Abstract
Cells harvest energy from ionic gradients by selective ion transport across membranes, and the same principle is recently being used for osmotic power generation from salinity gradients at ocean-river interfaces. Common to these ionic gradient conversions is that they require intricate nanoscale structures. Here, we show that natural submarine serpentinite-hosted hydrothermal vent (HV) precipitates are capable of converting ionic gradients into electrochemical energy by selective transport of Na+, K+, H+, and Cl-. Layered hydroxide nanocrystals are aligned radially outwards from the HV fluid channels, constituting confined nanopores that span millimeters in the HV wall. The nanopores change the surface charge depending on adsorbed ions, allowing the mineral to function as a cation- and anion-selective ion transport membrane. Our findings indicate that chemical disequilibria originating from flow and concentration gradients in geologic environments generate confined nanospaces which enable the spontaneous establishment of osmotic energy conversion.
Similar content being viewed by others
Introduction
Gradients such as temperature, pressure, redox, and concentration are ubiquitous in nature. The conversion of these gradients into usable energy is a key process in both biology and industry. In nature, cells interconvert electrochemical, ion, and chemical gradients using respiratory protein complexes embedded in a membrane1,2. In synthetic chemistry, ionic gradients are recently also being harnessed as energy sources using two-dimensional materials and confined nanostructures3,4,5,6,7,8,9.
Submarine HVs are fascinating geologic systems surrounded by steep, long-lasting gradients and have been hypothesized to function as natural energy converters that could lead to the formation of the first cells10,11,12,13,14,15,16,17,18,19,20,21,22,23. HVs are formed by mineralization chemistry when the hot, reduced ion-rich vent fluid solutions are injected into cooler, oxidizing seawater, and have been discovered as arising from a diverse range of geologic activity24 – for example, along mid-ocean ridges25,26 (on-axis) and away from spreading centers27,28 (off-axis). Off-axis HVs are mainly formed by the serpentinization process, in which the hydrolysis of olivine ((Mg,Fe)2SiO4) generates alkaline fluids with diverse ion compositions17,29,30. Subsequent mixing of alkaline fluids with seawater produces structurally highly complex, porous precipitates rich in layered metal oxides, hydroxides, and carbonates that act as a diffusion barrier to maintain steep gradients between the two solutions28.
Here, we report the first detailed insights into the nanoscale structure and energy conversion functions of off-axis, serpentinite-hosted, HV precipitates. A combined analysis of synchrotron X-ray diffraction, optical, and electrical measurements revealed that the HV precipitate contains interconnected nanopores with variable surface charges, allowing it to convert diverse ionic gradients into electrical energy – a process called osmotic energy harvesting. Selective ion transport is critical for osmotic energy conversion in cells1,2 and energy utilization in engineering fields3,4,5,6,7,8,9. The spontaneous establishment of nano- to millimeter-scale ion transport channels discovered in geologic environments suggests an evolutionary relevance between HVs and cells12,13,14,15,16,17,18,19 and showcases a geomimetic approach toward material self-organization.
Results
Structure of HVs with nanopores
The HV precipitates examined here (Fig. 1a) were collected from the Shinkai Seep Field (SSF), which is located in the southern Mariana Trench, Pacific Ocean, at a depth of 5743 m (11°39.38′N, 143°2.73′E)31. The geochemistry in the SSF is primarily related to serpentinization, a process that forms serpentine from olivine. During the initial stages of HV formation in the SSF, a brucite-rich white chimney forms. In time, the mineral composition of the HV precipitates changes from predominantly brucite to carbonate-rich as the discharge fluid activity diminishes31. The detailed mineralogy of SSF HVs was described in our previous publication31. Briefly, the mineralogy varies with vent fluid flow and is classified into types I, II, and III. In this study, a Type I sample of approximately 84 cm in height was analyzed, which contains brucite as the major component (~ 80%), with calcium carbonate and magnesium carbonate as minor components (Supplementary Figs. 1, 2).
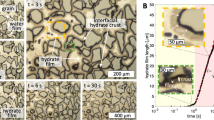
a Photograph of HV precipitates collected from the Shinkai Seep Field. b, c Cross-polarized optical microscope images of vertical (b) and horizontal slices of HV (cross-section, c). In the horizontal slice image, vent fluid channels and periodic line patterns in the HV wall are indicated as yellow asterisks and red arrows, respectively. d–g SEM images of a naturally cleaved HV fragment. d, e Inorganic walls with different cleavage directions. The walls show stratified structures, with the layers following the shape of the wall. f Magnification of the wall (boxed area in e) revealing a sublayer structure with consolidated features. g Sublayer structures in different locations of walls. Sublayers 2 to 5-µm thick (yellow square brackets) constituted the wall and exhibited a uniaxial texture. The uniaxial features result from the array of multiple nanocrystals shown in the inset. h TEM image of the crystals constituting the sublayers. i HAADF-STEM image of the crystal with nanopores visible inside.
Cross-polarized optical microscopy (POM; Fig. 1b, c) revealed a complex internal structure comprised of porous columnar structures that served as channels for discharging the vent fluid. The fluid channels were separated by 200 ~ 400-µm thick inorganic walls that were mainly composed of brucite (Mg(OH)2), a layered hydroxide crystal, (Fig. 1c and Supplementary Figs. 1, 2) and exhibited a periodic lamellar structure with a wall thickness of ~ 10 μm (Fig. 1c (red arrows), d, e). The sublayers of this structure consisted of consolidated plate-like brucite nanocrystals with a diameter of ~ 100 nm that were perpendicularly aligned to the surface of the channel (Fig. 1f–h). In an enlarged high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) image (Fig. 1i), individual crystals contained porous nanostructures (2 ~ 3 nm) that appeared as local density variations. The stacking of layered nanocrystals at multiple scales resulted in the formation of interconnected nanopores (Supplementary Figs. 3–5). Nitrogen adsorption-desorption isotherm analysis identified open-end slit-like nanopores (H3-type hysteresis) in the inorganic walls with a various pore size ranging from 2 to 100 nm (Supplementary Fig. 6).
