Abstract
This review screened 296 articles on wearable sensors for home monitoring of people with Parkinson’s Disease within the PubMed Database, from January 2017 to May 2023. A three-level maturity framework was applied for classifying the aims of 59 studies included: demonstrating technical efficacy, diagnostic sensitivity, or clinical utility. As secondary analysis, user experience (usability and patient adherence) was evaluated. The evidences provided by the studies were categorized and stratified according to the level of maturity. Our results indicate that approximately 75% of articles investigated diagnostic sensitivity, i.e. correlation of sensor-data with clinical parameters. Evidence of clinical utility, defined as improvement on health outcomes or clinical decisions after the use of the wearables, was found only in nine papers. A third of the articles included reported evidence of user experience. Future research should focus more on clinical utility, to facilitate the translation of research results within the management of Parkinson’s Disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Over the last 20 years, the digital transformation of medicine has evolved tremendously due to the development of wearable sensors as clinical support tools providing objective (digital) outcomes for clinical research and in remote monitoring applications for clinical care. The unwavering research interest in wearable sensors relies on their ability to generate accurate, quantitative, real-world, rater-independent, and user-derived measures for various medical applications.
Wearable sensors, or simply “wearables”, are not limited to a specific clinical setting, allowing for continuous or high sampling frequency assessments that are unsustainable by standard clinical evaluations. Consequently, the data generated by wearables can track the whole spectrum of changes in the user’s functional state and link impairment to clinical symptoms of patients. Furthermore, wearables can capture subtle, prodromal, or granular variations in motor symptoms that are clinically relevant but extremely difficult, if not impossible, to detect with current evaluation methods1,2. Finally, wearable technologies can be synergized with medical profiling, data mining, and machine learning to generate individualized health reports that inform patients, healthcare professionals, and society. Extracted data can complement patient-reported outcomes with digital objective biomarkers promptly detecting symptom deterioration. The recorded diagnostic information can support healthcare professionals in decision-making and provide a closed feedback loop during interventions. Finally, they can be used as objective and evidence-based indicators to regulate reimbursement within the healthcare ecosystem, providing additional value for payers and policymakers.
Parkinson’s Disease (PD) is an ideal target for the application of wearable sensors. It is the second-most common progressive neurodegenerative disease of the central nervous system3,4, known for its diverse clinical phenotypes, affecting both motor and non-motor domains. The former is mainly characterized by bradykinesia, rigidity, tremor, gait and balance impairment, while nonmotor symptoms expand across autonomic, neuropsychiatric, sensory and sleep domains, which may be attributed to aging rather than the underlying pathological mechanism5. For patients living with PD, this often means a confrontation with a variety of symptoms in their daily activities, a change in quality of life and interpersonal relationships, and in later life, the need for continuous care6. The progressive nature of PD and its heterogeneity across different patients makes the management complex and multi-dimensional5. For this reason, the medical workups are also constantly evolving in parallel with the patient’s journey, trying to balance short-term benefits with the long-term effects on the progression of the disease.
In this context, accurately tracking PD symptoms and their oscillation is crucial for optimal care. Typically, this is done during clinical assessments, where the patient is asked to perform a series of standardized tasks. At the same time, a trained neurologist visually evaluates the movements and provides a score for each symptom. In parallel, the non-motor sphere is evaluated via different questionnaires to obtain a comprehensive picture of the subject’s conditions.
However, recent studies have demonstrated that motor symptom severity can fluctuate rapidly, even multiple times in an average 30-min consultation2. Consequently, due to the excessive granularity needed, accurate tracking in time is not feasible through standard-of-care methods. These traditional methodologies, such as motor diaries and standard clinical examinations, need to be revised to decrease the rater-dependent variance and subjectivity, low accuracy, low sampling frequency, and low sensitivity-to-change7,8. For this reason, wearables raise the expectation that they will complement classical clinical examination, as they provide healthcare professionals with objective measures that may support clinical decisions or yield insights into the efficacy of clinical interventions.
PD is an ideal target for the application of wearables due to the variety of symptoms that appear throughout the stages of the disease (diagnostic target symptom of wearables) and their sensitivity to change following an intervention (monitoring target symptom for wearables)9. Monitoring target symptoms through wearables may help detect symptoms fluctuating over different timeframes ranging from months to years (progression of the disease), days to months (treatment kinetics), or even within minutes (motor fluctuations, fast-responding interventions). Thus, in remote monitoring settings, wearables must accurately measure, linking the information recorded with relevant clinical aspects and ultimately providing support to patients or healthcare professionals10.
