl]+. Cu foil was used as the working electrode, and the electrodes were functionalized with positively charged polydiallyldimethylammonium chloride (polyDDA). These electrodes changed the mobility of the Co anion through electrostatic stabilization, and the Co selectivity depended on the polyDDA loading. Using this method, final purities of ~96.4% Co and ~94.1% Ni were attained83. Conversely, Freitas & Garcia exclusively analyzed Co electrodeposition from LCO LIB cathode powder, and they specifically focused on pH effects, which eliminated the interference of Ni84. Starting with a solution primarily composed of 3 M HCl, which was used for the leaching operations, NaOH and boric acid (H3BO3) buffer were used for pH adjustment before Co electrodeposition. An Al working electrode was used for the experiments, which focused on ascertaining the most suitable electrochemical conditions for stable Co electrodeposition. Investigations into electrodeposition at a potential of −1.00 V (vs. Ag/AgCl/NaCl saturated reference electrode) and subsequent dissolution of the Co deposits in 0.5 M H2SO4 revealed that deposits formed above a pH of 4.0 were increasingly resistant to corrosion because of the non-inclusion of adsorbed hydrogen84.
The electrochemical processing of LIBs could take the form of electrolysis via the application of molten salt. Molten salt electrolysis involves the selective dissolution of certain battery components within a high-temperature molten salt solution, and it involves a combination of the dissolution, separation, and recovery steps, which are often separated in aqueous systems. Molten salts are excellent electrolytes due to their wide electrochemical window and high ionic conductivity85. These features make them ideally suited for the electrochemical manipulation of electrons, which can be used for metal reduction during the metal recovery and separation process86. In addition, the solubility of some compounds in molten salts can be manipulated, thereby allowing the recovery of insoluble compounds, such as Co and CoO86.
The cathode material, which is the subject of treatment, is made of an electrolytic cell cathode and immersed in a molten electrolyte, with various materials, such as carbon, LiFePO4, and Ni-Cu-Fe alloys, being used in some studies as the anode85,87,88,89. Some molten salts that have been utilized include Na2CO3-K2CO3, AlCl3-NaCl, NaCl-CaCl2, Na2CO3-K2CO3, and LiCl-KCl. Some works involving the application of molten salt systems include cathode material peeling from Al foil by melting PVDF in AlCl3-NaCl90, the recovery of Co and Li using Na2CO3-K2CO386, Mn reduction from LiMn2O4 in NaCl-CaCl288, and Co and Li recovery from LiCoO2 in LiCl-KCl91. A drawback is the elevated operating temperature, which can range from 160–750 °C depending on the application.
LCA and design considerations for lithium-ion battery recycling
The evaluation of the environmental performance of different processes is essential for obtaining a holistic picture of the application of recycling as a treatment for EoL LIBs. Life cycle assessment (LCA) is a tool that has been applied to ascertain certain information, such as the environmental implications of various processes, and has been similarly used to analyze EoL LIB treatment92. An LCA is a methodological framework used to estimate and analyze the attributable environmental impacts of a service, activity, or product, from conception to final disposal (including recycling)93. Depending on the LCA results, we consider the process from raw material extraction to a specific endpoint, such as the production of batteries (the “cradle-to-gate” approach), the final disposal of batteries (the “cradle-to-grave” approach), and the remanufacturing of batteries from waste (the “cradle-to-cradle” approach)94.
When carrying out any LCA, there are three main components that comprise an LCA that is compliant with International Organization for Standardization (ISO) 14040, as shown in Fig. 10. The goal and scope of the LCA are explicitly stated, wherein the material, process and/or service of the study are highlighted, the system boundary under consideration is defined, and the functional unit is presented. The life cycle inventory provides a detailed account of all required materials, energy, produced waste, and emissions based on the chosen functional unit. In the life cycle impact assessment, we then utilize the results from the inventory, which are converted to level indicators used to characterize impacts associated with the resource utilization and pollutants generated and released. Some level indicators that are used are the global warming potential (GWP), greenhouse gas emissions (GHG), water consumption (WC), mineral depletion potential (MDP), cumulative energy demand (CED), human toxicity potential (HTP), air pollutant emissions (APE) and particulate matter (PM)95,96.
According to Nordelöf and colleagues, modeling of the EoL treatment of a product may be carried out by applying two main approaches: the cutoff approach and the EoL recycling approach97. The cutoff approach accounts for collection and pretreatment and does not model the system to include the recovery, reuse, or repurposing of recycled materials. In the EoL recycling approach, material recovery is included above and beyond the collection. In addition, pretreatment is included in the cutoff approach. It is typically assumed that a unit quantity of the recovered material directly replaces an equivalent unit quantity of primary material used upstream in the production system97.
A major hindrance to LCA utilization in LIB studies is its non-standardized nature, wherein different researchers utilize different impact assessment techniques depending on their chemical compositions, thereby generating noncomparable and sometimes contradictory results98. Regardless, LCA still provides a technique for assessing the broad impacts of the treatment of LIBs upon reaching their EoL. Many LIB LCA studies addressing EoL treatment have focused mostly on NMC and LFP compositions, and pyrometallurgical, hydrometallurgical, and direct recycling techniques have been assessed99. Through LCA, it has been determined that conventional pyrometallurgical processing of EoL LIBs is predicted to have a larger GWP because of its higher carbon emission than hydrometallurgical processing100. This difference arises largely because of the energy requirement for pyrometallurgical processing, which results in the production of various direct and indirect carbon emissions. Studies aimed at addressing LIB pyrometallurgical processing emissions, such as the application of concentrated solar-powered molten reactors, are being performed62. Furthermore, Li recovery through conventional pyrometallurgical techniques is difficult because it is lost in flue dust and slag (from which Li recovery requires the processing of large quantities of slag), whereas hydrometallurgical processing allows for the recovery of Li either as a carbonate or a hydroxide101. The following is a summary of some works covering these aspects.
Jiang et al. conducted an LCA study with a focus on comparing hydrometallurgical and direct recycling processes for the recycling of NMC and LFP LIBs. The indicators that were applied in the study were the CED, GWP, HTP, and MDP. Their results indicated that the benefits of recycling were directly linked to recovery efficiency and electricity usage. No significant differences in the impacts associated with the use of the hydrometallurgical or direct recycling process were reported for LFP batteries. In contrast, NMC battery recycling via hydrometallurgy had relatively great impacts on all the indicator categories. The CED, GWP, HTP, and MDP ratios of the hydrometallurgical process to the direct recycling process were 2.4, 1.9, 1.5, and 1.4, respectively. This phenomenon was attributed to the need for additional energy and chemicals, such as H2SO4 and NaOH, for cathode component recovery and separation in the hydrometallurgical process95.
