Abstract
Multi-host parasites pose greater health risks to wildlife, livestock, and humans than single-host parasites, yet our understanding of how ecological and biological factors influence a parasite’s host range remains limited. Here, we assemble the largest and most complete dataset on permanently parasitic mammalian mites and build a predictive model assessing the probability of single-host parasites to become multi-hosts, while accounting for potentially unobserved host-parasite links and class imbalance. This model identifies statistically significant predictors related to parasites, hosts, climate, and habitat disturbance. The most important predictors include the parasite’s contact level with the host immune system and two variables characterizing host phylogenetic similarity and spatial co-distribution. Our model reveals an overrepresentation of mites associated with Rodentia (rodents), Chiroptera (bats), and Carnivora in the multi-host risk group. This highlights both the potential vulnerability of these hosts to parasitic infestations and the risk of serving as reservoirs of parasites for new hosts. In addition, we find independent macroevolutionary evidence that supports our prediction of several single-host species of Notoedres, the bat skin parasites, to be in the multi-host risk group, demonstrating the forecasting potential of our model.
Similar content being viewed by others
Introduction
Host specificity, quantified by host range breath and the relative susceptibility of hosts, is a fundamental property differentiating single-host (specialist) from multihost (generalist) parasites1. Among generalists, the most virulent parasites are those that exhibit equal adaptability to a wide range of hosts, exploiting multiple routes of transmission, and evolving complex patterns of virulence towards each species in their host range2,3,4. The probabilities of emerging infectious diseases are strongly associated with these multi-host pathogens5. In extreme cases, host-shifting to an immunologically naïve host species may lead to an epidemic followed by local or global extinction of the new host6,7,8. Remarkably, the source hosts may be largely unaffected in this case because they already have developed natural defenses against the pathogen. Host range also influences parasites’ survival strategies on a macroevolutionary time scale. Generally, multi-host parasites tend to persist longer in a community as diverse host species can maintain these parasites. In contrast, single-host parasites have a higher risk of extinction as their fitness and transmission entirely depend on the host’s survival8,9,10. Therefore, they tend to have low/intermediate levels of virulence11 or evolve into non-virulent commensals or even mutualistic symbionts over evolutionary time12. Long-term co-evolutionary interactions between hosts and parasites may favor one of these two strategies, promoting the evolution and maintenance of traits related to infectivity, transmission, and host utilization (in parasites) as well as parasite resistance or avoidance (in their hosts). As a result, parasite lineages may display predictable, trait-related host specificity patterns13,14 (Fig. 1). In addition, host-parasite associations strongly depend on host phylogenetic, geographic, and environmental factors15,16,17. Pathogen sharing, for example, could be strongly influenced by pairwise host phylogenetic similarity and spatial co-occurrence18. Thus, to predict a parasite host range, variables related to the pathogen, host, and environment should be considered simultaneously in order to build a robust probabilistic model14.
a single-host parasite, Cheyletiella yasguri, parasitizing the dog Canis familiaris. Yellow square is enlarged in b, mouthparts of Cheyletiella yasguri, showing piercing chelicerae (primary host immunity contact). c, multi-host parasite, Psoroptes ovis, parasitizing 26 host species. Yellow square is enlarged in d, mouthparts of Psoroptes ovis, showing chelate chelicerae (secondary host immunity contact).
Accurate prediction of a parasite’s host range can contribute to our understanding of which parasites are capable of a host shift and causing new diseases. This knowledge may translate into better forecasting infectious disease emergence and preventing parasite spillover, especially to humans and domestic animals19. Recent research that used a probabilistic modeling framework has identified several predictors of the host-parasite links, mainly focusing on mammalian viruses14,18,19,20,21,22,23,24,25,26,27. Despite progress extolled by these advanced modeling approaches, it still remains unclear whether host range predictions drawn from these studies are applicable to other host-pathogen systems. There are also two major but commonly overlooked technical challenges related to host-parasite data analyses: imbalanced classification and unobserved multi-host parasites. In imbalanced classification, one class is more prevalent than the other, potentially leading to a bias in model building and a deceptively high model accuracy. Despite the prevalence of imbalanced datasets, only a single study has explicitly accounted for this aspect so far28. More importantly, in host-parasite systems, not all host and infectious agent species are known, leading to the concept of ‘epidemiological dark matter’29. This lack of knowledge, particularly when true multi-host parasites are erroneously classified as single-hosts due to insufficient sampling (referred to as ‘unobserved multi-hosts’), can significantly impact the predictive power of analyses modeling host range expansion, host specificity, or host sharing. However, this issue has received little attention thus far, as most models assume that both single-and multihost classes are correctly labeled, but see Dallas et al. 30.
Here, we assemble a large dataset of all known acariform mite-mammal associations (1,984 mite and 1,432 mammal species) and conduct host range modeling in a global, pan-host, and pan-parasite context, which means all known target parasites and all their known hosts are included (unlike many mammal-virus analyses14, where avian hosts have been omitted by design). We focus only on permanent mammal associates—full-time parasites, staying on the host for the entire life-cycle, lacking a free-living or dispersal stage, and colonizing host individuals on contact (Fig. 1). Our dataset is one of the largest and most complete among compatible host-parasite databases available to date; for example, it has 92 primate-associated target mite species, while other databases reported much fewer species: 3431 or one species32. Among them is Sarcoptes scabiei, one of the most virulent multi-host mites, responsible for epidemics in wild and domesticated mammals and skin disease outbreaks in humans33.
We populate this dataset with a set of predictor variables related to parasites, their hosts, and the environment, which can aid in predicting mites’ host range expansion. For example, among these predictors, two variables are related to mites’ feeding specialization — mites with direct immune system interactions (e.g., hair follicular mites) are expected to have a lower establishment probability (and therefore narrower host range) compared to those feeding on non-immunogenic host tissue derivatives (e.g., fur mites). Similarly, ectoparasitic mites with diverse dispersal stages and broad geographic distributions are expected to have higher probabilities for successful transmission to new hosts, potentially contributing to broader host ranges. Hosts with many closely related mammal species provide more opportunities for their parasitic mites to invade additional host species due to similar immune evasion mechanisms25. Likewise, hosts living in regions with a high concentration of sympatric mammal species present direct opportunities for host shifting of their parasites, leading to broader host ranges. Other host properties, such as litter size, domestication status, and living in anthropogenically disturbed areas also offer further avenues for mite transfers and host range expansions. Finally, abiotic factors, such as temperature and humidity, may affect mite survival outside the host during transmission, thereby facilitating or prohibiting the transmission process, leading to broader or narrower host ranges, respectively.
To address the imbalanced nature of most host-parasite associations, we extend the previous modeling efforts14,18 by using down- and up-sampling strategies and a metric not influenced by single vs multi-host class imbalance. We also employ several strategies to alleviate the potential effect of unobserved multi-hosts: positive-unlabeled (PU) learning (explicitly assumes that labels in the multi-host class are known, while the other class is unlabeled and may contain both single-host and multi-host instances), weighting by publication counts (downweighs mite species with less sampling efforts as estimated by relevant publication numbers), and down- and up-sampling (maximizing the prediction of multi-hosts, which is the class of interest).
Based on a set of 13 predictor variables related to parasites, their hosts, and the environment, and accounting for the above challenges, such as class imbalance and potential influence of unobserved multi-hosts, we build a statistical model to predict the likelihood that a single-host parasite can extend its host range to become multi-host. To evaluate the predictive power of our best model, we conduct simultaneous k-fold cross-validation using an independent and unmodified test (holdout) dataset. Because our best model was based on resampling, using the unmodified test dataset for model evaluation should provide performance estimates that better reflect real-world situations. We then use our predictive model for forecasting to identify (i) a set of observed single-host species that have a high probability of becoming multi-host (high host switching / host range expansion risk), and (ii) a set of mammalian lineages that are ‘enriched’ with risk group mites. We then discuss whether our forecasting results, which identify potentially emerging epidemic risk-group parasites, could be supported by independent lines of evidence, such as host ecology promoting extensive parasite exchange, and whether related lineages have experienced host shifts on a macroevolutionary scale.
