Abstract
Ventilator-associated pneumonia (VAP) affects up to 20% of critically ill patients and induces significant antibiotic prescription pressure, accounting for half of all antibiotic use in the ICU. VAP significantly increases hospital length of stay and healthcare costs yet is also associated with long-term morbidity and mortality. The diagnosis of VAP continues to present challenges and pitfalls for the currently available clinical, radiological and microbiological diagnostic armamentarium. Biomarkers and artificial intelligence offer an innovative potential direction for ongoing future research. In this Review, we summarise the pathobiological heterogeneity and diagnostic challenges associated with VAP.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Ventilator-associated pneumonia (VAP) is a nosocomial infection of the lung parenchyma that occurs after 48 h of tracheal intubation and mechanical ventilation1,2,3,4. Within the Intensive Care Unit (ICU), VAP is the most frequent nosocomial infection, impacting 20–36% of critically ill patients. Incidence rates vary, ranging from 2 to 16 episodes per 1000 ventilator days, and are influenced by factors such as diagnostic criteria, preventative measures, and patient and geographical variations5,6,7,8. Intubation is the primary risk factor, associated with more than 95% of pneumonis in the ICU; however, patients with reduced consciousness, trauma, older age and illness severity also have an increased risk9,10,11.
Reported mortality rates for VAP span a wide range (24–76%); however, attributing mortality solely to VAP is complex due to the severity of underlying illnesses and diagnostic heterogeneity within ICU populations12,13. VAP can lead to the development of septic shock or acute respiratory distress syndrome if the treatment is delayed or inappropriate14. VAP results in substantial antibiotic prescription pressure, accounting for half of all antibiotics used in the ICU15,16,17,18,19,20. However, the prolific use of antibiotic therapy contributes to a vicious cycle of increasing multidrug-resistant (MDR) pathogens and mortality21. Treatment failure increases the risk of mortality and may occur in a third to two-thirds of VAP cases, with inappropriate antibiotic use the most common cause22,23. VAP increases hospital length of stay by an average of four to nine days and healthcare costs by £9000 per patient3,18,24,25,26. Despite preventative measures, there is a rising prevalence of MDR and persistently high mortality and healthcare costs associated with VAP. The pathobiological heterogeneity and diagnostic challenges associated with VAP remain of significant importance in global healthcare. In this review, we summarise the pathobiological heterogeneity and diagnostic challenges associated with VAP.
Pathobiology of VAP
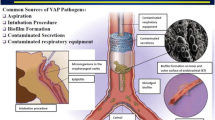
The pathobiology of VAP is multi-factorial due to alterations in the usual protective airway defences and changes in the patient microbiological flora and immune responses. There are several predisposing factors in ICU patients and some present therapeutic opportunities (Fig. 1).
1 External factors associated with ICU therapies include mechanical ventilation, sedation and paralysis agents and semi-recumbent positioning; 2 Endotracheal tube prevents glottis closure and provides direct communication to the lungs, allowing microaspiration of secretions from the nasal sinuses and oropharynx. The endotracheal cuff is at risk of deflation, movement and small folds, allowing pooled secretions to leak. A bacterial biofilm can form around the endotracheal tube, which can then become dislodged by movement or suctioning; 3 In response to invading pathogens, alveolar macrophages and neutrophils’ immune and inflammatory response lead to inflamed, oedematous and infected alveoli. Abbreviations: ICU = intensive care unit; AM = alveolar macrophages. Figure 1 was created with BioRender.com released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license (https://creativecommons.org/licences/by-nc-nd/4.0/deed.en).
Intensive care unit treatments and bacterial colonisation
The presence of an endotracheal tube (ETT) is a significant risk factor, as it breaches the natural protective barriers of the upper airway, allowing direct communication to the tracheobronchial tree19,27 (Fig. 1). The ETT’s high-volume, low-pressure protective cuff minimises gross aspiration but does not prevent micro aspirations. These phenomena include entrainment of pooled secretions, loss of cuff pressure and ETT movement27,28,29. The oropharynx, nasal sinuses and stomach are potential reservoirs of pooled secretions, which are susceptible to leaking below the cuff, resulting in silent micro-aspiration2,15,30,31. The ETT also causes epithelial damage and impedes cough, swallowing and mucociliary clearance mechanisms27,32,33. These mechanisms are further compromised by ICU therapies, such as sedation, paralysis, positive pressure ventilation and the influence of gravity associated with semi-recumbent positioning. Although necessary interventions, these ICU therapies can cause respiratory muscle weakness, mucociliary dysfunction and reduced airway humidification, which exacerbate the impairments to cough, tracheal mucus velocity and secretion clearance12,27,32,34.
VAP is also related to bacterial biofilm formation, found on the ETT surface of 95% of mechanically ventilated patients27,35,36,37 (Fig. 1). The biofilm acts as a reservoir for infective microorganisms, which can become dislodged, enter the distal airways and subsequently lead to VAP, with associations found between pathogens in tracheal secretions and the ETT biofilm27,35,36,37,38. Biofilm occurs within hours of intubation and is anticipated to precede VAP36. The longer the period of mechanical ventilation, the greater the risk of VAP10.
Microbiology, local innate immunity and inflammation associated with VAP
Microbiology
VAP is often polymicrobial with an increasing prevalence of antibiotic-resistant bacteria9. The most prevalent pathogens causing VAP are gram-negative bacteria (mainly Klebsiella, Acinetobacter, Pseudomonas, Escherichia coli and other Enterobacteriaceae) and some gram-positive species such as Staphylococcus aureus and Enterococci39,40. Candida colonisation is also common, presenting a risk for fungal VAP41. Based on bacteriological data, VAP can be classified as either early or late-onset; early occurs between 48 and 96 hours after ICU admission and late-onset occurs after 96 h42,43,44. Early-onset VAP is more likely to be caused by bacteria which are antibiotic-sensitive, such as Streptococcus pneumonia, enteric gram-negative bacilli or methicillin-susceptible Staphylococcus aureus, and therefore usually hold better prognosis44,45. Late-onset VAP is more likely to be caused by MDR pathogens, such as Pseudomonas aeruginosa, Acinetobacter baumanni, methicillin-resistant S. aureus, and as such, are associated with higher morbidity and mortality44,45,46. However, the timing of onset is not the only risk for MDR, as patients with pre-admission immunosuppression, prior antibiotic use or recent hospitalisation are also at greater risk of MDR or atypical infections, with emerging studies reporting similar MDR prevalence regardless of timings of onset44,45,47,48,49,50. In non-neutropenic patients, multicenter studies suggest a prevalence of Aspergillus infection up to 12%, which may be an emerging problem in ICUs due to the increasing use of immunosuppressive therapies51.
In early studies of patients with bacterial pneumonia, there is a reactivation of Cytomegalovirus, herpes simplex virus-1, and Epstein-Barr virus in up to 48% of patients, with an independent association with mortality52. These worrying trends in bacterial, fungal and viral infections should trigger new prospective large registry studies to standardise the diagnosis, identify new therapeutic opportunities, improve adherence to culture results, and improve prescription practices that plague intensive care units53.
The microbiome of the lung as a modulator of VAP
Although previously once considered sterile, the lungs are now recognised as hosting a diverse and dynamic microbiome that, under healthy conditions, maintains a symbiotic ecosystem and immune homoeostasis54,55. Numerous factors may cause microbiome dysbiosis during critical illness, including prior antibiotic use, mechanical ventilation or diet changes, which may exacerbate the risk of complications such as VAP54. Dysbiosis of the lung microbiota associated with VAP is linked to altered oropharyngeal microbiota, from which there is microaspiration or translocation to the lungs via the ETT, alongside gut-to-lung translocation and ICU-related factors such as antibiotic therapy55. Common ICU therapies, such as sedation and mechanical ventilation, impair cough reflexes and secretion clearance, decreasing bacterial elimination from the airways and increasing bacterial load56,57. During VAP, the lung microbiota becomes overwhelmed and dysbiotic, with low microbial diversity and high microbial burden39,58,59.