Alignment of brucite across the macro scale
The alignment of the brucite nanocrystals within the HV precipitates was further analyzed using synchrotron radiation X-ray diffraction (wide-angle X-ray scattering (WAXS)) scanning. Multiple areas of the horizontal slice of HV samples were scanned using a 5-μm X-ray beam, and a scattering intensity map was constructed from the total intensity of the diffractograms (Fig. 2a). Similar to that seen in the POM and SEM images, a band-like structure was observed, due to the periodic density changes of the brucite crystals within the HV wall (Fig. 2a and Supplementary Fig. 7a, b). Pronounced anisotropy of brucite 001 diffraction was detected, indicating that the (001) plane of brucite orients preferentially inside the HV wall (Fig. 2b, c and Supplementary Fig. 7c, d). The directionality of the preferential orientation of brucite (001) planes varied depending on the locations (Fig. 2b, c). A schematic of the layered (001) plane of brucite with the corresponding c-axis was shown below each WAXS pattern (Fig. 2c, bottom).
a An optical image of an HV slice (above) and its corresponding 3D map of scattering intensity (below). In the 3D intensity map, the scattering intensity at individual scanning points was integrated and converted into single values. b–e WAXS analysis on the HV wall sample. b Optical image of the scanning area. c Selected brucite 001 diffraction patterns at multiple points (red squares in b). Black double-headed arrows show the preferred orientation direction of the brucite (001) plane. Schematic images showing the preferred orientation of the brucite layers are shown below the diffraction patterns. The crystal structure of brucite consists of an edge-shared MgO6 layer in the (001) plane and a layered hydroxide stack along the c-axis. d Vector plots showing the direction and intensity of the preferred orientation of the (001) plane. The diffraction patterns at two different locations (line scans) were analyzed and plotted. The black lines in (b) show the location of the line scan. The color and length of the vectors represent the value of the highest scattering intensity of the asymmetric ring. The direction of the vector indicates the orientation of the strongest intensity axis of the asymmetric ring and shows the preferred orientation direction. e 2D map of the vector plot overlayed on the optical image shown in (b). f Schematic of the brucite (001) plane arrangement around the channel based on the WAXS/SAXS scanning and POM analysis. The corresponding c-axis of brucite is also shown.
We next extracted the diffraction intensities of the anisotropic rings and azimuthal angles of the highest intensity in the WAXS data and created a two-dimensional vector field map (Fig. 2d, e). The vector directions indicate the preferred orientation direction of the (001) plane along the [001] direction or c-axis, and the vector colors and lengths represent the relative amount of plane alignment. For example, the ring patterns of 3 and 4 (Fig. 2c) are represented by a red vector in the top right and a green vector in the bottom right, respectively. Notably, over the entire scanned area, the vector plot shows a continuous change in the direction of preferred orientation, suggesting that the brucite layers are arranged in a concentric manner radiating outwards from the fluid channel (Fig. 2e, f). The alignment of brucite nanocrystals along the channel continues throughout the entire sample, even when the wall structure bends, diverges, or converges (Fig. 2e and Supplementary Fig. 8a, b). The crystal alignment was also observed in the vertical HV slices (Supplementary Figs. 9, 10, and Supplementary Notes).
WAXS mapping using a larger, 100-μm X-ray beam size further confirmed that the continuous alignment of the nanocrystals in the walls extended to millimeter-scale (2.5 × 6.0 mm; Fig. 3a, b). Such long-range alignment of the brucite nanocrystals was corroborated by POM analysis conducted with a retardation plate (first order, 530 nm; Fig. 3c). The yellow and blue interference colors alternating along the angular direction of the inorganic wall represent the radial configuration of crystals surrounding the channel and are consistent with the schematic illustration presented in Fig. 2f.
a Optical image of a large HV wall sample area with overlaid vector plots generated from a single scan direction and plotted as white arrows. For the large-area scanning, a 100-μm beam was used. b Color map and vector plots for the entire sample area shown in (a). Based on the vector values from the plots, a color map was created. The angle (direction of alignment) and length (degree of preferred orientation) of the arrows are represented as the color hue and saturation, respectively. A color wheel representing the orientation-hue relationship is included on the bottom right. Similar contours in the HV sample wall and the constructed color map indicate the continuous alignment of brucite crystals in the inorganic wall. To clearly display the arrows, the width has been expanded from the original image. c A cross-polarized optical microscope image of a horizontal HV slice was obtained with a retardation plate (first order, 530 nm). The double-headed arrow indicates the slow axis of the retardation plate. A yellow subtractive interference color is produced as the c-axis of brucite (+ birefringence) is aligned perpendicular to the slow axis of the plate, and the blue additive color is displayed when the c-axis is parallel to the slow axis of the plate. The yellow and blue interference colors are dependent on the radial direction of the wall and indicate that the crystals form a radiating configuration. The c-axis of the crystals is shown in the image.
POM and WAXS analysis were also performed for the samples collected from multiple locations in the Type I sample. Thin sections were made from different parts of HV (the upper (35 sections), middle (10 sections), and lower (20 sections)). Essentially the same POM interference colors and anisotropic ring patterns were observed in all thin sections sampled from locations ranging from 26 cm to 84 cm apart (Supplementary Figs. 11, 12), confirming that the alignment observed above extends to almost entire parts of Type I HV. Small-angle X-ray scattering (SAXS) measurements of the HV wall showed ellipsoidal SAXS patterns attributable to nanoscale periodicity (Supplementary Fig. 13). Material characterization based on SEM, TEM, nitrogen adsorption-desorption isotherm, and WAXS/SAXS demonstrates the anisotropic alignment and multilayer stacking of plate-like brucite nanocrystals collectively, forming interconnected nanopores referred to as nanochannel inside the HV walls (Fig. 2f and Supplementary Figs. 3–6).
Ion adsorption and surface charge of HV minerals
In addition to the continuous alignment of nanocrystals in HV minerals (Figs. 1–3), the pores show variable surface charges depending on adsorbed ions. Brucite crystals rich in carbonate ions were detected at several locations in the inorganic HV wall (Fig. 4b, red spectrum; Fig. 4c, blue Raman map). In addition, crystals with different refractive indices were also observed on the surface at these locations (Fig. 4d) and were identified by Raman and X-ray diffraction mapping as Mg-carbonate crystals (Fig. 4a–c and Supplementary Fig. 14). The existence of both brucite with carbonate ions and Mg-carbonate crystals indicates that the interconnected HV walls are exposed to various inorganic ions that are present in the hydrothermal fluid and seawater. As brucite is able to adsorb a wide variety of inorganic ions32,33, the surface charge of HV precipitates can significantly change depending on the composition of the surrounding fluids (Fig. 4e, f). Indeed, Raman analysis of natural HV samples confirmed that brucite, which is the main constituent mineral of HVs, adsorbs K+, Ca2+, CO32-, NO3-, and SO42- (Fig. 4e). Furthermore, the zeta potential of the HV samples varied greatly depending on the type of adsorbed ion, from − 30 mV (CO32-) to + 25 mV (Ca2+), indicating that the electrostatic properties of the HV surface are markedly influenced by the interaction with inorganic ions present in the environment (Fig. 4f).
