Abstract
Adult zebrafish have an innate ability to recover from severe spinal cord injury. Here, we report a comprehensive single nuclear RNA sequencing atlas that spans 6 weeks of regeneration. We identify cooperative roles for adult neurogenesis and neuronal plasticity during spinal cord repair. Neurogenesis of glutamatergic and GABAergic neurons restores the excitatory/inhibitory balance after injury. In addition, a transient population of injury-responsive neurons (iNeurons) show elevated plasticity 1 week post-injury. We found iNeurons are injury-surviving neurons that acquire a neuroblast-like gene expression signature after injury. CRISPR/Cas9 mutagenesis showed iNeurons are required for functional recovery and employ vesicular trafficking as an essential mechanism that underlies neuronal plasticity. This study provides a comprehensive resource of the cells and mechanisms that direct spinal cord regeneration and establishes zebrafish as a model of plasticity-driven neural repair.
Similar content being viewed by others
Introduction
Mammalian spinal cord injuries (SCI) elicit complex multi-cellular responses that impede regeneration and cause permanent functional deficits in mammals1,2,3,4,5,6. Anti-regenerative neuron-extrinsic factors comprise chronic inflammation, fibrotic scarring, demyelination and the acquisition of a regeneration restricting extracellular milieu. These injury complications exacerbate the inherently limited ability of the mammalian spinal cord (SC) to replenish lost neurons via adult neurogenesis or to regrow lesioned axon tracts. Consequently, even the most groundbreaking regenerative therapies targeting select cell types or individual molecules have only yielded modest improvement in cellular and functional outcomes6,7. SCI studies have since pursued combinatorial strategies as a more promising therapeutic avenue8,9,10,11. We propose that comprehensive and simultaneous examination of neuronal and non-neuronal cells after SCI is fundamental to understanding and manipulating the multi-cellular complexities of neural injuries.
Unlike mammals, adult zebrafish have an innate ability to spontaneously recover from severe SCI. Following complete transection of SC tissues, zebrafish reverse paralysis and regain swim function within 6 to 8 weeks of injury12,13,14. Pro-regenerative injury responses involving immune and progenitor cells, neurons and glia, cooperate to achieve spontaneous and efficient repair in zebrafish15,16,17,18,19,20,21. Early after SCI, potent populations of progenitor cells, including central canal-surrounding ependymo-radial glial cells (ERGs), are activated to replenish lost neurons and glia16,18,20,21,22. Newly differentiated motor neurons and interneurons populate the regenerate tissue, as pre-existing neurons regrow axons across lesioned tissues19,20,21. Though less studied, glial cells are thought to enact instrumental pro-regenerative responses throughout the course of regeneration14,18,22,23. However, a holistic understanding of the cellular interactions that coordinate the pro-regenerative responses to direct SC regeneration in zebrafish is to be acquired.
The advent of single-cell transcriptomics provided the tools to achieve a refined understanding of molecular SCI responses across species and cell types. Multiple single-cell RNA sequencing (scRNA-seq) atlases have been generated for mouse SCI, rat SCI or human SC tissues24,25,26,27,28. Single-cell studies from mice revealed new insights into macroglial cell-cell interactions, revealed a rare population of lumbar spinocerebellar neurons that elicit a regeneration signature, or characterized astrocyte responses after injury26,27,28,29. However, single-cell studies from zebrafish SC tissues have been limited to isolated immune or progenitor motor neuron cells and to larval stages17,30. Thus, a complete resource of the regenerative cells and mechanisms from adult zebrafish is required to develop a molecular understanding of the cell identities that enable or limit spontaneous plasticity and regeneration.
The fundamental principles that determine or limit regenerative capacity across species have eluded scientists for ages. While pro-regenerative injury responses are overwhelmed by anti-regenerative complications in mammals, zebrafish cells exhibit increased potency and exclusively pro-regenerative signatures after SCI. Molecular and cellular studies of select cell types or regenerative pathways revealed key insights into the cellular contributions and molecular signatures associated with elevated regenerative capacity. Specifically, while ependymal cells elicit limited stem cell potential in adult mice31,32, zebrafish ERGs retain radial glial features and contribute to neurogenesis and gliogenesis after SCI16,18,20,21,22. The disparities in neuron progenitor capacities between zebrafish and mammals yielded an assumption that neurogenesis-based neural repair is unachievable in mammals and directed the community’s efforts toward plasticity-based neural repair strategies. However, while zebrafish have been an established model of neuron regeneration, it remains unclear whether zebrafish could contribute insights and applications into plasticity-driven repair mechanisms. Thus, how and why neuronal injury responses differ between zebrafish and mammals require comprehensive molecular investigation and cross-species comparisons.
This study presents an atlas of the dynamic responses across major spinal cell types during early, intermediate and late stages of regeneration in adult zebrafish. Single nuclear RNA sequencing (snRNA-seq) was performed at 0, 1, 3 and 6 weeks post-injury. Neurons elicit elevated signaling activity relative to the dozens of cell types that respond to injury. While SCI disrupts the excitatory/inhibitory (E/I) neuron balance, sequential neurogenesis of excitatory and inhibitory neurons restores the homeostatic neuronal landscape at late stages of regeneration. In addition to regenerating new neurons, a transient regenerative signature emerges in a population of injury-responsive neurons (iNeurons) at 1 week post-injury. EdU labeling showed iNeurons are injury surviving neurons that acquire a neuroblast-like transcriptional signature after SCI. iNeuron markers genes are required for functional SC repair, and dynamic vesicular trafficking is a central mechanism that promotes spontaneous neuronal plasticity. This study identifies multi-layered modes of regenerative neurogenesis and neuronal plasticity during innate SC regeneration, and establishes zebrafish as a platform to identify and manipulate regeneration- and plasticity-based modes of neural repair.
Results
Molecular identification and temporal dynamics of spinal cell types after injury
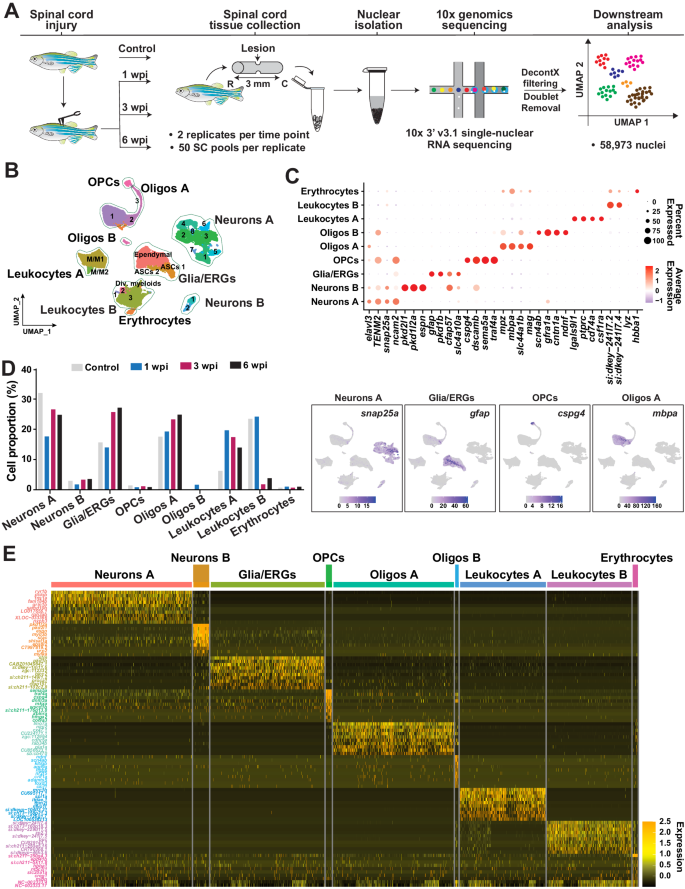
To determine the transcriptional identities of the cells that direct successful SC regeneration, we performed complete SC transections on adult zebrafish and dissected 3 mm sections of SC tissues surrounding the lesion site at 1, 3 and 6 weeks post-injury (wpi) for nuclear isolation. Corresponding tissue sections were collected from uninjured controls. For each time point, SC tissues were pooled from 50 animals and 2 pools of independent biological replicates were analyzed. Our dataset spans key regenerative windows including early injury-induced signals at 1 wpi, neuronal and glial regeneration at 3 wpi, and cellular remodeling at late stages of regeneration at 6 wpi14. Isolated nuclei were sequenced using 10x genomics platform (3’ v3.1 chemistry) and aligned to zebrafish genome GRCz11 with improved zebrafish transcriptome annotation33,34. Nuclei were subsequently filtered using the Decontx and DoubletFinder packages to exclude droplets that include doublet nuclei or a high proportion of ambient mRNA, respectively35,36. A second round of filtering was performed by thresholding covariates such as number of counts, genes and fraction of counts from mitochondrial genes per barcode. A total of 58,973 nuclei was obtained for downstream analysis using the Seurat package (Figs. 1A and S1A−C).
A Experimental pipeline to generate a single-cell atlas of zebrafish cells after SCI. SC tissue collection, nuclear isolation and single nuclear RNA-seq (snRNA-seq) were performed on wild-type fish at 1, 3 and 6 wpi. Uninjured SC nuclei were used as 0 wpi controls. 10x genomics sequencing with v3.1 chemistry was performed. Two biological replicates were used at each time point, and SC tissues from 50 animals were pooled into a single biological replicate. B Merged UMAP representation of the complete dataset. Two biological replicates, 4 time points and 58,973 cells were clustered into major spinal cell populations including Neurons A and B, glia/ependymo-radial glial cells (glia/ERGs), oligodendrocyte precursor cells (OPCs), oligodendrocytes (Oligos) A and B, Leukocytes A and B, and Erythrocytes. C Marker gene expression in the major cell types identified after SCI. Dot plot shows canonical markers are enriched in their respective cell types. Dot colors and diameters represent average gene expression and percent cells with at least one UMI detected per gene, respectively. Feature plots depicting the distributions of canonical markers corresponding to the major cell populations are shown. D Distribution of major cell types during SC regeneration. For each time point, cell proportions were normalized to the total number of cells present at that time point. E Heatmap showing top 10 differentially expressed (DE) genes in the major cell populations identified by snRNA-seq. Yellow and black represent highly enriched and low-expression genes, respectively. Source data are provided as a Source Data file.
Clustering of SC nuclei revealed 24 cell clusters with distinct molecular identities and temporal dynamics (Fig. S1D). Postulating that previously established cell type classifiers are likely biased toward cell identities present in the mammalian nervous system or in developing zebrafish, we optimized cell type identification by generating a custom-assembled Vertebrate CNS Marker (VNM) database. VNM compiled cell type markers commonly present in the central nervous system from over 13 transcriptomic datasets in addition to canonical markers from vertebrate species including zebrafish, mice and humans (Supplementary Data 1)17,24,27,37,38,39,40,41,42,43,44,45,46,47,48,49,50. Major spinal cell populations were then identified by cross-referencing differentially expressed (DE) markers for each cluster with our VNM database using a custom-made DE-Marker-Scoring algorithm (Supplementary Data 2, 3 and Fig. S1E). By grouping individual cell clusters into mega clusters, we report major cell types comprising neurons, glia/ERGs, oligodendrocyte precursor cells (OPCs), oligodendrocytes, leukocytes and erythrocytes. Two coarse clusters were identified for neurons (Neurons A and B), oligodendrocytes (Oligos A and B) and leukocytes (Leukocytes A and B) (Fig. 1B). VNM-based cell type classification was further confirmed by evaluating the expression of canonical marker genes for each coarse cluster (Fig. 1C). elavl3 and snap25a were enriched in neurons A and B, gfap and slc4a10a in glia/ERGs, cspg4 and sema5a in OPCs, mpz and mbpa in oligodendrocytes A, csfr1a and ptprc in leukocytes A, and hbba1 in erythrocytes (Figs. 1C and S1F). Although canonical cell markers were enriched in their respective clusters, the proportions of cells expressing these markers in leukocytes A, glia/ERGs and erythrocytes were relatively small. On the other hand, gene expression analysis of neurons B, oligodendrocytes B and leukocytes B did not show enrichment for classical markers. Further exploration into the specific markers that were identified by the DE-Marker-Scoring algorithm revealed that oligodendrocytes B expressed erbb3b and sox10, whereas subclusters of leukocytes B expressed markers of dividing myeloid cells (Fig. S1E, F and Supplementary Data 3). We noted that additional immune cell lineage genes were expressed in leukocytes A and B (Fig. S1G). The monocyte precursor marker zbtb14 was enriched in less than 10% of leukocytes B, whereas spi1a and spi1b were enriched in 30−50% of leukocytes A. In contrast, T cell markers (cd4-1, foxp3a and lck) and neutrophil markers (mpx and lyz) were enriched in less than 1% of leukocytes B, while 9% of leukocytes A expressed the myeloid marker itgam (orthologue of CD11b). Notably, we did not detect bona fide B cell markers in our dataset. Scatter plots representing the numbers of unique genes versus unique molecular identifiers (UMI) confirmed that the proportions of mitochondrial genes within each population does not exceed 5% (Fig. S1H). However, compared to all other cell populations, leukocyte B cells with the lowest UMIs showed higher mitochondrial gene content (Fig. S1H). On the other hand, although neurons B and oligodendrocytes B did not show enrichment for classical markers, both clusters showed negligible mitochondrial gene expression (Fig. S1H). We conclude that our VNM database is likely still biased toward commonly used mammalian cell markers that are not necessarily the best markers for adult zebrafish, and that our single-cell atlas provides a platform to identify improved CNS markers for adult zebrafish cells. To test this hypothesis, we examined whether our snRNA-seq dataset identified improved cell-specific DE markers (Fig. 1E and Supplementary Data 4). We found that glia/ERG cells expressed the cilia-associated cfap157 gene, indicative of the ciliated nature of central canal-lining ERGs. Neurons B expressed pkd2l1 and pkd1l2a, which are known markers of cerebrospinal fluid-contacting neurons51. Oligodendrocytes B expressed scn4ab and ndnf. Leukocytes A enriched for mrc1b (macrophage mannose receptor 1b precursor), CU695117.1 (protein phosphatase with EF-hand domain 2, ppef2) and itgae.1 (integrin, alpha E, tandem duplicate 1), whereas leukocytes B expressed unknown genes such as si:dkey-241l7.4 (zgc:171670, predicted to have signaling receptor activity), si:ch73-160p18.3 (zgc:101521) and si:dkey-229d11.5 (fd14c01). These studies indicated that extrapolating mammalian CNS or developmental zebrafish markers in adult zebrafish requires validation, and provided a platform to identify new and improved CNS markers in adult zebrafish.
