Abstract
Background
Sarcopenia is among the most common musculoskeletal illnesses, yet its underlying biochemical mechanisms remain incompletely understood. Identifying the relationship of inflammatory cytokines with sarcopenia components would help understand the etiology of sarcopenia. We performed a bi-directional Mendelian randomization study to explore the causal relationship between 41 inflammatory cytokines and sarcopenia-related traits.
Methods
The study was performed in two stages using bidirectional dual-sample Mendelian randomization. We obtained aggregated statistical data on inflammatory factors, low grip strength, and ALM from genome-wide association studies. To explore the causal association between exposure and outcomes, we primarily utilized the inverse variance weighted strategy. Furthermore, we conducted sensitivity analyses through the use of Mendelian randomization (MR) Egger, weighted median and simple mode methods. To evaluate robustness of the results and to identify and adjust for horizontal pleiotropy, we performed the MR Pleiotropy RESidual Sum and Outlier test, the MR Egger intercept test, and a leave-one-out analysis.
Results
The results displayed a potential association between interleukin-10 (OR: 1.046, 95% CI: 1.002–1.093, p = 0.042) and vascular endothelial growth factor (OR: 1.024, 95% CI: 1.001–1.047, p = 0.038) and the risk of low hand-grip strength. Moreover, interferon gamma-induced protein 10 (OR: 1.010, 95% CI: 1.000–1.019, p = 0.042) and macrophage colony-stimulating factor (OR: 1.010, 95% CI: 1.003–1.017, p = 0.003) were significantly linked to a higher risk of ALM.
Conclusion
We identified a causal relationship between multiple inflammatory factors and sarcopenia-related traits. Our study offers valuable insights into innovative methods for the sarcopenia prevention and treatment by regulating inflammatory factors.
Similar content being viewed by others
Introduction
Sarcopenia, a term first coined by Rosenberg in 1989, is defined as the age-related decline in muscle mass, accompanied by a higher proportion of slow fiber content and impaired muscle function. This systemic condition is marked by progressive dysfunction in skeletal muscles [1,2,3,4,5,6]. Sarcopenia significantly heightens the risk of falls and fractures, leading to disability and a decline in daily living capabilities [7]. Additionally, it is closely associated with activity disorders, falls, low bone density, and metabolic disturbances [2, 8,9,10,11]. The pathophysiology of sarcopenia is multifaceted, involving the downregulation of anabolic metabolic hormones such as insulin, sex steroids, and growth hormone, increased apoptosis in muscle cells, and up-regulated levels of circulating pro-inflammatory cytokines [12,13,14,15]. Sarcopenia is consistently linked to the age-related decline in muscle mass and function [16]. The loss of muscle mass, which may precede gradual diminution in muscle function, takes place when the rate of muscle protein breakdown exceeds the rate of protein synthesis, thus leading to a net negative balance [17]. The reduced sensitivity of muscle protein synthetic to dietary protein, also called ‘anabolic resistance’, is the main potential mechanism of the gradual loss of muscle mass during aging [17]. Two of the characteristic mammalian amino acid sensing pathways, mTORC1 and ATF4, are believed to play significant roles in the aging of skeletal muscle [18]. Increasing evidence highlights the crucial role of mitochondria in the progression of sarcopenia [19, 20]. Mitochondria, essential for cellular energy production, are central regulators in skeletal muscle and are thus vital in the aging process [21]. Recent research has shown that mitochondrial proteostasis and fission are disrupted in aged muscle. Both fission and mitophagy increase with age, alongside a reduction in mitochondrial content in both slow and fast muscle fibers [22]. These mechanisms are collectively associated with the progression of sarcopenia.
Chronic inflammation associated with aging may contribute to the progression of sarcopenia. Elevated levels of pro-inflammatory cytokines, such as interleukin-6 (IL-6), acute-phase C-reactive protein, and tumor necrosis factor α (TNF-α), have been implicated in muscle loss and physical impairment [23,24,25,26,27]. Past studies have illustrated that heightened IL-6 levels can hinder the synthesis of insulin-like growth factor 1(IGF-1) and disrupt muscle tissue metabolism [28]. Similarly, TNF-α can impede the Akt/mTOR pathway, activate the ubiquitin-proteasome system by transmitting reactive oxygen species and activating the forkhead box O, induce cell apoptosis via the lysosomal autophagy pathway, and trigger the phosphorylation of IKB kinase, ultimately leading to the breakdown of proteins and the inhibition of synthesis in skeletal muscle through increased nuclear entry of NF-KB via the NF-KB signaling pathway [25, 29,30,31]. Nevertheless, it remains debatable whether systemic inflammation is the primary inducement of sarcopenia or if it arises from disease development, subsequent infections, or medication utilization following the onset of sarcopenia. Despite efforts by observational studies to elucidate the relationship between inflammatory cytokines and muscle loss, the presence of unforeseen confounding variables and the potential for reverse causation complicate the interpretation of results, thereby hindering the establishment of a definitive causal relationship.