In their study, Yoo and colleagues analyzed a process focused on recovering Li in the form of LiOH and other cathode component materials from spent NMC batteries. The analysis was based on the EverBatt model102 and the Greenhouse gases, Regulated Emissions and Energy use in Technologies (GREET®) model103 on the basis of a kilogram of recovered LiOH. The battery recycling process was compared to that of LiOH production from Chilean brine (LCB) and from Australian ore (LAO). The results showed that the recycling process produces 37 and 72% less GHG emissions than LCB and LAO, respectively. The key contributor to GHG emissions while utilizing LCB is found to be Li2CO3. While utilizing LAO, the major contributor is the energy extracted from coal used for LiOH production. The water consumption results show 57 and 80% lower water usage levels than LCB and LAO, respectively. The factor contributing the most to the water consumption of the process to produce recycled LiOH is energy (electricity) use, which contributes to 70% of the life cycle water consumption96.
A major challenge that recyclers have encountered is the dismantling of LIBs to effectively recover and recycle great quantities of materials in pure states. It has become increasingly clear that cell design is an influencing factor in the effective dismantling of cells. With respect to EVs, cells aggregate into modules, after which the modules aggregate into packs104. Assembling packs, modules, and cells is important. The packs and modules are often glued while the cells are hermetically sealed. The use of adhesives in module and pack assembly ensures rigidity and introduces the challenge of dismantling. Organic solvents are often required for dissolution. Overall, various structures have been designed for safety and longevity that can affect recycling speed by impacting the disassembly process.
Marshall et al. highlighted a developed cell disassembly process using LMO and NCA (ratio of 3:1) pouch cells. The cells were first discharged, and then a ceramic scalpel was used to open the cell pouch with incisions made around the cell edges. The electrode stack was separated into different components: the cathode, anode, and other constituents. The electrodes were washed in an isopropanol-2-ol bath to remove salt and electrolyte, thereby preventing HF formation from electrolyte hydrolysis. The active BM was separated from the Cu and Al current collectors. An oxalic acid solution (0.02 M) was used for the separation of the BM from the anodes at ambient temperature and ultrasonication for 30 min, while the cathodes were ultrasonicated for 5 min in a 0.5 M oxalic acid solution at 50 °C105. A key finding is that the developed process is generally suitable for other cell chemistries; however, some aspects have been tailored to the reported chemistry and design. For example, a steel can requires a different dismantling approach than another component.
This research clearly shows how the design of the recycling process will become a major consideration in the future. A form of standardized design must be adopted to develop a recycling process with an efficient dismantling process. This design will significantly contribute to downstream processes with respect to the qualities of the recovered materials and the cost of the recycling process. If an overarching simplified comparison is to be made between the different processes, process design and selection can be further simplified. A comparison can be made with a focus on key indicators, which are termed green score indicators. These indicators include complexity, waste generation, and consumption. The green score pseudo technical approach was developed from a review of the literature showing an apparent dearth of a clear comparison matrix for the different EoL LIB recycling technologies. By mapping the LCA aspects onto the green score, waste generation may be considered to encompass GWP, GHG, APE, and PM, while consumption encompasses WC, MDP, and CED. Complexity is based only on the technical aspects previously outlined for each of the processes. A score of 10 (1-worst and 10-best) is assigned for each of the processes based on the summary of the literature provided, and the results are summarized in Table 3.
In terms of complexity, pyrometallurgy is the least complex process, as little to no sorting is needed for the feedstock. Furthermore, as pyrometallurgical processes are more established and less affected by chemistries than other processes, they boast robustness. Direct recycling has the next highest score because it eliminates the recovery process of each element that occurs in both pyrometallurgy and hydrometallurgy. Hydrometallurgy is the most complex because it involves several sequential steps to prepare EoL LIBs, extract target components, and recover them in various forms. Direct recycling is the method that generates the least waste because it does not extract or separate materials. The waste generated is attributed to excess material used during relithiation. Hydrometallurgical processes mostly produce large quantities of wastewater that must be treated. Thus, hydrometallurgy is assigned a score of 7. Pyrometallurgical procedures are assigned a score of 6. This score is assigned due to their contributions to the GWP, GHG, APE, and PM indicators. Technologies and approaches are being developed to improve the process by reducing gaseous emissions and energy density, thereby decreasing the contributions to the GWP and GHG indicators53,54. With respect to consumption, direct recycling utilizes the lowest amount of power and has the lowest MDP, but it ranks second to hydrometallurgical processing with respect to WC. Considering these aspects, direct recycling receives a score of 8. Hydrometallurgical processes consume power for temperature control and for commercial operations, an added power consumption is experienced during shredding. This process has a high WC and the second-highest MDP after direct recycling; therefore, a score of 7 is assigned to this process. Pyrometallurgical operations are by far the most energy intensive of the three approaches. However, these operations have the lowest WC and the highest MDP indicators. A score of 5 is assigned to pyrometallurgical processing. The ranking of the processes with respect to the total green score (from highest to lowest) is as follows: i) direct recycling, ii) hydrometallurgy, and iii) pyrometallurgy.
Economic considerations for lithium-ion battery waste management
A probe into the economics of each of the main approaches for handling LIB waste, as shown in Fig. 5, is carried out to provide an image of the current economic state of the market. Overall processes (which consist of multiple processing steps) that produce a final marketable product are selected: i) refurbishing and repurposing, ii) direct recycling, iii) indirect pyrometallurgy, and iv) indirect hydrometallurgy recycling. The economic analysis is built on a cost comparison with the basis of a tonne of EoL LIBs processed or treated. Notably, various sources provide data based on the units that best describe the desired process (marketable) product. The data gathered from the various sources are given in their original form before conversion and standardization in Table 4. Therefore, for comparison, conversions are applied based on information from sources, as indicated in the “notes” column of Table 5. An in-depth description of the calculation methods is given in Tables S2–S6 of the supplementary information.
After standardizing the data to provide a common basis for comparison (cost/tonne of EoL LIBs), the economics of each of the approaches highlighted in Table 4 are given in Table 5. The lowest cost is associated with direct pyrometallurgy treatment at USD 26 per tonne of EoL LIBs, while the highest cost is associated with refurbishing/repurposing at USD 7,452 per tonne of EoL LIBs. The repurposing/refurbishment process is greatly affected by the unit being treated. The refurbishment of relatively small units, such as cells, costs more than that of relatively large units, such as batteries.
