Abstract
The Na+-Cl− cotransporter (NCC) drives salt reabsorption in the kidney and plays a decisive role in balancing electrolytes and blood pressure. Thiazide and thiazide-like diuretics inhibit NCC-mediated renal salt retention and have been cornerstones for treating hypertension and edema since the 1950s. Here we determine NCC co-structures individually complexed with the thiazide drug hydrochlorothiazide, and two thiazide-like drugs chlorthalidone and indapamide, revealing that they fit into an orthosteric site and occlude the NCC ion translocation pathway. Aberrant NCC activation by the WNKs-SPAK kinase cascade underlies Familial Hyperkalemic Hypertension, but it remains unknown whether/how phosphorylation transforms the NCC structure to accelerate ion translocation. We show that an intracellular amino-terminal motif of NCC, once phosphorylated, associates with the carboxyl-terminal domain, and together, they interact with the transmembrane domain. These interactions suggest a phosphorylation-dependent allosteric network that directly influences NCC ion translocation.
Similar content being viewed by others
Introduction
Thiazide and thiazide-like diuretics, so categorized because the former bears a thiazide ring whereas the latter does not, were first developed more than six decades ago. They remain cornerstones of the clinical management of hypertension and fluid overload conditions due to, for example, congestive heart diseases, nephrotic syndrome, and liver cirrhosis1,2; there are more than 80 million combined prescriptions each year in the United States. Thiazide and thiazide-like diuretics promote salt loss and diuresis by inhibiting Na+ - Cl− cotransporter (NCC)-mediated salt (and obligatory water) retention in the kidney. These diuretic drugs, however, do have notable limitations. These include off-target inhibition of carbonic anhydrases that underlies insulin insensitivity and diabetes in some patients3,4, moderate potencies1,2,5, the presence of a sulfur atom which causes allergy in ∼3–8% of patients6, and photosensitivity associated with an increased risk of skin cancers in patients who need chronic management of their blood pressure7,8. An atomic-level understanding of how these diuretic drugs bind to their receptor sites and antagonize NCC would catalyze a rational approach to develop the next generation of diuretic therapeutics with improved specificity and potency as well as a more favorable side-effect profile. A recent NCC/polythiazide co-structure greatly enhanced our understanding of the pharmacology of thiazide diuretics9, but how thiazide-like diuretics (e.g., chlorthalidone and indapamide), which are anecdotally superior for preventing cardiovascular events10,11, act on and inhibit NCC remains mysterious. Moreover, hydrochlorothiazide is the most prescribed thiazide diuretic in the United States in the form of monotherapy or a single-pill combination with other medications, but we have yet to elucidate its site and mechanism of action.
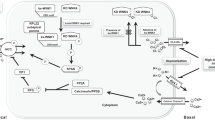
NCC is activated in response to hormones and other physiological stimuli by the WNKs-SPAK kinase cascade that leads to phosphorylation of several key threonine and serine residues located within an intracellular amino (N)-terminal segment of the transporter12,13,14. Gain-of-function mutations in WNK1, WNK4, or inactivating mutations in their upstream ubiquitin E3 ligase degrader CUL3/KLHL3 all lead to hyper-phosphorylation and activation of NCC, resulting in Familial Hyperkalemic Hypertension (FHHt, also called Gordon syndrome)15,16. WNKs-SPAK kinases are counteracted by phosphatases that dephosphorylate and inhibit NCC17. Notwithstanding a decisive role of kinases and phosphatases in tuning NCC activity, it remains obscure if (de)phosphorylation directly controls how rapidly NCC can alternate between transport states (e.g., outward-open, occluded, and inward-open) and hence how fast it can shuttle ions across the membrane. Alternatively, (de)phosphorylation could lead to the recruitment of cellular factors that in turn enhance NCC ion transportation and/or facilitate NCC trafficking to the plasma membrane.
To deepen our understanding of NCC pharmacology and phosphoregulation, we determined co-structures of human NCC individually bound with hydrochlorothiazide, chlorthalidone, and indapamide, which are the top three most prescribed thiazide diuretic drugs in the United States at 2.4, 2.9, and 2.7 Å resolution, respectively. We found that these drugs all occlude NCC ion translocation path by nestling in a pocket that overlaps with the ion binding sites, pinpointing an orthosteric site for future development of novel diuretics using structure-based computational approaches. We also reconstituted the WNK1-SPAK-NCC pathway in HEK293 cells, enabling us to purify authentically phosphorylated NCC (referred to as pNCC) for structure determination. The NCC N-terminal phosphoregulatory motif is disordered or only partially defined in structures when the transporter is not activated by kinases9,18. In contrast, our pNCC maps unambiguously showed that two threonine and one serine residues within the N-terminal motif each bear a PO43- group that enables them to engage in electrostatic interactions with neighboring arginine and lysine residues located on the carboxyl (C)-terminal domain (CTD); the two phosphor-threonine (pThr) and one phosphor-serine (pSer) help to anchor the phosphoregulatory motif to a concave surface on the CTD. The phosphorylation-dependent N-terminal motif/CTD association brings NCC cytosolic domains into close contact with an intracellular vestibule where ions escape into the cytosol. Our structure illustrates how phosphorylation of three residues fosters the formation of a new domain interface that we hypothesize stabilizes NCC in an active conformation that is conducive to rapid isomerization among its transport states. WNKs and SPAK inhibitors have been developed to reduce NCC phosphorylation as new anti-hypertensive drug leads, but they are fraught with unintended side effects because WNKs and SPAK have a myriad of other downstream effectors besides NCC19. Our pNCC structures now set the stage for computational design of mini-protein binders or small molecule compounds that could act as additional allosteric modulators of the NCC phosphoregulatory domain.
Results
Development of an ion flux assay for NCC in HEK293 cells
Currently available NCC ion flux assays show weak ion transport activity possibly due to inadequate phosphorylation and activation of NCC by the upstream WNKs-SPAK kinases9,20,21,22,23,24. To overcome this limitation, we generated a stable HEK293 cell line that co-expresses NCC and a Cl−-quenchable membrane-targeted YFP25. We then incubated these cells in a hypotonic, Cl− and K+ free buffer to activate the endogenous WNKs-SPAK signaling pathway prior to the initiation of ion flux assay (Fig. 1a, b)26. NCC is efficiently activated upon these treatments as indicated by a robust Na+-dependent Cl− influx that plateaus within 1 minute (Supplementary Fig. 1a, b). We further confirmed that the Cl− influx is inhibited by hydrochlorothiazide, indapamide, and chlorthalidone diuretic drugs, but not by loop diuretics such as bumetanide, azosemide, and torsemide (Fig. 1c and Supplementary Fig. 1c); loop diuretics were derived from the same parental molecule, sulfonamide, as thiazide and thiazide-like diuretics, but they inhibit the Na+ - K+ - Cl− cotransporters NKCC1 and NKCC2. NCC-mediated Cl− influx is attenuated by application of WNK463 (a WNKs inhibitor)27 or closantel (a SPAK inhibitor)28, and is enhanced when dephosphorylation is inhibited by calyculin-A (an antagonist of both PP1A and PP2A phosphatases)29 (Fig. 1b, d, and Supplementary Fig. 1d). In conclusion, we have developed a robust and convenient ion flux assay for NCC, enabling us to confirm that NCC activity is oppositely regulated by WNKs/SPAK kinases and phosphatases. Our assay provides a valuable tool to interrogate structure-function relationship, regulation, and pharmacology of NCC in mammalian cells; it can also be adapted to a high-throughput screening platform for the discovery of NCC modulators in the future.
a Schematics of cell-based Cl− flux assay for measuring NCC-mediated ion transport. In this assay, NCC is co-expressed with a membrane-anchored Cl−-sensitive YFP in a HEK293 stable cell line where NCC-mediated Cl− influx quenches YFP fluorescence. b NCC is regulated by (de)phosphorylation catalyzed by WNKs-SPAK kinases and phosphatases. c Average raw traces show that NCC activity is completely blocked by the thiazide-type drugs hydrochlorothiazide (HCTZ), indapamide, and chlorthalidone. d Cell-based Cl− flux assay enables interrogation of NCC phosphoregulation. Phosphatase inhibition (calyculin-A) potentiates, whereas kinase inhibition (WNK463 and closantel) attenuates NCC-mediated Cl− influx, as indicated by reduced rates of quenching of YFP fluorescence. NCC-mediated Cl− influx rates are measured as the slopes of fluorescence change in the first 30 s. Each circle represents one kinetic measurement of a single sample in a 96-well plate. Unpaired one-tailed Student’s t-tests are used for statistical analyses (n = 10; data are presented as mean value ± SD). Source data are provided as a Source Data file.
