Abstract
In the phase 3 KEYNOTE-355 study (NCT02819518), pembrolizumab plus chemotherapy demonstrated statistically significant and clinically meaningful improvements in progression-free survival (PFS) and overall survival (OS) versus placebo plus chemotherapy among patients with previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (TNBC) and programmed cell death ligand 1 (PD-L1) combined positive score (CPS) ≥ 10 tumors. We analyzed outcomes for the subgroup of patients enrolled in Asia in KEYNOTE-355. Patients received pembrolizumab 200 mg or placebo (2:1 randomization) every 3 weeks for 35 cycles plus investigator’s choice chemotherapy. Primary endpoints were PFS per Response Evaluation Criteria in Solid Tumors version 1.1 and OS. Among patients enrolled in Hong Kong, Japan, Korea, Malaysia and Taiwan (pembrolizumab plus chemotherapy, n = 113; placebo plus chemotherapy, n = 47), 117 (73.1%) had PD-L1 CPS ≥ 1 and 56 (35.0%) had PD-L1 CPS ≥ 10. Median time from randomization to data cutoff (June 15, 2021) was 43.8 (range, 36.8‒53.2) months (intent-to-treat [ITT] population). Hazard ratios (HRs [95% CI]) for PFS in the CPS ≥ 10, CPS ≥ 1, and ITT populations were 0.48 (0.24‒0.98), 0.58 (0.37‒0.91), and 0.66 (0.44‒0.99), respectively. Corresponding HRs (95% CI) for OS were 0.54 (0.28‒1.04), 0.62 (0.40‒0.97), and 0.57 (0.39‒0.84). Grade 3/4 treatment-related adverse events (AEs) occurred in 77.9% versus 78.7% of patients with pembrolizumab plus chemotherapy versus placebo plus chemotherapy. No grade 5 AEs occurred. Clinically meaningful improvement in PFS and OS with manageable toxicity were observed with pembrolizumab plus chemotherapy versus placebo plus chemotherapy in patients enrolled in Asia with previously untreated, inoperable or metastatic TNBC.
Trial registration: ClinicalTrials.gov, NCT02819518.
Similar content being viewed by others
Introduction
Metastatic triple-negative breast cancer (TNBC) accounts for ∼10% of all breast cancers globally and appears to have a similar incidence in Asia, albeit with considerable regional variability1,2,3,4. Programmed cell death ligand 1 (PD-L1) expression and high levels of tumor-infiltrating lymphocytes (TIL) were found to be prognostic for survival5,6,7,8,9. Immunotherapies targeting the programmed cell death 1 (PD-1) signaling pathway, such as the anti–PD-1 monoclonal antibody pembrolizumab, have demonstrated modest response rates in patients with metastatic TNBC as monotherapy10,11,12,13,14.
In the global KEYNOTE-355 study, patients with locally recurrent inoperable or metastatic TNBC received first-line treatment with either pembrolizumab or placebo plus paclitaxel, nab-paclitaxel, or gemcitabine plus carboplatin. Pembrolizumab plus chemotherapy significantly improved progression-free survival (PFS) and overall survival (OS) compared with placebo plus chemotherapy in patients with PD-L1‒positive tumors (combined positive score [CPS] ≥10)15,16. Median PFS was 9.7 months in the pembrolizumab plus chemotherapy group and 5.6 months in the placebo plus chemotherapy group (hazard ratio [HR], 0.65 [95% CI, 0.49‒0.86]; P = 0.0012). Median OS was 23.0 months and 16.1 months, respectively (HR, 0.73 [95% CI, 0.55‒0.95]; P = 0.0093). Based on PFS results from KEYNOTE-355, pembrolizumab plus chemotherapy was approved by regulatory agencies in several countries17,18 for patients with locally recurrent unresectable or metastatic TNBC whose tumors express PD-L1 (CPS ≥ 10).
Evidence suggests that there are important epidemiologic and biologic differences between Asian and non-Asian patients with TNBC that may affect response to treatment. For example, the probability of being diagnosed with TNBC decreases with age for White women in the United States but not for Asian American or East Asian women4. Additionally, driver mutations in the MYC and PTK2 genes have been reported to occur less frequently among patients from Japan than in a non-Asian patient population19, and patients from Korea with breast cancer have been reported to have increased TIL gene signatures compared with a non-Asian patient population20. Furthermore, certain patient characteristics (e.g., body mass index) have been reported to contribute towards difference in the toxicity profile among patients from Japan and Hong Kong versus patients from United States/Canada21, and polymorphisms in genes associated with drug clearance have been reported to contribute to chemotherapy pharmacokinetics among Asian versus non-Asian patients with breast cancer22.
Taken together, the available evidence highlights a need to evaluate the efficacy and safety of a broad range of anticancer therapies among Asian women with TNBC. The current analysis was conducted to better understand treatment outcomes with pembrolizumab among patients with TNBC enrolled in Asia in KEYNOTE-355.