Policy and government incentives
The government and policy-makers play pivotal roles in the support and adoption of LIB recycling. To date, most nations do not have government-affiliated centers focused on LIB recycling; however, several grants have been awarded to various organizations to help with the establishment and scaling of LIB recycling facilities106. It has been reported that Redwood Materials, a company located in Nevada U.S.A., secured a government loan commitment of USD 2 billion for the development of a LIB recycling complex107. Another LIB recycling company, Li-Cycle US Holdings, Inc., received a USD 375 million loan from the U.S. Department of Energy Loan Programs Office to finance the construction of a LIB resource recovery facility108. Major drawbacks in designing legislation directed at LIB recycling are the differences in battery design and utilization and in the level of maturity of LIB recycling technology. These characteristics are not managed in an established manner. Public awareness, information dissemination, and ease of access are also lacking. Figure 11 provides a summary of some of the policies enacted to promote LIB recycling across the globe.
A leading regulation that has been adopted by several countries across Europe, Asia, and North America, is the Extended Producer Responsibility (EPR). Manufacturers and retailers of LIB-utilizing products must collect the LIBs contained in the products and ensure their appropriate recycling109. The EU helped shape EPR regulations, with widespread adoption and continuous revision and improvement. While the EPR does not specifically address LIBs, it does highlight regulations for battery recycling. Industrial partners have influenced battery collection, sorting, and recycling. For example, the operator of Europe’s largest EV battery recycling plant, Hydrovolt, and the more established GRS Batterien, which was jointly established by the Electrical and Electronic Industries Association and battery manufacturers, have contributed to this discussion110.
As part of the European energy policy, national regulations regarding the management of waste batteries and associated accumulators have been introduced in EU member states. The EU regulations cover a multitude of different battery compositions, including LIBs. Through Directive 2006/66/EC, the polluter pays concept has been applied, enabling consumers to freely dispose of batteries at designated locations. The directive obligates EU member states to monitor and annually report waste and spent portable battery collection rates. Some provisions have since been made to aid in the implementation of Directive 2006/66/EC, such as Commission Regulation (EU) No 493/2012, which lays down details regarding waste batteries and accumulator recycling efficiency calculations. Moreover, Commission Regulation (EU) No 1103/2010 is considered, which provides guidelines related to the capacity labeling of automotive and portable rechargeable batteries and accumulators. To bridge the gap between the various sectors comprising the battery value chain—including industry, government, and the scientific community (including academia)—the European Battery Alliance (EBA) was established and launched in 2017. With the support of the European Investment Bank and the European Commission, the EBA has assisted in strategic action plans for a circular economy, such as the acquisition of raw materials by recycling111.
Even though the UK is no longer an EU Member State, the EU Batteries Directive was transposed to the UK legal scape. With regard to LIB recycling, the UK adopted elements of battery collection, disposal, and recycling from the EU Batteries Directive. Specifically, the Directive has addressed LIBs used in industry and banned their disposal by incineration or in landfills. Similar to the EU, the UK adopted the concept of “polluter pays,” wherein battery end-users can dispose of batteries at the end of use with battery producers or suppliers at no charge. A requirement is that records must be kept of the amounts of industrial batteries entering the UK market and of the amounts of batteries leaving the market through take-back programs by manufacturers and suppliers or through recycling through approved battery treatment operators inside or outside the UK borders112.
Apart from the EU and the UK, some other countries are enacting policies and legislation to improve LIB recycling. Some noteworthy nations are Japan and the US, which have LIB recycling markets that are still in flux. Japan has adopted several principles from the EU’s EPR program to promote resource recovery and the abatement of environmental consequences resulting from waste battery disposal113. The Promotion of Effective Utilization of Resources (PEUR) is a Japanese legislation that promotes the recycling of rechargeable batteries through partnerships between importers, manufacturers, and distributors of batteries (including LIBs) and battery-powered electronics114. In the US, the federal government has pushed for LIB recycling research and facility establishment through various grants to reduce the costs of EoL LIB collection and transportation, thereby improving the management of damaged and nondamaged EoL LIBs and improving battery design to facilitate the reuse, dismantling, recycling and recovery of materials and components. These strategies are designed to improve the overall EoL LIB treatment process while providing a secondary source of raw materials and thereby easing burdens on the material supply chain.
Future scope and gap analysis
Although LIB utilization is currently on the rise, an indirect method for reducing LIB waste and challenges faced by recycling is the modification of lithium-based battery technology and alternative high-capacity battery technologies. Li solid-state batteries, which utilize a Li metal anode and a solid matrix or solid-state electrolyte (SSE) for charge shuttling (not a liquid electrolyte), are promising alternatives to Li-based batteries115. Some solid matrices for Li solid-state batteries are solid polymers and solid inorganic electrolytes, which can be divided into oxide-, sulfide-, and halide-based SSEs116,117. This electrolyte change replaces the flammable liquid electrolyte and improves safety issues in downstream processing while increasing capacity and energy density118. With the utilization of Li metal, care still needs to be taken in handling spent Li solid-state batteries117.
An alternative ion battery to LIBs is the sodium-ion battery (SIB). SIBs have the same working principle and cell design as LIBs, with one key difference: the use of sodium (Na) instead of Li as the main chemical component. As Na is less expensive and more widely available than Li (>1000 times more abundant than Li), SIB costs might be lower than LIB costs119. However, owing to the lower average SIB service life (2–5 years) and the lower material valuation in SIBs, the economic recycling of EoL SIBs may prove to be more challenging than LIB recycling120. Apart from the changes in electrolytes and cathode materials, a modification in the anodes used in battery technology is a viable future prospect. Currently, graphite is chiefly utilized in LIB anodes. However, silicon (Si) is an alternative that is being explored for use as an anode. Si anodes may leverage the high Si abundance to make them cheaper than graphite anodes while providing higher energy densities and faster charging speeds121,122. While Si anodes may offer the aforementioned advantages, they often undergo extensive volume changes during cycling, leading to active material deterioration and current collector–active material contact loss; in addition, these anodes affect operational characteristics, such as solid electrolyte interface growth123,124,125.