Structures of phospho-activated NCC individually complexed with the three most prescribed thiazide diuretic drugs
In order to obtain an authentically phosphorylated NCC sample for structural studies, we reconstituted the WNK1 (bearing an activating S378D mutation in its kinase domain)30-SPAK-NCC cascade together with Mo25 (a scaffolding subunit of SPAK)31 in a stable HEK293 cell line (Supplementary Fig. 2a). To further ensure complete phosphorylation of NCC, we treated the cells in a hypotonic, Cl− and K+ free buffer supplemented with calyculin-A, a condition that we discovered maximumly activates NCC in ion flux assay (Fig. 1d). The resulting NCC sample (referred to as pNCC hereafter) was indeed phosphorylated as it was recognized by an antibody that has been used to detect phosphorylated Thr55 (pThr55) of NCC in western blot32 (Supplementary Fig. 2b). We incubated pNCC individually with indapamide, chlorthalidone, and hydrochlorothiazide, and determined the corresponding co-structures at 2.7, 2.9, and 2.4 Å resolution, respectively (Fig. 2, and Supplementary Figs. 3, 4, 5, 6,7, and 8).
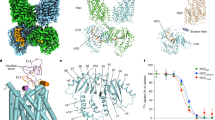
a, b Overall structure of NCC bound with indapamide shown in map (a) and ribbon diagram (b). c, d overall structure of NCC bound with chlorthalidone shown in map (c) and ribbon diagram (d). e, f Overall structure of NCC bound with hydrochlorothiazide shown in map (e) and ribbon diagram (f). Two NCC subunits are colored-coded blue and khaki. ATP, phosphoacceptors, indapamide, hydrochlorothiazide, and chlorthalidone are highlighted. The N-terminal phosphoregulatory region is shown in cyan with the phosphorylation sites highlighted in red.
We observed that Thr55, Thr60, and Ser73 each clearly bear a PO43- group in our pNCC/indapamide, pNCC/chlorthalidone, and pNCC/hydrochlorothiazide co-structures (Fig. 2, and Supplementary Fig. 9 and 10), defining the elusive phosphoregulatory apparatus of NCC. Our maps also unambiguously defined the poses of indapamide, chlorthalidone, hydrochlorothiazide, and adenosine triphosphate (ATP) in their respective binding sites (Fig. 2, and Supplementary Figs. 9 and 10). Our maps further identified ordered water molecules: some of them contribute to the binding of the diuretic drugs to their receptor sites and another fosters association between the transmembrane domain (TMD) and cytosolic domains upon phosphorylation (Supplementary Figs. 9 and 10).
The pNCC/indapamide structure assumes a dimeric architecture, and each subunit consists of an intracellular N-terminal domain (NTD) that harbors phosphoacceptor residues, a TMD composed of 12 transmembrane helices, and an intracellular CTD. The two NCC subunits associate extensively via CTDs, whereas their TMDs barely interact. Intriguingly, only one TMD of NCC is in complex with indapamide and is accordingly arrested in an outward-open state; this TMD is seen to tightly associate with cytosolic domains beneath (Fig. 2a). In contrast, the second TMD is in an apo state (i.e., without a bound indapamide molecule) and assumes an inward-open conformation (Fig. 2a); this TMD is detached from cytosolic domains and is resolved at lower resolution possibly because it is mobile with respect to the remaining parts of NCC (Supplementary Fig. 4b). Chlorthalidone and hydrochlorothiazide trap pNCC in a similar asymmetric dimer except that the second TMD, possibly in an apo state as well, becomes completely invisible in our final maps (Fig. 2c), albeit recognizable in lower resolution intermediate maps during data processing. Why indapamide (and possibly chlorthalidone and hydrochlorothiazide) only binds to one subunit and the significance of co-existing outward- and inward-open states in an NCC dimer are questions that await future investigations.
Indapamide, chlorthalidone, and hydrochlorothiazide target an orthosteric site located at the midway of NCC ion translocation path
Indapamide, chlorthalidone, and hydrochlorothiazide are the three most prescribed thiazide diuretics in the United States33, but their sites and mechanisms of action remain unclear. Our pNCC/indapamide, pNCC/chlorthalidone, and pNCC/hydrochlorothiazide co-structures showed that all these drugs nestle in a pocket located at roughly midpoint of the ion translocation path, and occlude an extracellular vestibule (Fig. 3g and Supplementary Fig. 11). Indapamide, chlorthalidone, and hydrochlorothiazide conform to the definition of orthosteric antagonists as these drugs compete with ion substrates for overlapping binding sites and interact extensively with residues lining the ion translocation path (Supplementary Fig. 12). Indapamide, chlorthalidone, and hydrochlorothiazide arrest NCC in an outward-open conformation, and in doing so, inhibit NCC as the transporter can no longer oscillate among its transport states to escort ions across the plasma membrane (Supplementary Fig. 11). Overall, indapamide, chlorthalidone, and hydrochlorothiazide share the same inhibitory mechanism with the loop diuretic drug bumetanide, the K+ - Cl- cotransporters (KCCs) antagonist VU0463271, which arrest NKCC1 and KCC1 in an almost identical outward-open state (Supplementary Fig. 13a), respectively9,34,35.
a A view of indapamide binding pocket highlights key coordinating residues. Density and chemical structure of indapamide are also shown. b N226A shows reduced sensitive to indapamide inhibition. c A view of chlorthalidone binding pocket highlights key coordinating residues. Density and chemical structure of chlorthalidone are also shown. d N226A shows reduced sensitivity to chlorthalidone inhibition. e A view of hydrochlorothiazide (HCTZ) binding pocket highlights key coordinating residues. Density and chemical structure of HCTZ are also shown. f N226A shows reduced sensitivity to HCTZ inhibition. g Indapamide, hydrochlorothiazide, and chlorthalidone bind to a deeper pocket in NCC than bumetanide binds to NKCC1. Also note, the sulfamoyl group of thiazide drugs (indapamide, hydrochlorothiazide, and chlorthalidone) and loop diuretics (bumetanide) assumes either an ‘up’ or ‘down’ pose, respectively. Unpaired one-tailed Student’s t-tests are used for statistical analyzes (n = 4; data are presented as mean value ± SD). Source data are provided as a Source Data file.
Indapamide nestles in the orthosteric site in a pose such that its sulfamoyl group on one end reaches deep into NCC ion translocation path, its bicyclic indole rings on the other end sit beneath the extracellular ion entryway, and its middle benzamide group resides in between. Indapamide interacts extensively with residues from TM1, TM3, TM6, TM8, and TM10, many of which project their side chains into the ion translocation path, including His233 that would otherwise coordinate a Na+ ion18 (Fig. 3a, Supplementary Fig. 12). At approximately the midpoint of the membrane, indapamide’s sulfamoyl group forms hydrogen bonds with Asn226 and Asn148. NCC with Asn226 mutated to alanine (N226A) retains ∼50% ion transport activity, but exhibits drastically reduced sensitive to inhibition by indapamide because this mutant is expected to be incapable of engaging with the sulfamoyl group (Fig. 3b, and Supplementary Figs. 14 and 15). Our results are consistent with the fact that the sulfamoyl group proves to be indispensable for the diuretic activity of both thiazide and thiazide-like drugs, preserved after extensive medicinal chemistry optimization efforts33. Moving above toward the extracellular side, indapamide’s aromatic benzamide ring participates in π–π stacking interactions with Phe535 and hydrophobic interactions with Cys471. Meanwhile, the oxygen atom in the benzamide group forms hydrogen bonds with Asn148 and His233. The chlorine atom in the benzamide group encroaches into the so-called Cl− site 2 and thus precludes Cl− from binding to this site via steric hindrance (Supplementary Fig. 12). This plausibly explains why increasing Cl− (and I−) concentration diminishes binding of thiazide diuretics20,36,37. Moving further up toward the extracellular side, indapamide’s bicyclic indole rings form π–π stacking interactions with His233.