Results
Patient population
The subgroup of patients enrolled in Asia included 160 patients randomized (pembrolizumab plus chemotherapy, n = 113; placebo plus chemotherapy, n = 47) between January 10, 2017 and May 23, 2018, in Hong Kong (n = 7), Japan (n = 87), Korea (n = 27), Malaysia (n = 21) and Taiwan (n = 18) (Fig. 1). Patient demographics and baseline disease characteristics were generally similar between the treatment groups (Table 1). At data cutoff for this analysis (June 15, 2021), median follow-up was 43.8 (range, 36.8‒53.2) months in the intention-to-treat (ITT) population.
Patients received a median of 10 (range, 1‒35) doses of pembrolizumab and 10 (range, 2‒35) doses of placebo. The median number of chemotherapy administrations in the pembrolizumab and placebo groups, respectively, were 22 (range, 2‒108) and 18 (range, 3‒109) for nab-paclitaxel; 18 (range, 12‒30) and 26 (range, 14‒33) for paclitaxel; 16 (range, 1‒83) and 16 (range, 2‒51) for gemcitabine; and 16 (range, 1‒83) and 16 (range, 2‒51) for carboplatin. Patients could discontinue chemotherapy without discontinuing pembrolizumab/placebo and vice versa. Median duration of exposure was 32 (range, 0–199) weeks in the pembrolizumab plus chemotherapy group and 30 (range, 3–146) weeks in the placebo plus chemotherapy group.
Efficacy
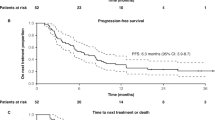
At data cutoff, 23/38 patients (60.5%) with PD-L1 CPS ≥ 10 in the pembrolizumab plus chemotherapy group and 14/18 patients (77.8%) in the placebo plus chemotherapy group had experienced progressive disease or died. Among patients with PD-L1 CPS ≥ 10, median PFS was 17.3 (95% CI, 7.6‒31.1) months with pembrolizumab plus chemotherapy and 5.6 (95% CI, 5.3‒9.0) months with placebo plus chemotherapy (HR, 0.48 [95% CI, 0.24‒0.98]; Fig. 2A), with 6-month PFS rates of 73.0% and 39.1%, respectively. Among patients with PD-L1 CPS ≥ 1, 56/81 patients (69.1%) and 32/36 patients (88.9%), respectively, had experienced progressive disease or died. Median PFS was 7.7 (95% CI, 6.3‒14.8) months versus 5.6 (95% CI, 5.3‒7.7) months (HR, 0.58 [95% CI, 0.37‒0.91]; Fig. 2B), with 6-month PFS rates of 64.2% and 48.3%, respectively. In the ITT population, 80/113 patients (70.8%) and 39/47 patients (83.0%), respectively, had experienced progressive disease or died. Median PFS was 8.8 (95% CI, 7.4‒10.3) months versus 6.7 (95% CI, 5.3‒7.7) months (HR, 0.66 [95% CI, 0.44‒0.99]; Fig. 2C), with 6-month PFS rates of 64.2% and 51.9%, respectively.
At data cutoff, 24/38 patients (63.2%) with PD-L1 CPS ≥ 10 in the pembrolizumab plus chemotherapy group and 16/18 patients (88.9%) in the placebo plus chemotherapy group had died. Among patients with PD-L1 CPS ≥ 10, median OS was 26.7 (95% CI, 18.7‒44.0) months versus 17.4 (95% CI, 11.5‒22.6) months (HR, 0.54 [95% CI, 0.28‒1.04]; Fig. 3A), with 12-month OS rates of 78.9% and 66.7% respectively. Among patients with PD-L1 CPS ≥ 1, 61/81 patients (75.3%) and 31/36 patients (86.1%), respectively, had died. Median OS was 22.0 (95% CI, 18.7‒26.7) months versus 16.9 (95% CI, 11.5‒19.2) months (HR, 0.62 [95% CI, 0.40‒0.97]; Fig. 3B), with 12-month OS rates of 79.0% and 63.9%, respectively. In the ITT population, 85/113 patients (75.2%) and 42/47 patients (89.4%), respectively, had died. Median OS was 24.1 (95% CI, 20.2‒27.5) months versus 17.2 (95% CI, 11.8‒19.2) months (HR, 0.57 [95% CI, 0.39‒0.84]; Fig. 3C), with 12-month OS rates of 79.6% and 63.8%, respectively.
The objective response rate (ORR) was 57.9% (95% CI, 40.8‒73.7%) in the pembrolizumab plus chemotherapy group and 38.9% (95% CI, 17.3‒64.3%) in the placebo plus chemotherapy group in patients with PD-L1 CPS ≥ 10, 53.1% (95% CI, 41.7‒64.3%) and 47.2% (95% CI, 30.4‒64.5%) in patients with PD-L1 CPS ≥ 1, and 49.6% (95% CI, 40.0‒59.1%) and 44.7% (95% CI, 30.2‒59.9%) in the ITT population, respectively (Supplementary Table 1). Results for PFS, OS, and ORR in patients with PD-L1 CPS < 10 are provided in Supplementary Table 2.
