Abstract
Beige fat activation involves a fuel switch to fatty acid oxidation following chronic cold adaptation. Mitochondrial acyl-CoA synthetase long-chain family member 1 (ACSL1) localizes in the mitochondria and plays a key role in fatty acid oxidation; however, the regulatory mechanism of the subcellular localization remains poorly understood. Here, we identify an endosomal trafficking component sortilin (encoded by Sort1) in adipose tissues that shows dynamic expression during beige fat activation and facilitates the translocation of ACSL1 from the mitochondria to the endolysosomal pathway for degradation. Depletion of sortilin in adipocytes results in an increase of mitochondrial ACSL1 and the activation of AMPK/PGC1α signaling, thereby activating beige fat and preventing high-fat diet (HFD)-induced obesity and insulin resistance. Collectively, our findings indicate that sortilin controls adipose tissue fatty acid oxidation by substrate fuel selection during beige fat activation and provides a potential targeted approach for the treatment of metabolic diseases.
Similar content being viewed by others
Introduction
Thermogenic adipocytes (brown and beige adipocytes) are mitochondria-rich and play a crucial role in driving non-shivering thermogenesis and energy expenditure, thus making them a promising target to combat obesity and its concomitant metabolic disorders1,2. Beige fat locates in white adipose tissue (WAT) and is readily able to convert to a ‘brown-like state’ with prolonged cold exposure or β-adrenergic compounds3. Exposure to chronic cold, thermogenic fat can take up and utilize a large amount of energy substrates [i.e., glucose and free fatty acids (FFAs)]. New evidence has highlighted that these substrates feed alternative pathways in a time-specific manner to sustain brown and beige fat thermogenesis4. For example, beige fat activation is associated with metabolic adaptation by inducing a switch from carbohydrate metabolism to fatty acid oxidation5. Fatty acid oxidation is favored over glucose oxidation during the energy dissipation in beige adipocyte6. From an evolutionary perspective, such metabolic shifts are considered to be protective mechanisms to secure enough glucose for the brain, while other fuels, such as fatty acids are allocated to the peripheral metabolic organs5. This physiological response allows for sustained thermogenesis for beige fat upon chronic cold adaptation and improves metabolic homeostasis. However, the molecular mechanism of beige fat prioritizes fatty acid oxidation for heat generation remains poorly understood.
The endolysosomal system is a promising and under-explored therapeutic target that controls the trafficking and distribution of key metabolic proteins. Some of the core machinery involved in this system are altered in metabolic diseases7. For example, published RNA sequencing datasets of adipose tissue from rats fed a high-fat diet (HFD) for 20 weeks (GSE 13271) found that genes encoding components of the endomembrane system, vesicle and endosome membrane were enriched among the differentially expressed genes, suggesting that endocytosis and endocytic trafficking are altered during obesity in adipose tissue8. Sortilin is one such endosomal component. As a transmembrane protein found in many cell types, it is involved in intracellular trafficking of diverse endogenous and exogenous protein substrates between the trans-Golgi network (TGN), endosomes and lysosomes9; it is also known to play an important role in lipid metabolism10. For example, sortilin has an essential role in the secretion of ApoB-containing very low-density lipoprotein (VLDL). Additionally, sortilin colocalizes with PCSK9 in the trans-Golgi network and facilitates its secretion from primary hepatocytes11. Sortilin, also known as neurotensin receptor 3 (NTSR3), is a type I transmembrane protein belonging to the family of Vps10p-domain receptors12. Its soluble ligand factor, neurotensin (NTS), is secreted from lymphatic vessels in brown adipose tissue (BAT), and is known to play an inhibitory role in adaptive thermogenesis13. NTS has three receptors (NTSR1, NTSR2 and NTSR3/sortilin). NTSR2 has been shown to mediate thermogenic inhibition via neurotensin13. However, the role of sortilin in adipocyte thermogenesis is not yet known. Here, we demonstrated that adrenergic activation modified the components of endolysosomal pathway during chronic cold stimulated beige fat activation. Moreover, sortilin level decreased during adrenergic activation in adipocytes and returned to baseline after stimulus removal. Depletion of sortilin induced significant browning in mouse and human white adipocytes. Mice with adipocyte-specific sortilin deletion or a chemogenic sortilin inhibitor were resistant to HFD-induced obesity and insulin resistance. Mechanistically, we showed that sortilin is a critical regulator of the subcellular distribution of ACSL1. We used mass spectrometry-based proteomics to identify ACSL1-sortilin protein interactions. ACSL1 is reported to localize both to the mitochondria and cytosol in adipocytes14. When localized to the outer mitochondrial membrane, ACSL1 generates long-chain acyl-CoA destined for β-oxidation (FA + CoA + ATP → acyl-CoA +AMP + PPi)15. Sortilin facilitated the translocation of mitochondrial ACSL1 to endosome/lysosome. Relative to controls, the mitochondria of sortilin-depleted adipocytes were enriched for ACSL1. ACSL1 has been identified as a key regulator of AMPK activation16, which is known to upregulate UCP1 expression through PGC1α to enhance white fat browning17,18. Overall, we uncover a fundamental mechanism by which endolysosomal system-mediated mitochondrial ACSL1 enrichment controls white fat browning. This study sheds light on the research to harness substrate’s metabolism in thermogenic fat to develop a potential targeted approach for the treatment of metabolic diseases.
Results
Dynamic regulation of sortilin expression during white fat browning and its association with human metabolic status
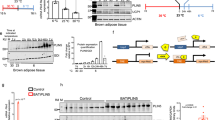
In order to elucidate the role of the endosomal system during white fat browning, we maintained wild-type (WT) mice at either room temperature (RT) or cold stimulation (4 °C) for 3 days and then performed bulk RNA sequencing to examine the differentially expressed genes in inguinal white adipose tissue (ingWAT) in the two groups (Fig. 1a). The gene-annotation enrichment analysis found that the majority of the cluster I genes were related to endosome expression (246 genes) (Fig. 1b-c) and function, specifically including endosome to lysosome transport (12 genes) (Fig. 1d). Seven of the twelve genes were decreased during cold stimulation and the highest expression level in white fat was Sort1 (coding protein sortilin) (Fig.1e). An essential characteristic of beige fat is its dynamic regulation of UCP1-positive and multilocular adipocytes by cold stimuli and UCP1-negative unilocular adipocytes when re-acclimated to ambient temperature19. We further verified the dynamic change of Sort1 in RNA-seq by a different experimental paradigm (Fig. 1f). We found that sortilin expression was significantly reduced in ingWAT following three days of cold exposure or seven days of β3-AR agonist CL316, 243 treatment (Fig. 1g, h and Supplementary Fig. 1a) and then restored upon removal of the stimulus. Interestingly, sortilin expression was inversely correlated to that of UCP1 (Fig. 1g, h). The expression of sortilin in heart and muscle were much lower than that in ingWAT. Importantly, sortilin expression in ingWAT changed in response to temperature changes, while no change in sortilin expression was observed in heart and muscle in response to cold stimulation (Supplementary Fig. 1b). These data suggest sortilin expression is dynamically regulated during white fat browning. To elucidate the relationship between sortilin and obesity, we quantified mRNA expression of sortilin in the abdominal adipose tissues of human patients, along with their circulating metabolite profile (Fig. 1i). We found sortilin level was significantly upregulated in patients with higher body mass index (BMI ≥ 25 kg/m2) (Fig. 1j) and positively correlated with waist circumference and BMI (Fig. 1k, l). Moreover, upregulated sortilin expression was significantly correlated with low-density lipoprotein C (LDL-C) (Fig. 1m) but not with TG, high-density lipoprotein C (HDL-C), blood glucose, alanine aminotransferase (ALT) and creatinine (Fig. 1n, o and Supplementary Fig. 1c, d). In summary, sortilin was reduced by adrenergic activation but elevated after reacclimating to room temperature. Importantly, the enrichment of sortilin in white fat was positively associated with obesity and its established circulating metabolites in human patients.
a Workflow of cold challenge for WT mice. b Heatmap of the RNA-seq transcriptome in ingWAT from RT or cold (4 °C) mice (n = 3 per group). All of the listed genes were significantly different (false-discovery rate (FDR) < 0.05). c GO (Gene ontology) cellular component analysis in ingWAT from RT and cold mice. d GO biological process analysis in ingWAT from RT and cold mice. e The FPKM expression of endosomal genes involved in Endosome to lysosome transport in ingWAT from RT and cold mice (n = 3 per group). f Workflow of cold challenge and rewarm for WT mice. g Left: Immunoblot analysis of sortilin and UCP1 in ingWAT of WT mice after cold challenge and rewarm. The right panel showed the quantification normalized to α-Tubulin. n = 6 per group. h The mRNA analysis of sortilin and UCP1 in ingWAT of WT mice after cold challenge and rewarm. n = 6 per group. i BMI of human subjects. n = 16 (BMI < 25 kg/m2), n = 14 (BMI ≥ 25 kg/m2). j Detection of the mRNA expression of sortilin in human omental adipose tissues. n = 14 (BMI < 25 kg/m2), n = 12 (BMI ≥ 25 kg/m2). k–o Analysis of the correlation between sortilin expression in human omental adipose tissues and waist circumference (k), BMI (l), LDL-C (m), blood glucose (n), and TG (o). n = 26 for waist circumference analysis, n = 26 for BMI analysis, n = 22 for LDL analysis, n = 25 for blood glucose analysis, and n = 23 for TG analysis respectively. Statistical significance was assessed by two-tailed Student’s t test. Values were presented as Pearson’s r correlation coefficient (k–o). Data were presented as mean ± SEMs. Source data and uncropped blots are available as a Source Data file.
