Abstract
Enhancing drought tolerance in crops and understanding the underlying mechanisms have been subject of intense research. The precise function and molecular mechanisms of B-box zinc finger proteins (BBX) remain elusive. Here, we report a natural allele of BBX18 (BBX18TT) that encodes a C-terminal truncated protein. While most wild tomato germplasms contain the BBX18CC allele and show more drought tolerant, modern cultivated tomatoes mostly carry BBX18TT allele and are more drought sensitive. Knockout of BBX18 leads to improved drought tolerance in transgenic plants of cultivated tomato. Ascorbate peroxidase 1 (APX1) is identified as a BBX18-interacting protein that acts as a positive regulator of drought resistance in tomato. Chromatin immunoprecipitation sequencing analyses reveal that BBX18 binds to a unique cis-acting element of the APX1 promoter and represses its gene expression. This study provides insights into the molecular mechanism underlying drought resistance mediated by the BBX18-APX1 module in plants.
Similar content being viewed by others
Introduction
The growth and development of plants are frequently influenced by the environment, with abiotic stress (such as drought, high temperature, low temperature, high salt concentration, and heavy metal toxicity) being a crucial determinant of plant distribution and growth. Drought not only directly induces water scarcity in plants, thereby impacting their water and nutrient uptake, but also engenders alterations in the soil environment surrounding plants, leading to increased soil salinity and compaction1. Among all abiotic stresses, drought exerts the most profound impact on soil and plants. Climate change is anticipated to escalate both the frequency and severity of drought events, thereby becoming a pivotal constraint on agricultural production2. Therefore, there is an urgent imperative to expedite the genetic enhancement of crop drought resistance through the utilization of novel biotechnological tools employing optimal genes. To expedite the breeding progress of drought-resistant varieties, it is imperative to explore and elucidate the genetic basis and underlying molecular mechanisms governing drought resistance in plants.
Transcription factors (TFs) are engaged not only in direct interactions with other associated proteins but also exhibit specific binding affinities to the cis-elements within the gene promoter region, thereby exerting their regulatory control over gene transcription through activation or inhibition. Consequently, manipulation of TFs can effectively modulate the functions of multiple genes, making it a more potent approach for enhancing crop stress resistance as compared to the introduction or modification of individual functional genes. A family of B-box (BBX) proteins represent a distinct class of zinc finger TFs that have garnered increasing attention in recent years due to their multifunctionality, pivotal roles in plant growth and development, and responsiveness to environmental stimuli3. BBX proteins are characterized by the presence of one or two conserved BBX domains at their N-termini and, in certain cases, a CONSTANS (CO), CO-like, and TOC1 (CCT)- domain at their C-termini4. BBX domains play crucial roles in the regulation of transcription and protein interactions4, while CCT domains are involved in the control of transcriptional processes and nuclear transport5.
Members of the BBX gene family have been identified in various plant species, including Arabidopsis thaliana (32 BBX genes), rice (30 genes), Moso bamboo (27 genes), buckwheat (28 genes), cabbage (43 genes), tomato (31 genes), and salvia (27 genes)6. BBX proteins have been shown to play crucial roles in diverse growth and developmental processes of plants, including photomorphogenesis, flowering regulation, signal transduction pathways, as well as responses to abiotic and biotic stresses. The BBX gene, AtBBX1, was studied in Arabidopsis thaliana and has been found to regulate flowering time by controlling the expression of downstream genes7,8,9. Subsequently, other Arabidopsis BBX genes such as BBX4, BBX6, BBX7, and BBX32 have also been discovered to play roles in regulating flowering time10,11,12,13. Furthermore, multiple BBX genes (BBX1, BBX4, BBX10, BBX19, BBX20, BBX21-24, and 28–29) have been implicated in photomorphogenic development. The proteins encoded by these genes are ubiquitinated by Constitutive Photomorphogenic Protein 1 (COP1) and subsequently degraded through the action of the 26S proteasome system when the plants are kept in darkness4,14. BBX proteins have also been shown to promote plant adaptation to abiotic stresses. For instance, overexpression of BBX24 in Arabidopsis thaliana exhibits enhanced salt tolerance as compared to the wild type plants grown under high salt conditions, leading to a significant increase in the root length of transgenic plants15. Overexpression of CmBBX24 in chrysanthemums not only extends the duration of flowering but also enhances their tolerance to cold and drought conditions9. Heterologous expression of the chrysanthemum CmBBX22 gene in Arabidopsis thaliana has been shown to positively regulate drought resistance in transgenic plants16. However, the same CmBBX22 gene negatively regulates drought resistance in chrysanthemums17. In addition, apple MdBBX10 has been demonstrated to enhance salt and drought tolerance by modulating the abscisic acid (ABA) signaling pathway and regulating the accumulation of reactive oxygen species (ROS)18. The MdBBX7 gene positively regulates plant drought resistance through its interaction with an E3 ubiquitin ligase, which leads to ubiquitination and subsequent degradation of its protein19.
With the advancement of research, the molecular function and regulatory network of BBX proteins in various plant species have been elucidated, garnering increasing attention in the field of plant molecular genetics. In Arabidopsis, BBX21 interacts with BBX22 and ELONGATED HYPOCOTYL 5 (HY5) and binds to the promoter regions of their respective genes, resulting in activation of their transcription20,21,22. Moreover, both BBX21 and HY5 can bind to the promoter region of BBX11 to facilitate its transcriptional activity, while BBX11, in turn, binds to the HY5 promoter to activate its gene transcription23. Thus, these three genes (BBX21, HY5, and BBX11) form a precise positive feedback loop that regulates plant photomorphogenesis.
The expression of tomato DIHYDROFLAVONOL-4-REDUCTASE (SlDFR) is activated by SlBBX20 through its binding to G-box1 in the promoter region of SlDFR, thereby promoting anthocyanin biosynthesis. In addition, SlBBX20 directly activates the expression of PHYTOENE SYNTHASE 1 (PSY-1) to regulate carotenoid biosynthesis, and its protein is degraded via the 26S proteasome-mediated ubiquitination24. A transcription factor module consisting of SlBBX20/21-SlHY5 has been shown to regulate tomato photomorphogenesis by triggering the transcription activation of downstream genes25. Therefore, the specific network formed by SlBBX20/21 and HY5 plays a crucial role in precisely controlling plant growth and development.
Under drought stress, plant cells generate substantial amounts of ROS, which directly inflict a wide range of damage to the plants. Peroxidase plays a pivotal role in the antioxidant defense system and effectively eliminates peroxides generated by oxygen reduction reactions, including H2O2, O2-, etc. Among them, ascorbate peroxidase (APX) is an essential enzyme in organisms for their survival from oxidative stresses. APX belongs to class I heme-containing peroxidases and utilizes ascorbic acid (AsA) as a specific electron donor to facilitate the conversion of H2O2 to H2O and O226. The presence of AsA can contribute to the removal of hydrogen peroxide and the enhancement of stress resistance in plants. Numerous studies have demonstrated that APX, functioning as an antioxidant enzyme, can lead to a reduction in cell osmotic potential by accumulating other osmolyte compounds such as proline and soluble sugars26,27.
Expression of APX genes exhibits a positive response to salt stress; for instance, APX3 and APX5 show significant responses to salinity, drought, and thermal stress28. The overexpression of celery AgAPX1 in Arabidopsis thaliana has been found to result in elevated levels of AsA, increased antioxidant capacity, and enhanced drought resistance29. SlAPX1 is one of the three cytosolic APXs found in the tomato APX family. Overexpression of the parasite Leishmania major LmAPX, which shares high sequence homology with tomato SlAPX1, in transgenic tobacco plants leads to significantly higher APX enzyme activity, accompanying with reduced H2O2 content, elevated proline concentration, and higher net photosynthetic rates. These changes effectively protect the plants from oxidative stress30.
Tomato, an essential vegetable crop globally, originated in the arid regions of the Andes in South America. Wild tomatoes (S. cheesmaniae, S. chilense, S. pennellii, S. pimpinellifolium, and S. lycopersicum var. cerasiforme) exhibit strong drought resistance31,32; however, through human domestication and natural variation, most cultivated tomatoes have become sensitive to drought33. In this work, we use gene chip analysis to investigate drought resistance acclimation and identify a naturally existing variant of BBX18 that is involved in the regulation of drought resistance in tomatoes. The BBX18 protein is found to interact with APX1 and bind to the cis-acting elements of the APX1 promoter to regulate its gene expression. This study has unveiled a mechanism underlying drought resistance regulation, providing valuable insights for studying drought tolerance in other plant species.
Results
SNP-265 in BBX18 is a crucial SNP site for transcription activation activity
Previously, two drought-tolerant introgression lines (IL2-5 and IL9-1) were identified from a population of Solanum pennellii introgression lines, using S. lycopersicum cv. M82, a drought-sensitive cultivar, is the recurrent parent34. We reasoned that genes that were differentially expressed exclusively in the drought-tolerant lines under drought stress might be caused by the two inserted chromosome segments of S. pennellii, which possibly contain drought-tolerance quantitative trait loci (QTLs). Analysis of these differentially expressed genes (DEG) revealed that they were typically found within the chromosome segments that were inherited from S. pennellii. Among these genes, the expression of SlBBX18 was found to be significantly decreased in IL2-5 as compared to M82 and IL9-1 under drought stress (Supplementary Fig. 1). Using the physical sequence database of the tomato genome and IL map (SGN: https://solgenomics.net/), SlBBX18 (Solyc02g084420.2) was located on chromosome 2 at SL2.50ch02: 47529657–47531609 and was flanked by restriction fragment length polymorphism (RFLP) markers TG151 and TG462, with the former being at SL2.50ch02: 53173050–53173593 (545 bp) and the latter at SL2.50ch02: 45844190–45844906 (717 bp). Furthermore, BBX18 was physically positioned within the bacterial artificial chromosome (BAC) clone C02SLm0132H19, which was mapped to the IL segment of chromosome 2-I in the tomato genome. All these pieces of evidence strongly suggest that SlBBX18 is localized in the IL2-5 region of the tomato genome and likely represents a major QTL for drought resistance. To investigate the role of BBX18 in drought stress response, we obtained full coding sequences of BBX18 from two different tomato species: S. lycopersicum M82 (referred to as SlBBX18) and S. pennellii LA0716 (referred to as SpBBX18). The sequences of SlBBX18 and SpBBX18 exhibited a remarkable degree of similarity, sharing a 99.6% identity. Notably, two single nucleotide polymorphisms (SNP-265 and SNP-328) were detected at positions 265 (T: C) and 328 (T: C) (Supplementary Figs. 2, 1a). Interestingly, the variation of T at position 265 of SlBBX18 resulted in premature translation termination due to the introduction of a stop codon (TGA), while the variation at position 328 led to an amino acid substitution (Supplementary Figs. 2, 1b).
a Schematic diagram of BBX18 alleles. There were two nucleotide changes in the coding region between SpBBX18 from wild tomato (S. pennelli) and SlBBX18 from cultivated tomato species (S. lycopersicum). The C328T change would lead to an amino acid residue substitution at position 110 from proline (328 CCT) in SpBBX18 to serine (328 TCT) in SlBBX18. The C265T change would lead to the substitution at amino acid residue position 89 from arginine (265 CGA) in SpBBX18 to a translation stop codon (265 TGA) in SlBBX18, which would truncate the C-terminal half of BBX18 protein in SlBBX18. The N-terminus of both alleles contains a double B-box domain. b Transcriptional activation activity analysis of different segments of SpBBX18 and SlBBX18 in yeast. c Subcellular localization of SpBBX18 and SlBBX18. The proteins SpBBX18 and SlBBX18 were fused with GFP, and ERF-RFP was employed as a marker protein. Three independent experiments were conducted. d Transcriptional activation activity analysis of C265T and C328T of SpBBX18 in yeast. The nucleotide C at positions 265 and 328 in SpBBX18 was mutated to T via the PCR-based site-directed mutagenesis method, resulting in C265T and C328T. e Transcriptional activation activity analysis of SpBBX18 and SlBBX18 in plant cells. Upper: Schematic diagram of effector and reporter genes. SlBBX8 and SpBBX18 were each fused in-frame with the yeast Gal4 DNA-binding domain. Below: The LUC:REN ratios of tobacco (Nicotiana benthamiana) leaves transformed with the corresponding effector constructs. A One-way ANOVA test was used to test the significance of the difference, bars represent means ± SD (n ≥ 3 biological replicates). Source data are provided as a Source Data file.
