Abstract
N-hydroxy pipecolic acid (NHP) plays an important role in plant immunity. In contrast to its biosynthesis, our current knowledge with respect to the transcriptional regulation of the NHP pathway is limited. This study commences with the engineering of Arabidopsis plants that constitutively produce high NHP levels and display enhanced immunity. Label-free proteomics reveals a NAC-type transcription factor (NAC90) that is strongly induced in these plants. We find that NAC90 is a target gene of SAR DEFICIENT 1 (SARD1) and induced by pathogen, salicylic acid (SA), and NHP. NAC90 knockout mutants exhibit constitutive immune activation, earlier senescence, higher levels of NHP and SA, as well as increased expression of NHP and SA biosynthetic genes. In contrast, NAC90 overexpression lines are compromised in disease resistance and accumulated reduced levels of NHP and SA. NAC90 could interact with NAC61 and NAC36 which are also induced by pathogen, SA, and NHP. We next discover that this protein triad directly represses expression of the NHP and SA biosynthetic genes AGD2-LIKE DEFENSE RESPONSE PROTEIN 1 (ALD1), FLAVIN MONOOXYGENASE 1 (FMO1), and ISOCHORISMATE SYNTHASE 1 (ICS1). Constitutive immune response in nac90 is abolished once blocking NHP biosynthesis in the fmo1 background, signifying that NAC90 negative regulation of immunity is mediated via NHP biosynthesis. Our findings expand the currently documented NHP regulatory network suggesting a model that together with NHP glycosylation, NAC repressors take part in a ‘gas-and-brake’ transcriptional mechanism to control NHP production and the plant growth and defense trade-off.
Similar content being viewed by others
Introduction
Plant defense responses are initiated by the recognition of pathogen-associated molecular patterns (PAMPs) or effectors molecules, which as a consequence result in activation of PAMP-triggered immunity or effector-triggered immunity, respectively1. In recent years, outstanding strides have been made in dissecting the regulatory mechanisms and components necessary for plant defense. Following attack by pathogens, salicylic acid (SA) accumulates in plants2,3. External application of SA or its chemical analogs can enhance the plants resistance to various diseases, whereas plants lacking SA biosynthesis, perception, or signal transduction display severely compromised resistance and failure in systemic acquired resistance (SAR) establishment4,5,6. Apart from SA, several additional metabolites have been implicated in plant defense response, including methyl salicylate (MeSA)7, azelaic acid (AzA)8, glycerol-3-phosphate (G3P)9, dehydroabietinal (DA)10, N-hydroxy pipecolic acid (NHP)11,12, β-aminobutyric acid (BABA)13, L-glutamate14, and melatonin15.
NHP is a non-protein amino acid derived from lysine that systemically accumulates in plants during pathogen infection and is essential for plant defense activation and SAR establishment11,12. Significant progress has been made in understanding the NHP biosynthetic pathway. It starts from L-lysine and involves three enzymes: AGD2-LIKE DEFENSE RESPONSE PROTEIN 1 (ALD1), SAR-DEFICIENT 4 (SARD4), and FLAVIN MONOOXYGENASE 1 (FMO1). Lysine is converted by ALD1 to form 2,3-dehydropipecolic acid, which is then reduced by SARD4 to generate pipecolic acid (Pip)16,17,18. Next, FMO1 hydroxylates Pip to form NHP11,12. The expression of these three NHP biosynthetic genes was strongly induced in plants in response to pathogen infection, resulting in high levels of NHP accumulation19. Plants with ALD1 and FMO1 loss-of-function mutations block NHP biosynthesis, making them more susceptible to pathogens and entirely compromised in SAR response11,12,16. Moreover, NHP and its biosynthetic genes have been found in a variety of plant species, and external treatment with synthetic NHP or overaccumulation of NHP through transiently overexpressing ALD1 and FMO1 can enhance disease resistance against various pathogens in different plants20,21. Furthermore, exogenous application of NHP in soil can restore the defense response in NHP deficient ald1 and fmo1 Arabidopsis mutants, partially rescue the response in salicylic acid induction-deficient 2 (sid2), a mutant in ISOCHORISMATE SYNTHASE 1 (ICS1), but fails to restore the defense response in the non-expression of pr1 (npr1) mutant22. The transcript levels of NHP, SA, phytoalexin and camalexin biosynthetic genes, as well as a series of defense-related genes could be effectively activated by exogenous NHP treatment22. Additionally, NHP can be further converted to NHP-glycoside (NHPG) and NHP-glucose ester, both of which are also considerably induced by pathogen infection23. The UDP-glycosyltransferase 76B1 (UGT76B1) is the enzyme catalyzing the glycosylation of NHP to NHPG reducing the levels of free and active NHP and SA upon pathogen infection24,25,26,27,28. Moreover, UGT76B1-mediated NHP homeostasis is critical to balance growth and defense trade-off in plants24. Therefore, NHP is a widespread and important metabolite in the activation of plant defense and SAR.
Over the last several years, significant progress has been made in understanding the regulation of NHP biosynthesis. Following pathogen infection, NHP levels were found to be much higher in sid2-1 and npr1 mutants, but much lower in enhanced disease susceptibility (eds1) and phytoalexin-deficient 4 (pad4) mutants23. MITOGEN-ACTIVATED PROTEIN KINASE (MAPK) activation was observed to trigger the expression of ALD1 and FMO1, resulting in higher levels of NHP29. Constitutive expression of Ca2+- DEPENDENT PROTEIN KINASES 5 (CPK5) in Arabidopsis elevated the expression of NHP biosynthetic genes as well as NHP levels30. Apart from these, several transcriptional regulators have been demonstrated to play critical roles in controlling NHP biosynthesis. For example, pathogen induced expression of ALD1, SARD4, and FMO1 was greatly decreased in sar deficient1 (sard1) and cam-binding protein 60-like g (cbp60g) double mutant (sard1/cbp60g)31. SARD1 and CBP60g were shown to regulate NHP biosynthesis by direct binding to the promoters of NHP biosynthetic genes ALD1, SARD4, and FMO131. WRKY33 is another positive regulator of NHP biosynthesis, which acts directly on the ALD1 promoter29. Pathogen induced NHP as well as the expression of ALD1 and FMO1 were significantly impaired in the wrky33 mutant29. The transcription factors TGACG-BINDING FACTOR 1, TGA4 and WRKY70 have been reported to positively control the expression of SARD1 and CBP60g, which act as positive regulators in NHP biosynthesis32,33. Conversely, CALMODULIN-BINDING TRANSCRIPTION ACTIVATORS (CAMTA1/2/3) were suggested to negatively impact NHP biosynthesis indirectly by suppressing the transcription of SARD1 and CBP60g34. Moreover, the protein complex comprising ASI1-IMMUNOPRECIPITATED PROTEIN 3 (AIPP3), ASI1-IMMUNOPRECIPITATED PROTEIN 3 (PHD2) and CARBOXYL-TERMINAL DOMAIN (CTD) PHOSPHATASE-LIKE 2 (CPL2), responsible for recognizing the histone 3 Lys 27 trimethylation (H3K27me3) and mediating the transcriptional repression, is involved in suppression of FMO1 expression and NHP biosynthesis35. TGA transcriptional regulators (2/5/6 and 1/4), along with NPR1, are essential for regulating the transcriptional response induced by NHP22,36. However, a direct negative regulator controlling NHP biosynthesis was not identified so far.
In this study, we identified a NAC-type transcription factor (i.e., NAC90) that negative controls the NHP biosynthetic pathway. NAC90 was previously reported as a negative regulator of leaf senescence by directly inhibiting SA biosynthesis37. Here, we showed that NAC90 protein abundance was significantly enhanced in Arabidopsis plants constitutively producing high NHP levels. We discovered that through interaction with two other NAC factors (i.e., NAC36 and NAC61) NAC90 negatively regulates plant immunity via direct suppression of both NHP and SA biosynthesis. Blocking NHP or SA biosynthesis suppressed the constitutive activation of immune response in the nac90 mutant background. Activity of this NAC-type triad of negative regulators complements the recently reported role of NHP glycosylation through UGT76B1 in balancing NHP levels and consequently the plant growth and defense trade-off.
Results
Engineering constitutive and high level NHP production in plants
We previously reported transient expression of the three NHP biosynthetic genes ALD1, SARD4, and FMO1 from Arabidopsis in N. benthamiana leaves24. As a continuation of this previous research, we generated stable transformants of Arabidopsis and tomato (Solanum lycopersicum) plants (termed pNHP and tpNHP, respectively) expressing the three Arabidopsis genes. Both Arabidopsis and tomato transgenic lines displayed severe phenotypes. The pNHP plants developed a smaller rosette (reduced diameter) and showed an early senescence phenotype as compared to wild-type (WT) Arabidopsis plants (Fig. 1a, b; Supplementary Fig 1a). Moreover, even though tpNHP plants developed flowers, they failed to set fruit. Liquid chromatography-mass spectrometry (LC-MS) analysis showed significant accumulation of NHP in pNHP and tpNHP leaves while it was not detectable in WT ones. (Fig. 1c and Supplementary Fig. 1b). The glycosylated form of NHP (NHPG) also accumulated to considerable amount in the transgenic plants (Supplementary Fig. 1c, d). In our previous study we showed that exogenous application of NHP could enhance SA accumulation in Arabidopsis. To confirm this SA enhancement in vivo (i.e., not through exogenous NHP application), we examined SA production in pNHP plants and found that its level was significantly enhanced (Fig. 1d). Next, we assayed the expression of NHP biosynthetic genes (ALD1, SARD4, and FMO1) and the defense marker gene- PATHOGENESIS-RELATED GENE 1 (PR1) and found that all of them were dramatically enhanced in pNHP as compared to WT plants (Fig. 1e, f, g, h). These results suggested that engineering NHP production can constitutively activate immune responses in Arabidopsis and tomato plants.
a Phenotypes of NHP producing Arabidopsis plants. Red arrows indicate yellow leaves in pNHP plants. b Small size of leaves and earlier senescence phenotypes in pNHP plants. c, d Abundance of NHP and SA in leaves of WT and pNHP plants. e–h Transcript levels of defense-related genes in the leaves of WT and pNHP plants. Leaves of WT and pNHP plants (pNHP-1 and pNHP-2) at 28 days were subjected to metabolite analysis by Quattro LC Triple Quad mass spectrometer and transcript analysis by RT-PCR. AGD2-LIKE DEFENSE RESPONSE PROTEIN 1, ALD1; SAR DEFICIENT 4, SARD4; FLAVIN MONOOXYGENASE 1, FMO1; PATHOGENESIS-RELATED GENE 1, PR1. WT, wild-type Arabidopsis plant; pNHP, Arabidopsis plants overexpressing the NHP biosynthetic pathway genes. Two different pNHP independent lines are shown (marked as 1 and 2). In (a), and (b), bar = 1 cm. Values showed here were the average of three biological replicates and error bars represent the SDs of three independent biological replicates (n = 3). ND, not detectable. Asterisks indicate significant changes compared to WT samples as calculated by two-tailed t test (*p value < 0.05; **p value < 0.01; ***p value < 0.001, p values are shown in the Source Data file).