Chlorthalidone assumes a similar sulfamoyl group down and bicyclic indole rings up pose as does indapamide when viewed parallel to the membrane (Fig. 3c). Chlorthalidone’s sulfamoyl group engages in the same set of interactions with Asn226 and Asn148 as described above for indapamide. Indeed, the N226A mutant also exhibits drastically decreased sensitive to inhibition by chlorthalidone (Fig. 3d and Supplementary Fig. 15). Chlorthalidone’s benzene ring lacks the oxygen and nitrogen atoms found in indapamide’s benzamide group but preserves the capability to participate in π–π stacking interactions with Phe535. Chlorthalidone’s bicyclic indole rings, however, bear an extra hydroxyl group as compared to those of indapamide, and this hydroxyl group engages in hydrogen-bonding interactions with His233 in a manner that is analogous to the oxygen atom in indapamide’s benzamide group. Of note, chlorthalidone’s indole rings are almost flipped by 180° as compared to those of indapamide to enable favorable interactions with NCC (Fig. 3a, c).
Hydrochlorothiazide binds to the same pocket as do indapamide and chlorthalidone, adopting as similar sufamoyl group down and thiazide ring up pose. At approximately midway of the membrane, hydrochlorothiazide’s sulfamoyl group engages in polar interactions with Asn148 on TM1, Asn226 on TM3, and Asn358 on TM6 (Fig. 3e and Supplementary Fig. 12). The N226A mutant similarly exhibits dramatically reduced sensitivity to hydrochlorothiazide inhibition (Fig. 3f and Supplementary Fig. 15). Moving up toward the extracellular side, hydrochlorothiazide’s benzothiadiazine group participates in π–π stacking interactions with Phe535 via its bicyclic ring; the dioxo substitutions and nitrogen atom of benzothiadiazine form hydrogen bonds with His233 on TM3 and Thr351 on TM6, respectively (Fig. 3e and Supplementary Fig. 12). We note that Thr351 and Cys471 within the thiazide pocket of human NCC are replaced with Ile370 and Phe491 in eNCCβ, respectively. We suspect that these two bulky substitutions would sterically hinder thiazide diuretics binding and render eNCCβ insensitive to these drugs38,39 (Supplementary Fig. 16).
Overall, thiazide and thiazide-like diuretic drugs utilize analogous chemical groups to interact with a similar set of residues lining the NCC ion translocation path. For instance, both classes of diuretics use their shared sulfamoyl group to interact with Asn226 and Asn148, and rely on a benzothiadiazine or benzene ring to engage in π–π stacking interactions with Phe535, and employ an oxygen atom substitution of benzothiadiazine, or benzamide ring, or indole rings to form hydrogen bond with His233 (Supplementary Fig. 17). Of note, His233 not only coordinates Na+ in the so-called Na+ site 1 in the context of NCC transport cycle, but also is co-opted as a key anchor residue for thiazide and thiazide-like diuretics binding. This explains why Na+ competes with thiazide and thiazide-like diuretics for binding to their respective sites20. Of note, we also observed an ordered water molecule that resides within hydrogen-bonding distance from several atoms of indapamide (Supplementary Fig. 12); this water molecule also participates in hydrogen-bonding interaction with Thr351, thus hinting at a water-mediated ligand/protein interaction that should be considered in future computation-based drug discovery efforts. Overall, NCC/thiazide co-structures stand in contrast to our NKCC1/bumetanide co-structure34, in which Tyr383 (equivalent to His233 in NCC) remains to coordinate a K+ ion and has a negligible role in drug binding (Fig. 3g).
CTD of NCC bears an ATP-binding site
In our NCC/indapamide and NCC/hydrochlorothiazide maps, a non-protein density with a shape resembling an ATP molecule was observed in a polar pocket in the CTD (Fig. 4a). ATP-binding site has been reported in KCC1 and NCC9,40, but our 2.4−2.7 Å maps precisely defined ATP’s pose and its interactions with residues on NCC. When nestled within the polar pocket in NCC, ATP assumes a compact conformation with its γ-phosphate group packed against the ribose group (Fig. 4a). Such a compact ATP conformation is often observed in allosteric ATP-binding sites in other proteins41. Conversely, ATP tends to be fully extended within sites when it is used as a substrate by kinases and other enzymes41. In the polar pocket, ATP’s adenine group interacts with Val676, Leu647, and His675, and its ribose group forms hydrogen bonds with the main chain nitrogen atom of Gly740 and with the sidechain of Arg654, while its three phosphate moieties participate in polar interactions with Arg654, Asn780, and Lys742 (Fig. 4b). Substitution of Arg654 with alanine (R654A) drastically decreased NCC ion transport activity (Fig. 4c). Alanine substitution of other ATP-coordinating residues (i.e., His675, Asn780, and Lys742) showed no statistically significant effect on NCC ion transport activity (Supplementary Fig. 18a). Together, these data suggest that Arg654 plays a dominant role in binding ATP possibly because it simultaneously interacts with the adenine, ribose, and phosphate moieties of ATP. Of note, mutations of Arg654 are associated with Gitelman syndrome42, providing a human genetic evidence in support of our structure and associated functional studies; Gitelman syndrome represents a phenotypic ‘mirror image’ of FHHt and is characterized by salt wasting and low blood pressure. Intriguingly, we found that R654A mutant exhibits a reduced phosphorylation level as compared to the wildtype transporter (Fig. 4d). It remains to be determined whether ATP-binding stabilizes NCC in a conformation that is more permissive for kinases to act on.
a ATP nestles in a polar pocket in CTD. Well resolved ATP density enables modeling of the entire molecule. b An enlarged view highlights ATP-coordinating residues on NCC. c, d The ATP-binding mutant R654A shows no ion flux activity (a) and exhibits reduced phosphorylation level (d). In c each circle represents one kinetic measurement of a single sample in a 96-well plate. Unpaired one-tailed Student’s t-tests are used for statistical analyzes (n = 6; data are presented as mean value ± SD). In d western blot image (top) and statistical quantification of phosphorylation level of R655A (bottom). Unpaired one-tailed Student’s t-tests are used for statistical analyzes (n = 6; data are presented as mean value ± SD). Source data are provided as a Source Data file.
NCC phosphorylation catalyzes formation of new domain interfaces
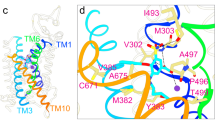
NCC and the related NKCC1 and NKCC2 transporters share a conserved regulatory mechanism whereby (de)phosphorylation of several key threonine and serine residues located in their NTD43,44,45, catalyzed by the opposing actions of WNKs-SPAK kinases and phosphatases, tunes their ion transport activities in response to physiological cues (e.g., vasopressin hormone)46,47,48. Mutation of the phosphoacceptor residue Thr60 in NCC causes Gitelman syndrome49, underscoring a fundamental role of NCC phosphoregulation in kidney salt handling and blood pressure/volume homeostasis. Our three NCC/thiazide maps unambiguously define the elusive N-terminal phosphoregulatory segment (residues Cys52 to Leu93) which is seen to interact extensively with the swapped CTD of the second subunit and the intracellular loop 1 (ICL1; residues Thr193-Gly211) connecting the TM2 and TM3 helices (Fig. 5a). In the N-terminal motif, three phosphoacceptors (i.e., Thr55, Thr60, and Ser73) are each conjugated with a negatively charged PO43- group that enables them to interact with adjacent positively charged arginine and lysine residues on CTD and TMD (Fig. 5a, c). For instance, pThr55 and pThr60 form salt bridges with Arg881 and Lys962 protruding from the swapped CTD, respectively, while pSer73 participates in electrostatic interactions with Lys196 and Lys198 located at the ICL1 of TMD.
a Phosphorylation of Thr55, Thr60, and Ser73 strengthens NTD-CTD and NTD-TMD association. b Weakening phosphorylation-induced interactions abolishes or reduces NCC ion transport rates, measured as the slopes of fluorescence change in the first 20 s. Each circle represents one kinetic measurement of a single sample in a 96-well plate. Unpaired one-tailed Student’s t-tests are used for statistical analyzes (n = 6; data are presented as mean value ± SD). c pThr55, pThr60, and pSer73 are docked into corresponding densities. Source data are provided as a Source Data file.