Fat-specific loss of sortilin promotes white fat browning
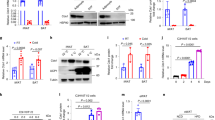
Accordingly, we asked whether sortilin depletion would affect the browning in white fat. To answer this question, we examined the expression of sortilin before and after the adipogenic differentiation of immortalized inguinal WAT-SVF cells from mice, (abbreviated as “iWAT cells”). Sortilin expression was markedly increased in differentiated iWAT cells comparison to the undifferentiated SVF cells (Supplementary Fig. 2a, b). We constructed a lentivirus to deplete sortilin in iWAT cells. Fully differentiated sortilin-depleted iWAT cells contained similar TG content as the control cells (Fig. 2a). Moreover, the mRNA expression of Pparγ, Fabp4 and Lep in sortilin-depleted white adipocytes was comparable with that in the control cells during adipogenic differentiation (Fig. 2b and Supplementary Fig. 2c), indicating that depletion of sortilin did not impede adipocyte differentiation. But depletion of sortilin significantly increased both the level of thermogenic gene markers (Ucp1, Pgc1α, Dio2, Elov3 and Cidea) and the protein abundance of UCP1 and PGC1α (Fig. 2b, c) in shSort1 adipocytes. The basal and maximal oxygen-consumption rates (OCRs) were also increased in shSort1 adipocytes (Fig. 2d). We also used siRNA to knockdown sortilin (siSORT1) in differentiated subcutaneous fat derived SVF cells from human (HumanWAT cells). Both the level of thermogenic gene markers (UCP1, CIDEA and PRDM16) and basal OCR were increased in sortilin-depleted HumanWAT cells (Fig. 2e, f). We next examined whether sortilin depletion promoted white fat browning in vivo. We generated adipocyte conditional sortilin-deletion (Sort1adipoKO) mice and the deletion of sortilin in adipose tissue but not in the liver was confirmed by qPCR and immunoblot (Fig. 2g-i). The level of thermogenic gene markers (Ucp1, Pgc1α, Dio2, Elov3 and Cidea) and the protein abundance of UCP1 and PGC1α were significantly increased in Sort1adipoKO mice (Fig. 2j, k). Sort1adipoKO mice displayed a higher core temperature during cold exposure and were more resistant to cold-induced hypothermia than Sort1fl/fl mice (Fig. 2l). Indirect calorimetry analysis indicated that Sort1adipoKO mice showed similar oxygen consumption (VO2) at 30°C. When housing temperature decreased to 22°C, this minor cold stimulation boosted a more obvious induction of VO2 in Sort1adipoKO mice than that in the control mice, while no difference was observed in their food intake and locomotor activity (Fig. 2m and Supplementary Fig. 2d, e). In turn, mice exhibited a lower respiratory exchange ratio (RER) compared with the controls (Fig. 2n), suggesting a preference for lipid metabolism in oxygen consumption following sortilin depletion. To further verify these metabolic differences, we employed a mouse model with ingWAT-specific depletion of sortilin. To perform this loss-of-function study in vivo, we used an adeno-associated virus (AAV) that expressed short hairpin RNA directly targeting sortilin (shSort1) (Supplementary Fig. 2f). Direct injection of AAVshSort1 into the ingWAT of wild-type mice resulted in an approximately 60% lower expression of sortilin in ingWAT compared to that in the mice received an AAV with a scrambled control shRNA (AAVshcon) (Supplementary Fig. 2g, h). Consistent with Sort1adipoKO, mice with ingWAT-specific depletion of sortilin (AAVshSort1) also exhibited an increase in thermogenic genes and UCP1 and PGC1α protein levels (Supplementary Fig. 2i, j). Consistent with previous findings, AAVshSort1 mice were more resistant to cold-induced hypothermia and AAVshSort1 mice possessed a higher systemic oxygen consumption than control mice when kept at 22°C from 30°C with similar food intake and locomotor activity (Supplementary Fig. 2k–n). These results demonstrate that sortilin deletion promotes white fat browning.
In vitro experiment, iWAT cells transfected with shRNA targeting Sort1 or scrambled control. Cells were induced to adipogenic differentiation for 8 days, namely shSort1 and shcon adipocytes, respectively. a Left: Oil Red O staining of shSort1 and shcon adipocytes. Scale bar: 100 μm. Right panel showed the TG concentration normalized to protein at 8 days of adipogenic differentiation. n = 3 per group. b, c The expression of thermogenic genes (b) and Immunoblot analysis of UCP1 and PGC1α (c) in differentiated shcon and shSort1 iWAT cells. Quantification is shown right in (c). n = 3 per group. d Left: OCRs in differentiated shcon and shSort1 iWAT cells. Calculation was shown right. n = 3 per group. e The expression of thermogenic genes was evaluated in differentiated subcutaneous fat-derived SVF cells from human (humanWAT cells). Cells were transfected with siRNA targeting SORT1 (siSORT1) or scrambled control (sicon) for 48 h. n = 3 per group. f Basal OCR in differentiated sicon and siSORT1 HumanWAT cells. n = 3 per group. g Overview of Sort1adipoKO mice model. h The mRNA expression analysis of sortilin in fat and liver from Sort1fl/fl and Sort1adipoKO mice. n = 6 per group. i Left: immunoblot analysis of sortilin in ingWAT, epiWAT, BAT and liver in Sort1fl/fl and Sort1adipoKO mice. Quantification was shown right. n = 6 per group. j The expression of thermogenic genes in ingWAT of Sort1fl/fl and Sort1adipoKO mice. n = 6 per group. (k) Immunoblot analysis of UCP1 and PGC1α in ingWAT of Sort1fl/fl and Sort1adipoKO mice. Quantification was shown right. n = 6 per group. l The rectal temperature of Sort1fl/fl and Sort1adipoKO mice fed chow diet in response to a cold challenge (4 °C) from thermoneutrality (30 °C) for at least 7 days. n = 6 per group. m, n Whole-body oxygen consumption (VO2) (m) and respiratory exchange ratio (RER) (n) of Sort1fl/fl and Sort1adipoKO mice fed chow diet at thermoneutrality (30 °C) or room temperature (22 °C). The right panel showed the quantification. n = 6 per group. Statistical significance was assessed by two-tailed Student’s t test. Data were presented as mean ± SEMs. Source data and uncropped blots are available as a Source Data file.
Fat-specific loss of sortilin increases brown fat thermogenic activity
We next examined whether sortilin depletion affects brown fat activity. In Sort1adipoKO mice, a higher back skin temperature was observed compare to control mice. And BAT in control mice was classically brown-red, whereas BAT was more intensely reddish-brown in Sort1adipoKO mice (Supplementary Fig. 3a). Thermogenic genes (Ucp1 and Pgc1α) level and proteins (UCP1 and PGC1α) expression in BAT isolated from Sort1adipoKO mice were significantly higher than control mice (Supplementary Fig. 3b, c). In vitro, sortilin depletion also increased thermogenic genes and proteins in differentiated immortalized BAT-SVF cells (abbreviated as “iBAT cells”) (Supplementary Fig. 3d, e). The increased level of OCR in differentiated sortilin-depleted iBAT cells was also evident, indicating enhanced brown fat thermogenesis (Supplementary Fig. 3f).
Fat-specific loss of sortilin restrains the development of HFD-induced obesity
To investigate whether fat-specific loss of sortilin mice improves systemic metabolism, we subjected Sort1adipoKO mice and littermates to a HFD challenge for 10 weeks. Sort1adipoKO mice gained significantly less body weight than Sort1fl/fl mice (Fig. 3a, b), mainly due to reduced fat mass rather than decrease in lean mass (Fig. 3c). Consistently, the weights of adipose tissues, including BAT, ingWAT and epididymal WAT (epiWAT), as well as liver tissue, were markedly lower in Sort1adipoKO mice compared to Sort1fl/fl mice (Fig. 3d). The diameters of adipocytes in ingWAT and epiWAT were significantly smaller in Sort1adipoKO mice than in Sort1fl/fl mice (Fig. 3e). Quantitative measurements of adipose tissues using micro-CT also revealed reduced volumes of both subcutaneous and visceral fat in sortilin-depleted mice relative to controls (Fig. 3f). Furthermore, glucose tolerance test (GTT) and insulin tolerance test (ITT) revealed that Sort1adipoKO mice exhibited more efficient clearance of plasma glucose than Sort1fl/fl mice, as evidenced by reduced areas under the curve (AUC) of GTT and ITT, respectively (Fig. 3g, h). In addition, lipid accumulation and TG content in the liver were lower in Sort1adipoKO mice than in Sort1fl/fl mice (Fig. 3i, j). Unlike those of HDL-C and FFAs, the concentrations of plasma TG, cholesterol and LDL-C were all significantly lower in Sort1adipoKO mice compared to Sort1fl/fl mice (Fig. 3k). These findings suggest that Sort1adipoKO imparts metabolic resilience to obesity.
a Representative photo of Sort1fl/fl and Sort1adipoKO mice at 12 weeks of HFD. b Body weight of Sort1fl/fl and Sort1adipoKO mice fed HFD. n = 6 per group. c Body composition (fat/lean mass) of Sort1fl/fl and Sort1adipoKO mice at 12 weeks of HFD. n = 6 per group. (d) Weights of fat tissues and liver in Sort1fl/fl and Sort1adipoKO mice at 12 weeks of HFD. n = 6 per group. e Representative H&E staining images and calculated adipocyte size of dissected tissues in Sort1adipoKO and Sort1fl/fl mice at 12 weeks of HFD. Scale bar: 50 µm. The right panel showed the quantification. One point indicated the value of one mouse’s mean adipocyte size quantification. n = 6 per group. f Representative micro-CT images (left) and quantification of adipose tissue volumes (right) of Sort1adipoKO and Sort1fl/fl mice at 12 weeks of HFD. Scale bar: 10 mm. n = 6 per group. g, h Intraperitoneal glucose tolerance test (GTT) (g) and insulin tolerance test (ITT) (h) in Sort1adipoKO and Sort1fl/fl mice at 12 weeks of HFD. n = 6 per group. i Triglyceride content in liver tissues of Sort1fl/fl and Sort1adipoKO mice at 12 weeks of HFD. n = 6 per group. j Representative HE-staining images of liver from Sort1fl/fl and Sort1adipoKO mice at 12 weeks of HFD. Scale bar: 50 µm. n = 6 per group. k Serum lipid profile of Sort1fl/fl and Sort1adipoKO mice at 12 weeks of HFD. n = 6 per group. Statistical significance was assessed by two-tailed Student’s t test. Data were presented as mean ± SEMs. Source data are available as a Source Data file.