The innate immune response of the lung parenchyma
For pneumonia to develop, microbes, typically bacteria, must first overcome mechanical barriers, such as cough, swallowing and the mucociliary escalator37. This is followed by tracheal and lung colonisation and expansion of pathobionts that exceed the lungs’ capacity to maintain immune resistance and tissue resilience59. Alveolar macrophages (AMs) are a diverse phagocyte group in the lower respiratory tract. Under healthy conditions, AMs maintain alveolar homoeostasis and are the most abundant innate immune cells during local infection, providing the first line of defence against invading respiratory pathogens60. AMs adapt to provide phagocytosis and apoptosis of microbes. Furthermore, AMs orchestrate coordinated innate immune response of cytokine activity through NF-kB transcription, including interleukins (IL-1α, IL-1β, IL-6, IL-8) and tumour necrosis factor (TNF-a) that recruit neutrophils to the site of infection. AMs act as antigen-presenting cells, stimulating lymphocyte cells and triggering cellular and humoral responses, subsequently leading to inflammation of the lung parenchyma, leaking capillaries and exudative congestion58,59.
In the event of pathogenic microbes overwhelming the local immune defences of the lung, the AMs and epithelial cells release additional cytokines, which facilitate the migration of neutrophils into the lung airspaces. In VAP positive for bacterial culture, there is a recruitment of neutrophils to the lungs, resulting in phagocytosis, degranulation, release of reactive oxygen species (ROS) and formation of neutrophil extracellular traps (NETs)61,62,63. NETs contain histones and antimicrobial peptides, which can kill invading pathogens associated with VAP61,62,63,64. Neutrophils and derived markers suggestive of a compartmentalised proinflammatory state can be used to diagnose bacterial pneumonia in VAP but require meticulous standardised bronchoalveolar lavage, and the resources and expertise are lacking in most centres, precluding its use in routine clinical practice65.
Localised inflammation
Critical illness is a heterogeneous status of simultaneous proinflammation and immune suppression66,67. In addition, mechanical ventilation is known to induce a localised inflammatory response of pulmonary cytokine upregulation, termed biotrauma68. Biotrauma generates persistent local inflammation and may increase the VAP risk68. Determining the causal mediators of inflammation and their attributable contribution to VAP is often clinically challenging. However, recent studies have demonstrated a significant increase in pulmonary biomarkers interleukin-1 beta (IL-1β), IL-8, matrix metalloproteinase-8 (MMP-8), MMP-9 and human neutrophil elastase (HNE) and serum CRP and IL-6 in the instance of VAP40,65,69. Although neutrophils play an important role in the innate immune response to VAP, the release of NETs can also contribute to localised and persistent inflammation61,62,63,64,70. This persistent inflammation can lead to lung injury and increase the risk of mortality61,70.
Histological definitions of VAP
VAP has been defined as the presence of consolidation with leucocyte accumulation in bronchioles and alveoli14,71,72,73. VAP has been categorised into four stages of severity73: early-stage bronchiolitis characterised by purulent mucus plugs with a proliferation of polymorphonuclear leucocytes (PMNL) within the bronchioles and bronchiolar wall. This is ensured by focal bronchopneumonia, with scattered neutrophilic infiltrates in the terminal bronchioles and alveoli. A confluent bronchopneumonia follows with focal infiltrates that extend into adjacent lobes. A lung abscess is the final step, with an extension of confluent bronchopneumonia, additional tissue necrosis, and the destruction of the surrounding lung architecture. Histological studies suggest that VAP is heterogeneous, with concurrent coexisting phases14. Early-phase pneumonia with capillary congestion and fibrinous exudate with PMNL in the alveolar spaces progresses to intermediate-phase pneumonia with alveoli containing fibrin, PMNL and a few erythrocytes. In the advanced phase, the macrophages engulf cellular debris when PMNL fills the alveoli, followed by a phase of resolution where the macrophagic activity of mononuclear cells eliminates inflammatory exudate.
Overall, VAP is a non-homogenous and diffuse process, often involving both lungs and mainly the lower lobes14. This histological pattern may be due to the distribution of bacteria from central to distal airways by the flow and volume generated by mechanical ventilation. However, this complex and dynamic presentation can explain why diagnostic methods are challenging.
Diagnosis of VAP
Despite significant work on diagnosing pneumonia and published international guidelines (Supplementary Information File), the definition and diagnosis of VAP remain controversial, with substantial variations in reference standards17,19,25,37. This confusion means that VAP incidence rates in the same patient cohort varied from 4% to 42% due to differing diagnostic criteria4,11,44,74,75,76,77,78. The diagnosis of VAP lacks sensitivity and specificity, forcing the clinician to balance the risks of unnecessary antibiotics against possible patient harm due to delayed therapy8,17,19,79,80. These diagnostic uncertainties and the absence of clear, accepted actionable evidence nationally and internationally lead to a reluctance to adhere to antimicrobial hygiene, adding to antibiotic pressure in VAP53. To reduce mortality, timely antimicrobial therapy is essential8,79,80. It is also imperative to diagnose other infections often wrongly attributed to pneumonia and identify confounders such as fluid overload and acute respiratory distress syndrome (ARDS). Hence, the diagnosis of VAP must be sensitive, specific and timely.
Histopathological examination of inflamed and infected lung tissue is considered the most accurate diagnostic tool for diagnosing VAP37,81. However, a lung biopsy is not practical or appropriate for the critically ill, and post-mortem examination is too late to influence treatment25,37. Clinical guidelines, therefore, attempt to amalgamate clinical, radiological and microbiological criteria to support the diagnosis of VAP11,44,74,75,76 (Table 1)17.
Clinical diagnosis of VAP: the pitfalls
Clinical examination is essential but has a sensitivity of 66.4% (95% CI 40.7–85.0) and specificity of 53.9% (95% CI 34.5–72.2) in the diagnosis of VAP8. Classical clinical features clinicians use for diagnosing VAP, such as fever, purulent secretions and leukocytosis, have a sensitivity of 69% and a specificity of 75% in post-mortem and prospective clinical studies71. These clinical features often formulate the foundation of VAP diagnosis, combined with radiological and microbiological investigations37. However, the preliminary signs of inflammation, including fever, tachycardia and leucocytosis, are nonspecific to VAP and can be seen in any process associated with the systemic inflammatory response of critical illness19. Although inconclusive and attributable to any condition that releases cytokines, fever and leucocytosis should prompt further clinical investigation12,19,37.
Cough, sputum production, tachypnoea and hypoxia
In critically unwell patients, the cough may be suppressed by sedation, positive pressure ventilation, artificial airway or disease progression (e.g., traumatic brain injury or stroke)37,82,83. A high burden of purulent tracheal secretions may suggest lower respiratory tract infection, yet in intubated patients, and this may be due to a suppression of cough reflex, bacterial colonisation upon airway devices and impaired mucociliary clearance18,27. Tracheal secretions may have other potential sources, such as tracheobronchitis and may be exacerbated by smoking history or underlying respiratory diseases15,19,84. As outlined in international guidelines, a change in subjective sputum characteristics (such as colour or consistency) contributes to the diagnosis of VAP44,85.
Tachypnoea and dyspnoea as an aid to VAP diagnosis have limited utility in the ICU as these features can be masked by sedation and ventilatory support. In the spontaneously breathing ICU patient, breathlessness may be multi-factorial and driven by other causes that may mimic the broad symptoms of pneumonia, such as delirium, pain, agitation or respiratory muscle weakness. Changes to oxygenation, ventilation and gas exchange may signify an inflammatory process of the lung parenchyma pointing towards a VAP but can be a feature of a non-infective aetiology, such as ARDS37. VAP and ARDS are both associated with hypoxaemia; however, their diagnosis has significant overlap and direct association, with pneumonia being the leading cause of ARDS, whilst ARDS also increases the risk of VAP74.