Results
Model selection
We evaluated the overall performance of five models using five-fold cross-validation and then assessed their predictive power with an independent test dataset (Table 1). A logistic regression analysis using generalized linear model served as the baseline (Model Id=1 in Table 1), while the four other models employed various strategies to address the issues related to class imbalance and potential unobserved multi-hosts: (2) weighting by publication counts to down-weight less-sampled and studied mite species, employing either down- (3) or up-sampling (4) during each iteration of the cross-validation procedure to enhance the prediction accuracy of the minority class (multihost), and (5) using a positive-unlabeled learning model with AdaSampling and an SVM classifier, assuming that only the positive class (multihost) is labeled, while the negative class is an unlabeled mixture of true single-hosts and unobserved multi-hosts.
The baseline model (Model Id=1 in Table 1) exhibited deceptively higher overall accuracy (0.810), which was influenced primarily by the majority class (singlehost), as indicated by high specificity (0.940) and low sensitivity (0.475). The two resampling procedures, up and down-sampling (Model Ids 3&4 in Table 1), largely removed this imbalance, resulting in sensitivities of 0.664-0.680 and specificities of 0.773–0.779, thus significantly improving the prediction of the category of interest, multihost. The PU model (Model Id=5 in Table 1) optimized the prediction of the multihost class, achieving the highest sensitivity (0.705) but the lowest specificity (0.753) among the resampling and weighting-based models (Model Ids 5 vs. 2–4 in Table 1). Based on the Area Under the ROC Curve (AUC) and F1 score, the down-sampled model (Model Id = 3 in Table 1) performed best, with an AUC of 0.799 vs 0.774–0.788 and an F1 score of 0.613 vs 0.586-0.610 (Table 1; Fig. 2a), indicating that this model has the best overall prediction for both multihost and singlehost classes. Therefore, we selected the down-sampled model as our preferred model. Alternatively, we also compared its forecasting results with those of the PU model, which maximized the accurate prediction of the multihost class.
a, Performance of five models (no-sampling, weighting, down-, up-sampling, PU learning) estimated using a Receiver Operating Characteristic (ROC) analysis; the down-sampled model has the largest ROC area under curve (AUC) and is considered as our preferred model. Detailed model statistics are given in Table 1. b Exponentiated coefficient estimates (dots) and their standard errors (bars) of the preferred model; positive (red) or negative (blue) effects of the 13 factors on predicting the multihost category; data are given as odds ratios; bars show standard errors; bars crossing the x-axis at zero are not statistically significant; two-sided z-score statistical significance is indicated as follows: *** p < 0.001; ** p < 0.01; * p < 0.05. c–j Relationships between the outcome (multihost) and a set of predictors based on the preferred model; categorical predictors are shown as bars, continuous predictors are shown as lines; predictor variables may increase or decrease the odds of becoming multi-host, for example, the variable Co-Distributed Potential Hosts (g) increases the odds of becoming multi-host at its whole range, while the natural spline variable Average Temperature (mean monthly temperature x0.1 °C) increases (range from −118.3 to +117.5) or decreases (range from +117.5 to +269.6) the odds of becoming multi-host; the effect of each variable is shown on a scale of odds ratios as the remaining explanatory variables are held constant (blue lines); 95% confidence bands are shown as shaded blue; partial residuals (the sum of the residuals and predictor terms) are shown as dots; bottom and top rugs correspond to the observations of class 0 residuals (singlehost) or 1 (multihost), respectively; ns(var, 2) denotes a two-degree-of-freedom (df=2) natural spline. Source data are provided as a Source Data file.
The preferred model
The likelihood ratio test suggests that our preferred model is significantly different from the intercept-only model (p < 2.2e-16), indicating that the independent variables, as a whole, contribute significantly to the prediction of the mite host range. Lower Akaike’s Information Criterion (AIC) and residual deviance values vs. those for the intercept-only model also indicate an overall good model fit (AIC 601.71 vs 808.8, residual deviance 563.71 vs. 806.8). Standard errors for the coefficient estimates were substantially less than 2.0, except for the spline variable ns(Average Temperature, 2)1 (Table 2), indicating the absence of a high multicollinearity. Three categorical and four continuous predictors were significant, one continuous predictor was marginally significant, while other variables were nonsignificant, including Domesticated Host (Table 2; Fig. 2b).
Factors affecting the odds of becoming multi-host
Individual model coefficients for mite-related predictors were positive for all levels of mite-related independent variables, Host Immunity Contact Level (primary vs secondary or none), Parasitism (ectoparasitic vs endoparasitic), Mite Biogeographic Region (multiregion vs singleregion), Mite Dispersal Stage (immatures vs female or female+immatures), Precopulatory Guarding (absent vs present) (Ids 1-5 in Table 2, Fig. 2b, Supplementary Fig. 1 a-e), indicating their positive effect on becoming multihost. For example, all else being equal, the odds of a mite with secondary host immune system contact being multihost are Exp (2.03) = 7.60 times higher than those with primary contact (where 2.03 is the model coefficient estimate, Id=1.1 in Table 2). All model coefficients were statistically significant (p < 0.05), except for Mite Dispersal Stage and Precopulatory Guarding (Table 2). Thus, having secondary or none host immunity contact level (or having chelate chelicerae), being an ectoparasite, and having a multi-region geographic distribution all increase the odds of a single-host mite becoming multi-host (Fig. 2 c-e). These results agree with our a priori expectations (see the Material and Methods section).
Of the five host-related predictors (Ids 6-10 in Table 2, Supplementary Fig. 1 f–j), only two, Co-Distributed Potential Hosts, Phylogenetically Similar Potential Hosts, were statistically significant (Ids 6&7 in Table 2, Fig. 2f, g), while the second natural spline basis function of Host Body Mass log was marginally significant (Id=10.2 in Table 2, Fig. 2h). This suggests that a mite is statistically more likely to become multi-host if its observed host(s) (i) have many geographic overlaps with other potential hosts and (ii) are phylogenetically similar to many other potential hosts on the mammalian tree of life, regardless of whether these hosts co-occur. Each additional sympatric potential host increases the odds of becoming multi-host by 3.6%, and each additional phylogenetically similar potential host increases the chance of becoming multi-host by 86% (Ids 6&7 in Table 2). However, other host-related variables (or natural spline functions) had non-significant coefficients, therefore, their effect on the outcome variable is uncertain (Ids 8, 9 &10.1 in Table 2, Supplementary Fig. 1 h-j).
Two climatic variables, Average Precipitation and Average Temperature, were natural spline (df=2) variables (Ids 11-12 in Table 2, Fig. 2i, Supplementary Fig. 1 k, l). Briefly, a natural spline function, is a flexible curve-fitting technique that smooths the data without imposing strict assumptions about the relationship between the predictor variable and the outcome variable. This approach is especially useful when the relationship is nonlinear or when the effect of the predictor can change directionality. For example, in scenarios where midrange temperatures are optimal and have a positive effect on the outcome, while both low and high temperatures diminish this effect, a natural spline can effectively capture this nonlinear relationship. The marginal effect of the variable Average Temperature (mean monthly temperature x0.1 °C) showed this pattern—it was fit by a hump-shaped curve with an upturn (scarce data), a peak around 11.8 °C (117.5*0.1), and a downturn (dense data) (Fig. 2i). In other words, in areas with average temperatures above 11.8 °C, the odds of becoming multi-host decrease, while in areas having lower temperatures, the effect of this variable is negative, which agrees with our a priori expectation. The Average Precipitation variable (mean monthly precipitation, mm) was fitted as a shallow, concave downward arch with a peak at 173 mm/month (Supplementary Fig. 1 k). This contrasts with our a priori expectation of a linear, positively correlated relationship with the outcome. However, this predictor was not statistically significant (Id=11 in Table 2).