To examine the temporal dynamics of SC cells during regeneration, we determined the numbers and relative proportions of nuclei within each coarse cluster in the integrated dataset (Figs. 1D and S1I). Neurons A, which comprised 32% of the nuclei harvested from uninjured SCs, comprised 18% of total nuclei at 1 wpi and recovered up to 25% at 6 wpi. Neurons B represented a smaller proportion of neurons and showed little variation during the course of regeneration. The proportions of leukocytes A increased from 6% of control nuclei prior to injury to 20% at 1 wpi and gradually decreased to 14% at 6 wpi. We observed an expansion in the relative abundance of glia/ERGs between 1 and 3 wpi, whereas OPCs maintained similar proportions at all time points. Oligodendrocytes A, which accounted for 18% of uninjured nuclei, gradually increased to 25% of total nuclei at 6 wpi. In contrast, Oligodendrocytes B cells were transiently present at 1 wpi and comprised 2% of total nuclei at that time point. These studies provided a comprehensive resource of the cellular architecture that directs successful SC regeneration and revealed a gradual recovery in neuronal proportions during innate SC repair.
Neurons are active signaling hubs during spinal cord regeneration
To infer molecular modes of intercellular communication during SC regeneration, we surveyed signaling pathways that are differentially active between 0 and 6 wpi (Figs. 2 and S2). We first converted our dataset of zebrafish genes to their human orthologues using the BioMart package from Ensembl. The R package “CellChat” was then used to quantitatively analyze global intercellular communication networks based on the expression of ligands, receptors and cofactors52. CellChat computes the communication probabilities of ligand-receptor interactions associated with each signaling pathway, and integrates the cumulative probabilities of all signaling pathways that are outgoing from or received (incoming) by each cell population. In control SC tissues, the cumulative strengths of all signaling pathways outgoing from neurons A and B were the highest relative to other cell types, indicating active neuronal signaling under homeostatic conditions (Fig. 2A, B). After SCI, outgoing signaling increased in most cell types, indicating tissue-wide responses are activated after injury (Fig. 2A, B). Notably, the strengths of signaling pathways outgoing from neurons A and B continued to be elevated across all time points and increased more than 2.5 fold between 0 and 6 wpi (Fig. 2A, B). This elevated neuronal signaling is not merely due to the inherent neurotransmission properties of neurons since major neurotransmitters are non-protein-coding genes that are excluded by CellChat53. On the other hand, the cumulative strengths of all incoming signaling pathways received by OPCs were the highest among spinal cell types after SCI (Figs. 2B and S2A). Incoming signaling received by neurons A doubled between 0 and 6 wpi (Fig. 2B). Mapping of ligand-receptor interactions predicted cell-cell signaling interactions are highest between neurons and OPCs at 6 wpi (Fig. 2C). Confirming CellChat analysis, trajectory inference showed OPCs and neurons showed high transcriptional activity after SCI54 (Fig. S2B). These results indicated neurons and OPCs have elevated and dynamic transcriptional and signaling activities during zebrafish SC regeneration.
A Relative strengths of outgoing signaling pathways in major spinal cell populations at 0, 1, 3 and 6 wpi. Bar graphs at the top of each heatmap show cumulative signaling strengths per cell population. Bar graphs at the right of each heatmap show cumulative signaling strength per pathway. Pathways highlighted in yellow are specifically enriched at one time point. Pathways highlighted in magenta are enriched after injury. B Cumulative strengths of signaling pathways outgoing from or received by major cell types. C Circle plots represent cell-cell interaction strengths of signaling pathways outgoing from Neurons A, B and OPCs at 6 wpi. Arrow thickness is proportional to the cumulative strengths of all predicted interactions between clusters. D Chord diagrams showing intercellular communication of signaling pathways that are enriched in neurons after spinal cord injury (SCI). Arc diameters are proportional to cumulative signaling strengths. Bar graphs at the bottom of each chord diagram represent the relative contribution of different ligand-receptor pairs predicted for each signaling pathway. Source data are provided as a Source Data file.
Global and neuron-specific analyses of injury-induced signaling pathways
Global CellChat analysis revealed active signaling pathways in control, 1, 3 and 6 wpi SC tissues. Tracking the strengths of individual signaling pathways across time points indicated dynamic intercellular communication during SC regeneration (Figs. 2A and S2A). In addition to dynamic regulation of signaling pathways present in control SC tissues, 7 pathways were specifically activated at 1, 3 or 6 wpi relative to uninjured controls. Globally-induced signaling pathways included chemokine signaling (CXCL), cell adhesion signaling networks (FN1 and ESAM), extracellular matrix (ECM) and growth factor mediated signaling networks (SEMA6 and PDGF) and developmental signaling pathways (FGF) (Figs. 2A and S2A).
To identify molecular pathways that account for elevated neuron signaling, we analyzed pathways predicted to be outgoing from or received by neurons A and B (Figs. 2D and S2C). Including both incoming and outgoing signaling, 32 to 35 pathways were predicted to be activated by neurons between 0 and 6 wpi (Fig. S2C). In addition to homeostatic signaling pathways increasing in strengths after injury, 5 injury-induced pathways were either activated or received by neurons after injury (FGF, WNT, PDGF, FN1 and SEMA6) (Fig. 2D). SCI triggered reactivation of growth factor and developmental signaling, substantiating FGF, WNT and PDGF as regulators of neuron survival, axon regrowth and synapse formation23,55,56,57,58,59,60,61,62,63. Specifically, neurons B are a major source of FGF18, which is predicted to interact with multiple FGFR1/2/3-expressing cells. On the other hand, neurons A were predicted recipients of WNT signaling, whereas both neurons A and B were at the receiving end of PDGF signaling. Ligand-receptor interactions involve WNT7A-FZD2/6/LRP5 and PDGFA-PDGFRB, whereby glia/ERG- and OPC-derived WNT7A and PDGFA are received by FZD2/6/LRP5- and PDGFRB-expressing neurons. Highlighting the requirement for extracellular matrix (ECM) remodeling and signaling to enact a pro-regenerative response64,65,66,67, multiple cell types including neurons A express Fibronectin 1 (FN1), which was predicted to direct strong interactions with SDC4-, ITGAV-, ITGA5- and ITGB1-expressing cells. Conversely, glia/ERGs are a major source of outgoing SEMA6A signaling, received by PLXNA4-expressing neurons to regulate axon guidance11,68,69,70,71. These studies revealed complex multicellular communication networks that coordinate signaling between neurons, glia and non-neural injury-responding cells during SC repair. Further validation and mechanistic insights into these signaling pathways are required to understand their exact function during SC regeneration.
Neuronal E/I balance is restored during successful spinal cord repair
To explore the cellular dynamics that support neuronal repair and functional recovery after SCI, we examined neuronal cell types and states prior to and after injury. Subcluster analysis identified 30 distinct neuron clusters between 0 and 6 wpi (Figs. 3A, S3A, B and Supplementary Data 5). Balanced E/I transmission is a central feature of CNS function as altered E/I balance results in severe behavioral defects in humans72. Despite eliciting features of cellular regeneration, SCI in mouse neonates causes impaired locomotor recovery due to imbalanced E/I73,74,75. With zebrafish recovering swim function after SCI, we postulated that balanced E/I transmission is a hallmark of functional SC repair in zebrafish. To test this hypothesis, we first performed a bioinformatic characterization of the excitatory and inhibitory properties of the neuron clusters identified in our dataset (Fig. 3B). Gene expression for classical markers of glutamatergic (slc17a6a, slc17a6b), GABA/glycinergic (slc6a5, gad1a, gad1b, gad2), cholinergic (chata, slc5a7a), serotonergic (slc6a4a, tph2) and catecholaminergic (slc6a2) neurons were analyzed76 (Fig. S3C). Neuron subclusters were then grouped as excitatory, inhibitory or ‘Other’ based on the expression profiles of neurotransmitter-related genes (Figs. 3B and S3C−E). The proportions of excitatory (30%) was lower than inhibitory (56%) neurons in uninjured SC tissues (Fig. 3C), resulting in a baseline E/I ratio of 0.5 (Fig. 3D). in silico calculation of E/I ratios suggested an imbalance toward an excitatory phenotype at 1 wpi and recovery to baseline by 6 wpi (Fig. 3D). We note that our in silico E/I ratio estimates did not allow for statistical testing. Moreover, our snRNA-seq data lacks spatial information and includes tissue portions that are both proximal and distal to the lesion, ostensibly diluting E/I changes localized proximal to the lesion. Nonetheless, these studies prompted us to calculate E/I ratios in vivo.
A UMAP plot showing 30 neuron subclusters identified with 0.6 resolution parameter. B Classification of neuron clusters based on neurotransmitter properties. Clusters enriched for at least one of the glutamatergic and GABA/glycinergic markers are identified as excitatory and inhibitory, respectively. Clusters that express serotonergic, cholinergic or a mix of glutamatergic and glycinergic markers are labeled as “Other”. One neuron cluster that does not express any of these marker genes is labeled “Unidentified”. C Pie charts show the proportions of excitatory, inhibitory and “Other” neuron populations at 0, 1, 3 and 6 wpi. D in silico calculation of E/I ratios during SC regeneration. E, F Immunostaining for RFP, GFP, HuC/D and Hoechst in SC cross sections from Tg(vglut2a:RFP) and Tg(gad1b:GFP) zebrafish at 0, 1, 3 and 6 wpi. White arrows point to RFP+ HuC/D+ neurons in E and GFP+ HuC/D+ neurons in (F). Insets depict double channels for RFP and HuC/D in E and for GFP and HuC/D in (F). Single channel insets of vglut2a:RFP or gad1b:GFP are shown in dorsal and ventral SC domains, respectively. G, H Quantification of glutamatergic and GABAergic neurons after SCI. Percent vglut2a+ HuC/D+ neurons (ANOVA p: 0.0086) and gad1b+ HuC/D+ neurons (ANOVA p: 0.0043) were normalized to the total number of HuC/D+ neurons. I in vivo quantification of E/I ratios during SC regeneration. Ratios of glutamatergic to GABAergic neurons were calculated (ANOVA p: 0.0268). Data points in all bar charts indicate individual animals and sample sizes are indicated in parentheses. SC cross sections 450 µm rostral to the lesion were analyzed. Brown-Forsythe and Welch ANOVA were performed in (G, H) with Dunnett’s T3 multiple comparisons performed across time points with 95% CI. Ordinary one-way ANOVA with Tukey multiple comparisons was performed in (I). All statistical tests are two-sided. In all bar charts, data are presented as mean values +/- SEM. *p ≤ 0.05, **p ≤ 0.01; Scale bars, 50 µm. Source data are provided as a Source Data file.
To validate our bioinformatic E/I balance calculations, we generated dual transgenic reporter fish that simultaneously label glutamatergic excitatory neurons (vglut2a:RFP) and GABAergic inhibitory neurons (gad1b:GFP)77. Complete SC transections were performed on double transgenic animals and SC tissues were collected for histological examination at 0, 1, 3 and 6 wpi (Fig. 3E, F). Absolute numbers and relative proportions of RFP, GFP and HuC/D expressing neurons were quantified in SC cross sections 450 μm rostral to the lesion to evaluate the E/I landscape during regeneration. Compared to control SC tissues, the profiles of vglut2a + excitatory neurons (vglut2a + HuC/D+) doubled in proportion at 1 wpi and were maintained near 1 wpi levels until 6 wpi (Figs. 3G and S3F). On the other hand, the profiles of gad1b+ inhibitory neurons (gad1b+ HuC/D+) increased by 50% at 6 wpi relative to controls (Figs. 3H and S3G). Notably, the absolute numbers of HuC/D+ neurons drastically increased at 1 and 3 wpi, indicating extensive neuronal remodeling during SC regeneration (Fig. S3H). Thus, consistent with our snRNA-seq findings, in vivo studies confirmed an early surge in excitatory neurons at 1 wpi and a recovery of the E/I balance toward baseline levels by 6 wpi (Fig. 3I).