Mendelian randomization (MR) is a research method which utilizes genetic variations as instrumental variables (IVs) to estimate association between exposure and outcomes [32]. By exploiting the random allocation of alleles during meiosis, MR reduces the influence of confounding variables and can even establish causal relationships. Consequently, MR provides stronger evidence for causal inference compared to observational studies or randomized controlled trials [33]. Moreover, MR circumvents the impact of residual confounding factors, ensuring that the strength of association results is more reliable. In our study, effective genetic variations were extracted from the published summary data of a genome-wide association study (GWAS) on 41 inflammatory cytokines. We aimed to explore their correlation with sarcopenia-related traits. Additionally, we performed reverse MR analysis to assess the impact of sarcopenia-related traits on cytokines. Our study aims to reveal the potential causal association between inflammatory cytokines and sarcopenia, which will provide valuable insights for the development of innovative therapeutic strategies.
Material and methods
Study design
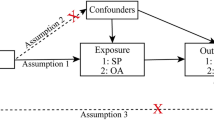
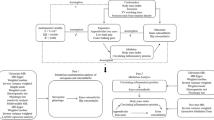
This study utilized a bidirectional MR study design to explore the potential causal relationship between inflammatory cytokines and sarcopenia-related traits, namely low grip strength and appendicular lean mass (ALM). MR analysis may minimize biases that are commonly present in traditional epidemiological observational studies, thus establishing a stronger correlation. MR analysis operates under three key assumptions: relevance, independence, and exclusion limitations [34]. These assumptions require that the chosen genetic variation is associated with the risk factors under investigation (relevance), but not with any confounding factors that may influence the outcome (independence) and that there are no alternative pathways connecting the genetic variation to the outcome, other than through the risk factors of interest (exclusion limitations). In the initial design of our study, we set a conventional significance threshold of P < 5e−8 for our genome-wide association analysis. However, to enhance the robustness of our findings and due to the exploratory nature of our study involving multiple inflammatory markers, we adjusted the threshold to P < 5e−6. This adjustment was made to balance the discovery of potential associations with the risk of type I errors, especially given the complex nature of the traits being studied and the relatively limited number of genetic variants that met the stricter threshold. The study flowchart is presented in Fig. 1A, B.
Data sources and instruments
Inflammatory cytokines We obtained the most comprehensive genetic predictors related to cytokines from a GWAS meta-analysis of three independent cohorts: YFS, FINRISK 1997, and FINRISK 2002. The meta-analysis included a total of 8293 Finnish participants [35]. We adjusted for the correlation between genetic variation and inflammatory cytokines by taking into account age, body mass index, and gender. The criterion for the selection of IVs was a whole genome p-value less than 5 × 10−6, specifically focusing on single nucleotide polymorphisms (SNPs) related to inflammatory cytokines. To make sure that the selected IVs were conditionally independent, we set a strict condition (chain imbalance threshold r2 < 0.001 and distance 10,000 kb). The strength of the association between IVs and inflammatory cytokines was assessed using the F-statistic. A threshold of F > 10 was used to mitigate potential bias from weak IVs. To minimize the impact of pleiotropy-driven confounding, we excluded IVs related to confusion or risk factors associated with sarcopenia, including older age, lower socio-economic status, less physical activity, and poor diet by utilizing PhenoScanner V2 (http://www.phenoscanner.medschl.cam.ac.uk/). Ultimately, we failed to find a significant association between SNPs and the four variables mentioned above.
Sarcopenia-related traits Data on low grip strength at a summary level was obtained from a comprehensive meta-analysis of GWAS, which involved 256,523 European participants with cancer (ebi-a-GCST90007526). The critical thresholds for low grip strength were <30 kg for males and <20 kg for females. Aggregated statistical data for ALM was derived from a GWAS study, which comprised 450,243 participants from the UK Biobank study (ebi-a-GCST90000025). We have selected the most recent and extensive GWAS research available. Supplementary Table S1 provides a comprehensive overview of the GWAS dataset for low grip strength and ALM. The secondary analysis included MR analysis of 41 inflammatory factors and whole body lean mass to increase the robustness of this study. Data regarding the lean body mass were obtained from the up-to-date largest meta-analysis of GWAS, which identified five single nucleotide polymorphisms (SNPs) associated with whole body lean mass in 38,292 individuals of European ancestry [36]. The choice of confounding factors such as age, socio-economic status, physical activity levels, and dietary habits was based on existing literature indicating their significant influence on inflammation and sarcopenia-related traits [37, 38]. For example, previous studies have shown that older age and lower socio-economic status are associated with increased inflammatory responses and reduced physical capabilities [39], respectively. Physical activity and diet are well-documented determinants of both inflammatory markers and muscle function, impacting the physiological pathways involved in sarcopenia [40, 41]. These factors were considered in our analysis to adjust for potential indirect pathways affecting the outcomes not through the exposure of interest but due to these confounders.
Statistical analysis
To ensure reliable and robust results, we utilized a combination of statistical methods, applying Bonferroni correction to adjust for multiple comparisons. The inverse variance weighted (IVW) method was conducted as the main method due to its effectiveness. Nevertheless, it assumes that all genetic variations act as effective IVs, which may not hold in practice [42]. To mitigate this concern, we also implemented several robust methods that do not necessitate all genetic variations to be effective IVs. These Supplementary Methods, namely MR Egger, simple mode tests, and weighted median, were utilized to provide consistent estimates of causal parameters. Additionally, the MR-PRESSO method was used to identify abnormal genetic variants with horizontal pleiotropy, under the assumption that over 50% of IVs are effective [43]. To determine the strength of the correlation between IV exposure, we approximated F-statistics based on aggregated level data. If F > 10, we assumed that the correlation was sufficiently strong to avoid weak IV bias. Finally, we utilized the Cochrane Q statistic of IVW to quantify the heterogeneity between each SNP estimate.