The log-transformed, Average Human Population Density log (a proxy for anthropogenic habitat disturbance) had a significant positive linear effect on the dependent variable (Fig. 2 j), which was expected a priori.
Predictions from the model: unobserved multi-host mites
When a mite species is labeled as singlehost in our database, it is still possible that it has other unobserved host species due to varying sampling efforts. To detect potentially unobserved multi-hosts (a risk group), we combined our prediction and test (holdout) datasets and calculated counts of correct and incorrect classifications for each class of the dependent variable (Fig. 3a, b). Unobserved multi-hosts were entered into the analysis in the singlehost category but classified as multihost by the model along with truly misclassified single-hosts (Fig. 3a, b). There were 220 counts of singlehost classified as multihost and 120 counts in the category of multihost classified as singlehost (Fig. 3b). To be conservative in estimating potential host shifts, out of 220 counts of singlehost classified as multihost, we only included those with a model prediction probability above 0.7 resulting in a subset of 86 instances included in the multi-host risk group. We then analyzed whether certain host orders are ‘enriched’ in this group. Per-host-order risk analyses identified Rodentia, Chiroptera and Carnivora as host orders with disproportionately high numbers of unobserved multi-hosts, while marsupials (Diprotodontia) lack any single-host mite species predicted in the multi-host risk group (Fig. 3c, Table 3). Among these mites, were five sarcoptid skin mites parasitizing molossid or vespertilionid bats: Notoedres ovatus (host Mops condylurus), N. yunkeri (Molossus molossus), N. anisothrix (M. molossus), N. helicothrix (Cynomops planirostris), and Notoedres eptesicus (Eptesicus brasiliensis). These mites are currently cited in the literature as single-host parasites, but our preferred model predicted them to be multi-host with high probabilities, ranging from 0.702 to 0.932 (Fig. 3b, Supplementary Data 1). Our auxiliary PU learning model, which assumes that only the multihost class is correctly labeled, predicted these five species in the multi-host risk group with even higher probabilities, 0.872-0.994 (Supplementary data 2).
a Distribution of prediction score grouped by known outcome, singlehost (0) and multihost (1); b Confusion matrix with cutoff at 0.5, high epidemic risk-group mites (potential multi-hosts, probability threshold at 0.7) and potential risk-epidemic parasites (Notoedres) are emphasized; 1=Notoedres helicothrix, 2 = N. ovatus, 3 = N. yunkeri, 4 = N. anisothrix; 5 = N. eptesicus. Grey shadings represent violin plots of the distributions of predicted likelihood of multihost of all the mite species. c, High epidemic risk-group mites per host order. Positive values (percentages) indicate higher than average likelihood of an observed single-host actually being a multi-host parasite or having the potential of being multi-host. The classification probability threshold was set to 0.7. Source data are provided as a Source Data file.
Mite-sharing patterns among hosts
We further investigated how patterns of mite sharing among mammalian hosts are shaped by the host pairwise geographical overlaps and phylogenetic distances (PD). The mite-host association network was projected to build a unipartite host-host network from our database, in which an edge represents a host pair that shares one or more mite species. Logistic regression using a generalized linear model was then used to predict the probability of mite-sharing between host pairs given their geographic overlap and phylogenetic distances (see Material and Methods for details). Only hosts that share at least one mite species with other hosts were kept for the mite-sharing analyses. Overall, the probability of mite-sharing increases with smaller phylogenetic distances and larger geographic overlap significantly (Fig. 4; Supplementary Table 1), with a negative interaction between the two predictors. We found that phylogenetic distance between host pairs is the most important factor in determining mite-sharing probabilities (Fig. 4a). Regardless of the degree of geographic overlap, the sharing probability quickly drops to zero when phylogenetic distance is larger than 100 (this threshold roughly corresponds to be within the same order, Fig. 4b). Alternatively, allopatric but highly phylogenetically similar (PD < 10) host pairs still have more than a 50% chance of sharing mites (Fig. 4b). The sharing probability increases to over 0.75 when the host pair is fully sympatric, i.e., geographic overlap = 1.
a Mite sharing probability (colored lines) decreases with host phylogenetic distance at five levels of host geographic overlap. b Mite sharing probability (colored lines) increases with host geographic overlap at eight levels of host phylogenetic distance. The shaded bands are standard errors of predicted sharing probability. Source data are provided as a Source Data file.
Discussion
Much of recent research has been focused on predicting the likelihood of emerging infections using host specificity or host range expansion as one of the risk factors, directly relating host shifting abilities and infection of novel hosts5,14,34. A variety of parasite, host, ecological and evolutionary determinants have been suggested as potential predictors of host range of parasitic organisms: host immunity20, species abundance21,22, body size23, sex24, geographic range, sympatry and host phylogenetic similarity14,18,25, parasite transmission strategy26, various environmental conditions27, and human population size within a parasite species geographical range18. Incorporating these factors to describe the level of host specificity in a quantitative modeling framework can be beneficial for forecasting infectious disease emergence, parasite spillover events, and can also help in preventing or reducing transmission of multi-host pathogens to humans and domestic and wild animals19.
Although forecasting host range expansion in single-host parasites is important from an epidemiological perspective, this task is challenging even with the use of advanced probabilistic modeling approaches. First, new epidemics are rare events despite many potentially dangerous parasites identified by the model. An epidemic depends on many factors, including the ability of the host immune system to effectively defend the host against a new pathogen, the ability of the parasite to evade the immune surveillance of a novel host, the host population size and contact frequency. An accurate representation of these predictors involves a detailed understanding of host-parasite biology. Second, as parasites may have hidden potential to infect novel hosts, a model trained on the current associations may not have sufficient predictive power when applied to future unobserved events. In addition, single-host parasites may be unobserved multi-host parasites, which could introduce noise during model building, resulting in diminishing accuracy in predicting the multi-host class. Third, failure to account for the imbalanced observations of single- and multi-host pathogens may lead to models with classification accuracy biased toward the majority class (i.e., single-host pathogens).
Our model emphasizes an accurate prediction of the risk group (multi-host) by using down-sampling, a technique that equalizes both single- and multi-host categories by randomly removing observations from the majority class (single-host), thus potentially reducing the noise (unobserved multi-hosts classified as single-hosts). This technique also accounts for class imbalance which is frequently present in host-parasite datasets. Our down-sampling approach was found to be superior to other noise-reducing approaches, such as positive-unlabeled (PU) learning, up-sampling or weighting records by previous research effort (Table 1: AUC, F1). As estimated by independent holdout validation, for the down-sampled model (Model Id=3 in Table 1), metrics related to multi-host class prediction (sensitivity=0.680) was the highest among all other models (except for the PU learning model), while still having a reasonable accuracy in predicting the single-host class (specificity=0.779). In contrast, the baseline model (no noise reduction) classified the minority class (multihost) worse than a random guess (Model Id=1 in Table 1; sensitivity=0.475), while having a deceptively high overall accuracy (0.805), which was driven by the majority class (Model Id=1 in Table 1; specificity=0.940).