Safety
Treatment-related adverse events (AEs) of any grade occurred in 110/113 patients (97.3%) who received pembrolizumab plus chemotherapy and 46/47 patients (97.9%) who received placebo plus chemotherapy (Table 2). Decreased neutrophil count, decreased white blood cell count and anemia were the most common treatment-related AEs in both treatment groups. Grade 3 or 4 treatment-related AEs were reported for 88 patients (77.9%) who received pembrolizumab plus chemotherapy and 37 patients (78.7%) who received placebo plus chemotherapy. No deaths were attributed to treatment-related AEs. Twenty-six patients (23.0%) and 5 patients (10.6%), respectively, discontinued ≥1 components of study treatment because of a treatment-related AE.
Immune-mediated AEs and infusion reactions were reported for 36/113 patients (31.9%) receiving pembrolizumab plus chemotherapy and 5/47 patients (10.6%) receiving placebo plus chemotherapy. The most common immune-mediated AEs in the pembrolizumab plus chemotherapy group were hypothyroidism, adrenal insufficiency, and hyperthyroidism (Table 2). Immune-mediated AEs and infusion reactions were mostly grade 1 or 2; grade 3 or 4 immune-mediated AEs and infusion reactions occurred in 7 patients (6.2%) in the pembrolizumab plus chemotherapy group (adrenal insufficiency, n = 2; severe skin reaction, n = 2; hepatitis n = 1; infusion reaction, n = 1; pneumonitis, n = 1). No patient in the placebo plus chemotherapy group had a grade 3 or 4 immune-mediated AE or infusion reaction. No deaths were attributed to immune-mediated AEs or infusion reactions in either treatment group.
Discussion
Clinically meaningful improvements in PFS and OS were observed among patients with locally recurrent inoperable or metastatic TNBC who were enrolled in Asia in the KEYNOTE-355 trial who received first-line treatment with pembrolizumab plus chemotherapy compared with patients who received placebo plus chemotherapy. HRs for both PFS and OS favored the pembrolizumab plus chemotherapy group in patients enrolled in Asia overall and among patients with PD-L1 CPS ≥ 10 and CPS ≥ 1 tumors.
We did not separately analyze results in the subgroup of patients who were enrolled outside of Asia for comparison with our findings because that was not the objective of the current analysis. Comparison of our findings with those of the global population from KEYNOTE-355 is therefore limited as the subgroup of patients enrolled in Asia was included in both populations. Recognizing the limitations, the results suggest that benefit with pembrolizumab plus chemotherapy in patients enrolled in Asia with PD-L1 CPS ≥ 10 TNBC was at least as favorable as seen in the global population, with some evidence suggesting that the magnitude of benefit may be greater. However, it must be noted that the 95% CIs in these groups were wider than, and overlapped with, those for the global population. The current results also showed HRs for PFS and OS that favored pembrolizumab plus chemotherapy among patients with PD-L1 CPS ≥ 1 tumors and in the ITT population. Any such potential differences between the global population and the subgroup of patients enrolled in Asia might be driven by differences in driver gene mutations19, genetic polymorphisms22, and/or immunological factors (such as TILs) between these groups23. The finding that benefit was greater among patients enrolled in Asia with higher tumor PD-L1 expression was consistent with the overall study results and with an exploratory subgroup analysis of the KEYNOTE-119 study, which reported a numeric improvement in OS with pembrolizumab monotherapy versus chemotherapy in previously treated patients with CPS ≥ 20 with metastatic TNBC who were enrolled in the Asia-Pacific region24.
Results from the current analysis and the global population of KEYNOTE-355 are supported by earlier findings from the phase 1 KEYNOTE-173 and phase 3 KEYNOTE-522 trials, which demonstrated clinical benefit associated with addition of pembrolizumab to chemotherapy as neoadjuvant treatment for early-stage TNBC25,26,27. In KEYNOTE-522, the pathological complete response rate at the time of definitive surgery for patients with previously untreated stage II or III TNBC was 64.8% with pembrolizumab plus chemotherapy and 51.2% with placebo plus chemotherapy25. The HR for event-free survival (EFS) was 0.63 (95% CI, 0.48‒0.82)26. Median EFS was not reached in either treatment group, with 18-month EFS rates of 91.3% and 85.3%, respectively.
Consistent with our findings, the results from a subgroup analysis of patients enrolled at Japanese centers in the phase 3 IMpassion130 study also demonstrated improved outcomes with atezolizumab in advanced TNBC. Median PFS was 7.4 months with atezolizumab plus chemotherapy and 4.6 months with placebo plus chemotherapy (HR, 0.47 [95% CI, 0.25‒0.90]) in the ITT population28. Among patients with PD-L1‒positive TNBC (assessed using a different assay to that used in the current study), median PFS was 10.8 and 3.8 months, respectively (HR, 0.04 [95% CI, <0.01 to 0.35]). Median OS in the ITT population was not estimable with atezolizumab plus chemotherapy and 16.8 months with placebo plus chemotherapy (HR, 0.44 [95% CI, 0.16‒1.24]). In the PD-L1‒positive subgroup, median OS was not estimable and 13.3 months, respectively (HR, 0.12 [95% CI, 0.01‒0.99]). In the global population of IMpassion130, a statistically significant improvement was demonstrated for PFS (HR, 0.80 [95% CI, 0.69‒0.92]; P = 0.002) but not for OS (HR, 0.84 [95% CI, 0.69‒1.02]; P = 0.08) with the addition of atezolizumab to chemotherapy29.