Sensitivity analysis
Multiple methods were utilized to conduct a sensitivity analysis, ensuring the robustness and reliability of the results [44]. Firstly, we employed the Cochran Q test to explore the heterogeneity among various SNP estimates. A statistically significant Cochran Q test indicates apparent heterogeneity in the analysis results. Secondly, the Mendelian random Pleiotropy RESidual Sum and Outlier (MR-PRESSO) test was utilized to verify the results obtained from the IVW model. This method corrects for the influence of outliers, and if any outliers are identified, they are removed for reanalysis. Thirdly, the MR Egger intercept test was conducted to examine the level of pleiotropy in SNPs. A p-value of <0.05 for the intercept term suggests the presence of pleiotropy in the IVs. However, if the p-value of the intercept term is not <0.05, there is no indication of horizontal pleiotropy in the selected IVs. Additionally, we constructed funnel and forest plots to visually identify any potential horizontal pleiotropy in the MR analysis. A p-value < 0.05 was statistically significant, suggesting a potential causal relationship in the MR analysis. All statistical analyses were carried out using the “TwoSampleMR” package in R software version 4.3.1.
Results
Effect of 41 inflammatory cytokines on low hand-grip strength and ALM
When the threshold for whole genome significance was set to 5 ×10−8, we found that nine out of the 41 available systemic inflammatory regulatory factors had three or more effective genetic variations. However, considering the low genetic variance and limited number of SNPs, we opted to use a higher threshold (P < 5 ×10−6 and r2 < 0.001) to ensure a sufficient number of SNPs for further MR analysis. By utilizing these stricter criteria, we dug out a total of 428 SNPs correlated with 41 cytokines. It is worth noting that the F-statistics of IVs exceeded 10, suggesting their robustness. More detailed information is presented in Supplementary Table S2.
Figure 2A, B illustrate the relationship between 41 cytokines from the IVW method and low grip strength and ALM. After Bonferroni correction, two specific cytokines, interleukin-10 (IL-10) and vascular endothelial growth factor (VEGF), demonstrated suggestive associations with the risk of decreased hand-grip strength. The odds ratios (OR) for IL-10 and VEGF were 1.046 (95% confidence interval (CI): 1.002–1.093, p = 0.042) and 1.024 (95% CI: 1.001–1.047, p = 0.038), respectively. Furthermore, interferon gamma-induced protein 10 (IP-10) and macrophage colony-stimulating factor (M-CSF) presented a higher likelihood of being associated with ALM, with OR of 1.010 (95% CI: 1.000–1.019, p = 0.042) and 1.010 (95% CI: 1.003–1.017, p = 0.003), respectively. Supplementary Data, which provided an overview of the genetic variation relationship between low grip strength and ALM, are presented in Supplementary Tables S3 and S4.
We performed pleiotropic testing on the IVs in the GWAS directory, but none of the SNPs were found to be pleiotropic (p > 0.05). For IVs with heterogeneity, the IVW random effects model was utilized; otherwise, the IVW fixed effects model was utilized. The results of the horizontal pleiotropy and heterogeneity tests are displayed in Tables 1 and 2. Furthermore, no abnormal SNPs were identified using the MR-PRESSO test. Additionally, the MR Egger intercept failed to find any evidence of directed pleiotropy effects (Tables 1 and 2; Supplementary Tables S5 and S6). If the IVW method result is significant (p < 0.05), even if the results of other methods are not significant, and no pleiotropy and heterogeneity was identified, it can be regarded as a positive result, provided that the beta values of the other methods are in the same direction [45]. No indications of heterogeneity or horizontal pleiotropy were observed in the scatter, funnel, and leave-one-out plots (Supplementary Figs. 1–6). Secondary Mendelian randomization analyses were performed to explore the human genetic evidence for effects of 41 cytokines and whole body lean mass. After Bonferroni correction, there is a suggestive association between interleukin-9 (IL-9) and the risk of whole body lean mass increase. The odds ratio (OR) of IL-9 is 0.578 (95% confidence interval (CI): 0.370–0.905, p = 0.017) (Supplementary Fig. 7).
Effect of low hand-grip strength and ALM on 41 inflammatory cytokines
The reverse MR analysis using the IVW method revealed no statistically significant correlation between low grip strength and IL-10 with VEGF, expressed as beta coefficients (Beta: −0.001, 95% CI: −0.001 to −0.002, P = 0.987; Beta: 0.035, 95% CI: 0.031–0.039, P = 0.571) (Supplementary Table S7). Meanwhile, there is also no significant correlation between ALM and IP-10 and M-CSF (Beta: −0.017, 95% CI: −0.016 to −0.018, P = 0.596; Beta: 0.021, 95% CI: 0.020–0.022, P = 0.551), and suggested an opposite direction of association. The results did not demonstrate a significant difference. The results of the IVs and those obtained from other methods are presented in Supplementary Tables S7 and S8. The MR Egger regression analysis found no evidence of potential directed pleiotropy among the SNPs (Supplementary Table S8). We performed an additional scan of the IVs in the GWAS directory and found no evidence of SNP pleiotropy. Reverse MR analysis using IVW method showed no statistically significant correlation between whole body lean mass and 41 inflammatory cytokines.