Considering the current status quo of battery technology, the primary motivation for LIB recycling is the growth of the LIB industry in the next several decades, which indicates a growth in waste processing. Therefore, innovation in recycling methods has been on the rise over the past ten years. The optimization of these methodologies—including heat treatment, leaching, and precipitation—will be of utmost importance, and research should be focused on ensuring a consistent supply of LIB constituent materials. In addition to the actual processes required for recycling, supporting structures, such as dependable collection and sorting, information dissemination, and robust handling channels, need to be established. A concerted effort by the US Department of Energy aimed at improving this aspect has been made through the provision of funding for research into the following aspects: i) improving consumer involvement in battery recycling; ii) programs targeting retailers and local and state consumers in terms of their electronic battery collection, transportation, recycling and reprocessing; and iii) cost reduction for recycling consumer electronics batteries, with a focus on less expensive collection and preprocessing to render the batteries nontoxic126. Novel recycling approaches show promise for economical and environmentally friendly extraction.
Currently, there are several limitations to battery recycling capacity. A premier imperfection is the large amount of waste generated with respect to the mass of batteries. A potential avenue is to repurpose used batteries at their EOL. Up to 70% of the original capacity of a used battery can be integrated into a new energy storage system127. Current and future national and global initiatives may be focused on environmental impacts and climate change related to all aspects of energy production. There are several ecological ramifications of recycling that make it a less appealing process. Primarily, a large amount of chemical waste is generated from hydrometallurgical methods, and high-temperature loads are coupled with emissions associated with pyrometallurgical processing. Research directed at addressing these shortcomings is being performed, with a focus on chemical waste reduction via closed-loop processing and green chemicals. Moreover, power supply technologies are emerging to manage energy demand and emissions. In the short term, these methods can be performed sequentially to reduce the harmful effects of each technology, with relatively low temperatures and fume control measures being employed to reduce heat treatment effects. However, the basicity of the generated wastewater and the potential for reagent recovery pose problems for future research.
To simplify the comparison of the three overarching processes (pyrometallurgy, hydrometallurgy, and direct recycling), a pseudo-technical green score approach based on LCA aspects has been described and applied to each process. With this green score, a numerical value is assigned based on three broad categories (complexity, waste generation, and power consumption). The green score is envisioned as a simplified matrix that can be condensed from more complex data and help all legislators and policy-makers in decision-making. Due to its nature, the green score can expand and reflect key parameters of interest across various industries and simplify data presentation.
Economic analyses of LIB recycling and waste management by different methods can be simplified by utilizing a common basis that clearly reflects the associated costs of each process. To highlight this benefit, the conversion of costs associated with various EoL LIB and waste LIB treatment processes are presented in this work. Costs are standardized to reflect the treatment cost per tonne of LIB. The results show that pyrometallurgy provides the lowest cost per tonne of LIB processed, while hydrometallurgy has the highest cost per tonne of LIB processed. Costs are largely influenced by the feedstock and the complexity of the method applied. This influence can be observed when considering the application of direct or indirect pyrometallurgy and the energy source and when considering various hydrometallurgical operations that require different processing chemicals. Overall, the LIB and overall battery landscape will continue to evolve with the development of new energy sources, processing technologies, and policies that will continuously affect LIB waste management and recycling.
References
Sun, Y.-K. A rising tide of Co-free chemistries for li-ion batteries. ACS Energy Lett. 7, 1774–1775 (2022).
Mohammadi, F. & Saif, M. A comprehensive overview of electric vehicle batteries market. e-Prime-Adv. Electr. Eng. Electr. Energy. 3, 100127 (2023).
Li, M., Lu, J., Chen, Z. & Amine, K. 30 years of lithium‐ion batteries. Adv. Mater. 30, 1800561 (2018).
Blomgren, G. E. The development and future of lithium ion batteries. J. Electrochem. Soc. 164, A5019 (2016).
Agrawal, R. & Pandey, G. Solid polymer electrolytes: materials designing and all-solid-state battery applications: an overview. J. Phys. D. Appl. Phys. 41, 223001 (2008).
Xue, C., Zhou, H., Wu, Q., Wu, X. & Xu, X. Impact of incentive policies and other socio-economic factors on electric vehicle market share: A panel data analysis from the 20 countries. Sustainability 13, 2928 (2021).
Yadlapalli, R. T., Kotapati, A., Kandipati, R. & Koritala, C. S. A review on energy efficient technologies for electric vehicle applications. J. Energy Storage 50, 104212 (2022).
Richa, K., Babbitt, C. W., Gaustad, G. & Wang, X. A future perspective on lithium-ion battery waste flows from electric vehicles. Resour. Conserv. Recy. 83, 63–76 (2014).
Islam, M. T. & Iyer-Raniga, U. Lithium-ion battery recycling in the circular economy: a review. Recycling 7, 33 (2022).
Manthiram, A. An outlook on lithium ion battery technology. ACS Cent. Sci. 3, 1063–1069 (2017).
Purwanto, A. et al. NCA cathode material: synthesis methods and performance enhancement efforts. Mater. Res. Express 5, 122001 (2018).
Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 11, 1550 (2020).
Helbig, C., Bradshaw, A. M., Wietschel, L., Thorenz, A. & Tuma, A. Supply risks associated with lithium-ion battery materials. J. Clean. Prod. 172, 274–286 (2018).
Ryu, H.-H., Sun, H. H., Myung, S.-T., Yoon, C. S. & Sun, Y.-K. Reducing cobalt from lithium-ion batteries for the electric vehicle era. Energ. Environ. Sci. 14, 844–852 (2021).
U.S. Geological Survey. Mineral commodity summaries 2023. U.S. Geological Survey, 210 (2023).
Mohsin, M., Picot, A. & Maussion, P. A new lead-acid battery state-of-health evaluation method using electrochemical impedance spectroscopy for second life in rural electrification systems. J. Energy Storage 52, 104647 (2022).
Pang, Z. et al. A new method for determining SOH of lithium batteries using the real-part ratio of EIS specific frequency impedance. J. Energy Storage 72, 108693 (2023).
Hannan, M. A., Lipu, M. H., Hussain, A. & Mohamed, A. A review of lithium-ion battery state of charge estimation and management system in electric vehicle applications: challenges and recommendations. Renew. Sust. Energ. Rev. 78, 834–854 (2017).
Hlal, M. I., Ramachandaramurthy, V. K., Sarhan, A., Pouryekta, A. & Subramaniam, U. Optimum battery depth of discharge for off-grid solar PV/battery system. J. Energy Storage 26, 100999 (2019).