Mice with specific depletion of sortilin in ingWAT (AAVshSort1) were subjected to HFD challenge, which revealed that AAVshSort1 mice featured decreased adiposity compared to AAVshcon mice, as shown by decreased body weights, adipose tissue weights, and fat mass (Supplementary Fig. 4a–d). Additionally, AAVshcon mice exhibited improvements in insulin resistance and hepatic steatosis (Supplementary Fig. 4e–i). Taken together, these data suggest that sortilin depletion in adipocytes can prevent HFD-induced obesity and metabolic comorbidities.
Sortilin depletion-mediated white fat browning depends upon activation of AMPK signaling
To identify the mechanism by which sortilin depletion promotes the browning of white fat, we performed RNA-seq in differentiated shcon and shSort1 iWAT cells in vitro (Fig. 4a). shSort1 cells had increased levels of thermogenic and oxidative phosphorylation associated genes (Supplementary Fig. 5a). KEGG analysis revealed the majority of the cluster I genes were related to AMPK signal17 and then MAPK signal20 and lipolysis21, all of which are well known to enhance white fat browning (Fig. 4b). We verified the above data both in vivo and in vitro. Relative to controls, both sortilin-depleted adipocytes and adipose tissues showed increased phosphorylation of AMPK (p-AMPKThr172), p38 MAPK (p-p38 MAPKThr180/Tyr182) and HSL (p-HSLSer660) levels (Fig. 4c, d and Supplementary Fig. 5b). To further investigate the role of AMPK, MAPK signal and lipolysis in these findings, we treated shSort1 cells with an AMPK inhibitor, Compound C (CC), or an AMPK dominant-negative mutant (AMPK-DN). Both AMPK inhibitor or AMPK-DN severely impaired sortilin depletion-mediated UCP1 induction (Fig. 4e, f). In contrast, p38 MAPK inhibitors (SB203580 and SB202190) and the lipase inhibitors, including Atglistatin (ATGL inhibitor) and CAY10499 (HSL inhibitor), efficiently suppressed the phosphorylation of p-HSL and p-p38 MAPK (Fig. 4g-i). However, blockade of p-p38MAPK or inhibition of lipolysis did not affect sortilin depletion-mediated UCP1 upregulation (Fig. 4g-i). Given these findings, we conclude that thermogenic activity of sortilin depletion-mediated browning relies upon AMPK signaling, but it is likely independent of signaling by MAPK and lipolysis. As changes in AMP or ADP are the classical activators of AMPK, we found that sortilin depletion had increased the level of AMP:ATP ratios in ingWAT of Sort1adipoKO mice (Fig. 4j). Furthermore, Fructose-1,6-bisphosphate (FBP), an AMP/ADP-independent mechanism that triggers AMPK activation also increased (Fig. 4k and Supplementary Fig. 5c). Taken together, these data suggest that sortilin depletion-mediated white fat browning depends upon activation of AMPK signaling.
a Workflow of shcon and shSort1 adipocytes for mRNA-seq. n = 3 per group. b Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis of global activated pathway in shSort1 adipocytes. c Immunoblots (proteins indicated) in shcon and shSort1 adipocytes. The right panel showed the quantification normalized to α-Tubulin, AMPK, p38 MAPK or HSL. n = 3 per group. p/T AMPK: phosphorylated AMPK/Total AMPK; p/T p38 MAPK: phosphorylated p38 MAPK/Total p38 MAPK; p/T HSL: phosphorylated HSL/Total HSL. d Immunoblots (proteins indicated) in ingWAT of Sort1fl/fl and Sort1adipoKO mice. The right panel showed the quantification normalized to α-Tubulin, AMPK, p-38 MAPK or HSL. n = 6 per group. e Immunoblots (proteins indicated) in shcon and shSort1 adipocytes incubated with or without AMPK inhibitor CC (10 μM) for 2 h. The lower panel showed the quantification normalized to α-Tubulin or AMPK. n = 4 per group. f Immunoblots (proteins indicated) of shcon and shSort1 adipocytes that were reverse-transfected with plasmid expressing AMPK-DN or control mock for 24 h. The lower panel showed the quantification normalized to α-Tubulin or AMPK. n = 4 per group. g, h Immunoblots (proteins indicated) in shcon and shSort1 adipocytes incubated with or without p-38 MAPK inhibitor SB203580 (10 μM) (g) or SB202190 (5 μM) (h) for 2 h. The lower or right panels showed the quantification normalized to α-Tubulin or p-38 MAPK. n = 4 per group. i Immunoblots (proteins indicated) in shcon and shSort1 adipocytes incubated with or without lipase inhibitor (Atglistatin, ATGL inhibitor 10 μM and CAY10499, HSL inhibitor 20 μM) (h) for 2 h. The right panel showed the quantification normalized to α-Tubulin or HSL. n = 4 per group. j The AMP/ATP and ADP/ATP ratio in ingWAT of 8-week-old Sort1fl/fl and Sort1adipoKO mice fed chow diet. n = 7 for Sort1fl/fl group, n = 9 for Sort1adipoKO group. k Metabolites levels of glycolysis and tricarboxylic acid cycle (TCA) in ingWAT of 8-week-old Sort1fl/fl and Sort1adipoKO mice fed chow diet. n = 7 for Sort1fl/fl group, n = 9 for Sort1adipoKO group. Statistical differences were determined by two-tailed Student’s t-test; Data were presented as mean ± SEMs. Source data and uncropped blots are available as a Source Data file.
Sortilin interacts with ACSL1 and controls its protein degradation
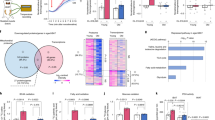
To identify the potential effector mediating the effects of sortilin-depletion on AMPK signaling activation and white fat browning, we detected precipitated proteins by sortilin from differentiated iWAT cells in vitro by liquid chromatography-tandem mass spectrometry (LC/MS-MS). Those proteins presented in the control group were all excluded. A total of 394 proteins were coprecipitated with sortilin (Fig. 5a, b). KEGG analysis revealed that the pathway with the highest enriched proteins was the Metabolic pathway (Supplementary Fig. 6a). Eukaryotic Orthologous Groups (KOG) analysis revealed 101 proteins were described as Metabolism function. They were enriched in Energy production and conversion and then Lipid transport and metabolism (Fig. 5c). The majority of sortilin is localized to the intracellular space and engaged in trafficking between trans‐Golgi, endosomes, lysosomes and less well‐defined transport vesicles22,23. Proteins located in mitochondrion inner membrane or matrix and extracellular matrix proteins were excluded. We finally obtained 32 candidate proteins (Supplementary Table 1). According to the abundance, the top 10 proteins were G3P, ACSL1, HBE, FAS, KPYM, DHE3, NB5R3, DHB12, DHB4 and VDAC2 (Fig. 5d). To verify these 10 candidates, we asked whether siRNA-mediated knockdown of their respective mRNAs was capable of compromising sortilin depletion-promoted effects on white adipocytes browning (Supplementary Fig. 6b–k). Only knockdown of ACSL1 substantially reduced sortilin depletion-mediated the increase in phosphorylated AMPK and the upregulation of the UCP1 protein (Fig. 5e–g). This suggests that the activation of AMPK and the induction of UCP1 by sortilin depletion is dependent on ACSL1. To verify the interaction between sortilin and ACSL1 identified via LC/MS-MS (Supplementary Fig. 6l), we used both sortilin-and ACSL1-specific antibodies to pull down the associated proteins and then confirmed their presence using western blot. Sortilin antibodies were able to pull down ACSL1 proteins and vice versa (Supplementary Fig. 6m, n). The physical interaction between sortilin and ACSL1 was further confirmed in differentiated iWAT cells via immunofluorescence assays (Supplementary Fig. 6o). We further probed the functional interactions between sortilin and ACSL1 and found that sortilin depletion markedly enhanced the protein expression of ACSL1 (Fig. 5h, i). However, sortilin depletion had little effect on ACSL1 mRNA levels in vivo and in vitro (Fig. 5j, k). Furthermore, cycloheximide (CHX) chase assays showed that sortilin depletion significantly extended the half-life of the ACSL1 protein (Fig. 5l). And in sortilin-depleted adipocytes, mitochondrial ACSL1 abundance was significantly increased compared to control adipocytes while cytosolic ACSL1 level did not (Fig. 5m). These data indicate that sortilin interacts with ACSL1 and controls its protein degradation.