Radiological diagnosis of VAP
Radiological investigations aim to identify the presence of alveolar inflammation and secretions. Pneumonia results in new or progressive infiltrates, resulting in an air-bronchogram due to alveolar opacification and air-filled bronchi with lobar and sub-lobar consolidation37. Areas of pulmonary opacities, areas of increased attenuation described as ground-glass infiltrates and the appearance of an air-bronchogram are all features of pneumonia on imaging86.
Radiological investigations also aid the differentiation between VAP and ventilator-associated tracheobronchitis (VAT). VAT presents with similar clinical and laboratory criteria to VAP yet is distinguished by the absence of pulmonary infiltrates on imaging as it does not involve the lung parenchyma44,87,88. VAT and VAP are closely related, as VAT is often a precursor to VAP15,89. Early recognition and differentiation between subtypes of ventilator-associated respiratory infections by radiological examination provides an opportunity for earlier, targeted intervention and antibiotic therapy89. Techniques such as computed tomography (CT), plain chest X-ray (CXR) or lung ultrasound (LUS) are usually combined with clinical criteria to support a diagnosis of VAP11,15,75.
Computed tomography
CT is the gold examination standard, providing a three-dimensional anatomical and pathological orientation without anatomy overlap and high resolution of different tissue densities25,37,90,91. Although helpful, CT is not routinely utilised to diagnose VAP in ICU patients due to transportation risks, the requirement for additional sedation and paralysis to facilitate CT and the radiation burden15,37,90,92. In our quaternary referral hospital, with trained staff performing intrahospital transfers to CT, up to 30% of patients are reported to have adverse events due to the transfer, in line with published literature93,94,95. Hence, it is reserved for VAP with diagnostic difficulties15.
Chest X-ray
In a recent UK survey, over 75% of ICU consultants reported they would request a CXR if clinically indicated by an acute change in clinical symptoms suspicious of VAP17. CXRs are portable and enable bedside imaging, avoiding the risks associated with CT transfer. However, although relatively inexpensive and practical, the interpretation of CXR imaging is often challenging in critically ill ICU patients due to the overlap of other common pathologies which present with lung infiltrates, such as pulmonary contusions, atelectasis, pulmonary oedema or ARDS12,15. CXR provides an isolated, two-dimensional image of a complex three-dimensional structure in which several tissues overlay one another, making interpretation challenging96,97. CXR findings should be compared to previous imaging to establish new or progressive changes75. The optimal approach for CXR requires posterior-anterior and lateral images in the erect posture96, which is impossible in the critically ill patient, necessitating an anterior-posterior view96. Imaging quality and interpretation are also confounded by factors such as body habitus, inspiration stage, disease progression and artefacts98.
A recent systematic review reported that CXR had a sensitivity of 88.9% (95% CI 73.9–95.8) and a specificity of 26.1% (95% CI 15.1–41.4) with a significant discrepancy in interpretation, increasing the likelihood of late or missed diagnosis8. A comparison of CT and CXR suggested poor cross-validation between CXR and CT chest, with less than 45% of opacities on CT observed on CXR and 26.9% vice versa15,86,98. CXR may still be considered a valuable adjunct for diagnosing VAP12,25.
Lung ultrasound
Lung Ultrasound (LUS) provides a rapid, point-of-care, noninvasive bedside assessment with no radiation risk78,99,100. LUS has minimal operator dependence and high interobserver agreement after training and at different levels of expertise; however, visualisation can be challenging in patients with obesity, subcutaneous emphysema and chest wall oedema100. Increasing evidence supports the use of LUS for detecting and monitoring VAP, identified by subpleural consolidation or dynamic bronchogram99,101,102. Equally, such features are associated with any source of lung consolidation and are not specific to VAP alone78,101. When combined with clinical signs and symptoms, LUS provides earlier and superior diagnostic performance than CXR in critically ill mechanically ventilated patients, including those with VAP103,104,105,106. Although the results of small-scale observational studies are promising for LUS, there has been minimal application to its benefit on patient outcomes. Earlier diagnosis using LUS, rather than CXR, has been associated with reduced ventilator-free days yet has not been shown to expedite VAP resolution nor improve antibiotic-free days or mortality104. Although there is increasing utility in the ICU, LUS is not currently recognised as a validated assessment tool in published guidelines for diagnosing VAP and requires further large-scale validation studies in critically ill patients on cost-effectiveness and patient outcomes.
Microbiological diagnosis of VAP
Accurate diagnosis techniques that identify the responsible infectious organisms are required to guide the most appropriate treatment regime19,37. A UK survey identified that all ICU consultants seek microbiology confirmation before the commencement of antibiotics for VAP, yet there is wide variation in techniques, availability and expertise17. The optimal method of microbiological diagnosis is a further source of controversy19,37,75,107.
Endotracheal aspirates
the most prominent method for aetiological diagnosis of VAP is noninvasive techniques, specifically endotracheal aspirates (ETA)108,109. ETA is a simple, safe and efficient technique that can be performed by most ICU staff19. Although it avoids oropharyngeal contamination, it cannot differentiate from colonising organisms in the airway devices, leading to inconclusive results12,19. While evidence suggests ETA has high false favourable rates and is associated with increased antibiotic use, the quantitative analysis provides a reliable alternative to invasive techniques19,107. A recent systematic review and meta-analysis concluded that ETA had a sensitivity of 75.7% (95% CI 51.5–90.1) and specificity of 67.9% (95% CI 40.5–86.8) for the diagnosis of VAP8. Despite this, national guidelines advocate noninvasive ETA sampling based on its comparable outcomes with invasive techniques, including mortality, length of stay and duration of mechanical ventilation74,76. Although the accuracy of ETA remains controversial, ETA imposes minimal risk due to its simple, noninvasive nature19,76.
Bronchoalveolar lavage (BAL)
The invasive techniques that are commonly used to obtain distal airway samples by a fibreoptic bronchoscope include bronchoalveolar lavage (BAL) and protected specimen brushing (PSB)19,75,107,108. BAL and PSB bypass the upper airway contamination to collect samples from the lower respiratory tract75,107,108. BAL and PSB methods are only routinely available in some hospitals and require specialist training15,25. BAL and PSB result in a loss of positive end-expiratory pressure, risks of accidental extubation, bronchospasm and hypoxia, with the additional need for sedation or neuromuscular blocking agents12.
Non-directed (without a bronchoscope) bronchial lavage techniques (NBL) allow cultures to be obtained without the complexities and risks accompanied by using a bronchoscope110,111. NBL is a safe and inexpensive method that can be performed by a range of clinicians, including respiratory therapists or physiotherapists, increasing routine availability110,111. NBL techniques have comparable diagnostic accuracy to BAL and PSB112.
Evidence suggests no reduction in mortality, duration of mechanical ventilation or ICU length of stay when BAL and PSB were compared to ETA in patients with VAP8,25,108,113. PSB is claimed to have a sensitivity of 61.4% [95% CI 43.7–76.5] and specificity of 76.5% [95% CI 64.2–85.6], while BAL has a sensitivity of 71.1% [95% CI 49.9–85.9] and specificity of 79.6% [95% CI 66.2–85.9]8. It is important to note that the reliability of such results is confounded by the lack of local, national, and international reference standards25. Because of the controversial evidence and risk of false positive and false negative results, findings should be interpreted cautiously8.
Qualitative vs quantitative microbiological analysis
BAL, PSB and ETA can be analysed quantitatively or qualitatively108. Quantitative analysis provides a threshold count of bacterial growth, allowing differentiation between infection and colonisation, whereas qualitative analysis identifies the presence or absence of pathogenic cultures (107). The rationale for using quantitative cultures of respiratory secretions sampled from patients with suspected VAP is to differentiate infectious organisms from simple colonisation and to optimise antibiotic therapy19,108. In early studies, evaluating a rapid multiplex PCR test for timely decision-making, bacterial resistance detection and antimicrobial treatment adaptation in adult patients was associated with antibiotic de-escalation. These findings should be welcomed in VAP, as they can reduce antibiotic prescription pressure in ICU settings114,115,116,117. In the UK ICU setting, only up to 30% of intensive care units have access to quantitative reporting services for VAP. Additionally, clinicians in over a quarter of these units are inadequately trained in performing the procedure17.