a–c Raman measurement of the HV wall near a fluid channel. a Overlay of the Raman map with the corresponding SEM image. The Raman map is a superimposed image of three specific peaks. The Raman map shows that the brucite and Mg-carbonate crystals are discretely located in the wall, while carbonate ions were present in all regions, indicating the adsorption of carbonate ions by the brucite. b Raman spectra of surface crystals (blue) and HV wall (red) at the positions shown in (a, c). c Map of three specific Raman peaks, red: 442.6 cm−1 (brucite lattice vibration, A1g, width 47.5 cm−1), blue: 1109.7 cm−1 (carbonate, width 73.4 cm−1), and green: 3420.7 cm−1 (interlayered water, width 98.8 cm−1). The corresponding peaks are shown in the spectra in (b). d A HV slice showing the different crystals inside the HV wall. (i–iii) Consecutive enlargements of the slice. Brucite and calcite crystals are localized at specific areas in the millimeter scale range. From the microscale observation of the HV slice, crystals with different refractive indices covering the surface of several channels were identified (red arrows). e, f Raman spectra (e) and zeta potential (f) of HV samples after adsorption of various ions. For the analysis, a powdered HV sample was mixed with various ions that are commonly found in seawater: Ca2+, CO32−, K+, NO3−, and SO42−. The peaks from the HV sample are indicated in gray and the other Raman peaks attributable to the adsorbed ions are highlighted using the same color of each spectrum. The peaks around 280 and 444 cm−1 did not shift in all the spectra, whereas the peaks around 1100 cm−1 varied with the type of ion, indicating that ions were exchanged inside the HV wall. The adsorbed ions significantly changed the surface charge of the HV particles. Mean ± s.d. (n = 3).
Osmotic energy conversion in HVs
Nanopores, structural alignment, and variable surface charges found in HVs are hallmarks of materials associated with osmotic energy conversion3,4,5,6,7,8,9. Materials with high surface charge density and confined nanopores have been designed for industrial power generation systems from salinity gradients between seawater and river water3,4,5. Motivated by the findings above, we tested the osmotic energy conversion in the HV.
A two-chamber electrochemical cell equipped with an Ag/AgCl electrode (Fig. 5a, b) was used to record the current-voltage (I–V) response of the HV samples. The HV samples were exposed to various concentrations of KCl, and the current response was plotted (Fig. 5c). At high KCl concentrations ranging from 1 M to 1 mM, the conductance of HVs is proportional with the salt concentration, indicating that electrolyte properties dictate ionic conductivity. However, the conductance remained constant even when the KCl concentration was decreased below 1 mM KCl, which is a significant deviation from the bulk characteristic of ion transport seen at higher concentrations. This plateau in the conductance is indicative of surface charge-governed ion transport34 and, therefore, suggests that ion transport behaviors are primarily regulated by electric fields generated at solid-electrolyte interfaces in intra- and inter-brucite crystal spaces (Fig. 5d, e)35.
a A photograph of the HV-PDMS membrane sample used for the measurement. b Experimental setup for measuring the current-voltage response of the HV samples. For conductance measurement, both sides were filled with a KCl solution of the same concentration. c Electrical conductance of HV sample as a function of KCl concentrations with pH 10. d Schematics showing the continuous arrangement in HV inorganic wall, comprising multilayers of the brucite (001) plane at the angstrom level, plate nanocrystal stacks at the nanometer level, and a radial alignment structure extending to the millimeter level. This configuration results in continuous channels, allowing the HV walls to function as a selectively permeable membrane for ion transport. e Schematics showing osmotic power generation. Upon exposure to KCl, an electric double layer (EDL) is formed on the surface of brucite crystals, exhibiting varying Debye screening lengths (λD) depending on KCl concentration. Blue represents the EDL formed on the brucite surface by exposure to the ion concentration gradient. The prevalent pore size in HV walls is measured to be 60 nm, which means that the 30 nm EDL formed by 0.1 mM KCl completely overlaps inside the HV nanopore. Effective overlap of EDLs within narrow nanopores establishes a screening barrier that is permeable only to ions with specific charges. Acting as a selective membrane, the HV wall allows selective movement of counterions from a high concentration to a low concentration, generating an ionic current.
The HV samples were next exposed to geologically plausible chemical gradient conditions by placing them at the interface of two KCl solutions (0.1 mM and 0.1 M). The HV samples were adsorbed with either CO32− or Ca2+, and the I–V response of the samples was recorded (Fig. 6a, b, N = 15, Supplementary Fig. 15). When carbonate ions were adsorbed to the HV nanopore walls, a negative surface charge was generated. Further, when the HV nanopores were exposed to KCl, electrical double layers (EDLs) were created within the nanopores depending on the KCl concentration (Fig. 5e, Debye screening length = 0.96 and 30.42 nm for 0.1 M and 0.1 mM KCl, respectively). In the HV mineral wall, the dominant pore size, created by the multilayer stacking of brucite nanocrystals, is 60 nm (Supplementary Fig. 6b and Fig. 5d, e), indicating that a 30 nm EDL formed by 0.1 mM KCl is completely overlapped inside the HV nanopore. The overlap of EDL within the narrow nanopores effectively results in a screen barrier permeable only to ions with a specific charge. This allows selective ion transport based on surface charge-governed ion transport mechanism34. Furthermore, the continuous arrangement of crystals, together with the slit-like pores (Figs. 1, 2, 5d and Supplementary Figs. 3–6, 13), enables the HV walls to act as a cation- and anion-selective ion transport membrane and thus harvesting electrical energy from diverse ionic gradients present in the environments as will be demonstrated below.
a Schematic illustration of the experimental setup for the ion selectivity measurements. The anion- and cation-selective HV samples were placed between reservoirs, and the HVs were exposed to alternating salt concentrations (0.1 mM / 0.1 M / 0.1 mM). b Osmotic energy conversion under a KCl gradient. Current-voltage curves were measured under a 1000-fold salinity gradient (0.1 mM / 0.1 M KCl) for CO32−-adsorbed (blue dots) and Ca2+-adsorbed (red dots) HV samples. The redox potential of the electrode was subtracted from the measured potential, and the diffusion potential (Vos) and current (Ios) are shown in the figure. The opposite polarity is generated from the different charge selectivity of HV samples. c Diffusion potential of a series of connected HVs as a function of time. A combination of anion- and cation-selective HV pairs was placed under NaCl gradient conditions (0.1 mM / 0.1 M), as illustrated in the schematic on the top. Multiple connections of HVs enhanced the diffusion potential. The electrolyte was not refreshed during the measurement. d, e Concentration changes of Na+ (d) and Cl− (e) ions over time at locations 1 and 2 were measured and plotted. f Osmotic power generation in the presence of pH (pH 10/ pH 4) and pD (pD 10/ pD 4) gradients. The cation-selective (CO32−-adsorbed) HV sample was used for the analysis.