Discussion
In our study, a dual-sample MR approach was employed to investigate the potential causal correlation between 41 inflammatory cytokines and sarcopenia-related traits. Our findings revealed that genetically predicted levels of IL-10 and VEGF were associated with low grip strength. Additionally, we identified IP-10 and M-CSF as risk factors for ALM. In the secondary Mendelian analysis, our findings revealed that genetically predicted levels of IL-9 was associated with whole body lean mass.
IL-10 inhibits the activity of human macrophages and monocytes, as well as the production of pro-inflammatory cytokines, thereby acting as an anti-inflammatory cytokine to suppress inflammation and immune responses [27]. A study in 2014 indicated a correlation between IL-10 gene variants and grip strength. The haplotype IL-10 gene variant, which reflects a pro-inflammatory immune response, is linked to higher muscle strength, while the IL-10 gene variant reflecting the anti-inflammatory immune response is linked to lower muscle strength. However, it remains unclear whether IL-10 directly contributes to muscle tissue repair or indirectly affects defense against infectious diseases [46]. In 2018, Rong et al. performed a cross-sectional study, which revealed that elderly patients with sarcopenia exhibited elevated levels of inflammatory cytokine IL-6, anti-inflammatory cytokine IL-10, and IL-6/IL-10 ratio [47]. However, the cross-sectional design of the study cannot identify the causal relationship between sarcopenia and IL-6 level, IL-10 level, or IL-6/IL-10 ratio. In our study, MR analysis revealed a potential detrimental effect of IL-10 on grip strength, leading us to conclude that IL-10 might contribute to the pathogenesis of low grip strength. However, the findings of observational studies should be validated through additional research. Notably, our reverse MR analysis displayed no significant causal relationship between low grip strength and IL-10 levels. Further research is necessary to elucidate the precise underlying mechanism through which IL-10 contributes to the development of low grip strength. The application of recombinant IL-10 in clinical experiment to treat autoimmune and neurodegenerative diseases is considered safe. Taken together, supplementation with IL-10 may restrain the production of IL-6 and thereby reduce the ratio of IL-6/IL-10, which could be a potential treatment strategy for preventing sarcopenia in the elderly. While we have adjusted for several key confounders known to influence both inflammation and sarcopenia-related outcomes, other potential confounders such as genetic predispositions, chronic disease status, or medication usage were not explicitly included in our model. Future studies may consider these factors to further elucidate their roles in the pathways linking inflammation to muscle function and mass.
VEGF, which was identified, isolated, and cloned as early as 25 years ago, primarily acts on endothelial cells [48]. It is particularly important for the growth and maturation of new blood vessels and the promotion of pathological angiogenesis associated with tumor growth. Its pathogenic effect is primarily mediated through its impact on vascular permeability and neovascularization [49]. Our findings indicate that genetically predicted elevated circulating VEGF levels are associated with an increased risk of low grip strength. Similarly, a cohort study involving 28 patients with nonmetastatic colon cancer showed significant upregulation of VEGF in patients with low skeletal muscle area or poor cancer prognosis [50]. These findings illustrate that systemic inflammation may be associated with the adverse impact of sarcopenia on cancer prognosis. However, our reverse MR analysis failed to find any significant causal correlation between low grip strength and VEGF levels. These data indicate that VEGF has potential to be a therapeutic target for sarcopenia, but further research is necessary to confirm the underlying biological mechanisms.
Furthermore, our study revealed that IP-10 and M-CSF had a significant negative causal effect on ALM. A previous study utilized Mendelian Randomization (MR) to explore the causal relationships between circulating cytokines and sarcopenia-related traits through genetic data. The findings indicated that elevated levels of genetically determined IP-10 were associated with a reduced risk of sarcopenia [51]. In addition, previous study evaluated the concentration of circulating inflammatory cytokines in adipose tissue samples surrounding tumors from seven cases of weight-stable cancer and nine cases of cachexia. The study revealed an upward trend in the concentration of the chemotactic factor IP-10 when stimulated by interferon [52]. IP-10 exhibited a positive correlation with regulated on activation, normal T cell expressed and secreted (CCL5) (RANTES) and a negative correlation with the anti-apoptotic, anti-angiogenic, and anti-inflammatory factor Granulocyte colony-stimulating factor (GCSF) [53, 54]. IP-10 functions as a chemotactic factor, potentially contributing to the pro-inflammatory tumor environment in cancer cachexia patients. Previous studies have revealed the elevated expression levels of circulating M-CSF in several autoimmune diseases, such as arthritis, pulmonary fibrosis, kidney inflammation, inflammatory bowel disease, obesity, and cancer metastasis [55, 56]. Neutralizing M-CSF and/or blocking colony stimulating factor 1 (CSF-1R) can improve inflammation, autoimmune, and cancer metastasis in various mouse models [57, 58]. For example, multiple studies have demonstrated that in a mouse model of lupus nephritis (MRL-Fas (lpr) mice), the loss of M-CSF leads to kidney inflammation, while an increase in systemic M-CSF levels accelerates disease onset. Therefore, these findings strongly indicate that the M-CSF/CSF-1R signaling pathway could be a potential therapeutic target for multiple malignances [59]. The increased risk of ALM caused by M-CSF levels may be attributed to the promotion of macrophage recruitment and the triggering of pro-inflammatory responses. However, further research is needed to confirm the potential biological mechanisms. Meanwhile, our reverse MR analysis found no significant causal correlation between ALM and levels of IP-10 and M-CSF.