However, these findings should be interpreted with caution, as there is still no overall evidence that quantitative cultures reduce mortality, ICU length of stay, period of mechanical ventilation or rates of antibiotic change compared to qualitative cultures in patients with VAP74,108. Quantitative cultures may also be challenging in low- and middle-income countries. Guidelines recommend that semiquantitative cultures diagnose VAP as it require fewer laboratory resources and expertise and provides more rapid results than quantitative cultures44.
Scoring systems for the diagnosis of VAP
The clinical, radiological and microbiological assessment tools discussed need to provide a definitive diagnosis of VAP, each presenting its strengths and weaknesses, as summarised in Table 2. Scoring systems are frequently used in numerous areas of medical practice to overcome the pitfalls and controversy of individual diagnostic methods, enabling clinicians to simplify and quantify complex clinical situations118. Scoring systems can provide an efficient and standardised method of assessment, diagnosis, risk stratification and outcome prediction in acute and emergency care medicine119,120.
Clinical pulmonary infection score
The Clinical Pulmonary Infection Score (CPIS) is the most relevant scoring system for VAP and has been widely documented12,44,121,122. CPIS combines clinical, radiological and microbiological criteria into a pragmatic scoring system to provide a single, quantifiable result. The tool calculates a score of 0 to 12, with a score equal to or greater than six, signifying a high likelihood of VAP (Table 3)44,122,123.
The main limitation is the variation in reference standards and modifications in the CPIS criteria, with varied opinions of sensitivity and specificity compared to bronchoscopic BAL, non-bronchoscopic BAL and post-mortem histology44,123. A CPIS score greater than six signifying VAP has a sensitivity of 73.8% (95% CI 50.6–88.5) and a specificity of 66.4% (95% CI 43.9–83.3)8. CPIS seem to provide benefits in monitoring and early detection and as an early warning system, as it potentially allows early antimicrobial therapy75,124. Although a pragmatic combination of frequently available criteria, the accuracy and reliability of CPIS are controversial. The widespread use of the CPIS as a diagnostic tool for VAP should be adopted with caution and in the context of clinical research8,121,123,125.
Biomarkers for the diagnosis of VAP
The use of biomarkers in ICU has increased significantly in recent years to provide an objective and quantifiable characteristic of a biological process126,127. Biomarkers indicate a normal or pathological process or a pharmacological response126. The WHO defines a biomarker as any substance or process that can be measured in the body or by its products128. Biomarkers are usually proteins detected in any biological sample, including serum, bronchoalveolar lavage fluid and exhaled breath condensate, therefore acting as a potential diagnostic method for numerous infections, including VAP126,127,129,130.
Many biological markers have been studied to improve the rapidity and performance of current diagnostic procedures of VAP, with almost 200 different biomarkers related to infectious diseases being reported53,65,130,131,132,133,134,135,136,137,138. There is increased recognition that procalcitonin (PCT), a soluble triggering receptor expressed on myeloid cells (sTREM-1), C-reactive protein (CRP) and proinflammatory cytokines such as IL-8 and IL-1β, may be elevated in non-infectious and infectious inflammatory causes (see Table 4)65,76,127. However, they are inconsistent in diagnosing VAP, altered by antibiotic usage and with variable sensitivities and specificities127,137,139,140,141,142,143,144,145,146,147. They are helpful for early identification of infection, timely initiation of antimicrobial therapy, and prompt evaluation of treatment course or duration. However, currently, an ideal biomarker has to be identified. Recent guidelines, therefore, do not recommend using biomarkers for diagnosing VAP, although suggestive that they may offer guidance on the duration of therapy76,148. The utility of biomarkers as predictors of VAP has yet to be demonstrated.
Bringing the multimodal diagnosis with Artificial Intelligence
Artificial intelligence (AI) is a rapidly growing concept, offering many innovative and sophisticated possibilities within modern healthcare149,150. With the increased availability of electronic health records, AI can process and interpret vast and complex data, which is opportunistic for disease identification, outcome prediction, and phenotyping within the field of VAP149,150. AI uses supervised, semi-supervised or unsupervised machine learning to interpret complex information confounding variables and pre-existing conditions to support diagnosis and disease identification150. Previous studies have demonstrated the benefit of AI for successful image interpretation, including CT scan analysis for COVID-19-induced pneumonia and traumatic brain injury150,151. AI can potentially expedite and enhance CT or CXR interpretation and aid decision-making without microbial sampling, yet further validation is required in clinical practice models152,153,154,155,156.
AI has also enabled algorithms to cluster symptoms to establish phenotypes and endophenotypes to predict deterioration in COVID-19, ARDS, pneumonia, and sepsis. In small studies, automated machine learning for VAP prediction was deemed superior to the CPIS for patients at high risk of VAP just 24 hours after intubations150,157. Although offering huge potential to alleviate practitioner decision-making, diagnostic uncertainty and potential treatment delay, further research and investment are required before the widespread implementation of AI in acute care medicine. In a recent systematic review, over 95% of AI studies in the ICU have been retrospective149. Prospective clinical trials are needed to improve their validity, usability, and trustworthiness. Secondly, there remain concerns regarding data privacy and the safety of information sharing150. Further resources, investment, and research are required before widespread implementation for the diagnosis, prognosis, and guided clinical management of VAP.
Healthcare inequalities—VAP in low-income countries
Healthcare-associated infections are a serious problem in low-income and middle-income countries, affecting a quarter of hospitalised patients158. The pooled incidence of VAP is greater in lower-income and middle-income countries than in high-income countries159. However, reporting and surveillance vary significantly in developing countries, with limited prevention interventions160. In many areas of low and middle-income with rapidly growing economies, such as across Asia, more people are accessing healthcare due to increasing provision, yet with a lack of regulation and stewardship of infection prevention and control and antibiotic stewardship159. In developing countries, VAP is associated with high mortality rates and increased ICU length of stay, contributing to an additional burden of scarce resources and excess costs160,161. In developing countries, the costs attributable to VAP are 5 times greater than in other patients161. Innovation with AI may reduce the cost of these patients.
Conclusion
The diagnosis of VAP continues to present a clinical conundrum125. With a variance in diagnostic methods and reference standards, the existing literature offers a limited comparison to measure VAP’s progression and treatment response. Although considered a definitive marker, histological diagnosis is not viable for investigation for diagnosis and prognosis of VAP in ICU.
With limited consensus in clinical research, a pragmatic approach to diagnosis is required, encompassing clinical expertise and experience with the breadth of clinical information available. A combination of the available clinical, radiological and microbiological methods should be sought after and collectively evaluated, with an appreciation of the advantages and disadvantages of each diagnostic method (as outlined in Table 2)162. Future clinical studies should investigate the antibiotic pressures caused by the VAP and its diagnosis in critically ill patients. A suggested algorithm to aid a pragmatic clinical diagnosis of VAP is demonstrated in Fig. 2. Clinical tests should ensure the least possible harm, considering the risks of time delay, invasive procedures and location beyond the specialist care of the ICU environment. Rapid bedside testing of sensitive and specific pulmonary cytokines would benefit from further exploration to improve antibiotic stewardship and diagnostics.
An algorithm for the diagnosis of VAP has been outlined; firstly, using clinical symptoms and features of infection and respiratory deterioration to inform initial suspicion in ventilated patients and to prompt completion of the Clinical Pulmonary Infection Score (CPIS). If uncertainty remains regarding the diagnosis of VAP due to missing data from the CPIS, clinicians are then prompted to complete sputum sampling and chest imaging using available methods to determine diagnosis. Antibiotic therapy is recommended on confirmed diagnosis of VAP.
Further research in the diagnosis of VAP with new investigations and existing usage of data with AI may provide a holistic and pragmatic approach.
References
Kollef, M. H. What is ventilator-associated pneumonia and why is it important? Respir. Care 50, 714–721 (2005).