Proteomics of pNHP plants results in the identification of NAC90 as a putative regulator of NHP biosynthesis
To understand the changes in the proteome of NHP over-producing Arabidopsis, we performed a label-free proteomics analysis of leaves derived from pNHP-1 and pNHP-2 (independent transgenic lines) and WT plants (Supplementary Fig. 2a). A total of 4257 proteins were identified in this experiment (Fig. 2a, Supplementary Data 1); out of which 585 defined as Differentially Accumulated Proteins (DAP; twofold significant difference set as threshold, p value < 0.05). To assess the reproducibility of the label-free proteomics experiment, Principal Component Analysis (PCA) and Pearson correlation were performed to analyze the different groups of samples. The PCA plot and correlation maps revealed marked distinction between groups and high correlation values among replicates (Supplementary Fig. 2b, c), indicating good repeatability of protein profiling in both wild type (WT) and pNHP plants. Compared to WT samples, 686 proteins were upregulated and 76 were downregulated in pNHP-1, while 632 proteins were up-regulated and 82 were down-regulated in pNHP-2 (Supplementary Fig. 2d). Overall, a total of 534 proteins were significantly up-regulated, and 51 proteins were down-regulated in both pNHP-1 and pNHP-2 lines (Fig. 2a; see proteins list in Supplementary Data 1).
a Label-free proteomics identified 585 proteins showing differential abundance in pNHP plants as compared to WT ones. A total of 4257 proteins (pink color) were identified and quantified. Of these, 534 (yellow color) and 51 (brown color) proteins were up-regulated or down-regulated in pNHP relative to WT plants. b Venn diagram showing overlapping transcripts/proteins between the differentially accumulated proteins found in this study and NHP response genes obtained from available transcriptomics data22 (ArrayExpress accession number E-MTAB-10230). The blue, orange, and brown colors represent the number of NHP-induced genes (NHP+), differentially accumulated proteins in pNHP plants (pNHP), and NHP-repressed genes (NHP-), respectively. c A heatmap displaying 585 differentially accumulated proteins in pNHP plants relative to their expression in WT samples. The color scale represents log2 fold change. Each row in the color heat map represents a single protein. Two different pNHP independent lines are shown (marked as 1 and 2). d GO annotation of proteins detected as differentially accumulating in pNHP plants as compared to the WT ones. e Relative abundance of the NAC90 protein in WT and pNHP plants. Values showed here were the average of three biological replicates and error bars represent the SDs of three independent biological replicates (n = 3). Asterisks indicate significant changes compared to WT samples as calculated by two-tailed t test (***p value < 0.001, p values are shown in the Source Data file).
To identify DAPs that are also transcriptionally altered by exogenous NHP treatment, we compared the lists of DAPs and NHP-responsive genes22. Notably, 291 DAPs were associated with genes affected by exogenous NHP; 281 proteins corresponding to genes upregulated by exogenous NHP (NHP+) and 16 proteins linked to genes downregulated by exogenous NHP (NHP-) (Fig. 2b and Supplementary Data 2). Moreover, we also compared the SAR responsive genes with the identified DAPs and found that 402 DAPs in the pNHP-1 and pNHP-2 proteomics experiment are encoded by SAR responsive genes (351 SAR+ and 51 SAR-) (Supplementary Fig. 2e and Supplementary Data 3)18. Next, we explored Gene Ontology (GO) categories enrichment among the DAPs. The results showed that “Response to stimulus”, “Response to chemical”, “Response to biotic stimulus” and “Defense response” were highly enriched in the DAPs (Fig. 2d). Moreover, “systemic acquired resistance”, and “cell death” which are central for plant immunity were also highly enriched categories among the DAPs (Fig. 2d). In addition, a set of known proteins involved in defense response were identified as DAPs, including PR1, PR5, ACCELERATED CELL DEATH 6 (ACD6), DOWNY MILDEW RESISTANCE 6 (DMR6), NUDIX HYDROLASE 6, MITOGEN-ACTIVATED PROTEIN KINASE (MPK4), and RPM1 INTERACTING PROTEIN 4 (RIN4) (Supplementary Data 1). Furthermore, ENHANCED DISEASE SUSCEPTIBILITY 1 (EDS1), PHYTOALEXIN-DEFICIENT 4 (PAD4), and MPK3 involved in NHP and SA biosynthesis and signal transduction, were found to be among the DAPs (Supplementary Data 1). Several transcription factors, including CBP60b and NAC90, were found to be significantly induced in pNHP plants, (Fig. 2e, Supplementary Fig. 2f and Supplementary Data 1). CBP60b has been reported to regulate plant immunity through CBP60g38,39. NAC90 was reported to bind to the promoter of ICS1 and negatively regulate SA biosynthesis during senescence37. Here, NAC90 protein abundance was dramatically induced (more than 100 times) in pNHP plants and it also appeared among the list of NHP- and SAR- induced genes (see above). We thus decided to focus on the characterization of the NAC90 transcription factor and its involvement in NHP biosynthesis and pathogen response.
Expression of NAC90 is induced by NHP and modulated by numerous key defense-regulators
Consistent with the changes in protein abundance, NAC90 transcript level was also significantly enhanced in both pNHP-1 and pNHP-2 plants as compared to WT leaves (Fig. 3a). Since published transcriptomics data showed that NAC90 transcript was induced by pathogens, SA, Pip, and NHP treatments12,18,22,40, we examined its levels in response to bacterial pathogen (Psm ES4326), NHP, and SA application. Samples harvested at 24-hour post inoculation (hpi) showed that NAC90 expression was strongly up-regulated following these treatments (Fig. 3b). NAC90 transcript level was also evaluated in a series of mutants defective in NHP and SA defense response pathways including the NHP-deficient fmo1, sid2, npr1, eds1, and pad4 mutants. The results showed that the basal (i.e., mock treatment) transcript level of NAC90 in fmo1, eds1, and pad4 was lower than in WT (Fig. 3c). Following Psm ES4326 infection, the induction of NAC90 transcript level was severely reduced in fmo1, sid2, npr1, eds1, and pad4 mutants (Fig. 3c). These results indicate that FMO1, ICS1, NPR1, EDS1, and PAD4 positively modulate the expression of NAC90 following pathogen infection. The UDP glycosyltransferase UGT76B1 serves as a negative regulator of defense immunity by controlling the levels of NHP and SA following pathogen infection24,25,26,27. We analyzed NAC90 transcript level in the ugt76b1 mutant background that over-accumulates NHP and SA. A substantial increase of NAC90 in the ugt76b1 mutant relative to WT suggested that UGT76B1 activity negatively affects the expression of NAC90 (Fig. 3c).
a Transcript levels of NAC90 in 4-week-old WT and pNHP plants. b Transcript levels of NAC90 in WT Arabidopsis plants in response to Psm ES4326, NHP, and SA treatments. Leaves of 4-week-old WT Arabidopsis plants were treated with 10 mM MgCl2 (Mock), Psm ES4326 (OD600 = 0.001) (Psm), 1 mM NHP (NHP), and 200 μM SA (SA) solution, harvested at 24 h post treatments, and subjected for qRT-PCR analysis. c, d Transcript levels of NAC90 in different SAR deficient mutants. Leaves of 4-week-old WT and mutant plants (AGD2-like defense response protein 1, ald1; flavin monooxygenase 1, fmo1; salicylic acid induction-deficient 2, sid2; non-expression of PR1, npr1; enhanced disease susceptibility 1, eds1; phytoalexin-deficient 4, pad4; sar deficient 1, sard1; Calmodulin-binding protein 60 g, cbp60g) were treated with 10 mM MgCl2 (Mock) and Psm ES4326 (OD600 = 0.001), harvested at 24 h post treatments (hpi), and subjected for transcript levels analysis. Ten mM MgCl2 was used as mock control (Mock). e Transactivation of NAC90 promoters with SARD1 and CBP60g. All the values showed in (a, b, c, and d) were the average of three biological replicates and error bars represent the SDs of three biological replicates (n = 3). Values showed in (e) were the average of four biological replicates and error bars represent the SDs of four independent biological replicates (n = 4). Asterisks indicate significant changes compared to WT or Mock samples as calculated by two-tailed t test (*p value < 0.05; **p value < 0.01; ***p value < 0.001, p values are shown in the Source Data file).