Xiong, R., Tian, J., Mu, H. & Wang, C. A systematic model-based degradation behavior recognition and health monitoring method for lithium-ion batteries. Appl. Energy 207, 372–383 (2017).
Li, R., Hong, J., Zhang, H. & Chen, X. Data-driven battery state of health estimation based on interval capacity for real-world electric vehicles. Energy 257, 124771 (2022).
Zou, Y., Hu, X., Ma, H. & Li, S. E. Combined state of charge and state of health estimation over lithium-ion battery cell cycle lifespan for electric vehicles. J. Power Sources 273, 793–803 (2015).
Xiong, R., Li, L. & Tian, J. Towards a smarter battery management system: a critical review on battery state of health monitoring methods. J. Power Sources 405, 18–29 (2018).
Ezpeleta, I. et al. Characterisation of commercial Li‐ion batteries using electrochemical impedance spectroscopy. ChemistrySelect 7, e202104464 (2022).
Galeotti, M., Cinà, L., Giammanco, C., Cordiner, S. & Di Carlo, A. Performance analysis and SOH (state of health) evaluation of lithium polymer batteries through electrochemical impedance spectroscopy. Energy 89, 678–686 (2015).
Xiong, R., Li, L., Li, Z., Yu, Q. & Mu, H. An electrochemical model based degradation state identification method of Lithium-ion battery for all-climate electric vehicles application. Appl. Energy 219, 264–275 (2018).
Chen, L., Lü, Z., Lin, W., Li, J. & Pan, H. A new state-of-health estimation method for lithium-ion batteries through the intrinsic relationship between ohmic internal resistance and capacity. Measurement 116, 586–595 (2018).
Cai, L. et al. Multiobjective optimization of data-driven model for lithium-ion battery SOH estimation with short-term feature. IEEE Trans. Power Electron 35, 11855–11864 (2020).
Jiang, S. & Song, Z. Estimating the state of health of lithium-ion batteries with a high discharge rate through impedance. Energies 14, 4833 (2021).
Bhar, M., Ghosh, S., Krishnamurthy, S., Kaliprasad, Y. & Martha, S. K. A review on spent lithium-ion battery recycling: from collection to black mass recovery. RSC Sustain. 1, 1150–1167 (2023).
Werner, D., Peuker, U. A. & Mütze, T. Recycling chain for spent lithium-ion batteries. Metals 10, 316 (2020).
Alipanah, M., Saha, A. K., Vahidi, E. & Jin, H. Value recovery from spent lithium-ion batteries: a review on technologies, environmental impacts, economics, and supply chain. Clean. Technol. Recy. 1, 152–184 (2021).
Richa, K., Babbitt, C. W. & Gaustad, G. Eco‐efficiency analysis of a lithium‐ion battery waste hierarchy inspired by circular economy. J. Ind. Ecol. 21, 715–730 (2017).
Saxena, S., Le Floch, C., MacDonald, J. & Moura, S. Quantifying EV battery end-of-life through analysis of travel needs with vehicle powertrain models. J. Power Sources 282, 265–276 (2015).
Haram, M. H. S. M. et al. Feasibility of utilising second life EV batteries: applications, lifespan, economics, environmental impact, assessment, and challenges. Alex. Eng. J. 60, 4517–4536 (2021).
Neubauer, J., Smith, K., Wood, E. & Pesaran, A. Identifying and overcoming critical barriers to widespread second use of PEV batteries. National Renewable Energy Laboratory (NREL), Golden, CO (United States), (2015).
Zhao, Y. et al. A review on battery market trends, second-life reuse, and recycling. Sustain. Chem. 2, 167–205 (2021).
Ahmadi, L., Young, S. B., Fowler, M., Fraser, R. A. & Achachlouei, M. A. A cascaded life cycle: reuse of electric vehicle lithium-ion battery packs in energy storage systems. Int. J. Life Cycle Assess. 22, 111–124 (2017).
DeRousseau, M., Gully, B., Taylor, C., Apelian, D. & Wang, Y. Repurposing used electric car batteries: a review of options. JOM 69, 1575–1582 (2017).
Martinez-Laserna, E. et al. Technical viability of battery second life: a study from the ageing perspective. IEEE Trans. Ind. Appl. 54, 2703–2713 (2018).
Hua, Y. et al. Toward sustainable reuse of retired lithium-ion batteries from electric vehicles. Resour. Conserv. Recy. 168, 105249 (2021).
Sloop, S. E. et al. Cathode healing methods for recycling of lithium-ion batteries. Sustain. Mater. Technol. 22, e00113 (2019).
Li, X., Zhang, J., Song, D., Song, J. & Zhang, L. Direct regeneration of recycled cathode material mixture from scrapped LiFePO4 batteries. J. Power Sources 345, 78–84 (2017).
Hanisch, C. et al. Recycling of lithium-ion batteries: a novel method to separate coating and foil of electrodes. J. Clean. Prod. 108, 301–311 (2015).
Lombardo, G., Ebin, B., Foreman, M. R. S. J., Steenari, B.-M. & Petranikova, M. Incineration of EV lithium-ion batteries as a pretreatment for recycling–determination of the potential formation of hazardous by-products and effects on metal compounds. J. Hazard. Mater. 393, 122372 (2020).
Zhang, G. et al. A sustainable process for the recovery of anode and cathode materials derived from spent lithium-ion batteries. Sustainability 11, 2363 (2019).
Zachmann, N., Petranikova, M. & Ebin, B. Electrolyte recovery from spent lithium-ion batteries using a low temperature thermal treatment process. J. Ind. Eng. Chem. 118, 351–361 (2023).
Hu, X. et al. Complex gas formation during combined mechanical and thermal treatments of spent lithium-ion-battery cells. J. Hazard. Mater. 431, 128541 (2022).
Yang, Y., Huang, G., Xu, S., He, Y. & Liu, X. Thermal treatment process for the recovery of valuable metals from spent lithium-ion batteries. Hydrometallurgy 165, 390–396 (2016).
Lombardo, G., Ebin, B., St. J. Foreman, M. R., Steenari, B.-M. & Petranikova, M. Chemical transformations in Li-ion battery electrode materials by carbothermic reduction. ACS Sustain. Chem. Eng. 7, 13668–13679 (2019).
Haldar, S. K. Mineral exploration: principles and applications. (Elsevier, 2018).
Yue, Y. et al. Recovering valuable metals from spent lithium ion battery via a combination of reduction thermal treatment and facile acid leaching. ACS Sustain. Chem. Eng. 6, 10445–10453 (2018).