The ICL1 represents the most conserved stretch of amino acid sequence in cation-chloride cotransporters (CCCs), and in NKCC1 and KCC1, it gates ion exit at the intracellular side34,35,50. Of note, amino acid sequences of ICL1 (and the preceding cytoplasmic end of TM2) differ in three NKCC2 isoforms, partly dictating their distinct ion transport characteristics that are tailored to fulfill their specialized roles in specific regions of the kidney51,52. In our NCC structures, ICL1, alongside other structural elements such as the intracellular gate formed by Lys477 and Asp362, occludes an intracellular path for ion escape into cytosol (Supplementary Figs. 19d and 20). A comparison of inward- and outward-open NCC structures showed that the ICL1 undergoes subtle but notable movements as NCC proceeds along the transport cycle (Supplementary Fig. 19d). It is therefore conceivable that the N-terminal phosphoregulatory segment, once phosphorylated and complexed with CTD, could accelerate NCC isomerization among transport states via interactions with the ICL1. Weakening these phosphorylation-induced interactions by alanine substitutions (i.e., T55A, T60A, S73A, K196A, K198A, R881A, K962A, and R965A) either completely inactivates NCC or substantially decreases its ion transport activity in ion flux assay (Fig. 5b and Supplementary Fig. 18b). Our structure-inspired mutagenesis studies agree with and extend previous reports that only examined the phosphoacceptor residues44. In conclusion, pThr55, pThr60, and pSer73 function as footholds for the N-terminal phosphoregulatory segment to engage with CTD and TMD: pThr55 and pThr60 help to anchor the phosphoregulatory segment to a concave surface on CTD, while pSer73 promotes the association of the resulting NTD/CTD structure with TMD. Together, these phosphorylation-induced domain interfaces may stabilize NCC in an active conformation that is conducive to rapid ion flux.
Plasticity in mode of assembly of NCC dimers
Our pNCC/indapamide dimer unexpectedly showed that the two subunits are not related by two-fold symmetry as often observed in CCCs53; it further revealed that two transport states (i.e., outward- and inward-open states) and two modes of TMD engagement with cytosolic domains can co-exist in an NCC dimer. On the one side, one TMD (subunit A), which is arrested by indapamide in an outward-open state, meets and tightly associates with the phosphorylated NTD (pNTD)/CTD structure positioned just beneath the membrane via polar interactions with a buried surface area of 682 Å2 (Fig. 6a). On the other side, the second TMD (subunit B) adopts an apo and inward-open state and barely contacts cytosolic domains. In the former case, the TMD/cytosolic domain interface primarily involves ICL1 and ICL5 (residues Asn553-Asn565 connecting the TM10 and TM11 helices) of TMD, a short loop centered around pSer73 of NTD, and CTD in particular its extreme C-terminal tail (C-tail; residues Arg1008-Gln1020) (Fig. 6a). Here, residues Arg558, Asn553, and Ser554 on ICL5 establish polar interactions with residues Tyr847 and Arg851 on CTD (Fig. 6b). Asn194 on ICL1 interacts with CTD residue Arg886 (Fig. 6b). The C-tail assumes an extended configuration, associates extensively with pNTD as it threads its way toward TMD where it meets and interacts with ICL1 and ICL5 via its terminal residues Thr1016 and Tyr1018. Of note, on the other side where TMD and cytosolic domains barely contact, the C-tail is completely disengaged from ICL1 (Fig. 7d). Perhaps the most revealing evidence of phosphorylation fostering formation of the TMD/cytosolic domain interface is that pS73’s PO43- group establishes polar interactions with Lys196 and Lys198 on ICL1, either through direct electrostatic attraction between opposite charges or via a bridging water molecule (Fig. 5a). Weakening the TMD/cytosolic domain interface by mutations (i.e., Y1018A, R851A, R558A, and R886A) manifestly decreases NCC ion transport activity (Fig. 6c and Supplementary Fig. 18c), confirming the functional significance of allosteric interactions between TMD and cytosolic domains seen in our structure.
a The TMD-cytosolic domain interface primarily involves NTD, C-tail, ICL1, and ICL5. b A zoomed view highlights contacts at the TMD-cytosolic domain interface. c Weakening the TMD and cytosolic domain interface abolishes or reduces NCC ion transport rates, measured as the slopes of fluorescence change in the first 20 s. Each circle represents one kinetic measurement of a single sample in a 96-well plate. Unpaired one-tailed Student’s t-tests are used for statistical analyzes (n = 6; data are presented as mean value ± SD). Source data are provided as a Source Data file.
a Superimposition of one subunit of NKCC1/bumetanide and two individual subunits of NCC/indapamide highlights that the loop connecting TM12 and scissor helix can assume distinct conformations. b, c The TM12/scissor helix loop associates with C-tail and ICL1 (b) and weakening these interactions reduces NCC ion transport rate (c). In c Cl− influx rates are measured as the slopes of fluorescence change in the first 20 s. Each circle represents one kinetic measurement of a single sample in a 96-well plate. Unpaired one-tailed Student’s t-tests are used for statistical analyzes (n = 12; data are presented as mean value ± SD). d The TM12/scissor helix loop can disengage from ICL1 and assumes an alternative conformation stabilized by interactions with CTD. Source data are provided as a Source Data file.
The coexistence of tight and weak TMD/cytosolic domain interfaces in NCC coincides with formation of a dimeric architecture distinct from NCC/polythiazide and NKCC1/bumetanide co-structures9,34. Comparison of NCC/indapamide, NCC/polythiazide, and NKCC1/bumetanide structures showed that their C-terminal domain dimers superimpose quite well (Supplementary Fig. 19a). However, a flexible loop, which connects TM12 and the CTD scissor helix, adopts different conformation in these structures, giving rise to drastically distinct placement of their TMDs within the lipid bilayer (Fig. 7a and Supplementary Fig. 19b). In the NCC subunit in which TMD associates extensively with cytosolic domains, the loop interacts with the ICL1 helix of the same subunit and C-tail of the swapped CTD via hydrogen bond and cation-π interactions as also observed in the NKCC1 transporter34 (Fig. 7b). In the second NCC subunit, this loop is completely disengaged from ICL1 and primarily interacts with the swapped CTD (Fig. 7d). Of note, Trp611 in the loop can alternatively form cation-π interactions with Arg208 (ICL1) in the former or Arg641 (CTD) in the latter, which may drive this loop to assume two distinct conformations (Fig. 7b, d). Of note, the R641G variant in homozygous state leads to the salt-wasting Gitelman syndrome, whereas in heterozygous state protects against hypertension54. Site-directed mutations (i.e., H636A, N610A, W611A, R208A, and Q1020A) designed to weaken these interactions all attenuate NCC ion transport activity (Fig. 7c and Supplementary Fig. 18d). Together, human genetics and mutagenesis studies support that dynamic interactions among the TM12/scissor helix loop, ICL1, and C-tail are critical to NCC function.