a Workflow of IP by sortilin and IgG antibody for protein capture by LC-MS/MS in differentiated iWAT cells. b Representative photo of Bis-Tris Gel which was stained with Coomassie Blue Staining in (a). c KOG analysis of proteins pulldown by sortilin in (a). d List of the top 10 abundance candidate proteins pulldown by sortilin. e Immunoblots of p-AMPK and UCP1 protein levels in differentiated Sort1adipoKO-iWAT cells and differentiated Sort1fl/fl-iWAT cells (called as control). Differentiated Sort1adipoKO-iWAT cells were transfected with corresponding siRNAs. f, g The quantification of p/T AMPK (f) and UCP1 (g) in (e). h, i Immunoblots of UCP1 and ACSL1 in ingWAT of Sort1fl/fl or Sort1adipoKO mice (h) and shcon or shSort1 adipocytes (i). Quantifications are shown below. j mRNA expression of ACSL1 and UCP1 in ingWAT of Sort1fl/fl and Sort1adipoKO mice. k mRNA expression of ACSL1 and UCP1 in differentiated Sort1adipoKO-iWAT cells (called as KO) and differentiated Sort1fl/fl-iWAT cells (called as control). l CHX chase assay showed the degradation of ACSL1 in shcon and shSort1 adipocytes. Experiment was independently repeated 3 times and the quantification was shown at below. m ACSL1 expression in mitochondrial and cytosol fraction of differentiated control or KO cells in vitro. Quantifications were shown right. n Workflow of ingWAT-specific ACSL1 overexpression: mice were injected with AAVAcsl1 and scramble (AAVcon) in bilateral ingWATs. o Thermogenic genes were evaluated in ingWAT of AAVcon and AAVAcsl1 mice. p The rectal temperature of AAVcon and AAVAcsl1 mice in response to a cold challenge (4 °C) from RT. q Immunoblots of p/T AMPK and UCP1 in ingWAT of AAVcon and AAVAcsl1 mice. Quantification was shown right. r, s VO2 (r) and RER (s) of AAVcon and AAVAcsl1 mice fed chow diet at RT. The right panel showed the quantification. (t) Body weight of AAVcon and AAVAcsl1 mice fed HFD. n = 3 each group in (f), (g), (i), and (k–m). n = 6 each group in (h, j, o–q, t). n = 8 each group in (r), (s). Statistical significance was assessed by two-tailed Student’s t test. Data were presented as mean ± SEMs. Source data and uncropped blots are available as a Source Data file.
ACSL1 promotes white fat browning and systematic energy metabolism
It was previously reported that ACSL1 expression has been identified as a key regulator of AMPK activation16,24,25. We asked whether the increased ACSL1 could promote the activation of AMPK signaling and the browning of white fat. Consistent with UCP1 expression, ACSL1 expression was upregulated in ingWAT of cold-exposed mice and then down to a similar level with mice in room temperature (Supplementary Fig. 6p). Moreover, ACSL1 mRNA level in human abdominal adipose tissues was significantly negatively correlated with BMI (Supplementary Fig. 6q). To have a better understanding of the in vivo role of ACSL1 on metabolic performances from a genetic point of view, we generated mice model with ingWAT specific ACSL1 overexpression (AAVAcsl1) (Fig. 5n). AAVAcsl1 mice characterized an approximately 5-fold increase in ACSL1 mRNA level of ingWAT (Fig. 5o). AAVAcsl1 mice featured increased thermogenic genes, enhanced resistance to cold challenge and elevated phosphorylation of AMPK (p-AMPK) and UCP1 protein abundance (Fig. 5o–q). They also possessed increased oxygen consumption and reduced RER (Fig. 5r, s). When subjected to HFD, AAVAcsl1 mice showed a significant decrease in body weight and improved glucose and insulin resistance compared to AAVcon mice (Fig. 5t and Supplementary Fig. 6r, s).
ACSL1 acts downstream of sortilin depletion-mediated the browning of white fat
We then set out to unravel whether ACSL1 acts downstream of sortilin depletion-mediated browning of white fat. We constructed a silence expression of sortilin in WT or Acsl1KO mice by ingWAT local injection (Fig. 6a). In WT mice, ingWAT-specific sortilin depletion mice had enhanced resistance capacity to cold challenge (Fig. 6b); Acsl1KO mice were cold intolerance, and ingWAT-specific sortilin depletion did not enhance the impaired cold tolerance capacity in Acsl1KO mice (Fig. 6b). When kept at 30 °C, both WT and Acsl1KO mice with or without ingWAT-specific sortilin depletion showed similar expression levels of thermogenic genes (Ucp1 and Pgc1α) and AMPK phosphorylation in ingWAT as well as VO2 consumption (Fig. 6c–e). But when kept at RT, an increased expression levels of thermogenic genes (Ucp1 and Pgc1α) and AMPK phosphorylation were observed in ingWAT-of WT mice with ingWAT-specific sortilin depletion but not in that of Acsl1KO mice after ingWAT-specific sortilin depletion (Fig. 6c, d). Consistently, ingWAT-specific sortilin depletion in WT mice at RT could lead to an enhanced oxygen consumption, which was not observed in Acsl1KO mice after ingWAT-specific sortilin depletion (Fig. 6e). Upon stimulation of CL-316, 243 (a β3-adrenoceptor agonist), ingWAT-specific sortilin depletion in WT mice kept at 30 °C caused a significantly increased oxygen consumption, which was also not observed in Acsl1KO mice (Fig. 6e). These data highlight the effect of sortilin depletion on white fat browning and this effect relies upon ACSL1. To further confirm ACSL1 as the effector for sortilin depletion-mediated browning of white fat, we silenced the expression of ACSL1 in both Sort1fl/fl and Sort1adipoKO mice by tail vein injection of AAVshAcsl1 (Fig. 6f). In AAVshAcsl1 injection mice, AAVshAcsl1 led to nearly 70% reduction of ACSL1 expression in ingWAT (Fig. 6h, i). Consistently with Acsl1KO mice, with AAVshAcsl1 injection, both Sort1fl/fl and Sort1adipoKO mice were intolerant to cold challenge (Fig. 6g). When kept at 30 °C, levels of thermogenic genes (such as Ucp1 and Pgc1α) and AMPK phosphorylation in ingWAT of these mice as well as their VO2 consumption were comparable (Fig. 6h–j). However, when kept at RT, Sort1adipoKO mice showed an increased expression levels of thermogenic genes and an enhanced AMPK phosphorylation in ingWAT compared to the Sort1fl/fl mice, which were blunted after an AAVshAcsl1 injection in these mice (Fig. 6h, i). Moreover, an elevated VO2 consumption was also observed in Sort1adipoKO mice in comparison with the control mice, which was also blunted after an AAVshAcsl1 injection in these mice (Fig. 6j). If given CL-316, 243 treatment at 30 °C, Sort1adipoKO mice displayed an increased oxygen consumption with AAVcon injection, but not after AAVshAcsl1 injection (Fig. 6j). Overall, above data suggest that ACSL1 acts downstream of sortilin-depletion mediated the browning of white fat.
a Workflow of ingWAT-specific sortilin/ACSL1 double depletion models: WT and Acsl1KO mice were injected with AAVshSort1 or scramble virus (AAVshcon) in bilateral ingWATs. Mice were divided into four groups. b The rectal temperature of mice fed chow diet in response to a cold challenge (4 °C) from RT. n = 6 per group. c The expressions of thermogenic genes (Ucp1 and Pgc1α) were evaluated in ingWAT of mice kept at 30 °C or RT (22 °C). n = 6 per group. d Immunoblots analysis of ACSL1, UCP1 and p-AMPK were evaluated in ingWAT of mice kept at 30 °C or RT (22 °C). n = 6 per group. The right panel showed the quantification normalized to α-Tubulin or AMPK. e Whole-body VO2 of mice kept at RT (22 °C) or 30 °C with CL-316,243 stimulation (1 mg/kg). The arrow indicated the time of drug administration. n = 6 per group. f Workflow of ingWAT-specific sortilin/ACSL1 double depletion models: Sort1fl/fl and Sort1adipoKO mice were injected with AAVshAcsl1 or scramble virus (AAVshcon) through tail vein. g The rectal temperature of mice fed chow diet in response to a cold challenge (4 °C) from RT. n = 6 per group. h The expressions of thermogenic genes (Ucp1 and Pgc1α) were evaluated in ingWAT of mice kept at 30 °C or RT (22 °C). n = 6 per group. i Immunoblots analysis of ACSL1, UCP1, and p-AMPK were evaluated in ingWAT of mice kept at 30 °C or RT (22 °C). The lower panel showed the quantification normalized to α-Tubulin or AMPK. n = 6 per group. j Whole-body VO2 of mice fed chow diet were kept at RT (22 °C) or 30 °C with CL-316, 243 stimulation (1 mg/kg). The arrow indicated the time of drug administration. n = 6 per group. Statistical significance was assessed by two-tailed Student’s t test. Data were presented as mean ± SEMs. Source data and uncropped blots are available as a Source Data file.
Sortilin regulates the subcellular distribution of ACSL1
Considering the established role of sortilin as a sorting protein, in order to further evaluate how sortilin depletion increased ACSL1 protein level, we then investigated whether sortilin regulated the subcellular distribution of ACSL1 in differentiated iWAT cells. Sortilin depletion increased the colocalization of ACSL1 with TOM20, a mitochondrial marker, but reduced its colocalization with EEA1 (early endosome antigen 1) and LAMP1(lysosome-associated protein 1), indicating a decrease translocation of ACSL1 from mitochondria to endosome and lysosome by sortilin (Supplementary Fig. 7a). In order to verify this finding, we treated sortilin-depleted and control cells with selective lysosomal inhibitors NH4Cl (250 μM) and chloroquine (20 μM). Both of these treatments reversed the downregulation of ACSL1 by sortilin (Supplementary Fig. 7b, c), but the inhibitors for autophagy (3-MA) and proteasome (MG132) showed no effect on sortilin-mediated ACSL1 degradation (Supplementary Fig. 7d, e). Besides, there was no difference in ubiquitinated ACSL1 in both sortilin-depleted and control adipocytes (Supplementary Fig. 7f). The small GTPase Rab7 is localized in late endosome compartments which is defined as the maturation of endosomes and necessary for the trafficking of cargos in the final fusion step with lysosomes26. Knocking down of Rab7 was able to significantly diminish sortilin depletion-mediated degradation of ACSL1 (Supplementary Fig. 7g). Overall, these findings suggest that sortilin mediates the degradation of mitochondrial ACSL1 in an endolysosomal pathway.