Previous studies have evaluated the correlation between several circulating cytokines and the risk of cachexia. For example, Yuan et al. reported that elevated levels of IL-6 and H2O2 could increase the expression of miR-21 in primary myoblasts, leading to decreased muscle vitality and myogenic potential [60]. Similarly, Grosicki et al. found that the increase in circulating IL-6 was an important factor in the decline in skeletal muscle strength, function, quality, and training-mediated adaptability. Reducing IL-6 presents an appealing treatment method to preserve skeletal muscle health [61]. Leary et al. researched mouse cell lines and mouse models and revealed that IL-15 promoted muscle production and protected against inflammation-mediated skeletal muscle atrophy in sarcopenia and cachexia. Thus, IL-15 can help reduce inflammatory skeletal muscle loss [62]. Nevertheless, our current study did not find evidence linking IL-6, IL-15, and sarcopenia. This discrepancy may stem from variations in the selection of aggregated data between IVs and GWAS. However, only 2 SNPs showed significance, and subsequent research should be conducted in different GWAS databases. Our study firstly performed the comprehensive and systematic MR investigation into the relationship between cytokines and sarcopenia-related traits. Additionally, we conducted a reverse MR analysis, which serves as supplementary evidence for our initial research.
However, our research must acknowledge several limitations. In this study, we opted to focus on low hand grip strength and appendicular lean mass as indicators of sarcopenia based on their widespread recognition and frequent use in large-scale genetic studies, which provide robust datasets. While whole-body lean mass and walking speed are indeed relevant metrics as per the European Working Group on Sarcopenia in Older People (EWGSOP) latest consensus, the data availability for these traits in the genomic databases we accessed was limited, which influenced our choice of outcome variables.We acknowledge that the inclusion of whole-body lean mass and walking speed could have provided a more comprehensive assessment of sarcopenia, aligning with the latest guidelines by the EWGSOP. Future studies could aim to incorporate these additional sarcopenia-related traits as more genetic data become available, potentially providing a broader understanding of the genetic determinants of sarcopenia across different phenotypes. Firstly, it is significant to note that all participants in our study were of European ancestry, which may introduce potential racial bias. Secondly, due to the characteristic of MR analysis, we cannot determine if our study is influenced by weak IVs. Although all genetic tools used in our study were closely associated with the exposure (F-values > 10), it is still possible that bias may be present. The primary MR analyses were performed by the multiplicative random-effects inverse-variance weighted (IVW) method, which provides the most precise estimates though assuming that all SNPs are valid instruments. The weighted median method can provide consistent estimates when more than 50% of the weight comes from valid instrument variants, MR-Egger regression can generate estimates after accounting for horizontal pleiotropy albeit with less precision. Thirdly, the data were obtained from two large-scale GWAS. Unfortunately, owing to the lack of specific demographic and clinical information of the study patients, subgroup analysis was not possible. Fourthly, while cytokines may not have a direct causal role in the progression of sarcopenia-related features, they could still impact survival or disease progression [63, 64]. However, our MR analysis did not address such associations, and it is necessary to verify these potential associations in larger cohorts and GWAS. Therefore, further research should examine whether cytokines contribute to the invasiveness of sarcopenia-related traits.
Conclusions
Our research findings highlight the significance of diverse inflammatory cytokines on muscle physiology, offering a foundation for investigating the correlation between circulating inflammatory cytokines and sarcopenia-associated characteristics. Subsequent studies should explore the underlying mechanisms that connect circulating inflammatory cytokines and muscle tissue while assessing their potential as viable therapeutic targets.
Data availability
The data generated or analyzed during this study are available in this published article and its supplementary information files.
References
Evans WJ. What is sarcopenia? J Gerontol A Biol Sci Med Sci. 1995;50:5–8. https://doi.org/10.1093/gerona/50a.special_issue.5.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–23. https://doi.org/10.1093/ageing/afq034.
Bauer J, Morley JE, Schols A, Ferrucci L, Cruz-Jentoft AJ, Dent E, et al. Sarcopenia: A Time for Action. An SCWD Position Paper. J Cachexia Sarcopenia Muscle. 2019;10:956–61. https://doi.org/10.1002/jcsm.12483.
Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019;393:2636–46. https://doi.org/10.1016/S0140-6736(19)31138-9.
Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc. 2020;21:300–7.e302. https://doi.org/10.1016/j.jamda.2019.12.012.
Liu JC, Dong SS, Shen H, Yang DY, Chen BB, Ma XY, et al. Multi-omics research in sarcopenia: Current progress and future prospects. Ageing Res Rev. 2022;76:101576. https://doi.org/10.1016/j.arr.2022.101576.
Yuan S, Larsson SC. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism. 2023;144:155533. https://doi.org/10.1016/j.metabol.2023.155533.
Waters DL, Hale L, Grant AM, Herbison P, Goulding A. Osteoporosis and gait and balance disturbances in older sarcopenic obese New Zealanders. Osteoporos Int. 2010;21:351–7. https://doi.org/10.1007/s00198-009-0947-5.