Among the 29 BBX genes in tomato, BBX18 belongs to the group I subfamily and contains two concatenated B-box domains35. These domains are characterized by the presence of a zinc binding motif, consisting of conserved Cys and His residues35. To provide supporting evidence for the transcription factor function of SlBBX18 and SpBBX18, we investigated the subcellular localization of a fusion protein consisting of SlBBX18 and SpBBX18 fused to a green fluorescent protein (SlBBX18-GFP and SpBBX18-GFP) in tobacco (Nicotiana benthamiana) leaves. Both SlBBX18-GFP and SpBBX18-GFP fusion proteins were localized predominantly in the nucleus, while BBX18 fluorescent signals were also detected in the cytoplasm (Fig. 1c). This nucleus localization of SlBBX18 and SpBBX18 is consistent with their function as transcription factors. We subsequently conducted a transactivation assay in yeast by individually inserting the sequences encoding the open reading frame (ORF) of SlBBX18 and SpBBX18 into the expression vector pGBKT7. Subsequently, each of the distinct GAL4 DNA-binding domain fusion proteins was expressed in yeast strain AH109. When subjected to the selection medium (SD/-Ade/-His/-Trp/), cells transformed with the negative control and pBD-SpBBX18 plasmid failed to grow, while those transformed with the positive plasmid and pBD-SlBBX18 displayed normal growth (Fig. 1b). This suggests that SlBBX18 functions as a transcriptional activator in yeast, whereas SpBBX18 does not exhibit this function. To elucidate the transcriptional activation domain of BBX18 in tomato, we divided the ORF of SlBBX18 and SpBBX18 into their N-terminal (1–267 nt) and C-terminal (268–549 nt) regions for expression in yeast cells. Our results revealed that the N-terminus of SlBBX18 functions as a transcriptional activator, whereas its C terminus lacks this activity (Fig. 1b). Notably, two SNPs were identified between the sequences of SlBBX18 and SpBBX18. To pinpoint the SNP site responsible for transcription activation, we employed PCR-based site-directed mutagenesis. Specifically, we changed SNP 265 (C) and 328 (C) of SpBBX18 to 265 (T) and 328 (T). The resulting constructs pBD-SpBBX18-C265T and pBD-SpBBX18-C328T were expressed in yeast strain AH109 for transactivation assays. As depicted in Fig. 1d, pBD-SpBBX18-C265T exhibited transcription activation, whereas pBD-SpBBX18-C328T did not lead to transcription activation. These results suggest that SlBBX18 and SpBBX18 display distinct characteristics to activate transcription in yeast cells, highlighting their essential role in transcriptional activation. To investigate the involvement of SlBBX18 and SpBBX18 in plant transcriptional regulation, we constructed expression vectors that would produce recombinant fusion proteins of full-length SlBBX18 and SpBBX18 with the Gal4 DNA-binding domain (DBD), as described in the previous study36. Pairs of reporter- and effector-expressing vectors were co-infiltrated into tobacco leaves for transcriptional activation assays. The results showed that co-expression of SlBBX18 increased the reporter gene activity by approximately two-fold as compared to the control of Gal4 DBD alone (Fig. 1e). Interestingly, when SpBBX18 was used to replace SlBBX18, the reporter gene activity was significantly repressed (Fig. 1e). This confirms that the observed transcription activity is specifically conferred by SlBBX18 rather than SpBBX18. Based on these findings, we conclude that SlBBX18 and SpBBX18 exhibit distinct features in terms of transcriptional activation in both yeast and plants. Furthermore, we identified the SNP-265 in BBX18 as a crucial SNP site responsible for the observed transcription activation activity.
Drought tolerance and BBX18 allele was negatively selected during tomato evolution
The C-to-T change of SNP-265 introduces a stop codon (TAG) and causes premature translational termination of the BBX18 gene, which seems to be the critical SNP of BBX18. A total of 321 tomato germplasm samples37 were subjected to SNP analysis of BBX18, including 53 accessions of S. pimpinellifolium (PIM), 108 accessions of S. lycopersicum var. cerasiforme (CER), and 160 accessions of S. lycopersicum (BIG) (Fig. 2a). CER represents a domestication lineage derived from PIM, while BIG represents an improved lineage derived from CER. Approximately 98% of the wild tomato species and 69% of the early domesticated cherry tomatoes exhibited homozygosity for the BBX18CC allele, while only 28% of cultivated tomatoes displayed homozygosity for BBX18CC, with 67% being homozygous for BBX18TT alleles (Fig. 2a). Subsequently, the analysis of BBX18 variation revealed that the BBX18 locus did not undergo selection in candidate tomato domestication sweeps (Fig. 2b).
a Frequency distribution of the three genotypes (265CC, 265TT, and 265TC) of BBX18 in a collection of 321 tomato germplasm accessions, including 53 from Solanum pimpinellifolium (PIM), 108 from S. lycopersicum var. cerasiforme (CER), and 160 from S. lycopersicum big-fruit (BIG) tomato species. b Scan for selection signature surrounding the BBX18 locus on chromosome 2. Top: nucleotide diversity (π) of PIM species (green line), CER species (blue line), and big-fruit cultivars (orange line). Middle: π ratio of the CER species to BIG cultivars. The horizontal dashed line indicates the genome-wide top 5% ratio cutoff for candidate domestication sweeps. Bottom: π ratio of the PIM species to CER species. The horizontal dashed line indicates the genome-wide top 5% ratio cutoff for candidate domestication sweeps. The vertical red arrow indicates the chromosomal location of BBX18. c Phenotypes of drought resistance in 7 accessions of PIM, 15 accessions of CER, and 16 accessions of BIG cultivars. n = 6, 3 replicates. “TS” indicates the tomato accession number in our previous study37. d Survival rate (%) of seedlings of the three tomato groups after drought treatment. PIM (n = 7), CER (n = 14), BIG (n = 17). The minima, maxima, center, bounds of the box, and whiskers and percentile were shown in the box plots. e Survival rate (%) of seedlings carrying the alleles of 265CC and 265TT in BBX18. PIM (n = 7), CER (n = 14), BIG (n = 17). The minima, maxima, center, bounds of the box, and whiskers and percentile were shown in the box plots. Significant statistical difference was calculated according to a two-tailed Student’s t test. Source data are provided as a Source Data file.
To investigate the contribution of BBX18 in cultivated tomatoes, we assessed the impact of allelic variation at BBX18 on drought resistance in 38 tomato accessions among the 321 tomato germplasm samples (Fig. 2c). After drought treatment, we evaluated the survival rate (SR) as an indicator of plant’s resistance to drought stress. The average SR observed in the PIM group was significantly higher than that in the CER and BIG groups, suggesting that the trait for drought resistance was likely favored during tomato domestication (Fig. 2c). Furthermore, tomato accessions with the BBX18CC genotype exhibited significantly greater SR as compared to those with the BBX18TT genotype (Fig. 2d). Hence, our results suggest that BBX18 has been subjected to natural selection during tomato evolution.
BBX18 negatively regulates drought stress tolerance in tomato
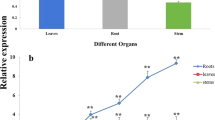
The transcript profiles of BBX18 were investigated in various plant organs, including root, stem, leaf, flower, and fruit in both M82 and S. pennellii genotypes. As illustrated in Supplementary Fig. 3, BBX18 exhibited prominent expression levels primarily in the above-ground tissues of the plant, particularly in the stem, leaf, and flower. The expression patterns of BBX18 were analyzed by qRT-PCR under various abiotic stresses (salt, drought, and methyl viologen (MV)) and abscisic acid (ABA) hormone treatment. The transcript level of BBX18 was found to rapidly decrease at 0.5 h after exposure to drought, salt, MV, and ABA treatments in both M82 and S. pennellii genotypes. However, a continuous induction was observed thereafter. Notably, the maximum increase in transcript abundance occurred at 6 h in M82 but only at 1 h in S. pennellii after drought treatment. Similarly, after treatments with salt, MV, and ABA, peak expression levels of BBX18 were observed earlier in S. pennellii than in M82 (Supplementary Fig. 4). These findings suggest that BBX18 functions as an initial negative regulator during abiotic stress responses in both M82 and S. pennellii genotypes. However, they also highlighted distinct expression patterns between the two genotypes with earlier gene expression peaks observed in S. pennellii.
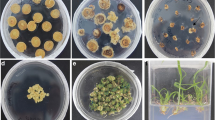
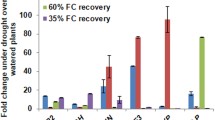
To investigate the role of BBX18 in regulating drought stress tolerance, we conducted overexpression of SpBBX18 (OE), RNA interference (Ri) suppression of SlBBX18, and knockout of SlBBX18 using CRISPR Cas9 (CR) in tomato. Transgenic lines with significantly altered expression levels of BBX18 as compared to the wild-type plants were selected for further analysis (Fig. 3a and Supplementary Fig. 5a). Homozygous CR mutant lines were isolated by PCR-based genotyping. Functional analysis of BBX18 transgenic plants revealed that following a 7-day drought treatment, tomato plants of RNAi and CR knockout lines significantly improved drought resistance, while plants of OE lines were more sensitive to drought stress (Fig. 3b and Supplementary Fig. 5b). Subsequently, after subjecting the plants to 25 days of drought treatment followed by rehydration, the survival rates were much higher in the CR knockout lines at 93% for CR8 and 90% for CR1 and RNAi lines at 62% for Ri8 and 64% for Ri14 than in the control at 39%. In contrast, the survival rates were much lower in the OE lines, with 12% for OE3 and 9% for the OE10 line (Fig. 3b and Supplementary Fig. 5c). To further investigate the role of genes in drought resistance in field conditions, we selected BBX18-knockout (bbx18) lines for yield analysis during two distinct growth cycles under controlled watering conditions. Our findings revealed no significant difference in yield between the bbx18 and WT under normal watering conditions. However, following water control, a substantial reduction in tomato yield was observed, with bbx18 exhibiting significantly higher yields per plant than the WT control (Supplementary Fig. 6). These results indicate that the knockout of BBX18 can enhance tomato’s drought resistance and increase its yield under drought stress.
a Gene structure of BBX18 and genomic DNA sequences of BBX18 knockout lines (CR1 and CR8). Nucleotide sequences of the guide RNAs (gRNAs) are highlighted in red. The knockout of SlBBX18 was carried out using CRISPR-Cas9 on cultivated tomato (S. lycopersicum) Ailsa Craig (WT) plants. Transgenic lines with disruption of reading frames in the target region of SlBBX18 were identified by genomic DNA sequencing of the target region. Protospacer adjacent motifs (PAMs) are framed. The dash line (-) indicates the one-bp deletion and the two-bp deletion in the target region of BBX18 in CR1 and CR8 lines, respectively. b Seedling phenotypes of BBX18 knockout lines (CR1 and CR8) and overexpression lines (OE 3 and OE 10) after drought treatment. WT served as the control. c Activities of ascorbate peroxidase (APX), ascorbate oxidase (AAO), catalase (CAT), and peroxidase (POD), and contents of ascorbic acid (ASA) and proline were measured in tomato seedlings of different transgenic lines grown under normal growth condition (control, gray bars) and after drought treatment (drought, yellow bars). Significant differences were statistically analyzed with two-way ANOVA by GraphPad Prism 9. Bars represent means ± SD (n ≥ 3 biological replicates). P-values were observed compared to the WT. Source data are provided as a Source Data file.