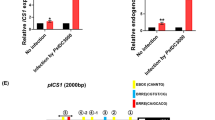
SARD1 and CBP60g are two master regulators that directly control SA and NHP biosynthesis and play important roles in plant immunity31,34. We therefore examined NAC90 expression in sard1, cbp60g, and sard1 cbp60g plants. As shown in Fig. 3d, following Psm ES4326 infection, NAC90 expression was notably induced in WT, and this induction was significantly reduced in sard1 and sard1 cbp60g but not in cbp60g plants, suggesting that SARD1 may regulate NAC90 expression. Furthermore, examining the 2,000 bp upstream region of the NAC90 gene sequence identified three GAAATT motifs, which are like the previously reported SARD1 binding elements31 (Supplementary Fig. 3a). In addition, SARD1 chromatin immunoprecipitation sequencing revealed a high signal upstream of the NAC90 translation start site31 (Supplementary Fig. 3b), suggesting that NAC90 is the direct target gene of SARD1. To further support this assumption, we performed dual-luciferase reporter assays in N. benthamiana leaves by co-expressing a reporter construct of the firefly luciferase (LUC) driven by the NAC90 upstream region (NAC90upsr:LUC) and an effector construct expressing the SARD1 or CBP60g protein (Supplementary Fig. 3c). The results suggested that SARD1 could activate the NAC90 upstream region (Fig. 3e), further supporting that NAC90 is directly regulated by SARD1.
Growth phenotypes and immunity-related transcriptome changes of NAC90 altered plants
To further explore the function of NAC90, we identified two Arabidopsis mutants with T-DNA insertions in its coding region [nac90-1 (SALK_206203) and nac90-2 (SALK_203643)]. (Supplementary Fig. 4a). We also generated NAC90 constitutive overexpression lines (NAC90-1 and NAC90-2) in which the NAC90 coding sequence is fused to a 3×Flag tag. NAC90 transcript was barely detectable in the leaves of these two nac90 mutants, whereas both overexpression lines displayed a significantly higher transcript levels as compared to WT ones (Fig. 4a, b). Western blotting with anti-Flag confirmed the abundance of NAC90 protein expression in both NAC90-1 and NAC90-2 lines (Fig. 4c). Phenotypically, in addition to the previously reported early senescence phenotype in nac90 mutants37, these plants also exhibited small rosette size. Conversely, NAC90 overexpression lines developed enlarged rosettes as compared to WT ones (Fig. 4d). Consistent with these phenotypes, the nac90 mutants above-ground parts weight and rosettes diameter were considerably reduced as compared to those of WT plants, whereas they were notably bigger in the NAC90 overexpression plants (Supplementary Fig. 4b, c).
a, b Transcript levels of NAC90 in leaves of 3-week-old wild-type (WT), nac90 mutants (nac90-1 and nac90-2) and NAC90 overexpression (NAC90-1 and NAC90-2) plants. c Western blot (Anti-Flag) showing NAC90 protein abundance in the leaves of 4-week-old WT and NAC90 overexpression plants (NAC90-1 and NAC90-2). d Phenotype of WT, nac90-1, nac90-2, and NAC90 overexpression (OE-NAC90-1 and OE-NAC90-2) plants. Bar = 1 cm. Red triangles indicate senescence leaves in both nac90-1 and nac90-2 mutants. Bar = 1 cm. e Go annotation analysis of differentially expressed genes in both nac90 mutants (nac90-1 and nac90-2). f Heatmap showing differentially expressed genes identified in the transcriptome data of both nac90 mutants (nac90-1 and nac90-2). The color scale represents log2 fold change. Each row in the color heatmap represents a single gene. AGD2-LIKE DEFENSE RESPONSE PROTEIN 1, ALD1; NAC DOMAIN CONTAINING PROTEIN 61, NAC061; NAC DOMAIN CONTAINING PROTEIN 36, NAC036; SAR DEFICIENT 1, SARD1; SENESCENCE-ASSOCIATED GENE 13, SAG13; FLAVIN MONOOXYGENASE 1, FMO1; CHITINASE, CHI; ENHANCED DISEASE SUSCEPTIBILITY 5, EDS5; CALMODULIN-BINDING PROTEIN 60 g, CBP60g; PATHOGENESIS-RELATED GENE 1, PR1. Values showed here were the average of three biological replicates and error bars represent the SDs of three biological replicates (n = 3). Asterisks indicate significant changes compared to WT samples as calculated by two-tailed t test (***p value < 0.001, p values are shown in the Source Data file).
To investigate the impact of NAC90 knockout on gene expression, we performed transcriptome analysis on 5-week-old of WT and nac90-2 leaves. A total of 1480 Differentially Expressed Genes (DEGs, 2-fold significant difference set as threshold, p value < 0.05) including 818 upregulated and 662 downregulated in the nac90-2 mutant (Supplementary Fig. 4d and Supplementary Data 4). We also re-analyzed the transcriptome data examining global gene expression in the nac90-1 mutant37, using a threshold of a twofold significant difference (p value < 0.05) and found that 716 genes, including 497 upregulated and 219 downregulated, were differentially expressed in the nac90-1 mutant (Supplementary Fig. 4d and Supplementary Data 5). We next compared the transcriptome datasets from the two nac90 mutant alleles and identified 179 common DEGs (including 32 downregulated and 147 upregulated genes) relative to the WT plants leaves (Supplementary Fig. 4e and Supplementary Data 6). To determine the specific cellular processes associated with these 179 DEGs, we performed enrichment analysis of Gene Ontology (GO) biological processes. We found that the functional classes “Response to stimulus”, “Defense response”, “Response to bacterium”, and “Response to hormone” were the four enriched groups containing the numerous defense-related genes (Fig. 4e). In addition, 31 and 11 DEGs were classified as “Response to salicylic acid” and “Leaf senescence” processes, respectively. Among these 42 (i.e., 31 + 11) DEGs, those encoding defense markers (PR1, CHI, and SAG13)37, NHP biosynthesis (ALD1 and FMO1), isochorismate transport (EDS5)41, and the two pathogen-induced master regulators of NHP and SA biosynthesis (SARD1 and CBP60g), identified in previous transcriptome data40, were substantially up-regulated in both nac90-1 and nac90-2 mutants (Fig. 4f). Notably, two other NAC-type transcription factors (NAC61 and NAC36) were also upregulated in both knockout mutant lines (Fig. 4f), suggesting that they might play a role in plant immunity.
NAC90 is a negative regulator of NHP biosynthesis and defense response
The transcriptome data described above and previous report showed that the expression of ALD1, FMO1 and ICS1 was significantly upregulated in both nac90 mutants (Fig. 4f)37. The results were confirmed by Real Time-PCR (RT-PCR) assays showing that transcript levels of ALD1, SARD4, FMO1, and ICS1 were significantly higher in the nac90 mutants than in WT, but lower in NAC90 overexpression lines (Fig. 5a–d). Following Psm attack, the transcript levels of ALD1, SARD4, FMO1, and ICS1 in nac90 mutants and NAC90 overexpression plants were dramatically lower compared to WT leaves (Fig. 5a–d). We next analyzed the upstream region of these genes and found that they contained from 4 and up to 10 NAC transcription factor binding motifs (Supplementary Fig. 5a), suggesting that NAC90 may bind to the promoters of these genes. To further investigate whether NAC90 could directly bind to the upstream regions of NHP biosynthetic genes in vivo, we performed chromatin immunoprecipitation coupled with quantitative RT-PCR (ChIP-qPCR) analysis for NHP biosynthetic genes. ICS1 was previously reported to be directly repressed by NAC90 and hence used as a positive control in this experiment37. Compared to the negative control IgG, the putative promoter fragments of ALD1 and FMO1 were significantly enriched by Anti-Flag, indicating that NAC90 can bind to these regulatory regions (Fig. 5e and Supplementary Fig. 5b). NAC90 does not appear to bind the putative SARD4 promoter although a trend in enrichment was detected (which was not statistically significant). We next performed dual-luciferase reporter assays in N. benthamiana leaves co-transfected with reporter constructs of firefly luciferase driven by the upstream regions of NHP biosynthetic genes (i.e., ALD1upsr::LUC, SARD4upsr::LUC, and FMO1upsr:: LUC) and an effector construct expressing the NAC90 protein (Supplementary Fig. 5c). After two days, a significant reduction in expression driven by the putative promoter regions of the three NHP biosynthetic genes and ICS1 was observed in the samples with NAC90 (Fig. 5f and Supplementary Fig. 5d). The results indicated that NAC90 directly binds to the promoters of NHP and SA biosynthetic genes to repress their expression.
a–d Transcript levels of NHP (FMO1, ALD1, and SARD4) and SA (ICS1) biosynthetic genes in the leaves of 4-week-old WT, nac90-1, nac90-2, and NAC90 overexpression (NAC90-1 and NAC90-2) plants upon Psm ES4326 infection. e Chromatin immunoprecipitation-qPCR assay indicated that NAC90 directly binds to the upstream region of NHP biosynthetic genes. f Dual luciferase promoter activity assays showed that NAC90 represses the expression of NHP biosynthetic genes. g, h Abundance of NHP (g) and SA (h) in the leaves of 4-week-old WT, nac90-1, nac90-2, and NAC90 overexpression (NAC90-1 and NAC90-2) plants upon Psm ES4326 infection. i Growth of Psm ES4326 in leaves of WT, nac90-1, nac90-2, and NAC90 overexpression (NAC90-1 and NAC90-2) plants. j Symptoms of leaves treated with Psm ES4326 observed in WT, nac90-1, nac90-2, and NAC90 overexpression (NAC90-1 and NAC90-2) plants. k Transcript levels of PR1 in the leaves of 4-week-old WT, nac90-1, nac90-2, and NAC90 overexpression (NAC90-1 and NAC90-2) plants upon Psm ES4326 infection. Values showed here were the average of three biological replicates and error bars represent the SDs of three biological replicates (n = 3). Asterisks indicate significant changes compared to WT or control samples as calculated by two-tailed t test (*p value < 0.05; **p value < 0.01; ***p value < 0.001, p values are shown in the Source Data file).