Neurogenesis of excitatory and inhibitory neurons after spinal cord injury
We explored the cellular bases behind the recovery of E/I balance during zebrafish SC repair. The observed E/I ratio changes could be due to sequential neurogenesis of excitatory then inhibitory neurons after SCI. Alternatively, E/I ratios could reflect plasticity and changes in the neurotransmitter properties of pre-existing neurons. To distinguish between these possibilities, we first performed a cell proliferation assay. Dual transgenic fish for vglut2a:RFP and gad1b:GFP were subjected to SCI and a single EdU pulse for 24 h prior to SC tissue collection. SC tissues were harvested at 1, 3 and 6 wpi for co-labeling with the proliferation marker EdU (Figs. 4A, B and S4A). SC tissues from uninjured animals that received a single EdU injection for 24 h were harvested as controls. In striking manifestation of the regenerative capacity of zebrafish SC tissues, EdU incorporation was markedly elevated at 1 wpi relative to 0, 3 and 6 wpi (Fig. S4B, C). The proportions and absolute numbers of newly generated glutamatergic neurons (vglut2a+ EdU+) peaked at 1 wpi (Figs. 4C and S4D, E), supporting a surge of regenerating excitatory neurons after injury. As the profiles of GABAergic neurons showed a gradual increase up to 6 wpi (Figs. 3G, H and S4F) and due to low numbers of EdU+ cells at 3 and 6 wpi, we were unable to evaluate newly generated GABAergic neurons with a single EdU pulse (Figs. 4D and S4F, G). To overcome this limitation and evaluate the numbers of regenerating glutamatergic and GABAergic neurons after SCI, we performed SC transections on vglut2a:RFP and gad1b:GFP dual transgenic fish followed by daily EdU injections for 1 or 3 wpi (Figs. 4E, F and S4H). Uninjured control animals received daily EdU injections for 1 week prior to SC tissue collection. Compared to single EdU pulses, daily EdU labeling allowed us to estimate the total and cumulative profiles of regenerating neurons. To account for anatomical differences along the proximo-distal axis of lesioned SC tissues, RFP, GFP and EdU expressing cells were quantified from SC sections 150, 450 and 750 μm rostral to the lesion. The numbers and proportions of EdU+ cells sharply increased at 1 wpi and showed less pronounced additive EdU incorporation at 3 wpi (Fig. S4I, J). The profiles of newly formed glutamatergic (EdU+ vglut2a+) neurons markedly increased at 1 and 3 wpi relative to controls (Fig. 4G and S4K). The profiles of regenerating GABAergic (EdU+ gad1b+) neurons were significantly elevated at 1 wpi compared to controls, and continued to increase at 3 wpi compared to 1 wpi (Fig. 4H and S4L). As proximal tissue sections (150 μm from lesion) showed elevated neurogenesis across time points, differential dynamics of glutamatergic and GABergic neuron regeneration were most prominent in more distal SC sections (750 μm from lesion) (Fig. 4G, H). At these levels, the profiles of newly formed glutamatergic (EdU+ vglut2a+) neurons sharply increased at 1 wpi and stabilized between 1 and 3 wpi (Fig. 4G), whereas GABAergic (EdU+ gad1b+) continuously increased from 1 to 3 wpi (Fig. 4H). These in vivo studies showed glutamatergic neurogenesis precedes a continuous and slower regeneration timeline for GABAergic neurons, suggesting that sequential neurogenesis of different neuron subtypes around the lesion accounts for the dynamic changes in E/I properties during zebrafish SC regeneration.
A Experimental timeline to assess cell proliferation. SC cross-sections 450 µm rostral to the lesion were used for quantification. B Staining for RFP, GFP, EdU and Hoechst in Tg(vglut2a:RFP; gad1b:GFP) zebrafish at 0, 1, 3 and 6 wpi. Insets marked by dotted lines represent dual channels for RFP and EdU or for GFP and EdU. Yellow arrowheads indicate vglut2a+ EdU+ neurons. Green arrowheads indicate gad1b+ EdU+ neurons. C, D Quantification of vglut2a+ EdU+ neurons (ANOVA p < 0.0001) and gad1b+ EdU+ neurons (ANOVA p: 0.0449) at 0, 1, 3 and 6 wpi. Percent cells were normalized to the total number of nuclei in SC sections. E Experimental timeline to assess the cumulative profiles of regenerating neurons at 1 and 3 wpi. Tissue sections 150, 450 and 750 μm rostral to the lesion were analyzed. F Staining for RFP, GFP, EdU and Hoechst in Tg(vglut2a:RFP; gad1b:GFP) zebrafish at 0, 1 and 3 wpi. Insets marked by dotted lines represent dual channels for RFP and EdU or for GFP and EdU. Yellow arrowheads indicate vglut2a+ EdU+ neurons. Green arrowheads indicate gad1b+ EdU+ neurons. G, H Quantification of regenerating glutamatergic neurons (vglut2a+ EdU+) (ANOVA p < 0.0001) and regenerating GABAergic neurons (gad1b+ EdU+) (ANOVA p < 0.0001). For each section, percent cells were normalized to the number of vglut2a+ neurons in (G) and to gad1b+ neurons in (H). Data points in all bar charts indicate individual animals and sample sizes are indicated in parentheses. Brown-Forsythe and Welch ANOVA were performed in (C, D) with Dunnett’s T3 multiple comparisons performed across time points with 95% CI. Two-way ANOVA was performed in (G, H) with Tukey’s multiple comparisons with 95% CI. In all bar charts, data are presented as mean values +/- SEM. All statistical tests are two-sided. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. Scale bars, 50 µm. Source data are provided as a Source Data file.
Identification of injury-responsive iNeurons during early stages of spinal cord repair
To delve into the distribution of specific neuronal populations prior to and after SCI, we calculated the numbers and relative proportions of each neuron subcluster between 0 and 6 wpi (Figs. 5A, 3A and S3A, B). We also applied established marker genes from zebrafish larval neurons17 onto our marker scoring algorithm, identifying 7 major neuron subpopulations with varying cellular dynamics during regeneration (Figs. 5B, S5A−C and Supplementary Data 5, 6). Out of 30 neuronal clusters identified in our integrated dataset, cell proportions decreased >2-fold in 9 clusters (N0, 5, 10, 14, 15, 24, 25, 28 and 29), representing interneurons, V0, V2a interneurons, progenitor-like neurons and motor neurons. On the other hand, 4 neuronal clusters expanded >2-fold after SCI (N18, 20, 23 and 27) (Fig. 5A). The numbers and proportions of N18 neurons increased 2.5-fold at 3 wpi and returned to control levels at 6 wpi (Fig. 5A). Consistent with delayed GABAergic neurogenesis (Figs. 3 and 4), Cluster N18 cells were identified as GABAergic V2b interneurons (Figs. 3B, 5B and S5A). The profiles of N20 neurons sharply increased 6.7-fold at 1 wpi compared to all other time points. Intriguingly, N20 was the only neuron cluster corresponding to neuroblast-like neurons, suggesting a pro-regenerative role for N20 neurons after SCI (Figs. 5A, B and S5A). Yet, the exact identities and role of N20 during SC repair are unknown. N23 neurons were injury-induced, accounting for 2% of total neurons at 1 wpi and decreasing to 0.6% of neurons at 6 wpi (Fig. 5A). N23 neurons were identified as motor neurons, which regenerate via neurogenesis in adult zebrafish20. N27 neurons increased 2.9-fold at 1 wpi compared to other time points and were identified as serotonergic interneurons (Figs. 3B and 5B). We conclude that cluster N27 corresponds to intraspinal serotonergic neurons, which promote excitatory axon regrowth and coordinated locomotor recovery in zebrafish78. This analysis revealed known and unanticipated neuronal dynamics during innate SC repair.
A Dynamics of neuronal subclusters at 0, 1, 3 and 6 wpi. Cell proportions for each cluster and time point were normalized to the total number of neurons analyzed at that time point. Clusters that either increased or decreased by >2-fold in proportion are highlighted in red and blue, respectively. B UMAP plot showing the predicted cluster identity for each neuron subpopulations. V0, V2a, V2b, interneurons, motor neurons are shown along with clusters identified as neuroblast-like or progenitor-like neurons. Cluster N20 was the only cluster identified as neuroblast-like neurons. C UMAP plot showing a calculated “Regeneration Score” for each neuron subclusters. -log10(p value) of each cluster is represented in a gradient scale of gray (min = 0) to red (max = 6). Cluster N20 showed the highest regeneration score compared to all other neurons. D Feature plots show the expression of the regeneration associated genes gap43 and atf3 in cluster N20. E Gene ontology of N20 iNeuron DE markers. Twenty of the most enriched terms are shown. X-axis represents −log10(p value) for each term. F Circle plots represent cell-cell interaction strengths at 1 wpi. Arrow thickness is directly proportional to the cumulative strengths of all predicted interactions between clusters. G Signaling pathways outgoing from or received by iNeurons at 1 wpi. Bar graphs at the top of each heatmap show cumulative signaling strengths per cell population. Bar graphs at the right of each heatmap show cumulative signaling strength per pathway. One-tailed hypergeometric test is performed in (C, E). Source data are provided as a Source Data file.
To estimate the regenerative potential of spinal neurons after SCI, we assigned a “regeneration score” to individual neuron clusters by cross referencing the DE markers for each cluster with a compiled list of regeneration associated genes (RAGs). RAGs were defined based on their association with the term “regeneration” in the Amigo database (Supplementary Data 7). Strikingly, compared to all other neuron subtypes, cluster N20 showed the highest regeneration score (Figs. 5C and S5D) and expressed known RAG genes including gap43 and atf3 (Fig. 5D)79,80,81,82,83,84,85,86,87,88. Gene ontology comparisons showed that terms associated with neuron development, synaptic organization and axon extension were enriched in N18, 20, 23 and 27 (Fig. S5E). However, RAG enrichment was most pronounced and significant for cluster N20 (Fig. S5E). GO terms enriched in N20 neurons included neuron development, axon guidance and vesicle-mediated transport (Fig. 5E). We propose that cluster N20 represents a transient and unique population of injury-responsive neurons (iNeurons) that are likely to contribute to functional SC repair.
iNeurons are signaling hubs during spinal cord regeneration
To evaluate the role of N20 iNeurons during SC regeneration, we determined the extent and identities of the signaling pathways that are initiated from and received by iNeurons using CellChat. To specifically identify iNeurons (N20 cells) within the rest of the SC microenvironment, we first tracked iNeurons in the integrated dataset that includes all SC cells (Fig. S5D). Cells from clusters 4 and 5 from of the integrated dataset accounted for the majority of iNeurons and were renamed iNeurons for CellChat analysis. Compared to the global cell-interactions derived from the integrated dataset, the cumulative and reciprocal signaling strengths were highest between iNeurons and OPCs at 1 wpi (Fig. 5F). The cumulative strengths of outgoing and incoming signaling were markedly lower in the rest of the neuronal cells compared to iNeurons (Fig. 5F). These findings suggested that iNeuron signaling accounts for the majority of neuronal signaling at 1 wpi.
To examine the molecular features of the crosstalk between iNeurons and OPCs, we identified major signaling networks that are either incoming or outgoing from iNeurons (Figs. 5G and S5F, G). CellChat analysis showed iNeurons have a high probability to transduce NCAM1/2 and NRXN3 signaling to OPCs and other neurons. TIGIT, NRXN1/2, CDH4 and CADM1/3 were also predicted as incoming and outgoing iNeuron signals. Consistent with the ontology of iNeuron expressed genes, we observed an enrichment for reciprocal Neuregulin (NRG)-ERBB signaling from iNeurons to OPCs. ErbB signaling was recently shown to regulate OPC transformation and spontaneous remyelination after SCI in mice89. We found NRG1 and NRG2 ligands are likely to activate of the ERBB3/4 receptor mediated signaling between iNeurons and OPCs (Fig. S5F). These results showed iNeurons are potent centers of molecular signaling during SC regeneration and suggested that reciprocal signaling between iNeurons and OPCs is a principal feature of innate SC repair.
in vivo mapping of iNeurons during SC regeneration
To validate the emergence of an iNeuron transcriptional signature in regenerating SC tissues, we performed HCR in situ hybridization for select markers that are uniquely expressed in iNeurons compared to all other spinal cell types in the integrated dataset (Fig. S6A). To enable differential labeling of regenerating and pre-existing neurons, wild-type fish were subjected to SCI and daily EdU injections. syt11b and vamp4 in situ hybridization and staining for HuC/D and EdU were performed at 0, 1 and 3 wpi (Fig. 6A−F). To test whether iNeuron markers are preferentially expressed in neurons, integrated densities of in situ hybridization signals were quantified in total SC tissues and in HuC/D+ neurons (Fig. 6A−F). At 1 wpi, synaptotagmin XI b (syt11b) expression increased 3.6-fold in total SC tissues and 4.3-fold in HuC/D+ neurons (Fig. 6B, C). syt11b expression was reduced to wild-type levels by 3 wpi (Fig. 6B, C). The numbers of syt11b+ cells were also significantly increased at 1 wpi and accounted for 14.5% of HuC/D+ neurons at that timepoint (Fig. S6B, C). vesicle-associated membrane protein 4 (vamp4) transcripts were upregulated at 1 and 3 wpi, with 2.3- to 2.5-fold increase in total SC tissues and HuC/D+ neurons at 1 wpi (Fig. 6E, F). The syt11b paralog, syt11a, was not identified as a unique iNeuron marker. However, as zebrafish paralogs have been shown to play redundant functions upon genetic perturbation18,90,91, we examined the expression of syt11a after SCI (Fig. S6D−H). Like syt11b, syt11a transcripts were upregulated in neurons after injury. However, unlike syt11b, syt11a expression was not restricted to neurons including expression in cells surrounding the central canal at 1 wpi (Fig. S6D−H). These findings validated syt11b and vamp4 as markers of zebrafish iNeurons during SC regeneration.