During the last decades, different types of wearable sensors have been developed by researchers to address the aforementioned challenges. Inertial Measurement Units (IMUs) have found an immediate application in investigating motor symptoms such as tremor, dyskinesia, bradykinesia, and mobility impairments. Pressure sensors have also been used to evaluate gait and balance disturbances of patients. Furthermore, wearables have been utilized to better understand the impact of PD on biopotential signals, generating electrocardiograms, electromyograms, and electroencephalograms. Finally, in recent years, electrochemical biosensors have been gaining popularity to monitor α-synuclein and other biomarkers of the disease from blood samples11.
Wearables can accurately collect a huge amount of relevant medical information from patients. However, from a practical point of view, their application in the management of PD is still limited.
Looking at the literature on wearables and PD as a joint research topic, since 2000, exclusively in the PubMed Central database (RRID: SCR_004846), 950 papers related to wearables and PD have been published (see Fig. 1). This number is expected to exceed one thousand units by the end of 2023. However, when looking at healthcare procedures, a barrier to the broader implementation of wearables in PD management becomes evident. In real-world care, treatment adjustments are generally based exclusively on doctors’ experience, relying on information collected during short in-person neurological visits. Even though some wearables reached the maximum “Technological readiness” level TRL9 and have gained regulatory approval12, their precise scope within PD management remains unclear13.
Number of articles published from 2000 to May 2023 focusing on wearables sensors and PD (screening search terms: Parkinson and wearables); screening performed using PubMed as a data-source (RRID: SCR_004846). In dashed red line the estimation for the full 2023. In total the research revealed 950 papers. This number is expected to exceed one thousand unit by the end of the year.
One of the possible causes of this translational gap is a lack of what in this manuscript is called “clinical utility evidence”14 defined as: “an improvement on health outcomes, diagnosis, treatment management or prevention demonstrated after the use of the wearable sensor”. In fact, the value of wearables for patients and doctors is ultimately determined by the improvements they can generate in terms of health outcomes and treatment management, rather than the technical accuracy of their measurements only. Interestingly, different evidence evaluation frameworks have already conceptualized this “clinical utility” concept while addressing the development phases of diagnostic tests in healthcare15 or aspects of digital transformation of technology in general16.
In general, clinical utility should be measured within the standard of care. However, quantifying the impact of wearables in this context may be extremely challenging, due to the huge variability in terms of patients, settings, care pathways, and healthcare professionals. Research studies can provide a valuable option to reduce this heterogeneity. In fact, they present more structured assessment protocols and inclusion/exclusion criteria, which allow to investigate more easily the clinical utility of these technologies.
For this reason, in this review, we want to evaluate the clinical utility evidences generated by research studies that focus on wearable sensors in PD applied to the home monitoring setting. The final goal is not only clarifying their applicability in term of disease management strategy, but also to better quantify the impact of these technologies on clinical decisions and their effectiveness on improving health outcomes. For this reason, we tailored the existing evidence models towards the evaluation of clinical utility of wearables and reviewed the included studies based on their maturity level. We narrowed our search to articles targeting wearables for home monitoring in people with PD.
In our review process, we categorized eligible articles on wearables by three hierarchical efficacy evidence levels: technical efficacy, diagnostic sensitivity, and clinical utility.
In addition, as secondary analysis, we also evaluated user-experience related aspects presented in the studies. This because the health outcomes obtained might be deeply influenced by the usability of the system and the concordance/adherence of the patients. A detailed description of the methods that we applied, including our evaluation framework and article assignment criteria, can be found in the final section of this review.
Results
Article selection
Within the large spectrum of studies on wearable sensors in PD, we identified 296 articles addressing their home monitoring applicability. From these articles, we identified through PubMed between January 2017 and May 2023, 59 studies were eligible for inclusion after screening and full-text eligibility assessment. Interestingly, albeit no exclusion criteria were applied on the type of sensor technology, only a limited number of papers adopted alternative measurement tools to inertial measurement units. More precisely, surface electromyography, pressure sensors and audio stimuli, were utilized in one study each, while 2 articles used GPS to track participants mobility.