By linking various predictors describing the parasite morphology, distribution, host and parasite ecology, the number of phylogenetically similar hosts and co-distributed hosts, as well as climatic and anthropogenic habitat disturbance, our model provides a unique window into the determinants affecting the ability of a single-host parasite for host shifting and thus becoming multi-host. In order of importance (Table 2b: absolute values of column ‘z-value’), the likelihood for becoming multi-host increases in mites: (i) having a large number of potential phylogenetically similar hosts (Id=7 in Table 2); (ii) feeding with chelate chelicerae on host tissues having no direct contact to the host immune system but an immune response still can be mounted if the tissue is damaged (e.g., outermost layer of the epidermis consisting of dead keratinocytes) (Id=1.1 in Table 2); (iii) having a large number of co-distributed potential hosts (Id=6 in Table 2); (iv) spatially distributed in more than one biogeographic region (Id=3 in Table 2); (v) inhabiting host tissue derivatives which have no contact to the host immunity (e.g., fur and other hair derivatives) (Id=1.2 in Table 2); (vi) being an ectoparasite (Id=2 in Table 2); (vii) living in areas with a larger anthropogenic habitat destruction/larger human population density (Id=13 in Table 2); (viii) living in warm rather than hot regions (applies only to areas with an average monthly temperature of 21.8 °C and above) (Id=12.2 in Table 2); (ix) associated with less massive hosts (marginally significant, applies only to hosts with an average mass of 53.3 g and above) (Id=10.2 in Table 2). In this list, the first most important variable, Potential Phylogenetically Similar Hosts, characterizes phylogenetic affinities of potential hosts. The second most important predictor (ii) is related to both mite proximity to the host immune system and mite mouthpart morphology, the two mite properties that are nearly perfectly correlated to each other. Similarly, the majority of statistically significant predictors identified by our model were related to mite (ii, iv-vi) or host properties (i, iii, ix), while only a single predictor was related to each climate (viii) and habitat disturbance (vii). In contrast to the literature, which suggests that host domestication status10,11,26 and host body mass14 or size23 are important risk factors of host range expansion, our study found that these two predictors are not statistically significant (Ids 9&10 in Table 2). In case of host domestication, the effect of this variable on the outcome was positive (as expected), however, as most host shifts onto domesticated animals have been made by multi-host parasites, the model coefficient was estimated non-significant for this variable. Similarly, in mammalian viruses, host domestication status was also found to be non-significant due to a small sample size, which limited the predictive power of the model14. In case of host body mass, a positive effect is expected as larger mammals can house a larger species richness of parasites23,35. However, very large and massive mammals may have smaller population sizes and isolated geographic ranges, therefore limiting parasite transmission. Our study agrees with this result, albeit this relationship was only marginally significant (Id=10.2 in Table 2), and this negative effect of host body mass on pathogen sharing has also been noticed in the literature18. Overall, our preferred model suggests that the relationships between the probability of host range expansions and various parasite and host properties, as well as abiotic factors, are stronger than previously thought.
With a sensitivity of 0.680 and specificity of 0.780 (Model Id=3 in Table 1), our model can effectively forecast the risk of a host shift in single-host parasites for mite species associated with hosts that frequently come into close contact with humans, such as captive and domesticated mammals. Risk assessment is also needed for invasive mammal species expanding their geographic ranges following climate change, habitat loss, or through direct human activities. These hosts can carry unwanted and potentially dangerous acarine parasites, which may shift to wild or domesticated animals and humans themselves8.
In host-risk forecasting, our model identified rodents (Rodentia), bats (Chiroptera) and Carnivora as hosts harboring the highest number of risk-group mites i.e., potentially unobserved multi-host species (Fig. 3c; Table 3). As these three orders are species-rich, they provide more opportunities for host shifts among phylogenetically similar hosts (Fig. 4a). In addition, cross-species contacts promoting horizontal mite transmission are expected in these three host orders. In Carnivora, horizontal pathogen exchange can be facilitated through predation on various mammalian species which harbor an array of diverse acarine parasites forming natural associations with their hosts. In bats, mite pathogen exchange may be facilitated through the formation of large multi-species aggregations in the same roosting sites36. Put differently, these two host orders exhibit certain ecological characteristics that promote mite sharing.
When used for forecasting of risk-group mites, our preferred model identified 86 species at a probability threshold of 0.7 (Supplementary Data 1). Among these potentially multi-hosts parasites are five species of sarcoptid skin mites, Notoedres ovatus and N. yunkeri, N. anisothrix, N. helicothrix, and N. eptesicus. Currently, each of these species have been found on a different host species of molossid/vespertilionid bats, however our model predicts with a high probability, 0.866 − 0.930, that they are actually multi-hosts (Fig. 3b; Supplementary Data 1). There is some indirect evidence suggesting that host range expansion is plausible for these five single-host parasites. The genus Notoedres originated as a bat parasite, however, its crown group contains species resulting from historical host shifts from bats to other mammalian host orders, such as Rodentia (Notoedres muris, N. musculi) and Carnivora (N. cati)37. In other words, in the past, inter-ordinal host shifts followed by a speciation event occurred on a macroevolutionary scale in this genus. By analogy, one may conclude that these single-host mites predicted as multi-host by our model may potentially shift to other hosts and potentially cause an epidemic in new hosts. This scenario is likely assuming increased contact of molossid/vespertilionid bats and other mammalian lineages via predation and/or geographic range expansion via climate change or habitat disturbance.
In conclusion, we assembled the largest and the most complete dataset to date on mites permanently parasitic on mammals and developed a predictive model to analyze a set of determinants influencing the likelihood of single-host parasites transitioning into multi-host parasites. Our model accounted for potentially unobserved host-parasite links and class imbalances, identifying statistically significant predictors related to parasites, hosts, climate, and habitat disturbance. This analysis provided valuable insights into the ecological and epidemiological aspects of mammalian acarine parasites and potential disease transmission dynamics. When applied to forecast epidemic risk-group parasites, our model revealed that rodents (Rodentia), bats (Chiroptera) and Carnivora harbor a disproportionately large number of single-host parasites with the potential to become multi-host, including the sarcoptid skin mites of the genus Notoedres, posing significant epidemic risks. Our study is one of the major attempts to analyze host specificity patterns in mammalian acarine parasites in a predictive, quantitative framework. However, more empirical and experimental studies are clearly needed to understand the general properties underpinning host-parasite interactions.
Methods
Mites
We selected acariform mites forming permanent (full-time) host associations with mammals (i.e., they do not have a free-living or specialized dispersal stage like chiggers or ticks) to be included in the database. Acariform mites include two major and distantly related lineages, Prostigmata and Astigmata, showing multiple independent origins of parasitism38 and thus allowing phylogenetically independent comparisons, e.g., most prostigmatan families (n = 6) have colonized mammals independently, and astigmatan mammal mites are bi- or triphyletic39. Among these mites are relatively benign human-specific (single-host) symbionts, such as follicular mites, Demodex folliculorum and D. brevis40. Demodex mites are common components of healthy skin, acting as mostly harmless symbionts41. They have had a long history of co-evolution with mammals, and likely have mechanisms to evade and manipulate the host immune system42. In contrast to mostly non-pathogenic and single-host Demodex, the multi-host mite Sarcoptes scabiei, also belonging to an ancient lineage showing codivergence since the time of the marsupial/placental dichotomy37, causes a highly contagious skin disease. This mite disease affects more than 200 million people, particularly in resource-poor tropical regions43. In immunocompromised humans or when host-shifted from humans to other mammals, the disease may develop into a crusted form, which is often lethal44. Multi-host skin mite parasites (Otodectes, Psoroptes, Chorioptes, Sarcoptes) pose threats to domesticated and wild mammals, including endangered species44,45. Because all developmental stages in our target taxa live on (or inside) the host body, there is a limited possibility for dispersal or host shifts other than by direct body contact, such as mother to offspring vertical transmission, mating, or within-species social contact46. However, interspecific (among-species) mite transmission may be facilitated by hosts sharing the same habitat (e.g., bat roosting sites) or predator-prey interactions (e.g., hyaenas and hedgehogs) (our data). Because these mites lack a vector transmission, our analysis should not be confounded by an additional layer of complexity associated with traits of vector organisms.