A syt11b HCR in situ hybridization and staining for HuC/D, EdU and Hoechst. SC cross sections from wild-type zebrafish subjected to SC transections and daily EdU injections are shown. Control SCs received daily EdU injections for 7 days before collection. Dotted lines delineate central canal edges. B, C syt11b integrated density was calculated in whole SC sections (ANOVA p: 0.0007) and in HuC/D+ neurons (ANOVA p: 0.0009). D vamp4 HCR in situ hybridization and staining for HuC/D, EdU and Hoechst were performed as described in (A). E, F vamp4 integrated density was calculated in whole SC sections (ANOVA p-value: 0.0012) and in HuC/D+ neurons (ANOVA p: 0.0043). G Numbers of iNeurons and proportions of EdU+ iNeurons at 0, 1 and 3 wpi. Statistical analysis was performed for iNeuron numbers (gray) (ANOVA p: 0.0040) and the proportions of EdU+ iNeurons (maroon) (ANOVA p: 0.1403). H Numbers of newborn neurons and proportions of syt11b+ iNeurons at 0, 1 and 3 wpi. Statistical analysis was performed for newborn neurons numbers (gray) (ANOVA p < 0.0001) and the proportions of syt11b+ iNeurons (maroon) (ANOVA p: 0.1403). I syt11b HCR in situ hybridization in Tg(vglut2a:RFP; gad1b:GFP) zebrafish at 0, 1 and 3 wpi. Violet and yellow arrowheads indicate vglut2a+ syt11b+ neurons and gad1b+ syt11b+ neurons, respectively. J Quantification of syt11b transcripts in vglut2a+ and gad1b+ neurons (ANOVA p < 0.0001). For all quantifications, SC cross sections 450 µm rostral to the lesion were analyzed. Data points in all bar charts indicate individual animals and sample sizes are indicated in parentheses. Brown-Forsythe and Welch ANOVA were performed in (B, C, E, F, G and H) with Dunnett’s T3 multiple comparisons performed across different time points with 95% CI. Two-way ANOVA was performed in (J) with Tukey’s multiple comparisons with 95 % CI. All statistical tests are two-sided. In all bar charts, data are presented as mean values +/- SEM. ns; p > 0.05, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. Scale bars, 50 µm. Source data are provided as a Source Data file.
iNeurons are surviving neurons that elicit spontaneous plasticity after injury
The emergence of an iNeuron transcriptional profile at 1 wpi raised a central question related to their cell of origin and pro-regenerative signature. We postulated that iNeurons could either represent newly formed regenerating neurons, or a population of spontaneously plastic neurons that survive injury and support immediate repair. To distinguish between these possibilities, we quantified the proportions of iNeurons that express syt11b, HuC/D and EdU (Fig. 6G, H). In these experiments, HuC/D+ EdU+ cells represent regenerating neurons, HuC/D+ EdU- cells represent pre-existing neurons and syt11b expression was used to mark iNeurons. We first evaluated the extent of EdU incorporation within the iNeuron population (Fig. 6G). This analysis confirmed a transient and significant increase in iNeurons at 1 wpi compared to 0 or 3 wpi. However, at 1 wpi, regenerating neurons accounted for only 9% of iNeurons (Fig. 6G). To validate these results, we examined the emergence of iNeurons among regenerating neurons (Fig. 6H). Expectedly, the profiles of newborn neurons continuously increased from 0 to 1 then 3 wpi, indicating potent neurogenic responses are activated during innate SC repair (Fig. 6H). However, at 1 wpi, syt11b labeled only 6.4% of regenerating neurons (Fig. 6H). These results indicated that over 90% of iNeurons are pre-existing neurons that survive SCI.
Since the majority of iNeurons are pre-existing in control SC tissues, we examined whether iNeurons represent a specific population of neurons with elevated survival capacity or spontaneous plasticity. To address this question, we performed syt11b HCR in situ hybridization on vglut2a:RFP and gad1b:GFP dual transgenic fish, which simultaneously label glutamatergic and GABAergic neurons (Fig. 6I, J). Neuron subtypes not labeled with vglut2a:RFP or gad1b:GFP were labeled as “other”. The integrated density of syt11b transcript signals broadly increased at 1 wpi (Fig. S6I), including upregulated expression in vglut2a + and gad1b+ neurons (Fig. S6J, K). Notably, only 37.5% and 20.7% of syt11b transcripts co-localized to glutamatergic and GABAergic neurons at 1 and 3 wpi, indicating that “other” neuron subtypes express the majority of syt11b during regeneration (Fig. 6J). We also performed syt11b HCR in situ hybridization along with Hb9, isl1:GFP or Pax2 staining (Fig. S6L), and found that 30-40% of Hb9+, isl1:GFP+ and Pax2+ neurons expressed syt11b at 1wpi (Fig. S6M). On the other hand, Hb9+, isl1:GFP+ and Pax2+ iNeurons accounted for 6.8, 13, and 16.8% of iNeurons, respectively (Fig. S6M). These findings indicated that iNeurons do not represent a specific neuron subtype and suggested that multiple zebrafish neurons survive SCI and elicit an iNeuron transcriptional and regenerative signature during early stages of SC repair.
iNeurons are integrated in the spinal circuitry after spinal cord injury
We performed two sets of tracing experiments to trace axons projecting across the lesion using either rostral (Fig. 7A−C) or caudal labeling (Fig. S7A−C) with Biocytin at 1 wpi. For rostral labeling, Biocytin-soaked gelfoam was applied at the brainstem level at 1 wpi and 3 h prior to SC tissue collection. In addition to Biocytin, syt11b HCR in situ hybridization and HuC/D staining were performed for simultaneous labeling of iNeurons and total neurons, respectively (Fig. 7A). We found that 28−33% of HuC/D+ neurons between 450 and 750 μm caudal to the lesion were labeled (Fig. 7B), indicating integration of caudal neurons into the spinal circuitry as early as 1 wpi. 20−38% of rostrally-labeled caudal neurons (Biocytin+ HuC/D+) expressed syt11b (Biocytin+ HuC/D+ syt11b+) (Fig. 7C), suggesting syt11b+ iNeurons are part of the integrated SC circuitry at 1 wpi. To validate these results, we also applied Biocytin caudal to the lesion at 1 wpi (Fig. S7A). Caudal tracing labeled 18−20% of HuC/D+ neurons 450 and 750 μm rostral to the lesion (Biocytin+ HuC/D+) (Fig. S7B), with ~40% of these neurons expressing syt11b (Biocytin+ HuC/D+ syt11b+) (Fig. S7C). These studies confirmed that iNeurons establish connections within the regenerating SC circuitry as early as 1 wpi.
A−C Rostral-to-caudal neuronal tracing at 1 wpi. Biocytin was applied 4 mm rostral to the lesion and co-labeled with syt11b and HuC/D. SC cross sections 750 μm caudal to the lesion are shown. Dotted lines delineate central canal edges. Biocytin-labeled neurons were normalized to total neurons in (B). Biocytin-labeled iNeurons were normalized to traced neurons in (C). D Pipeline for CRISPR/Cas9 mutagenesis of iNeuron marker genes and regeneration assessment. E Swim endurance in CRISPR/Cas9 targeted animals at 4 wpi. Uninjected control siblings were used (ANOVA p < 0.0001). F Swim behavior in targeted animals at 4 wpi (ANOVA p < 0.0001). Swim distance was tracked under water current velocities of 0, 10 and 20 cm/s. G Glial bridging in targeted animals at 4 wpi. Gfap+ bridges are shown at the lesion site of atf3, vamp4 and syt11a/b crispants relative to controls. Percent bridging represents the cross-sectional area of the glial bridge at the lesion (0 µm) relative to the intact SC 750 µm rostral to the lesion (ANOVA p: 0.0049). H Anterograde axon tracing in targeted animals at 4 wpi. Biocytin was applied rostrally and analyzed 600 μm (proximal) (ANOVA p < 0.0001) and 1500 μm (distal) (ANOVA p < 0.0001) caudal to the lesion. Representative biocytin traces are shown at the proximal level. Axon growth was first normalized to biocytin 450 and 750 µm rostral to the lesion, and then to labeling in wild-type controls. Data points in all bar charts indicate individual animal and sample sizes are indicated in parentheses. Brown-Forsythe and Welch ANOVA were performed in (G) with Dunnett’s T3 multiple comparisons performed across different time points with 95% CI. Ordinary one-way ANOVA was performed in (E) with Dunnett’s multiple comparison with 95% CI. Two-way ANOVA with Tukey’s multiple comparisons (95% CI) was performed in (F, H). In all bar charts, data are presented as mean values +/- SEM. All statistical tests are two-sided. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. Scale bars, 50 µm. Source data are provided as a Source Data file.
iNeuron genes are required for functional recovery after spinal cord injury
To evaluate the roles of iNeuron marker genes during SC regeneration, we employed CRISPR/Cas9 mutagenesis, SCI and functional swim assays (Fig. 7D)12,91. Among the top DE markers of N20 iNeurons, 10 genes were filtered as unique iNeuron markers and chosen for mutagenesis (hdac10, mthfd1l, si:ch211-153j24.3, fhdc5, shootin3, syt11b, fam171b, chrnb2b, fblim1, vamp4) (Fig. S7D). atf3 was selected as a positive control79. In addition to targeting syt11b, we individually and combinatorially targeted the syt11a and syt11b paralogs to account for putatively redundant effects on regeneration. For efficient and simultaneous gene targeting in zebrafish, we used a recently adapted CRISPR/Cas9 protocol that achieves near complete mutagenesis in F0 injected adults12,91. CRISPR/Cas9 targeted animals (crispants) were raised to adulthood and mutagenesis rates were examined by capillary electrophoresis (Fig. S7E). Adult crispants with >90% mutagenesis were subjected to SCI, and swam against increasing water current velocities inside an enclosed swim tunnel to screen for functional regeneration defects. At 4 wpi, swim endurance was significantly reduced in atf3 and vamp4 crispants (Fig. 7E). Targeting syt11a and syt11b paralogs individually did not alter swim function. However, swim endurance was diminished in syt11a;syt11b dual crispants, indicating the syt11 paralogs were functionally redundant during SC repair (Fig. 7E). Importantly, overall growth and behavior were comparable between crispant fish and their control siblings prior to SCI, indicating the swim defects observed in these crispants are injury-induced. To validate swim capacity defects in iNeuron gene crispants, we tracked the swim behavior of atf3, vamp4 and syt11a;syt11b crispants under at 0, 10 and 20 cm/s current velocities (Fig. 7F). Compared to uninjected control siblings and in the absence of water flow, swim distance was 50−55% reduced in atf3 and syt11a;syt11b crispants and 25% reduced in vamp4 crispants (Fig. 7F). Swim distance was significantly lower in atf3, vamp4 and syt11a/b at 10 and 20 cm/s water current velocities (Fig. 7F). These results indicated the iNeuron markers atf3, vamp4 and syt11a/b are required for functional SC repair in zebrafish.
To date, cellular growth across the lesion has served as a primary readout of cellular regeneration in zebrafish. Glial bridging and axon tracing assays were performed to evaluate the extents of glial and axonal regeneration in iNeuron gene crispants. By Gfap immunostaining, the cross-sectional area of glial bridges was 65% reduced in atf3 crispants at 4 wpi, but was not significantly affected in vamp4 or syt11a;syt11b crispants compared to controls (Fig. 7G). At this time point, anterograde axon tracing using Biocytin showed comparable axon regrowth from hindbrain neurons into proximal and distal SC sections between vamp4, syt11a;syt11b and control animals (Fig. 7H). Consistent with their defective glial bridging, axon regrowth into the proximal and distal sections of caudal SC tissues was markedly impaired in atf3 crispants compared to controls (Fig. 7H). Axon regrowth was also significantly reduced in atf3 crispants compared to either vamp4 or syt11a;syt11b crispants (Fig. 7H). Thus, vamp4 and syt11a/b are required for functional SC repair but dispensable for cellular regeneration across the lesion.
These studies indicated iNeurons play essential regeneration-independent plasticity-based roles after SCI, and suggested vesicular trafficking is required for the recovery of local neuronal circuitry during innate SC repair.
Discussion
This study presents a single-cell atlas of innate SC repair in adult zebrafish, uncovers neurons as potent signaling hubs after SCI, identifies cooperative modes of regeneration- and plasticity-based neuronal repair, and shows essential roles for vesicular trafficking in spontaneous neuronal plasticity.
The single-cell dataset generated in this study offers a comprehensive resource to determine regenerative cell identities and mechanisms in highly regenerative vertebrates. The use of snRNA-seq is ideal to profile the majority of spinal cell types in zebrafish. Single-cell RNA sequencing is inherently biased against larger cell types such as neurons and astrocytes92,93,94. snRNA-seq reduces the challenges of cell dissociation, retrieving large numbers of neurons and glia. While dissection- and dissociation-induced gene expression changes in control samples present a longstanding difficulty for regeneration studies, our protocol minimizes background activation of injury-induced genes by flash-freezing SC tissues from individual animals during tissue collection. Finally, large numbers of nuclei were obtained by pooling frozen SC tissues, minimizing confounding problems associated with single-cell sequencing and allowing us to identify small cell populations93,94,95,96,97. Overall, this dataset faithfully represents the proportions and dynamics of major cell populations during zebrafish SC regeneration.
Candidate approaches have unveiled central roles for several signaling pathways during SC regeneration17,23,98,99,100. Yet, an integrated assessment of regenerative signaling pathways and cell-cell interactions were missing. Using CellChat analysis, our dataset identified a myriad of cell-specific and injury-induced signaling pathways52. Notably, comprehensive assessment of cell-cell signaling networks uncovered neurons as potent sources of signaling in homeostatic and lesioned SC tissues. Injury-induced neuronal signaling pathways include developmental pathways like FGF and WNT, extracellular matrix components like FN1, and axon guidance molecules like SEMA. Concerted activation of these pathways is instrumental for neuron survival, neurogenesis, axon regrowth, proper axon guidance and synapse targeting16,21,23,55,60,62,101. As CellChat analysis is limited to short-range autocrine or paracrine signaling pathways and is based on the expression of protein-coding transcripts, our analysis excludes long-range neuronal transmission and does not account for non-peptide neurotransmitters such as glutamate or GABA53. Thus, our findings that neurons in general, and specifically iNeurons, are hubs of intercellular signaling compared to other spinal cell types is independent of neurotransmission. Further studies of injury-induced pathways and neurotransmitter-mediated signaling will shed light into signaling pathways that direct regeneration- or plasticity-driven SC repair.