Since NAC90 directly represses the expression of NHP and SA biosynthetic genes, we next investigated the levels of Pip, NHP, and SA in leaves of WT, nac90 mutants and NAC90 overexpression lines. LC-MS analysis showed that levels of these three metabolites were significantly elevated in nac90 mutants as compared to leaves of WT and NAC90 overexpression lines. In contrast, the levels of Pip, NHP, and SA in NAC90 overexpressing leaves were much lower than those in WT ones (Fig. 5g, h, and Supplementary Fig. 5e). In addition, the levels of NHPG and salicylic acid beta-glucoside (SAG), the glycosylated derivatives of NHP and SA, were also much higher in the leaves of nac90 mutants than in WT ones, whereas they were significantly lower in NAC90 overexpression lines (Supplementary Fig. 5f, g). Following Psm ES4326 infection, the above five metabolites were strongly induced in WT, nac90 mutants, and NAC90 overexpression plants. The induction of Pip, NHP, SA, and NHPG was significantly compromised in nac90 mutants and NAC90 overexpression plants (Fig. 5g, h, and Supplementary Fig. 5e, f, g).
We then investigated whether alteration of NAC90 expression affects plant immunity. We treated Arabidopsis leaves of WT, nac90 mutants, and NAC90 overexpression plants with Psm ES4326 and analyzed bacterial growth three days later. The Psm ES4326 bacterial titers in nac90 mutants were significantly lower than those in WT plants leaves (Fig. 5i, j), consistent with previous reports40. In contrast, Psm ES4326 growth was notably higher in NAC90 overexpression plants (Fig. 5i, j). Expression analysis results showed that PR1 (a defense marker gene) was significantly enhanced in nac90 mutants corroborating previous report37(Fig. 5k). Conversely, PR1 expression was suppressed in NAC90 overexpression plants compared to the WT (Fig. 5k). Following Psm ES4326 infection, the induction of PR1 was significantly reduced in NAC90 overexpression plants, while no difference was observed between WT and nac90 mutants (Fig. 5k). The results indicated that nac90 mutants display constitutive activation of immune response, which is consistent with the previous report40. Taken together, the data suggests that NAC90 functions as a negative regulator of plant immunity via suppressing NHP and SA biosynthesis.
Knockout of FMO1 suppresses the constitutive activation of immune response in nac90 mutant
The above results showed that nac90 mutants displayed constitutive activation of immune response and accumulated higher levels of NHP and SA (Fig. 5). To examine whether the NHP or SA biosynthesis pathway is responsible for the activation of immune response in nac90 mutants, fmo1 or sid2 mutants were crossed with nac90-1 to create nac90-1 fmo1 and nac90-1 sid2 double mutants. As shown in Fig. 6a and Supplementary Fig. 6a, the dwarfed morphology of nac90-1 can be completely abolished and resemble WT plants in the nac90-1 fmo1 double mutant and could be partially reverted to a WT phenotype in the nac90-1 sid2 double mutant. Next, we examined Pip, NHP, SA, NHPG, and SAG levels in these plants. The results showed that nac90-1 plants accumulated a considerable higher level of Pip, NHP, NHPG, SA and SAG compared to WT, fmo1, nac90-1 fmo1, and nac90-1 sid2 plants leaves (Fig. 6b, c and Supplementary Fig. 6b–d). Following Psm ES4326 infection, levels of Pip, NHP and NHPG were significantly induced in WT, nac90, sid2, and nac90-1 sid2 plants. Similar to previously reports12,22, the induction of NHP in sid2 and nac90-1 sid2 plants was significantly higher than that in WT and nac90-1 plants (Fig. 6b and Supplementary Fig. 6b, c). In terms of SA and SAG, their levels were also strongly induced in WT, nac90-1, fmo1, and nac90-1 fmo1 plants but dramatically suppressed in sid2 and nac90-1 sid2 plants (Fig. 6c and Supplementary Fig. 6d). Furthermore, we investigated disease resistance in these genotypes and found that the enhanced disease resistance against Psm ES4326 in nac90-1 was abolished in the nac90-1 fmo1 and nac90-1 sid2 double mutants (Figs. 6d, e). In agreement with these findings, RT-PCR analysis showed that the increased transcript levels of PR1 observed in nac90-1 was also repressed in the nac90-1 fmo1 and nac90-1 sid2 double mutants (Fig. 6f). In addition, we could not detect the nac90-1 early senescence phenotype in the nac90-1 fmo1 and nac90-1 sid2 double mutant. Thus, we provide several points of evidence that FMO1 and ICS1 are required for the constitutive activation of immune responses and early senescence in the nac90-1 plants.
a FMO1 determines the size of the rosette in nac90-1 plant. Four-week-old wild-type (WT), fmo1, nac90-1, nac90-1 fmo1, and nac90-1 sid2 plants was examined for their morphology. Bar = 1 cm. b, c Abundance of NHP (b) and SA (c) in the leaves of 4-week-old WT, fmo1, nac90-1, nac90-1 fmo1, and nac90-1 sid2 plants upon Psm ES4326 infection. d Growth of Psm ES4326 in the leaves of WT, fmo1, nac90-1, nac90-1 fmo1, and nac90-1 sid2 plants. e Symptoms detected in leaves of WT, fmo1, nac90-1, nac90-1 fmo1, and nac90-1 sid2 plants treated with Psm. Leaves were treated with Psm ES4326 (OD600 = 0.001) and photographed 3 dpi. Bar = 1 cm. f Transcript levels of PR1 in leaves of WT, fmo1, nac90-1, nac90-1 fmo1, and nac90-1 sid2 plants upon Psm ES4326 infection. Values showed here were the average of three biological replicates and error bars represent the SDs of three biological replicates (n = 3). Different letters above the bars denote significant differences among treatments (one-way ANOVA with Tukey’s test, p values are shown in the Source Data file).
The NAC triad cooperates to repress NHP biosynthesis and plant immunity
To identify additional regulators involved in NHP biosynthesis, we performed gene co-expression analysis using the three NHP biosynthetic genes (ALD1, SARD4, and FMO1) as baits against the expression data of all transcription factors from the previously reported transcriptomics experiments of (i) Psm-treated WT, (ii) Psm-treated ald1 and sid2 mutants18, and (iii) Pip-treated fmo1 mutants12. Consequently, one hundred transcription factors were co-expressed with all three baits with an r value greater than 0.9. Consistent with previous report22, NAC and WRKY were the two largest families among these transcription factors (Fig. 7a). As WRKY33 was shown to be a positive regulator of NHP biosynthesis29, we focused on NAC-type factors. Phylogenetic analysis of this this family revealed that NAC90, NAC61, and NAC36 (all three co-expressed) belong to the same subgroup (Supplementary Fig. 7). In addition, NAC61 and NAC36 were significantly induced by Psm ES4326, NHP, and SA treatments (Supplementary Fig. 8a and b), as well as upregulated in both nac90-1 and nac90-2 mutants as noted above (Fig. 4f). The results were corroborated by mining previous transcriptome data18,22,40.
a Co-expression analysis identified potential transcriptional regulators of NHP biosynthesis. Each triangle in the color nightingale rose charts represents a single gene family. b Phenotype of wild-type (WT), nac61 mutants (nac61-1 and nac61-2), and nac36 mutants (nac36-1 and nac36-2). Bar = 1 cm. c Growth of Psm ES4326 on leaves of 4-week-old WT, nac36 mutants. d, e Abundance of NHP (d) and SA (e) in leaves of 4-week-old WT, nac61 mutants (nac61-1 and nac61-2), and nac36 mutants (nac36-1 and nac36-2) upon Psm ES4326 infection. f Transcript levels of PR1 in leaves of 4-week-old WT, nac61 mutants (nac61-1 and nac61-2), and nac36 mutants (nac36-1 and nac36-2) plants upon Psm ES4326 infection. Values showed here were the average of three biological replicates and error bars represent the SDs of three biological replicates (n = 3). Asterisks indicate significant changes compared to WT samples as calculated by Student’s t test (*p value < 0.05; **p value < 0.01; ***p value < 0.001).
To investigate the role of NAC61 and NAC36 in plant immunity, we generated nac61 and nac36 mutants (using CRISPR/Cas9). As shown in Fig. 7b, c, similar to the nac90 mutants, nac36 mutants (i.e., nac36-1 and nac36-2) displayed reduced growth and early senescence, whereas no differences in morphology were observed in the nac61 mutants (i.e., nac61-1 and nac61-2) (Fig. 7b). Furthermore, the nac36 mutants displayed enhanced resistance against Psm ES4326 infection, whereas the disease resistance phenotype of the nac61 mutants was consistent with earlier findings, showing no significant difference compared to the WT (Fig. 7c)40. In nac36 mutants, there was a significant increase in the levels of Pip, NHP, NHPG, SA, and SAG (Fig. 7d, e, and Supplementary Fig. 9a–c). Upon infection with Psm ES4326, a significant reduction in the induction of NHP, SA, and NHPG was observed, while the induction of SAG was augmented in these mutants (Fig. 7d, e, and Supplementary Fig. 9a–c). Conversely, no evident alteration was detected in the levels of Pip, NHP, NHPG, SA, or SAG in the nac61 mutants in response to either Mock or Psm ES4326 infection (Fig. 7d, e, and Supplementary Fig. 9a, b, c). Consistent with the defense-related metabolites, the transcript levels of PR1, FMO1, ALD1, SARD4, and ICS1 were also significantly elevated in nac36 mutants, as compared to WT plants. Conversely, induction of FMO1, ALD1, SARD4, and ICS1 transcripts was notably reduced in nac36 in response to Psm ES4326 infection (Fig. 7f, Supplementary Fig. 9d–g), indicating that NAC36 functions as a negative regulator of NHP biosynthesis.