Fu, Y. et al. Microwave reduction enhanced leaching of valuable metals from spent lithium-ion batteries. J. Alloy. Compd. 832, 154920 (2020).
Zhao, Y., Liu, B., Zhang, L. & Guo, S. Microwave-absorbing properties of cathode material during reduction roasting for spent lithium-ion battery recycling. J. Hazard. Mater. 384, 121487 (2020).
Fu, Y. et al. Improved hydrometallurgical extraction of valuable metals from spent lithium-ion batteries via a closed-loop process. J. Alloy. Compd. 847, 156489 (2020).
Peng, C., Liu, F., Wang, Z., Wilson, B. P. & Lundström, M. Selective extraction of lithium (Li) and preparation of battery grade lithium carbonate (Li2CO3) from spent Li-ion batteries in nitrate system. J. Power Sources 415, 179–188 (2019).
Lin, J. et al. Conversion mechanisms of selective extraction of lithium from spent lithium-ion batteries by sulfation roasting. ACS Appl. Mater. Interfaces 12, 18482–18489 (2020).
Barrios, O. C., González, Y. C., Barbosa, L. I. & Orosco, P. Chlorination roasting of the cathode material contained in spent lithium-ion batteries to recover lithium, manganese, nickel and cobalt. Miner. Eng. 176, 107321 (2022).
Windisch-Kern, S., Holzer, A., Ponak, C. & Raupenstrauch, H. Pyrometallurgical lithium-ion-battery recycling: approach to limiting lithium slagging with the InduRed reactor concept. Processes 9, 84 (2021).
Gallo, A., Marzo, A., Fuentealba, E. & Alonso, E. High flux solar simulators for concentrated solar thermal research: a review. Renew. Sust. Energ. Rev. 77, 1385–1402 (2017).
Halmann, M., Steinfeld, A., Epstein, M. & Vishnevetsky, I. in Encyclopedia of Aluminum and Its Alloys, Two-Volume Set (Print) 2771–2777 (CRC Press, 2018).
Nuraeni, B. A., Nababan, D. C., Putera, A. & Rhamdhani, M. A. In: TMS Annual Meeting & Exhibition. 187–199 (Springer).
Shaw-Stewart, J. et al. Aqueous solution discharge of cylindrical lithium-ion cells. Sustain. Mater. Technol. 22, e00110 (2019).
Ojanen, S., Lundström, M., Santasalo-Aarnio, A. & Serna-Guerrero, R. Challenging the concept of electrochemical discharge using salt solutions for lithium-ion batteries recycling. Waste Manag. 76, 242–249 (2018).
Wu, L., Zhang, F.-S., He, K., Zhang, Z.-Y. & Zhang, C.-C. Avoiding thermal runaway during spent lithium-ion battery recycling: a comprehensive assessment and a new approach for battery discharge. J. Clean. Prod. 380, 135045 (2022).
Kaas, A., Wilke, C., Vanderbruggen, A. & Peuker, U. A. Influence of different discharge levels on the mechanical recycling efficiency of lithium-ion batteries. Waste Manag. 172, 1–10 (2023).
Natarajan, S. & Aravindan, V. Recycling strategies for spent Li-ion battery mixed cathodes. ACS Energy Lett. 3, 2101–2103 (2018).
Lei, S. et al. Strengthening valuable metal recovery from spent lithium-ion batteries by environmentally friendly reductive thermal treatment and electrochemical leaching. ACS Sustain. Chem. Eng. 9, 7053–7062 (2021).
Li, L. et al. Recovery of metals from spent lithium-ion batteries with organic acids as leaching reagents and environmental assessment. J. Power Sources 233, 180–189 (2013).
Meshram, P., Pandey, B. D. & Mankhand, T. R. Recovery of valuable metals from cathodic active material of spent lithium ion batteries: Leaching and kinetic aspects. Waste Manag. 45, 306–313 (2015).
Zheng, X. et al. Spent lithium-ion battery recycling—Reductive ammonia leaching of metals from cathode scrap by sodium sulphite. Waste Manag. 60, 680–688 (2017).
Paulino, J. F., Busnardo, N. G. & Afonso, J. C. Recovery of valuable elements from spent Li-batteries. J. Hazard. Mater. 150, 843–849 (2008).
Li, L. et al. Recovery of valuable metals from spent lithium-ion batteries by ultrasonic-assisted leaching process. J. Power Sources 262, 380–385 (2014).
Jegan Roy, J., Srinivasan, M. & Cao, B. Bioleaching as an eco-friendly approach for metal recovery from spent NMC-based lithium-ion batteries at a high pulp density. ACS Sustain. ACS Sustain. Chem. Eng. 9, 3060–3069 (2021).
Bahaloo-Horeh, N. & Mousavi, S. M. Enhanced recovery of valuable metals from spent lithium-ion batteries through optimization of organic acids produced by Aspergillus niger. Waste Manag. 60, 666–679 (2017).
Biswal, B. K. et al. Biological leaching and chemical precipitation methods for recovery of Co and Li from spent lithium-ion batteries. ACS Sustain. Chem. Eng. 6, 12343–12352 (2018).
Gao, W. et al. Lithium carbonate recovery from cathode scrap of spent lithium-ion battery: a closed-loop process. Environ. Sci. Technol. 51, 1662–1669 (2017).
Meshram, P., Pandey, B. & Mankhand, T. Hydrometallurgical processing of spent lithium ion batteries (LIBs) in the presence of a reducing agent with emphasis on kinetics of leaching. Chem. Eng. J. 281, 418–427 (2015).
Sa, Q. et al. Synthesis of high performance LiNi1/3Mn1/3Co1/3O2 from lithium ion battery recovery stream. J. Power Sources 282, 140–145 (2015).
Dhiman, S. & Gupta, B. Partition studies on cobalt and recycling of valuable metals from waste Li-ion batteries via solvent extraction and chemical precipitation. J. Clean. Prod. 225, 820–832 (2019).
Xu, X. et al. Role of Li‐ion depletion on electrode surface: underlying mechanism for electrodeposition behavior of lithium metal anode. Adv. Energy Mater. 10, 2002390 (2020).
Lu, J., Dreisinger, D. & Glück, T. Manganese electrodeposition—a literature review. Hydrometallurgy 141, 105–116 (2014).
Kim, K., Raymond, D., Candeago, R. & Su, X. Selective cobalt and nickel electrodeposition for lithium-ion battery recycling through integrated electrolyte and interface control. Nat. Commun. 12, 6554 (2021).