In the presence of a KCl gradient, the CO32--adsorbed HV sample generated the open circuit voltage (Voc) of 229 mV (Supplementary Fig. 15a, blue circle). Subtracting the redox potential of the Ag/AgCl electrode (Vredox, 163 mV; Method, Supplementary Table) yielded a pure osmotic potential (Vos, diffusion potential) of 66 mV shown in Fig. 6b (blue circle). The Vos and diffusion current (Ios) increased proportionally with the ionic concentration difference (Supplementary Fig. 16a, b), confirming that the detected electrical energy originates from the ionic gradient. Notably, a negative Vos value of − 46 mV was observed when Ca2+ ions were adsorbed (Fig. 6b, red circle). This change in the Vos indicates that the surface charge resulting from CO32− and Ca2+ adsorption plays a key role in the selective transport of cations and anions in HV nanopores. In other words, changing the sign of Vos indicates that the dominant charge carrier is shifted from K+ to Cl−upon switching the adsorbed ions from CO32− to Ca2+ due to the change in polarity of the EDLs. Furthermore, due to the cation- and anion-selective nature of HV walls, the series connection of positively and negatively charged HV sample pairs increased diffusion potentials, demonstrating that this configuration allows the HV precipitates to harness the chemical potential originating from both K+ and Cl− gradient and thus amplify the Vos (Fig. 6c). When the direction of the HV sample was rotated 90° from the natural HV configuration, Ios was lower than that of the HV’s natural direction, suggesting that the arrangement of brucite in HV precipitates facilitates selective ion transport (Supplementary Fig. 17 and Supplementary Notes).
Selective ion transport within HV precipitates was further verified by the direct measurement of Na+ and Cl− ions over time (Fig. 6a, d, e and Supplementary Fig. 16c). Cation- (CO32−-adsorbed) and anion-selective (Ca2+-adsorbed) HV samples were fixed between two reservoirs filled with either low (0.1 mM) or high (0.1 M) salt concentrations and the concentration changes of the Na+ and Cl- ions were monitored with respect to time (Fig. 6d, e). In the case of Na+ ion transport through CO32−-adsorbed HV nanopores, the concentration of Na+ ions in the low-concentration reservoir gradually increased over time. In contrast, there was minimal change in the Na+ ion concentration across the Ca2+-adsorbed HV sample. Instead, the Cl− increased steadily on this side of the reactor, providing compelling evidence for selective ion transfer within the HV precipitates.
One of the most characteristic features of serpentinite-hosted HV systems is the presence of a large pH gradient between the seawater (pH ~ 7) and vented fluids (pH 9~11). Experimental simulation of alkaline HV systems has been reported in the context of the origin of life on the ancient Earth, using reducing high pH fluid (pH 10 ~ 12) and oxidative low pH seawater (pH 4~6) simulant22,36,37. To examine the conversion of a pH gradient to electrochemical energy, the cation-selective (CO32−-adsorbed) HV sample was placed between reservoirs containing pH 10 and 4 solutions, and the electrical response was measured (Fig. 6f, gray circle). Under these conditions, 55 mV of Vos and − 2 nA of Ios were generated. The cation-selective HV sample was also subjected to a deuterium gradient (pD), and then I–V response was monitored (Fig. 6f, red circle). The Ios decreased by 2.3 fold compared to the pH gradient case, while the potential was similar, indicating that proton movement was the source of the generated current. In contrast, when the anion-selective HV sample was subjected to the same test, a reduced H/D kinetic isotope effect was observed, suggesting that hydroxide is involved in the power generation (Supplementary Fig. 18).
Discussion
The findings from the present study provide experimental evidence for geochemically driven self-organization of the inorganic structure and energy conversion function of serpentinite-hosted SSF HV systems. The periodic banding pattern found in the HV samples can be interpreted within the framework of combined chemical (reaction) and physical (diffusion) processes38,39,40. Reactions involving transport phenomena such as diffusion or flow intrinsically provide spatially varying concentrations41,42. Especially in systems with internal feedback, such as precipitation accompanied by a pH change, ionic concentrations, show spatial and temporal oscillation, resulting in periodic patterns in the structure43,44. The formation of HVs generated by the injection of alkaline fluids is a flow-driven formation system that include spatially varying concentration differences. The repetitive banded texture in the HV samples (Figs. 1 and 2) and the periodic formation of areas rich in OH bonds within brucite crystals (Supplementary Fig. 19) provide support for a reaction-diffusion mechanism in HV formation.
In addition to the periodic pattern, it is worth noting the anisotropic crystal arrangement observed in nearly the entire part of the HV samples (Figs. 2 and 3 and Supplementary Figs. 7–13). Previous studies have demonstrated the alignment of anisotropic particles, such as two-dimensional graphene oxide sheets, underflow conditions45,46. Anisotropic particles typically align along the flow direction (shear flow-induced alignment)45,46,47. Contrary to the shear flow-induced orientation, the two-dimensional brucite sheets align perpendicularly to the flow direction (Supplementary Fig. 10). We speculate diffusiophoresis, a phenomenon in which charged particles move along a concentration gradient of ions, such as NaCl, KCl, protons, and hydroxides48,49,50,51, acts as a driving force for the alignment. Namely, in serpentinite-hosted HV systems, a concentration gradient is established between the hot, reduced ion-rich vent fluid solutions and oxidizing seawater36,37,52,53, generating an electric field perpendicular to the flow direction. The resulting diffusiophoretic field would provide a directional driving force to the charged precipitates and promote the concentric arrangement of nanocrystals around the channels (Supplementary Notes).
Spontaneous formation of selective ion channels in HVs has direct implications for the origin of life on Earth and beyond. HV research over the last 40 years has profoundly advanced our views of life and Earth17,54, and the similarity of the chemical gradients present in HVs to those found across cell membranes gives rise to hypotheses regarding the chemical evolution of biomolecules, energy metabolisms, and cellular functions in the prebiotic ocean of Earth10,11,12,13,14,15,16,17,18,19,20,21,22,23. Our results provide a physicochemical insight into the argument for how osmotic energy conversion, a vital function in modern life, can occur abiotically in a geological setting12,13,14,15,16,17,18,19. As we have shown, the chemical disequilibria created by the serpentinization process produce mineral precipitates with confined nanopores and charged surfaces with directional alignment, which are the basis for osmotic energy conversion. The formation of selective ion transport channels may be derived from the interplay of physicochemical processes such as diffusiophoresis48,49,50,51, electrostatic interactions, and surface charge-governed ion transport34 that are inherently imparted to HV systems. As ionic gradients are ubiquitous in nature and could have existed on the early Earth55, functionality attendant with osmotic energy conversion may readily emerge on the modern and early Earth. The recent discovery of ongoing hydrothermal activity on icy satellites such as the Saturnian moon Enceladus56 could also extend the possibilities for osmotic energy conversion to astrobiological perspectives57.