Host-parasite database
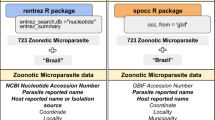
Using the literature, particularly recent reviews47,48, we compiled a taxonomic database of parasitic acariform mites, including the following information: (1) valid mite name, authority, and year; (2) unique host records per mite species; (3) mite taxonomy (family, parvorder); (4) biogeographic region; and (5) credibility of host record (see below). Our database has 3489 unique host-parasite records representing 1998 mite and 1486 mammalian species (Supplementary Data 3). Based on interviewing authors about their sampling procedures and the repeatability of a particular association, we marked 113 records as potential sample cross-contamination in the field, laboratory or museum (low credibility). After quality control (e.g., missing data, uncertainty in host identification, low credibility records) and synchronizing the host taxonomy with the latest source, the Mammal Diversity Database (MDD) v1.1049, our dataset had 3350 unique host-parasite records representing 1,984 mite species (22 families, 2 parvorders) and 1432 mammal species (118 families, 22 orders). A host name lookup table was created to retrieve data from four external sources, all using different host taxonomies: host classification, domestication status, and biogeographic region (MDD v1.10)49; host phylogeny50; host traits and environmental data (PanTHERIA)51; shape files describing spatial distribution of mammalian hosts (MDD v1.2)52; and Google Scholar publication count per mite species accessed on May 20 2023 (custom Python script: see Code Availability). After removing observations with missing data (mostly due to external host databases) and after summarizing our final dataset by unique mite species (as required by downstream analyses), our analysis dataset had 1445 unique observations (Supplementary Data 4).
Dependent variable
The variable multihost has two categories describing the mite host range, either No (singlehost, 0) or Yes (multihost, 1). There were 1032 (71. 4%) single-host and 413 (28.6%) multi-host mite species, indicating that our dataset is moderately imbalanced with respect to single-host mite species, which is the majority category. Therefore, when the imbalanced nature of host-parasite association is not accounted for, a random mite record has a higher probability of being classified as single-host.
Predictors
We coded 14 variables belonging to three general groups: (i) mite-related: Host Immunity Contact Level, Chelicerae, Parasitism, Mite Bioregion, Mite Dispersal Stage, Precopulatory Guarding; (ii) host-related: Co-Distributed Potential Hosts (see below), Potential Phylogenetically Similar Hosts (see below), Average Host Litter Size, Domesticated Host, Average Host Body Mass log, (iii) climatic: Average Precipitation, Average Temperature, and (iv) habitat disturbance: Average Human Population Density log. The mite-related predictors were coded using our data and the following main sources38,53,54,55. Co-Distributed Potential Hosts and Phylogenetically Similar Potential Hosts were calculated using a custom script (see below), while the remaining host-related and environmental variables were imported from the PanTHERIA database51. Our analysis is a mite species-level analysis, i.e., each mite species (or subspecies) has a set of values related to the mite itself, host(s), or the environment. In the case of multi-host mites, we averaged the values of corresponding host-related predictor variables to ensure that the combined effect of all hosts is appropriately represented. For two variables, Co-Distributed Potential Hosts and Phylogenetically Similar Potential Hosts, we used maximum values because the effect of these variables is expected to be most pronounced at maximum values, which will be explained in the following section.
Two variables, Host Immunity Contact Level and Chelicerae, were nearly perfectly correlated (see below), so only the former was used in downstream analyses. Therefore, our final dataset consisted of the following 13 predictor variables, with variable names italicized for readability:
1. Host Immunity Contact Level describes the three levels of contact with the host immunity: (1) Primary—mites directly contact elements of the host immune system while feeding; (2) Secondary—mites feed superficially on outer (dead) epidermal tissue; immune response occurs subsequently, usually because of a combination of host abrasion/scratching and mite presence, resulting in oozing inflammatory lipid exudates and lymph secretions (which can also be consumed by mites); (3) None—mites feed on host tissue derivatives with no immune response, e.g., sebaceous secretions. For example, myobiid mites pierce the skin and feed on the lymph and intercellular fluids of the host56 (Primary). The skin mite Psoroptes ovis abrades the stratum corneum, depositing allergens as they progress, and this combination of skin abrasion, allergen deposition and self-grooming behavior by the host in response to the pruritis triggers the subsequent activation of a cutaneous inflammatory response53,54 (Secondary). Fur mites (Listrophoridae, Atopomelidae, Chirodiscidae) feed on materials accumulated on the hair surface: shed dead skin, sebaceous gland secretions, fungal spores, hyphae, and pollen57 (None). Mites with the states Secondary or None are expected to have a higher likelihood of establishing on a new host species (host range is broader) as they do not have direct contact with the host immune system, while mites with the state Primary are expected to have narrower host ranges. The variable Chelicerae describes the shape of the mite chelicerae (a mouthpart): chelate or piercing. All prostigmatan mites treated here have piercing chelicerae, while psoroptidid astigmatan mites, with the exception of the family Lemurnyssidae, have chelate chelicerae. The values Host Immunity Contact Level: Primary and Piercing Chelicerae are perfectly correlated with each other; if Host Immunity Contact Level is Secondary or None then chelicerae are always chelate. Thus, to avoid collinearity, the variable Chelicerae was excluded from further analyses. It should be noted that due to the nature of statistical data, predictor variables are not expected to perfectly separate the groups of the dependent variable. For example, even though mites with chelate chelicerae are expected to be multi-host, a small proportion of mites with chelate chelicerae may still be strictly host-specific (single-host), such as several species of the genus Chirobia37.
2. Parasitism describes whether the mite is ectoparasitic (lives outside the host body) or endoparasitic (lives inside the host body). Examples are fur mites living on the host hair (Ectoparasitic) and species of Gastronyssidae and Ereynetidae living in the nasal cavities of their hosts (Endoparasitic). Ectoparasitic mites are expected to have a higher likelihood of being transferred on contact between different host species (broader host range) than endoparasitic mites (narrower host range). The four species-level taxa of Opsonyssus (O. brutsaerti indica, O. pseudoindicus, O. eidoloni, O. pteropodi) could be considered both endoparasites and ectoparasites due to their habitat on the outer surface of host eyeballs. Here, we conservatively coded them as ectoparasitic. Our sensitivity analysis using alternative coding (Endoparasitic) yielded very similar results in terms of coefficient estimates and classification accuracy (Supplementary Table 2), suggesting that our model is robust to different interpretations of the parasitism type in Opsonyssus.
3. BiogeographicRegionMite describes the known geographic distributions of mite species (not to be confused with host geographic distribution). We used several biogeographic coding schemes (MA=Madagascar, PM=Papua New Guinea, SA=Sahara Desert): 10 categories (Afrotropic, Afrotropic_MA, Australasia, Australasia_PM, Ethiopian_SA, Indomalaya, Nearctic, Neotropic, Palearctic, Multiregion), 6 categories (Afrotropic, Australasia, Holarctic, Indomalaya, Neotropic, Multiregion), and 2 categories (Singleregion, Multiregion). Multiregion refers to mites that are distributed in more than one biogeographic zone. We used the 2-category scheme in our final analyses because the 10- or 6-category schemes did not significantly improve the group separation (single- vs. multi-host mites) in the dependent variable. Mites having the Multiregion state are expected to have wider host ranges because they have adapted to a wider range of environments and have higher probabilities of being exposed to higher diversity of host species, while the reverse is expected for Singleregion mites.
4. Mite Dispersal Stage refers to the specific life stage during which mites primarily disperse (in contrast, adult males are always mobile and are not categorized within this variable). In most mite lineages, dispersal occurs through all life stages, including adult females and immatures (F_Imm), which are capable of walking on the host. However, in several mite groups, females may have modified characteristics rendering them sedentary, while dispersal is primarily achieved by immature stages, often just larvae (Imm). For example, in the genus Gastronyssus, females live in the host’s stomach and are elongated, while larvae are found in the mouth and nasal cavities, indicating that this immature stage is the dispersal stage58. Similarly, in the sarcoptid genera Rousettocoptes, Tychosarcoptes, Chirobia, Teinocoptes, females are physogastric (extreme enlargement of the body) and cannot move, while dispersal is accomplished by larvae37. Finally, some lineages disperse only as adult females (F). For example, immature female stages of the chirodiscid subfamily Labidocarpinae lack functional legs and only acquire them at the final molt, when they become adult females59. Even though the legless labidocarpine immatures cannot disperse on their own, they still can be dispersed by males as part of precopulatory guarding behavior (see below). Mites that exhibit dispersal across all stages (F_Imm) may achieve greater establishment success, suggesting that species possessing this trait are likely to have broader host ranges, while species having a restricted set of dispersal stages (Imm) or (F) are expected to have narrower host ranges.