Sortilin depletion promotes white fat browning conferred by inhibitor AF38469
AF38469 is an orally bioavailable inhibitor of sortilin currently used for the treatment of cholesterol reduction in western diet-fed mice27,28. We investigated the effects of AF38469 on white fat both in vitro and in vivo (Fig. 7a). AF38469 treatment did not alter the TG content and the expression of adipogenic genes (Pparγ, Fabp4 and Lep) during adipogenesis in vitro (Supplementary Fig. 8a, b). However, AF38469 treatment caused a significant elevation in the expression of thermogenic genes and OCRs (Supplementary Fig. 8b, c). Mice after AF38469 treatment exhibited an upregulation of ACSL1, p-AMPK and UCP1 in ingWAT (Fig. 7b). When kept at 30 °C, mice with AF38469 treatment displayed similar VO2 as the control mice. When housing temperature decreased to 22 °C, mice with AF38469 treatment showed significantly higher O2 consumption than their littermate controls, while no difference was observed in food intake and locomotor activity between the groups (Fig. 7c and Supplementary Fig. 8d, e). With respect to systematic metabolism, AF38469-treated mice showed significantly lower body weight, adipose tissues weight and fat mass when subjected to a HFD (Fig. 7d–g). In addition, AF38469-treated mice showed improved glucose tolerance and insulin sensitivity relative to the control mice fed HFD (Fig. 7h, i). In summary, sortilin inhibition using commercially available drugs promotes white fat browning and has been shown to be protective against critical metrics to metabolic dysregulation associated with obesity.
a Chemical structure formula of sortilin inhibitor AF38469. b Immunoblots of ACSL1, p-AMPK, and UCP1 in ingWAT of AF38469 or vehicle treated mice. The right panel showed the quantification normalized to α-Tubulin or AMPK. n = 6 per group. c Whole-body VO2 of vehicle or AF38469 treated mice kept at 30 °C or RT (22 °C). Right panel showed the quantification. n = 6 per group. d Representative photo of vehicle or AF38469-treated mice at 12 weeks of HFD. e Body weight of vehicle or AF38469-treated mice fed HFD. n = 6 per group. f Fat tissue weights of vehicle or AF38469-treated mice at 12 weeks of HFD. n = 6 per group. g Body composition (fat/lean mass) of vehicle or AF38469-treated mice at 12 weeks of HFD. n = 6 per group. h, i GTT and ITT of vehicle or AF38469-treated mice at 12 weeks of HFD. n = 6 per group. Statistical significance was assessed by two-tailed Student’s t test). Data were presented as mean ± SEMs. Source data and uncropped blots are available as a Source Data file.
Discussion
Beige fat-mediated thermogenesis has been shown leveraged to protect from hypothermia and is regarded as a promising approach to combat obesity1,29. In this study, we found that sortilin, directly targeted mitochondrial ACSL1 to impair fatty acid oxidation and adipocyte thermogenesis by sending mitochondrial ACSL1 to the endolysosomal pathway for degradation. Beige fat prioritizes fatty acid oxidation for heat generation during chronic cold adaption. Upon cold stimulation, sortilin expression in white adipocytes decreased. Sortilin depletion increased mitochondrial ACSL1 expression and thus activated AMPK signaling to promote white adipocyte browning to sustain thermogenesis. Substrate use and fate in thermogenic fat varies with the conditions of thermogenic challenge to meet the timely metabolic demands. We found sortilin acted as an endosomal effector to regulate and control this process. Besides, genetic depletion of sortilin or administration of the sortilin inhibitor AF38469 to mouse models of diet-induced obesity reduced weight gain and enhanced insulin resistance. These shed light on a way to harness the substrate’s metabolism in thermogenic fat to develop a potential targeted approach for the treatment of metabolic diseases.
The dynamic processes regulating activity of the endosomal pathway control the distribution of more than 5,000 integral membrane proteins-many of which are metabolic transporters, signaling receptors and ligands30. Organelle proteomics showed that the livers of mice fed a HFD undergo a redistribution of their subcellular structures, strongly suggesting intracellular transport changes in disease state such as obesity31. Furthermore, RNA sequencing datasets from rats fed a HFD revealed that genes encoding components of the endosomal membrane were differentially enriched8. In this study, we found the dynamic expression of sortilin during the browning of white fat and sortilin showed as a potential target for obesity therapy. This aligns with previous studies and laterally highlights the pivotal role of sortilin as an endosomal component target for metabolic homeostasis and diseases. Emerging evidence has connected the endosomal system with mitochondria, providing a cellular link between endosomal-mediated uptake of nutrients and their use in mitochondrial respiration7. In this study, we found that sortilin regulated mitochondrial ACSL1 protein abundance to control nutrient utilization and energy expenditure. This finding highlights the bidirectional crosstalk between endosomes and mitochondria. Additionally, we found the small GTPase Rab7 was implicated in the transport of ACSL1 to lysosomes. Rab7, localized in late endosomal compartments, is necessary for the trafficking of cargos to lysosomes26. Several members of the Rab family have also been shown to have therapeutic potential for metabolic homeostasis and diseases. Depletion of Rab8a in hepatocytes of leptin-deficient ob/ob mice strongly reduced liver steatosis and decreased circulating levels of non-esterified fatty acids and triglycerides32. Rab24, a Rab protein associated with late endosomes and autophagy, regulated mitochondrial activity in the liver, thereby influencing liver steatosis and insulin sensitivity33. These data emphasized the importance of Rab members in obesity-related metabolic disease. Future research elucidating the role of other endosomal components, like Rab7 for example, in thermogenesis and obesity-related metabolic disease is warranted.
AMPK in adipocyte is required for cold and β-adrenergic-mediated browning of white fat17. ACSL1 expression has been identified as a key regulator of AMPK activation16,24,25 in both adipocytes and myocytes. Reconciling these findings, in ACSL1 overexpression mouse models, AMPK signaling should be probably activated, which in turn promotes white fat browning. Interestingly, we did not observe an opposing phenotype in ACSL1 deficient mice, which exhibited similar p-AMPK levels relative to control mice under basal conditions. This may be at least partially due to other metabolic intermediates, FBP for example, also acts as an activator for the AMPK signal, because ACSL1 deficient mice downregulate FAO but compensate with enhanced glycolysis15. Critically, ACSL1 deficiency mice still maintain normal thermogenic genes level compared to control mice in this study, which is consistent with previous studies15. However, mice lacking carnitine palmitoyl transferase 2 (CPT2), the ACSL1 activated-enzyme which converts carnitine to fatty acyl-CoA, fail to upregulate thermogenic genes in response to CL-316, 243-induced stimulation in BAT34. This is likely due to decreased carnitine levels, which is necessary to maintain the phenotype and function of BAT35. These data suggest that an accumulation of metabolites from fuels catabolism in mitochondria may link metabolic capacity to nuclear encoded mitochondrial gene expression to regulate adipocyte thermogenesis or even influence the acquisition of mature adipocyte properties.
The severity of the obesity epidemic warrants further aggressive intervention. Currently, six major FDA-approved drugs have been approved as anti-obesity medications36. These drugs can be classified into two types: anorectics to suppress appetite and pancreatic lipase inhibitors to reduce intestinal fat absorption. Unfortunately, most of them have undesirable adverse effects36. Previous studies have implemented cold stimuli or adrenergic signaling activation as a useful way to activate brown and beige fat for obesity treatment37. However, these treatments have limited applications because of various health concerns and potential cardiovascular hazards38,39. In the present study, we demonstrated that sortilin depletion promoted human white adipocyte browning in vitro. Genetic depletion of sortilin or administration of the sortilin inhibitor AF38469 to mouse models of diet-induced obesity reduced body weight and enhanced insulin resistance relative to controls. Of clinical significance, we revealed the association of decreased sortilin abundance in visceral adipose tissues with lower human BMI and improved metabolic traits. This study offers evidence for the efficacy of the small molecule sortilin inhibitor, AF38469, in treating obesity and metabolic diseases.
Overall, the benefits mediated by sortilin inhibition were particularly striking in the context of enhancing adipocyte metabolism. Our work highlights this receptor as a critical negative regulator of adipocyte thermogenesis and offers preliminary evidence that modulating endosomal pathways may be a viable approach to treat metabolic disorders.