To further investigate the physiological and biochemical changes induced by the different expression levels of BBX18, we quantified the contents of proline (Pro), ASA, and the activities of several antioxidant-related enzymes, including peroxidase (POD), catalase (CAT), ascorbate peroxidase (APX), and ascorbate oxidase (AAO), before and after subjecting the plants to drought stress. Our findings revealed that under well-watered conditions, there were no significant differences in the physiological indices between the wild-type and BBX18 transgenic lines. However, following 17 days of drought treatment, all CR lines exhibited a significant increase in Pro content as compared to the WT lines, while OE lines showed reduced levels of Pro content (Fig. 3c). The contents of ASA displayed similar patterns to those of proline with increased levels in the CR lines but reduced levels in the OE lines (Fig. 3c). After drought treatment, all lines demonstrated significantly enhanced activities of POD, CAT, and APX enzymes; however, the activities of these three enzymes were significantly lower in the OE lines than in the WT control, whereas the activity of AAO showed an opposite trend, having lower enzyme activities in the CR lines and higher activities in the OE lines than the WT control (Fig. 3c). These results collectively suggest that the BBX18 knockout in the bbx18 lines leads to enhancement of the activities of POD, CAT, and APX enzymes in transgenic plants under drought stress, thereby improving their resistance to drought stress. Furthermore, the proline and ASA contents were increased significantly in the CR lines under drought stress. The increases in Pro and ASA contents, along with altered activity levels of antioxidant enzymes, ultimately led to the improvement of the ability of transgenic plants of the CR lines to withstand water scarcity.
BBX18 interacts with ascorbate peroxidase 1 (APX1)
To elucidate the molecular mechanism underlying drought resistance involving BBX18, we employed a comprehensive approach utilizing the full-length SpBBX18 protein to screen for interacting protein partners through immunoprecipitation followed by mass spectrometry (IP-MS). We also used the yeast two-hybrid (Y2H) analysis as a supplementary method to identify interacting proteins and subsequently validated these interactions using the STRING database. The IP-MS analysis revealed 269 potential interacting proteins with BBX18 (Fig. 4). Functional enrichment analysis indicated that these genes were primarily associated with solute transport, redox homeostasis, transcription factors, photosynthesis, as well as transcription and post-transcriptional regulation. These results highlighted the crucial roles of BBX18 as a transcription factor in regulating diverse metabolic pathways in tomato plants, particularly those related to solute transport and osmotic stress response mechanisms. Furthermore, this IP-MS analysis also identified other interacting proteins of BBX18, such as Late Embryogenesis Abundant (LEA), Sugar Transport Protein (STP), and Tonoplast Intrinsic Protein (TIP). To complement the IP-MS analysis, we used BBX18 as bait to screen for interacting proteins of the tomato cDNA library expressed in the Y2H. The results from this screen identified a total of 270 proteins as candidates of interactors of BBX18 (Fig. 4). Analysis of these candidates revealed that they were significantly enriched in the redox homeostasis pathways involving AAO and APX1 enzymes, the transcription regulation pathway involving BBX5 protein, and the post-transcriptional regulation pathway involving ubiquitin E2 (UBE2) enzyme (red lines in Fig. 4). Of particular interest was the interaction between BBX18 and APX1 because this interaction was detected in both the IP-MS and the Y2H analyses (gray and red lines in Fig. 4). We decided to investigate the importance of this interaction in more details.
Interactome of the Mediator complex network was constructed with the Cytoscape software. The gray edges represent interactions with BBX18 detected by IP-MS experiments, the blue dashed lines indicate interactions reported in plants (String database), and the red edges indicate interactions identified through the yeast two-hybrid (Y2H) screening. Source data are provided as a Source Data file.
We validated the direct interaction between BBX18 and APX1 in planta using bimolecular fluorescence complementation (BiFC) analysis. 24 h post Agrobacterium-infiltration, yellow fluorescence from the reconstituted YFP was observed, indicating the physical association between BBX18 and APX1 in planta. In contrast, no yellow fluorescence was detected in the negative controls when the empty vectors or vectors containing one truncated protein were used (Fig. 5a). We confirmed the interaction between SlAPX1 and BBX18 using the yeast point-to-point verification system. Subsequently, due to the amino acid variations between SlBBX18 and SpBBX18, we tested the full-length sequences of SlBBX18 and SpBBX18 as well as their different segments to interact with SlAPX1. These results confirmed that the interaction between SlAPX1 and either SlBBX18 or SpBBX18 occurred specifically through the N-terminal double B-box domain, which was shared by both SlBBX18 and SpBBX18 proteins, and that the C-terminal CCT domain of BBX18 was not required for the interaction (Fig. 5b).
a BiFC assay showing the interaction of SlBBX18 and SpBBX18 with SlAPX1 in N. benthamiana leaves. SlBBX18 and SpBBX18 were expressed as fusion proteins with the N-terminus of YFP (YFPn), while SlAPX1 was fused with the C-terminus of YFP (YFPc). They were co-expressed in tobacco leaf cells, and if there was interaction between BBX18 and APX1, yellow fluorescence would be detected. YFPn and YFPc, and truncating of BBX18 and APX1 were used as negative controls. The yellow fluorescent images (YFP) and bright-field images (BF) were superimposed to create merged images (Merge). Two independent experiments were conducted. b Y2H assays for interactions between BBX18 and its N terminus or C terminus with SlAPX1. Yeast cells harboring the appropriate plasmids were grown on SD/–Leu/–Trp. The same cells were tested for protein-protein interactions on SD/–Leu/–Trp/–His/–Ade solid medium for cell growth, or on SD/–Leu/–Trp/–His/–Ade/+X-Gal for X-Gluc staining. c Co-IP assays showing interactions of SlBBX18, SpBBX18 and SpBBX18-C328T with SlAPX1 in N. benthamiana leaves. Anti-FLAG (@-Flag) and anti-HA (@-HA) antibodies were used for immunoprecipitation in (upper) and (below), respectively. Two independent experiments were conducted. Source data are provided as a Source Data file.
We conducted experiments to examine the subcellular localization of SlAPX1, co-subcellular localization of SpBBX18 and SlAPX1, and BiFC experiments in tomatoes using split YFP tags for SlAPX1 and SpBBX18. The results demonstrated the cytoplasmic localization of APX1 and the co-localization of BBX18 and APX1 in the cytoplasm (Supplementary Fig. 7). To further validate the interaction between APX1 and BBX18 in tomato cells, we expressed SpBBX18 and SlAPX1 proteins that were fused with the N-terminus and C-terminus of YFP, respectively, in tomato protoplasts. Yellow fluorescent signals were reconstituted and observed in the cytoplasm, confirming their interaction in tomato cells (Supplementary Fig. 8).
Concurrently, co-immunoprecipitation (Co-IP) was employed to validate the interaction between BBX18 and its domains with APX1. We constructed vectors for expression of tagged recombinant proteins in plant cells, including SlBBX18-Flag, SpBBX18-T328C-Flag, SpBBX18-Flag, APX1-HA, and GFP-HA (a negative control). Agrobacterium strains carrying the expression constructs were mixed and used for transient expression in tobacco leaf cells. Two days post infiltration with the mixture of Agrobacterium strains, total leaf protein was extracted for the enrichment of tagged recombinant proteins using anti-Flag labeled antibodies that were immobilized to magnetic beads. The experimental groups included co-expression of SlBBX18-Flag, SpBBX18-T328C-Flag, or SpBBX18-Flag with APX1-HA, while the negative control group contained co-expression of SlBBX18-Flag and GFP-HA. Proteins that were co-purified with the anti-Flag antibody were examined using both the Flag antibody and HA antibody on immunoblots (Fig. 5c). The Co-IP results further confirmed the interaction between BBX18 and APX1 (Fig. 5c). Taken together, our results demonstrated that the full-length proteins of SlBBX18 and SpBBX18, as well as their N-termini that contain the double B-box domain, interact with SlAPX1 (Fig. 5b, c).
To validate the effect of their interaction, we expressed SlAPX1 and SpBBX18 recombinant proteins in E. coli cells and purified them separately (Supplementary Fig. 9a). Ascorbate peroxidase activity of purified APX1 proteins was assayed in the absence or presence of BBX18. As illustrated in Supplementary Fig. 9b, the presence of SpBBX18 exerted a significant inhibitory effect on the ascorbate peroxidase activity of recombinant APX1. These findings provide support for the hypothesis that the binding of BBX18 to APX1 leads to a reduction in its APX activity.
BBX18 directly negatively regulates the expression of APX1
As a transcriptional activator, BBX18 potentially binds to the promoter of downstream genes. To identify potential binding sites of promoters by BBX18, we generated transgenic lines overexpressing HA-tagged BBX18, using construct CaMV 35S::SpBBX18-HA. We conducted ChIP assays using HA antibodies to pull down DNA-protein complexes associated with BBX18. The DNA fragments from the ChIP complexes were isolated and used for the construction of libraries, which were sequenced through high-throughput sequencing. Our results showed that the identified putative cis-acting elements (ChIP peaks) were distributed throughout the tomato chromosomes (Supplementary Fig. 10a), with a majority of these elements concentrated in gene promoter regions (Supplementary Fig. 10b). This analysis also presented a pioneering discovery of plant-specific cis-acting elements that were potentially regulated by BBX proteins, such as GGRCCM (C1), CACDTG (C2), GAAARWGA (C3), ATTTAWT (C4) and others (Fig. 6a). All these elements, except C2, were reported24, to be cis-elements recognized by a BBX protein. We conducted a comprehensive screening within the tomato genome and identified a total of 1033 potential target genes regulated by BBX18. The GO enrichment analysis revealed that most of these target genes are involved in DNA binding and transcriptional regulation, while others participate in various regulatory pathways, highlighting the crucial functions of BBX18 in plants (Fig. 6b). In summary, this study unveiled cis-acting elements recognized by BBX18 and identified a list of 1033 potential target genes, whose expression could be regulated by BBX18.
a Putative SpBBX18-binding motifs predicted by DREME. ChIP assays were performed on leaves of transgenic plants expressing HA-tagged SpBBX18. DNA fragments bound by SpBBX18 were isolated using an anti-HA antibody and sequenced. Identification of binding motifs was conducted using Model-based Analysis of ChIP-Seq (MACS). The cis-elements 3 (C3) contains a nucleotide sequence of GAAARWGA, where R indicates A or G (A/G) and W denotes T or A (T/A). Two C3 cis-elements were found in the promoter of the APX1 gene and used for binding with BBX18 transcription factor (see Fig. 7a). b GO enrichment of genes related to the SpBBX18 binding peaks. c Visualization of SpBBX18 binding peaks in the promoters of SlAPX1, SlBBX3 and SlBBX24. Black lines indicate probe locations of the cis-elements 1–4 (C1-4). d ChIP-qPCR validation for the binding sites on promoters of APX1, BBX3, and BBX24. Values are expressed as the means of triplicates ± SD. NC indicates no peak position (The specific sequence is shown in Supplementary Data 2) as the negative control. Source data are provided as a Source Data file.