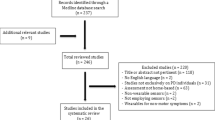
The PRISMA flow diagram generated through the review process can be found in Fig. 2, with an overview of excluded studies17.
Article assignment
Articles were assigned to the EELs based on their research questions. The distribution of the papers reviewed, represented in Fig. 3, shows that 56 studies out of 59 included evidence results, while three papers focused exclusively on wearables usability and/or adherence18,19,20. Five articles were assigned in multiple EELs due to overlapping aspects being evaluated: four were assigned to both Technical Efficacy and Diagnostic Sensitivity levels21,22,23,24, and one was assigned to Diagnostic Sensitivity and Clinical Utility25. Regarding User Experience, 21 articles reported evidence of usability and adherence within their results, and two exclusively assessed the usability level26,27. The using environment of the research studies analyzed in this review is the home setting. However, in the supplementary tables of this manuscript we also reported separately the results obtained in lab environment, when present.
Level I. technical efficacy
Research questions on technical efficacy were investigated in six articles21,22,23,24,28,29. They assessed the accuracy of sensor-based mobility measures without connecting the outcomes to clinical scores or patient phenotypes. Overall, the primary focus of EEL-I articles was to detect movements of interest in a real-world environment, such as walking bouts and activities of daily living. The most common goal shared by three out of the six articles was accurately detecting gait segments in the unsupervised environment23,28,29.
Patient numbers varied significantly across studies, with a minimum of four patients22 up to a maximum of 25 people with PD21,23. Full results are presented in Supplementary Table 1.
Level II. diagnostic sensitivity
The second level was associated with research questions on diagnostic sensitivity. A total of 46 articles were included, which accounted for >75% of those reviewed. Study types and research questions varied significantly across the group, spanning from assessing the capability of gait sensors to differentiate the mobility of PD versus healthy controls or other neurodegenerative diseases26,30,31,32,33,34 to comparing sensor-derived parameters with symptoms23,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49 and clinical scores21,50,51. Differences across PD patient phenotypes were also investigated across fallers vs. non-fallers43,44 and freezers vs. non-freezers45,46.
Additionally, three manuscripts presented results using machine learning models for prediction. Shah et al.31 and L. Evers et al.30, while discriminating PD versus HC, achieved an AUC of 0.89 and 0.76, respectively, while Mancini et al.45 classified freezers vs. non-freezers with an AUC equal to 0.90. The impact of the “Real-World” noise was also assessed but difficult to quantify. Powers et al.25 reported 8% of false positives in their study due to manual teeth brushing and 2% linked to playing musical instruments. Furthermore, it was observed that measures in the clinic are often not representative of the patient’s conditions at home41, as patients’ gait at home was slower, strides shorter, and shuffling gait more present. The remaining results are presented in Supplementary Table 2.
Level III. clinical utility
Clinical utility as the EEL-III included nine articles (15%)25,52,53,54,55,56,57,58,59. No increasing trend was /observed in the six years considered (0 articles until May 2023, 1 paper in 2022, 4 studies from 2021, 2 from 2019, 0 from 2018, and 2 from 2017. See Table S3 for details). Three articles presented interventional studies where remote monitoring wearable sensors improved outcomes through individualized measures within training or rehabilitation settings57,58,59. Two assessed auditory stimuli for mobility training57,58. The remaining studies explored more traditional treatment programs25,52,53,54,55,56.
The utility of wearables information in the context of clinical decisions was assessed in 6 articles25,52,53,54,55,56. The contribution of sensor-based measurements in providing sufficient additional knowledge to justify therapy changes varied across the studies, from 6% (6 out of 100 participants) in25 up to 43% (85 out of 200 participants) in ref. 56. The medical impact of wearables was quantified in two studies54,56, which both observed statistically significant improvements in terms of clinical scores when sensor measurements support doctors and healthcare professionals. Complete study outcomes are listed in Supplementary Table 3.
Types of efficacy evidence provided
Four macro types of analytical evidence have been used to prove the different EELs. Statistical methods were the most exploited tools to compare groups, diagnoses, and interventions. Primarily, descriptive statistic of the distributions examined was presented together with an associated p-value. Rarely were Cohen-d values for the effect size or Area Under the Curve (AUC) values presented.