Zolfaghari, P. S. & Wyncoll, D. L. The tracheal tube: gateway to ventilator-associated pneumonia. Crit. Care 15, 310 (2011).
Safdar, N., Dezfulian, C., Collard, H. R. & Saint, S. Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit. Care Med. 33, 2184–2193 (2005).
Torres, A. et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia. Eur. Respiratory J. 50, 1700582 (2017).
Barbier, F., Andremont, A., Wolff, M. & Bouadma, L. Hospital-acquired pneumonia and ventilator-associated pneumonia: recent advances in epidemiology and management. Curr. Opin. Pulm. Med. 19, 216–228 (2013).
Timsit, J. F., Esaied, W., Neuville, M., Bouadma, L. & Mourvllier, B. Update on ventilator-associated pneumonia. F1000Res 6, 2061 (2017).
Semet, C. The ongoing challenge of ventilator-associated pneumonia: epidemiology, prevention, and risk factors for mortality in a secondary care hospital intensive care unit. Infect. Prev. Pract. 5, 100320 (2023).
Fernando, S. M. et al. Diagnosis of ventilator-associated pneumonia in critically ill adult patients-a systematic review and meta-analysis. Intensive Care Med. 46, 1170–1179 (2020).
Lynch, J. P.III. Hospital-acquired pneumonia: risk factors, microbiology, and treatment. CHEST 119, 373S–384SS (2001).
Wu, D., Wu, C., Zhang, S. & Zhong, Y. Risk factors of ventilator-associated pneumonia in critically III patients. Front Pharm. 10, 482 (2019).
ECDC. European Centre for Disease Prevention and Control. European surveillance of healthcare- associated infections in intensive care units – HAI-Net ICU protocol Stockholm: ECDC. https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/healthcare-associated-infections-HAI-ICU-protocol.pdf. (2015).
Chastre, J. & Fagon, J. Y. Ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 165, 867–903 (2002).
Melsen, W. G. et al. Attributable mortality of ventilator-associated pneumonia: a meta-analysis of individual patient data from randomised prevention studies. Lancet Infect. Dis. 13, 665–671 (2013).
Fabregas, N. et al. Histopathologic and microbiologic aspects of ventilator-associated pneumonia. Anesthesiology 84, 760–771 (1996).
Craven, D. E. & Hjalmarson, K. I. Ventilator-associated tracheobronchitis and pneumonia: thinking outside the box. Clin. Infect. Dis. 51, S59–S66 (2010).
Vincent, J. L. et al. The prevalence of nosocomial infection in intensive care units in Europe. results of the European Prevalence of Infection in Intensive Care (EPIC) study. EPIC International Advisory Committee. Jama 274, 639–644 (1995).
Browne, E. et al. A national survey of the diagnosis and management of suspected ventilator-associated pneumonia. BMJ Open Respir. Res 1, e000066 (2014).
Rello, J. et al. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest 122, 2115–2121 (2002).
Hunter, J. D. Ventilator associated pneumonia. Bmj 344, e3325 (2012).
Li, Y., Liu, C., Xiao, W., Song, T. & Wang, S. Incidence, risk factors, and outcomes of ventilator-associated pneumonia in traumatic brain injury: a meta-analysis. Neurocrit Care 32, 272–285 (2020).
Teixeira, P. J. Z., Seligman, R., Hertz, F., Cruz, D. & Fachel, J. Inadequate treatment of ventilator-associated pneumonia: risk factors and impact on outcomes. J. Hospital Infect. 65, 361–367 (2007).
Domínguez A. A., Arango M. V. & Torres A. Treatment failure in patients with ventilator-associated pneumonia. Semin. Respir. Crit. Care Med. 27, 104–14 (2006).
Gursel, G., Aydogdu, M., Ozyilmaz, E. & Ozis, T. N. Risk factors for treatment failure in patients with ventilator-associated pneumonia receiving appropriate antibiotic therapy. J. Crit. Care 23, 34–40 (2008).
Storms, A. D. et al. Rates and risk factors associated with hospitalization for pneumonia with ICU admission among adults. BMC Pulm. Med. 17, 208 (2017).
Al-Omari, B. et al. Systematic review of studies investigating ventilator associated pneumonia diagnostics in intensive care. BMC Pulm. Med. 21, 196 (2021).
Luckraz, H. et al. Cost of treating ventilator-associated pneumonia post cardiac surgery in the National Health Service: Results from a propensity-matched cohort study. J. Intensive Care Soc. 19, 94–100 (2018).
Mietto, C., Pinciroli, R., Patel, N. & Berra, L. Ventilator associated pneumonia: evolving definitions and preventive strategies. Respir. Care 58, 990–1007 (2013).
Young, P. J., Pakeerathan, S., Blunt, M. C. & Subramanya, S. A low-volume, low-pressure tracheal tube cuff reduces pulmonary aspiration. Crit. Care Med. 34, 632–639 (2006).
Carter, E. L. et al. Strategies to prevent ventilation-associated pneumonia: the effect of cuff pressure monitoring techniques and tracheal tube type on aspiration of subglottic secretions: an in-vitro study. Eur. J. Anaesthesiol. 31, 166–171 (2014).
Niederman, M. S. The clinical diagnosis of ventilator-associated pneumonia. Respir. Care 50, 788–796 (2005). discussion 807-12.
Jackson, L. & Owens, M. Does oral care with chlorhexidine reduce ventilator-associated pneumonia in mechanically ventilated adults? Br. J. Nurs. 28, 682–689 (2019).
Goetz, R. L., Vijaykumar, K. & Solomon, G. M. Mucus clearance strategies in mechanically ventilated patients. Front Physiol. 13, 834716 (2022).
Konrad, F., Schreiber, T., Brecht-Kraus, D. & Georgieff, M. Mucociliary transport in ICU patients. Chest 105, 237–241 (1994).
Lorente, L., Lecuona, M., Jiménez, A., Mora, M. L. & Sierra, A. Ventilator-associated pneumonia using a heated humidifier or a heat and moisture exchanger: a randomized controlled trial [ISRCTN88724583]. Crit. Care 10, R116 (2006).
Deem, S. & Treggiari, M. M. New endotracheal tubes designed to prevent ventilator-associated pneumonia: do they make a difference? Respir. Care 55, 1046–1055 (2010).
Adair, C. G. et al. Implications of endotracheal tube biofilm for ventilator-associated pneumonia. Intensive Care Med. 25, 1072–1076 (1999).
Morris A. C. Management of pneumonia in intensive care. J. Emgy Crit. Care Med. 2 https://doi.org/10.21037/jeccm.2018.11.06 (2018).
Biel, M. A. et al. Reduction of endotracheal tube biofilms using antimicrobial photodynamic therapy. Lasers Surg. Med. 43, 586–590 (2011).
Delle Rose, D. et al. Clinical predictors and microbiology of ventilator-associated pneumonia in the intensive care unit: a retrospective analysis in six Italian hospitals. Eur. J. Clin. Microbiol Infect. Dis. 35, 1531–1539 (2016).
Hellyer, T. P. et al. Diagnostic accuracy of pulmonary host inflammatory mediators in the exclusion of ventilator-acquired pneumonia. Thorax 70, 41–47 (2015).
Azoulay, E. et al. Candida colonization of the respiratory tract and subsequent pseudomonas ventilator-associated pneumonia. Chest 129, 110–117 (2006).
Mandelli, M., Mosconi, P., Langer, M. & Cigada, M. IS PNEUMONIA DEVELOPING IN PATIENTS IN INTENSIVE CARE ALWAYS A TYPICAL “NOSOCOMIAL” INFECTION? Lancet 328, 1094–1095 (1986).
Ben Lakhal, H., M’Rad, A., Naas, T. & Brahmi, N. Antimicrobial Susceptibility among Pathogens Isolated in Early- versus Late-Onset Ventilator-Associated Pneumonia. Infect. Dis. Rep. 13, 401–410 (2021).
ATS. American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 171, 388–416 (2005).