Apart from confined nanopore involvement in selective ion transport, the nanoconfined structures formed in HVs also provide fascinating chemical reactors that have the potential to significantly improve reaction rate, selectivity, and chemical complexity. Reactions in confined nano- and micro spaces differs substantially from bulk reactors, partially due to the efficient concentration of reactants, stabilization of intermediates, and confined water58,59,60,61,62,63,64. These properties have led to improved efficiency of epoxidation in zeolites63 and multistep biocatalytic cascade reactions in porous metal oxide electrodes65. Furthermore, relevant to the findings in this study, confined spaces identified in natural clay minerals have been proposed to be involved in the abiotic synthesis of the aromatic amino acids, tryptophan66. Although how aromatic amino acids are abiotically synthesized via fluid-rock interactions remains elusive, the confined space generated by layered nanocrystals may facilitate the dehydration reaction even in water-rich environments due to the high population of tightly bound, ice-like water with an anomalously low dielectric constant67.
Geochemically driven self-organization of inorganic materials provides valuable insights into engineering applications. One such example is selective ion transport membranes, which are a critical component of industrial systems for harvesting energy from salinity gradients between seawater and river water, known as blue energy harvesting5. Significant advances in this research area have been recently made through the utilization of two-dimensional layered nanomaterials and their directional alignment to fabricate ion transport channels3,4,5. Unlike synthetic materials, in the HV system, the self-assembly of brucite, a two-dimensional layered material, occurs spontaneously by fluid flow and concentration gradients. Such spontaneous formation of intricate nanostructures and functions may offer new synthetic methodologies for the water-energy challenges and be beneficial for mechanical properties such as toughness and stability68. As the formation of ordered materials in a continuous flow regime is understudied, flow-driven precipitations in serpentinite-hosted HVs shed new light on a geomimetic approach for material synthesis and functionalization using Earth’s crust elements.
By performing a comprehensive material analysis of HVs, we have found the formation of nano- to millimeter-scale ion transport channels that provide new perspectives on the multifaceted roles that serpentinite-hosted HVs may play. These findings open up an intriguing connection between the origin of life on Earth and beyond and the emerging field of chemistry and physics in nanospaces. The current understanding of the material properties derived from confined nanospaces in geologic environments is still at an early stage, leaving much room for further exploration and discovery.
Methods
Chemicals
MgCl2·6H2O (97.0%), CaCl2·2H2O (99.9%), NaNO3 (99.0%), Na2CO3 (99.8%), KCl (99.5%), KOH (85.0%), HCl (35.0–37.0%), NaClO·5H2O (39.0 % (as Cl)) were purchased from FUJIFILM Wako Pure Chemical Corporation. Na2SO4 (99%) and NaCl (99.5%) were obtained commercially from Junsei Chemicals. For aqueous solution preparation, high-purity deionized water (18.2 MΩ cm−1) was used. D2O (99.9 atom % D), KOD solution (40 wt. % in D2O, 98 atom % D), and DCl solution (35 wt. % in D2O, ≥ 99 atom % D) were purchased from Sigma-Aldrich. PDMS (SYLGARD 184 Silicone Elastomer Kit) and Ag wire (φ0.50 mm, 99.99%) were provided by Dow Corning and Nilaco, respectively.
Materials
The hydrothermal vent (HV) sample was collected using the Shinkai 6500 manipulators during dive #1404 on cruise YK14-13 in 2014 (ID; 12541-14001_6K1404-R05). The sample was sectioned onboard immediately after recovery and stored at 4 °C. The SSF is located on the forearc slope of a subduction zone and differs in tectonic setting from the Lost City Hydrothermal Field, the most well-studied off-axis, alkaline HV site. However, both vent systems are associated with serpentinization, forming similar precipitates in texture and mineral composition. Therefore, this study used the SSF samples to represent alkaline HV precipitates.
Sample preparations
At the time of HV collection in the deep sea, the investigated HV had a height of approximately 84 cm, divided into three parts (upper: ~ 28 cm, middle: ~ 26 cm, lower: ~ 30 cm) for further analysis (Supplementary Fig. 1a). The HV parts were cut into multiple pieces for inspection using an MC-420 diamond cutting saw (Maruto Instrument Co., Ltd.) and a razor blade. The gentle pressure applied during cutting induced cracks in the sample, and the spontaneously cut fragments were also collected and observed. To observe HVs with a polarized light microscope, samples were embedded into epoxy resin (E205, Nichika). The samples were then cut and polished, and then mounted on glass slides and further polished to the desired thickness (tens of microns thick of thin section). Multiple thin sections were made using samples collected from various locations in each HV part, upper, middle, and lower. For the synchrotron X-ray scanning, slices of HV samples (~ 120 µm) were prepared by embedding the HVs in a UV-curable resin, which was then cut and polished to the desired thickness. Cross-sectional analysis of HV-polymer samples was observed after milling the samples using an Ar ion beam milling system (Leica EM TIC 3X).