Our results show that pembrolizumab plus chemotherapy has a manageable safety profile among patients enrolled in Asia with TNBC. Consistent with the global population15, treatment-related AEs of any grade were reported for 97% of patients who received pembrolizumab plus chemotherapy and 98% who received placebo plus chemotherapy. In the subgroup of patients enrolled in Asia, grade 3 or 4 treatment-related AEs occurred at slightly higher rates (78% and 79% of patients, respectively) than were seen in the global population (68% and 67%, respectively). This is not unexpected as prior evidence has reported differences in hematological toxicities between Asian patients and non-Asian patients, including a higher incidence of neutropenia due to taxane-based therapy compared with non-Asian patients30. In the subgroup of patients enrolled in Asia, immune-mediated AEs were reported for 32% of patients receiving pembrolizumab plus chemotherapy and 11% receiving placebo plus chemotherapy. The corresponding rates were 26% and 6%, respectively, in the global population. Median duration of treatment was similar among patients enrolled in Asia versus that in the global population (pembrolizumab plus chemotherapy group: 32 weeks vs 26 weeks; placebo plus chemotherapy, 30 weeks vs 23 weeks)15.
This analysis provides important information describing the activity of pembrolizumab plus chemotherapy in patients enrolled in Asia with locally recurrent inoperable or metastatic TNBC31. However, given that KEYNOTE-355 was not powered to detect statistically significant differences among the subgroup of patients enrolled in Asia, caution is warranted in interpreting the results. The global analysis found a statistically significant and clinically meaningful treatment difference for PFS and OS among patients with tumor PD-L1 CPS ≥ 10 but not for those with CPS ≥ 115,16. Consequently, statistical significance was not assessed for the global ITT population. Numeric differences in PFS and OS outcomes were observed between treatment groups for all subgroups (CPS ≥ 10, CPS ≥ 1, and ITT) in both the current subgroup analyses in patients enrolled in Asia and in the global analyses, with the greatest differences observed among the CPS ≥ 10 subgroup. Additionally, in patients enrolled in Asia with PD-L1 CPS < 10, median PFS and 6-month PFS rates were similar between the two treatment groups and the median OS and 6-month OS rate was higher in the pembrolizumab plus chemotherapy group. However, no formal statistical testing was performed in this subgroup in either the global population or in the patients enrolled in Asia. Our results also highlight a critical need for a more ethnically diverse population in future immunotherapy trials as the majority of patients (∼70%) enrolled in the global population of KEYNOTE-355 were of White race15.
In summary, the present results show clinically meaningful improvements in PFS and OS with pembrolizumab plus chemotherapy in the subgroup of patients enrolled in Asia with locally recurrent inoperable or metastatic TNBC. These findings support the use of pembrolizumab plus chemotherapy as a standard-of-care treatment regimen for Asian patients with PD-L1‒positive (CPS ≥ 10), locally recurrent inoperable or metastatic TNBC, consistent with the global population from KEYNOTE-355.
Methods
Study design and participants
KEYNOTE-355 (ClinicalTrials.gov, NCT02819518) was a phase 3, randomized, placebo-controlled, multicenter, international trial. Detailed methods were previously published15,16. Briefly, eligible patients (≥18 years) had previously untreated, locally recurrent inoperable or metastatic, centrally confirmed TNBC as defined by the American Society of Clinical Oncology College of American Pathologists guidelines32,33; ≥1 measurable lesion per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 as assessed by the investigator; de novo metastasis or completion of treatment with curative intent ≥6 months before the first disease recurrence; Eastern Cooperative Oncology Group performance status of 0‒1; and adequate organ function. Patients were ineligible if they were receiving systemic steroids; had active central nervous system metastases; had a diagnosis of immunodeficiency or received immunosuppressive therapy in the previous week; had class II to IV congestive heart failure or myocardial infarction within 6 months of randomization; had active autoimmune disease in the previous 2 years; had any active infection requiring systemic therapy; history of noninfectious pneumonitis requiring glucocorticoids or current pneumonitis; or history of interstitial lung disease. All patients provided a new tumor sample for immunohistochemistry determination of TNBC and PD-L1 status; however, patients were eligible to enroll regardless of tumor PD-L1 status.
The study was conducted in accordance with the Declaration of Helsinki and the International Council on Harmonisation Good Clinical Practice guidelines. An institutional review board or independent ethics committee at each site approved the protocol (Supplementary Table 3). Patients provided written informed consent.