Giantsou, E. et al. Both early-onset and late-onset ventilator-associated pneumonia are caused mainly by potentially multiresistant bacteria. Intensive Care Med. 31, 1488–1494 (2005).
Vallés, J. et al. Excess ICU mortality attributable to ventilator-associated pneumonia: the role of early vs late onset. Intensive Care Med. 33, 1363–1368 (2007).
Restrepo, M. I. et al. Comparison of the bacterial etiology of early-onset and late-onset ventilator-associated pneumonia in subjects enrolled in 2 large clinical studies. Respiratory Care 58, 1220–1225 (2013).
Khan, R. et al. The impact of onset time on the isolated pathogens and outcomes in ventilator associated pneumonia. J. Infect. Public Health 9, 161–171 (2016).
Gunalan, A., Sastry, A. S., Ramanathan, V. & Sistla, S. Early- vs late-onset ventilator-associated pneumonia in critically ill adults: comparison of risk factors, outcome, and microbial profile. Indian J. Crit. Care Med. 27, 411–415 (2023).
TROUILLET, J.-L. et al. Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am. J. Respiratory Crit. Care Med. 157, 531–539 (1998).
Loughlin, L. et al. Pulmonary aspergillosis in patients with suspected ventilator-associated pneumonia in UK ICUs. Am. J. Respiratory Crit. Care Med. 202, 1125–1132 (2020).
Huang, L. et al. Viral reactivation in the lungs of patients with severe pneumonia is associated with increased mortality, a multicenter, retrospective study. J. Med Virol. 95, e28337 (2023).
Hellyer, T. P. et al. Biomarker-guided antibiotic stewardship in suspected ventilator-associated pneumonia (VAPrapid2): a randomised controlled trial and process evaluation. Lancet Respir. Med. 8, 182–191 (2020).
Martin-Loeches, I. et al. The importance of airway and lung microbiome in the critically ill. Crit. Care 24, 537 (2020).
Fernández-Barat, L., López-Aladid, R. & Torres, A. Reconsidering ventilator-associated pneumonia from a new dimension of the lung microbiome. EBioMedicine 60, 102995 (2020).
Fenn, D. et al. Composition and diversity analysis of the lung microbiome in patients with suspected ventilator-associated pneumonia. Crit. Care (Lond., Engl.) 26, 203 (2022).
Zakharkina, T. et al. The dynamics of the pulmonary microbiome during mechanical ventilation in the intensive care unit and the association with occurrence of pneumonia. Thorax 72, 803–810 (2017).
Torres, A. et al. Pneumonia. Nat. Rev. Dis. Prim. 7, 25 (2021).
Quinton, L. J., Walkey, A. J. & Mizgerd, J. P. Integrative physiology of pneumonia. Physiological Rev. 98, 1417–1464 (2018).
Joshi, N., Walter, J. M. & Misharin, A. V. Alveolar macrophages. Cell. Immunol. 330, 86–90 (2018).
Mikacenic, C. et al. Neutrophil extracellular traps (NETs) are increased in the alveolar spaces of patients with ventilator-associated pneumonia. Crit. Care 22, 358 (2018).
Cheng, O. Z. & Palaniyar, N. NET balancing: a problem in inflammatory lung diseases. Front. Immunol. 4, 1 (2013).
Porto, B. N. & Stein, R. T. Neutrophil extracellular traps in pulmonary diseases: too much of a good thing? Front. Immunol. 7, 311 (2016).
Narasaraju, T. et al. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am. J. Pathol. 179, 199–210 (2011).
Conway Morris, A. et al. Diagnostic importance of pulmonary interleukin-1beta and interleukin-8 in ventilator-associated pneumonia. Thorax 65, 201–207 (2010).
Greathouse, K. C. & Hall, M. W. Critical illness-induced immune suppression: current state of the science. Am. J. Crit. Care 25, 85–92 (2016).
Muszynski, J. A., Thakkar, R. & Hall, M. W. Inflammation and innate immune function in critical illness. Curr. Opin. Pediatr. 28, 267–273 (2016).
Halbertsma, F. J., Vaneker, M., Scheffer, G. J. & van der Hoeven, J. G. Cytokines and biotrauma in ventilator-induced lung injury: a critical review of the literature. Neth. J. Med. 63, 382–392 (2005).
Grover, V. et al. A biomarker panel (Bioscore) incorporating monocytic surface and soluble TREM-1 has high discriminative value for ventilator-associated pneumonia: a prospective observational study. PLOS ONE 9, e109686 (2014).
Horn, K. J., Fulte, S., Yang, M., Lorenz, B. P. & Clark, S. E. Neutrophil responsiveness to IL-10 impairs clearance of Streptococcus pneumoniae from the lungs. J. Leukoc. Biol. 115, 4–15 (2023).
Fàbregas, N. et al. Clinical diagnosis of ventilator associated pneumonia revisited: comparative validation using immediate post-mortem lung biopsies. Thorax 54, 867–873 (1999).
Torres, A., Fábregas, N., Arce, Y. & López-Boado, M. A. Histopathology of ventilator-associated pneumonia (VAP) and its clinical implications. Infection 27, 71–76 (1999).
Rouby, J. J. et al. Nosocomial bronchopneumonia in the critically ill. histologic and bacteriologic aspects. Am. Rev. Respir. Dis. 146, 1059–1066 (1992).
Rotstein, C. et al. Clinical practice guidelines for hospital-acquired pneumonia and ventilator-associated pneumonia in adults. Can. J. Infect. Dis. Med Microbiol 19, 19–53 (2008).
Masterton, R. G. et al. Guidelines for the management of hospital-acquired pneumonia in the UK: report of the working party on hospital-acquired pneumonia of the British Society for antimicrobial chemotherapy. J. Antimicrob. Chemother. 62, 5–34 (2008).
Kalil, A. C. et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 63, e61–e111 (2016).
Ego, A., Preiser, J. C. & Vincent, J. L. Impact of diagnostic criteria on the incidence of ventilator-associated pneumonia. Chest 147, 347–355 (2015).
Wang, G., Ji, X., Xu, Y. & Xiang, X. Lung ultrasound: a promising tool to monitor ventilator-associated pneumonia in critically ill patients. Crit. Care 20, 320 (2016).
Kuti, E. L., Patel, A. A. & Coleman, C. I. Impact of inappropriate antibiotic therapy on mortality in patients with ventilator-associated pneumonia and blood stream infection: a meta-analysis. J. Crit. Care 23, 91–100 (2008).
Martin-Loeches, I. et al. Resistance patterns and outcomes in intensive care unit (icu)-acquired pneumonia. validation of European Centre For Disease Prevention And Control (ECDC) and the Centers for Disease Control and Prevention (CDC) classification of multidrug resistant organisms. J. Infect. 70, 213–222 (2015).
Mackenzie, G. The definition and classification of pneumonia. Pneumonia 8, 14 (2016).
Mangram, A. J. et al. Trauma-associated pneumonia: time to redefine ventilator-associated pneumonia in trauma patients. Am. J. Surg. 210, 1056–1061 (2015).
Cavalcanti, M. et al. Risk and prognostic factors of ventilator-associated pneumonia in trauma patients. Crit. Care Med. 34, 1067–1072 (2006).
Niederman, M. S. Hospital-acquired pneumonia, health care-associated pneumonia, ventilator-associated pneumonia, and ventilator-associated tracheobronchitis: definitions and challenges in trial design. Clin. Infect. Dis. 51, S12–S17 (2010).
Horan, T. C., Andrus, M. & Dudeck, M. A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control 36, 309–332 (2008).
Self, W. H., Courtney, D. M., McNaughton, C. D., Wunderink, R. G. & Kline, J. A. High discordance of chest x-ray and computed tomography for detection of pulmonary opacities in ED patients: implications for diagnosing pneumonia. Am. J. Emerg. Med. 31, 401–405 (2013).
Nseir, S. et al. Nosocomial tracheobronchitis in mechanically ventilated patients: incidence, aetiology and outcome. Eur. Respiratory J. 20, 1483–1489 (2002).