SC tissues in adult zebrafish retain potent progenitor cells with radial glial features and established contribution to neurogenesis after SCI16,20,21. SC regeneration is marked by early imbalance and late restoration of baseline E/I activity. Our data support a model in which excitatory neurons expand and repopulate regenerate SC tissues, while slower and continued neurogenesis of inhibitory neurons underlie the recovery of E/I balance at 6 wpi. These findings are consistent with published studies using a two-cut SCI model in adult zebrafish78, and with the observation that gad1b:GFP and vglut2a:RFP expression domains are mutually exclusive in our SC sections. Genetic lineage tracing showed excitatory to inhibitory neurotransmitter switching has adverse effects on functional recovery after adult mouse SCI75. In zebrafish, fast motor neurons were shown to upregulate glutamate expression after injury or exercise102. Although we cannot rule out similar mechanisms taking place during regeneration, we found that staggered increase in the profiles of newly generated excitatory and inhibitory neurons account for the recovery of E/I balance after SCI. We propose that in depth molecular evaluation of spinal progenitors is needed to better understand the potency and neurogenic capacities of spinal progenitors during SC regeneration.
While zebrafish is an established model of neuron regeneration, our study establishes zebrafish as a renewed model to understand and manipulate fundamental mechanisms of plasticity-based neuronal repair. Neurogenesis-based neural repair is thought to be unattainable in mammals. Instead, the mammalian SCI field has directed its efforts to develop plasticity-based repair strategies. We propose that pre-existing zebrafish neurons upregulate an iNeuron transcriptional signature that includes regeneration associated genes, and that iNeurons elicit spontaneous plasticity during early stages of SC repair. While our dual-grade, biocytin-mediated tracing experiments established the integration of iNeurons in the spinal circuitry at 1 wpi, finer tracing tools are needed for detailed mapping of iNeuron connectivity. The emergence of iNeurons after SCI is consistent with a recently identified population of embryonically derived dormant neurons that immediately respond to SCI in larval and adult zebrafish15. Similarly to the dormant precursor neurons reported by Vandestadt et al., more than 90% of iNeurons are not newly generated. Zebrafish iNeurons also share transcriptional similarities with a rare population of mouse spinal neurons that express regeneration associated genes including Atf3 and Sox11 after mouse SCI26. Cross-species transcriptional comparisons and follow-up in vivo studies are required to determine the extents of anatomical and functional similarities between these neuronal populations. Although the majority of iNeurons are injury-surviving neurons that acquire an iNeuron signature at 1 wpi, 9% of iNeurons incorporated EdU in our proliferation assays. Supporting the premise that a minor fraction of syt11b+ neurons are newborn, we noted that EdU+ iNeurons have smaller soma that is characteristic of newborn neurons and different from the majority of iNeurons. The origin, type and function of EdU+ iNeurons remain to be determined. At 1 wpi, iNeuron signaling accounts for the majority of total neuronal signaling, eliciting particularly strong interactions with OPCs. We propose that future zebrafish studies will contribute insights and applications into mechanisms of plasticity-driven neural repair.
Unbiased characterization of the molecular players that direct iNeuron functions after SCI showed multiple components of vesicular trafficking are required for functional SC repair in zebrafish. Syt11a/b and Vamp4 are vesicle-associated proteins that play different roles in neuronal synapses. Syt11 is a non-canonical SNARE that inhibits spontaneous neurotransmission and bulk endocytosis, whereas Vamp4 promotes Calcium-dependent excitatory neurotransmission release and bulk endocytosis103,104,105,106,107,108. Our CRISPR/Cas9 studies showed syt11a/b and vamp4 are required for the recovery of swim function after SCI in zebrafish. These findings are consistent with recent mammalian studies showing that inhibition of the presynaptic release machinery is important for axon regrowth109, and that Syt11 knockout in excitatory forebrain neurons impairs synaptic plasticity and memory108. While our data reveals vesicular trafficking is an essential mechanism that underlies spontaneous plasticity and functional recovery after SCI, future cell-specific manipulations that enable iNeuron lineage tracing or ablation are needed to better understand their plasticity and requirement during regeneration. We propose that zebrafish provide a valuable model to investigate the origin and function of iNeurons during SC regeneration, elucidate the role of vesicular trafficking in spontaneous plasticity, and identify new manipulations to promote plasticity after SCI.
Methods
Zebrafish
Adult zebrafish of the Ekkwill, Tubingen and AB strains were maintained at the Washington University Zebrafish Core Facility. All animal experiments were approved by Washington University School of Medicine Institutional Animal Care and Use Committee. Size matched (~2 cm) male and female animals of ~4 months of age were used. SC transection surgeries and regeneration analyses were performed in a blinded manner. For all experiments, 2 to 4 independent experimental replicates were performed using different clutches of animals. Within each experiment, experimental fish and control siblings of similar size and equal sex distribution were used. Transected animals from control and experimental groups were housed in equal numbers (4−7 fish) in 1.1-liter tanks. The following previously published zebrafish strains were used: Tg(vglut2a:RFP)77, Tg(gad1b:GFP)77 and Tg(isl1a:GFP)110.
Spinal cord transection
Zebrafish were anaesthetized in 0.2 g/L of MS-222 buffered to pH 7.0. Fine scissors (World Precision Instruments, NS-500376) were used to make a small incision that transects the SC 4 mm caudal to the brainstem region. Complete transection of the SC was visually confirmed at the time of surgery. Injured animals were also assessed at 2 or 3 dpi to confirm loss of swim capacity post-surgery.
Isolation of spinal cord nuclei from adult zebrafish
For nuclear isolation, a previously described method to isolate nuclei from mouse SC tissues was adapted to zebrafish SCs34. Three mm SC tissue, flanking the lesion site were collected from 50 adult zebrafish at time points 1, 3 and 6 wpi. Corresponding segments of 3 mm SC tissue were collected from uninjured control animals. For tissue lysis, the detergent mechanical lysis protocol described by Matson et al.34 was performed. SC tissues were homogenized at low setting for 15 s. Density gradient separation using sucrose solution was used to sediment the nuclei from the supernatant. Final nuclear lysates were resuspended using 100 µl of resuspension solution (1x PBS + 2% BSA + 0.2 U/µl RNase inhibitor - New England Biolabs, Cat#: M0314S). Hoechst staining was performed to assess the quality of isolated nuclei based on their shape. Samples, in which more than 70% of nuclei were scored as ‘healthy’, were submitted for single nuclear RNA-sequencing.
Single nuclear RNA-sequencing
For snRNA-seq, 30 µl of resuspension solution containing isolated nuclei at a concentration of ~1000 nuclei/µl was submitted to the Genome Technology Access Center in the McDonnell Genome Institute of Washington University. Two biological replicates were used for each time point at 0, 1, 3 and 6 wpi. cDNA was prepared after GEM generation and barcoding, followed by the GEM-RT reaction and bead cleanup steps. cDNA was amplified for 11−13 cycles then purified using SPRIselect beads. Purified cDNA samples were then run on a Bioanalyzer to determine cDNA concentration. GEX libraries were prepared as recommended by the 10x Genomics Chromium Single Cell 3’ Reagent Kits User Guide (v3.1 Chemistry Dual Index) with appropriate modifications to the PCR cycles based on the calculated cDNA concentration. For sample preparation on the 10x Genomics platform, the Chromium Next GEM Single Cell 3’ Kit v3.1, 16 rxns (PN-1000268), Chromium Next GEM Chip G Single Cell Kit, 48 rxns (PN-1000120), and Dual Index Kit TT Set A, 96 rxns (PN-1000215) were used. The concentration of each library was accurately determined through qPCR utilizing the KAPA library Quantification Kit according to the manufacturer’s protocol (KAPA Biosystems/Roche) to produce cluster counts appropriate for the Illumina NovaSeq6000 instrument. Normalized libraries were sequenced on a NovaSeq6000 S4 Flow Cell using the XP workflow and a 50 × 10 × 16 × 150 sequencing recipe according to manufacturer protocol. A median sequencing depth of 50,000 reads/cell was targeted for each Gene Expression Library.
Aligning snRNA-seq reads
After sequencing, the Illumina output was processed using the CellRanger (v6.0.0) recommended pipeline to generate gene-barcode count matrices. A custom reference genome was made with the “cellranger mkref” command, using the fasta file of zebrafish reference genome GRCz11 constructed from the Ensemble genome build (https://useast.ensembl.org/Danio_rerio/Info/Index) and the sorted Gene Transfer Format file (v4.3.2) from the improved zebrafish transcriptome annotation33. Base call files for each sample from Illumina were demultiplexed into FASTQ reads. Then, the “cellranger count” pipeline was used to align sequencing reads in FASTQ files to the custom reference genome. Both exon and intron sequences were included during the alignment. The filtered gene-barcode count matrices generated by “cellranger count” was used for downstream analysis.
Quality control
DecontX package35 (celda v1.16.1) was used to remove droplets containing aberrant counts of ambient mRNA. The function “decontx” was used on the raw “RNA” counts to obtain “decontX” count data. Contamination score was assigned to each cell after running “decontx”. Cells with contamination scores greater than 0.75 were filtered out and only cells with contamination scores less than 0.75 were included for downstream analysis. Furthermore, decontX counts were used as default for pre-processing and normalization. After ambient RNA removal, DoubletFinder36 (v2.0.3) was used to identify doublets formed from transcriptionally distinct cells. Optimal pK values were calculated from the outputs of function “paramsweep_v3”. Then, “doubletFinder_v3” function was used to predict doublet cells, where 50 principal components and pN value of 0.25 were given as input along with the previously calculated pK values (0.26). All the cells predicted as doublets were removed.
Integrated analysis of snRNA-seq dataset
Datasets were integrated and analyzed using Seurat (v4.1.1) package with R (v4.2.1)111,112. Each sample count matrix was filtered for genes that were expressed in at least 3 cells and cells expressing at least 200 genes, followed by cell quality assessment using commonly used QC matrixes113. Cells having a unique number of genes between 200 and 4000 and a mitochondrial gene percentage <5 were used for downstream processing. Each dataset was independently normalized and scaled using the “SCTransform” function, which is an improved method for normalization, that performs a variance-stabilizing transformation using negative binomial regression114. Standard integration workflow of Seurat was used to identify shared sources of variation across experiments as well as mutual nearest neighbors115,116. Integration features were selected based on the top 4000 highly variable features using “SelectIntegrationFeatures” function (nfeatures = 4000), which was used as input for the “anchor.features” argument of the “FindIntegrationAnchors” function. PCA analysis was performed on the 4000 variable features and the top 50 principal components selected based on the elbow plot heuristic, which measures the contribution of variation in each component. These 50 principal components were used in “FindNeighbors” and “FindClusters” functions to perform graph-based clustering on a shared nearest neighbor graph117,118. Louvain algorithm was used for modularity optimization in cell clustering using “FindClusters” function. The resolution parameter (res = 0.4) that determines the granularity of clustering was selected by visually inspecting clusters with resolutions ranging between 0.1 and 2.0 as well as clustree graphs119. Uniform Manifold Approximation and Reduction (UMAP) was used for non-linear dimensional reduction of the first 50 principal components and visualize the data using “RunUMAP” function120. Data was graphed using different plot functions, such as “DimPlot”, “VlnPlot”, “FeaturePlot”, “Dotplot” and “DoHeatmap”, to view the cell cluster identity and marker gene expression. Cell proportions were extracted using the “table” and “prop.table” functions. Differential gene expression for individual clusters was identified using Wilcoxon rank sum test in the “FindAllMarkers” function. Marker genes detected in at least 25% of the clustered cells and with a logFC threshold of 0.25 were selected. Only positive markers were reported.
Subset analysis of neuron clusters
Neuronal cells identified from the complete dataset were subclustered using the “subset” function for subcluster analysis. The neuron subset was again normalized and scaled using the “SCTransform” function with glmGamPoi method121. Fifty principal components were used and the resolution parameter was set to 0.6. Downstream analysis was done as described above for the integrated analysis. The top DE markers generated for each neuron subcluster using “FindAllMarkers” function is given in Supplementary Data 5.
Cluster identification using differentially expressed markers
A “CNS markers” database of previously published markers of the different cell types that comprise the vertebrate brain and/or SC tissues was compiled17,24,27,37,38,39,40,41,42,43,44,45,46,47,48,49,50. For each cell cluster, every marker gene identified as a top differentially expressed (DE) marker of that cluster was cross-referenced with our compiled VNM database (Supplementary Data 1) using our “DE-Marker-Scoring” algorithm122. For every matching marker gene, one point was given to the respective cluster under the column name with matching cell identity. Iteration over every marker gene was performed to generate a scoring matrix with varying points for each cluster against the different cell identities compiled in the VNM database (Supplementary Data 2, sheet: scoring). The “phyper” function in R was then used to calculate one-tailed binomial probabilities using hypergeometric distribution for the total score obtained from each cluster against each cell identity in the database (Supplementary Data 2, sheet: Binomial probability). −log10 of probability values were obtained for plotting the heatmap (Supplementary Data 2, sheet: −log10P). The resulting values were scaled from 0 to 100 and plotted as a heatmap using GraphPad prism. Each cluster was given an identity based on the maximum −log10 P score obtained in the heatmap. The top DE markers of clusters with ambiguous scores were manually searched in the literature40,41 (https://brainrnaseq.org/) to confirm cluster identity. “RenameIdents” function was used to assign identity to each cluster. To confirm the assigned cluster identities, enrichment of classical markers of respective cell types were tested using Dot plot.