Methods
Animals
All animal experiments were performed in accordance with procedures approved by the Ethics Committee of Animal Experiments of Tongji Hospital, affiliated with Huazhong University of Science & Technology. Wild type (WT) C57BL/6 J mice and ACSL1 whole-body knockout mice (Acsl1KO) were purchased from GemPharmatech Co., Ltd. (no. N000013 and no. T014817). Sort1flox/flox mice in the C57BL/6 J background were purchased and made by Cyagen Co., Ltd. (no. S-CKO-05173). In brief, a conditional KO region containing exon 2 and exon 3 of Sort1 gene were amplified and assembled into a targeting vector. The final targeting vector was confirmed by digestion with multiple restriction enzymes and full sequencing, then transformed into C57BL/6 J mice embryonic stem cells by electroporation. Correct embryonic stem cell clones were identified and injected into C57BL/6 J mouse blastocysts. Mice with a targeted deletion of sortilin in adipose tissues (Sort1adipoKO) were generated by crossing the Sort1flox/flox mice with Adiponectin-Cre mice (no. C001186, Cyagen). Littermates expressing no Cre (WT mice) were used as a control group throughout the experiments. Mice were kept at room temperature under a 12-hour light/dark cycle at ambient room temperature (23 ± 1 °C) and 40–50% humidity if not otherwise stated, and were allowed free access to chow and water. For studies with specific diets, 5-week-old C57BL/6 J were given high fat (60% fat, Cat# D12492, Research Diet) diet for a total period of 8-12 weeks to generate HFD-induced obesity mice. For sortilin inhibition studies, mice were given AF38469 (no. HY-12802, MCE) 5-10 μg/day/mouse in their drinking water as previous described40. In general, AF38469 was dissolved at a concentration of 0.4 μg/ml in drinking water. Water was changed weekly. According to the daily water intake of the mice, the estimated intake of AF38469 ranged from 5 to 10 μg/day/mouse. For CL316, 243 (no. C5976, Sigma) treatment, mice received a single intraperitoneal injection or continuous injection for 7 days (1 mg/kg body weight). Mice were euthanized by carbon dioxide (CO2) asphyxiation inhalation, and cervical dislocation was performed as a secondary euthanasia procedure, and then the tissues were isolated.
Human samples
We extracted omental adipose tissues from omentum margin (n = 26) and abdominal subcutaneous adipose tissues (n = 4) from patients undergoing gallstone operation in Tongji Hospital in 2022. The procedure was approved by the Ethical Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (Approval No:TJ-IRB20220975). Subcutaneous fat was located at near right midclavicular line during the operation, where previous study has shown that thermogenic fat depots of human is located in Ref. 1. Informed consent was obtained from each individual and individuals’ clinical biochemical data was taken from medical records. Omental fat was immediately snap frozen in liquid nitrogen and stored at −80 °C until analyses. Fresh subcutaneous fat was used to isolated human SVFs immediately. The age, sex and body mass index of each individual were provided in Supplementary Table 4.
Cell lines
SVF cells derived from mice fat (ingWAT and interscapular BAT) and human abdominal subcutaneous fat were prepared, as previously described41. In general, mouse fat from 8 to 10 weeks old C57BL/6 J male mice and human subcutaneous fat were dissected, minced and digested in Dulbecco’s Modified Eagle Medium (DMEM)-F12 (no. 11320033, Gibco) with 10 mg/mL BSA (no. 9048-46-8, BIU4O) and 1 mg/mL type I collagenase (no. BS163, Biosharp) for 40 min at 37 °C. The digested solution was filtered through 70 μm cell strainers and then centrifuged at 150 g for 5 min. The red blood cells were lysed by red blood cell lysis buffer (no. R1010, Solarbio). Mice SVF cells were suspended in DMEM-F12 with 10% FBS (no. 10099141 C, Gibco) and then immortalized by infecting retrovirus expressing SV40-Large T antigen (ObiO Co., Ltd.) according to the cell immortalization protocol42. Human SVF cells were directly prepared for differentiation. Immortalized SVFs derived from ingWAT were induced to adipogenic differentiation as previously described43. In general, cells were grown to 100% confluency and differentiated with 1 nM T3 (no. T2877, Sigma), 0.5 μM rosiglitazone (no. R2408, Sigma), 5 μg/mL insulin (no. I5523, Sigma), 0.5 mM 3-isobutyl-1-methylxanthine (IBMX) (no. I7018, Sigma), 2 μg/mL dexamethasone (no. D4902, Sigma) and 0.125 mM Indomethacin (no. I7378, Sigma) for two days and subsequently cultured in maintenance medium supplemented with 1 nM T3, 0.5 μM rosiglitazone and 5 μg/mL insulin. The maintenance medium was changed every two days and mature adipocytes were collected on the eighth day. Immortalized SVFs derived from BAT were induced as previously described44. In general, when 100% confluency was reached, SVF cells were treated with 1 nM T3, 1 μM rosiglitazone, 5 μg/mL insulin, 0.5 mM IBMX, 2 μg/mL dexamethasone and 0.125 mM Indomethacin for 2 days. Cells were re-fed every 2 days with 1 μM rosiglitazone, 5 μg/mL insulin and 1 nM T3, and were fully differentiated by day 8 post-induction. For human SVF cells adipogenic differentiation45, cells were induced with 1 μM rosiglitazone and 5 μg/mL insulin. Cells were fully differentiated four weeks after induction until the lipid droplets appeared.
Antibodies
The following antibodies were used in this study: anti-Sortilin (1:2000 diluted, no.12369-1, Proteintech); anti-ACSL1 (1:2000 diluted, no.13989-1, Proteintech); anti-α-Tubulin (1:10000 diluted, no.11224-1, Proteintech); anti-FABP4 (1:10000 diluted, no.12802-1, Proteintech); anti-β-Actin (1:10000 diluted, no.20536-1, Proteintech,); anti-TOM20 (1:2000 diluted, ET1609-25, HUABIO); anti-UCP1 (1:1000 diluted, ab234430, Abcam); Rab7 (1:2000 diluted, No.55469-1, Proteintech); AMPK (1:1000 diluted, no.2532, CST); p-AMPK-Thr172 (1:1000 diluted, no.2531, CST); p38MAPK (1:1000 diluted, no.9212, CST); p-p38MAPK-Thr180/Tyr182 (1:1000 diluted, no.4511, CST); HSL (1:1000 diluted, no.A15686, ABclonal), p-HSL-Ser660 (1:1000 diluted, no.AP1432, ABclonal); PGC1α (1:1000 diluted, no.A12348, ABclonal). For western blot, all the primary antibodies were diluted in 5% non-fat milk in Tris-buffered saline and Tween 20 (TBST). Secondary antibodies were used at a 1:5000 dilution in 5% non-fat milk in TBST.
Immunofluorescence
Cells cultured on glass slides were fixed in 4% paraformaldehyde for 15 min. After washed three times with PBS, cells were permeabilized with 0.1% Triton X-100 (Sigma-Aldrich, T9284-100ML) in PBS for 10 min, and blocked in 5% BSA for 30 min at room temperature. Cells were then stained with primary antibodies against sortilin (1:400 diluted, No.12369-1, Proteintech), ACSL1 (1:100 diluted, No.13989-1, Proteintech), LAMP1 (1:100 diluted, ab208943, Abcam), EEA1 (1:1000 diluted, No. 28347-1, Proteintech) or TOM20 (1:1000 diluted, ET1609-25, HUABIO) overnight at 4 °C. Cells were washed and incubated with secondary antibodies for 1 h at room temperature. DAPI was used to visualize the nuclei. Images were taken by Leica confocal microscope and signal intensity was processed for the quantification of colocalization factors (Pearson’s R value) by ImageJ.
Immunoprecipitation
Pierce Classic Magnetic IP/Co-IP Kit (88804, Thermo Scientific) was used for sortilin/ACSL1 immunoprecipitation. In brief, fresh cells were washed by PBS for three times and lysed with NP-40 lysis buffer (AR0107, BOSTER, China). Cell lysates were scraped and collected into one vial. And then cell lysates were centrifuged (14,000 g at 4 °C for 2 min) to remove debris. The supernatant protein sample (500 µg) was immunoprecipitated with 10 µg anti-sortilin antibody (No. 12369-1, Proteintech) or anti-ACSL1 antibody (No. 13989-1, Proteintech) overnight at 4 °C. Subsequently, 20-40 µL of fully suspended protein A/G magnetic beads were added and incubated at room temperature for 1 h. The samples were washed extensively six times and subjected to liquid chromatography-tandem mass spectrometry (LC/MS-MS) analysis by BGI Co. Ltd (Shenzhen, China) or resolved in the SDS-PAGE loading buffer and subjected to western blotting.
Western blotting
Cells or tissues were lysed in RIPA buffer (B1025, BIU4O) with protease inhibitor cocktail (HY-K0010, MCE) and phosphatase inhibitor cocktails (HY-K0021 and HY-K0021, MCE). After centrifuging the lysates at 14,000 g for 15 min, the supernatant was used for protein quantification by BCA protein assay kits (AR0197, BOSTER, China). Each protein sample was subjected to SDS-PAGE (50–100 μg) and transferred to PVDF. Then, the corresponding primary and secondary antibodies were incubated to visualize the protein. Blots were imaged and processed with Gelview 6000 Pro (BLT Technology, China). Western blots were quantified by densitometry analysis using ImageJ software and with quantification.
Isolation of mitochondrial and cytosolic fractions
Mitochondrial and cytosolic fractions were isolated via differential centrifugation using the Mitochondria Isolation and Protein Extraction Kit (PK10016, Proteintech) according the industrialized instructions. All fractionation steps were conducted at 4 °C. In brief, the cells sample were homogenized in solution A, and the homogenate was added gently to the top layer of an equal volume of solution B. The mixture was centrifuged at 600 g for 10 min to remove nuclei and unbroken cells. The supernatant was collected and further centrifuged at 10,000 g for 10 min. After centrifugation, the supernatant was transferred to another centrifuge tube and designated as the cytoplasm fraction. Whereas, the pellet was resuspended in a lysis buffer and used as the crude mitochondrial fraction.
Cycloheximide assay
Cells were treated with cycloheximide (no. HY-12320, MCE) at a final concentration of 20 μg/mL. Cells were collected at the indicated time points. Cell lysates were analyzed by immunoblotting for ACSL1. The intensity of ACSL1-specific protein expression was quantified by ImageJ software and normalized to that of β-Actin signals.