NAC-type transcription factors form homodimer or heterodimers to regulate target gene expression42,43. To examine whether NAC90, NAC61, and NAC36 can interact with each other, we performed a coimmunoprecipitation assay. As shown in Fig. 8a, NAC61-HA and NAC36-HA could be co-precipitated with NAC90-Flag or NAC36-Flag by an anti-Flag antibody. We next performed a split luciferase complementation imaging assay in N. benthamiana leaves. Strong luminescence was observed in the co-transformations of NAC90 and NAC61, NAC61 and NAC36, as well as NAC36 and NAC90, whereas no signals were detected in the combination of negative controls (Fig. 8b, c, Supplementary Fig. 10). These indicated that the NAC triad can form heterodimers between each other.
a Co-immunoprecipitation experiment analyzing the interactions between the NAC90, NAC61, and NAC36 proteins. Total protein was extracted from N. benthamiana leaves agroinfiltrated with different combinations of NAC90-Flag and NAC61-HA, NAC90-Flag and NAC36-HA, as well as NAC36-Flag and NAC61-HA, and co-immunoprecipitated with anti-Flag. The immunoprecipitated proteins (IP) were analyzed by immunoblotting (IB) using anti-HA antibody (α-HA). b, c Split luciferase complementation assays showing the interactions between the NAC90, NAC36 and NAC61 proteins. Images were acquired at 2 days post infiltration. Red circles indicate infiltrated areas. d Phenotype of wild-type (WT), nac90-1, nac36-1, nac61-1, nac90 nac36, nac90 nac61, and nac61 nac36 plants. Bar = 1 cm. e Abundance of NHP and SA in leaves wild-type (WT), nac90-1, nac36-1, nac61-1, nac90 nac36, nac90 nac61, and nac61 nac36 plants treated with Psm ES4326. Values showed here were the average of three biological replicates and error bars represent the SDs of three biological replicates (n = 3). Different letters above the bars denote significant differences among treatments (one-way ANOVA with Tukey’s test, p values are shown in the Source Data file). f Representation of the “gas-and-brake” model of NHP and SA biosynthetic pathways regulation including negative regulation by the NAC protein triad as revealed in this study.
To investigate the significance of these interactions, we generated a set of double mutants including nac90 nac36, nac90 nac61, and nac61 nac36. We also attempted to generate a triple mutant but were not successful in obtaining such a combination. As depicted in Fig. 8d, nac90 nac36 double mutants exhibited a marginally smaller rosettes phenotype compared to nac90-1, nac36-1, nac90 nac61, and nac61 nac36 mutants. No significant difference was observed among nac90-1, nac36-1, nac90 nac61, and nac61 nac36 mutants. Additionally, the transcript levels of FMO1, ALD1, SARD4, and ICS1 were notably upregulated in nac90 nac36, nac90 nac61, and nac61 nac36 mutants. However, a significant downregulation of these transcripts was observed in the nac90 nac36, nac90 nac61, and nac61 nac36 mutants in response to Psm ES4326 infection (Supplementary Fig. 11–d). In accordance with the transcript changes, nac90 nac36 mutants exhibited elevated NHP levels relative to WT, nac90, nac36, nac61, nac90 nac61, and nac61 nac36 mutants (Fig. 8e). Upon infection of Psm ES4326, a significant suppression in NHP induction was observed in nac90, nac36, nac90 nac36, nac90 nac61, and nac61 nac36 mutants compared to both WT and nac61 (Fig. 8e).
With respect to SA accumulation, nac90 nac36, nac90 nac61, and nac61 nac36 showed relatively higher levels than WT, nac90, nac36, and nac61 mutants. The induction of SA levels was suppressed in nac90 nac36, nac61 nac36, nac90, and nac36 mutants upon infection (Fig. 8e). Likewise, PR1 transcript was significantly elevated n nac90 nac36, nac61 nac36, and nac90 nac61 mutants compared to WT, nac90, nac36, and nac61. Following Psm ES4326 infection, a dramatic increase in PR1 induction was observed in nac90 nac36 and nac61 nac36 relative to other plants (Supplementary Fig. 11h). Subsequently, disease resistance was analyzed among the different double mutant lines. Quantification of Psm ES4326 titers revealed a greater reduction in bacterial growth in the nac90 nac61 as compared to WT and the single mutants, consistent with previous findings40. Similarly, the nac90 nac36 and nac61 nac36 double mutants displayed superior resistance to Psm ES4326 infection compared to the WT and single mutants, which also exhibited enhanced disease resistance relative to the WT plants (Supplementary Fig. 11i). Collectively, these findings suggest that the NAC transcription factor triad discovered here jointly contributes to the suppression of NHP biosynthesis and the negative regulation of plant immunity.
Discussion
Activation of plant defense leads in some responses to simultaneous biosynthesis of NHP and SA, a pair of signaling molecules with essential functions in plant immunity44. However, constitutive production of NHP and SA cannot endure infinitely as they activate defense programs that negatively correlate with plant growth. Considerable progress has been made in understanding the mechanisms that govern plant defense and growth. Many of these processes are controlled by hormone signaling pathways and interconnecting regulatory networks. In our previous work, we reported that UGT76B1-mediated NHP glycosylation plays a critical role in regulating the levels of NHP and SA, resulting in balancing the trade-off between plant growth and defense24. In the present study, we discovered that a triad of NAC transcription factors functions as negative regulators of NHP biosynthesis and plays a vital role in controlling this trade-off by directly repressing NHP accumulation. Together with additional transcriptional activators, this triad takes part in the mechanism mediated by NHP glycosylation. Yet, the above activities are likely part of a more intricate process controlling NHP levels in time and space that might include, non-coding RNAs, epigenetic, and post -transcriptional and -translational mechanisms.
Pathogen attack results in a significant increase in the levels of NHP and SA metabolites, which in turn activate plant immunity to enhance resistance against pathogens44. Substantial evidence indicates the presence of shared and co-regulated molecular mechanisms between SA and NHP44. EDS1 and PAD4 are essential components required for expression of SA and NHP biosynthetic genes, accumulation of NHP and SA, as well as the execution of their functions12,45. The production of SA and NHP can also be regulated by Ca2+ through the modulation of CPKs, CBP60a/CBP60g, and CAMTA1/2/3 activities44. SARD1 and CBP60g serve as the master regulators in plant immunity as they exhibit direct binding abilities to the promoters of both SA (ICS1 and PBS3) and NHP biosynthetic genes31. UGT76B1 is a key enzyme responsible for glycosylation of both NHP and SA and inactive pathogen-induced accumulation of NHP and SA24,25,26,27,28. Apart from the above co-regulated mechanisms, regulation of the SA and NHP pathways varies in several cases. For instance, despite the fact that the SA receptor NPR1 is essential for NHP-mediated immunity22,46, there was no discernible interaction found between NPR1 and NHP46. WRKY33 directly binds to the promoter of ALD1 to enhance NHP biosynthesis, but represses SA biosynthesis29. In this study, we found that a NAC triad plays a significant role as in suppressing the accumulation of both NHP and SA. This is achieved through the direct inhibition of their biosynthetic genes.
Earlier studies showed that exogenous NHP application leads to alteration in the expression of over 1500 genes in Arabidopsis, categorized as NHP response genes22. These were found to be associated with a variety of defense responses, including NPR1-dependent priming of SA accumulation as well as the biosynthesis of phytoalexins and branched-chain amino acids22. Likewise, SAR response also alters the expression of thousands genes18. In this study, we performed proteomics analysis of pNHP plants and identified 585 DAPs between pNHP and WT plants. Notably, the presence of these DAPs in pNHP plants is attributed to continuous high levels of NHP that accumulate over several weeks of plant development. Comparing the list of these DAPs with NHP and SAR responsive genes revealed a significant overlap. Notably, many of these proteins are key players in immune regulation, including PR1, PR5, EDS1, PAD4, RIN4, ACD6, and DMR6, as well as MPK3, MPK4, MPK5, MPK647,48,49,50,51, indicating that they are encoded by genes induced by both SAR and NHP responses. In our study of plants constitutively producing NHP, we found a significant link between cell death and NHP-induced plant immunity. Additionally, this observation was consistent with the pNHP plants phenotype and our earlier work, showing that overaccumulation of NHP triggers cell death in N. Benthamiana and Arabidopsis leaves24,52.
As we found NAC90 to act as a negative regulator, we questioned its hierarchy in the established regulatory network controlling NHP biosynthesis (Fig. 8f). The results showed that following pathogen infection NAC90 expression was considerably reduced in the fmo1, sid2, npr1, eds1, pad4, and sard1 mutants, suggesting that these genes are required for NAC90 function. Additionally, ChIP-sequencing31 and promoter activity assays provided evidence for NAC90 being a direct target of SARD1. Furthermore, the transcript levels of NHP and SA biosynthetic genes were significantly enhanced in the nac90 mutant background and suppressed in NAC90 overexpression plants, suggesting a regulatory negative feedback loop between NAC90 and these genes. We also detected higher levels of Pip, NHP, and SA in nac90 mutants, and the opposite in NAC90 overexpressing plants in the absence of pathogen infection. Moreover, we found that NAC90 directly represses the expression of ALD1, FMO1, and ICS1 genes. NAC transcription factors were reported earlier to act together as negative regulators of downstream pathways. In one example, NAC019, NAC055, and NAC072 negatively regulate SA accumulation by directly suppressing ICS1 and activating SALICYLIC ACID GLUCOSYLTRANSFERASE, a gene encoding an enzyme involved in SA glycosylation53. In addition, a NAC troika (NAC017, NAC082, and NAC90) negatively controls leaf senescence by co-ordinately suppressing both SA and reactive oxygen species pathways37. Here, we showed that the transcript levels of NAC36 and NAC61 were significantly enhanced in both nac90 mutants, and they can interact with each other. Similarly, we also observed higher levels of NHP and SA, as well as enhanced resistance against Psm ES4326 in nac36 mutants. However, we did not find any differences in the resistance against Psm ES4326 in nac61 mutants. Considering these different findings, it is possible that the NHP and SA levels in nac61 mutants are insufficient to initiate defense response. Thus, these three transcription factors exhibit partial functional redundancy and together act to suppress the biosynthesis of NHP and SA. The inference is further substantiated by the enhanced resistance displayed by the double mutants, accompanied by augmented transcript abundance of genes involved in NHP and SA biosynthesis, as well as higher levels of NHP and SA even in the absence of pathogen infection. However, when subjected to pathogen attack, a decrease in the activation of NHP and SA biosynthesis gene expression and their corresponding metabolites levels was noticed in the nac90, nac36, nac90 nac36, nac90 nac61, and nac61 nac36 mutants. This reduction might be caused by two factors; first, enhanced constitutive resistance, leading to reduced infection and lower pathogen stimulus, thereby reducing induction of the immune response, a phenomenon also observed in many constitutive defense mutants. Additionally, this reduction might also be attributed to repression exerted by the enhanced expression and feedback of NAC factors that were not targeted for mutation and remained native in these plant genotypes (either NAC90, NAC61, or NAC36, see Supplementary Fig. 12). These findings also suggest a complex interplay among NAC family members in modulating plant defense responses.