Freitas, M. & Garcia, E. Electrochemical recycling of cobalt from cathodes of spent lithium-ion batteries. J. Power Sources 171, 953–959 (2007).
Zhang, B. et al. A paired electrolysis approach for recycling spent lithium iron phosphate batteries in an undivided molten salt cell. Green. Chem. 22, 8633–8641 (2020).
Zhang, B. et al. A green electrochemical process to recover Co and Li from spent LiCoO2-based batteries in molten salts. ACS Sustain. Chem. Eng. 7, 13391–13399 (2019).
Li, H. et al. Electrochemical mechanism of recovery of nickel metal from waste lithium ion batteries by molten salt electrolysis. Materials 14, 6875 (2021).
Liang, J. et al. The electrochemical mechanism of preparing Mn from LiMn2O4 in waste batteries in molten salt. Crystals 11, 1066 (2021).
Zhao, J. et al. Extraction of Co and Li2CO3 from cathode materials of spent lithium-ion batteries through a combined acid-leaching and electro-deoxidation approach. J. Hazard. Mater. 379, 120817 (2019).
Wang, M., Tan, Q., Liu, L. & Li, J. Efficient separation of aluminum foil and cathode materials from spent lithium-ion batteries using a low-temperature molten salt. ACS Sustain. Chem. Eng. 7, 8287–8294 (2019).
Mirza, M. et al. Recovery of cobalt from lithium-ion batteries using fluidised cathode molten salt electrolysis. Electrochim. Acta 391, 138846 (2021).
Sadhukhan, J. & Christensen, M. An in-depth life cycle assessment (LCA) of lithium-ion battery for climate impact mitigation strategies. Energies 14, 5555 (2021).
Rebitzer, G. et al. Life cycle assessment: Part 1: framework, goal and scope definition, inventory analysis, and applications. Environ. Int. 30, 701–720 (2004).
Chen, Q. et al. Investigating carbon footprint and carbon reduction potential using a cradle-to-cradle LCA approach on lithium-ion batteries for electric vehicles in China. J. Clean. Prod. 369, 133342 (2022).
Jiang, S. et al. Environmental impacts of hydrometallurgical recycling and reusing for manufacturing of lithium-ion traction batteries in China. Sci. Total Environ. 811, 152224 (2022).
Yoo, E., Lee, U., Kelly, J. C. & Wang, M. Life-cycle analysis of battery metal recycling with lithium recovery from a spent lithium-ion battery. Resour. Conserv. Recy. 196, 107040 (2023).
Nordelöf, A. et al. Methodological approaches to end-of-life modelling in life cycle assessments of lithium-ion batteries. Batteries 5, 51 (2019).
Arshad, F. et al. Life cycle assessment of lithium-ion batteries: a critical review. Resour. Conserv. Recy. 180, 106164 (2022).
Mohr, M., Peters, J. F., Baumann, M. & Weil, M. Toward a cell‐chemistry specific life cycle assessment of lithium‐ion battery recycling processes. J. Ind. Ecol. 24, 1310–1322 (2020).
Kallitsis, E., Korre, A. & Kelsall, G. H. Life cycle assessment of recycling options for automotive Li-ion battery packs. J. Clean. Prod. 371, 133636 (2022).
Liu, C., Lin, J., Cao, H., Zhang, Y. & Sun, Z. Recycling of spent lithium-ion batteries in view of lithium recovery: a critical review. J. Clean. Prod. 228, 801–813 (2019).
Dai, Q. et al. EverBatt: A closed-loop battery recycling cost and environmental impacts model. Argonne National Laboratory (ANL), Argonne, IL (United States) (2019).
Wang, M. et al. Greenhouse gases, regulated emissions, and energy use in technologies model (2021 Excel). Argonne National Laboratory (ANL), Argonne, IL (United States) (2021).
Warner, J. T. The handbook of lithium-ion battery pack design: chemistry, components, types and terminology. (Elsevier, 2015).
Marshall, J. et al. Disassembly of Li ion cells—characterization and safety considerations of a recycling scheme. Metals 10, 773 (2020).
Liz, T. Argonne is helping U.S. companies advance battery recycling technology and strengthen the nation’s battery supply chain, Argonne National Laboratory (ANL), Argonne, IL (United States, 2023).
Paul, L. & David, S. U.S. set to loan Redwood Materials $2 bln for EV materials plant, Reuters (2023).
Jigar, S. LPO announces a conditional commitment for loan to Li-Cycle’s U.S. Battery resource recovery facility to recover critical electric vehicle battery materials. Energy.Gov (2023).
Pazoki, M. & Zaccour, G. Extended producer responsibility: regulation design and responsibility sharing policies for a supply chain. J. Clean. Prod. 236, 117516 (2019).
Nilsson, M. et al. Understanding policy coherence: analytical framework and examples of sector–environment policy interactions in the EU. Environ. Policy Gov. 22, 395–423 (2012).
Dobrowolski, Z., Sułkowski, Ł. & Danielak, W. Management of waste batteries and accumulators: quest of European Union goals. Energies 14, 6273 (2021).
Malinauskaite, J., Anguilano, L. & Rivera, X. S. Circular waste management of electric vehicle batteries: legal and technical perspectives from the EU and the UK post Brexit. Int. J. Thermofluids 10, 100078 (2021).
Kim, H., Jang, Y.-C., Hwang, Y., Ko, Y. & Yun, H. End-of-life batteries management and material flow analysis in South Korea. Front. Environ. Sci. Eng. 12, 1–13 (2018).
Morita, Y., Saito, Y., Yoshioka, T. & Shiratori, T. Estimation of recoverable resources used in lithium-ion batteries from portable electronic devices in Japan. Resour. Conserv. Recy. 175, 105884 (2021).
Ye, L. & Li, X. A dynamic stability design strategy for lithium metal solid state batteries. Nature 593, 218–222 (2021).
Chen, R., Li, Q., Yu, X., Chen, L. & Li, H. Approaching practically accessible solid-state batteries: stability issues related to solid electrolytes and interfaces. Chem. Rev. 120, 6820–6877 (2019).
Jacob, M., Wissel, K. & Clemens, O. Recycling of solid-state batteries—challenge and opportunity for a circular economy? Mater. Futures 3, 012101 (2024).
Sun, Y.-K. Promising all-solid-state batteries for future electric vehicles. ACS Energy Lett. 5, 3221–3223 (ACS Publications, 2020).