Correlations analysis, described generally as correlation coefficients and relative p-values, were the most common approaches for the analysis of symptom severity. In some cases, confidence intervals (CI) and R2 values were also reported. Intraclass Correlation Coefficient (ICC) was unanimously applied to evaluate test-retest reliability.
Articles based on machine learning metrics, all provided with measures beyond pure accuracy. If the AUC was overall the most frequent outcome, sensitivity, specificity, F1 score, and mean absolute error were used in the different papers reviewed.
Finally, qualitative/descriptive statistics were utilized in the reviewed papers. It is interesting to mention that this type of approach was used only in EEL-I when feasibility was tested or when describing clinical decision support in EEL-III.
For detailed results on the analytical evidence types, see Tables 1, 2, and 3.
User Experience
Twenty-one articles included user experience outcomes about the usability of wearables or participants’ adherence. Two articles investigated exclusively whether the patients considered a device usable26,27, and 11 articles focused only on adherence to the study protocol19,25,32,37,48,54,55,57,58,60,61, and 8 evaluated both18,20,22,40,52,57,59,62.
The duration of remote data collection across the different studies spanned from 2 days to 16 months. The small sample size of the cohort was the most frequent limitation, with only two papers that enrolled >50 participants.
The largest study that assessed usability and adherence was from Lima et al., which included 953 PD participants in North America (NAM) and the Netherlands (NL), monitored for 6 or 13 weeks20,33,36. Overall, 84% of participants contributed sensor data, although this amount was affected by the platform usability score and self-reported user depression. Participant’s adherence to the study protocol ranged from 62% (14.8 h/participant/day) up to 68% (16.3 h/participant/day) in the different countries. A general decreasing trend in time for adherence was observed in both cohorts, with the daily accelerometer data recording a reduction of 23% in the NL after 13 weeks and 27% in NAM after 6 weeks.
Burg et al. reported on 388 people with early-stage PD where usability was measured as the participant’s ability to perform the protocol test during the in-clinic visit, obtaining almost a perfect score (100% for tremor and upper extremity bradykinesia and 98.5% for gait)40. Adherence was evaluated in terms of median wear-time (21.1 h/day), and the percentage of per-protocol remote assessments completed was 59%. Similar to the previous study, the adherence rate decreased with time from 80% of participants who had at least one virtual motor exam during the first week to just 40% in week 52, with a dropout rate of 5.4%.
In general, all papers reviewed presented good results regarding adherence and usability. Participants reported positive experiences with wearables and found them easy to use and incorporate into their daily routines. Nevertheless, long-term data collection still measured a substantial decrease in adherence with time. Detailed user experience results are summarized in Supplementary Table 4.
Discussion
The primary aim of this manuscript was to assess the evidence of clinical utility and general usability of wearables for home-monitoring applications to understand if these are two critical elements that limit the translation of research results into the patient’s journey in real-world healthcare scenarios. This was evaluated through a systematic review, which categorized the level of maturity of wearable technologies in research studies by assigning tailored Efficacy Evidence Levels for each paper.
Most manuscripts published in literature on wearable sensors in PD focus exclusively on technology development under a laboratory test environment. Consequently, they were excluded from this review during the screening phase. However, we could identify 59 manuscripts that transfer wearable technology into home monitoring, which adds to the technical measurement aspects, the real-world context, and non-standardized clinical assessments.
The division of the articles with our framework highlighted how the majority (>80%) is oriented toward technical efficacy or diagnostic sensitivity. Consequently, it does not directly generate evidence of improvement in health outcomes, diagnosis, treatment management, or prevention. Only nine articles could be categorized into the EEL-III, clinical utility level. Two major clinical contexts were associated with these studies. The authors either presented an improvement in patients’ condition after sensor-based training interventions, or demonstrated the utility of wearables for healthcare professionals during their clinical decisions.
The interventional studies testing the effect of wearables technologies revealed that sensors could play an essential role in this type of trial with closed-loop feedback, individualized training parameters, and primary outcome measures. The crucial factor observed was training intensity. Protocols with a minimum training frequency of 150 min per week for at least 6 weeks yielded considerably higher benefits than a lower training frequency63. These findings align with previously published research on rehabilitation training64.