5. Precopulatory Guarding describes the presence or absence (y/n) of precopulatory guarding, i.e., an adult male grasps an immature female (unreceptive or nymphal) and carries it for an extended period of time prior to mating60. The presence of precopulatory guarding increases the likelihood of a successful transmission since both sexes can be transmitted to a host individual in a single colonization event (wider host range), while species exhibiting no precopulatory guarding are expected to have narrower host ranges.
6. Co-Distributed Potential Hosts (n_CPH_max) characterizes the number of mammal species that have spatial overlap with the host/hosts associated with a mite species. To calculate this quantity, we first computed an m-by-m matrix of all pairwise geographic overlap of mammals that have distribution information in the MDD v.1.10 database49, where ‘m’ indicates the total number of mammalian host species. Geographic overlap was assessed using pairwise Jaccard Similarity Coefficient,
To calculate the n_CPH_max for each mite species, we followed these steps: (1) for a single-host mite, we matched the corresponding matrix row representing its host and determined n_CPH_max by summing the number of Jaccard similarity values exceeding 0.5; (2) for a multi-host mite, we repeated step 1 for each host and retained the maximum count. The n_CPH_max measure provides more probabilistic metrics to estimate the available potential hosts in the mites’ geographic distribution. We used the maximum count because that host serves as the most likely reservoir of parasites for new hosts (see Code Availability for custom code).
7. Potential Phylogenetically Similar Hosts (n_PSH10_max) characterizes the number of mammal species that are phylogenetically closely related to the parasite host/hosts. It was calculated in a similar manner to n_CPH_max, but using pairwise phylogenetic distances between mammal pairs from the latest mammal supertree50. The n_PSH10_max measure counts the number of mammal species whose phylogenetic distances to the parasite host are smaller than 10. For multi-host mites, n_PSH10_max is chosen to be the maximum count among the hosts. This measure provides a probabilistic metric to estimate all the available potential hosts on the mammal tree that are phylogenetically similar. Unlike raw phylogenetic distances and related metrics, our metric is agnostic to the current parasite’s host range status, particularly whether if the parasite is single- or multi-host (see Code Availability for custom code).
8. Average Host Litter Size describes the number of host offspring born per litter per female; PanTHERIA: 15-1_LitterSize51. We expect that larger litter sizes increase the parasite dispersal through the vertical route of transmission (parent to offspring) and, in addition, large litter sizes may be correlated with host fitness and the propensity to support large parasite loads30. Hence, this variable is likely to be positively correlated with the elevated probability of host range expansion.
9. Domesticated Host (y, n) accounts for empirical observations that pathogens associated with domesticated animals experience frequent host switches resulting from the increased opportunity for cross-infestation in anthropogenic settings10,61,62. Here, we used a list of domesticated hosts as defined in MDD v.1.1049, amended with common peri-domestic pest animals, such as the black rat Rattus rattus and humans themselves, which are often the source of mites secondarily infesting domestic animals63.
10. Average Host Body Mass log describes the host body mass (g); extracted from the PanTHERIA database, variable 5-1_AdultBodyMass_g51. In our analyses, raw values were converted to natural logarithms. We expect nonlinear/bi-directional relationships with the dependent variable: in the low to middle value range of this variable, larger mammals may harbor larger population sizes of parasites, facilitating transmission, and therefore promoting the evolution of multi-host parasites (positive correlation with the dependent). However, very large and massive mammals may themselves have smaller population sizes and isolated geographic ranges, therefore limiting parasite transmission (negative correlation with the dependent). When applied to massive animals, this negative effect of host body mass was noted in the literature51.
11. Average Precipitation is the mean monthly precipitation (mm) within the geographic range of the host; PanTHERIA: 28-1_Precip_Mean_mm51. We expect that precipitation positively affects the mite transmission probability as mites are prone to desiccation while outside the host — higher humidity extends the short period when mites can survive in the environment.
12. Average Temperature, mean monthly temperature (x0.1 °C) within the geographic range of the host; PanTHERIA: 28−2_Temp_Mean_01degC51. This variable is expected to be negatively correlated with the dependent as higher temperatures may promote mite desiccation and decrease survival while being transmitted across hosts.
13. Average Human Population Density log, 5th percentile human population density (persons per km2); PanTHERIA: 27-3_HuPopDen_5p_n/km251. This variable is a proxy for anthropogenic habitat disturbance. Environmental disturbance is expected to promote host switches (host range expansion) as there are increased opportunities to encounter new hosts64.
Host range model
To determine which factors affect host range expansion in a single-host mite, we ran a logistic regression using Generalized Linear Model (glm) in caret65, with some of the continuous variables transformed into polynomial variables using natural spline function in splines for R v4.2.266. Natural splines can fit smooth regression curves to nonlinear data, with the number of spline curves controlled by the user through the degree of freedom parameter or set automatically via cross-validation. Generalized Additive Model (gam) is similar to Spline Regression, but it selects parameters for smooth basis functions automatically, sometimes leading to overfitting / unrealistically wiggly smooth functions, especially in areas with little data67. We could not alleviate this issue by increasing the basis dimension parameter (k) and, therefore, use Natural Spline Regression over gam here. After the removal of 19 records from captive hosts (natural host distribution is unknown), we randomly split our dataset into train (n = 1024, 70.9%) and test (n = 421, 29.1%) subsets. Because our data had disparate frequencies of the observed host range classes (class imbalance: single-host 71.4%, multi-host 28.6%), we simultaneously subsampled (up- and down-sampling) and resampled (5-fold cross-validations, 5 repeats) our train dataset to estimate the performance of different models based on AUC-ROC (area under the Receiver Operating Characteristic curve) metric in caret65. For comparison, we used the baseline unweighted model, a weighted model, and a positive-unlabeled (PU) learning model with AdaSampling68.
The weighted model weights records by Google Scholar publication counts for each mite species as follows: 0.2 + (totalPubs > =10)*0.8. This model accounts for the fact that some mite species received more research efforts than others, and thus their records of association with hosts are more complete than other species. This formula gives full weights to mite species with more than 10 publications on Google Scholar and 0.2 weights to the species that have less than 10 publications. This weighting scheme was designed to mitigate the impact of unobserved multi-hosts, which are likely to be associated with a lower sampling effort, hence having lower publication counts.
A positive-unlabeled (PU) learning model explicitly assumes that labels in the positive class are known while the negative class is unlabeled and contains a mixture of both positive and negative instances69. This contrasts with traditional supervised learning (such as spline regression), where both positive and negative instances are assumed to be accurately labeled. In our case, the positive class is multi-host, which should be correctly labeled because once a mite species is credibly classified as multi-host, the multi-host status of this mite species will remain unchanged with additional sampling. In contrast, the negative class is unlabeled and contains both true single-hosts and true multi-hosts erroneously classified as single-hosts due to insufficient sampling (unobserved multi-hosts). PU learning algorithms typically aim to estimate the probability that an unlabeled instance belongs to the positive class, leveraging information from the labeled positive instances and the unlabeled data69.
For model selection, we used several metrics: ROC-AUC, Accuracy, Kappa, Sensitivity, Specificity, and F1. Of them, the former three provide a measure of overall model performance (still can be biased to class imbalance, especially Accuracy), while the latter three evaluate the positive class performance (multihost). For our main metric, ROC-AUC, we evaluated 95% confidence intervals in the R package pROC integrated with caret. We used our final model to predict the test (holdout) dataset, i.e., records that were not used in model inference, and further analyzed records observed as singlehost but predicted as multihost by the model. Mites in this category have the highest risk probability to become multi-host (high host switching / host expansion risk) given the data. We then calculated the ‘enrichment’ of the multi-host risk group among different mammal orders for each host order by subtracting the percentages of observed singlehost which were predicted as multihost by the model at different classification thresholds; from these values, we subtracted the percentages of observed singlehost records per host order. Values above zero (above the average) are considered as high-risk single-host parasites that are likely to become multi-hosts.