Hwang, J.-Y., Myung, S.-T. & Sun, Y.-K. Sodium-ion batteries: present and future. Chem. Soc. Rev. 46, 3529–3614 (2017).
Zhao, Y. et al. Recycling of sodium-ion batteries. Nat. Rev. Mater. 8, 623–634 (2023).
Kim, K. et al. Improving the cyclability of silicon anodes for lithium-ion batteries using a simple pre-lithiation method. J. Power Sources 459, 228066 (2020).
Chen, H. et al. A mechanically robust self-healing binder for silicon anode in lithium ion batteries. Nano Energy 81, 105654 (2021).
Son, Y. et al. Exploring critical factors affecting strain distribution in 1D silicon‐based nanostructures for lithium‐ion battery anodes. Adv. Mater. 30, 1705430 (2018).
Li, X. et al. Mesoporous silicon sponge as an anti-pulverization structure for high-performance lithium-ion battery anodes. Nat. Commun. 5, 4105 (2014).
Zhang, L. et al. A yolk–shell structured silicon anode with superior conductivity and high tap density for full lithium‐ion batteries. Angew. Chem. Int. Ed. 58, 8824–8828 (2019).
U. S. Department of Energy. Vol. DE-FOA-0002897 Bipartisan Infrastructure Law (BIL) Consumer Electronics Battery Recycling, Reprocessing, and Battery Collection (ed. Department of Energy) 9–18 (2023).
Hossain, E. et al. A Comprehensive review on second-life batteries: current state, manufacturing considerations, applications, impacts, barriers & potential solutions, business strategies, and policies. IEEE Access 7, 73215–73252 (2019).
Chen, L. et al. Process for the recovery of cobalt oxalate from spent lithium-ion batteries. Hydrometallurgy 108, 80–86 (2011).
Suzuki, T., Nakamura, T., Inoue, Y., Niinae, M. & Shibata, J. A hydrometallurgical process for the separation of aluminum, cobalt, copper and lithium in acidic sulfate media. Sep. Purif. Technol. 98, 396–401 (2012).
Jingu, K., Senanayake, G. & Sohn, J. Recovery of cobalt sulfate from spent lithium ion batteries by reductive leaching and solvent extraction with Cyanex 272. Hydrometallurgy 100, 168–171 (2010).
Nan, J., Han, D., Yang, M., Cui, M. & Hou, X. Recovery of metal values from a mixture of spent lithium-ion batteries and nickel-metal hydride batteries. Hydrometallurgy 84, 75–80 (2006).
Zhang, L. et al. Lithium recovery from effluent of spent lithium battery recycling process using solvent extraction. J. Hazard. Mater. 398, 122840 (2020).
Rallo, H., Benveniste, G., Gestoso, I. & Amante, B. Economic analysis of the disassembling activities to the reuse of electric vehicles Li-ion batteries. Resour. Conserv. Recy. 159, 104785 (2020).
Bruno, M. & Fiore, S. Low-cost and environmentally friendly physic-mechanical pre-treatments to recycle lithium iron phosphate cathodes. J. Environ. Chem. Eng. 12, 12106 (2024).
Park, K. et al. Direct cathode recycling of end-of-life Li-ion batteries enabled by redox mediation. ACS Sustain. Chem. Eng. 9, 8214–8221 (2021).
Reinhart, L. et al. Pyrometallurgical recycling of different lithium-ion battery cell systems: economic and technical analysis. J. Clean. Prod. 416, 137834 (2023).
Thompson, D. et al. To shred or not to shred: a comparative techno-economic assessment of lithium ion battery hydrometallurgical recycling retaining value and improving circularity in LIB supply chains. Resour. Conserv. Recy. 175, 105741 (2021).
Vanderbruggen, A. et al. Automated mineralogy as a novel approach for the compositional and textural characterization of spent lithium-ion batteries. Miner. Eng. 169, 106924 (2021).
Diekmann, J. et al. Ecological recycling of lithium-ion batteries from electric vehicles with focus on mechanical processes. J. Electrochem. Soc. 164, A6184 (2016).
Padhi, A. K., Nanjundaswamy, K. S. & Goodenough, J. B. Phospho‐olivines as positive‐electrode materials for rechargeable lithium batteries. J. Electrochem. Soc. 144, 1188 (1997).
Miao, Y., Hynan, P., Von Jouanne, A. & Yokochi, A. Current Li-ion battery technologies in electric vehicles and opportunities for advancements. Energies 12, 1074 (2019).
Hannan, M. A., Hoque, M. M., Hussain, A., Yusof, Y. & Ker, P. J. State-of-the-art and energy management system of lithium-ion batteries in electric vehicle applications: issues and recommendations. IEEE Access 6, 19362–19378 (2018).
U.S. Geological Survey. Mineral commodity summaries 2015. In: U.S. Geological Survey 196 (2015).
U.S. Geological Survey. Mineral commodity summaries 2016. In: U.S. Geological Survey 202 (2016).
U.S. Geological Survey. Mineral commodity summaries 2017. In: U.S. Geological Survey 202 (2017).
U.S. Geological Survey. Mineral commodity summaries 2018. In: U.S. Geological Survey 200 (2018).
U.S. Geological Survey. Mineral commodity summaries 2019. In: U.S. Geological Survey 200 (2019).
U.S. Geological Survey. Mineral commodity summaries 2020. In: U.S. Geological Survey 200 (2020).
U.S. Geological Survey. Mineral commodity summaries 2021. In: U.S. Geological Survey 200 (2021).
U.S. Geological Survey. Mineral commodity summaries 2022. In: U.S. Geological Survey 202 (2022).
Acknowledgements
This review has been made possible because of the partial funding support to Prichard M. Tembo by the University of Nevada, Reno Chemical and Materials Engineering Department and information gathered during a study conducted through the NSF I-Corps program under grant number 2323629. Funding support for Catherine Dyer through the NSF EPSCoR program is acknowledged. Finally, the authors would like to acknowledge the initial discussions with Rachel Werner.
Author information
Authors and Affiliations
Contributions
P.M.T. and V.S. conceived and designed the study outline and prepared the figures. P.M.T., C.D. and V.S. reviewed the literature, analyzed the data, and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tembo, P.M., Dyer, C. & Subramanian, V. Lithium-ion battery recycling—a review of the material supply and policy infrastructure. NPG Asia Mater 16, 43 (2024). https://doi.org/10.1038/s41427-024-00562-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41427-024-00562-8
- Springer Japan KK