When investigating clinical decision support, the primary aim of digital technologies was to provide doctors with a better overview of the individual patient’s motor symptom severity and fluctuation. This allowed a personalized treatment selection and a more accurate evaluation of the therapy effects in time. However, the final impact of sensors on health outcomes has been assessed quantitatively only in two studies. In the work from A. Farzanehfar et al.56, sensor-based therapy modification led to a decrease of 6 MDS-UPDRS III and 12 total MDS-UPDRS points compared to regular assessment. Similarly, Isaacson et al.54 observed a significant least square mean improvement of 2.6 points in UPDRS II and a descriptive improvement of 4 points in UPDRS III. The remaining four studies in the EEL-III group presented qualitative results on clinical utility.
This imbalance between quantitative vs. qualitative results highlights a critical gap in the assessment of clinical utility. In the future, it would be essential having harmonized conditions and study designs to assess and compare the clinical utility of the different digital technologies. Ideally, for the specific case of Parkinson’s, the wearables effect should be evaluated on long-term disease progression, rapid changes due to interventional trials, and standard-of-care treatment cycles65.
A better evaluation framework for clinical utility of studies would lead not only to an improved care for patients, but also to validated metrics to assess wearables outcomes and support clinical decision-making. It is important mentioning in this regard, the results of a recent scoping review on the clinical adoption of gait analysis technologies (which was not specific to the clinical and usability settings covered in this manuscript)66. This work identified as major barriers to adoption of sensors, as reported by healthcare professionals, the underlying interpretability of the measurements in the clinical decision context, and the lack of literacy available with reference data to establish objective comparisons. These findings highlight the necessity of more numerous and structured clinical utility studies.
Within the diagnostic sensitivity category EEL-II, the studies reviewed presented a broad range of targeted motor symptoms within heterogeneous applications, participants, and sample sizes.
Studies converge in claiming the feasibility of “symptoms monitoring” and “treatment monitoring”: continuously tracking in the home environment clinically relevant information about the severity of patient motor symptoms25,30,35,37,47,61,62,67. This tracking is affected by “Real-World” noise, i.e., movements from unsupervised daily activities mimicking PD symptoms, which may lead to errors in the sensor report and false alarms. A robust strategy to filter this type of interference still needs to be found.
Also, studies consistently agree that measurements in the unsupervised environment are not just an extension of what is observed in the lab but a new perspective that substantially differs. Nonetheless, links and correlations were found across the two settings21,25,41,68, highlighting the importance of having lab measurements and unsupervised scripted tasks in the home environment as a reference.
When investigating patients’ stratification, wearable sensors demonstrated sufficient sensitivity to capture variations between inter and intra-disease69. Combined with artificial intelligence, sensor-derived data and digital outcomes can discriminate PD vs. HC and different PD patient sub-types, such as fallers vs. non-fallers43,44 or freezers vs. non-freezers45,46. Long-term, digital outcomes could be essential in diagnosing and identifying potentially harmful patient risk factors. However, especially in this field, it is essential to mention how the proper evaluation of the results should revolve primarily on clinical utility efficacy aspects rather than the pure numerical performance of the algorithm. It must be proven that these smart models can still provide additional value to healthcare professionals in real-world applications, which present additional constraints compared to a research environment.
Surprisingly, only a few articles included analyses associated with non-motor symptoms despite the complex multidimensional nature of Parkinson’s disease. A possible explanation is the additional hardware and software required to evaluate the non-motor sphere (e.g., symptom questionnaires, patient diaries, or qualitative interviews), consequently limiting the number of validated devices on the market that monitor motor and non-motor symptoms. This gap represents a huge growth opportunity for the future, particularly if we consider the extreme relevance of non-motor symptoms from a clinical perspective and their significant impact on the quality of life of people with PD.
The articles in the Technical efficacy group focused on proving feasibility or identifying movements and segments of interest, such as walking periods, whose characteristics can be linked to clinically relevant information in follow up studies. This category was the smallest as expected. In fact, more controlled and less noisy environments are usually preferred when evaluating this type of research questions. All the works in this category showed promising results. In the work of Reykov et al.23, walking segments in the unsupervised environment were detected with a specificity and sensitivity of 91% for PD patients. Excellent performance was achieved also in the study of Nouriani et al.24 where walking, standing, and turning have been detected with an accuracy of above 99%. However, a common limitation was the small sample size, with the biggest study that enrolled 25 people with PD.