Kemmler W, von Stengel S. Exercise frequency, health risk factors, and diseases of the elderly. Arch Phys Med Rehabil. 2013;94:2046–53. https://doi.org/10.1016/j.apmr.2013.05.013.
Tournadre A, Vial G, Capel F, Soubrier M, Boirie Y. Sarcopenia. Jt Bone Spine. 2019;86:309–14. https://doi.org/10.1016/j.jbspin.2018.08.001.
Geiker NRW, Molgaard C, Iuliano S, Rizzoli R, Manios Y, van Loon LJC, et al. Impact of whole dairy matrix on musculoskeletal health and aging-current knowledge and research gaps. Osteoporos Int. 2020;31:601–15. https://doi.org/10.1007/s00198-019-05229-7.
Li W, Yue T, Liu Y. New understanding of the pathogenesis and treatment of stroke-related sarcopenia. Biomed Pharmacother. 2020;131:110721. https://doi.org/10.1016/j.biopha.2020.110721.
Papadopoulou SK. Sarcopenia: A Contemporary Health Problem among Older Adult Populations. Nutrients. 2020;12. https://doi.org/10.3390/nu12051293.
Xie WQ, He M, Yu DJ, Wu YX, Wang XH, Lv S, et al. Mouse models of sarcopenia: classification and evaluation. J Cachexia Sarcopenia Muscle. 2021;12:538–54. https://doi.org/10.1002/jcsm.12709.
Cho MR, Lee S, Song SK. A Review of Sarcopenia Pathophysiology, Diagnosis, Treatment and Future Direction. J Korean Med Sci. 2022;37:e146. https://doi.org/10.3346/jkms.2022.37.e146.
Scott D, Blizzard L, Fell J, Jones G. The epidemiology of sarcopenia in community living older adults: what role does lifestyle play? J Cachexia Sarcopenia Muscle. 2011;2:125–34.
Moore DR. Keeping older muscle “young” through dietary protein and physical activity. Adv Nutr. 2014;5:599S–607S.
Moro T, Ebert SM, Adams CM, Rasmussen BB. Amino Acid Sensing in Skeletal Muscle. Trends Endocrinol Metab. 2016;27:796–806. https://doi.org/10.1016/j.tem.2016.06.010.
Broskey NT, Greggio C, Boss A, Boutant M, Dwyer A, Schlueter L, et al. Skeletal muscle mitochondria in the elderly: effects of physical fitness and exercise training. J Clin Endocrinol Metab. 2014;99:1852–61. https://doi.org/10.1210/jc.2013-3983.
Del Campo A. Mitophagy as a new therapeutic target for sarcopenia. Acta Physiol. 2019;225:e13219. https://doi.org/10.1111/apha.13219.
Viña J, Gomez-Cabrera MC, Borras C, Froio T, Sanchis-Gomar F, Martinez-Bello VE, et al. Mitochondrial biogenesis in exercise and in ageing. Adv Drug Deliv Rev. 2009;61:1369–74. https://doi.org/10.1016/j.addr.2009.06.006.
Murgia M, Toniolo L, Nagaraj N, Ciciliot S, Vindigni V, Schiaffino S, et al. Single Muscle Fiber Proteomics Reveals Fiber-Type-Specific Features of Human Muscle Aging. Cell Rep. 2017;19:2396–409. https://doi.org/10.1016/j.celrep.2017.05.054.
Bruunsgaard H, Skinhoj P, Pedersen AN, Schroll M, Pedersen BK. Ageing, tumour necrosis factor-alpha (TNF-alpha) and atherosclerosis. Clin Exp Immunol. 2000;121:255–60. https://doi.org/10.1046/j.1365-2249.2000.01281.x.
Bano G, Trevisan C, Carraro S, Solmi M, Luchini C, Stubbs B, et al. Inflammation and sarcopenia: A systematic review and meta-analysis. Maturitas. 2017;96:10–15. https://doi.org/10.1016/j.maturitas.2016.11.006.
Bian AL, Hu HY, Rong YD, Wang J, Wang JX, Zhou XZ. A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-alpha. Eur J Med Res. 2017;22:25. https://doi.org/10.1186/s40001-017-0266-9.
Beheshti I, Sone D, Maikusa N, Kimura Y, Shigemoto Y, Sato N, et al. Pattern analysis of glucose metabolic brain data for lateralization of MRI-negative temporal lobe epilepsy. Epilepsy Res. 2020;167:106474. https://doi.org/10.1016/j.eplepsyres.2020.106474.
Pan L, Xie W, Fu X, Lu W, Jin H, Lai J, et al. Inflammation and sarcopenia: A focus on circulating inflammatory cytokines. Exp Gerontol. 2021;154:111544. https://doi.org/10.1016/j.exger.2021.111544.
Pototschnig I, Feiler U, Diwoky C, Vesely PW, Rauchenwald T, Paar M, et al. Interleukin-6 initiates muscle- and adipose tissue wasting in a novel C57BL/6 model of cancer-associated cachexia. J Cachexia Sarcopenia Muscle. 2023;14:93–107. https://doi.org/10.1002/jcsm.13109.
Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57:M326–332. https://doi.org/10.1093/gerona/57.5.m326.