Randomization and study treatment
Patients were randomized 2:1 to receive pembrolizumab 200 mg or placebo intravenously (IV) every 3 weeks plus the investigator’s choice of open-label chemotherapy. Chemotherapy dosing regimens consisted of nab-paclitaxel 100 mg/m2 IV on days 1, 8, and 15 every 28 days; paclitaxel 90 mg/m2 IV on days 1, 8, and 15 every 28 days; or gemcitabine 1000 mg/m2 with carboplatin AUC 2 on days 1 and 8 every 21 days. Pembrolizumab was continued for up to 35 administrations (∼2 years) or until confirmation of progressive disease, unacceptable toxicity, consent withdrawal, or physician decision. Chemotherapy was continued at the investigator’s discretion.
Randomization was done using a central interactive voice response system with an integrated web-response system (Oracle; Redwood City, CA). Randomization used a block method (block size of 6) and was stratified according to chemotherapy received (taxane or gemcitabine-carboplatin), tumor PD-L1 expression (CPS ≥ 1 or < 1), and prior treatment with the same class of chemotherapy in the neoadjuvant or adjuvant setting (yes or no). Patients, investigators, the sponsor, and other study site staff were blinded to treatment assignment and tumor PD-L1 status.
Endpoints
The dual primary endpoints were PFS (per RECIST version 1.1) by blinded independent central review (BICR) and OS among the ITT population (all randomized patients) and among patients with PD-L1–positive tumors (CPS ≥ 10 and ≥ 1). After enrollment and the first interim analysis were complete, the primary endpoints were amended to include assessments of PFS and OS in patients with tumor CPS ≥ 10. This decision was based on data from other clinical studies that showed greater clinical benefit in patients with higher PD-L1 expression12,15,16. Secondary endpoints included ORR per RECIST version 1.1 by BICR in the ITT population and in those with PD-L1–positive tumors (CPS ≥ 10 and ≥ 1), and safety.
Assessments
Baseline tumor PD-L1 status was assessed at a central laboratory (Q2 Solutions; Valencia, CA) using PD-L1 IHC 22C3 PharmDx (Agilent Technologies, Inc.; Carpinteria, CA). PD-L1 status was determined according to the CPS, calculated as the number of PD-L1‒positive tumor cells, lymphocytes, and macrophages, divided by the total number of tumor cells, multiplied by 10034.
Tumor imaging was done every 8 weeks through week 24, then every 9 weeks through week 52, and every 12 weeks thereafter. Response was assessed per RECIST version 1.1 by BICR. Patients who had progressive disease or who began new anticancer therapy were contacted every 12 weeks to monitor survival.
Adverse events were monitored throughout the study and for 30 days after treatment had ended (90 days for serious AEs). AEs were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. Immune-mediated AEs were based on a predefined list of MedDRA terms.
Statistical analyses
The study was powered to test hypotheses in the global population; no alpha was assigned to the exploratory analyses of patients enrolled in Asia; therefore, the results reported herein are considered descriptive only. PFS and OS were estimated using the nonparametric Kaplan-Meier method. An unstratified Cox proportional hazard model with the Efron method of tie handling was used to calculate HRs with 95% CIs. The randomization stratification factors were also used for all stratified analyses. Statistical analyses were done using SAS version 9.4 (Cary, NC). A full description of statistical analyses for the primary and secondary hypotheses have been previously published15,16.
Data availability
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD) is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the US and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
References
Alcantara, V. S., Lim, G. H., Lim, S. H., Sultana, R. & Lee, J. A. Incidence and prognosis of non-metastatic triple negative breast cancer (TNBC) among different races in Southeast Asia. J. Surg. Oncol. 115, 523–537 (2017).
Kulkarni, A. et al. Meta-analysis of prevalence of triple-negative breast cancer and its clinical features at incidence in Indian patients with breast cancer. JCO Glob. Oncol. 6, 1052–1062 (2020).
Boyle, P. Triple-negative breast cancer: epidemiological considerations and recommendations. Ann. Oncol. 23, vi7–12 (2012).
Lin, C. H. et al. Contrasting epidemiology and clinicopathology of female breast cancer in Asians vs the US population. J. Natl. Cancer Inst. 111, 1298–1306 (2019).
Loi, S. et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann. Oncol. 25, 1544–1550 (2014).
Adams, S. et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J. Clin. Oncol. 32, 2959–2966 (2014).
Gatalica, Z. et al. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol. Biomarkers Prev. 23, 2965–2970 (2014).
Mittendorf, E. A. et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol. Res. 2, 361–370 (2014).
Loi, S. et al. Tumor-infiltrating lymphocytes and prognosis: a pooled individual patient analysis of early-stage triple-negative breast cancers. J. Clin. Oncol. 37, 559–569 (2019).
Heeke, A. L. & Tan, A. R. Checkpoint inhibitor therapy for metastatic triple-negative breast cancer. Cancer Metastasis Rev. 40, 537–547 (2021).
Adams, S. et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann. Oncol. 30, 405–411 (2019).
Nanda, R. et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J. Clin. Oncol. 34, 2460–2467 (2016).