Agrafiotis, M., Siempos, I. I. & Falagas, M. E. Frequency, prevention, outcome and treatment of ventilator-associated tracheobronchitis: Systematic review and meta-analysis. Respiratory Med. 104, 325–336 (2010).
Craven, D. E., Hudcova, J. & Lei, Y. Diagnosis of ventilator-associated respiratory infections (VARI): microbiologic clues for tracheobronchitis (VAT) and pneumonia (VAP). Clin. Chest Med. 32, 547–557 (2011).
Mahmoud, M., Towe, C. & Fleck, R. J. CT chest under general anesthesia: pulmonary, anesthetic and radiologic dilemmas. Pediatr. Radio. 45, 977–981 (2015).
Long, L., Zhao, H. T., Zhang, Z. Y., Wang, G. Y. & Zhao, H. L. Lung ultrasound for the diagnosis of pneumonia in adults: A meta-analysis. Med. (Baltim.) 96, e5713 (2017).
Lichtenstein, D. et al. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology 100, 9–15 (2004).
Nonami, S. et al. Incidence of adverse events associated with the in-hospital transport of critically ill patients. Crit. Care Explor 4, e0657 (2022).
Jia, L., Wang, H., Gao, Y., Liu, H. & Yu, K. High incidence of adverse events during intra-hospital transport of critically ill patients and new related risk factors: a prospective, multicenter study in China. Crit. Care 20, 12 (2016).
Beckmann, U., Gillies, D. M., Berenholtz, S. M., Wu, A. W. & Pronovost, P. Incidents relating to the intra-hospital transfer of critically ill patients. Intensive Care Med. 30, 1579–1585 (2004).
Delrue L., et al. Difficulties in the interpretation of chest radiography. https://springerlink.fh-diploma.de/chapter/10.1007/978-3-540-79942-9_2#citeas.(Berlin: Springer; 2011).
Chavez, M. A. et al. Lung ultrasound for the diagnosis of pneumonia in adults: a systematic review and meta-analysis. Respir. Res 15, 50 (2014).
Cardinale, L., Volpicelli, G., Lamorte, A. & Martino, J. Revisiting signs, strengths and weaknesses of standard chest radiography in patients of acute dyspnea in the emergency department. J. Thorac. Dis. 4, 398–407 (2012).
Staub, L. J., Biscaro, R. R. M. & Maurici, R. Accuracy and applications of lung ultrasound to diagnose ventilator-associated pneumonia: a systematic review. J. Intensive Care Med. 33, 447–455 (2018).
Lichtenstein, D. A. BLUE-Protocol and FALLS-Protocol: two applications of lung ultrasound in the critically ill. Chest 147, 1659–1670 (2015).
Mongodi, S. et al. Lung ultrasound for early diagnosis of ventilator-associated pneumonia. Chest 149, 969–980 (2016).
Bouhemad, B., Dransart-Rayé, O., Mojoli, F. & Mongodi, S. Lung ultrasound for diagnosis and monitoring of ventilator-associated pneumonia. Ann. Transl. Med. 6, 418 (2018).
Xirouchaki, N. et al. Lung ultrasound in critically ill patients: comparison with bedside chest radiography. Intensive Care Med. 37, 1488–1493 (2011).
Pradhan, S., Shrestha, P. S., Shrestha, G. S. & Marhatta, M. N. Clinical impact of lung ultrasound monitoring for diagnosis of ventilator associated pneumonia: a diagnostic randomized controlled trial. J. Crit. Care 58, 65–71 (2020).
Gaber, S., Tayeh, O., Wahab, K. A., Mohamed, N. & Essawy, T. Early detection of ventilator-associated pneumonia: bedside tools. Egypt. J. Crit. Care Med. 7, 74–79 (2020).
Nafae, R., Eman, S. R., Mohamad, N. A., El-Ghamry, R. & Ragheb, A. S. Adjuvant role of lung ultrasound in the diagnosis of pneumonia in intensive care unit-patients. Egypt. J. Chest Dis. Tuberculosis 62, 281–285 (2013).
Masterton, R. et al. Hospital-acquired pneumonia guidelines in Europe: a review of their status and future development. J. Antimicrob. Chemother. 60, 206–213 (2007).
Berton, D. C., Kalil, A. C. & Teixeira, P. J. Quantitative versus qualitative cultures of respiratory secretions for clinical outcomes in patients with ventilator-associated pneumonia. Cochrane Database Syst. Rev. 2014, Cd006482 (2014).
Koulenti, D. et al. Spectrum of practice in the diagnosis of nosocomial pneumonia in patients requiring mechanical ventilation in European intensive care units. Crit. Care Med. 37, 2360–2368 (2009).
Bonvento, B. et al. Non-directed bronchial lavage is a safe method for sampling the respiratory tract in critically ill patient. J. Intensive Care Soc. 20, 237–241 (2019).
Perkins, G. D. et al. Safety and tolerability of nonbronchoscopic lavage in ARDS. Chest 127, 1358–1363 (2005).
Flanagan, P. et al. The diagnosis of ventilator-associated pneumonia using non-bronchoscopic, non-directed lung lavages. Intensive care Med. 26, 20–30 (2000).
Shorr, A. F., Sherner, J. H., Jackson, W. L. & Kollef, M. H. Invasive approaches to the diagnosis of ventilator-associated pneumonia: a meta-analysis. Crit. Care Med. 33, 46–53 (2005).
Monard, C. et al. Multicenter evaluation of a syndromic rapid multiplex PCR test for early adaptation of antimicrobial therapy in adult patients with pneumonia. Crit. Care 24, 434 (2020).
Maataoui, N. et al. Impact of rapid multiplex PCR on management of antibiotic therapy in COVID-19-positive patients hospitalized in intensive care unit. Eur. J. Clin. Microbiol. Infect. Dis. 40, 2227–2234 (2021).
Srivastava, S. et al. Utility of a multiplex pathogen detection system directly from respiratory specimens for treatment and diagnostic stewardship. Microbiol Spectr. 12, e0375923 (2024).
Vaz, A. P. et al. [Positive bronchoalveolar lavage and quantitative cultures results in suspected late-onset ventilator associated penumonia evaluation-retrospective study]. Rev. Port. Pneumol. 17, 117–123 (2011).
Fleig, V., Brenck, F., Wolff, M. & Weigand, M. A. Scoring systems in intensive care medicine: principles, models, application and limits. Anaesthesist 60, 963–974 (2011).
Oprita B., Aignatoaie B., Gabor-Postole D. A. Scores and scales used in emergency medicine. practicability in toxicology. J Med Life. 2014; 7 Spec No. 3(Spec Iss 3):4-7.
Bouch, D. C. & Thompson, J. P. Severity scoring systems in the critically ill. Continuing Educ. Anaesth. Crit. Care Pain. 8, 181–185 (2008).
Shan, J., Chen, H. L. & Zhu, J. H. Diagnostic accuracy of clinical pulmonary infection score for ventilator-associated pneumonia: a meta-analysis. Respir. Care 56, 1087–1094 (2011).
Pugin, J. et al. Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic “blind” bronchoalveolar lavage fluid. Am. Rev. Respir. Dis. 143, 1121–1129 (1991).
Schurink, C. A. M. et al. Clinical pulmonary infection score for ventilator-associated pneumonia: accuracy and inter-observer variability. Intensive Care Med. 30, 217–224 (2004).
Gaudet, A. et al. Accuracy of the clinical pulmonary infection score to differentiate ventilator-associated tracheobronchitis from ventilator-associated pneumonia. Ann. Intensive Care 10, 101 (2020).
Zilberberg, M. D. & Shorr, A. F. Ventilator-associated pneumonia: the clinical pulmonary infection score as a surrogate for diagnostics and outcome. Clin. Infect. Dis. 51, S131–S135 (2010). Suppl 1.
Strimbu, K. & Tavel, J. A. What are biomarkers? Curr. Opin. HIV AIDS 5, 463–466 (2010).