Paik JK, Chae JS, Kang R, Kwon N, Lee SH, Lee JH. Effect of age on atherogenicity of LDL and inflammatory markers in healthy women. Nutr Metab Cardiovasc Dis NMCD. 2013;23:967–72. https://doi.org/10.1016/j.numecd.2012.08.002.
Wu SE, Hsu JC, Chang YL, Chuang HC, Chiu YL, Chen WL. Benzo[a]pyrene exposure in muscle triggers sarcopenia through aryl hydrocarbon receptor-mediated reactive oxygen species production. Ecotoxicol Environ Saf. 2022;239:113599. https://doi.org/10.1016/j.ecoenv.2022.113599.
Emdin CA, Khera AV, Kathiresan S. Mendelian Randomization. JAMA. 2017;318:1925–6. https://doi.org/10.1001/jama.2017.17219.
Burgess S, Scott RA, Timpson NJ, Davey Smith G, Thompson SG, Consortium E-I. Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol. 2015;30:543–52. https://doi.org/10.1007/s10654-015-0011-z.
Borg K, Tjalve H. Uptake of 63Ni2+ in the central and peripheral nervous system of mice after oral administration: effects of treatments with halogenated 8-hydroxyquinolines. Toxicology. 1989;54:59–68. https://doi.org/10.1016/0300-483x(89)90078-4.
Ahola-Olli AV, Wurtz P, Havulinna AS, Aalto K, Pitkanen N, Lehtimaki T, et al. Genome-wide Association Study Identifies 27 Loci Influencing Concentrations of Circulating Cytokines and Growth Factors. Am J Hum Genet. 2017;100:40–50. https://doi.org/10.1016/j.ajhg.2016.11.007.
Zillikens MC, Demissie S, Hsu YH, Yerges-Armstrong LM, Chou WC, Stolk L, et al. Large meta-analysis of genome-wide association studies identifies five loci for lean body mass. Nat Commun. 2017;8:80. https://doi.org/10.1038/s41467-017-00031-7.
Hernández-Lepe MA, Miranda-Gil MI, Valbuena-Gregorio E, Olivas-Aguirre FJ. Exercise Programs Combined with Diet Supplementation Improve Body Composition and Physical Function in Older Adults with Sarcopenia: A Systematic Review. Nutrients. 2023;15. https://doi.org/10.3390/nu15081998.
Colleluori G, Villareal DT. Aging, obesity, sarcopenia and the effect of diet and exercise intervention. Exp Gerontol. 2021;155:111561. https://doi.org/10.1016/j.exger.2021.111561.
Dowd JB, Zajacova A, Aiello A. Early origins of health disparities: burden of infection, health, and socioeconomic status in U.S. children. Soc Sci Med. 2009;68:699–707. https://doi.org/10.1016/j.socscimed.2008.12.010.
Kim S, Kim S, Hong KH. Association of Combining Diet and Physical Activity on Sarcopenia and Obesity in Elderly Koreans with Diabetes. Nutrients. 2024;16. https://doi.org/10.3390/nu16070964.
Hashimoto Y, Takahashi F, Okamura T, Hamaguchi M, Fukui M. Diet, exercise, and pharmacotherapy for sarcopenia in people with diabetes. Metabolism. 2023;144:155585. https://doi.org/10.1016/j.metabol.2023.155585.
Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37:658–65. https://doi.org/10.1002/gepi.21758.
Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693–8. https://doi.org/10.1038/s41588-018-0099-7.
Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity Analyses for Robust Causal Inference from Mendelian Randomization Analyses with Multiple Genetic Variants. Epidemiology. 2017;28:30–42. https://doi.org/10.1097/EDE.0000000000000559.
Zagkos L, Dib MJ, Pinto R, Gill D, Koskeridis F, Drenos F, et al. Associations of genetically predicted fatty acid levels across the phenome: A mendelian randomisation study. PLoS Med. 2022;19:e1004141. https://doi.org/10.1371/journal.pmed.1004141.
Beenakker KG, Koopman JJ, van, Bodegom D, Kuningas M, Slagboom PE, et al. Variants of the IL-10 gene associate with muscle strength in elderly from rural Africa: a candidate gene study. Aging Cell. 2014;13:862–8. https://doi.org/10.1111/acel.12244.
Rong YD, Bian AL, Hu HY, Ma Y, Zhou XZ. Study on relationship between elderly sarcopenia and inflammatory cytokine IL-6, anti-inflammatory cytokine IL-10. BMC Geriatr. 2018;18:308 https://doi.org/10.1186/s12877-018-1007-9.
Ferrara N, Adamis AP. Ten years of anti-vascular endothelial growth factor therapy. Nat Rev Drug Discov. 2016;15:385–403. https://doi.org/10.1038/nrd.2015.17.
Velde Vande, Cleveland C. DW. VEGF: multitasking in ALS. Nat Neurosci. 2005;8:5–7. https://doi.org/10.1038/nn0105-5.
Radev RN. Prevention of the aspiration syndrome in obstetrical anesthesiology. Akush Ginekol. 1987;44–6..
Di W, Luyao Y, Chengwei Y, Valtonen AM, Juha-Pekka K, Ying G. Exploring the causal link between circulating cytokines and sarcopenia traits: A Mendelian randomization analysis. Environ Toxicol. 2024;39:3434–47. https://doi.org/10.1002/tox.24206.