Adams, S. et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann. Oncol. 30, 397–404 (2019).
Winer, E. P. et al. Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): a randomised, open-label, phase 3 trial. Lancet Oncol. 22, 499–511 (2021).
Cortes, J. et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 396, 1817–1828 (2020).
Cortes, J. et al. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N. Engl. J. Med. 387, 217–226 (2022).
Keytruda (pembrolizumab). Full Prescribing Information, Merck & Co., Inc., Rahway, NJ, USA, 2021.
European Medicines Agency. Keytruda. https://www.ema.europa.eu/en/medicines/human/EPAR/keytruda. Accessed March 13, 2024.
Nagahashi, M. et al. Actionable gene alterations in an Asian population with triple-negative breast cancer. JCO Precis Oncol 2, PO.17.00211 (2018).
Kan, Z. et al. Multi-omics profiling of younger Asian breast cancers reveals distinctive molecular signatures. Nat Commun. 9, 1725 (2018).
Han, H. S. et al. Racial differences in acute toxicities of neoadjuvant or adjuvant chemotherapy in patients with early-stage breast cancer. Eur. J. Cancer 47, 2537–2545 (2011).
Lal, S. et al. Novel SLC22A16 polymorphisms and influence on doxorubicin pharmacokinetics in Asian breast cancer patients. Pharmacogenomics 8, 567–575 (2007).
Pan, J. W. et al. The molecular landscape of Asian breast cancers reveals clinically relevant population-specific differences. Nat. Commun. 11, 6433 (2020).
Im, S. et al. Pembrolizumab (pembro) vs chemotherapy (chemo) for previously treated metastatic triple-negative breast cancer (mTNBC): KEYNOTE-119 Asia-Pacific subpopulation. Ann. Oncol. 31, S1257–S1269 (2020).
Schmid, P. et al. Pembrolizumab for early triple-negative breast cancer. N. Engl. J. Med. 382, 810–821 (2020).
Schmid, P. et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N. Engl. J. Med. 386, 556–567 (2022).
Schmid, P. et al. Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: results from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann. Oncol. 31, 569–581 (2020).
Iwata, H. et al. Subgroup analysis of Japanese patients in a phase 3 study of atezolizumab in advanced triple-negative breast cancer (IMpassion130). Jpn. J. Clin. Oncol. 49, 1083–1091 (2019).
Schmid, P. et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 379, 2108–2121 (2018).
Lu, Y. S. et al. An overview of the treatment efficacy and side effect profile of pharmacological therapies in Asian patients with breast cancer. Target. Oncol. 16, 701–741 (2021).
Wang, C. et al. Triple negative breast cancer in Asia: an insider’s view. Cancer Treat. Rev. 62, 29–38 (2018).
Hammond, M. E., Hayes, D. F., Wolff, A. C., Mangu, P. B. & Temin, S. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Oncol. Pract. 6, 195–197 (2010).
Wolff, A. C. et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J. Clin. Oncol. 25, 118–145 (2007).
Kulangara, K. et al. Clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch. Pathol. Lab. Med. 143, 330–337 (2019).
Acknowledgements
Funding for this research was provided by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. We thank the patients and their families and caregivers for participating in this study, along with all investigators and site personnel. Medical writing assistance was provided by Autumn Kelly, MA, of ICON plc (Blue Bell, PA, USA). This assistance was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. Sherene Loi is supported by the National Breast Cancer Foundation of Australia Endowed Chair and the Breast Cancer Research Foundation, New York. Seock-Ah Im had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All collaborators of this study who have fulfilled the criteria for authorship required by Nature Portfolio journals have been included as authors, as their participation was essential for the design and implementation of the study. Roles and responsibilities were agreed among collaborators ahead of the research. This work includes findings that are locally relevant, which have been determined in collaboration with local partners. This research was not severely restricted or prohibited in the setting of the researchers and does not result in stigmatization, incrimination, discrimination, or personal risk to participants. Local and regional research relevant to our study was taken into account in citations. Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. These results were presented in part at the 19th Annual Meeting of the Japanese Society of Medical Oncology; Kyoto, Japan; February 17‒19, 2022 (abstract PS2-2).