Filtering differentially expressed markers for candidate gene selection
To identify candidate genes that are uniquely expressed in N20 iNeurons, top DE markers were cross-checked and filtered against the top DE markers of all other neuron subclusters. Enrichment and percent expression of these unique genes were then confirmed using Dot plots. Genes that were enriched or expressed in other neuronal or non-neuronal subclusters, in addition to their expression in iNeurons, were omitted. Genes that are exclusively enriched in iNeurons were selected to generate crispants.
Cell-cell interaction assay
The R package CellChat (v1.4.0) was used to evaluate regenerative cell-cell interactions after SCI52. CellChat models the probability of cell-cell communication by integrating our gene expression data with a database of known interaction between signaling ligands, receptors, and their cofactors (CellChatDB). CellChatDB is manually curated from literature-supported ligand-receptor interactions in human and mouse, only. Therefore, we converted the normalized RNA data matrix of our zebrafish dataset into human orthologues using Ensemble biomaRT package123. The following rules were applied while converting the data matrix: (1) one-to-one orthologue mapping was performed whenever possible, (2) For genes with one to several human orthologues, the corresponding zebrafish RNA data value was copied to every mapped gene in humans, (3) RNA data values were eliminated for zebrafish genes that did not have any human orthologue, (4) RNA data values of paralogous zebrafish genes were added and the cumulative data value was assigned to the human orthologue122.The converted RNA data was then used to create CellChat object using “createCellChat” function, followed by the recommended preprocessing functions with default parameters for the analysis of individual datasets. Truncated mean method with 10% trimmed observation was used to compute average gene expression per cell group for the complete dataset (Figs. 2 and S2). The default trimean method was used to compute average gene expression for the CellChat analysis of the neuron subset (Figs. 5 and S5). CellChatDB.human was used to infer cell-cell communication. All categories of ligand-receptor interactions in the database were used in the analysis. Communications involving less than 10 cells were excluded. The “netAnalysis_computeCentrality” function was used to calculate network centrality scores at each time point. Functions such as “netVisual_circle”, “netAnalysis_contribution”, “netVisual_aggregate”, “netVisual_bubble” and “netAnalysis_signalingRole_heatmap” were used to generate different plots used in this paper.
Trajectory inference analysis
Pseudotime analysis was performed using CellRank (v1.5.1) package in Python (v3.8.10)124,125. Before analysis, the Seurat object was converted to an AnnData in a two-step process using the R package SeuratDisk (v0.9020). First, the Seurat object was saved as an h5Seurat file and converted to an AnnData file, which was used as an input for pseudotime analysis using CellRank. Basic pre-processing of the AnnData file was performed with the default recommended settings using the scvelo (v0.2.4) and scanpy (v1.9.1) packages. Genes expressed in at least 10 cells were included and 30 principal components and neighbors were used for preprocessing. CytoTRACEkernel was used for pseudotime computation and direct KNN graph-edges to point into the direction of increasing differentiation status54. The transition matrix was calculated by setting the threshold_scheme parameter to “soft” to reconstruct cellular trajectories using VIA algorithm126. This transition matrix was projected onto a UMAP plot to obtain a velocity graph.
Gene ontology
Gene ontology analysis was performed using Metascape127. Input and analysis species were set as D. rerio. Express analysis was performed for gene ontology. Metascape identified all statistically enriched terms (including GO biological processes, Reactome gene set and KEGG pathway), and calculated accumulative hypergeometric p values and enrichment factors. Significant terms were hierarchically clustered into a tree based on Kappa-statistical similarities among their gene memberships. A kappa score of 0.3 was applied to cast the tree into term clusters. The most enriched term in each cluster was chosen as the representative term.
Regeneration score
Genes associated with the GO term “regeneration” in the database Amigo were identified. As a result, Regeneration Associated Genes (RAG) present in the following terms were included: axon extension involved in regeneration_GO_0048677, Axon regeneration_GO_0031103, dendrite regeneration_GO_0031104, Positive regulation of axon regeneration_GO_0048680, Positive regulation of neuron projection regeneration_GO_0070572, Regeneration_GO_0031099 (Supplementary Data 7). A regeneration score for each neuron cluster was calculated based on the DE expressed markers obtained through “FindAllMarkers” function. All upregulated marker genes detected in at least 25% of the clustered cells and with a logFC threshold of 0.25 were considered top DE markers. Our previously mentioned “DEMarkerScoring” algorithm was then used to cross-reference and score each cluster based on the similarity between Top DE markers and RAG. -log10P value was used to generate the UMAP plot with the regeneration score. Raw values are detailed given in Supplementary Data 5 (sheet: Cluster ID).
Histology
Sixteen µm cross cryosections of paraformaldehyde fixed SC tissues were used. Tissue sections were mainly imaged using a Zeiss LSM 800 confocal microscope (Axio Imager.Z2, Objective: Plan-Apochromat 20x/0.8 M27, Detector: Multialkali-PMT) for immunofluorescence and hybridization chain reaction (HCR) RNA in situ hybridization. For glial bridging and axon-regrowth assay (Fig. 7G, H), images were taken using Zeiss slide scanner (Axio Scan.Z1, Objective: Plan-Apochromat 20x/0.8 M27, Imaging device: OrcaFlash).
The HCR RNA in situ hybridization protocol was adapted from Molecular Instruments128,129. Briefly, tissue sections were hydrated in PBS, dehydrated stepwise into 100% ethanol, then rehydrated stepwise into PBT (0.1% Tween-20 in PBS). Sections were pre-treated either with 0.2% TritonX-100 in PBS for 5 min or boiled in Citrate Buffer (10 mM Citric Acid, 0.05% Tween-20, pH 6.0) for 10 min. For blocking, sections were incubated in Hybridization buffer (Molecular Instruments) for 1 h at 37 °C. For hybridization, sections were incubated in pre-warmed DNA probe sets diluted to 0.0015 pmol/uL in Hybridization buffer for 48 h at 37 °C. Washes were then performed with Wash Buffer (Molecular Instruments) at 37 °C followed by 5x SSCT (3 M NaCl, 0.3 M Sodium Citrate, 0.1% Tween-20, pH 7.0). For signal amplification, sections were incubated in Amplification buffer (Molecular Instruments) for 1 h at room temperature. Prior to amplification, h1 and h2 hairpins were snap-cooled in individual tubes by heating to 95 °C and allowing tubes to return to ambient temperature slowly. For amplification, h1 and h2 snap-cooled hairpins were mixed together and diluted 1:50 in Amplification buffer. Amplification proceeded overnight at room temperature in the dark. Samples were then washed in 5x SSCT, 5x SSC, and PBT at room temperature before proceeding to immunohistochemistry. HCR RNA probes used in this study (syt11a, syt11b, and vamp4) were designed using a python script. Probe sets were ordered as 50 pmol opools from IDT. Oligo sequences for each probe set are provided in Supplementary Data 9.
For immunohistochemistry, tissue sections were hydrated in PBT then treated with blocking agent (5% goat serum in PBT) for 1 h at room temperature. For nuclear antigens, sections were treated with 0.2% TritonX-100 in PBT for 5 min and washed thoroughly in PBT prior to the blocking step. Sections were incubated overnight at 4 °C with respective primary antibodies diluted in blocking agent, then washed in PBT, and treated for 1 h in secondary antibodies diluted in blocking agent at room temperature. For EdU staining, sections are incubated with freshly prepared EdU staining solution (100 mM Tris at pH 8.5 - SIGMA T6066, 1 mM CuSO4 – SIGMA C1297, 200 µM Fluorescent azide – Thermo Fischer Scientific and 100 mM Ascorbic Acid - SIGMA A5960) for 30 min in the dark at room temperature130. Following washes, sections were incubated in 1 µg/mL of Hoechst (Thermo Scientific, H3570) for 3 min, washed in PBT and mounted in Fluoromount-G mounting media (SouthernBiotech, 0100-01). Primary antibodies used in this study were chicken anti-GFP (AVES, GFP-1020, 1:1000), rabbit anti-dsRed (Takara, 632496, 1:250), mouse anti-HuC/D (Invitrogen, A21271, 1:500), Mouse anti-81.5C10 (MNR2/HB9/Mnx1, DSHB, 1:50), rabbit anti-Pax2 (BioLegend, 901002, 1:500). Secondary antibodies (Invitrogen, 1:250) used in this study were Alexa Flour 488, Alexa Flour 594, and Alexa Flour 647 goat anti-rabbit or goat anti-mouse antibodies.
Rostral and caudal axon tracing
Two sets of axon tracing experiments were performed at 1 wpi14. For rostral labeling, fish were anaesthetized using MS-222 and fine scissors were used to transect the cord 4 mm rostral to the lesion site. For caudal labeling, fish were anaesthetized using MS-222 and fine scissors were used to transect the cord 4 mm caudal to the lesion site. For both sets of tracing experiments, Biocytin-soaked Gelfoam Gelatin Sponge was applied at the new injury site (Gelfoam, Pfizer, cat# 09-0315-08; Biocytin, saturated solution, Sigma, Cat# B4261). Fish were euthanized 3 h post-treatment and Biocytin was histologically detected using Alexa Fluor 594-conjugated Streptavidin (Thermo Fisher, cat# S-11227). syt11b HCR in situ hybridization and HuC/D staining were performed on the same sections. The numbers of Biocytin+ HuC/D+ and Biocytin+ HuC/D+ syt11b+ neurons were calculated 150, 450 and 750 μm rostral and caudal to the lesion. The numbers of Biocytin+ HuC/D+ were normalized to HuC/D+ neurons to estimate the extent of labeling. The numbers of Biocytin+ HuC/D+ syt11b+ neurons were then normalized to Biocytin+ HuC/D+ neurons to estimate the extent of iNeuron integration in the labeled circuitry. We note that in the axon tracing experiments performed at 1 wpi (Figs. 7 and S7), we observed ectopic Biocytin labeling around the ependyma on the non-labeled side of the cord distal to the lesion site (caudal ependymal cells distal to the lesion were Biocytin+ following rostral tracing; rostral ependymal cells distal to the lesion were Biocytin+ following caudal tracing). This labeling is not usually observed by axon tracing at 4 or 6 wpi.
CRISPR/Cas9 mutagenesis
crRNA guide RNA sequences were selected using CHOPCHOP. Only sequences with no off-target sites with three or fewer mismatches elsewhere in the genome were selected. To maximize the effect of small indels, target sites were chosen that lie within essential domains (Supplementary Data 8). Alt-R tracrRNA and crRNA gRNAs (IDT, Cat# 1072534) were reconstituted using manufacturer’s specifications as 100 μM stocks, then annealed at a final concentration of 50 μM. Alt-R S.p. Cas9 nuclease V3 (IDT, Cat# 1081059) was diluted in Cas9 dilution buffer (1 M HEPES at pH 7.5, 2 M KCl) to a working concentration of 25 μM. Annealed dgRNA duplexes were diluted 1:1 in duplex buffer (IDT, Cat# 11-05-01-03) to a working concentration of 25 μM. Equal volumes of dgRNA were added to Cas9 protein and incubated at 37 °C for 5 min. For dual targeting of syt11a and syt11b, Cas9 protein was added in equal molar amounts to the total concentration of dgRNA. Tubingen wild-type embryos were injected with 1 nL of CRISPR/Cas9 solution at the one-cell stage and grown to adulthood for genotyping, SC surgeries and functional analysis. The full sequence for each guide RNAs is provided in Supplementary Data 8.
Genotyping by capillary electrophoresis
Capillary electrophoresis was used to calculate the indel frequency for each CRISPR/Cas9 target site. For DNA extraction, whole 4 dpf larvae or ~3 mm of excised adult tail fins were added to 20 μL (larvae) or 50 μL (adult fin) of fin lysis buffer (10 mM Tris at pH 7.0, 50 mM KCl, 1 mM EDTA at pH 8.0, 0.3% Tween-20, freshly added Proteinase K at 200 µg/ml). DNA samples were incubated at 55 °C for 60 min and then, followed by a 15-min incubation at 95 °C. 150−250 bp PCR products were generated using NEB Taq Polymerase (Cat# M0273) with gene-specific primers (Supplementary Data 8) in a volume of 10 μL in Pryme PCR semi-skirted PCR plates (MidSci, Cat# AVRT1). Samples were diluted to 24 μL in TE dilution buffer (Agilent, DNF-495-0060) and loaded into the 5200 Fragment Analyzer System (Agilent, Cat# M5310AA). Capillary electrophoresis was carried out using the Agilent Fragment Analyzer Qualitative DNA Kit (Cat# DNF-905-K1000) according to the manufacturer’s specifications.
To calculate indel frequency, 2 wild-type control siblings were PCR amplified. The size of wild-type products was determined using these control samples. PCR products with peaks within 1 bp of control amplicon size were termed wild types (non-indel). Indel frequency was calculated by dividing the signal intensity of total non-indel peaks by the cumulative intensity of all peaks in each sample. Because of significant noise due to primers (<100 bp) and non-specific products (>300 bp) in wild-type samples, only signals between 100 and 300 bp were used to calculate indel frequency.
Swim endurance assay
Zebrafish were exercised in groups of 8−15 in a 5 L swim tunnel device (Loligo, Cat# SW100605L, 120 V/60 Hz). After 5 min of acclimation (with no flow) inside the enclosed tunnel, fish were swum at 9 cm/s and 10 cm/s for 5 min each to evaluate their swimming capability at bare minimum velocity. Water current velocity was then increased every two minutes and fish swam against the current until they reached exhaustion. Exhausted animals were removed from the chamber without disturbing the remaining fish. Swim time at exhaustion was recorded for each fish.