In parallel to evidence, in this manuscript, we evaluated “User experience” through adherence and usability. Overall, wearables received a high usability rating and were well accepted by the patients in the different cohorts. However, the duration of the studies evaluated was limited in some cases26,27, which calls for long-term evaluation of these aspects.
Furthermore, the drop in the adherence in time remains a challenge to be addressed in future work. In the context of wearable technologies, the potential bias due to the ‘novelty effect’ must be considered70. However, reasons for discontinuation of use need to be systematically identified and tackled, including, e.g., privacy concerns, lack of digital health literacy71, motivations behind unwillingness of long-term/continuous use72,73. Family problems can also play a role. In this case, also caregivers need to be included in the overall usage concept of technical devices, allowing for flexibility given heterogenous personal care needs. To achieve long-term adherence and fit to people’s everyday life, patient-centered approaches to design and evaluate body worn devices are highly recommended, e.g., through co-design and value-based studies74, taking into consideration needs for informed decision-making and autonomy75,76.
Finally, it is important to highlight that only 21 out of 59 articles provided usability or adherence outcomes, most of which were feasibility studies with small sample sizes. Furthermore, measurement methods for user experience were highly variable across articles, with a limited number using standardized usability evaluations and the rest using self-developed, non-validated user questionnaires. For this reason, it is essential to consistently introduce usability assessments as a regular practice, irrespective of the cohort under investigation or the type of study undertaken, while using systematic criteria for evaluating and iterating on user experience and by conducting both quantitative and qualitative research in a systematic way17. Patient engagement strategies and a patient-centered design for user experience should be explicitly implemented for long data acquisition periods to mitigate the drop in concordance/adherence observed in many longitudinal projects. In future work, an iterative approach to designing and evaluating a holistic user experience likely prevent expensive changes to mature prototypes that showed low adherence in clinical studies.
To complete this discussion section, it is important to highlight also two main limitations of the current work, which are essential to the correct interpretation of the clinical utility results of this manuscript. First, part of the devices eventually integrated within standard of care procedures, are developed completely inside the manufacturer validation pipeline. Consequently, their development does not generate research studies that can be evaluated in this review.
Second, our EEL framework was primarily developed to categorize the paper screened in this review. A comprehensive and accurate evaluation of the clinical utility of wearables would require a “Health Technology Assessment” approach that explores multiple domains in parallel to evidence and user experience, see Fig. 4. Additionally, this evaluation should be carried not in research studies, as performed in this manuscript, but within the multiple Real-Word clinical pathways of the people with PD. However, this was beyond the scope of our manuscript.
Recently, the (state-of-the-art) management strategy of PD started a profound transformation following the advent of Precision Medicine. Generic treatments have been replaced by individualized interventions tailored on the necessities of the single patient, and subjective evaluations substituted by objective data driven assessments. The combination of innovative technologies and algorithms, such as wearable sensors and machine learning models, triggered this change making new types of information available to healthcare professionals. This has impacted the decision-making process, which has shifted toward a “Quadruple Decision-Making Model” that is not anymore exclusively based on professional expertize, but combines expert opinion, patient preferences, scientific evidence, and big data approaches76.
At the same time, this transformation is slowly but steadily shifting the role itself of wearables technologies. They are not expected anymore to simply replicate of what is done in regular hospital assessments. They are requested to provide a different perspective and added value. In summary, they need to provide clinical utility evidence.
The results of this manuscript, shows that wearable sensors, such as PKG, MM4PD, Kinesia 360, Ambulosono among others (see also the work of C). Moreau et al. for an anthology of the most used body-warn sensors for PD13, can already play a relevant role as clinical decision support tools for neurologists or during individualized rehabilitation programs. Consequently, the low amount of clinical utility evidence observed in the research papers screened, seems to be motivated more by a lack of study designs investigating these factors, rather than the technological readiness of the systems. To maximize the potential of wearable sensors and their positive impact on the care strategy of PD is essential investigate deeper the clinical utility of these technologies and to generate validated reference metrics that can be used in the different care pathways.