Author information
Authors and Affiliations
Contributions
Conception, design or planning of the study: Seock-Ah Im, Javier Cortes, Chi-Feng Chung, Wilbur Pan, Vassiliki Karantza, Hope S. Rugo, Peter Schmid. Acquisition of the data: Seock-Ah Im, David W. Cescon, Mastura Md Yusof, Hiroji Iwata, Norikazu Masuda, Toshimi Takano, Chiun-Sheng Huang, Chi-Feng Chung, Koichiro Tsugawa, Yeon Hee Park, Koji Matsumoto, Kenichi Inoue, Ava Kwong, Sherene Loi, Hope S. Rugo. Analysis of the data: Seock-Ah Im, Chi-Feng Chung, Yeon Hee Park, Wei Fu, Wilbur Pan, Vassiliki Karantza, Peter Schmid. Interpretation of the results: Seock-Ah Im, Javier Cortes, David W. Cescon, Mastura Md Yusof, Hiroji Iwata, Norikazu Masuda, Chiun-Sheng Huang, Chi-Feng Chung, Koichiro Tsugawa, Yeon Hee Park, Koji Matsumoto, Kenichi Inoue, Ava Kwong, Sherene Loi, Wei Fu, Wilbur Pan, Vassiliki Karantza, Hope S. Rugo, Peter Schmid. Drafting of the manuscript: Seock-Ah Im, Chi-Feng Chung, Yeon Hee Park, Wei Fu, Peter Schmid. Reviewing or revising the manuscript for important intellectual content: Javier Cortes, David W. Cescon, Mastura Md Yusof, Hiroji Iwata, Norikazu Masuda, Toshimi Takano, Chiun-Sheng Huang, Chi-Feng Chung, Koichiro Tsugawa, Yeon Hee Park, Koji Matsumoto, Kenichi Inoue, Ava Kwong, Sherene Loi, Wei Fu, Wilbur Pan, Vassiliki Karantza, Hope S. Rugo, Peter Schmid. Provision of study materials/patients: Seock-Ah Im, David W. Cescon, Mastura Md Yusof, Hiroji Iwata, Toshimi Takano, Chiun-Sheng Huang, Chi-Feng Chung, Koichiro Tsugawa, Yeon Hee Park, Koji Matsumoto, Ava Kwong, Hope S. Rugo. Final approval and accountability: all authors.
Corresponding author
Ethics declarations
Competing interests
Seock-Ah Im: reports advisory role for AstraZeneca, Daiichi-Sankyo, GSK, Hanmi, Idience, Lilly, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD), Novartis, Pfizer, Bertis and Roche; and grants for AstraZeneca, Boryung Pharm, Daewoong Pharm, Daiichi-Sankyo, Eisai, Pfizer, and Roche. Javier Cortes: Consulting/Advisor: Roche, Celgene, Cellestia, AstraZeneca, Seattle Genetics, Daiichi Sankyo, Erytech, Athenex, Polyphor, Lilly, MSD, GSK, Leuko, Bioasis, Clovis Oncology, Boehringer Ingelheim, Ellipses, Hibercell, BioInvent, Gemoab, Gilead, Menarini, Zymeworks, Reveal Genomics, and Expres2ion Biotechnologies. Honoraria: Roche, Novartis, Celgene, Eisai, Pfizer, Samsung Bioepis, Lilly, MSD, Daiichi Sankyo, and AstraZeneca. Research funding to institution: Roche, Ariad Pharmaceuticals, AstraZeneca, Baxalta GMBH/Servier Affaires, Bayer Healthcare, Eisai, F. Hoffman-La Roche, Guardanth Health, MSD, Pfizer, Piqur Therapeutics, Puma C, and Queen Mary University of London. Stock: MedSIR, Nektar Pharmaceuticals, and Leuko (relative). Travel, accommodation, expenses: Roche, Novartis, Eisai, Pfizer, Daiichi Sankyo, AstraZeneca, Gilead, and MSD. Patents: Pharmaceutical Combinations of a Pi3k Inhibitor and a Microtubule Destabilizing Agent.Javier Cortés Castán, Alejandro Piris Giménez, Violeta Serra Elizalde. WO 2014/199294 A. ISSUED; Her2 as a predictor of response to dual HER2 blockade in the absence of cytotoxic therapy.Aleix Prat, Antonio Llombart, Javier Cortés.US 2019/ 0338368 A1. LICENSED. David W. Cescon: Consultancy/Advisory: AstraZeneca, Exact Sciences, Eisai, Gilead, GlaxoSmithKline, Inflex, Inivata/Neogenomics, Lilly, MSD, Novartis, Pfizer, Roche, and SAGA. Research support (to institution): AstraZeneca, GlaxoSmithKline, Inivata, Knight, Merck, Pfizer, and Roche. Member of a trial steering committee: AstraZeneca, Merck, and GlaxoSmithKline. Holds a patent (US62/675,228) for methods of treating cancers characterized by a high expression level of spindle and kinetochore associated complex subunit 3 (ska3) gene. Mastura Md Yusof: Received honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Merck, MSD, Specialised Therapeutics, Zuellig Pharma, Novartis, Pfizer, Roche, Eisai, Celgene; has received grant funding (to institution) from Astellas, AstraZeneca, Arcus, Genentech, Mundi Pharma, Novartis, Pfizer, Roche. Hiroji Iwata: Honoraria - AstraZeneca; Chugai Pharma; Daiichi Sankyo; Eisai; Kyowa Hakko Kirin; Lilly Japan; Novartis; Pfizer. Consulting or Advisory Role - AstraZeneca; Chugai Pharma; Daiichi