To further investigate the working mechanism of BBX18, we performed RNA-seq of BBX18-OE, BBX18-RNAi and wild-type plants. Under normal growth conditions, we observed only a limited number of genes exhibiting differential expression patterns between the BBX18-OE or BBX18-RNAi transgenic lines and the wild-type control plants. However, following drought treatment, the abundance of DEGs significantly increased (Supplementary Fig. 11a). Pathway enrichment analysis revealed that most of these DEGs were enriched in the metabolic pathways after drought treatment, including biosynthesis of secondary metabolites, flavonoid biosynthesis, ascorbate, and aldarate metabolism, and peroxisome pathways (Supplementary Fig. 11b). Furthermore, by integrating the ChIP-Seq data with the RNA-seq analysis, we identified APX1 and BBX genes (such as BBX3 and BBX24) as potential targets of BBX18 (Fig. 6c). The ChIP- qPCR analysis confirmed the presence of BBX18-binding peaks in APX1, BBX3, and BBX24 (Fig. 6d). Consistent with this finding is the observation that the expression levels of APX1and BBX24 were negatively regulated by BBX18, and the expression of BBX3 was positively regulated by BBX18 (Supplementary Fig. 12a, b). In consistence with the observed expression patterns under drought conditions, the expression levels of APX1 and the two BBX genes were significantly upregulated in the BBX18- CR and RNAi lines, suggesting that they may serve as the targets of BBX18. To investigate if the regulation of APX1 and the two BBX genes by BBX18 was direct or indirect, we performed EMSA assays, and our data showed that BBX18 could bind directly to the cis-elements (C1–C4) (Supplementary Fig. 12c). These findings indicate a possible role of BBX18 in regulating the expression of the BBX gene family, especially the expression of APX1, whose protein product was shown to interact with BBX18.
We have successfully identified two C3 motifs in the promoter sequence of SlAPX1. To confirm whether BBX18 could directly bind to the SlAPX1 promoter, we conducted the yeast one-hybrid (Y1H) assay. Our results showed that SlBBX18-C, SpBBX18, and SpBBX18-C could bind to the -1222 bp promoter of SlAPX1 (Fig. 7a–d). To further investigate whether the two mutations of BBX18 and segments of BBX18 could affect the activation of SlAPX1, we co-expressed SlBBX18, SpBBX18, and their N- or C-terminus (as effector), respectively, with the different lengths of the promoter (SlAPX1-P0, P1 and P2) that were used to drive the expression of the LUC reporters in dual-luciferase reporter assays (Fig. 7a, b). As shown in Fig. 7c, the expression of the LUC reporter by SlAPX1-P1 and P2 was not significantly different from the control, whereas the LUC activity under the SlAPX1-P0 promoter was significantly lower than that in the control, suggesting that the full-length promoter of APX1 (SlAPX1-P0) that contained two C3 cis-elements was repressive to the expression of the reporter gene. When SpBBX18, SlBBX18-C, or SpBBX18-C were co-infiltrated with SlAPX1-P0, respectively, the LUC activities of SlAPX1 pro:LUC were significantly decreased. Importantly, the SlAPX1-P0 LUC activities were not significantly different using SlBBX18 and SlBBX18-N as effectors (Fig. 7c). These results indicated that the promoter of SlAPX1 is a target of SlBBX18 and the C3 motifs in the SlAPX1 promoter may serve as specific cis-elements for BBX18-C. To further validate these findings, we conducted EMSA to confirm the specificity of BBX18 with the C3 motif. We observed that SpBBX18 could bind directly to the C3 cis-elements of APX1, causing the DNA fragment to shift in mobility on EMSA (Fig. 7d). However, SlBBX18 could not bind to the C3 cis-elements of APX1, because SlBBX18 contained only the N-terminal region but lacked the C-terminal domain due to the premature translation termination. Interestingly, when a T265C mutation was introduced in SlBBX18, which would remove the premature stop codon and allow for the translation of a full-length protein, the mutated SlBBX18 (T265C) regained the ability to bind to the C3 cis-elements of APX1 (Fig. 7d). This additional evidence provides strong supporting evidence that the C-terminus of BBX18 is the protein domain that binds to the C3 cis-acting element of the APX1 promoter.
a Schematic diagrams of reporters and effectors in the dual luciferase system for assays of the transactivation activities of BBX18 on the APX1 promoter. SlBBX18, SpBBX18, and their N- or C-terminal segments were expressed as the effectors, while firefly luciferase (LUC) was expressed as the reporter under the control of the full-length APX1 promoter (P0) or fragments (P1 and P2) of its promoter. Renillia luciferase activity (REN) served as the internal control in the dual luciferase system. b Schematic diagrams of P0 or P1 and P2 of the SlAPX1 gene. Two C3 cis-elements (GAAARWGA) were present in the full-length promoter but were absent in P1 and P2. c Relative LUC/REN ratios. The LUC: REN ratio of Nicotiana benthamiana leaves transformed with the empty effector construct 62SK (pGreen II 62-SK), and reporter construct 0800 (pGreen II 0800-SK). The two-tailed Student’s t-test was used to test the significance of the difference. Data are shown as means ± SD (n = 4 biological replicates). d Assays of transactivation activity of BBX18 and its N- and C-terminus in yeast cells. The antibiotic aureobasidin A (AbA) resistance marker was expressed as the reporter under the control of the full-length APX1 promoter (P0). The binding of effectors to the promoter of APX1 would allow the expression of resistance to antibiotic AbA. Yeast cells harboring the plasmids were grown on SD/-Leu media and tested for antibiotic AbA resistance on SD/-Leu/+AbA media. e EMSAs for verification of the binding between BBX18 and the C3 cis-elements (GAAARWGA) of the SlAPX1 promoter. In SlBBX18-T265C, the nucleotide residue T at position 265 was changed back to C, as in SpBBX18. Recombinant proteins of SlBBX18, SlBBX18-T265C, and SpBBX18 were purified and used to incubate with a double-stranded oligonucleotide probe that contained the C3 cis-elements prelabeled with 6-carboxyfluorescein (FAM) for detection. The ‘+’ and ‘-’ signs indicate the presence and absence, respectively. This representative image is from one of two independent experiments that yielded consistent results. Source data are provided as a Source Data file.
SlAPX1 positively regulates drought tolerance and is modulated by BBX18 in tomato
Our findings suggested that BBX18 not only interacted with APX1 at the protein level but also bound to the cis-acting elements in the promoter of the APX1 gene to regulate its expression. Therefore, we focused on elucidating the regulatory relationship between these two genes. SlAPX1 is a member of the APX gene family and encodes a cytoplasmic APX that contains a highly conserved ascorbate peroxidase domain consisting of 250 amino acids. SlAPX1 shows high amino acid sequence homology with orthologs from other species based on phylogenetic analysis. We cloned the full-length SlAPX1 cDNA for the construction of overexpression (OE) vectors and CRISPR-Cas9 knockout vectors. Using these vectors, we successfully generated stable transgenic lines of tomato via Agrobacterium-mediated genetic transformation. Phenotypic characterization and physiological and biochemical analyses revealed that overexpression of SlAPX1 significantly enhanced drought tolerance in tomatoes (Supplementary Fig. 13a). After drought treatment, plants of the SlAPX1-OE lines exhibited reduced levels of H2O2 and O2− enrichment as compared to the wild type plants, as evidenced by the decreased conductivity, malondialdehyde (MDA) content, and AAO activity in the OE lines. Conversely, the chlorophyll content, photosynthetic rate, AsA content, and activities of superoxide dismutase (SOD), ascorbate peroxidase (APX), and peroxidase (POD) were all higher than those observed in the wild-type control group (Supplementary Fig. 13b). These findings suggest that overexpression of the SlAPX1 gene leads to enhancement of plant metabolic responses through modulation of enzyme activities, which ultimately results in the improvement of drought tolerance in tomato plants.
To further investigate the genetic interaction and regulatory relationship between BBX18 and APX1 in plant drought resistance, we carried out a genetic crossing experiment using the bbx18 plant, the APX1 overexpression (APX1) plant, and APX1 knockout (apx1) (Supplementary Fig. 14a). We performed genotyping of the F2 plants using PCR and sequencing verification and successfully identified plants carrying both the homozygous bbx18, APX1, and apx1 loci. Plants of the selected lines were subjected to simulated drought conditions through mannitol treatment on the growth medium. The results showed that there was no significant difference in the degree of drought resistance between the bbx18/APX1-OE plants and the bbx18 plants. However, the drought resistance in both lines was significantly higher than that of the APX1-OE plants, which in turn exhibited significantly greater drought resistance (with increased values in plant height, root length, and plant weight) than the wild-type control plants (Fig. 8a, b). Similar results were obtained when the plants of different lines were subjected to the drought treatment from withholding water to the soil (Fig. 8c). The bbx18/APX1 and bbx18 plants exhibited higher survival rates than APX1-OE plants and the WT plants (Fig. 8d). The expression of the APX1 gene and the activity of the APX enzyme in both bbx18/APX1 and APX1-OE plants were elevated as compared to those in the bbx18 and wild-type control. However, the expression of APX1 in bbx18 and wild-type control showed no significant difference, indicating that SlBBX18 knockout did not affect the expression of APX1. Nonetheless, the APX enzyme activity in bbx18/APX1 and bbx18 plants was significantly higher than in APX1-OE plants (Fig. 8e). Moreover, the drought resistance of bbx18/apx1 double mutant has been restored compared to apx1 (Supplementary Fig. 14b). This evidence supports for a regulatory role of BBX18 on APX1. We then measured the activity of ROS in bbx18, apx1, and bbx18/apx1 plants. Our findings indicate that there were no significant differences in ROS activity under normal growth conditions. However, following drought treatment, both bbx18 and bbx18/apx1 exhibited reduced accumulation of ROS (H2O2 and O2−) compared to the wild-type control (WT) and apx1, while apx1 showed a significantly higher accumulation of ROS than WT. Similar results were obtained by different methods using 3,3’-diaminobenzidine (DAB) and nitro blue tetrazolium (NBT) staining (Supplementary Fig. 14c, d). This suggests that SlBBX18 modulates the activity of the APX enzyme but does not influence the expression of the APX1 gene.
a Morphological characteristics of the wild type (WT) plants and transgenic lines of BBX18-knockout (bbx18#1 and bbx18#8), SlAPX1 overexpression (APX1#3 and APX1#12), and two double mutant lines (bbx18#1/APX1#3 and bbx18#8/APX1#12) grown on regular MS media (MS) or MS media containing 200 mM mannitol for simulated drought treatment. b The plant height, root length, and plant weight of seedlings grown on regular MS media (MS) or MS media containing 200 mM mannitol. Significant differences were statistically analyzed with two-way ANOVA by GraphPad Prism 9. Bars represent means ± SD (n ≥ 3 biological replicates). P-values were observed compared to the wild type. c Morphological characteristics of the wild type (WT) plants and transgenic lines of BBX18-knockout (bbx18#1 and bbx18#8), SlAPX1 overexpression (APX1#3 and APX1#12), and double mutants (bbx18#1/APX1#3 and bbx18#8/APX1#12) grown on soil and subjected to drought treatment (withholding water) at different time points (0–10 days) and after rehydration for 3 days (R3d) following the 10 days drought treatment. d The survival rate analysis was conducted after drought treatment and rehydration. A total of 16 seedlings per replicate, with 4 replicates, ultimately recovered after rehydration, indicating their ability to survive. Bars represent means ± SD (n ≥ 3 biological replicates). e The expression of the APX1 gene and the activity of the APX enzyme were analyzed in normal growth (Control) and drought conditions for 10 days. Significant differences were tested by the two-way ANOVA. Bars represent means ± SD (n ≥ 3 biological replicates). P values were observed compared to the wild type. Source data are provided as a Source Data file.