Discussion
We propose a tentative phosphoregulatory model for NCC, which we believe is also applicable to NKCC1 and NKCC2, two other Na+-dependent CCCs (Supplementary Fig. 21). When NCC is dephosphorylated and inactive as represented by PDB 7YG0, its NTD cannot stably associate with CTD because pThr55-, pThr60-, and pSer73-mediated electrostatic interactions are missing. Cytosolic NTD and CTD, when separated from each other, are incapable of associating with and regulating the ion translocation path housed in TMDs. Inactive NCC also features strong association of two TMDs within the lipid bilayer. Our NCC/indapamide, NCC/hydrochlorothiazide, and NCC/chlorthalidone co-structures possibly represent NCC trapped in a partially one-subunit activated state in which the second subunit is en route to assume the active conformation. Here, only one pNTD is seen to associate with CTD and the resulting NTD/CTD structure engages with the TMD above, fostering formation of an interface that couples ICL1, C-tail, pNTD, and the TM12/scissor helix loop. In the meantime, the second TMD unit barely contacts the cytosolic domains and it also loosely associates with the first TMD unit in the membrane; a lack of contact with the remaining parts of NCC may explain why this TMD unit exhibits lower local resolution in the NCC/indapamide map and is completely invisible in the NCC/chlorthalidone and NCC/hydrochlorothiazide maps. In the fully activated state possibly represented by our NKCC1/bumetanide co-structure34, the N-terminal phosphoregulatory segment in both subunits are seen to associate with CTD, and the resulting two NTD/CTD structures each engage with one TMD unit above; formation of two symmetric TMD/cytosolic domain interfaces leads to separation of two TMD units within the lipid bilayer. Whether all these NCC and NKCC1 dimers exist in native cell and if they convert from one form to another in response to actions of kinases and phosphatases await future studies, for example, using single-molecule fluorescence energy transfer (smFRET) which has been successfully employed to monitor conformational changes of CFTR at single-molecule precision55.
Thiazide and loop diuretics promote natriuresis and diuresis by inhibiting two distinct ion transport systems in the kidney, NCC and NKCC2, respectively56, although they were developed from the same parental compound called sulfonamide based on the serendipitous discovery that sulfonamide, used as an antibiotic at the time, exhibits diuretic properties57. How can thiazide and loop diuretics discriminate NCC and NKCC2 given that both classes of drugs evolved from sulfonamide and retain the sulfamoyl group and that NCC and NKCC2 share high sequence and structure similarity? Breakthroughs in NCC and NKCC1 (an NKCC2 ortholog that is equally inhibited by loop diuretics such as bumetanide) structural pharmacology have started to shed light on this long-standing mystery9,34. Thiazide and loop diuretics assume an almost opposite pose in their respective receptor sites when the shared sulfamoyl group is used as a reference (Fig. 3g). In NCC co-structures, the sulfamoyl group of hydrochlorothiazide, indapamide, and chlorthalidone points downward and almost reaches halfway into the membrane. Thiazide and thiazide-like diuretics all bear a chorine atom that clashes with the Cl− site 2. In contrast, our NKCC1/bumetanide co-structure showed that bumetanide occupies a more superficial pocket with its sulfamoyl group pointing toward the extracellular side and its carboxyl group facing down to coordinate a co-occluded K+ ion. Atomic-level understanding of thiazide and loop diuretics pharmacology now makes it possible to rationally design NCC and NKCC2 orthosteric site-targeting small molecule therapeutics with improved potency and fewer side effects (e.g., sulfur-free compounds for treating patients who are contraindicated because of sulfur allergy6). In the same vein, by exploring an exceptionally vast chemical space afforded by in silico docking based on co-structures of NCC and NKCC1 bound with diuretic drugs, it may be feasible to discover compounds that simultaneously inhibit both NCC and NKCC2; such dual antagonism is desirable to resolve drug-resistant edema that often afflicts patients with congestive heart diseases and liver cirrhosis1,2,58.
Protein phosphorylation is a ubiquitous post-translational modification that functions as a molecular switch for the regulation of a broad range of biological processes, including ion transport across cellular membranes through channels and transporters. Deciphering how kinases tune the activities of their channel and transporter clients using a structural approach remains a technical challenge because it requires the preparation of membrane protein samples that are authentically phosphorylated by their cognate kinases. Our integrated approach of co-expressing NCC together with its cognate kinases, coupled with activating conditions validated by a cell-based assay, could serve as a roadmap for mechanistic studies of phosphoregulation of other transporters and ion channels. We showed that NCC phosphorylation fosters allosteric coupling among distinct domains of the transporter to directly accelerate the ion transport rate, although we cannot formally rule out that recruitment of as-yet unknown partners to the phosphoacceptor sites leads to NCC activation. In general, our finding that phosphorylation acts as an allosteric switch in NCC is conceptually similar to regulation of the ATP-binding cassette proteins CFTR and Ycf1 by kinases59,60. Both proteins have evolved a regulatory (R) domain inserted between two nucleotide-binding domains (NBDs), and in basal state, the R domain sterically hinders dimerization of the two NBDs, a prerequisite step along their activation path; once phosphorylated by kinases, the R domain is dislodged from its auto-inhibitory position to allow dimerization of NBDs and subsequent activation-associated conformational changes. Our pNCC structures now set the stage to target the NCC phosphoregulatory interfaces to generate diuretic therapeutics for the treatment of hypertension.
Methods
Expression and purification of human NCC in phosphorylated state
We used full-length human NCC (GenBank: KAI4055015.1) with an N-terminal Twin-Strep tag for both structural and functional studies (Supplementary Fig. 2a). We further used a bi-directional PiggyBac transposon vector to co-express NCC together with kinases and mbYFPQS in HEK293T/17 SF (ATCC ACS-4500) stable cell lines61. For structural studies, we cloned Twin-Strep-NCC into the multiple cloning site I (MCSI) of the vector, and the WNK1 (1-483 S378D)-Mo25-mbYFPQS-SPAK expression cassette into the MCSII of the vector; WNK1, Mo25, and SPAK are all human genes, and mbYFPQS is sensitive to [Cl−] and bears an N-terminal myristylation sequence for membrane targeting25, and these four genes are separated by a P2A sequence62. For functional studies, the wildtype Twin-Strep-NCC and mutants were cloned into the MCSI site and mbYFPQS was cloned into the MCSII site.
To prepare authentically phosphorylated NCC for structural studies, the stable cell line co-expressing NCC and kinases was grown in suspension in Freestyle 293 expression medium (Invitrogen, Carlsbad, CA) at 37 °C in an orbital shaker; protein expression was induced by adding 1 μg/ml doxycycline when the cell density reached ∼1.5 × 106/ml. 20 μM indapamide or 20 μM chlorthalidone was added during protein expression to suppress cell death caused by constitutive WNK1 and NCC activation. Cells were harvested 36 h post induction, and then suspended into a hypotonic, Cl− and K+ free buffer composed of 20 mM HEPES (pH=7.4), 45 mM (NMDG)2SO4, 0.5 μM Calyculin-A supplemented with 100 μM indapamide, or 100 μM chlorthalidone, or 100 μM hydrochlorothiazide for another 2 h with gentle shaking in 37 °C. Cells were then broken with a Dounce homogenizer, and membranes were harvested by centrifugation at 100,000 × g for 1 h and resuspended in a buffer composed of 20 mM HEPES (pH = 7.4), 75 mM K2SO4,100 μM indapamide or 100 μM chlorthalidone, and flash frozen in liquid nitrogen and stored at −80 °C until use. Our initial attempt to determine a wildtype pNCC bound with hydrochlorothiazide was not successful. We therefore used NCC_S344E mutant to overcome the obstacle with the rationale that this mutant may bind hydrochlorothiazide more tightly than the wildtype NCC, analogous to the equivalent NKCC1_A492E mutant that has been shown to exhibit enhanced binding affinity for loop diuretics.