Sankyo; Kyowa Hakko Kirin; Lilly Japan; Novartis; Pfizer. Research Funding - AstraZeneca, Bayer, Chugai Pharma, Daiichi Sankyo, Eisai, GlaxoSmithKline, Kyowa Hakko Kirin, Lilly Japan, MSD, Nihonkayaku, Novartis, and Pfizer (all to institution). Norikazu Masuda: Leadership - Japan Breast Cancer Research Group Association (JBCRG). Honoraria - AstraZeneca; Chugai Pharma; Eisai; Lilly Japan; Pfizer. Research Funding – AstraZeneca, Chugai Pharma, Daiichi Sankyo, Eisai, Eli Lilly, Kyowa-Kirin, MSD, Novartis, Pfizer, and Sanofi (all to institution). Toshimi Takano: Lecture fees from Daiichi-Sankyo, Chugai, Kyowa Kirin, Eisai, Pfizer, Eli Lilly, and Celltrion. Research funding from Daiichi-Sankyo, Chugai, Eisai, Ono, and MSD. Chiun-Sheng Huang: Research grants: Aston Sci, AstraZeneca, Daiichi Sankyo, EirGenix, Eli Lilly, Gilead, MSD, Novartis, OBI Pharma, Pfizer, Roche, and Seagen. Honoraria: AstraZeneca, Daiichi Sankyo, Eli Lilly, Novartis, Pfizer, and Roche. Advisory fees: AstraZeneca, Daiichi Sankyo, Eli Lilly, Novartis, Pfizer, and Roche. Chi-Feng Chung: Honoraria: Daiichi Sankyo, Roche, Lotus, EirGenix, AstraZeneca, and Novartis. Koichiro Tsugawa: Honoraria: Pfizer and Kyowa Hakko Kirin. Research funding (to institution): Daiichi Sankyo. Yeon Hee Park: Consultancy or advisory fees: AstraZeneca, Eisai, Eli Lilly Export S.A. Puerto Rico Branch, Novartis, Pfizer, and Roche. Research funding: AstraZeneca, Merck, Novartis, Pfizer, and Roche. Koji Matsumoto: Honoraria: Chugai, Kyowa Hakko Kirin, and MSD. Research funding (to institution): Daiichi Sankyo, Eisai, Gilead Sciences, Lilly Japan, and MSD. Kenichi Inoue: Research funding: AstraZeneca, Chugai Pharma, Daiichi Sankyo, Eisai, Eli Lilly, Kyowa-Kirin, MSD, Novartis, Pfizer, Taiho, Ono, Astellas, and Sanofi (all to institution). Ava Kwong: Honoraria (for giving educational lectures and attending conferences): AstraZeneca, Pfizer, and Stryker Roche. Grant funding (to institution for clinical trials and research): AstraZeneca, Pfizer, MSD (Asia) Limited, and Roche. Sherene Loi: research funding to her institution from Novartis, Bristol-Myers Squibb, Merck, Puma Biotechnology, Eli Lilly, Nektar Therapeutics, Astra Zeneca, and Seattle Genetics. She has acted as consultant (not compensated) to Seattle Genetics, Novartis, Bristol-Myers Squibb, Merck, AstraZeneca, Eli Lilly, Pfizer, Gilead Therapeutics, and Roche-Genentech. She has acted as consultant (paid to her institution) to Aduro Biotech, Novartis, GlaxoSmithKline, Roche-Genentech, Astra Zeneca, Silverback Therapeutics, G1 Therapeutics, PUMA Biotechnologies, Pfizer, Gilead Therapeutics, Seattle Genetics, Daiichi Sankyo, Merck, Amunix, Tallac Therapeutics, Eli Lilly, and Bristol-Myers Squibb. Wei Fu: employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA; owns stock in Merck & Co., Inc., Rahway, NJ, USA. Wilbur Pan: employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA; owns stock in Merck & Co., Inc., Rahway, NJ, USA. Vassiliki Karantza: employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA; owns stock in Merck & Co., Inc., Rahway, NJ, USA. Hope S. Rugo: Research support to institution: Astellas Pharma Inc.; AstraZeneca; Daiichi Sankyo, Inc.; F. Hoffmann-La Roche AG/Genentech, Inc.; Gilead Sciences, Inc.; GlaxoSmithKline; Eli Lilly; Merck & Co., Inc.; Novartis Pharmaceuticals Corporation; OBI Pharma; Pfizer; Pionyr Immunotherapeutics; Sermonix Pharmaceuticals Inc.; Taiho Oncology, Inc., and Veru Inc. Consultancy/advisory support: Puma, NAPO, Blueprint, and Scorpion Therapeutics. Peter Schmid: has been a consultant to/received honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Merck, Novartis, Pfizer, Puma, Roche, Eisai, and Celgene; has received grant funding (to institution) from Astellas, AstraZeneca, Genentech, Novartis, Oncogenex, Roche, and Medivation.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Im, SA., Cortes, J., Cescon, D.W. et al. Results from the randomized KEYNOTE-355 study of pembrolizumab plus chemotherapy for Asian patients with advanced TNBC. npj Breast Cancer 10, 79 (2024). https://doi.org/10.1038/s41523-024-00679-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41523-024-00679-7
- Springer Nature Limited