Discussion
Drought and salinity are major abiotic stresses that greatly limit crop yield and crop distribution in modern agriculture, encompassing the combined effects of various other abiotic stresses on agricultural productivity. Tomatoes trace their origins to the arid regions of the Andes in South America. Despite exhibiting robust resilience to drought in their wild species, extensive human cultivation and natural diversification have rendered most cultivated tomato varieties highly susceptible to drought stress induced by water scarcity. In this study, we investigated the nucleotide diversity of 321 natural accessions of tomato and identified the C-to-T substitution at nucleotide position 265 of SlBBX18 (265C-to-T allele) as a naturally existing allele that has apparently arisen under natural selection during tomato evolution. This variant potentially represents one of the key drought resistance genes discovered using our previous introgressive line-based identification method34. The differential expression of BBX18 between M82 and S. pennellii genotypes was evident during the early stages of various treatments in S. pennellii (0.5 h), with relatively low expression levels observed as compared to that in M82 (Supplementary Fig. 4). Stress-inducible genes play crucial roles in both the initial stress responses and the establishment of plant stress tolerance38. The diverse expression patterns observed may thus potentially enhance the drought resistance in tomatoes. Furthermore, we conducted an analysis of drought tolerance in 38 different tomato varieties at the seedling stage and observed a reduction in their ability to withstand drought (Fig. 2c, d). These findings contradict earlier observations suggesting that domestication actually enhances cold tolerance in tomatoes39. Importantly, our results indicate that there exists a negative selection acting upon the 265 C-to-T allele of BBX18 throughout tomato evolution (Fig. 2c, e).
All BBX proteins from plants possess a conserved B-box domain, a type of zinc finger domain. Some BBX proteins also feature a CCT domain. The presence of BBX genes has been observed in all eukaryotic genomes examined thus far3. Previous studies have demonstrated that the fragment located between the B-box and CCT domains in CmBBX249, CmBBX2217, and BBX16/COL7 exhibits transcriptional activation activity in yeast cells. The CmBBX24 protein harbors two B-box domains at the N-terminus and lacks a CCT domain, while its activation domain is located in the C-terminal region9. Similarly, the CmBBX22 protein functions as a transcription activator, with its C-terminal region playing a crucial role in mediating transcriptional activation17. Moreover, the segments of CmBBX8 located between the conserved B-box and the CCT domain are responsible for its transcriptional activation activity, while the CCT domain alone does not possess any transcriptional activation activity40. Our observation of tomato BBX18 supports the hypothesis that the transactivation domain (TAD) of BBX proteins is present between the B-Box domain and the CCT domain (Fig. 1). In particular, our result is consistent with a previous study which identifies a predicted 9 amino acid TAD of BBX21 that enhances transcription in yeast41. In this study, while SpBBX18 did not exhibit transcriptional activation activity in yeast, a single nucleotide mutation in SlBBX18 resulted in its transcriptional activation activity. Moreover, when transiently expressed in tobacco, SlBBX18 significantly upregulated the expression of the reporter gene, whereas SpBBX18 notably downregulated its expression (Fig. 1e). These findings suggest that the N-terminal and C-terminal domains of BBX18 protein are reciprocally regulated. The C-terminal domain of BBX proteins has been reported to determine both the positive and negative regulations in Arabidopsis. Specifically, the C-terminal domains of BBX24 and BBX21 play opposing roles in photomorphogenesis, highlighting their crucial involvement in the transcriptional regulation of anthocyanin biosynthesis genes42. Taken together, these findings demonstrate that the activation domain of BBX proteins is located between the N-terminal and C-terminal regions, while the B-box and CTT domains are capable of regulating the expression of other genes.
The distribution of the BBX protein family is widespread across various plant species, and members of this family play various and crucial roles in regulating plant growth and development. These encompass pivotal processes such as seedling photomorphogenesis, anthocyanin biosynthesis, photoperiod-based control of flowering, and modulation of hormonal pathways3. Recent research findings have also indicated that the impact of BBX proteins on plant responses to abiotic stress can exhibit either positive or negative effects. In Arabidopsis, AtBBX18 has been identified as a negative regulator of thermotolerance by modulating the expression levels of heat-stress-responsive genes, including DGD1, Hsp70, Hsp101, and APX243. The growth promotion of roots under salt stress is facilitated by the positive regulation of AtBBX24/STO, another B-box protein in Arabidopsis15. The positive regulation of flowering time and cold resistance, as well as the negative regulation of drought resistance in chrysanthemums, are attributed to CmBBX24 and CmBBX22, respectively9,17. In apples, MdBBX10 and MdBBX7 have been found to positively regulate drought resistance. In tomatoes, SlHY5 directly binds to the promoter region of SlBBX31, thereby inducing transcriptional activation of SlBBX31 and conferring enhanced resistance to cold temperatures39. In this study, we found that BBX18 negatively regulated drought resistance in tomatoes. Because the similarity between tomato BBX18 and the reported BBX genes (e.g., MdBBX7, CmBBX22, and CmBBX24) associated with drought resistance is relatively low (Supplementary Fig. 15), the discovery of the role of BBX18 in drought resistance in tomato is potential and has implications for future improvement of drought resistance in breeding programs. We have uncovered a regulatory mechanism in plants, wherein the combined action of BBX18 interacting with APX1 (Fig. 5) and binding to the C3 cis-acting element of the APX1 promoter (Fig. 7) leads to the repression of the APX1 gene expression (Supplementary Fig. 12a), thereby enhancing the sensitivity to drought stress (Fig. 9). This discovery bears resemblance to the regulatory mechanism observed in the BBX21 and HY5 genes in Arabidopsis. AtBBX21 has been shown to directly bind to the promoter regions of BBX22 and interact with HY5, which then activates transcription of BBX2220,21,22. Notably, it has been observed that the binding to the T/G-box by BBX21 in the HY5 promoter occurs independently of HY521. Furthermore, both BBX21 and HY5 exhibit the ability to bind to the BBX11 promoter to promote its transcription, and in turn, BBX11 binds to the HY5 promoter to activate its transcription23. A similar regulatory pattern of regulation has also been shown in tomatoes, where SlBBX20 and SlBBX21, respectively, interact with SlHY5 and bind to the promoter region of SlHY5 to activate its transcription25.
Wild tomato (Solanum pennellii) plants are more tolerant to drought than cultivated tomatoes (S. lycopersicum). The BBX18 gene from the wild tomato (SpBBX18) encodes a full-length transcription factor, whereas its ortholog from the cultivated tomato (SlBBX18) was found to contain a nucleotide substitution (C265T) at position 265 that introduces a premature stop codon (TAG) and would lead to the synthesis of an N-terminal truncated protein of BBX18. The C-terminus of SpBBX18 possesses transcriptional repressor activity that inhibits the expression of the APX1 gene. In wild tomatoes, SpBBX18 binds to the two tandem C3 cis-elements (GAAARWGA) in the promoter of APX1 and inhibits its gene expression. In cultivated tomato, SlBBX18 is a truncated protein that has the N-terminal half with the double B-box domain, but lacks the C-terminus and does not possess transcriptional repressor activity, allowing the normal expression of APX1. The interaction between the N-terminus of SlBBX18 and APX1 reduces its ascorbate peroxidase activity, thereby rendering sensitivity to drought stress in cultivated tomatoes.
Numerous studies have also demonstrated the involvement of the N-terminus of the BBX proteins in protein-protein interactions, while their C-terminus functions as a binding site for the cis-regulatory element for gene expression regulation3,44, which aligns with our findings in this work. The N-terminal B-box domain of BBX18 was found to be responsible for interacting with the APX1 protein, whereas the C-terminal CCT domain of BBX18 regulates gene expression by binding to the C3 cis-acting element of APX1. Yeast two-hybrid assays have been used to demonstrate the negative impact of CmBBX19 on drought tolerance in chrysanthemum, as only the B-box domain of CmBBX19 has been shown to interact with CmABF344. The N-terminal region of BBX proteins has been found to be sufficient for protein-protein interactions between BBX28 and CO or between BBX29 and CO45. The two B-box motifs in MdBBX7 have also been shown to be essential for the interaction between MdBBX7 and MdMIEL119. Hence, B-box motifs often facilitate protein-protein interactions, and it is plausible that an unidentified motif exists within the C-terminal region. CmBBX22 functions as a transcriptional activator, with its transcriptional activation being mediated through its C-terminal region17. In Arabidopsis, the divergence of the C-terminal domains has been shown to result in functional diversity among the B-box proteins in structural group IV42. Consequently, further investigation into the motifs and residues present in the C-terminal regions of BBX proteins is warranted in future research endeavors.
Drought tolerance in plants is a crucial quantitative trait that is governed by numerous genes in crops. In the last decades, significant advances have been made in elucidating the genetic and molecular mechanisms underlying drought tolerance in plants, particularly model species. Given its status as an important model crop, tomato is an ideal plant species for future investigations into the roles and molecular mechanisms of BBX genes in drought resistance. B-box proteins are a highly diverse group of zinc finger transcription factors and regulators that form extensive families across various plant species. As an exemplary transcription factor, BBX18 exhibits typical characteristics such as nuclear localization, along with distinct domains for both transcriptional activation and DNA binding functions. However, there is an urgent need for a more comprehensive characterization of the cis-acting elements involved in the regulation of BBX genes. For instance, SlBBX20 has been demonstrated to exhibit the ability to modulate PSY1 expression through specific interaction with a G-box motif present within its promoter region24. Similarly contributing to the regulation of plant development processes like photomorphogenesis is AtBBX21, which acts as a positive regulator by directly engaging with another G-box motif found within the promoter region of HY5, thereby promoting its expression46. In addition, MdBBX7 exhibits the binding affinity towards the promoter elements of MdGLK1, MdERF1, and MdERD15 containing CCTTG, CACGTG (G-box), or CACGTT sequences19. In this study, we conducted a systematic analysis of the cis-acting elements that could be bound by BBX18 (Fig. 6). Also, the interaction between the C terminus of BBX18 and the C3 cis-element resulted in the suppression of APX1 gene expression and enhancement of drought sensitivity in plants (Fig. 7). In addition to APX1, many other genes were also regulated by BBX18. They include different types of transcription factors such as BBX3 and BBX24, bZIP, ERF, MYB, and WRKY, as well as ubiquitin-related genes such as ubiquitin carboxyl-terminal hydrolase, ubiquitin-conjugating enzyme E2, and ubiquitin thioesterase (Fig. 7 and Supplementary Data 1). These genes are involved in the regulation of gene expression in various processes and the ubiquitination pathways in plants. The process of ubiquitination, which requires the function of the E1 enzymes (ubiquitin-activating enzymes), E2 enzymes (ubiquitin-conjugating enzymes), and E3 ligases (ubiquitin ligases), serves as a representative mechanism for regulating protein degradation. MdMIEL1 E3 ligase has the ability to facilitate the ubiquitination and subsequent degradation of MdBBX7 via the 26S proteasome pathway19. Similarly, SlBBX20 undergoes protein degradation through a pathway that is dependent on the 26S proteasome24. Thus, BBX genes play crucial roles in the regulation of ubiquitination-dependent protein degradation. It is possible that BBX18 may play a role in the ubiquitin-dependent pathway. Our IP-MS results demonstrate that BBX18 interacted with ubiquitination-related proteins, further highlighting its significance in regulating the plant ubiquitination pathway (Fig. 4). However, further research is required to fully comprehend the specific metabolic pathways regulated by BBX18-related ubiquitination. In addition, our analysis of protein interactions by IP-MS and Y2H revealed that BBX18 interacts with APX1 and other proteins associated with the resistance to oxidative stress, such as P450, AAO, sulfoxide reductase, and peptide methionine. In addition, tomato BBX18 was also shown to interact with other proteins implicated primarily in osmotic stress (Fig. 4). Therefore, this work has shed insight into the molecular mechanism underlying drought resistance mediated by BBX18 and provides targets for future genetic engineering projects aimed at improvement of drought tolerance in plants.