Mite-sharing model
The n-by-n mite-sharing matrix of hosts was constructed by projecting mite-host association records into a unipartite network, where an edge between a host pair represents the sharing of one or more parasites. Hosts that do not share any parasites with other hosts were excluded from the analyses. The final dataset was constructed by taking the intersection of host species that were present in all three matrices: the n-by-n mite-sharing matrix and the m-by-m matrices of all pairwise host geographic overlaps and phylogenetic distances (see above), and sub-setting the three matrices accordingly to be of the same size. The distribution of pairwise phylogenetic distances (PD) is very uneven due to the deep divergence between marsupial and placental mammals (PD > 300). We recoded these PDs to be 178 (i.e., the highest PD within placental mammals) so that models would not overfit the gap region between large PDs. We discretized the PD and geographic overlap (GO) and counted the cases of observations in each combination. 91% of the host pairs (833,454 out of 915,981 pairs) do not have geographic overlaps and are phylogenetically very divergent from each other (Fig. 4a, b, Supplementary Fig. 2 a, b). To avoid overfitting in the regions of extreme values, we sub-sampled each bin down to 1000 pairs (Supplementary Fig. 2 b). We used a generalized linear model in which the probability of observing mites sharing between hosts is a linear response of GO, natural splines of PD and their interactions. Specifically,
Using this model, we can predict the probability of mites sharing between any pair of mammal hosts given their PD and GO. The statistics of the model output was summarized in Supplementary Table 1. Our entire statistical pipeline is documented through R scripts (see code availability).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data generated in this study, including the main host-parasite database, are provided in Supplementary Data and have been deposited on the Zenodo database at https://doi.org/10.5281/zenodo.1113064870. See Methods for data sources used to compile the host-parasite database. All the source data for generating the figures are provided in Supplementary Information/Source Data File. Source data are provided with this paper.
Code availability
The scripts for data analyses and prediction are available on Zenodo at https://doi.org/10.5281/zenodo.1113064870.
References
Van Klinken, R. Host specificity testing: why do we do it and how we can do it better. Proceedings, host specificity testing of exotic arthropod biological control agents: the biological basis for improvement in safety, X international symposium on biological control of weeds, July 4–14, 1999, Bozeman, Montana, U.S.A. 1, 54–68 (2000).
Rigaud, T., Perrot-Minnot, M. J. & Brown, M. J. Parasite and host assemblages: embracing the reality will improve our knowledge of parasite transmission and virulence. Proc. R. Soc. B 277, 3693–3702 (2010).
Cressler, C. E., McLeod, D. V., Rozins, C., van den Hoogen, J. & Day, T. The adaptive evolution of virulence: a review of theoretical predictions and empirical tests. Parasitology 143, 915–930 (2016).
Regoes, R. R., Nowak, M. A. & Bonhoeffer, S. Evolution of virulence in a heterogeneous host population. Evolution 54, 64–71 (2000).
Woolhouse, M. E. & Gowtage-Sequeria, S. Host range and emerging and reemerging pathogens. Emerg. Infect. Dis. 11, 1842–1847 (2005).
Scheele, B. et al. Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science 363, 1459–1463 (2019).
Rudd, J. L. et al. Molecular epidemiology of a fatal sarcoptic mange epidemic in endangered San Joaquin kit foxes (Vulpes macrotis mutica). Parasite Vector 13, 456 (2020).
de Castro, F. & Bolker, B. Mechanisms of disease-induced extinction. Ecol. Lett. 8, 117–126 (2005).
Lafferty, K. D. Biodiversity loss decreases parasite diversity: theory and patterns. Philos. Trans. R. Soc. B 367, 2814–2827 (2012).
Cleaveland, S., Laurenson, M. K. & Taylor, L. H. Diseases of humans and their domestic mammals: pathogen characteristics, host range and the risk of emergence. Philos. Trans. R. Soc. B 356, 991–999 (2001).
Woolhouse, M. E. J., Taylor, L. H. & Haydon, D. T. Population biology of multihost pathogens. Science 292, 1109–1112 (2001).
Gibson, A. K. et al. The evolution of parasite host range in heterogeneous host populations. J. Evol. Biol. 33, 773–782 (2020).
Strobel, H. M., Stuart, E. C. & Meyer, J. R. A trait-based approach to predicting viral host-range evolvability. Annu. Rev. Virol. 9, 139–156 (2022).
Olival, K. J. et al. Host and viral traits predict zoonotic spillover from mammals. Nature 546, 646–650 (2017).
Locke, S. A., McLaughlin, J. D. & Marcogliese, D. J. Predicting the similarity of parasite communities in freshwater fishes using the phylogeny, ecology and proximity of hosts. Oikos 122, 73–83 (2013).
Cooper, N., Griffin, R., Franz, M., Omotayo, M. & Nunn, C. L. Phylogenetic host specificity and understanding parasite sharing in primates. Ecol. Lett. 15, 1370–1377 (2012).
Lafferty, K. D. The ecology of climate change and infectious diseases. Ecology 90, 888–900 (2009).
Albery, G. F., Eskew, E. A., Ross, N. & Olival, K. J. Predicting the global mammalian viral sharing network using phylogeography. Nat. Commun. 11, 2260 (2020).
Albery, G. F. et al. The science of the host-virus network. Nat. Microbiol. 6, 1483–1492 (2021).
Bradley, J. E. & Jackson, J. A. Measuring immune system variation to help understand host-pathogen community dynamics. Parasitology 135, 807–823 (2008).
Dyer, L. A. et al. Host specificity of Lepidoptera in tropical and temperate forests. Nature 448, 696–699 (2007).
Altizer, S., Nunn, C. L. & Lindenfors, P. Do threatened hosts have fewer parasites? A comparative study in primates. J. Anim. Ecol. 76, 304–314 (2007).
Esser, H. J. et al. Host body size and the diversity of tick assemblages on Neotropical vertebrates. Int. J. Parasitol.: Parasites Wildl. 5, 295–304 (2016).
Christe, P. et al. Host sex and ectoparasites choice: preference for, and higher survival on female hosts. J. Anim. Ecol. 76, 703–710 (2007).
Davies, T. J. & Pedersen, A. B. Phylogeny and geography predict pathogen community similarity in wild primates and humans. Proc. R. Soc. B 275, 1695–1701 (2008).
Pedersen, A. B., Altizer, S., Poss, M., Cunningham, A. A. & Nunn, C. L. Patterns of host specificity and transmission among parasites of wild primates. Int. J. Parasitol. 35, 647–657 (2005).
Wells, K. & Clark, N. J. Host specificity in variable environments. Trends Parasitol. 35, 452–465 (2019).
Ahmadzadeh, A. et al. Challenges with extreme class-imbalance and temporal coherence: A study on solar flare data. In 2019 IEEE international conference on big data (Big Data), pp. 1423–1431 (Ieee, 2019).
Buhnerkempe, M. G. et al. Eight challenges in modelling disease ecology in multi-host, multi-agent systems. Epidemics 10, 26–30 (2015).
Dallas, T., Park, A. W. & Drake, J. M. Predicting cryptic links in host-parasite networks. PLoS Comput. Biol. 13, e1005557 (2017).
Nunn, C. L. & Altizer, S. M. The global mammal parasite database: An online resource for infectious disease records in wild primates. Evol. Anthropol. 14, 1–2 (2005).
Wardeh, M., Risley, C., McIntyre, M. K., Setzkorn, C. & Baylis, M. Database of host-pathogen and related species interactions, and their global distribution. Sci. Data 2, 150049 (2015).