In vitro compound treatment
To investigate the effect of the compounds, cells were treated with vehicle or the candidate compounds. For the inhibitors’ treatment, cells were pre-incubated with AMPK inhibitor compound C (CC, 10 μM, no. HY-13418A, MCE); the lipase inhibitors, including ATGL inhibitor Atglistatin (10 μM, no. HY-15859, MCE) and HSL inhibitor CAY10499 (20 μM, no. HY-119283, MCE); p38 MAPK inhibitors SB203580 (10 μM, no. HY-10256, MCE) and SB202190 (5 μM, no. HY-10295, MCE); lysosomal inhibitors NH4Cl (250 μM, no. HY-Y1269, MCE) and chloroquine (20 μM, no. HY-17589A, MCE); autophagy inhibitor 3-MA (20 μM, no. HY-19312, MCE) and proteasome inhibitor MG132 (20 μM, no. HY-13259, MCE). For AMPK-DN plasmids transfection, cells were transfected with plasmids expressing AMPK-DN (purchased from ObiO Biotechnology) by using Lipofectamine 3000 Transfection Reagent (no. L3000008, Invitrogen/Life Technologies) following the manufacturer’s instructions.
Adeno-associated virus (AAV) production and delivery
AAV vector-mediated overexpression of mouse Acsl1 active form and shRNA targeting Acsl1 were designed and synthesized by ObiO Co., Ltd. (Shanghai, China); shRNA targeting Sort1 was prepared from Genechem Co., Ltd. (Shanghai, China). For local adenovirus infection, mice were anesthetized with Isoflurane. A total of 1 × 109 Vg/μL of shSort1-AAV or Acsl1 active-AAV and scrambled controls (total volume 20 μL) were slowly injected into inguinal fat pads of mice with a 10 μL-Hamilton syringe13. For whole body delivery, a total of 1 × 1011 Vg/μL of shAcsl1-AAV (total volume 100 μL) and scrambled controls were injected by tail vein.
shRNA construction and virus infection
The lentiviral shRNA clone was designed and synthesized by Genechem Co., Ltd. (Shanghai, China). The shRNA sequences targeting Sort1 were as follows: 5′- ACCTGACAACAAATGGGTA-3′. For shRNA virus infection, SVF cells were incubated at 50–60% confluence with the virus (MOI 40 according the industrialized instructions) supplemented with 10 μg/mL polybrene for 24 h. Subsequently, stable cell lines were obtained by selection with the puromycin (Gibco) at 1 μg/mL.
siRNA transfection
All siRNAs were designed and synthesized by Servicebio Co., Ltd. Cells were transfected with siRNA using Lipofectamine 3000 Transfection Reagent (no. L3000008, Invitrogen/Life Technologies) according to the manufacturer’s instructions. The optimal final concentration of siRNA was 20 nM, and the protein expression analysis was determined by immunoblotting after 48 h. Nonspecific siRNA was used as a control. siRNA target sequences are listed in Supplementary Table 3.
Metabolic analysis in mice
Oxygen consumption, food intake, total locomotor activity and RER were measured in Comprehensive Lab Animal Monitoring System (CLAMS, Columbus Instruments). Mice were housed in metabolic cage system with ad libitum access to food and water. The lean/fat mass composition of mice was measured using the Echo-magnetic resonance imaging (MRI) Composition Analyzer (Echo Medical System). Adipose tissue volume imaging in vivo was scanned using Bruker Micro-CT Skyscan 1276 system (Kontich, Belgium). Density measurements were calibrated to the manufacturer’s calcium hydroxyapatite (CaHA) phantom. Analysis was performed using the manufacturer’s evaluation software. Reconstruction was accomplished by NRecon (version 1.7.4.2). 3D images were obtained from contoured 2D images by methods based on distance transformation of the grayscale original images (CTvox; version 3.3.0). 3D and 2D analysis were performed using software CT Analyzer (version 1.20.3.0). For core-body temperature recording experiments, rectal temperature was monitored by thermometer for indicated hours after cold exposure. For thermal image of BAT, thermal imaging photos were taken using an infrared thermal camera (T1010, FLIR). For glucose tolerance test, mice were fasted 6 h and injected intraperitoneally with a glucose solution in saline (1.25 g/kg body weight). For insulin tolerance test, mice received an intraperitoneal injection of insulin at 1.25 U/kg body weight. Blood glucose levels were measured at 0 min, 15 min, 30 min, 60 min, 90 min and 120 min after injection with an automated reader (Bayer).
Cell triglyceride (TG), serum lipid and liver triglyceride analysis
Cellular TG levels were measured via commercially available TG ELISA kits. TG content was normalized to total cellular protein. Serum parameters were measured via commercially available ELISA kits including serum TG, total cholesterol (TC), high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL) and free fatty acids (FFA) (no. A110-1-1, no. A111-1-1, no. A112-1-1, no. A113-1-1 and no. A042-2-1, NJBI). To measure liver triglyceride contents, liver tissues were homogenized with NP-40 lysis buffer. Tissue lysates were centrifuged, and the supernatant liquid were obtained. The triglyceride levels were determined by TG kit and normalized to liver protein content using BCA protein assay kit.
Oil Red O staining
Cells were fixed with 4% paraformaldehyde for 15 min and then washed twice with PBS. After rinsed with 60% isopropanol, cells were stained with a filtered Oil Red O working solution (no. G1015, Servicebio) for 20 min at room temperature. Cells were washed twice with PBS and then for photograph.
Histological and adipocytes size quantitative analysis
Dissected adipose and liver tissues were fixed in 4% paraformaldehyde and embedded in paraffin. 5 μm tissue sections were stained with Hematoxylin and Eosin (H&E) according to manufacturer’s instructions. The images were acquired by optical microscope and adipocytes size was measured by ImageJ (approximately 100 adipocytes per slide).
Coomassie brilliant blue staining
Protein samples were mixed with loading buffer and boiled for 5 min. After separation in a 12% SDS-PAGE gel, Coomassie Blue staining (no. P1305, Solarbio) was used to visualize the protein.
OCR assays
Oxygen consumption rate (OCR) was measured using an Agilent Seahorse XFe24 Analyzer and Cell Mito Stress Test Kit (Agilent). In brief, SVF cells were seeded onto an XFe24 cell culture microplate and differentiated into mature adipocytes. Cells at day 8 of differentiation were equilibrated in XF DMEM for 1 h in a 37 °C incubator without CO2. The XFe24 plate was then transferred to the Seahorse XFe24 analyzer to collect data. To determine ATP production and spare respiratory capacity, oligomycin (final concentration: 2 μM), carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP, final concentration: 2 μM), and rotenone/actinomycin A (final concentration: 1 μM) were then sequentially added into the microplate by automatic pneumatic injection, followed by the measurement of OCR. Total proteins of each well were extracted and quantified after OCR measurement. Data were analyzed using Seahorse Wave Desktop Software (Agilent).
Targeted metabolites by LC-MS/MS
Approximately 100 mg of frozen adipose tissue samples were extracted in 1 mL of ice-cold methanol/acetonitrile/water (2:2:1, v/v/v). The samples were sonicated for 30 min at ice and repeat. They were incubated at −20 °C for 1 h to precipitate protein. After centrifugation (10 min, 12,000 rpm, 4 °C), the supernatant was transferred to clean plastic microtubes, and then vacuum drying. For UHPLC-ESI-MS/MS analysis, the samples were dissolved in 200 μL of 50% ACN (vol/vol) and transferred to insert-equipped vials. The sample extracts were analyzed using an UPLC-Orbitrap-MS system (UPLC, Vanquish; MS, QE). Data were acquired on the Q-Exactive using Xcalibur 4.1 (Thermo Scientific), and processed using TraceFinder 4.1 Clinical (Thermo Scientific). Quantified data were output into excel format.
RNA sequencing
For high-throughput sequencing analysis of adipocytes and adipose tissues, total RNA was isolated using TRI Reagent (no. T9424, Sigma) and the quality of the extracted total RNA samples was examined with RNA 6000 Nano kit (no. 5067-1511, Agilent). The samples were sent to MGI Tech Co., Ltd. (Shenzhen, China) for mRNA sequencing. Gene expression analysis was performed by mRNA sequencing on a BGISEQ500 (MGI Tech Co.).
RNA extraction and quantitative PCR
Total RNA was extracted from cells or tissues with RNAiso Plus, and 1 μg total RNA was reverse transcribed to cDNA using the HiScript II Q RT SuperMix (no. R222-01, Vazyme, Nanjing, China) according to the manufacturers’ instructions. Quantitative Real-time PCR was performed using the ChamQ Universal SYBR qPCR Master Mix (no. Q711-02, Vazyme) on the Thermo Fisher Scientific QuantStudio 1. Experiments were repeated three times and gene expression levels were calculated using the delta Ct method after normalization to 36B4 or 18S rRNA expression. Sequences of primers used for real-time PCR are listed in Supplementary Table 2.
Quantification and statistical analysis
Statistical analyses were performed with Prism software version 8.4.3 (GraphPad Software). All experiments were replicated at least three independent times. Averaged values were shown as mean ± SEMs. Comparisons of two groups were made by conducting a two-tailed Student’s t-test. The statistical methods of each experiment were indicated in the figure legends.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All methods and data supporting the findings are available within the manuscript or supplementary information. The RNA-seq data generated in this study have been deposited in the National Center for Biotechnology Information Sequence Read Archive database under accession code PRJNA114989. The protein mass spectrometry raw data has been deposited in ProteomeXchange partner repository under accession code PXD055014. Source data are provided with this paper in Source Data file. Uncropped blots are available in Source Data file. Source data are provided with this paper.
References
Cohen, P. & Kajimura, S. The cellular and functional complexity of thermogenic fat. Nat. Rev. Mol. Cell Biol. 22, 393–409 (2021).
Betz, M. J. & Enerbäck, S. J. D. Human brown adipose tissue: what we have learned so far. Diabetes 64, 2352–2360 (2015).