NHP triggers an upregulation of various genes associated with defense response, which includes those responsible for its own biosynthesis11. Additionally, UGT76B1-mediated glycosylation reduces the levels of free NHP, which subsequently leads to a decrease in expression levels of ALD1, SARD4, and FMO124. These indicate the existence of a self-amplifying feedback mechanism within the NHP biosynthesis pathway11. NHP also promotes the expression of genes that are central to SA synthesis and a group of regulators (EDS1, PAD4, CBP60g, and SARD1), which in turn enhance gene expression required for the biosynthesis of both NHP and SA22. Thus, NHP and SA production is orchestrated by numerous feedback loops mechanisms. The findings in our study further amplify the complexity of NHP and SA biosynthesis. The data suggests that NAC90 is regulated by SARD1 and is activated in response to pathogens, as well as by SA and NHP. In turn, it acts as a sort of “hand brake” downregulating the expression of genes involved in the biosynthesis of NHP and SA, thereby curbing the accumulation of these molecules following pathogen attack.
Taken together, we propose a model in which a NAC triad plays a significant role in the trade-off between plant growth and defense response. Under normal conditions, these transcription factors suppress the expression of NHP biosynthetic genes, leading to trace amounts of NHP and SA produced in plants, which can promote plant development and growth. Upon pathogen attack, the NHP and SA biosynthesis pathways are activated and both metabolites accumulate to high levels. Consequently, defense response is enhanced but also early senescence and growth inhibition is triggered. However, at a certain threshold, the three NAC factors are transcriptionally induced by these two metabolites and function as a “hand brake” to decrease NHP and SA levels via transcriptional repression of the corresponding biosynthetic genes (Fig. 8f). This negative control of gene expression prevents harmful effects of excessive NHP and SA levels. Discovery of the regulatory NAC proteins here expands the array of molecular genetic tools currently available for manipulating crop plants to better sustain pathogen attacks with reduced penalty in terms of biomass and yield.
Methods
Plant material and growth condition
Arabidopsis (Arabidopsis thaliana) ecotype Columbia-0 (Col-0) was used as wild-type (WT). The nac90-1 and nac90-2 T-DNA insertion mutants (SALK_206203 and SALK_203643, respectively) were obtained from the Arabidopsis Biological Resource Center (ABRC) and genotyped by PCR using gene- and T-DNA- specific oligonucleotides. Seeds of nac90-1, nac90-2, fmo112, sid254, npr154, pad445, and eds145 were surface sterilized with 70% ethanol for 4 min and 3% (v/v) bleach and then washed with sterile water. Subsequently, seeds were sown on half MS agar medium with 1 % sucrose and stratified at 4 °C for 2 days. Arabidopsis seedlings with two true leaves were transplanted to soil in a long day growth chamber (16 h light). Four to five weeks-old adult plants were used for all the experiments.
Bacterial strains, growth, and infection
Pseudomonas syringae pv. maculicola (Psm) strain ES4326 was grown at 28 °C in King B agar medium containing 50 μg L−1 rifampicin and 50 μg L−1 kanamycin55. For bacterial infection, an overnight log phase culture was collected by centrifugation at 4000 g, washed three times with 10 mM MgCl2 and diluted to a final optical density of 0.001 (at OD600 nm) for infection56.
Vectors construction and plant transformation
For pNHP plants generation, the pAtNHP vector24 was transformed into the A. tumefaciens GV3101 strain used to transform Arabidopsis. Transformants were screened on MS medium containing Kanamycin. Homozygous pNHP plants were obtained in the T2 generation and subsequently used for assays. The open reading frame of NAC90 was amplified from Arabidopsis leaves cDNA by PCR using specific oligonucleotides and cloned into the overexpression vector pALPHA2-NptII-UBQ10pro-CCD-Ter10 using golden gate reaction. For CRISPR plasmid construction, four guide RNAs specifically targeting the NAC61 and NAC36 were designed using CRISPR-P (http://cbi.hzau.edu.cn/crispr/)57,58. After two rounds of amplification, the four sgRNA expression cassettes were cloned into the pAGM55261-CCD binary vector using the golden gate ligation method59. The overexpression and CRISPR vectors were introduced to the A. tumefaciens GV3101 strain using the heat shock method and next transformed to Arabidopsis by the flower dipping method60. Primers used for plasmid construction were listed in Supplementary Data 7.
Label-free proteomics
Label-free proteomics was performed as described previously61. In brief, leaves were harvested from 28 d old WT, pNHP1, and pNHP2 plants and milled into fine power in liquid nitrogen. About 100 mg leaf powder was lysed in 300 μl lysis buffer (4% (w/v) sodium dodecyl sulfate, 100 mm Tris/HCl pH|7.6, 0.1 M dithiothreitol), boiled at 95 °C 5 min, and then centrifugated at 16,000 g for 15 min. Subsequently, the supernatants were collected and subjected to protein concentrations determination using Thermo Scientific Pierce 660 nm protein assay kit. Fifty micrograms of protein from each sample were reduced, alkylated and digested on a centrifugal filter unit (10 kDa MWCO) according to the filter-aided sample preparation (FASP) protocol62. The tryptic peptides were acidified with trifluoroacetic acid, desalted, and dried using a speed vacuum. The label-free proteomics experiment was performed in three independent biological replicates. The peptides were dissolved in 97:3 H2O:acetonitrile + 0.1% formic acid, and then separated using an HSS T3 nano-column (75 μm internal diameter, 250 mm length, 1.8 μm particle size; Waters) at 0.35 μL min−1. Peptides were then eluted using the following gradient: 4–35% B in 105 min; 35–90% B in 5 min; maintained at 90% for 5 min; and then back to initial conditions. The mass spectrometry analysis was performed using a Nano-UPLC system equipped with a quadrupole orbitrap mass spectrometer (Q Exactive Plus, Thermo Scientific). Data was acquired in a data dependent acquisition mode using the Top20 method. MS1 mass range was 300–1750 m/z, resolution was set to 60 000 (at 400 m/z), AGC target 1e6 and maximum injection time was set to 120 ms. MS2 resolution were set to 17,500, isolation window of 2 m/z, underfill ratio 1.3%, AGC target of 5e5 and maximum injection time of 100 ms. Dynamic exclusion was 120 ms. MS raw data was processed using the Proteome Discoverer 3.1 software (Thermo Fisher Scientific) for protein identification and quantification with 1% false discovery rate (FDR). The following parameters were used for searching the Sequest HT search engine against TAIR 10 proteins dataset.: (1) maximum two missed tryptic cleavages; (2) peptide length 6–144 amino acids; (3) precursor mass tolerance 10 ppm; (4) fragment mass tolerance 0.02 Da; and (5) fixed and variable modifications like carbamidomethylation of Cys and oxidation of Met. The data were filtered for at least two peptides matching the protein. For protein quantification, the abundance of proteins was evaluated by estimating the peak areas of their MS1 precursor ions. The relative protein abundance was normalized by employing a total peptide amount-based approach across LC runs using a normalization algorithm (total intensity count) of the Proteome Discoverer 3.1 software. To identify proteins that were significantly different between WT and pNHP plants, a twofold cut-off was used as a determinant. GO terms were analyzed in the TAIR website (https://www.arabidopsis.org/tools/go_term_enrichment.jsp).
Metabolite extraction and quantification
Metabolites were extracted from leaf samples according to previously reported method24. Briefly, 100 mg Arabidopsis and tomato leaf tissue were flash frozen in liquid nitrogen immediately after collection, ground to fine power using a ball mill (Retsch MM400), resuspended in 400 μl of 80% MeOH: H2O (v/v), sonicated for 20 min at room temperature, and centrifugated at 13,000 g for 15 min. The supernatants were harvested, filtered through a 0.22 μm polyvinylidene fluoride (PVDF) syringe-driven filter (Millipore, Billerica, MA), and subjected to liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis. The analyses of metabolites were conducted on Xevo TQ-S micro Triple Quadrupole Mass Spectrometry (Waters) connected to UPLC instrument (Premier system, Waters). MS was operating in a Multiple Reaction Monitoring (MRM) mode for relative quantification of NHP and SA derivatives (Pip, NHP, NHPG, SA, and SAG). A 1.8 μm, 2.1 mm × 100 mm ACQUITY UPLC HSS T3 column (Waters) was used for metabolites separation. The mobile phase A consisted of 0.1% formic acid in 1% aqueous acetonitrile and mobile phase B was 0.1% formic acid in 100% acetonitrile. The following linear gradient was used with a flow rate of 0.3 ml/min (percentages indicate percentage A): 0–3 min (99.9%), 3–9 min (99.9–92%), 9–9.2 min (92–0%), 9.2–11.8 min (0–0%), 11.8–12 min (0–99.9%), 12–14 min (99.9%). Mass spectra were collected within a mass range of 50–1600 in the positive ionization mode for NHP derivatives and in the negative ionization mode for SA derivatives. The following instrument settings were applied: capillary = 1.5 kV and cone = 15 V for positive ionization (1 kV and 27 V for negative ionization); source temperature, 140 °C; desolvation, 450 °C; desolvation gas flow, 800 l/h. Argon was used as the collision gas. NHP was relatively quantified in positive mode using quantification transition (QT) (146.08 > 110.06) and verification transition (VT) (146.08 > 100.07). For NHPG, QT was 308.13 > 146.08, and VT was 308.13 > 110.06. SA was relatively quantified in negative mode using transition 137.02 > 93.03, and VT 93.03 > 65.03. For SAG, QT was [299.07 > 137.02], VT was [299.07 > 93.03]. TargetLynx (Water, UK) was used for data analysis and relative quantification. A mixture of Pip, NHP, NHPG, SA, and SAG standards was processed along the samples and served as a positive quality control.