Recognition of the genetic and molecular mechanisms underlying plants’ resistance to environmental stimuli empowers researchers to devise innovative strategies for enhancing their stress tolerance. While abiotic stress tolerance is controlled by multiple genes, individual genes encoding crucial transcriptional regulators possess the potential to augment plant adaptation through the modulation of gene networks. Numerous studies have demonstrated the feasibility of manipulating the BBX genes to confer enhanced tolerance against diverse stresses. Manipulation of BBX gene expression has been shown to bolster stress tolerance in various plant species, including Arabidopsis, chrysanthemums, apples, and rice3. The CRISPR/Cas9 system is commonly utilized in plants to induce targeted mutations for investigating gene function and offering novel possibilities for crop enhancement47,48. One potential application involves the suppression of BBX18, a negative regulator of drought resistance, the knockout of which was demonstrated to enhance drought tolerance in tomatoes. To validate our hypothesis, we conducted a comprehensive analysis of the impacts of BBX18 knockout on plant growth in a field greenhouse over a two-year period. As illustrated in Supplementary Fig. 6a, a significant reduction in watering input by 1/3 led to the majority of the initial set of fruit from wild-type tomatoes falling, along with a decrease in plant biomass, resulting in a notable decline in tomato yield. However, under normal growth conditions, there was no discernible difference between tomato plants with BBX18 knockout and the wild-type controls. Upon drought treatment, the yield plummeted by nearly 45%, yet the yield of BBX18 knocked out plants was notably higher than that of the wild type (Supplementary Fig. 6b). This demonstrates the paramount significance of water in promoting plant growth and production, and the BBX18 gene presents an opportunity to utilize CRISPR/ Cas9 knockout techniques to generate transgene-free, mutant tomato offspring with improved drought resistance.
Methods
Plant materials, growth condition, and drought treatment
Tomato cultivars S. lycopersicum cv. M82 and S. pennellii (LA0716), as well as tomato introgression lines (ILs), were obtained from the Tomato Genetics Resource Center (TGRC). Tomato species used for drought tolerance experiments and nucleotide diversity (π) analysis were collected from 53 Solanum pimpinellifolium (PIM) accessions, 108 S. lycopersicum var. cerasiforme (CER) accessions, 160 S. lycopersicum big-fruit (BIG) accessions and 17 modern commercial hybrids (F1)37. To explore the selection region around BBX18, SNPs near the BBX18 locus (Chr2:41.0–43.5 Mb, SL2.40) in the tomato genome were identified. The level of π was obtained using a 50-kb window with a step size of 5 kb in PIM, CER, and BIG tomato species. The ratios of nucleotide diversity between PIM and CER (π_PIM/π_CER), and between CER and BIG (π_CER/π_BIG) were also calculated. The top 5% of ratios were used as the cutoff for sweeps49. The simulated drought conditions through mannitol treatment. Tomato seeds were germinated in MS medium, and the uniformly sprouted seedlings were sub-cultured in MS media containing 200 mM mannitol for the treatments, no-stress treatment was used as the control50. For drought treatment, tomato seeds were sown into the soil. After the seedlings reached the stage of three true leaves, they were transplanted into individual pots for further drought treatment. The entire process occurred in a controlled greenhouse with a 16-h light/8-h dark cycle and a constant temperature of around 25 °C (with a range of 5 °C, especially at night)51. The field drought treatment was conducted in the greenhouse of Southwest University, Chongqing, China, during the spring of 2022 and 2023, respectively. Forty-five days after sowing, wild-type and gene-knockout plants were transplanted into pots in the greenhouse. The plants were then assigned to either a normal watering group or a drought treatment group, with the latter receiving 1/3 of the water quantity administered to the former via drip irrigation. Each group consisted of 3 replicates, each containing 20 plants. The yield of the plants was analyzed 85 days post-transplanting, with a yield analysis conducted on uniformed 12 plants selected from each of the 20 plants in the respective groups. AsA content and APX and AAO enzyme activities were determined following the established methods52,53. The trichloroacetic acid (TCA) was used for the extraction of ASA, and the AsA standard (Sigma-Aldrich, USA, A7631) was prepared, and its absorbance at 550 nm was measured using an Infinite M200 (Tecan, Switzerland, Infinite M200 Pro). APX and AAO activities were determined by the analysis of the AsA oxidation rate, which was recorded at 290 and 265 nm, respectively. CAT and POD activities were determined by the analysis of the H2O2 oxidation rate, which was recorded at 240 and 470 nm, respectively. The proline content was determined by quantifying the absorbance at a wavelength of 520 nm using a sulfosalicylic acid solution for extraction50.
Transcriptional activation, PCR-based site-directed mutagenesis, and subcellular localization
For transactivation experiments in yeast, SlBBX18 and SpBBX18 with their N-terminus and C-terminus were introduced to pGBKT7 through PCR amplification, respectively, resulting in the fusion vectors pBD-SlBBX18, pBD-BBX18N, pBD-SlBBX18C, pBD-SpBBX18, and pBD-SpBBX18C. PCR-based, site-directed mutagenesis was performed using pBD-SpBBX18 as a template, with one base-pair substitution introduced at positions 265 (T to C) and 328 (T to C) of SpBBX18, respectively, using primer design facilitated by Primer Spanner (http://ps1.biocloud.org.cn/index.php). Following the PCR reaction, the resulting product was digested with Dpn I and incubated at 37 °C for 8 h. Successful transformation into Escherichia coli was confirmed through PCR detection and subsequent DNA sequencing verification. The extracted plasmid was then transformed into yeast AH109 for transcriptional activation identification. Empty vector pGBKT7 was used as a negative control, while the pGAL4 vector served as a positive control. These fusion vectors were transformed into yeast strain AH109. All yeast vectors and reagents for yeast experiments were obtained from Clonetech, CA, USA. Transcriptional activation activity was assessed by observing the growth of yeast cells harboring these constructs on SD/-Trp medium as well as SD/-Ade/-His/-Trp + X-α-Gal medium.
For transactivation experiments in plants, SlBBX18 and SpBBX18 were cloned into the effector vector following PCR amplification and the construction of specific reporter and effector vectors36. Co-transformation of effector and reporter vectors into tobacco (N. benthamiana) leaf epidermis cells was achieved through Agrobacterium-mediated infiltration. Analysis of firefly luciferase (LUC) and Renilla luciferase (REN) activities was conducted using the Dual-Luciferase Reporter Assay System Kit (Promega, USA).
By utilizing gene-specific primers that contained homologous recombination sites, the complete coding sequences of SlBBX18 and SpBBX18 were amplified by PCR and inserted into vectors containing the coding sequence for green fluorescent protein (GFP), generating fusion vectors of SlBBX18 -GFP and SpBBX18 -GFP. The plasmids were then transformed into Agrobacterium tumefaciens strain GV3101. ERF-RFP was used as a marker for the nucleus. We transiently infected four-week-old tobacco leaves with a mixture of Agrobacterium strains carrying ERF-RFP, followed by incubation at 25 °C for 2 days. Using a Leica SP8 confocal microscope, we observed the fluorescence signals of GFP and RFP in tobacco leaves excited at wavelengths of 515 nm and 561 nm, respectively.
Plasmid construction and plant transformation
The full-length coding sequences of SlBBX18 (Solyc02g084420) and SpBBX18 (Sopen02g029130) were amplified by PCR from M82 and S. pennellii, respectively, as well as SlAPX1 (Solyc06g005160) from M82. The target sequences were then introduced into pMV (a modified pBI121 vector) for over-expression, incorporating a 3 x HA tag under the CaMV35S promoter. All primer sequences can be found in Supplementary Data 2, similarly hereinafter. The pHellsgate 2 vector (Invitrogen, CA, USA) for BBX18-RNAi construction via combination reaction50. Two guide RNAs (gRNAs, target1, and target2) corresponding to the coding sequences for the N- and C-termini of BBX18 and SlAPX1 were designed using the CRISPR-p v2.0 tool (http://cbi.hzau.edu.cn/CRISPR2/) and cloned into CRISPR-Cas9 vector pTX by homologous recombination54. The homologous recombination reaction systems and procedures used in this study followed the instructions provided in the Hieff Clone® Plus One Step Cloning Kit (Yisheng, Shanghai, China). All plasmids were introduced into Agrobacterium tumefaciens LB4404 and used for stable transformation into S. lycopersicum cv. Ailsa Craig through Agrobacterium-mediated transformation. Transgenic lines were confirmed by PCR and sequencing analysis of genomic DNA. All experiments were conducted using homozygous lines from the T2 or T3 generation. Transgenic lines of BBX18 CR1, BBX18 CR8, APX1 OE3, and APX1 OE12 were used for genetic crossings to create double mutant/OE lines. The presence of transgenes in the double mutant lines was confirmed by PCR and sequencing of genomic DNA.
RNA-seq and real-time PCR
Leaves from the WT, BBX18 OE, and Ri lines were subjected to 17-day drought conditions and then collected for RNA extraction. Plants with regular watering served as a control. Total RNA was used for reverse transcription and cDNA library construction. Different libraries were constructed for RNA samples from the WT, OE, and Ri lines that were subjected to drought treatment (DT) or regular watering control conditions (CK), thus generating the WTCK, OECK, RiCK, WTDT, OEDT, and RiDT cDNA libraries. The libraries were sequenced using the Illumina BGI platform (BGI, China), and the filtered reads were aligned to the tomato genome (ITAG2.4).
Treatments of leaves with hormones and oxidative reagents were established55. Briefly, leaves of WT seedlings at the five-leaf stage were sprayed with abscisic acid (ABA, 100 μM), and methyl viologen (MV, 100 μM). In addition, the soil was irrigated with polyethylene glycol (PEG, 100 mM) and NaCl (100 mM) for the drought and salt treatment, and the control was sprayed with distilled water.
For qRT-PCR reactions, RNA extraction using TRIzol solution (Sangon, Shanghai, China), and cDNA synthesis was adopted a PrimeScript RT reagent Kit (TaKaRa, Dalian, China) according to the manufacturer’s instructions. The SYBR Green Real-time PCR system (Bao Guang, China) was used for qRT-PCR assays, and the PCR products were monitored using the CFX96 PCR system.
Y2H and BiFC assays
The full-length ORF sequence of SpBBX18 was amplified by PCR and then inserted into the pGBKT7 vector containing the GAL4 DNA-binding domain to construct pGBKT7-SpBBX18 as bait. Mating of the yeast bait strain with the yeast strain carrying the tomato cDNA expressing library was employed to screen for interaction proteins50. Positive clones obtained were further screened using X-α-GAL, and the nucleotide sequences of the interacting clones were amplified using SMARTIII and CDS III primers. Positive clones were carefully selected and subjected to sequencing in order to identify potential protein interactors with BBX18. To further investigate their interrelationship, full-length cDNA sequences encoding the interaction proteins were cloned and ligated into pGADKT7 vector, which was subsequently co-transformed with pGBKT7-BBX18 into yeast cells for point-to-point validation. The plasmid combination of pGBKT7-53 + pGADT7-recT served as a positive control, while pGBKT7-SlAPX1 + pGADT7 served as a negative control. The combinations of pGBKT7-SlAPX1 + pGADT7-SlBBX18, pGBKT7-SlAPX1 + pGADT7-SpBBX18, pGBKT7-SlAPX1 + pGADT7-SlBBX18-N, pGBKT7-SlAPX1 + pGADT7-SlBBX18-C, and pGBKT7-SlAPX1 + pGADT7-SpBBX18-C were used for co-transformation experiments. The recombinant plasmids were co-transfected into yeast strain AH109 and incubated at 30 oC for 2-3 days on SD/-Leu/-Trp medium. Yeast cell suspensions were adjusted to the concentration of OD600 = 0.2 and subsequently diluted using 0.9% NaCl to different concentrations of 1 ×, 10 ×, and 100 ×. The diluted yeast cell suspensions were then cultured on SD/-Leu/-Trp medium as well as SD/-Ade/-His/-Leu/-Trp + X-α-Gal medium at 30 oC for 2–3 days.