Since the introduction of new devices within the medical procedures often comes with changes in professional roles and tasks77, new types of specialized trainings will be required (as was the case in articles reviewed50), and as offered by professional healthcare networks in some countries78. Firstly, for healthcare professionals to use novel devices and integrate the sensor information into their care routines. Secondarily, for patients to learn how to properly handle these new tools.
To conclude, this manuscript presents a review of the clinical utility evidences of wearable sensors in research studies focusing on people with PD in the home environment. The results showed that within the large production of articles centered on this topic, only a very limited number of studies generate evidence about clinical utility. This phenomenon could explain, in part, the gap between technical readiness and usage within standard of care that is observed for wearable technologies in PD.
As already mentioned, analyzing the limitations of the EEL framework, the fully comprehensive evaluation of the impact of wearables in PD management would require a more complex and multi perspective approach. However, it is important to consider that research trials, thanks to the possibility of shaping the study designs and selecting inclusion/exclusion criteria of the participants, offer a more controlled and suitable environment to investigate pure clinical utility. They can create the ideal conditions to limit the interference of external factors while maximizing the impact of wearable tools on healthcare outcomes. For this reason, they play a vital role in the generation of clinical utility evidence.
Ultimately, the results of our work strongly suggest that the scarcity of clinical utility evidence is induced primarily by the lack of study designs tailored to quantify improvement in health outcomes, and not by existing technical hardware/software limitations. Wearables nowadays can generate accurate and relevant diagnostic information (as shown by the EEL-II) that, if used, clearly provides a benefit to patients and doctors (EEL-III). For this reason, we encourage future researchers to focus more on clinical utility studies, generating results that will shape the future care strategies for Parkinson’s Disease.
Methods
To evaluate and assign the selected research articles to different maturity levels based on the given evidence, we generated an “Efficacy Evidence Level” model (EEL) tailored towards the three essential milestones wearables need to achieve. They have to undergo a prove of technical precision and accuracy, a validation of their in home-based applications, and the proof of clinical utility for their measures or parameters, i.e., being beneficiary for individual patients and/or their healthcare professionals. The EEL model builds on two evaluation frameworks with a more general comprehensive evaluation scope (Fig. 5). In 1991, Fryback and Thornbury (FT) proposed a model to categorize diagnostic tests in general, where their maturity was classified into six levels of efficacy, spanning from technical efficacy if the test correctly measures what it is supposed to in a laboratory setting, up to social efficacy, where cost-benefit and cost-effectiveness have to be proven15. Adapted versions of this model have been utilized in more recent works, such as the 2020 review by K. van Leeuwen et al., who classified AI-based commercially available products in radiology79. In 2020, a different approach was proposed by Goldsack et al.16: the V3 framework evaluates the maturity level of biometric monitoring technologies according to 4 validation classes and presents Clinical Utility as the maximum level of maturity, i.e., if it has been demonstrated an improvement on health outcomes, diagnosis, treatment management or prevention.
For feasibility reasons, we tailored the evaluation items of the two models to the clinical utility of wearables for home monitoring in PD. Thus, our framework is composed of three hierarchical EELs defined as follows:
-
1.
Technical efficacy: the first EEL evaluates the accuracy of the information being measured by the sensor.
-
2.
Diagnostic sensitivity: the second EEL analyzes how well sensor-derived information correlate or are sensitive to change compared to clinical parameters or patient phenotype. Four application domains can be foreseen: distinguishing across different conditions, stratifying across patients intra-disease, evaluating symptoms severity, and evaluating changes in patient conditions on long or short timeframes (disease progression and treatment cycles, respectively).
-
3.
Clinical utility: the third level of EEL considers to which extent the data measured by the sensors can be translated into practical knowledge supporting personalized clinical decisions and positive effects on the individual patient’s clinical outcome.
The definitions for the first two levels were inspired by the FT model, whereas the clinical utility EEL was adjusted to the V3 clinic grade accuracy scale, as the latter fits better to evaluate the evidence of wearable technologies from feasibility studies.
Together with the evidence level, a separate analysis focusing on user experience has been performed. User Experience can be independently evaluated at different levels of maturity. In fact, multiple reviewed articles from all the EELs investigated these aspects. For this reason, user experience has been analyzed in parallel to the primary evidence efficacy framework.