Palazzo, S. J., Simpson, T. & Schnapp, L. Biomarkers for ventilator-associated pneumonia: review of the literature. Heart Lung 40, 293–298 (2011).
WHO. World Health Organization. Environmental Health Criteria 222. Biomarkers In Risk Assessment: Validity And Validation Geneva 2001; https://www.inchem.org/documents/ehc/ehc/ehc222.htm (2021).
Kumar, A. & Lodha, R. Biomarkers for diagnosing ventilator associated pneumonia: is that the way forward? Indian J. Pediatrics 85, 411–412 (2018).
Tekerek, N. U., Akyildiz, B. N., Ercal, B. D. & Muhtaroglu, S. New biomarkers to diagnose ventilator associated pneumonia: pentraxin 3 and surfactant protein D. Indian J. Pediatrics 85, 426–432 (2018).
Xu C., Li S., Wang Y., Zhang M., Zhou M. Biomarkers in intensive care unit infections, friend or foe? J. Crit. Care Med. 40, 465–475 (2019).
Hellyer, T. P. The evaluation of a biomarker-based exclusion of ventilator-associated pneumonia to improve antibiotic stewardship. a multi-centre validation study and randomised controlled trial. J. Intensive Care Soc. 20, 245–247 (2019).
Charles, P. E. et al. Serum procalcitonin for the early recognition of nosocomial infection in the critically ill patients: a preliminary report. BMC Infect. Dis. 9, 49 (2009).
Müller, F. et al. Procalcitonin levels predict bacteremia in patients with community-acquired pneumonia: a prospective cohort trial. Chest 138, 121–129 (2010).
Luyt, C. E. et al. Usefulness of procalcitonin for the diagnosis of ventilator-associated pneumonia. Intensive Care Med. 34, 1434–1440 (2008).
Dallas, J. et al. Diagnostic utility of plasma procalcitonin for nosocomial pneumonia in the intensive care unit setting. Respir. Care 56, 412–419 (2011).
Ramirez, P. et al. Sequential measurements of procalcitonin levels in diagnosing ventilator-associated pneumonia. Eur. Respir. J. 31, 356–362 (2008).
Duflo, F. et al. Alveolar and serum procalcitonin: diagnostic and prognostic value in ventilator-associated pneumonia. Anesthesiology 96, 74–79 (2002).
Tanrıverdi, H. et al. Prognostic value of serum procalcitonin and C-reactive protein levels in critically ill patients who developed ventilator-associated pneumonia. Ann. Thorac. Med. 10, 137–142 (2015).
Jensen, J. U. et al. Procalcitonin-guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomized trial. Crit. Care Med. 39, 2048–2058 (2011).
Bopp, C. et al. Soluble TREM-1 is not suitable for distinguishing between systemic inflammatory response syndrome and sepsis survivors and nonsurvivors in the early stage of acute inflammation. Eur. J. Anaesthesiol. 26, 504–507 (2009).
Bouchon, A., Dietrich, J. & Colonna, M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J. Immunol. 164, 4991–4995 (2000).
Palazzo, S. J., Simpson, T. A., Simmons, J. M. & Schnapp, L. M. Soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) as a diagnostic marker of ventilator-associated pneumonia. Respir. Care 57, 2052–2058 (2012).
Anand, N. J., Zuick, S., Klesney-Tait, J. & Kollef, M. H. Diagnostic implications of soluble triggering receptor expressed on myeloid cells-1 in BAL fluid of patients with pulmonary infiltrates in the ICU. Chest 135, 641–647 (2009).
Determann, R. M. et al. Serial changes in soluble triggering receptor expressed on myeloid cells in the lung during development of ventilator-associated pneumonia. Intensive Care Med. 31, 1495–1500 (2005).
Horonenko, G. et al. Soluble triggering receptor expressed on myeloid cell-1 is increased in patients with ventilator-associated pneumonia: a preliminary report. Chest 132, 58–63 (2007).
Gibot, S. et al. Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. N. Engl. J. Med. 350, 451–458 (2004).
Torres, A., Artigas, A. & Ferrer, R. Biomarkers in the ICU: less is more? no. Intensive Care Med. 47, 97–100 (2021).
van de Sande, D., van Genderen, M. E., Huiskens, J., Gommers, D. & van Bommel, J. Moving from bytes to bedside: a systematic review on the use of artificial intelligence in the intensive care unit. Intensive Care Med. 47, 750–760 (2021).
Liang, Y. et al. Early prediction of ventilator-associated pneumonia in critical care patients: a machine learning model. BMC polm. 22, 250 (2022).
Becker J., et al. Artificial intelligence-based detection of pneumonia in chest radiographs. Diagnostics (Basel). 12, 1465 (2022).
Chumbita M., et al. Can artificial intelligence improve the management of pneumonia?. J. Clin. Med. 9, 248 (2020).
Kermany, D. S. et al. Identifying medical diagnoses and treatable diseases by image-based deep learning. Cell 172, 1122–31.e9 (2018).
Stephen, O., Sain, M., Maduh, U. J. & Jeong, D.-U. An efficient deep learning approach to pneumonia classification in healthcare. J. Healthc. Eng. 2019, 4180949 (2019).
Heckerling, P. S., Gerber, B. S., Tape, T. G. & Wigton, R. S. Prediction of community-acquired pneumonia using artificial neural networks. Med. Decis. Mak. 23, 112–121 (2003).
Hwang, E. J. et al. Deep learning for chest radiograph diagnosis in the emergency department. Radiology 293, 573–580 (2019).
Giang, C. et al. Predicting ventilator-associated pneumonia with machine learning. Med. (Baltim.) 100, e26246 (2021).
Bardossy, A. C., Zervos, J. & Zervos, M. Preventing hospital-acquired infections in low-income and middle-income countries: impact, gaps, and opportunities. Infect. Dis. Clin. North Am. 30, 805–818 (2016).
Bonell, A. et al. A systematic review and meta-analysis of ventilator-associated pneumonia in adults in asia: an analysis of national income level on incidence and etiology. Clin. Infect. Dis. 68, 511–518 (2018).
Arabi, Y., Al-Shirawi, N., Memish, Z. & Anzueto, A. Ventilator-associated pneumonia in adults in developing countries: a systematic review. Int J. Infect. Dis. 12, 505–512 (2008).
Mathai, A. S., Phillips, A., Kaur, P. & Isaac, R. Incidence and attributable costs of ventilator-associated pneumonia (VAP) in a tertiary-level intensive care unit (ICU) in northern India. J. Infect. Public Health 8, 127–135 (2015).
Sanders, S., Doust, J. & Glasziou, P. A Systematic review of studies comparing diagnostic clinical prediction rules with clinical judgment. PLOS ONE 10, e0128233 (2015).
Fine, M. J. et al. The hospital admission decision for patients with community-acquired pneumonia. results from the pneumonia patient outcomes research team cohort study. Arch. Intern Med. 157, 36–44 (1997).
Lim, W. S. et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 58, 377–382 (2003).
Ewig, S. et al. Prognostic analysis and predictive rule for outcome of hospital-treated community-acquired pneumonia. Eur. Respir. J. 8, 392–397 (1995).
Spindler, C. & Ortqvist, A. Prognostic score systems and community-acquired bacteraemic pneumococcal pneumonia. Eur. Respir. J. 28, 816–823 (2006).
Author information
Authors and Affiliations
Contributions
F.H. and T.V. contributed to the manuscript's conception, design, preparation, and review. C.C., A.M., N.G., B.P., B.T., J.W., F.G.S., Z.A., and N.A.D. contributed to manuscript preparation and review. All agreed on the final content of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests
Peer review
Peer review information
Nature Communications thanks Antoni Torres, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Howroyd, F., Chacko, C., MacDuff, A. et al. Ventilator-associated pneumonia: pathobiological heterogeneity and diagnostic challenges. Nat Commun 15, 6447 (2024). https://doi.org/10.1038/s41467-024-50805-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-50805-z
- Springer Nature Limited