Swim behavior assay
Zebrafish were divided into groups of 5 in a 5 L swim tunnel device (Loligo, Cat# SW100605L, 120 V/60 Hz). Each group was allowed to swim for a total of 15 min under zero to low current velocities (5 min at 0 cm/s, 5 min at 10 cm/s, and 5 min at 15 cm/s). The entire swim session was recorded using high-speed camera (iDS, USB 3.0 color video camera) with following settings: aspect ratio, 1:4; pixel clock, 344; frame rate, 70 frames/s; exposure time, 0.29; aperture, 1.4 to 2; maximum frames, 63,000. Movies were converted to 20 frames/s and analyzed using a customized Fiji macro122. For each frame, animals/objects >1500 px2 were identified, and the XY coordinates were derived for each animal/object. Each frame was independently analyzed, and animal/object tracking was completed using a customized R Studio script122. The script aligned coordinates, calculated swim metrics considering three separate frame windows (Frames 0−6000 at 0 cm/s; frames 6001−12,000 at 10 cm/s, and frames 12,001−18,001 at 20 cm/s). Distance swam was analyzed at 0, 10, and at 15 cm/s.
Axon regrowth assay
Fish were anaesthetized using MS-222 and fine scissors were used to transect the cord 4 mm rostral to the lesion site. Biocytin-soaked Gelfoam Gelatin Sponge was applied at the new injury site (Gelfoam, Pfizer, cat# 09-0315-08; Biocytin, saturated solution, Sigma, Cat# B4261). Fish were euthanized 3 h post-treatment and Biocytin was histologically detected using Alexa Fluor 594-conjugated Streptavidin (Thermo Fisher, cat# S-11227). Biocytin-labeled axons were quantified using the “threshold” and “particle analysis” tools in the Fiji software. Four sections per fish at 0.5 (proximal) and 2 (distal) mm caudal to the lesion core, and 2 sections 1 mm rostral to the lesion, were analyzed. Axon growth was normalized to the efficiency of Biocytin labeling rostral to the lesion for each fish. The axon growth index was then normalized to the control group for each experiment.
Glial bridging assay
Gfap immunohistochemistry was performed on serial cross sections. The cross-sectional area of the glial bridge and the area of the intact SC rostral to the lesion were measured using ImageJ software. Bridging was calculated as a ratio of these measurements.
Quantifications
Cell counting
Cell counting were performed using a customized Fiji script (adapting ITCN plugin: Image based Tool for counting nuclei122,). Orthogonal projections of individual image stacks were generated using Zen software. A customized Fiji script incorporated user-defined inputs to define channels (including Hoechst) and to outline SC perimeters. To quantify nuclei, the following parameters were set in ITCN counter: width – 15; minimal distance – 7.5; threshold – 0.4. Once nuclei were identified, user-defined thresholds of individual cell markers were used to mask the image and identify nuclei located inside the masked regions. X/Y coordinates were extracted for each nucleus for cell counting. Raw counts and X/Y coordinates from Fiji were processed using a customized R script122. Markers that share nuclei with the same X/Y coordinates were considered overlapping.
Quantifying HCR in situ hybridization signal
HCR in situ hybridization signals were quantified using a customized Fiji script122.Orthogonal projections of individual image stacks were generated using the Zen software (Zeiss). ROIs around SC borders were defined to exclude non-SC tissues and user-defined thresholds were set to create an inverted mask. Background noise was either manually corrected or defined by thresholding a dedicated, unstained background channel. Integrated density was quantified for fluorescence signals inside the quantification mask using the “Analyze Particles” command. To calculate the HCR in situ hybridization signal inside neurons, an inverted mask was created after thresholding HuC/D+ neurons, then added to the final quantification mask. Fluorescence signals inside this newly generated mask were considered neuron-specific.
For all statistical data, data points represent individual animals and N numbers are indicated. Statistical tests are reported in figure legends for each panel. Multiple comparisons were performed within groups and only significant observations were reported.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Both raw and processed files of the snRNA-seq data generated in this study are deposited in Gene Expression Omnibus under accession code GSE235395. Additionally, snRNA-seq datasets for both the integrated cell types and the neuron subset are available to exploring here: https://shinypath.wustl.edu/SCI_Atlas/. All other source data are provided as Source Data file with this paper. Source data are provided with this paper.
Code availability
All the scripts used in this study are available in the GitHub repository named Zebrafish_SCI_Atlas122.
References
Tran, A. P., Warren, P. M. & Silver, J. New insights into glial scar formation after spinal cord injury. Cell Tissue Res. 387, 319–336 (2022).
Brennan, F. H. & Popovich, P. G. Emerging targets for reprograming the immune response to promote repair and recovery of function after spinal cord injury. Curr. Opin. Neurol. 31, 334–344 (2018).
Fouad, K., Popovich, P. G., Kopp, M. A. & Schwab, J. M. The neuroanatomical-functional paradox in spinal cord injury. Nat. Rev. Neurol. 17, 53–62 (2021).
He, Z. & Jin, Y. Intrinsic control of axon regeneration. Neuron 90, 437–451 (2016).
Milich, L. M., Ryan, C. B. & Lee, J. K. The origin, fate, and contribution of macrophages to spinal cord injury pathology. Acta Neuropathol. 137, 785–797 (2019).
Sofroniew, M. V. Dissecting spinal cord regeneration. Nature 557, 343–350 (2018).
Park, K. K. et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 322, 963–966 (2008).
Anderson, M. A. et al. Required growth facilitators propel axon regeneration across complete spinal cord injury. Nature 561, 396–400 (2018).
DePaul, M. A., Lin, C. Y., Silver, J. & Lee, Y. S. Combinatory repair strategy to promote axon regeneration and functional recovery after chronic spinal cord injury. Sci. Rep. 7, 9018 (2017).
Nakamura, Y. et al. Modulation of both intrinsic and extrinsic factors additively promotes rewiring of corticospinal circuits after spinal cord injury. J. Neurosci. 41, 10247–10260 (2021).
Squair, J. W. et al. Recovery of walking after paralysis by regenerating characterized neurons to their natural target region. Science 381, 1338–1345 (2023).
Burris, B., Jensen, N. & Mokalled, M. H. Assessment of swim endurance and swim behavior in adult Zebrafish. J. Vis. Exp. 177, e63240 (2021).
Jensen, N. O. et al. Functional trajectories during innate spinal cord repair. Front. Mol. Neurosci. 16, 1155754 (2023).
Mokalled, M. H. et al. Injury-induced ctgfa directs glial bridging and spinal cord regeneration in zebrafish. Science 354, 630–634 (2016).
Vandestadt, C. et al. RNA-induced inflammation and migration of precursor neurons initiates neuronal circuit regeneration in zebrafish. Dev. Cell 56, 2364–2380.e2368 (2021).
Briona, L. K., Poulain, F. E., Mosimann, C. & Dorsky, R. I. Wnt/ss-catenin signaling is required for radial glial neurogenesis following spinal cord injury. Dev. Biol. 403, 15–21 (2015).
Cavone, L. et al. A unique macrophage subpopulation signals directly to progenitor cells to promote regenerative neurogenesis in the zebrafish spinal cord. Dev. Cell 56, 1617–1630.e1616 (2021).
Klatt Shaw, D. et al. Localized EMT reprograms glial progenitors to promote spinal cord repair. Dev. Cell 56, 613–626.e617 (2021).
Kuscha, V. et al. Lesion-induced generation of interneuron cell types in specific dorsoventral domains in the spinal cord of adult zebrafish. J. Comp. Neurol. 520, 3604–3616 (2012).
Reimer, M. M. et al. Motor neuron regeneration in adult zebrafish. J. Neurosci. 28, 8510–8516 (2008).
Saraswathy, V. M. et al. Myostatin is a negative regulator of adult neurogenesis after spinal cord injury in zebrafish. Cell Rep. 41, 111705 (2022).
Zhou, L. et al. Progenitor-derived glia are required for spinal cord regeneration in zebrafish. Development 150, dev201162 (2023).
Goldshmit, Y. et al. Fgf-dependent glial cell bridges facilitate spinal cord regeneration in zebrafish. J. Neurosci. 32, 7477–7492 (2012).
Yadav, A. et al. A cellular taxonomy of the adult human spinal cord. Neuron 111, 328–344.e327 (2023).
Yao, C. et al. Single-cell sequencing reveals microglia induced angiogenesis by specific subsets of endothelial cells following spinal cord injury. FASEB J. 36, e22393 (2022).
Matson, K. J. E. et al. Single cell atlas of spinal cord injury in mice reveals a pro-regenerative signature in spinocerebellar neurons. Nat. Commun. 13, 5628 (2022).
Milich, L. M. et al. Single-cell analysis of the cellular heterogeneity and interactions in the injured mouse spinal cord. J. Exp. Med. 218, e20210040 (2021).
Rodrigo Albors, A. et al. An ependymal cell census identifies heterogeneous and ongoing cell maturation in the adult mouse spinal cord that changes dynamically on injury. Dev. Cell 58, 239–255.e210 (2023).
Hou, J. et al. Heterogeneity analysis of astrocytes following spinal cord injury at single-cell resolution. FASEB J. 36, e22442 (2022).
Scott, K., O’Rourke, R., Winkler, C. C., Kearns, C. A. & Appel, B. Temporal single-cell transcriptomes of zebrafish spinal cord pMN progenitors reveal distinct neuronal and glial progenitor populations. Dev. Biol. 479, 37–50 (2021).
Ren, Y. et al. Ependymal cell contribution to scar formation after spinal cord injury is minimal, local and dependent on direct ependymal injury. Sci. Rep. 7, 41122 (2017).
Shah, P. T. et al. Single-cell transcriptomics and fate mapping of ependymal cells reveals an absence of neural stem cell function. Cell 173, 1045–1057.e1049 (2018).
Lawson, N. D. et al. An improved zebrafish transcriptome annotation for sensitive and comprehensive detection of cell type-specific genes. Elife 9, e55792 (2020).
Matson, K. J. E. et al. Isolation of adult spinal cord nuclei for massively parallel single-nucleus RNA sequencing. J. Vis. Exp. 140, e58413 (2018).
Yang, S. et al. Decontamination of ambient RNA in single-cell RNA-seq with DecontX. Genome Biol. 21, 57 (2020).
McGinnis, C. S., Murrow, L. M. & Gartner, Z. J. DoubletFinder: doublet detection in single-cell RNA sequencing data using artificial nearest neighbors. Cell Syst. 8, 329–337.e324 (2019).
Tambalo, M., Mitter, R. & Wilkinson, D. G. A single cell transcriptome atlas of the developing zebrafish hindbrain. Development 147, dev184143 (2020).
Tang, Q. et al. Dissecting hematopoietic and renal cell heterogeneity in adult zebrafish at single-cell resolution using RNA sequencing. J. Exp. Med. 214, 2875–2887 (2017).
Zeisel, A. et al. Molecular architecture of the mouse nervous system. Cell 174, 999–1014.e1022 (2018).
Zhang, Y. et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 34, 11929–11947 (2014).
Zhang, Y. et al. Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron 89, 37–53 (2016).
Baek, M., Menon, V., Jessell, T. M., Hantman, A. W. & Dasen, J. S. Molecular logic of spinocerebellar tract neuron diversity and connectivity. Cell Rep. 27, 2620–2635.e2624 (2019).
Guillemot, F. Spatial and temporal specification of neural fates by transcription factor codes. Development 134, 3771–3780 (2007).
Haring, M. et al. Neuronal atlas of the dorsal horn defines its architecture and links sensory input to transcriptional cell types. Nat. Neurosci. 21, 869–880 (2018).
Hayashi, M. et al. Graded arrays of spinal and supraspinal V2a interneuron subtypes underlie forelimb and hindlimb motor control. Neuron 97, 869–884.e865 (2018).
Hernandez-Miranda, L. R., Muller, T. & Birchmeier, C. The dorsal spinal cord and hindbrain: from developmental mechanisms to functional circuits. Dev. Biol. 432, 34–42 (2017).
Lu, D. C., Niu, T. & Alaynick, W. A. Molecular and cellular development of spinal cord locomotor circuitry. Front. Mol. Neurosci. 8, 25 (2015).
Rosenberg, A. B. et al. Single-cell profiling of the developing mouse brain and spinal cord with split-pool barcoding. Science 360, 176–182 (2018).
Rougeot, J. et al. RNAseq profiling of leukocyte populations in Zebrafish Larvae reveals a cxcl11 chemokine gene as a marker of macrophage polarization during mycobacterial infection. Front. Immunol. 10, 832 (2019).
Sathyamurthy, A. et al. Massively parallel single nucleus transcriptional profiling defines spinal cord neurons and their activity during behavior. Cell Rep. 22, 2216–2225 (2018).
Sternberg, J. R. et al. Pkd2l1 is required for mechanoception in cerebrospinal fluid-contacting neurons and maintenance of spine curvature. Nat. Commun. 9, 3804 (2018).
Jin, S. et al. Inference and analysis of cell-cell communication using CellChat. Nat. Commun. 12, 1088 (2021).
Zhao, W., Johnston, K. G., Ren, H., Xu, X. & Nie, Q. Inferring neuron-neuron communications from single-cell transcriptomics through NeuronChat. Nat. Commun. 14, 1128 (2023).
Gulati, G. S. et al. Single-cell transcriptional diversity is a hallmark of developmental potential. Science 367, 405–411 (2020).
Tome, D., Dias, M. S., Correia, J. & Almeida, R. D. Fibroblast growth factor signaling in axons: from development to disease. Cell Commun. Signal. 21, 290 (2023).
Tsata, V. et al. A switch in pdgfrb(+) cell-derived ECM composition prevents inhibitory scarring and promotes axon regeneration in the zebrafish spinal cord. Dev. Cell 56, 509–524.e509 (2021).
Ye, L. X. et al. Exogenous platelet-derived growth factor improves neurovascular unit recovery after spinal cord injury. Neural Regen. Res. 16, 765–771 (2021).