Characterization of HV
Cross-polarized optical microscope (POM) images were obtained using an IX-70 inverted microscope (Olympus) equipped with additional linear polarizers and a first-order retardation plate (530 nm). In order to obtain a high-resolution optical image of a wide area, images observed by a digital microscope were connected without magnification and color adjustment. Naturally cut samples were observed using a Quattro S SEM (Thermo Fisher Scientific) without coating. For TEM observation, the samples were crushed, and the powdered samples were loaded on the grid. Images were taken with a JEM-2100F TEM (JEOL) coupled with a high-angle annular dark-field (HAADF) detector. Powder X-ray diffraction patterns of bulk samples were measured using a SmartLab XE diffractometer (RIGAKU). The synchrotron X-ray scanning experiments were conducted using the RIKEN Targeted Proteins Beamline (BL32XU) and RIKEN Structural Biology I Beamline (BL38B1) in SPring-8 (Hyogo, Japan). Two beamlines with different beam sizes were used for the wide-angle X-ray scattering (WAXS) experiment. At the BL32XU beamline, the X-ray beam was 5 µm (Horizontal, H) × 5 µm (Vertical, V) with a 0.6300 Å wavelength. The sample-to-detector distance was 125.0 mm, and an EIGER X 9 M (Dectris) detector was used. Sliced samples were mounted on a goniometer and raster scanned in 5-μm steps using an X-ray beam with an exposure time of 10 ms per point. Scanning was performed over several regions of the HV slices at room temperature. In the case of BL38B1, the X-ray beam was collimated to a size of 120 µm (H) × 60 µm (V) and a wavelength of 1.0 Å. WAXS patterns were acquired using a Pilatus 3X 2 M (Dectris) detector69. The scattering vector (q = 4πsinθ/λ; 2θ, scattering angle; λ, a wavelength of the X-ray beam) and the position of the incident X-ray beam on the detectors were calibrated with FIT2D software70,71 based on silver behenate (d = 58.38 Å). The sample-to-detector distance was 312.689 mm. The sliced sample was mounted on a motorized stage and linear-scanned with 100-µm steps and 10 seconds of exposure time at each point. The scan was conducted over a large area (~7 × ~3 mm) at room temperature. In the case of small-angle X-ray scattering (SAXS), the same beamline, RIKEN Structural Biology I Beamline (BL38B1), was used for measurement. The sample-to-detector distance was 2.5450 m. The beam is collimated to the size of 100 µm(H) × 100 µm(V). The WAXS patterns were analyzed using DPDAK v.1.5.0, open-source software72. Information on the intensity and direction of the diffraction peaks was extracted using a Python program, which will be made available at github after acceptance. For the ion adsorption test, 5~10 mg of powder HV was dispersed in 1 M of each electrolyte solution (2 mL) adjusted to pH 10, and the solution was then sonicated for 1 h and allowed to sit overnight at room temperature. For Raman spectral acquisition of slice and ion adsorption samples, a Senterra Raman Microscope (Bruker Optics) was used. A 785 nm laser line with 25 mW of power was applied, and the exposure time varied from 5 to 60 s depending on the target. To obtain micro-Raman map images, a RAMANtouch (Nanophoton) system was used with a 532-nm laser line, 2 s of exposure time, and 9.6 mW power. The Raman was calibrated using Si peaks and cosmic ray reduction was applied for all Raman measurements. The surface charge of HV particles was evaluated using an ELSZ-2PL zeta-potential analyzer (Otsuka Electronics). Gas adsorption-desorption isotherms were obtained using a BELSORP-mini X surface area analyzer (Microtrac) with nitrogen gas.
HV-PDMS membrane fabrication
HV samples were first soaked in 1 M Na2CO3 or CaCl2 (adjusted to pH 10) overnight to adsorb carbonate or calcium ions. The ion-adsorbed HV sample was placed in a partially polymerized PDMS precursor liquid. A vacuum pump was used to infiltrate the PDMS into the HV samples to fill the macropores inside the HVs. The resulting HV-PDMS samples were kept at 40 °C to solidify the PDMS. HV-PDMS was inserted in the middle of the PDMS film to prevent the mixing of the electrolyte or two different solutions in a two-chamber electrochemical cell while allowing the transport of ions only through the HV samples. After the PDMS embedding process, the upper surface of the HV sample was cut to remove the polymer coating, and the left and right sides of the HV surface were exposed to the solution. Before each measurement, the HV-PDMS membranes were fully hydrated by immersion in a pH 10 solution for 2 h. Raman, WAXS, and cross-section SEM image analysis confirmed that the material properties of brucite remain intact during membrane fabrication processes (Supplementary Figs. 20–22).
Electrical measurements
For KCl ion transport, an HV pellet-PDMS membrane was used. The HV material was ground and pelletized and then inserted into a PDMS film containing a 1 cm hole in the center of the film. The HV pellet-PDMS membrane was clamped between two reservoirs (two-chambers electrochemical cell equipped with Ag/AgCl electrode at both reservoirs) containing the same concentration of KCl solution. Current-voltage (I–V) curves of the membrane were recorded at various salt concentrations.
For the power generation test, low and high concentrations of KCl, NaCl, or protons were filled in the left (working electrode placement) and right (counter electrode placement) reservoirs, respectively, and the I–V response of the HV-PDMS sample was then measured. To suppress the dissolution of the HV samples, various concentrations of salt solutions were adjusted to pH 10. For I–V measurement in a pH gradient, 0.1 mM KCl was added to both sides of the reservoir to prevent the formation of AgOH on the Ag/AgCl electrode. All electrical measurements were conducted using an HZ-7000 electrochemical workstation (Hokuto Denko) in a Faraday cage. A pair of Ag/AgCl electrodes was used for the measurement. Ag/AgCl electrode wires were prepared by chemical chlorination and rechlorination was performed after each measurement. The applied voltage scan range was from –0.3 V to +0.3 V at a scan rate of 50 mV/sec. The concentration of Na+ and Cl− ions were detected using Orion Versa Star Pro electrochemistry meters (Thermo Scientific) coupled with ROSS sodium ion and chloride ion selective electrodes. Before conducting the deuterium experiments, the dried HV samples were immersed overnight in a pD 10 solution titrated with KOD in D2O.
Calibration
The open circuit voltage (Voc) measured with Ag/AgCl wires is a result of the pure osmotic potential (Vos) of the HV and the redox potential (Vredox) of the electrode-solution interface upon exposure to a different concentration of salt and is represented by the equation: Voc = Vos + Vredox. Subtracting the redox potential from Voc gives the pure osmotic potential of the HVs. To measure the redox potential of the Ag/AgCl wire, a commercial reference electrode (filled with saturated KCl in a glass housing, Hokuto Denko) and the prepared wire were placed in a specific concentration of KCl, and the potential was recorded. The potential difference at the specific KCl concentrations was used to calculate the redox potential increase (see Supplementary Table). The measured current (Imeas) was adjusted by the following; Ios = (Vos/Voc) × Imeas
Data availability
The data generated in this study have been deposited in the Zenodo database73 under https://doi.org/10.5281/zenodo.12788138
Code availability
Python code for analysis is provided alongside with this paper. It can also be accessed at https://github.com/HideshiOoka/SI_for_Publications
References
Mitchell, P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 191, 144–148 (1961).
Schoepp-Cothenet, B. et al. On the universal core of bioenergetics. Biochim. Biophys. Acta 1827, 79–93 (2013).
Xiao, K., Jiang, L. & Antonietti, M. Ion transport in nanofluidic devices for energy harvesting. Joule 3, 2364–2380 (2019).
Macha, M., Marion, S., Nandigana, V. V. R. & Radenovic, A. 2D materials as an emerging platform for nanopore-based power generation. Nat. Rev. Mater. 4, 588–605 (2019).
Zhang, Z., Wen, L. & Jiang, L. Nanofluidics for osmotic energy conversion. Nat. Rev. Mater. 6, 622–639 (2021).
Guo, W. et al. Energy harvesting with single-ion-selective nanopores: a concentration-gradient-driven nanofluidic power source. Adv. Funct. Mater. 20, 1339–1344 (2010).
Kim, D.-K., Duan, C., Chen, Y.-F. & Majumdar, A. Power generation from concentration gradient by reverse electrodialysis in ion-selective nanochannels. Microfluid Nanofluid 9, 1215–1224 (2010).