Protein extraction and western blot
Western blot was conducted following the previously reported protocol63. Hundred mg leaf samples were ground and heated at 95 °C in 2× protein loading buffer for 5 min. Protein samples were collected after centrifugation, run on an 12% SDS-PAGE gel, transferred to PVDF membranes and blocked with 5% BSA in 1× TBST for 1 h at room temperature followed by incubation with the Anti-Flag (Abmart, M20008S) primary antibody in 5% BSA for 1 h. PVDF membranes were washed four times with 1× TBST and incubated with horseradish peroxidase-conjugated secondary antibody for 1 h. SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific) was used for chemiluminescence signal.
Dual luciferase promoter activity assays
Dual luciferase promoter activity assays were performed according to the reported method with several modifications64. About 2 kb upstream from the transcription start site of target genes was amplified from Arabidopsis leaves DNA and introduced upstream of the firefly luciferase reporter gene in the pCambia 1302 plasmid harboring a renilla luciferase gene under the control of the CaMV35S promoter as an internal control. The open reading frame of NAC90, SARD1, and CBP60g were amplified and cloned into the effector plasmid pALPHA2-Npt II- UBQ10-CCDB-Ter10 using the infusion method (Vazyme, China). The empty effector plasmid was used as the negative control. The destination reporters and effectors were co-transformed into N. benthamiana leaves by agroinfiltration and infiltrated leaves were harvested after 48 h at 22 °C and subjected for LUC/REN luciferase activities assay. All the primers used for dual luciferase promoter activity assays are listed in Supplementary Data 7.
Gene expression analysis
Leaves tissues were collected and flash frozen in liquid nitrogen immediately for total RNA isolation, total RNA was extracted from leaves tissues using the hot phenol method, treated with RNase-free DNAase I (Sigma), and then reversed transcribed to cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). Quantitative real-time PCR was performed with Fast SYBR Green reagent (Applied Biosystems) in an Applied Biosystems Quantstudio real-time PCR system (Applied Biosystems). Gene-specific primers used for qRT-PCR are listed in Supplementary Data 7. The fold change was calculated according to the formula 2–ΔΔct, and actin gene expression was used as a reference for normalization65.
Chromatin immunoprecipitation (ChIP) analysis
ChIP assays were performed according to the reported protocol with some modifications31. 4-g leaves of NAC90-3×Flag plants at 25 days were harvested, fixed in 1% formaldehyde under a vacuum for 10 min. Glycine (2 M) was added to a final concentration of 0.125 M and the leaves was vacuumed for another 5 min to stop the cross-linking. After that, the leaves tissues were washed with 300 ml of cold ddH20 and dried with paper. The chromatin complexes were isolated and sonicated to an average size of 0.3–1 kb as previously described66. The sonicated chromatin complexes were incubated with polyclonal anti-Flag (Cell Signaling, #82103) or pre-immune serum IgG (negative control, Cell Signaling, #8726) according to the previously described method31. The eluted chromatin complexes were reversed and the precipitated DNA was purified using a DNA purification kit (Cat #N1073, GDSbio, China). The purified DNA was quantified by real-time PCR using specific primers (Supplementary Data 7).
Phylogenetic tree construction
NAC protein sequences were extracted from the TAIR database (https://www.arabidopsisi.or) using HMM search based on the NAC domain (Pfam: PF02365) and alignment performed in Clustal X (version 2.1) with default multiple parameters and imported into the MEGA (Version X) software67. The phylogenetic tree was constructed using the neighbor-joining method with 1000 bootstrp replicates.
Split luciferase complementation imaging (LCI) assay
LCI experiments were performed according to the described method. The coding region of NAC90, NAC61, and NAC36 was amplified from the Arabidopsis DNA and purified with DNA purification kit (Cat #N1073, GDSbio, China). The fragments then were introduced into pCAMBIA1300-Cluc/Nluc to generate NAC90-Nluc, Cluc-NAC90, Cluc-NAC36, and NAC61-Nluc using the in-fusion method. Primers used for the LCI plasmids construction are listed in Supplementary Data 7. The destination plasmids were introduced into A. tumefacients strain GV3101 and transiently expressed in N. benthamiana leaves through agroinfiltration. Forty-eight hours post infiltration, leaves were sprayed with 1 mM luciferin solution (1% Triton X-100), kept in the dark for 5 min, and observed for luminescence.
Co-immunoprecipitation (CoIP) assay
CoIP assays were performed according to the described protocol68. Briefly, total proteins were extracted from agroinfiltrated N. benthamiana leaves using native extraction buffer and centrifuged twice at 16,000 g at 4 °C for 20 min. The supernatants were collected for input immunoblot analysis and immunoprecipitation. The supernatants were incubated with antibody anti-Flag and IgG conjugated magnetic beads (Cell Signaling, #82103) for 4 h with agitation. After that, beads were washed four times with native protein extraction buffer. Finally, the 2 × SDS loading buffer was added to the beads and boiled 5 min to release the proteins from the beads. The eluted proteins were immunoblotted using the anti-HA antibody (Abmart, M20003S).
Statistics and reproducibility
Statistical analyses were performed using the GraphPad Prism software. All data are presented as the mean ± SD. Significant differences among samples or genotypes were calculated using a two-tailed Student’s t test or one-way ANOVA with Tukey’s post hoc test. Details of the biological replicates used in various experiments are provided in the “Methods” section, as well as in the legends of the Main Figures and Supplementary Figs., wherever necessary. Source data are provided in the Source Data file. Samples were arranged randomly in related experiments. No data were excluded from our analyses. The results are reliable and reproducible.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The proteomics raw data generated in this study have been deposited in a ProteomeXchange Consortium member under the accession number PXD054472. The proteomics data generated in this study are provided in the Supplementary Information file. The RNA-seq data in this study are available in the NCBI Sequence Read Archive database under the accession number PRJNA996953 [https://www.ncbi.nlm.nih.gov/bioproject/?term=996953]. Source data are provided with this paper.
References
Jones, J. D. & Dangl, J. L. The plant immune system. Nature 444, 323–329 (2006).
Malamy, J., Carr, J. P., Klessig, D. F. & Raskin, I. Salicylic acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science 250, 1002–1004 (1990).
Métraux, J. et al. Increase in salicylic acid at the onset of systemic acquired resistance in cucumber. Science 250, 1004–1006 (1990).
Nawrath, C. & Métraux, J. Salicylic acid induction-deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell 11, 1393–1404 (1999).
Ward, E. R. et al. Coordinate gene activity in response to agents that induce systemic acquired resistance. Plant Cell 3, 1085–1094 (1991).
Wu, Y. et al. The Arabidopsis NPR1 protein is a receptor for the plant defense hormone salicylic acid. Cell Rep. 1, 639–647 (2012).
Park, S., Kaimoyo, E., Kumar, D., Mosher, S. & Klessig, D. F. Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science 318, 113–116 (2007).
Jung, H. W., Tschaplinski, T. J., Wang, L., Glazebrook, J. & Greenberg, J. T. Priming in systemic plant immunity. Science 324, 89–91 (2009).
Chanda, B. et al. Glycerol-3-phosphate is a critical mobile inducer of systemic immunity in plants. Nat. Genet. 43, 421–427 (2011).
Chaturvedi, R. et al. An abietane diterpenoid is a potent activator of systemic acquired resistance. Plant J. 71, 161–172 (2012).
Chen, Y. et al. N-hydroxy-pipecolic acid is a mobile metabolite that induces systemic disease resistance in Arabidopsis. Proc. Natl Acad. Sci. USA 115, E4920–E4929 (2018).
Hartmann, M. et al. Flavin monooxygenase-generated N-hydroxypipecolic acid is a critical element of plant systemic immunity. Cell 173, 456–469.e416 (2018).
Schwarzenbacher, R. E. et al. The IBI1 receptor of β-aminobutyric acid interacts with VOZ transcription factors to regulate abscisic acid signaling and callose-associated defense. Mol. Plant 13, 1455–1469 (2020).
Goto, Y. et al. Exogenous treatment with glutamate induces immune responses in Arabidopsis. Mol. Plant-Microbe Interact. 33, 474–487 (2020).
Zeng, H., Bai, Y., Wei, Y., Reiter, R. J. & Shi, H. Phytomelatonin as a central molecule in plant disease resistance. J. Exp. Bot. 73, 5874–5885 (2022).
Návarová, H., Bernsdorff, F., Döring, A. & Zeier, J. Pipecolic acid, an endogenous mediator of defense amplification and priming, is a critical regulator of inducible plant immunity. Plant Cell 24, 5123–5141 (2012).
Hartmann, M. et al. Biochemical principles and functional aspects of pipecolic acid biosynthesis in plant immunity. Plant Physiol. 174, 124–153 (2017).
Bernsdorff, F. et al. Pipecolic acid orchestrates plant systemic acquired resistance and defense priming via salicylic acid-dependent and-independent pathways. Plant Cell 28, 102–129 (2016).
Tian, H. & Zhang, Y. The emergence of a mobile signal for systemic acquired resistance. Plant Cell 31, 1414–1415 (2019).
Holmes, E. C., Chen, Y., Sattely, E. S. & Mudgett, M. B. An engineered pathway for N-hydroxy-pipecolic acid synthesis enhances systemic acquired resistance in tomato. Sci. Signal. 12, eaay3066 (2019).
Schnake, A. et al. Inducible biosynthesis and immune function of the systemic acquired resistance inducer N-hydroxypipecolic acid in monocotyledonous and dicotyledonous plants. J. Exp. Bot. 71, 6444–6459 (2020).