All protein purification steps were carried out at 4 °C unless stated otherwise. Membrane proteins were extracted for 2 h at 4 °C in a buffer composed of 20 mM HEPES (pH7.4), 75 mM K2SO4, 100 μM indapamide (or chlorthalidone), 3 mM lauryl maltose neopentyl glycol (LMNG-3), and 0.6 mM cholesteryl hemisuccinate tris salt (CHS), 5 μg/ml leupeptin, 1.4 μg/ml pepstatin A, and 2 μg/ml aprotinin. The supernatant was collected after centrifugation at 100,000 × g for 30 mins and then incubated with Strep-Tactin resin (IBA, Strep-tactin@XT 4flows) for 2 h. Contaminant proteins were removed by washing with 15 column volume of buffer composed of 20 mM HEPES (pH7.4), 75 mM K2SO4, 100 μM indapamide (or chlorthalidone, or hydrochlorothiazide), and 0.02%GDN. NCC protein was eluted from the resin with a buffer composed of 20 mM HEPES (pH7.4), 75 mM K2SO4, 100 μM indapamide (or chlorthalidone, or hydrocholorothiazide), 50 mM biotin, and 0.02%GDN. NCC was further separated with a Superose 6 column using a buffer composed of 20 mM HEPES (pH7.4), 75 mM K2SO4, 100 μM indapamide (or chlorthalidone, or hydrochlorothiazide), and 0.01% GDN; peak fractions corresponding to NCC were pooled and further supplemented with indapamide (or chlorthalidione, or hydrochlorothiazide) to a final concentration of 800 μM, and then concentrated for Cryo-EM analyzes.
Cl− influx assay in HEK293 cells
The NCC-mediated Cl− influx was measured using a membrane targeted yellow fluorescent protein (mbYFPQS) as a Cl− indicator25. Briefly, ∼1.0 × 105 Twin-Strep-NCC/mbYFPQS stable HEK293 cells were seeded per well in a poly-D-lysine treated, black-walled, clear-bottom 96-well plate, and 1 μg/ml doxycycline was added to induce protein expression. HEK293 cells only expressing mbYFPQS was included as a negative control in all experiments. 24–36 h post induction, the medium was replaced by 100 μl activation buffer (20 mM HEPES, 45 mM (NMDG)2SO4, 0.5 uM calyculin-A, 100 uM Dicoumarol), and incubated for 2–3 h prior to assay. The activation buffer was exchanged to 100 μl assay buffer (20 mM HEPES, 140 mM NaCl, pH7.4) to initiate NCC-mediated Cl− influx; 100 μM thiazide or thiazide-like drugs was also added to validate that observed Cl− influx was mediated by NCC. Fluorescence intensity was measured on a BioTek Synergy Neo2 HTS Multi-Mode Microplate Reader (excitation/emission wavelengths are 485 nm/535 nm). The rates of Cl− transport were calculated as the slopes of the fluorescent intensity change within the initial 30 s. Unpaired Student’s t-test was used to evaluate the significance of NCC-mediated, thiazide-sensitive transport activity.
Western blot
The HEK293 stable cells expressing wildtype NCC or designed mutants were seeded ∼5.0 × 105 cells per well in a standard 24-well culture plate; protein expression was induced by adding 1 μg/ml doxycycline. 36 h post induction, the cells were harvested and suspended in the activation buffer (20 mM HEPES, 45 mM (NMDG)2SO4, 0.5 μM calyculin-A) for 2 h. The cells were then harvested and lysed in PBS supplemented with 1% SDS followed by brief sonication. The cell debris was removed by centrifugation at 20,000 × g for 10 mins. 10 μl supernatant was loaded and separated on a SDS-PAGE gel. Anti-NCC pThr53 (Phosphosolutions; Cat. # p1311-53, 1:2000 dilution) and anti-Strep (IBA; Cat. # 2-1507-001, 1:500 dilution) were used to detect pNCC and total NCC, respectively.
Electron microscopy sample preparation and data collection
For cryo-EM, 3.5 μl of NCC sample at ∼3–5 mg/ml was applied to a glow-discharged Au 1.2/1.3 holey, 300 mesh gold grid and blotted for 2.5 s at 4 °C, 90% relative humidity on a Vitrobot Mark III (FEI) before being plunge-frozen in liquid ethane cooled by liquid nitrogen. Data were collected on a Krios (FEI) operating at 300 kV equipped with a K3 direct electron detector and a GIF energy filter at the University of Utah, National Center for Cryo-EM Access and Training (NCCAT), SLAC, NCI National Cryo-EM facility, and Pacific Northwest Cryo-EM Center (PNCC). Movies were recorded using SerialEM63, Leginon, or EPU, with a defocus range between −1.0 and −3.5 μm. Specifically, movies were recorded in super-resolution counting mode at a physical pixel size of 0.83 Å (NCC/chlorthalidone), or 0.825 Å (NCC/hydrochlorothiazide), or 0.86 Å (NCC/indapamide). The data were collected at a dose rate of 1.0–1.25 e − /Å2/frame with a total exposure of 40 frames, giving a total dose of 40−50 e − /Å2.
Image processing 3D reconstruction and model building
Movie frames were aligned, dose weighted, and then summed into a single micrograph using MotionCor264. CTF parameters for micrographs were determined using the program CTFFIND465. Approximately 2000 particles were manually boxed out in relion 4 to train a neuronal network model, which was then used to extract particles from all micrographs using TOPAZ66. For the NCC/indapamide dataset, a total of 2,148,162 particles were extracted and then subjected to one round of 2D classification in cryoSPARC 3.0 software67. ‘Junk’ particles that were sorted into incoherent or poorly resolved classes were rejected from downstream analyses. The remaining 1,302,442 particles from well resolved 2D classes were pooled and were used to calculate four de novo models in cryoSPARC 3.0 without imposing any symmetry followed by heterogenous refinement. The particles from the good class were subjected CTF correction and polishing in relion 4 software68,69 and then used to calculate a 2.8 Å map using non-uniform refinement in cryoSPARC 3.0. The resulting particles with orientation parameters were subjected to 3D classification without alignment in cryosparc 4.0 and the good class was used to calculate a 2.7 Å map.
For the NCC/chlorthalidone dataset, a total of 2,851,886 particles were extracted and subjected to one round of 2D classification in cryoSPARC 3.0, resulting in 652,609 good particles. Ab initio models and heterogeneous refinement were similarly carried out in cryoSPARC 3.0 as described for the NCC/indapamide dataset. The good class consisting of 73,255 particles was subjected to CTF refinement and Bayesian polishing in RELION 4, and yielded a final map of 2.9 Å resolution after non-uniform refinement in cryosparc 3.0.
For the NCC/hydrochlorothiazide dataset, a total of 3,281,696 particles were extracted and subjected to one round of 2D classification in cryoSPARC 3.0, resulting in 975,535 good particles. Ab initio models and heterogeneous refinement were similarly carried out in cryoSPARC 3.0 as described for the NCC/indapamide dataset. The good class consisting of 103,688 particles was subjected to CTF refinement and Bayesian polishing in RELION 4, and yielded a final map of 2.44 Å resolution after non-uniform refinement in cryosparc 3.0.
The maps were locally sharpened in cryoSPARC 4.0 with an overall b factor of approximately −52 Å2 (NCC/indapamide), or −70 Å2 (NCC/chlorthalidone), or −68 Å2 (NCC/hydrochlorothiazide) for model building in Coot 0.8.9.3 software70. The models were refined in real space using PHENIX 1.18 software71, and assessed in Molprobity72 as shown in Supplementary Table 1. UCSF Chimera was used to visualize and segment density maps, and figures were generated using Chimera. jsPISA was used to calculate buried surface area73. The structural model of eNCCβ is predicted using AlphaFold274.
To further refine the placement of the indapaminde, chlorthalidone, and hydrochlorothiazide ligands, the final maps and derived structures of each NCC/thiazide drug complex were processed for use with the ChemEM flexible-fitting docking program75. Briefly, ChemEM makes use of the experimentally determined density maps to perform biased molecular dynamics simulations to ‘dock’ ligands into the observed cryo-EM densities. Input for ChemEM was prepared by removing the ligand entries from the PDB files of the models produced from the Coot and PHENIX software packages and providing the ligand to the ChemEM engine as a SMILES string. ChemEM was then run with the preprocessing, pre-processing split, auto_split_point, fitting, and post-processing flags set to one. 5 post-processing solutions were requested for each ligand. Centroid locations for the binding pockets were assigned as the center of mass position of the unrefined ligand placement. It should be noted that these refinements were performed in the absence of explicit water molecules.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The cryo-EM maps have been deposited in the Electron Microscopy Data Bank (EMDB) with the accession codes EMD-43411 (NCC/indapamide), EMD-44979 (NCC/hydrochlorothiazide), and EMD-43412 (NCC/chlorthalidone). The atomic coordinates for the corresponding maps have been deposited in the Protein Data Bank (PDB; https://www.rcsb.org) with the accession codes 8VPN (NCC/indapamide), 9BWT (NCC/hydrochlorothiazide), and 8VPP (NCC/chlorthalidone). All other data and reagents that support the findings of this study are available from the corresponding authors upon request. Source data are provided in this paper.