Siria, A. et al. Giant osmotic energy conversion measured in a single transmembrane boron nitride nanotube. Nature 494, 455–458 (2013).
Feng, J. et al. Single-layer MoS2 nanopores as nanopower generators. Nature 536, 197–200 (2016).
Baross, J. A. & Hoffman, S. E. Submarine hydrothermal vents and associated gradient environments as sites for the origin and evolution of life. Orig. Life Evol. Biosph. 15, 327–345 (1985).
Wächtershäuser, G. Pyrite formation, the first energy source for life: a hypothesis. Syst. Appl. Microbiol. 10, 207–210 (1988).
Russell, M. J., Hall, A. J. & Turner, D. In vitro growth of iron sulphide chimneys: possible culture chambers for origin-of-life experiments. Terra Nova 1, 238–241 (1989).
Wächtershäuser, G. Groundworks for an evolutionary biochemistry: the iron-sulphur world. Prog. Biophys. Mol. Biol. 58, 85–201 (1992).
Russell, M. J. & Hall, A. J. The emergence of life from iron monosulphide bubbles at a submarine hydrothermal redox and pH front. J. Geol. Soc. Lond. 154, 377–402 (1997).
Martin, W. & Russell, M. J. On the origins of cells: a hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells. Philos. Trans. R. Soc. Lond. B Biol. Sci. 358, 59–85 (2003).
Martin, W. & Russell, M. J. On the origin of biochemistry at an alkaline hydrothermal vent. Philos. Trans. R. Soc. B 362, 1887–1925 (2007).
Martin, W., Baross, J., Kelley, D. & Russell, M. J. Hydrothermal vents and the origin of life. Nat. Rev. Microbiol. 6, 805–814 (2008).
Russell, M. J., Hall, A. J. & Martin, W. Serpentinization as a source of energy at the origin of life. Geobiology 8, 355–371 (2010).
Lane, N. & Martin, W. F. The origin of membrane bioenergetics. Cell 151, 1406–1416 (2012).
Nakamura, R. et al. Electrical current generation across a black smoker chimney. Angew. Chem. Int. Ed. Engl. 49, 7692–7694 (2010).
Kitadai, N. et al. Metals likely promoted protometabolism in early ocean alkaline hydrothermal systems. Sci. Adv. 5, eaav7848 (2019).
Hudson, R. et al. CO2 reduction driven by a pH gradient. Proc. Natl. Acad. Sci. USA 117, 22873–22879 (2020).
Preiner, M. et al. A hydrogen-dependent geochemical analogue of primordial carbon and energy metabolism. Nat. Ecol. Evol. 4, 534–542 (2020).
Tivey, M. Generation of seafloor hydrothermal vent fluids and associated mineral deposits. Oceanography 20, 50–65 (2007).
Francheteau, J. et al. Massive deep-sea sulphide ore deposits discovered on the East Pacific Rise. Nature 277, 523–528 (1979).
Spiess, F. N. et al. East Pacific Rise: hot springs and geophysical experiments. Science 207, 1421–1433 (1980).
Kelley, D. S. et al. An off-axis hydrothermal vent field near the Mid-Atlantic Ridge at 30 degrees N. Nature 412, 145–149 (2001).
Kelley, D. S. et al. A serpentinite-hosted ecosystem: the Lost City hydrothermal field. Science 307, 1428–1434 (2005).
Sleep, N. H., Meibom, A., Fridriksson, T., Coleman, R. G. & Bird, D. K. H2-rich fluids from serpentinization: geochemical and biotic implications. Proc. Natl. Acad. Sci. USA 101, 12818–12823 (2004).
Proskurowski, G. et al. Abiogenic hydrocarbon production at Lost City hydrothermal field. Science 319, 604–607 (2008).
Okumura, T. et al. Brucite chimney formation and carbonate alteration at the Shinkai Seep Field, a serpentinite-hosted vent system in the southern Mariana forearc. Geochem. Geophys. Geosyst. 17, 3775–3796 (2016).
Khan, A. I. & O’Hare, D. Intercalation chemistry of layered double hydroxides: recent developments and applications. J. Mater. Chem. 12, 3191–3198 (2002).
Estrada, C. F., Sverjensky, D. A. & Hazen, R. M. Selective adsorption of aspartate facilitated by calcium on brucite [Mg(OH)2]. ACS Earth Space Chem. 3, 1–7 (2018).
Stein, D., Kruithof, M. & Dekker, C. Surface-charge-governed ion transport in nanofluidic channels. Phys. Rev. Lett. 93, 035901 (2004).
Daiguji, H. Ion transport in nanofluidic channels. Chem. Soc. Rev. 39, 901–911 (2010).
Barge, L. M. et al. Characterization of iron-phosphate-silicate chemical garden structures. Langmuir 28, 3714–3721 (2012).
Moller, F. M., Kriegel, F., Kiess, M., Sojo, V. & Braun, D. Steep pH gradients and directed colloid transport in a microfluidic alkaline hydrothermal pore. Angew. Chem. Int. Ed. Engl. 56, 2340–2344 (2017).
Nakouzi, E. & Steinbock, O. Self-organization in precipitation reactions far from the equilibrium. Sci. Adv. 2, e1601144 (2016).
Epstein, I. R. & Xu, B. Reaction-diffusion processes at the nano- and microscales. Nat. Nanotechnol. 11, 312–319 (2016).
Barge, L. M. et al. From chemical gardens to chemobrionics. Chem. Rev. 115, 8652–8703 (2015).
Heaney, P. J. & Davis, A. M. Observation and origin of self-organized textures in agates. Science 269, 1562–1565 (1995).
Noorduin, W. L., Grinthal, A., Mahadevan, L. & Aizenberg, J. Rationally designed complex, hierarchical microarchitectures. Science 340, 832–837 (2013).
Montalti, M. et al. Local pH oscillations witness autocatalytic self-organization of biomorphic nanostructures. Nat. Commun. 8, 14427 (2017).
Garcia-Ruiz, J. M., Melero-Garcia, E. & Hyde, S. T. Morphogenesis of self-assembled nanocrystalline materials of barium carbonate and silica. Science 323, 362–365 (2009).
Xin, G. et al. Microfluidics-enabled orientation and microstructure control of macroscopic graphene fibres. Nat. Nanotechnol. 14, 168–175 (2019).
Zhao, C. et al. Layered nanocomposites by shear-flow-induced alignment of nanosheets. Nature 580, 210–215 (2020).
Hayter, J. B. & Penfold, J. Use of viscous shear alignment to study anisotropic micellar structure by small-angle neutron scattering. J. Phys. Chem. 88, 4589–4593 (2002).