Smith, K. F., Acevedo-Whitehouse, K. & Pedersen, A. B. The role of infectious diseases in biological conservation. Anim. Conserv. 12, 1–12 (2009).
Leroy, E. M. et al. Fruit bats as reservoirs of Ebola virus. Nature 438, 575–576 (2005).
Arneberg, P. Host population density and body mass as determinants of species richness in parasite communities: comparative analyses of directly transmitted nematodes of mammals. Ecography 25, 88–94 (2002).
Kunz, T. H. Roosting ecology of bats. In Ecology of bats. (ed. Kunz, T. H.) pp. 1–55 (Plenum Press, New York, 1982).
Klompen, J. S. H. Phylogenetic relationships in the mite family Sarcoptidae (Acari: Astigmata). Misc. Publ. Mus. Zool. Univ. Mich. 180, 1–154 (1992).
Krantz, G. W. & Walter, D. E. A Manual of Acarology (Texas Tech University Press, 2009).
Klimov, P. B. & OConnor, B. M. Origin and higher-level relationships of psoroptidian mites (Acari: Astigmata: Psoroptidia): evidence from three nuclear genes. Mol. Phylogenet. Evol. 47, 1135–1156 (2008).
Lacey, N., Kavanagh, K. & Tseng, S. C. Under the lash: Demodex mites in human diseases. Biochem. (Lond.) 31, 2–6 (2009).
Palopoli, M. F., Minot, S., Pei, D., Satterly, A. & Endrizzi, J. Complete mitochondrial genomes of the human follicle mites Demodex brevis and D. folliculorum: novel gene arrangement, truncated tRNA genes, and ancient divergence between species. BMC Genom. 15, 1124 (2014).
Forton, F. M. N. The pathogenic role of Demodex mites in rosacea: A potential therapeutic target already in erythematotelangiectatic rosacea? Dermatol. Ther. 10, 1229–1253 (2020).
Akuta, T. et al. Development of a rapid scabies immunodiagnostic assay based on transcriptomic analysis of Sarcoptes scabiei var. nyctereutis. Sci. Rep. 11, 6455 (2021).
Arlian, L. G. & Morgan, M. S. A review of Sarcoptes scabiei: past, present and future. Parasit. Vectors 10, 297 (2017).
Pedersen, A. B., Jones, K. E., Nunn, C. L. & Altizer, S. Infectious diseases and extinction risk in wild mammals. Conserv. Biol. 21, 1269–1279 (2007).
Palopoli, M. F. et al. Global divergence of the human follicle mite Demodex folliculorum: Persistent associations between host ancestry and mite lineages. Proc. Natl Acad. Sci. Usa. 112, 15958–15963 (2015).
Bochkov, A. V. A review of mammal-associated Psoroptidia (Acariformes: Astigmata). Acarina 18, 99–260 (2010).
Izdebska, J. N. & Rolbiecki, L. The biodiversity of demodecid mites (Acariformes: Prostigmata), specific parasites of mammals with a global checklist and a new finding for Demodex sciurinus. Diversity-Basel 12, 261 (2020).
Abreu, E. et al. Mammal Diversity Database (Version 1.10) [Data set]. zenodo. https://doi.org/10.5281/zenodo.7394529 (2022).
Upham, N. S., Esselstyn, J. A. & Jetz, W. Inferring the mammal tree: Species-level sets of phylogenies for questions in ecology, evolution, and conservation. PLoS Biol. 17, e3000494 (2019).
Jones, K. E. et al. PanTHERIA: a species-level database of life history, ecology, and geography of extant and recently extinct mammals. Ecology 90, 2648 (2009).
Marsh, C. J. et al. Expert range maps of global mammal distributions harmonised to three taxonomic authorities. J. Biogeogr. 49, 979–992 (2022).
Hamilton, K. A., Nisbet, A. J., Lehane, M. J., Taylor, M. A. & Billingsley, P. F. A physiological and biochemical model for digestion in the ectoparasitic mite, Psoroptes ovis (Acari: Psoroptidae). Int. J. Parasitol. 33, 773–785 (2003).
Burgess, S. T. et al. Transcriptomic analysis of the temporal host response to skin infestation with the ectoparasitic mite Psoroptes ovis. BMC Genom. 11, 624 (2010).
Arlian, L. G., Runyan, R. A. & Vyszenski-Moher, D. L. Water balance and nutrient procurement of Sarcoptes scabiei var. canis (Acari: Sarcoptidae). J. Med. Entomol. 25, 64–68 (1988).
Fain, A. Adaptation, specificity and host-parasite coevolution in mites (Acari). Int. J. Parasitol. 24, 1273–1283 (1994).
Wurst, E. Investigations on the anatomy and the behaviour of the fur mite Listrophorus leuckarti (Acari: Listrophoridae). Stuttg. Beitr. Nat. A 53, 1–68 (1993).
Bochkov, A. V., Zabludovskaya, S. & OConnor, B. M. Phylogeny and systematics of the endoparasitic astigmatid mites (Acari: Sarcoptiformes) of mammals: families Gastronyssidae, Lemurnyssidae, and Pneumocoptidae. Zootaxa 1951, 1–152 (2008).
OConnor, B. M. Cohort Astigmatina. In A Manual of Acarology. (eds Krantz, G.W. & Walter, D.E.) pp. 565–657 (Texas Tech University Press, Lubbock, Texas, 2009).
Witaliński, W., Dabert, J. & Walzl, M. G. Morphological adaptation for precopulatory guarding in astigmatic mites (Acari: Acaridida). Int. J. Acarol. 18, 49–54 (1992).
Taylor, L. H., Latham, S. M. & Woolhouse, M. E. Risk factors for human disease emergence. Philos. Trans. R. Soc. B 356, 983–989 (2001).
Wolfe, N. D., Dunavan, C. P. & Diamond, J. Origins of major human infectious diseases. Nature 447, 279–283 (2007).
Messenger, A. M., Barnes, A. N. & Gray, G. C. Reverse zoonotic disease transmission (zooanthroponosis): a systematic review of seldom-documented human biological threats to animals. PLoS One 9, e89055 (2014).
Hoberg, E. P. & Brooks, D. R. Evolution in action: climate change, biodiversity dynamics and emerging infectious disease. Philos. Trans. R. Soc. B 370, 20130553 (2015).
Kuhn, M. Building predictive models in R using the caret package. J. Stat. Softw. 28, 1–26 (2008).
R Development Core Team. R: A language and environment for statistical computing, reference index version 2.11.1. R Foundation for Statistical Computing, Vienna, Austria. (2010).
Venables, W. N. & Ripley, B. D. Modern Applied Statistics with S (Springer, 2002).
Yang, P. Y. et al. AdaSampling for positive-unlabeled and label noise learning with bioinformatics applications. IEEE Trans. Cybern. 49, 1932–1943 (2019).
Bekker, J. & Davis, J. Learning from positive and unlabeled data: a survey. Mach. Learn. 109, 719-760 (2020).
Klimov, P., & He, Q. Predicting host range expansion in parasitic mites using a global mammalian-acarine dataset. Zenodo. https://doi.org/10.5281/zenodo.11130648 (2024).
Acknowledgements
We thank Barry OConnor (University of Michigan, Ann Arbor, USA), Sergey Mironov, and the late Andrey Bochkov (Zoological Institute, Russian Academy of Sciences, Saint Petersburg, Russia) for comments and discussion. We also thank four anonymous reviewers for their insightful suggestions, which helped us improve the earlier versions of the manuscript.
Author information
Authors and Affiliations
Contributions
P.B.K. and Q.H. conceived the overall research plan, designed the experiments, analyzed the data, and wrote the manuscript. P.B.K. generated the host-parasite dataset.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Klimov, P.B., He, Q. Predicting host range expansion in parasitic mites using a global mammalian-acarine dataset. Nat Commun 15, 5431 (2024). https://doi.org/10.1038/s41467-024-49515-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-49515-3
- Springer Nature Limited