For Bimolecular fluorescence complementation (BiFC) analysis, the full and truncated sequences of SlBBX18, SpBBX18, and APX1were obtained via PCR amplification and cloned into pUC-SPYNE expressing the N-terminal 155 amino acids of yellow fluorescent protein (YFP) or pUC-SPYCE expressing the C-terminal 84 amino acids of YFP56. The resulting constructs were introduced into Agrobacterium tumefaciens stains GV3101. Agrobacterium cells containing different vectors were mixed to create the following paired combinations: YFPN + YFPC, SlBBX18:YFPN + APX1: YFPC, SpBBX18:YFPN + APX1: YFPC, SlBBX18-N:YFPN + APX1: YFPC, SlBBX18:YFPN + APX1-(1-600): YFPC, SpBBX18:YFPN + APX1-(1-600): YFPC, SlBBX18-N:YFPN + APX1-(1-600): YFPC. They were used for the co-infiltration of tobacco leaves. Laser confocal microscopy (Zeiss, LSM780, Germany) was employed to visualize YFP fluorescence.
Overexpression of SpBBX18 or APX1 and co-expression of SpBBX18 and APX1 simultaneously in tobacco leaves were performed as described above. Two days after Agrobacterium injection, tobacco leaves were sampled for APX activity analysis53.
Y1H and luciferase reporter analysis
The coding sequences of SlBBX18 and SpBBX18, along with their corresponding fragments, were amplified from tomato cDNA. The promoter sequence of SlAPX1 was amplified from tomato genomic DNA (gDNA). The coding sequences of SlBBX18 and SpBBX18 and their fragments were integrated into pGADT7 through homologous recombination techniques, generating plasmids pGADT7- SlBBX18, pGADT7- SpBBX18, pGADT7- SlBBX18-N, pGADT7- SlBBX18-C, and pGADT7- SpBBX18. The 1222 bp promoter sequence of SlAPX1 was ligated into the pAbAi vector through homologous recombination to obtain pAbAi-SlAPX1-Ps plasmids (pAbAi- SlAPX1-P0, pAbAi- SlAPX1-P0, pAbAi- SlAPX1-PS) and pAbAi-SlAPX1-Ps plasmids (pAbAi-SlAPX1-P1 and pAbAi-SlAPX1-P2). The constructed pAbAi-SlAPX1-Ps plasmid was linearized by restriction enzyme digestion and transferred into the yeast strain Y1HGold. After plasmid confirmation using PCR, the appropriate strain carrying the pAbAi-SlAPX1-Ps plasmid was selected for further analysis. Plasmids of pGADT7, pGADT7- SlBBX18, pGADT7- SpBBX18, pGADT7- SlBBX18-N, pGADT7- SlBBX18-C, and pGADT7- SpBBX18-C were introduced, respectively, into the Y1HGold strain harboring pAbAi- SlAPX1-Ps. The transformed cells were plated on SD/-Leu yeast medium and incubated for cell growth at 30 oC for 2–3 days. Positive clones were selected and further cultured on SD/-Leu media with varying concentrations of aureobasidin A (AbA) gradients to screen for the minimum AbA concentration that inhibited normal cell growth of yeast harboring pAbAi-SlAPX1-Ps + pGADT7. Ultimately, a concentration of 160 ng/mL AbA was established as the lowest inhibitory concentration for yeast cell growth. Yeast cell suspensions containing appropriate plasmids were adjusted to OD600 = 0.2 and then diluted with 0.9% NaCl to obtain a series of dilutions of 1 ×, 10 ×, and 100 ×. These diluted yeast solutions were then plated on both SD/-Leu and SD/-Leu + 160 ng/L AbA media and cultured at 30 °C for 2–3 days to assess their interactions based on the yeast growth.
Luciferase reporter assays were performed as described24. In summary, the different alleles of BBX18s (SlBBX18, SpBBX18, SlBBX18-N, SlBBX18-C, SpBBX18-C) were fused with the yeast Gal4 DNA binding domain (DBD) and then cloned into the pGreen II 62-SK vector as the effector. Five copies of core sequences (USAG sequence, shown in Fig. 1e: GAL4) were inserted upstream of the 35S minimal promoter containing the TATA box. These reporter elements were cloned into the pGreen II 0800-SK vector. The effector and reporter vector pairs were co-transformed into the tobacco leaf epidermis cells through Agrobacterium-mediated infiltration. The activities of LUC and REN were assessed 3 days after Agrobacterium infiltration using the Dual-Luciferase Reporter Assay System Kit (Promega, USA).
Electrophoretic mobility shift assays (EMSA)
EMSAs were conducted following the following protocols57. Different alleles of full-length and truncated BBX18 cDNAs were amplified and inserted into pGEX-4T-1. Different recombinant proteins were expressed in E. coli BL21 (DE3) strain after IPTG induction and purified using GST-tag agarose in accordance with the manufacturer’s instructions. DNA probes were synthesized and labeled with 6-carboxyfluorescein (FAM) (Tianyihuayu, Beijing, China). Proteins with GST tag and the DNA probes were incubated at 37 oC for 30 min in the binding buffer (50 mM Tris-HCl (pH 8.0), 100 mM KCl, 2.5 mM MgCl2, 1 mM dithiothreitol, and 10% glycerol). After the reaction, the product was separated by electrophoresis for 1 h in the dark. The resulting images were captured using Amersham Imager 600.
Protein extraction and IP assay
Full-length and truncated BBX18 (SlBBX18, SpBBX18, SpBBX18-C328T) cDNAs and APX1 were amplified and cloned into the pH7LIC vector. The resulting recombinant vectors were utilized to express Flag-tagged or HA-tagged proteins in tobacco leaves, respectively. Leaf samples were grinded, and the powders were resuspended in an extraction buffer. Protein extraction and co-immunoprecipitation (Co-IP) analysis were performed following the protocol below. Proteins from 0.3 g tobacco leaves were extracted in 1 mL extract buffer, followed by centrifugation, and the supernatants were filtered through 0.45-µm syringe filters. They were mixed with anti-DDDDK-tag mAb-Magnetic beads (M185-10; MBL). The beads were further boiled in an SDS sample buffer and prepared for protein separated and immunoblotting.
Chromatin immunoprecipitation (ChIP)
For the ChIP assays57, about 2 g leaf samples of four-week transgenic lines expressing the 3 × HA-tagged SpBBX18 were fixed with 1% (v/v) formaldehyde and then grinded into powders in liquid nitrogen. The leaf powders were used for ChIP assays using Protein A/G magnetic beads (Invitrogen, USA) and an anti-HA antibody (Sigma, USA). Bound DNA fragments were released into the elution buffer for subsequent qPCR and DNA sequencing by Novogene (Beijing, China).
ChIP-seq analysis
Clean reads of ChIP-seq were aligned to the tomato genome (ITAG2.4) using Bowtie2 (version 2.3.4.1). Uniquely aligned reads were employed to identify peaks using MACS2 with default parameters. The overlapping peaks were retained for further analysis. A 100 bp sequence, including 50 bp upstream and 50 bp downstream of the peak summit, was used for motif identification using MEME (Model-based Analysis of ChIP-Seq (MACS)) (https://meme-suite.org/)58. The reads per million (RPM) within a region around the peak summit (± 5 kb) were recorded using a 100-nt window size to calculate the average enrichment level of the peak. The IGV (Integrative Genomics Viewer) was utilized to visualize ChIP-seq read depths.
IP-MS/MS assay and data analysis
Target protein extraction and Co-IP assays were performed as described above. Mass spectrometry (MS) analysis of the Co-IP products was carried out by Shanghai Bioprofile Biotechnology (Shanghai, China). Protein digestion was performed with the filter-aided sample preparation (FASP) method59. Briefly, the digested proteins were loaded to a Q Exactive Plus mass spectrometer that was coupled to Easy nLC (Thermo Fisher, Waltham, MA, USA) for liquid chromatography, followed by tandem mass spectrometry (LC-MS/MS) assays. The obtained MS data were analyzed using MaxQuant software v1.6.0.16, with the following parameters: 4 for max missed cleavage, 4.5 ppm for precursor tolerance (main search), 20 ppm for precursor tolerance, and 20 ppm as mass tolerance for MS/MS. The search results were further filtered at a 1% false discovery rate (FDR) at peptide and protein, respectively. Different abundant proteins were screened with the criterion of different sequence coverage in different samples. The interaction network was loaded in Cytoscape (version 3.9.0), which was used to export graphics.
Tomato protoplast isolation and transfection
Protoplasts of wild-type tomato Alisa Craig (AC) were prepared and transfected according to the following method reported in Xue et al. with several modifications60. Briefly, protoplasts were isolated from the stems and leaves of two-week-old seedlings grown on MS medium. Approximately 20 seedlings were cut into strips of 5 mm long using sharp razors. The plant strips were placed in a 6-cm glass Petri dish with cell wall digestion solution (A solution containing 3% w/vol Cellulase, 1% w/vol Macerozyme, 0.4 M Mannitol, 10 mM MES pH = 5.7, 0.02 M KCl. The solution was heated to 55 °C and subsequently cooled to room temperature, followed by supplementation with 0.001 g/mL BSA and 20 mM CaCl2). The material was incubated at room temperature in the dark with gentle shaking (50 rpm) for 6 hr. W5 solution buffer (154 mM NaCl, 125 mM CaCl2, 5 mM KCl, 2 mM MES pH = 5.7), MMG buffer (0.4 M mannitol, 15 mM MgCl2, 4 mM MES pH = 5.7) and 40% PEG (0.2 M mannitol, 100 mM CaCl2, 40% g/V PEG 4000, Sigma) were used for subsequent protoplast collection and transfection experiments using 10 μL of plasmid (5–20 μg). Finally, the protoplasts were incubated at 28 °C in the dark for 16 h. Fluorescence from the protoplasts was captured by SP8 laser confocal microscope (Zeiss, LSM780, Germany).
In vitro assay of ascorbate peroxidase activity
The coding sequences of SpBBX18 and SlAPX1 were amplified and inserted into pMBL-His, and recombination proteins were expressed in E. coli BL21 (DE3) strain after IPTG induction, followed by purification using Ni-agarose (GE Healthcare, Amersham, Sweden)57. Purified proteins were separated on SDS-gels and stained with 0.1% Coomassie Blue R250 in a solution containing 10% acetic acid, 50% methanol, and 40% H2O for 30 mins. Ascorbate peroxidase activity of purified APX1 recombinant protein was measured according to the commercial kit from Solarbio (Solarbio, Beijing, China). A reaction mixture consisting of 50 μL of 0.4 M phosphate buffer (pH = 7.0), 50 μL of AsA at a concentration of 100 μg/mL, 50 μL of 3% H2O2, and finally, mixed proteins comprising SlAPX1 protein mix (10 μL), with tag protein (10 μL), and SpBBX18 protein (10 μL), respectively, was rapidly mixed well. After incubation for 10 and 130 s, absorption values at 290 nm were recorded.
Statistical analyses
Statistical significances between various groups were conducted according to the two-tailed Student’s t test or one-way ANOVA or two-way ANOVA followed by Duncan’s multiple range tests. All analyses were performed by GraphPad Prism 9 (http://www.graphpad.com/).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The ChIP-Seq and RNA-Seq raw data are available in the Sequence Read Archive database of NCBI under accession PRJNA1067194 and PRJNA1067321, respectively. Source data are provided in this paper.
References
Rahmati M., et al. On the impact of increasing drought on the relationship between soil water content and evapotranspiration of a grassland. Vadose Zone J. 19, e20029 (2020).
Dietz, K. J., Zörb, C. & Geilfus, C. M. Drought and crop yield. Plant Biol. 23, 881–893 (2021).
Talar, U. & Kiełbowicz-Matuk, A. Beyond Arabidopsis: BBX Regulators in crop plants. Int. J. Mol. Sci. 22, 2906 (2021).
Gangappa, S. N. & Botto, J. F. The BBX family of plant transcription factors. Trends Plant Sci. 19, 460–470 (2014).
Feng, Z. et al. Comprehensive identification and expression analysis of B-Box genes in cotton. BMC Genom. 22, 1–16 (2021).