Prieve, D. C., Anderson, J. L., Ebel, J. P. & Lowell, M. E. Motion of a particle generated by chemical gradients. Part 2. Electrolytes J. Fluid Mech. 148, 247–269 (1984).
Anderson, J. L. Colloid transport by interfacial forces. Annu. Rev. Fluid Mech. 21, 61–99 (1989).
Velegol, D., Garg, A., Guha, R., Kar, A. & Kumar, M. Origins of concentration gradients for diffusiophoresis. Soft Matter 12, 4686–4703 (2016).
Shim, S. Diffusiophoresis, diffusioosmosis, and microfluidics: surface-flow-driven phenomena in the presence of flow. Chem. Rev. 122, 6986–7009 (2022).
Barge, L. M. et al. From chemical gardens to fuel cells: generation of electrical potential and current across self-assembling iron mineral membranes. Angew. Chem. Int. Ed. Engl. 54, 8184–8187 (2015).
Glaab, F., Kellermeier, M., Kunz, W., Morallon, E. & Garcia-Ruiz, J. M. Formation and evolution of chemical gradients and potential differences across self-assembling inorganic membranes. Angew. Chem. Int. Ed. Engl. 51, 4317–4321 (2012).
Dick, G. J. The microbiomes of deep-sea hydrothermal vents: distributed globally, shaped locally. Nat. Rev. Microbiol. 17, 271–283 (2019).
Russell, M. J., Nitschke, W. & Branscomb, E. The inevitable journey to being. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368, 20120254 (2013).
Hsu, H. W. et al. Ongoing hydrothermal activities within Enceladus. Nature 519, 207–210 (2015).
Russell, M. J. et al. The drive to life on wet and icy worlds. Astrobiology 14, 308–343 (2014).
Derouane, E. G., André, J.-M. & Lucas, A. A. A simple van der waals model for molecule-curved surface interactions in molecular-sized microporous solids. Chem. Phys. Lett. 137, 336–340 (1987).
Koblenz, T. S., Wassenaar, J. & Reek, J. N. H. Reactivity within a confined self-assembled nanospace. Chem. Soc. Rev. 37, 247–262 (2008).
Grommet, A. B., Feller, M. & Klajn, R. Chemical reactivity under nanoconfinement. Nat. Nanotechnol. 15, 256–271 (2020).
Munoz-Santiburcio, D. & Marx, D. Chemistry in nanoconfined water. Chem. Sci. 8, 3444–3452 (2017).
Bregante, D. T. et al. Cooperative effects between hydrophilic pores and solvents: catalytic consequences of hydrogen bonding on alkene epoxidation in zeolites. J. Am. Chem. Soc. 141, 7302–7319 (2019).
Bregante, D. T. et al. The shape of water in zeolites and its impact on epoxidation catalysis. Nat. Catal. 4, 797–808 (2021).
Munoz-Santiburcio, D. & Marx, D. Confinement-controlled aqueous chemistry within nanometric slit pores. Chem. Rev. 121, 6293–6320 (2021).
Morello, G., Megarity, C. F. & Armstrong, F. A. The power of electrified nanoconfinement for energising, controlling and observing long enzyme cascades. Nat. Commun. 12, 340 (2021).
Menez, B. et al. Abiotic synthesis of amino acids in the recesses of the oceanic lithosphere. Nature 564, 59–63 (2018).
Fumagalli, L. et al. Anomalously low dielectric constant of confined water. Science 360, 1339–1342 (2018).
Aizenberg, J. et al. Skeleton of Euplectella sp.: structural hierarchy from the nanoscale to the macroscale. Science 309, 275–278 (2005).
Fujisawa, T. et al. Small-angle X-ray scattering station at the SPring-8 RIKEN beamline. J. Appl. Crystallogr. 33, 797–800 (2000).
Hammersley, A. P. ESRF Internal Report, ESRF98HA01T, FIT2D V9.129 Reference Manual V3.1. (1998).
Hammersley, A. P., Svensson, S. O. & Thompson, A. Calibration and correction of spatial distortions in 2D detector systems. Nucl. Instrum. Methods Phys. Res. A 346, 312–321 (1994).
Benecke, G. et al. A customizable software for fast reduction and analysis of large X-ray scattering data sets: applications of the new DPDAK package to small-angle X-ray scattering and grazing-incidence small-angle X-ray scattering. J. Appl. Crystallogr. 47, 1797–1803 (2014).
Lee, H.-E. et al. Dataset for the manuscript “Osmotic Energy Conversion in Serpentinite-Hosted Deep-Sea Hydrothermal Vents” https://doi.org/10.5281/zenodo.12788138 (2024).
Acknowledgements
The authors would thank the Shinkai 6500 operation team and the crew of the R/V Yokosuka for their professional support for sampling HV precipitates during the YK14-13 cruise. This study was conducted under the special use permit of the US Fish and Wildlife Service for studies in the Mariana Trench Marine National Monument (#12541-14001). The synchrotron radiation experiments were performed at RIKEN Targeted Proteins Beamline (BL32XU) and RIKEN Structural Biology I (BL38B1) in SPring-8 with the approval of RIKEN (Proposal No. 20210086). This work was supported by the RIKEN Special Postdoctoral Researchers (SPDR) fellowship to H.-E. L. and by JSPS Grant-in-Aid for Scientific Research to R. N. (22H05153), to S.E.M and R.N. (22K18278), and T. O. (15H05468 and 19K14830). We thank Daishi Inoue for the technical support and discussion on WAXS sample preparation.
Author information
Authors and Affiliations
Contributions
H.-E. L., S. E. M., and R. N. conceived the idea and designed the experiments. T. O. and Y. O. collected hydrothermal vent precipitates. T. O. and Masahiro Y. prepared specimens. H.-E. L. performed the experiments and characterization. H. O. and H.-E. L. processed the diffraction peaks and generated 2D plots. K. A., T. H., K. H., Y. K., H. M., and Masaki Y. carried out the X-ray experiments. D. H., K. A., and H.-E. L. analyzed the X-ray diffraction results. A. Y. conducted nitrogen adsorption/desorption analysis. H.-E. L. conducted electrical property measurements with contributions from R. N., A. Y., H. O., J.-E. L., and K. T. N. H. T. and H.-E. L. performed HV mimicking experiments to understand the alignment mechanism in HV. H.-E. L. and R. N. wrote the paper with contributions from S. E. M. and H. O., and feedback from all authors. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The Authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lee, HE., Okumura, T., Ooka, H. et al. Osmotic energy conversion in serpentinite-hosted deep-sea hydrothermal vents. Nat Commun 15, 8193 (2024). https://doi.org/10.1038/s41467-024-52332-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-52332-3
- Springer Nature Limited