Yildiz, I. et al. The mobile SAR signal N-hydroxypipecolic acid induces NPR1-dependent transcriptional reprogramming and immune priming. Plant Physiol. 186, 1679–1705 (2021).
Hartmann, M. & Zeier, J. l‐lysine metabolism to N‐hydroxypipecolic acid: an integral immune‐activating pathway in plants. Plant J. 96, 5–21 (2018).
Cai, J. et al. Glycosylation of N-hydroxy-pipecolic acid equilibrates between systemic acquired resistance response and plant growth. Mol. Plant 14, 440–455 (2021).
Bauer, S. et al. UGT76B1, a promiscuous hub of small molecule-based immune signaling, glucosylates N-hydroxypipecolic acid, and balances plant immunity. Plant Cell 33, 714–734 (2021).
Holmes, E. C., Chen, Y., Mudgett, M. B. & Sattely, E. S. Arabidopsis UGT76B1 glycosylates N-hydroxy-pipecolic acid and inactivates systemic acquired resistance in tomato. Plant Cell 33, 750–765 (2021).
Mohnike, L. et al. The glycosyltransferase UGT76B1 modulates N-hydroxy-pipecolic acid homeostasis and plant immunity. Plant Cell 33, 735–749 (2021).
Zeier, J. Metabolic regulation of systemic acquired resistance. Curr. Opin. Plant Biol. 62, 102050 (2021).
Wang, Y. et al. A MPK3/6-WRKY33-ALD1-pipecolic acid regulatory loop contributes to systemic acquired resistance. Plant Cell 30, 2480–2494 (2018).
Guerra, T. et al. Calcium‐dependent protein kinase 5 links calcium signaling with N‐hydroxy‐L‐pipecolic acid‐and SARD1‐dependent immune memory in systemic acquired resistance. N. Phytol. 225, 310–325 (2020).
Sun, T. et al. ChIP-seq reveals broad roles of SARD1 and CBP60g in regulating plant immunity. Nat. Commun. 6, 1–12 (2015).
Sun, T. et al. TGACG‐BINDING FACTOR 1 (TGA1) and TGA4 regulate salicylic acid and pipecolic acid biosynthesis by modulating the expression of SYSTEMIC ACQUIRED RESISTANCE DEFICIENT 1 (SARD1) and CALMODULIN‐BINDING PROTEIN 60g (CBP60g). N. Phytol. 217, 344–354 (2018).
Zhou, M. et al. WRKY70 prevents axenic activation of plant immunity by direct repression of SARD1. N. Phytol. 217, 700–712 (2018).
Sun, T. et al. Redundant CAMTA transcription factors negatively regulate the biosynthesis of salicylic acid and N-hydroxypipecolic acid by modulating the expression of SARD1 and CBP60g. Mol. Plant 13, 144–156 (2020).
Lan, J. et al. Epigenetic regulation of N‐hydroxypipecolic acid biosynthesis by the AIPP3‐PHD2‐CPL2 complex. J. Integr. Plant Biol. 65, 2660–2671 (2023).
Yildiz, I. et al. N‐hydroxypipecolic acid induces systemic acquired resistance and transcriptional reprogramming via TGA transcription factors. Plant Cell Environ. 46, 1900–1920 (2023).
Kim, H. J. et al. Time-evolving genetic networks reveal a NAC troika that negatively regulates leaf senescence in Arabidopsis. Proc. Natl Acad. Sci. USA 115, E4930–E4939 (2018).
Huang, W., Wu, Z., Tian, H., Li, X. & Zhang, Y. Arabidopsis CALMODULIN-BINDING PROTEIN 60b plays dual roles in plant immunity. Plant Commun. 2, 100213 (2021).
Li, L. et al. Arabidopsis CBP60b is a central transcriptional activator of immunity. Plant Physiol. 186, 1645–1659 (2021).
Hickman R. et al. Transcriptional dynamics of the salicylic acid response and its interplay with the jasmonic acid pathway. BioRxiv, 742742 (2019).
Rekhter, D. et al. Isochorismate-derived biosynthesis of the plant stress hormone salicylic acid. Science 365, 498–502 (2019).
Ernst, H. A., Nina Olsen, A., Skriver, K., Larsen, S. & Lo Leggio, L. Structure of the conserved domain of ANAC, a member of the NAC family of transcription factors. EMBO Rep. 5, 297–303 (2004).
Olsen, A. N., Ernst, H. A., Leggio, L. L. & Skriver, K. DNA-binding specificity and molecular functions of NAC transcription factors. Plant Sci. 169, 785–797 (2005).
Huang, W., Wang, Y., Li, X. & Zhang, Y. Biosynthesis and regulation of salicylic acid and N-hydroxypipecolic acid in plant immunity. Mol. Plant 13, 31–41 (2020).
Cui, H. et al. A core function of EDS1 with PAD4 is to protect the salicylic acid defense sector in Arabidopsis immunity. N. Phytol. 213, 1802–1817 (2017).
Liu, Y. et al. Diverse roles of the salicylic acid receptors NPR1 and NPR3/NPR4 in plant immunity. Plant Cell 32, 4002–4016 (2020).
Bethke, G. et al. Activation of the Arabidopsis thaliana mitogen-activated protein kinase MPK11 by the flagellin-derived elicitor peptide, flg22. Mol. Plant-Microbe Interact. 25, 471–480 (2012).
Feys, B. J., Moisan, L. J., Newman, M. & Parker, J. E. Direct interaction between the Arabidopsis disease resistance signaling proteins, EDS1 and PAD4. EMBO Rep. 20, 5400–5411 (2001).
Lu, H., Rate, D. N., Song, J. T. & Greenberg, J. T. ACD6, a novel ankyrin protein, is a regulator and an effector of salicylic acid signaling in the Arabidopsis defense response. Plant Cell 15, 2408–2420 (2003).
Mackey, D., Belkhadir, Y., Alonso, J. M., Ecker, J. R. & Dangl, J. L. Arabidopsis RIN4 is a target of the type III virulence effector AvrRpt2 and modulates RPS2-mediated resistance. Cell 112, 379–389 (2003).
Zeilmaker, T. et al. DOWNY MILDEW RESISTANT 6 and DMR 6‐LIKE OXYGENASE 1 are partially redundant but distinct suppressors of immunity in Arabidopsis. Plant J. 81, 210–222 (2015).
Cai, J. & Aharoni, A. Amino acids and their derivatives mediating defense priming and growth tradeoff. Curr. Opin. Plant Biol. 69, 102288 (2022).
Zheng, X. et al. Coronatine promotes Pseudomonas syringae virulence in plants by activating a signaling cascade that inhibits salicylic acid accumulation. Cell Host Microbe 11, 587–596 (2012).
Poraty-Gavra, L. et al. The Arabidopsis Rho of plants GTPase AtROP6 functions in developmental and pathogen response pathways. Plant Physiol. 161, 1172–1188 (2013).
Fan, J., Crooks, C. & Lamb, C. High‐throughput quantitative luminescence assay of the growth in planta of Pseudomonas syringae chromosomally tagged with Photorhabdus luminescens luxCDABE. Plant J. 53, 393–399 (2008).
Rufián, J. S., Rueda-Blanco, J., Beuzón, C. R. & Ruiz-Albert, J. Protocol: an improved method to quantify activation of systemic acquired resistance (SAR). Plant Methods 15, 1–8 (2019).
Lei, Y. et al. CRISPR-P: a web tool for synthetic single-guide RNA design of CRISPR-system in plants. Mol. Plant 7, 1494–1496 (2014).
Ma, X. et al. A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol. Plant 8, 1274–1284 (2015).
Grützner, R. et al. High-efficiency genome editing in plants mediated by a Cas9 gene containing multiple introns. Plant Commun. 2, 100135 (2021).
Zhang, X., Henriques, R., Lin, S., Niu, Q. & Chua, N. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protoc. 1, 641–646 (2006).
Szymanski, J. et al. Label‐free deep shotgun proteomics reveals protein dynamics during tomato fruit tissues development. Plant J. 90, 396–417 (2017).
Wiśniewski, J. R., Zougman, A., Nagaraj, N. & Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 6, 359–362 (2009).
Cai, J., Chen, T., Wang, Y., Qin, G. & Tian, S. SlREM1 triggers cell death by activating an oxidative burst and other regulators. Plant Physiol. 183, 717–732 (2020).
Hellens, R. P. et al. Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1, 1–14 (2005).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25, 402–408 (2001).
Wang, Y. et al. Tomato nuclear proteome reveals the involvement of specific E2 ubiquitin-conjugating enzymes in fruit ripening. Genome Biol. 15, 1–19 (2014).
Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547 (2018).
Cai, J., Qin, G., Chen, T. & Tian, S. The mode of action of remorin1 in regulating fruit ripening at transcriptional and post‐transcriptional levels. N. Phytol. 219, 1406–1420 (2018).
Acknowledgements
The work is supported by the ERC-2019-ADG project SIREM (884316). We thank the Adelis Foundation, the Leona M. and Harry B. Helmsley Charitable Trust, the Jeanne and Joseph Nissim Foundation for Life Sciences, the Tom and Sondra Rykoff Family Foundation Research, and the Raymond Burton Plant Genome Research Fund for supporting the Aharoni lab. A.A. is the incumbent of the Peter J. Cohn Professorial Chair.
Author information
Authors and Affiliations
Contributions
J.C. designed and performed the research and wrote the article. S.P. contributed to data analysis. Y.K. assisted with label-free proteomic analysis and operated the LC-MS. E.A. assisted with the generation of transgenic plants. S.M. and I.R. assisted with metabolomics data analysis and operated the LC-MS. Z.L. assisted with paper correction. A.A. designed the research and wrote the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Yanmei Chen, Jürgen Zeier, and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cai, J., Panda, S., Kazachkova, Y. et al. A NAC triad modulates plant immunity by negatively regulating N-hydroxy pipecolic acid biosynthesis. Nat Commun 15, 7212 (2024). https://doi.org/10.1038/s41467-024-51515-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-51515-2
- Springer Nature Limited