References
Malha, L. & Mann, S. J. Loop diuretics in the treatment of hypertension. Curr. Hypertens. Rep. 18, 27 (2016).
Sinha, A. D. & Agarwal, R. Thiazide diuretics in chronic kidney disease. Curr. Hypertens. Rep. 17, 13 (2015).
Baranauskiene, L., Skiudaite, L., Michailoviene, V., Petrauskas, V. & Matulis, D. Thiazide and other Cl-benzenesulfonamide-bearing clinical drug affinities for human carbonic anhydrases. PLoS One 16, e0253608 (2021).
Kucharczyk, P. et al. Thiazides attenuate insulin secretion through inhibition of mitochondrial carbonic anhydrase 5b in beta -islet cells in mice. J. Am. Soc. Nephrol. 34, 1179–1190 (2023).
Huang, X., Mees, D. E., Vos, P., Hamza, S. & Braam, B. Everything we always wanted to know about furosemide but were afraid to ask. Am. J. Physiol. Ren. Physiol. 310, F958–F971 (2016).
Earl, G., Davenport, J. & Narula, J. Furosemide challenge in patients with heart failure and adverse reactions to sulfa-containing diuretics. Ann. Intern Med 138, 358–359 (2003).
Haeberle, M. & Karle, C. A. Photodermatitis caused by torasemide. Contact Dermat. 84, 53–54 (2021).
Shao, S. C. et al. Associations of thiazide use with skin cancers: a systematic review and meta-analysis. BMC Med 20, 228 (2022).
Fan, M., Zhang, J., Lee, C. L., Zhang, J. & Feng, L. Structure and thiazide inhibition mechanism of the human Na-Cl cotransporter. Nature 614, 788–793 (2023).
Ishani, A. et al. Chlorthalidone vs. hydrochlorothiazide for hypertension-cardiovascular events. N. Engl. J. Med 387, 2401–2410 (2022).
Messerli, F. H. Chlorthalidone vs. hydrochlorothiazide for hypertension-cardiovascular events. N. Engl. J. Med 388, 1341–1342 (2023).
Kahle, K. T., Ring, A. M. & Lifton, R. P. Molecular physiology of the WNK kinases. Annu Rev. Physiol. 70, 329–355 (2008).
Ostrosky-Frid, M., Castaneda-Bueno, M. & Gamba, G. Regulation of the renal NaCl cotransporter by the WNK/SPAK pathway: lessons learned from genetically altered animals. Am. J. Physiol. Ren. Physiol. 316, F146–F158 (2019).
Uchida, S., Mori, T., Susa, K. & Sohara, E. NCC regulation by WNK signal cascade. Front Physiol. 13, 1081261 (2022).
Boyden, L. M. et al. Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature 482, 98–102 (2012).
Wilson, F. H. et al. Human hypertension caused by mutations in WNK kinases. Science 293, 1107–1112 (2001).
Carbajal-Contreras, H., Gamba, G. & Castaneda-Bueno, M. The serine-threonine protein phosphatases that regulate the thiazide-sensitive NaCl cotransporter. Front Physiol. 14, 1100522 (2023).
Nan, J. et al. Cryo-EM structure of the human sodium-chloride cotransporter NCC. Sci. Adv. 8, eadd7176 (2022).
Brown, A., Meor Azlan, N. F., Wu, Z. & Zhang, J. WNK-SPAK/OSR1-NCC kinase signaling pathway as a novel target for the treatment of salt-sensitive hypertension. Acta Pharm. Sin. 42, 508–517 (2021).
Monroy, A., Plata, C., Hebert, S. C. & Gamba, G. Characterization of the thiazide-sensitive Na(+)-Cl(-) cotransporter: a new model for ions and diuretics interaction. Am. J. Physiol. Ren. Physiol. 279, F161–F169 (2000).
Wilson, F. H. et al. Molecular pathogenesis of inherited hypertension with hyperkalemia: the Na-Cl cotransporter is inhibited by wild-type but not mutant WNK4. Proc. Natl Acad. Sci. USA 100, 680–684 (2003).
de Jong, J. C. et al. Effects of chemical chaperones on partially retarded NaCl cotransporter mutants associated with Gitelman’s syndrome in a mouse cortical collecting duct cell line. Nephrol. Dial. Transpl. 19, 1069–1076 (2004).
Rosenbaek, L. L., Rizzo, F., MacAulay, N., Staub, O. & Fenton, R. A. Functional assessment of sodium chloride cotransporter NCC mutants in polarized mammalian epithelial cells. Am. J. Physiol. Ren. Physiol. 313, F495–F504 (2017).
Valdez-Flores, M. A. et al. Functionomics of NCC mutations in Gitelman syndrome using a novel mammalian cell-based activity assay. Am. J. Physiol. Ren. Physiol. 311, F1159–F1167 (2016).
Watts, S. D., Suchland, K. L., Amara, S. G. & Ingram, S. L. A sensitive membrane-targeted biosensor for monitoring changes in intracellular chloride in neuronal processes. PLoS One 7, e35373 (2012).
Richardson, C. et al. Activation of the thiazide-sensitive Na+-Cl- cotransporter by the WNK-regulated kinases SPAK and OSR1. J. Cell Sci. 121, 675–684 (2008).
Yamada, K. et al. Small-molecule WNK inhibition regulates cardiovascular and renal function. Nat. Chem. Biol. 12, 896–898 (2016).
AlAmri, M. A., Kadri, H., Alderwick, L. J., Simpkins, N. S. & Mehellou, Y. Rafoxanide and closantel inhibit SPAK and OSR1 kinases by binding to a highly conserved allosteric site on their C-terminal domains. ChemMedChem 12, 639–645 (2017).
Ishihara, H. et al. Calyculin A and okadaic acid: inhibitors of protein phosphatase activity. Biochem Biophys. Res Commun. 159, 871–877 (1989).
Schiapparelli, P. et al. Phosphorylated WNK kinase networks in recoded bacteria recapitulate physiological function. Cell Rep. 36, 109416 (2021).
Filippi, B. M. et al. MO25 is a master regulator of SPAK/OSR1 and MST3/MST4/YSK1 protein kinases. EMBO J. 30, 1730–1741 (2011).
Pedersen, N. B., Hofmeister, M. V., Rosenbaek, L. L., Nielsen, J. & Fenton, R. A. Vasopressin induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter in the distal convoluted tubule. Kidney Int 78, 160–169 (2010).
Ernst, M. E. & Fravel, M. A. Thiazide and the thiazide-like diuretics: review of hydrochlorothiazide, chlorthalidone, and indapamide. Am. J. Hypertens. 35, 573–586 (2022).
Zhao, Y. et al. Structural basis for inhibition of the cation-chloride cotransporter NKCC1 by the diuretic drug bumetanide. Nat. Commun. 13, 2747 (2022).
Zhao, Y. et al. Structure of the human cation-chloride cotransport KCC1 in an outward-open state. Proc. Natl Acad. Sci. USA 119, e2109083119 (2022).
Beaumont, K., Vaughn, D. A. & Fanestil, D. D. Thiazide diuretic drug receptors in rat kidney: identification with [3H]metolazone. Proc. Natl Acad. Sci. USA 85, 2311–2314 (1988).
Tran, J. M., Farrell, M. A. & Fanestil, D. D. Effect of ions on binding of the thiazide-type diuretic metolazone to kidney membrane. Am. J. Physiol. 258, F908–F915 (1990).