Kajimura, S., Spiegelman, BruceM. & Seale, P. Brown and Beige Fat: Physiological Roles beyond Heat Generation. Cell Metab. 22, 546–559 (2015).
Sponton, C. H., de Lima-Junior, J. C. & Leiria, L. O. What puts the heat on thermogenic fat: metabolism of fuel substrates. Trends Endocrinol. Metab. 33, 587–599 (2022).
Chouchani, E. T. & Kajimura, S. Metabolic adaptation and maladaptation in adipose tissue. Nat. Metab. 1, 189–200 (2019).
Morigny, P., Boucher, J., Arner, P. & Langin, D. Lipid and glucose metabolism in white adipocytes: pathways, dysfunction and therapeutics. Nat. Rev. Endocrinol. 17, 276–295 (2021).
Gilleron, J. & Zeigerer, A. Endosomal trafficking in metabolic homeostasis and diseases. Nat. Rev. Endocrinol. 19, 28–45 (2023).
Xue, B. et al. Effects of High Fat Feeding on Adipose Tissue Gene Expression in Diabetic Goto-Kakizaki Rats. Gene Regul. Syst. Bio 9, 15–26 (2015).
Braulke, T. & Bonifacino, J. S. Sorting of lysosomal proteins. Biochim Biophys. Acta 1793, 605–614 (2009).
Kjolby, M. et al. Sort1, Encoded by the Cardiovascular Risk Locus 1p13.3, Is a Regulator of Hepatic Lipoprotein Export. Cell Metab. 12, 213–223 (2010).
Gustafsen, C. et al. The hypercholesterolemia-risk gene SORT1 facilitates PCSK9 secretion. Cell Metab. 19, 310–318 (2014).
Ouyang, S. et al. Mechanism underlying the regulation of sortilin expression and its trafficking function. J. Cell Physiol. 235, 8958–8971 (2020).
Li, J. et al. Neurotensin is an anti-thermogenic peptide produced by lymphatic endothelial cells. Cell Metab. 33, 1449–1465. e1446 (2021).
Ji, L. et al. AKAP1 Deficiency Attenuates Diet-Induced Obesity and Insulin Resistance by Promoting Fatty Acid Oxidation and Thermogenesis in Brown Adipocytes. Adv. Sci. (Weinh.) 8, 2002794 (2021).
Ellis, J. M. et al. Adipose acyl-CoA synthetase-1 directs fatty acids toward β-oxidation and is required for cold thermogenesis. Cell Metab. 12, 53–64 (2010).
Liu, Q., Gauthier, M. S., Sun, L., Ruderman, N. & Lodish, H. Activation of AMP-activated protein kinase signaling pathway by adiponectin and insulin in mouse adipocytes: requirement of acyl-CoA synthetases FATP1 and Acsl1 and association with an elevation in AMP/ATP ratio. FASEB J. 24, 4229–4239 (2010).
Mottillo, E. P. et al. Lack of Adipocyte AMPK Exacerbates Insulin Resistance and Hepatic Steatosis through Brown and Beige Adipose Tissue Function. Cell Metab. 24, 118–129 (2016).
Yan, M. et al. Chronic AMPK activation via loss of FLCN induces functional beige adipose tissue through PGC-1alpha/ERRalpha. Genes Dev. 30, 1034–1046 (2016).
Rosenwald, M., Perdikari, A., Rulicke, T. & Wolfrum, C. Bi-directional interconversion of brite and white adipocytes. Nat. Cell Biol. 15, 659–667 (2013).
Wang, Q. et al. IL-27 signalling promotes adipocyte thermogenesis and energy expenditure. Nature 600, 314–318 (2021).
Sveidahl Johansen, O. et al. Lipolysis drives expression of the constitutively active receptor GPR3 to induce adipose thermogenesis. Cell 184, 3502–3518 e3533 (2021).
Nielsen, M. S. et al. The sortilin cytoplasmic tail conveys Golgi-endosome transport and binds the VHS domain of the GGA2 sorting protein. EMBO J. 20, 2180–2190 (2001).
Mari, M. et al. SNX1 defines an early endosomal recycling exit for sortilin and mannose 6-phosphate receptors. Traffic 9, 380–393 (2008).
Lobo, S., Wiczer, B. M. & Bernlohr, D. A. Functional analysis of long-chain acyl-CoA synthetase 1 in 3T3-L1 adipocytes. J. Biol. Chem. 284, 18347–18356 (2009).
Goldenberg, J. R. et al. Preservation of Acyl Coenzyme A Attenuates Pathological and Metabolic Cardiac Remodeling Through Selective Lipid Trafficking. Circulation 139, 2765–2777 (2019).
Vitelli, R. et al. Role of the small GTPase Rab7 in the late endocytic pathway. J. Biol. Chem. 272, 4391–4397 (1997).
Schrøder, T. J. et al. The identification of AF38469: an orally bioavailable inhibitor of the VPS10P family sorting receptor Sortilin. Bioorg. Med Chem. Lett. 24, 177–180 (2014).
Chen, C., Li, J., Matye, D. J., Wang, Y. & Li, T. Hepatocyte sortilin 1 knockout and treatment with a sortilin 1 inhibitor reduced plasma cholesterol in Western diet-fed mice. J. Lipid Res. 60, 539–549 (2019).
Lee, P., Werner, C., Kebebew, E. & Celi, F. J. I. j. o. o. Functional thermogenic beige adipogenesis is inducible in human neck fat. Int J. Obes. (Lond.) 38, 170–176 (2014).
Cullen, P. J. & Steinberg, F. To degrade or not to degrade: mechanisms and significance of endocytic recycling. Nat. Rev. Mol. Cell Biol. 19, 679–696 (2018).
Krahmer, N. et al. Organellar Proteomics and Phospho-Proteomics Reveal Subcellular Reorganization in Diet-Induced Hepatic Steatosis. Dev. Cell 47, 205–221 e207 (2018).
Wu, L. et al. Rab8a-AS160-MSS4 regulatory circuit controls lipid droplet fusion and growth. Dev. Cell 30, 378–393 (2014).
Seitz, S. et al. Hepatic Rab24 controls blood glucose homeostasis via improving mitochondrial plasticity. Nat. Metab. 1, 1009–1026 (2019).
Lee, J., Ellis, J. M. & Wolfgang, M. J. Adipose fatty acid oxidation is required for thermogenesis and potentiates oxidative stress-induced inflammation. Cell Rep. 10, 266–279 (2015).
Ozaki, K., Sano, T., Tsuji, N., Matsuura, T. & Narama, I. Carnitine is necessary to maintain the phenotype and function of brown adipose tissue. Lab Invest. 91, 704–710 (2011).
Srivastava, G. & Apovian, C. M. J. N. R. E. Current pharmacotherapy for obesity. Nat. Rev. Endocrinol. 14, 12–24 (2018).
Harms, M. & Seale, P. J. N. m. Brown and beige fat: development, function and therapeutic potential. Nat. Med. 19, 1252–1263 (2013).
Larsen, T. M. et al. Effect of a 28-d treatment with L-796568, a novel β3-adrenergic receptor agonist, on energy expenditure and body composition in obese men. Am. J. Clin. Nutr. 76, 780–788 (2002).
Bhadada, S. V. et al. β3 receptors: Role in cardiometabolic disorders. Ther. Adv. Endocrinol. Metab. 2, 65–79 (2011). & Metabolism.
Rhost, S. et al. Sortilin inhibition limits secretion-induced progranulin-dependent breast cancer progression and cancer stem cell expansion. Breast Cancer Res. 20, 1–15 (2018).
Kajimura, S., Ruiz, L. & Liisberg Aune, U. Isolation and Differentiation of Stromal Vascular Cells to Beige/Brite Cells. J. Visualized Exp. 28, 50191 (2013).
Shinoda, K. et al. Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat. Med 21, 389–394 (2015).
Altshuler-Keylin, S. et al. Beige adipocyte maintenance is regulated by autophagy-induced mitochondrial clearance. cell Metab. 24, 402–419 (2016).
Yoneshiro, T. et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature 572, 614–619 (2019).
Chen, S. et al. The phytochemical hyperforin triggers thermogenesis in adipose tissue via a Dlat-AMPK signaling axis to curb obesity. Cell Metab. 33, 565–580.e567 (2021).
Acknowledgements
We thank all members of the Division of Endocrinology, Tongji hospital. We also thank all patients for donating the research samples. This work was supported by grants from the National Natural Science Foundation of China (82350610277, 82070859 to Y.C., 82270910 to Y.C., R.P.P. and D.P.L.), and the National Key R&D Program of China (2022YFA0806100 to Y.C.).
Author information
Authors and Affiliations
Contributions
M.Y. and Y.C. conceived the study and designed the experiments. Y.C. supervised the study. M.Y. and J.G. conducted the experiments and analyzed the data. Y.L.L., H.Y.W., Z.H.W., D.P.L., and R.H. assisted with animal euthanization and tissue sample collection. Y.Y.X., H.Y.D., and X.M.P. contributed analytic tools. W.S.W., J.D.L., Z.Z.Z., and X.F.Y provided materials and samples for experiments in vitro. M.Y. and Y. C. wrote the manuscript. Y. C., R.P.P., P.M., and S.K. edited the manuscript. All authors read and approved the revised manuscript. Y.C. supervised and led this work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Dominique Langin, Karen Reue and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, M., Ge, J., Liu, YL. et al. Sortilin-mediated translocation of mitochondrial ACSL1 impairs adipocyte thermogenesis and energy expenditure in male mice. Nat Commun 15, 7746 (2024). https://doi.org/10.1038/s41467-024-52218-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-52218-4
- Springer Nature Limited