Li, Y. et al. Genome-wide characterization of B-Box gene family in Salvia miltiorrhiza. Int. J. Mol. Sci. 24, 2146 (2023).
Robson, F. et al. Functional importance of conserved domains in the flowering-time gene CONSTANS demonstrated by analysis of mutant alleles and transgenic plants. Plant J. 28, 619–631 (2001).
Putterill, J., Robson, F., Lee, K., Simon, R. & Coupland, G. The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell 80, 847–857 (1995).
Yang, Y. et al. A zinc finger protein regulates flowering time and abiotic stress tolerance in chrysanthemum by modulating gibberellin biosynthesis. Plant Cell 26, 2038–2054 (2014).
Datta, S., Hettiarachchi, G., Deng, X.-W. & Holm, M. Arabidopsis CONSTANS-LIKE3 is a positive regulator of red light signaling and root growth. Plant Cell 18, 70–84 (2006).
Cheng, X. F. & Wang, Z. Y. Overexpression of COL9, a CONSTANS‐LIKE gene, delays flowering by reducing expression of CO and FT in Arabidopsis thaliana. Plant J. 43, 758–768 (2005).
Tripathi, P., Carvallo, M., Hamilton, E. E., Preuss, S. & Kay, S. A. Arabidopsis B-BOX32 interacts with CONSTANS-LIKE3 to regulate flowering. Proc. Natl. Acad. Sci. USA 114, 172–177 (2017).
Hassidim, M., Harir, Y., Yakir, E., Kron, I. & Green, R. M. Over-expression of CONSTANS-LIKE 5 can induce flowering in short-day grown Arabidopsis. Planta 230, 481–491 (2009).
Fan, X. Y. et al. BZS1, a B-box protein, promotes photomorphogenesis downstream of both brassinosteroid and light signaling pathways. Mol. Plant 5, 591–600 (2012).
Nagaoka, S. Takano T. Salt tolerance-related protein STO binds to a Myb transcription factor homologue and confers salt tolerance in Arabidopsis. J. Exp. Bot. 54, 2231–2237 (2003).
Liu, Y. et al. The heterologous expression of CmBBX22 delays leaf senescence and improves drought tolerance in Arabidopsis. Plant Cell Rep. 38, 15–24 (2019).
Liu, Y. et al. The BBX gene CmBBX22 negatively regulates drought stress tolerance in chrysanthemum. Hortic. Res. 9, uhac181 (2022).
Liu, X. et al. A B-box zinc finger protein, MdBBX10, enhanced salt and drought stresses tolerance in Arabidopsis. Plant Mol. Biol. 99, 437–447 (2019).
Chen, P. et al. Zinc-finger protein MdBBX7/MdCOL9, a target of MdMIEL1 E3 ligase, confers drought tolerance in apple. Plant Physiol. 188, 540–559 (2022).
Xu, D. COP1 and BBXs-HY5-mediated light signal transduction in plants. N. Phytol. 228, 1748–1753 (2020).
Xu, D., Jiang, Y., Li, J., Holm, M. & Deng, X. W. The B-Box domain protein BBX21 promotes photomorphogenesis. Plant Physiol. 176, 2365–2375 (2018).
Xu, D. et al. BBX21, an Arabidopsis B-box protein, directly activates HY5 and is targeted by COP1 for 26S proteasome-mediated degradation. Proc. Natl. Acad. Sci. USA 113, 7655–7660 (2016).
Zhao, X., Heng, Y., Wang, X., Deng, X. W. & Xu, D. A positive feedback loop of BBX11-BBX21-HY5 promotes photomorphogenic development in Arabidopsis. Plant Commun. 1, 100045 (2020).
Xiong, C. et al. A tomato B-box protein SlBBX20 modulates carotenoid biosynthesis by directly activating PHYTOENE SYNTHASE 1, and is targeted for 26S proteasome-mediated degradation. N. Phytol. 221, 279–294 (2019).
Yang, G. et al. Activation and negative feedback regulation of SlHY5 transcription by the SlBBX20/21-SlHY5 transcription factor module in UV-B signaling. Plant Cell 34, 2038–2055 (2022).
Noctor, G. & Foyer, C. H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Phys. 49, 249–279 (1998).
Bowler, C., Inze, D. & Montagu, M. V. Superoxide-Dismutase and Stress Tolerance. Annu Rev. Plant Phys. 43, 83–116 (1992).
Li, Z. Q., Li, J. T., Bing, J. & Zhang, G. F. [The role analysis of APX gene family in the growth and developmental processes and in response to abiotic stresses in Arabidopsis thaliana]. Yi Chuan 41, 534–547 (2019).
Liu J. X., et al. Isolation, purification and characterization of an ascorbate peroxidase from celery and overexpression of the AgAPX1 gene enhanced ascorbate content and drought tolerance in Arabidopsis. Bmc Plant Biol. 19, 488 (2019).
Wu, G. et al. Cloning of a cytosolic ascorbate peroxidase gene from Lycium chinense Mill. and enhanced salt tolerance by overexpressing in tobacco. Gene 543, 85–92 (2014).
Bolger, A. et al. The genome of the stress-tolerant wild tomato species Solanum pennellii. Nat. Genet. 46, 1034–1038 (2014).
Razdan, M.K., Mattoo, A.K. Genetic Improvement of Solanaceous Crops: Tomato, edit CRC Press 2, (2007).
Ebert A. W., Schafleitner R. Utilization of wild Relatives in the Breeding of Tomato and Other Major Vegetables. Crop wild relatives and climate change, 141–172 (2015).
Gong, P. et al. Transcriptional profiles of drought-responsive genes in modulating transcription signal transduction, and biochemical pathways in tomato. J. Exp. Bot. 61, 3563–3575 (2010).
Chu, Z. et al. Genomic organization, phylogenetic and expression analysis of the B-BOX gene family in tomato. Front. Plant Sci. 7, 1552 (2016).
Tiwari, S. B. et al. The flowering time regulator CONSTANS is recruited to the FLOWERING LOCUS T promoter via a unique cis‐element. N. Phytol. 187, 57–66 (2010).
Lin, T. et al. Genomic analyses provide insights into the history of tomato breeding. Nat. Genet. 46, 1220–1226 (2014).
Shinozaki, K. & Yamaguchi-Shinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 58, 221–227 (2007).
Zhu, Y. et al. A natural promoter variation of SlBBX31 confers enhanced cold tolerance during tomato domestication. Plant Biotechnol. J. 21, 1033–1043 (2023).
Wang, L. et al. CmBBX8 accelerates flowering by targeting CmFTL1 directly in summer chrysanthemum. Plant Biotechnol. J. 18, 1562–1572 (2020).
Bursch K., et al. Identification of BBX proteins as rate-limiting cofactors of HY5. Nat. Plants 6, 921–928 (2020).
Job, N., Yadukrishnan, P., Bursch, K., Datta, S. & Johansson, H. Two B-box proteins regulate photomorphogenesis by oppositely modulating HY5 through their diverse C-terminal domains. Plant Physiol. 176, 2963–2976 (2018).
Wang, Q., Tu, X., Zhang, J., Chen, X. & Rao, L. Heat stress-induced BBX18 negatively regulates the thermotolerance in Arabidopsis. Mol. Biol. Rep. 40, 2679–2688 (2013).
Xu Y., et al. A zinc finger protein BBX19 interacts with ABF3 to affect drought tolerance negatively in chrysanthemum. Plant J. 103, 1783–1795 (2020).
Wang, M. J., Ding, L., Liu, X. H. & Liu, J. X. Two B-box domain proteins, BBX28 and BBX29, regulate flowering time at low ambient temperature in Arabidopsis. Plant Mol. Biol. 106, 21–32 (2021).
Xu D., Jiang Y., Li J., Holm M., Deng X. W. The B-box domain protein BBX21 promotes photomorphogenesis. Plant Physiol. https://doi.org/10.1104/pp.17.01305 (2017).
Gao, C. X. Genome editing in crops: from bench to field. Natl Sci. Rev. 2, 13–15 (2015).
Voytas, D. F. & Gao, C. Precision genome engineering and agriculture: opportunities and regulatory challenges. PLoS Biol. 12, e1001877 (2014).
Li, R. et al. FIS1 encodes a GA2-oxidase that regulates fruit firmness in tomato. Nat. Commun. 11, 5844 (2020).
Wang, L. et al. Novel flavin-containing monooxygenase protein FMO1 interacts with CAT2 to negatively regulate drought tolerance through ROS homeostasis and ABA signaling pathway in tomato. Hortic. Res. 10, uhad037 (2023).
Li, J. et al. A tomato proline-, lysine-, and glutamic-rich type gene SpPKE1 positively regulates drought stress tolerance. Biochem. Biophys. Res. Commun. 499, 777–782 (2018).
Li, Y. et al. The C2H2 zinc-finger protein SlZF3 regulates AsA synthesis and salt tolerance by interacting with CSN5B. Plant Biotechnol. J. 16, 1201–1213 (2018).
Wang, L. et al. Characterization of genes involved in pear ascorbic acid metabolism and their response to bagging treatment during ‘Yali’fruit development. Sci. Hortic. 285, 110178 (2021).
Song, J. et al. Variation in the fruit development gene POINTED TIP regulates protuberance of tomato fruit tip. Nat. Commun. 13, 5940 (2022).
Li J., et al. SpPKE1, a Multiple stress-responsive gene confers salt tolerance in tomato and tobacco. Int. J. Mol. Sci. 20, 2478 (2019).
Walter, M. et al. Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J. 40, 428–438 (2004).
Wang, J. et al. NF‐Y plays essential roles in flavonoid biosynthesis by modulating histone modifications in tomato. N. Phytol. 229, 3237–3252 (2021).
Gao, F. et al. Blocking miR396 increases rice yield by shaping inflorescence architecture. Nat. Plants 2, 1–9 (2015).
Wiśniewski, J. R., Zougman, A., Nagaraj, N. & Mann, M. Universal sample preparation method for proteome analysis. Nat. methods 6, 359–362 (2009).
Xue, C. et al. Tuning plant phenotypes by precise, graded downregulation of gene expression. Nat. Biotechnol. 41, 1758–1764 (2023).
Acknowledgements
We are thankful to Dr. Wenhao Yan, Huazhong Agricultural University, China, for providing an experimental platform for ChIP experiments, and Dr. Pengwei Wang, Huazhong Agricultural University, China, for the valuable advice on cell biology experiments. We sincerely thank the Sharing Platform of Large-Scale Instruments & Equipments at the Academy of Agricultural Science, Southwest University, and the computing platform of the National Key Laboratory of Crop Genetic Improvement at Huazhong Agricultural University for providing the computational resources. This work was supported by Special Key Project of Technological Innovation and Application Development of Chongqing (No. CSTB2023TIAD-KPX0026, J.L.), National Natural Science Foundation of China (No. 31872123, J.L.), the Science and Technology Planning Project of Guangxi (GuikeAA22068088-1, J.H.), Fundamental Research Funds for the Central Universities (XDJK2020B060, J.L.,) and the Earmarked fund for China Agriculture Research System (CARS-23-A13, J.H.).
Author information
Authors and Affiliations
Contributions
J.L. and J.Z. conceived and supervised the study. J.L., G.A., Y.W., Y.D., X.H., Y.L., Q.Y., K.W., R.H. and C.C. performed experiments and collected the data. X.Z., Y.P. and L.W. analyzed the data. J.L. wrote the manuscript. B.O., Z.H. and J.Z. revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks David Des Marais, Xiangqiang Zhan, and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, J., Ai, G., Wang, Y. et al. A truncated B-box zinc finger transcription factor confers drought sensitivity in modern cultivated tomatoes. Nat Commun 15, 8013 (2024). https://doi.org/10.1038/s41467-024-51699-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-51699-7
- Springer Nature Limited