Moreno, E. et al. The European Eel NCCbeta gene encodes a thiazide-resistant Na-Cl cotransporter. J. Biol. Chem. 291, 22472–22481 (2016).
Moreno, E. et al. The European and Japanese eel NaCl cotransporters beta exhibit chloride currents and are resistant to thiazide type diuretics. Am. J. Physiol. Cell Physiol. 323, C385–C399 (2022).
Chi, G. et al. Phospho-regulation, nucleotide binding and ion access control in potassium-chloride cotransporters. EMBO J. 40, e107294 (2021).
Lu, S. et al. The structural basis of ATP as an allosteric modulator. PLoS Comput Biol. 10, e1003831 (2014).
Vargas-Poussou, R. et al. Spectrum of mutations in Gitelman syndrome. J. Am. Soc. Nephrol. 22, 693–703 (2011).
Darman, R. B. & Forbush, B. A regulatory locus of phosphorylation in the N terminus of the Na-K-Cl cotransporter, NKCC1. J. Biol. Chem. 277, 37542–37550 (2002).
Pacheco-Alvarez, D. et al. The Na+:Cl- cotransporter is activated and phosphorylated at the amino-terminal domain upon intracellular chloride depletion. J. Biol. Chem. 281, 28755–28763 (2006).
Gimenez, I. & Forbush, B. Regulatory phosphorylation sites in the NH2 terminus of the renal Na-K-Cl cotransporter (NKCC2). Am. J. Physiol. Ren. Physiol. 289, F1341–F1345 (2005).
Bachmann, S. & Mutig, K. Regulation of renal Na-(K)-Cl cotransporters by vasopressin. Pflug. Arch. 469, 889–897 (2017).
Rojas-Vega, L. & Gamba, G. Mini-review: regulation of the renal NaCl cotransporter by hormones. Am. J. Physiol. Ren. Physiol. 310, F10–F14 (2016).
Carbajal-Contreras, H. et al. Arginine vasopressin regulates the renal Na-Cl and Na-K-Cl cotransporters through with-no-lysine kinase 4 and inhibitor 1 phosphorylation. Am. J. Physiol. Renal. Physiol. 326, F285–F299 (2024).
Ma, J. et al. Genetic features of Chinese patients with gitelman syndrome: sixteen novel SLC12A3 mutations identified in a new cohort. Am. J. Nephrol. 44, 113–121 (2016).
Somasekharan, S., Tanis, J. & Forbush, B. Loop diuretic and ion-binding residues revealed by scanning mutagenesis of transmembrane helix 3 (TM3) of Na-K-Cl cotransporter (NKCC1). J. Biol. Chem. 287, 17308–17317 (2012).
Gimenez, I., Isenring, P. & Forbush, B. Spatially distributed alternative splice variants of the renal Na-K-Cl cotransporter exhibit dramatically different affinities for the transported ions. J. Biol. Chem. 277, 8767–8770 (2002).
Gimenez, I. & Forbush, B. The residues determining differences in ion affinities among the alternative splice variants F, A, and B of the mammalian renal Na-K-Cl cotransporter (NKCC2). J. Biol. Chem. 282, 6540–6547 (2007).
Zhao, Y. & Cao, E. Structural pharmacology of cation-chloride cotransporters. Membr. (Basel) 12, 1206 (2022).
Cruz, D. N. et al. Mutations in the Na-Cl cotransporter reduce blood pressure in humans. Hypertension 37, 1458–1464 (2001).
Levring, J. et al. CFTR function, pathology and pharmacology at single-molecule resolution. Nature 616, 606–614 (2023).
Ellison, D. H. Clinical pharmacology in diuretic use. Clin. J. Am. Soc. Nephrol. 14, 1248–1257 (2019).
Feit, P. W. Bumetanide-the way to its chemical structure. J. Clin. Pharm. 21, 531–536 (1981).
Jentzer, J. C., DeWald, T. A. & Hernandez, A. F. Combination of loop diuretics with thiazide-type diuretics in heart failure. J. Am. Coll. Cardiol. 56, 1527–1534 (2010).
Zhang, Z., Liu, F. & Chen, J. Conformational changes of CFTR upon phosphorylation and ATP binding. Cell 170, 483–491.e8 (2017).
Khandelwal, N. K. et al. The structural basis for regulation of the glutathione transporter Ycf1 by regulatory domain phosphorylation. Nat. Commun. 13, 1278 (2022).
Yusa, K., Zhou, L., Li, M. A., Bradley, A. & Craig, N. L. A hyperactive piggyBac transposase for mammalian applications. Proc. Natl Acad. Sci. USA 108, 1531–1536 (2011).
Kim, J. H. et al. High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice. PLoS One 6, e18556 (2011).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: Fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Bepler, T. et al. Positive-unlabeled convolutional neural networks for particle picking in cryo-electron micrographs. Nat. Methods 16, 1153–1160 (2019).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Zivanov, J., Nakane, T. & Scheres, S. H. W. A Bayesian approach to beam-induced motion correction in cryo-EM single-particle analysis. IUCrJ 6, 5–17 (2019).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr D. Biol. Crystallogr 66, 486–501 (2010).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D. Biol. Crystallogr 66, 213–221 (2010).
Williams, C. J. et al. MolProbity: more and better reference data for improved all-atom structure validation. Protein Sci. 27, 293–315 (2018).
Krissinel, E. Stock-based detection of protein oligomeric states in jsPISA. Nucleic Acids Res 43, W314–W319 (2015).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Sweeney, A., Mulvaney, T., Maiorca, M. & Topf, M. ChemEM: flexible docking of small molecules in cryo-EM structures. J. Med Chem. 67, 199–212 (2024).
Acknowledgements
This work was supported by the NIH grant R01 DK128592 to E.C., and the NIH grant R01 DK127268 to A.B. and H.S, and the NIH grant GM117230 to J.R. We thank Anita Orendt, Irvin Allen, Martin Cuma, and other staff members at the Utah Center for High Performance Computing for computational support. We are grateful to Barbie Ganser and David Belnap for data collection at the Electron Microscope Core at the University of Utah. The Electron Microscope Core at the University of Utah was supported by a grant from the Beckman Foundation. A portion of this research was supported by NIH grant U24GM129547 and performed at the PNCC at OHSU and accessed through EMSL (grid.436923.9), a DOE Office of Science User Facility sponsored by the Office of Biological and Environmental Research. Some of this work was performed at the National Center for CryoEM Access and Training (NCCAT) and the Simons Electron Microscopy Center located at the New York Structural Biology Center, supported by the NIH Common Fund Transformative High Resolution Cryo-Electron Microscopy program (U24 GM129539,) and by grants from the Simons Foundation (SF349247) and NY State Assembly. Some of this work was performed at the Stanford-SLAC Cryo-EM Center (S2C2), which is supported by the National Institutes of Health Common Fund Transformative High-Resolution Cryo-Electron Microscopy program (U24 GM129541). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Omar Davulcu, Janette Myers, Drew Gingerich, Ed Eng, Elina Kopylov, Patrick Mitchell, Lisa Dunn, Ian Fries, and other staff members at the PNCC, S2C2, and NCCAT for data collection and technical support.
Author information
Authors and Affiliations
Contributions
Conceptualization: Y.Z. and E.C. designed cryo-EM studies and Cl− influx assay, and B.F. and J.R. provided constructs and guidance for preparing active NCC samples; Investigation: Y.Z. carried out cryo-EM experiments and Cl- influx assay in HEK293 cells, M.S. performed docking experiments for the three thiazide diuretic drugs into NCC models/maps, and H.S. and A.B. collected cryo-EM datasets; Writing: E.C. and Y.Z. wrote the manuscript with the contribution of all other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Gerardo Gamba, and Jiangtao Guo for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhao, Y., Schubert, H., Blakely, A. et al. Structural bases for Na+-Cl− cotransporter inhibition by thiazide diuretic drugs and activation by kinases. Nat Commun 15, 7006 (2024). https://doi.org/10.1038/s41467-024-51381-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-51381-y
- Springer Nature Limited