Neto NIP, Murari ASP, Oyama LM, Otoch JP, Alcantara PSM, Tokeshi F, et al. Peritumoural adipose tissue pro-inflammatory cytokines are associated with tumoural growth factors in cancer cachexia patients. J Cachexia Sarcopenia Muscle. 2018;9:1101–8. https://doi.org/10.1002/jcsm.12345.
Tsai RK, Chang CH, Sheu MM, Huang ZL. Anti-apoptotic effects of human granulocyte colony-stimulating factor (G-CSF) on retinal ganglion cells after optic nerve crush are PI3K/AKT-dependent. Exp Eye Res. 2010;90:537–45. https://doi.org/10.1016/j.exer.2010.01.004.
Malashchenko VV, Meniailo ME, Shmarov VA, Gazatova ND, Melashchenko OB, Goncharov AG, et al. Direct anti-inflammatory effects of granulocyte colony-stimulating factor (G-CSF) on activation and functional properties of human T cell subpopulations in vitro. Cell Immunol. 2018;325:23–32. https://doi.org/10.1016/j.cellimm.2018.01.007.
Menke J, Iwata Y, Rabacal WA, Basu R, Stanley ER, Kelley VR. Distinct roles of CSF-1 isoforms in lupus nephritis. J Am Soc Nephrol. 2011;22:1821–33. https://doi.org/10.1681/ASN.2011010038.
Toh ML, Bonnefoy JY, Accart N, Cochin S, Pohle S, Haegel H, et al. Bone- and cartilage-protective effects of a monoclonal antibody against colony-stimulating factor 1 receptor in experimental arthritis. Arthritis Rheumatol. 2014;66:2989–3000. https://doi.org/10.1002/art.38624.
Ohno H, Uemura Y, Murooka H, Takanashi H, Tokieda T, Ohzeki Y, et al. The orally-active and selective c-Fms tyrosine kinase inhibitor Ki20227 inhibits disease progression in a collagen-induced arthritis mouse model. Eur J Immunol. 2008;38:283–91. https://doi.org/10.1002/eji.200737199.
Kubota Y, Takubo K, Shimizu T, Ohno H, Kishi K, Shibuya M, et al. M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J Exp Med. 2009;206:1089–102. https://doi.org/10.1084/jem.20081605.
Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol. 2008;8:533–44. https://doi.org/10.1038/nri2356.
Borja-Gonzalez M, Casas-Martinez JC, McDonagh B, Goljanek-Whysall K. Inflamma-miR-21 Negatively Regulates Myogenesis during Ageing. Antioxidants. 2020;9. https://doi.org/10.3390/antiox9040345.
Grosicki GJ, Barrett BB, Englund DA, Liu C, Travison TG, Cederholm T, et al. Circulating Interleukin-6 Is Associated with Skeletal Muscle Strength, Quality, and Functional Adaptation with Exercise Training in Mobility-Limited Older Adults. J Frailty Aging. 2020;9:57–63. https://doi.org/10.14283/jfa.2019.30.
O’Leary MF, Wallace GR, Bennett AJ, Tsintzas K, Jones SW. IL-15 promotes human myogenesis and mitigates the detrimental effects of TNFalpha on myotube development. Sci Rep. 2017;7:12997. https://doi.org/10.1038/s41598-017-13479-w.
Lee JD, McDonald TS, Fung JNT, Woodruff TM. Absence of Receptor for Advanced Glycation End Product (RAGE) Reduces Inflammation and Extends Survival in the hSOD1(G93A) Mouse Model of Amyotrophic Lateral Sclerosis. Mol Neurobiol. 2020;57:4143–55. https://doi.org/10.1007/s12035-020-02019-9.
Thonhoff JR, Berry JD, Macklin EA, Beers DR, Mendoza PA, Zhao W et al. Combined Regulatory T-Lymphocyte and IL-2 Treatment Is Safe, Tolerable, and Biologically Active for 1 Year in Persons With Amyotrophic Lateral Sclerosis. Neurol Neuroimmunol Neuroinflamm. 2022;9. https://doi.org/10.1212/NXI.0000000000200019.
Acknowledgements
We thank all the GWASs for making the summary data publicly available, and we are grateful for all the investigators and participants who contributed to those studies.
Funding
This work was financially supported by the capital health research and development of special (2022-2-7083); R&D Program of Beijing Municipal Education Commission (KM202010025005); Beijing Municipal Natural Science Foundation7222100 and National Natural Science Foundation (No. 82203576).
Author information
Authors and Affiliations
Contributions
LW and DY conceived and initiated the project. JW, YXX and LHW analyzed the data, and wrote the first draft of the manuscript. All authors contributed to the interpretation of the results and critical revision of the manuscript for important intellectual content and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This article contains human participants collected by several GWAS. All participants gave informed consent in all the corresponding original studies. Here, we only used the large-scale GWAS summary datasets, and not the individual-level data. Hence, ethical approval was not sought.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, J., Xiang, Y., Wu, L. et al. The association between inflammatory cytokines and sarcopenia-related traits: a bi-directional Mendelian randomization study. Eur J Clin Nutr (2024). https://doi.org/10.1038/s41430-024-01486-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41430-024-01486-w
- Springer Nature Limited