Cardozo, M. J., Mysiak, K. S., Becker, T. & Becker, C. G. Reduce, reuse, recycle - Developmental signals in spinal cord regeneration. Dev. Biol. 432, 53–62 (2017).
Diez Del Corral, R. & Morales, A. V. The multiple roles of FGF signaling in the developing spinal cord. Front. Cell Dev. Biol. 5, 58 (2017).
Garcia, A. L., Udeh, A., Kalahasty, K. & Hackam, A. S. A growing field: the regulation of axonal regeneration by Wnt signaling. Neural Regen. Res. 13, 43–52 (2018).
Guo, X.Y. et al. Subcutaneous administration of PDGF-AA improves the functional recovery after spinal cord injury. Front. Neurosci. 13, 6 (2019).
He, C. W., Liao, C. P., & Pan, C. L. Wnt signalling in the development of axon, dendrites and synapses. Open Biol. 8, 180116 (2018).
Shimada, T., Yoshida, T. & Yamagata, K. Neuritin mediates activity-dependent axonal branch formation in part via FGF signaling. J. Neurosci. 36, 4534–4548 (2016).
Bradbury, E. J. et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416, 636–640 (2002).
Zavvarian, M. M., Zhou, C., Kahnemuyipour, S., Hong, J., & Fehlings, M. G. The MAPK signaling pathway presents novel molecular targets for therapeutic intervention after traumatic spinal cord injury: a comparative cross-species transcriptional analysis. Int. J. Mol. Sci. 22, 12934 (2021).
Kolb, J. et al. Small leucine-rich proteoglycans inhibit CNS regeneration by modifying the structural and mechanical properties of the lesion environment. Nat. Commun. 14, 6814 (2023).
Monaghan, J. R. et al. Early gene expression during natural spinal cord regeneration in the salamander Ambystoma mexicanum. J. Neurochem. 101, 27–40 (2007).
Syed, D. S., Gowda, S. B., Reddy, O. V., Reichert, H. & VijayRaghavan, K. Glial and neuronal Semaphorin signaling instruct the development of a functional myotopic map for Drosophila walking. Elife 5, e11572 (2016).
Yoshida, Y. Semaphorin signaling in vertebrate neural circuit assembly. Front. Mol. Neurosci. 5, 71 (2012).
Carulli, D., de Winter, F. & Verhaagen, J. Semaphorins in adult nervous system plasticity and disease. Front. Synaptic Neurosci. 13, 672891 (2021).
Runker, A. E., Little, G. E., Suto, F., Fujisawa, H. & Mitchell, K. J. Semaphorin-6A controls guidance of corticospinal tract axons at multiple choice points. Neural. Dev. 3, 34 (2008).
Isaacson, J. S. & Scanziani, M. How inhibition shapes cortical activity. Neuron 72, 231–243 (2011).
Dudek, F. E. & Sutula, T. P. Epileptogenesis in the dentate gyrus: a critical perspective. Prog. Brain Res. 163, 755–773 (2007).
Gao, R. & Penzes, P. Common mechanisms of excitatory and inhibitory imbalance in schizophrenia and autism spectrum disorders. Curr. Mol. Med. 15, 146–167 (2015).
Bertels, H., Vicente-Ortiz, G., El Kanbi, K. & Takeoka, A. Neurotransmitter phenotype switching by spinal excitatory interneurons regulates locomotor recovery after spinal cord injury. Nat. Neurosci. 25, 617–629 (2022).
Horzmann, K. A. & Freeman, J. L. Zebrafish get connected: investigating neurotransmission targets and alterations in chemical toxicity. Toxics 4, 19 (2016).
Satou, C. et al. Transgenic tools to characterize neuronal properties of discrete populations of zebrafish neurons. Development 140, 3927–3931 (2013).
Huang, C. X. et al. An injury-induced serotonergic neuron subpopulation contributes to axon regrowth and function restoration after spinal cord injury in zebrafish. Nat. Commun. 12, 7093 (2021).
Wang, L. F. et al. Activating transcription factor 3 promotes spinal cord regeneration of adult zebrafish. Biochem. Biophys. Res. Commun. 488, 522–527 (2017).
Wang, Z., Reynolds, A., Kirry, A., Nienhaus, C. & Blackmore, M. G. Overexpression of Sox11 promotes corticospinal tract regeneration after spinal injury while interfering with functional recovery. J. Neurosci. 35, 3139–3145 (2015).
Williams, R. R., Venkatesh, I., Pearse, D. D., Udvadia, A. J. & Bunge, M. B. MASH1/Ascl1a leads to GAP43 expression and axon regeneration in the adult CNS. PLoS ONE 10, e0118918 (2015).
Zhang, Y. et al. Growth-associated protein GAP-43 and L1 act synergistically to promote regenerative growth of Purkinje cell axons in vivo. Proc. Natl Acad. Sci. USA 102, 14883–14888 (2005).
Curtis, R., Green, D., Lindsay, R. M. & Wilkin, G. P. Up-regulation of GAP-43 and growth of axons in rat spinal cord after compression injury. J. Neurocytol. 22, 51–64 (1993).
Doster, S. K., Lozano, A. M., Aguayo, A. J. & Willard, M. B. Expression of the growth-associated protein GAP-43 in adult rat retinal ganglion cells following axon injury. Neuron 6, 635–647 (1991).
Gey, M. et al. Atf3 mutant mice show reduced axon regeneration and impaired regeneration-associated gene induction after peripheral nerve injury. Open Biol. 6, 160091 (2016).
Jankowski, M. P. et al. Sox11 transcription factor modulates peripheral nerve regeneration in adult mice. Brain Res. 1256, 43–54 (2009).
Jing, X., Wang, T., Huang, S., Glorioso, J. C. & Albers, K. M. The transcription factor Sox11 promotes nerve regeneration through activation of the regeneration-associated gene Sprr1a. Exp. Neurol. 233, 221–232 (2012).
Kole, C. et al. Activating Transcription Factor 3 (ATF3) protects retinal ganglion cells and promotes functional preservation after optic nerve crush. Investig. Ophthalmol. Vis. Sci. 61, 31 (2020).
Bartus, K. et al. ErbB receptor signaling directly controls oligodendrocyte progenitor cell transformation and spontaneous remyelination after spinal cord injury. Glia 67, 1036–1046 (2019).
El-Brolosy, M. A. et al. Genetic compensation triggered by mutant mRNA degradation. Nature 568, 193–197 (2019).
Klatt Shaw, D., and Mokalled, M. H. Efficient CRISPR/Cas9 mutagenesis for neurobehavioral screening in adult zebrafish. G3 (Bethesda) 11, jkab089 (2021).
Wu, H., Kirita, Y., Donnelly, E. L. & Humphreys, B. D. Advantages of single-nucleus over single-cell RNA sequencing of adult kidney: rare cell types and novel cell states revealed in fibrosis. J. Am. Soc. Nephrol. 30, 23–32 (2019).
Bakken, T. E. et al. Single-nucleus and single-cell transcriptomes compared in matched cortical cell types. PLoS ONE 13, e0209648 (2018).
Lake, B. B. et al. A comparative strategy for single-nucleus and single-cell transcriptomes confirms accuracy in predicted cell-type expression from nuclear RNA. Sci. Rep. 7, 6031 (2017).
van den Brink, S. C. et al. Single-cell sequencing reveals dissociation-induced gene expression in tissue subpopulations. Nat. Methods 14, 935–936 (2017).
Ding, J. et al. Systematic comparison of single-cell and single-nucleus RNA-sequencing methods. Nat. Biotechnol. 38, 737–746 (2020).
Lacar, B. et al. Nuclear RNA-seq of single neurons reveals molecular signatures of activation. Nat. Commun. 7, 11022 (2016).
Zhang, H. et al. Erythropoietin signaling increases neurogenesis and oligodendrogenesis of endogenous neural stem cells following spinal cord injury both in vivo and in vitro. Mol. Med. Rep. 17, 264–272 (2018).
Dias, T. B., Yang, Y. J., Ogai, K., Becker, T. & Becker, C. G. Notch signaling controls generation of motor neurons in the lesioned spinal cord of adult zebrafish. J. Neurosci. 32, 3245–3252 (2012).
Reimer, M. M. et al. Sonic hedgehog is a polarized signal for motor neuron regeneration in adult zebrafish. J. Neurosci. 29, 15073–15082 (2009).
Wehner, D. et al. Wnt signaling controls pro-regenerative Collagen XII in functional spinal cord regeneration in zebrafish. Nat. Commun. 8, 126 (2017).
Bertuzzi, M., Chang, W. & Ampatzis, K. Adult spinal motoneurons change their neurotransmitter phenotype to control locomotion. Proc. Natl Acad. Sci. USA 115, E9926–E9933 (2018).
Wang, C. et al. Synaptotagmin-11 inhibits clathrin-mediated and bulk endocytosis. EMBO Rep. 17, 47–63 (2016).
Bakr, M. et al. The vSNAREs VAMP2 and VAMP4 control recycling and intracellular sorting of post-synaptic receptors in neuronal dendrites. Cell Rep. 36, 109678 (2021).
Li, W. R. et al. Synaptotagmin-11 inhibits spontaneous neurotransmission through vti1a. J. Neurochem. 159, 729–741 (2021).
Lin, P. Y. et al. VAMP4 maintains a Ca(2+)-sensitive pool of spontaneously recycling synaptic vesicles. J. Neurosci. 40, 5389–5401 (2020).
Nicholson-Fish, J. C., Kokotos, A. C., Gillingwater, T. H., Smillie, K. J. & Cousin, M. A. VAMP4 is an essential cargo molecule for activity-dependent bulk endocytosis. Neuron 88, 973–984 (2015).
Shimojo, M. et al. Synaptotagmin-11 mediates a vesicle trafficking pathway that is essential for development and synaptic plasticity. Genes Dev. 33, 365–376 (2019).
Hilton, B. J. et al. An active vesicle priming machinery suppresses axon regeneration upon adult CNS injury. Neuron 110, 51–69.e57 (2022).
Higashijima, S., Hotta, Y. & Okamoto, H. Visualization of cranial motor neurons in live transgenic zebrafish expressing green fluorescent protein under the control of the islet-1 promoter/enhancer. J. Neurosci. 20, 206–218 (2000).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177, 1888–1902.e1821 (2019).
R Core Team (2018). R: A language and environment for statistical computing.
Ilicic, T. et al. Classification of low quality cells from single-cell RNA-seq data. Genome Biol. 17, 29 (2016).
Hafemeister, C. & Satija, R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 20, 296 (2019).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
Haghverdi, L., Lun, A. T. L., Morgan, M. D. & Marioni, J. C. Batch effects in single-cell RNA-sequencing data are corrected by matching mutual nearest neighbors. Nat. Biotechnol. 36, 421–427 (2018).
Xu, C. & Su, Z. Identification of cell types from single-cell transcriptomes using a novel clustering method. Bioinformatics 31, 1974–1980 (2015).
Levine, J. H. et al. Data-driven phenotypic dissection of AML reveals progenitor-like cells that correlate with prognosis. Cell 162, 184–197 (2015).
Zappia, L. & Oshlack, A. Clustering trees: a visualization for evaluating clusterings at multiple resolutions. Gigascience 7, giy083 (2018).
Becht, E. et al. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. 37, 38−44 (2018).
Ahlmann-Eltze, C. & Huber, W. glmGamPoi: fitting Gamma-Poisson generalized linear models on single cell count data. Bioinformatics 36, 5701–5702 (2021).
Saraswathy, V. M., Zhou, L. & Mokalled, M. H. Single-cell analysis of innate spinal cord regeneration identifies intersecting modes of neuronal repair, Mokalledlab/zebrafish_sci_atlas: Sci_atlas_codes, https://doi.org/10.5281/zenodo.11585743 (2024).
Durinck, S., Spellman, P. T., Birney, E. & Huber, W. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat. Protoc. 4, 1184–1191 (2009).
Lange, M. et al. CellRank for directed single-cell fate mapping. Nat. Methods 19, 159–170 (2022).
Rossum, G. V. & Drake, F. L. Python 3 Reference Manual. (CreateSpace) (2009).
Stassen, S. V., Yip, G. G. K., Wong, K. K. Y., Ho, J. W. K. & Tsia, K. K. Generalized and scalable trajectory inference in single-cell omics data with VIA. Nat. Commun. 12, 5528 (2021).
Zhou, Y. et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 10, 1523 (2019).
Choi, H. M. T. et al. Third-generation in situ hybridization chain reaction: multiplexed, quantitative, sensitive, versatile, robust. Development 145, dev165753 (2018).
Schwarzkopf, M. et al. Hybridization chain reaction enables a unified approach to multiplexed, quantitative, high-resolution immunohistochemistry and in situ hybridization. Development 148, dev199847 (2021).
Salic, A. & Mitchison, T. J. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc. Natl Acad. Sci. USA 105, 2415–2420 (2008).
Acknowledgements
We thank V. Cavalli and A. Johnson for discussion and the Washington University Zebrafish Shared Resource for animal care. We thank S. Nandagopal, T. Tsai and S. Megason for the HCR probe design script. This research was supported by grants from the NIH (R01 NS113915 to M.H.M.) and a Postdoctoral Fellow Seed of Independence Grant from the Department of Developmental Biology at Washington University School of Medicine (to V.M.S.).
Author information
Authors and Affiliations
Contributions
V.M.S. and M.H.M. designed the study. V.M.S. and L.Z. generated the snRNA-seq dataset. V.M.S. performed all computational and in vivo analyses. V.M.S. and M.H.M. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Saraswathy, V.M., Zhou, L. & Mokalled, M.H. Single-cell analysis of innate spinal cord regeneration identifies intersecting modes of neuronal repair. Nat Commun 15, 6808 (2024). https://doi.org/10.1038/s41467-024-50628-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-50628-y
- Springer Nature Limited