Article selection and structured reviewing procedure
The systematic review of the articles was performed according to the PRISMA principles17. The research was conducted using the PubMed Central database (RRID: SCR_004846) and included studies with publication dates between January 2017 and May 2023. Only original, full-text articles published in English that described the usage of wearable sensors for home monitoring of people with PD were included in the review. All types of body worn technologies were considered for this review. However, manuscripts based exclusively on surveys without a real usage of devices were excluded. Specific search terms were used for a detailed literature review: (Parkinson*) AND (measure OR monitoring) AND (free-living OR daily living OR continuous OR 24-h OR home OR unsupervised) AND (sensors), located within the title, abstract, or full text.
The article filtering process was composed of two phases. First, text availability, titles, and abstracts were checked as a preliminary screening of the extracted links. Second, the full texts of the selected manuscripts were further analyzed to include articles for final review.
The modified AXIS appraisal tool method was utilized to analyze the risk of bias80,81. Each article was scored between 0 and 13, summing the number of positive answers in the reviewer’s assessment (see Supplementary Table 5). Papers with scores below 10 points were considered at medium-high risk of bias and consequently excluded from the review.
Five independent reviewers (GZ, MJ, OT, AC, SS) selected and evaluated the studies. In case of disagreement, the articles were discussed with non-reviewing co-authors. To be included in the review, the article had to meet the criteria listed below:
Inclusion criteria
-
Type of paper: original, full-text, peer-reviewed, journal or articles
-
Time frame: January 2017–May 2023
-
Participants: people with PD
-
Environment for assessment: Home monitoring
-
Information source: wearable sensors
Exclusion criteria
-
Type of paper: conference articles.
-
Studies conducted in artificial “home-like” environments.
-
Reviews, meta-analyses, and papers not reporting original data.
-
Article only describes the protocol or study design without concrete results.
-
Article without access to the full-text version.
-
Articles with an AXIS score below 10 points.
-
Surveys without usage of wearables
-
No body-worn sensors
EEL-framework categorization
Finally, the articles included were categorized according to the EEL framework based on their research questions. Papers investigating pure technical precision, without correlating sensor-derived measures with any medical parameters, were assigned to the “Technical Efficacy” group, EEL-I. In the EEL-II, “Diagnostic Sensitivity”, were included studies that applied statistical analysis, algorithms, or machine learning models to link sensor outcomes with clinical scores and patient profiles, cross-sectionally or longitudinally. Finally, in the “Clinical Utility” grade, EEL-III, were assigned papers where sensor-derived measures were utilized to enhance patient care. This was achieved either by improving decision-making from healthcare professionals through relevant information or actively tailoring interventions according to the device measurements.
Studies were assigned to more than one category when they presented multiple research questions associated with the different levels of our framework. Detailed results from the reviewed articles are shown in separate tables related to each level of the EEL framework (Supplementary Tables 1, 2 and 3).
User experience evaluation
In addition to categorizing the 3 EELs, we evaluated the user experience aspects presented in the reviewed studies. User Experience has been considered by reviewing adherence and usability results62,69. The WHO defines adherence as “the degree to which the person’s behavior corresponds with the agreed recommendations from a healthcare provider” and measured regarding data contribution and task completion rates82. According to ISO norms, usability is defined as “the degree to which a given user’s goal is achieved in terms of effectiveness, efficiency, and satisfaction”83. These two aspects are positively correlated and vital when considering the implementation and diffusion of remote monitoring wearable sensors in healthcare.
Data availability
The data source utilized to generate Fig. 1 and the PRISMA diagram are available in Zenodo repository (REF: 10.5281/zenodo.11349002).
References
Del Din, S., Godfrey, A., Mazzà, C., Lord, S. & Rochester, L. Free-living monitoring of Parkinson’s disease: lessons from the field. Mov. Disord. 31, 1293–1313 (2016).
Monje, M. H. G., Foffani, G., Obeso, J. & Sánchez-Ferro, Á. New sensor and wearable technologies to aid in the diagnosis and treatment monitoring of Parkinson’s disease. Annu. Rev. Biomed. Eng. 21, 111–143 (2019).
Adam, H. et al. An update on pathogenesis and clinical scenario for Parkinson’s disease: diagnosis and treatment. 3 Biotech 13, 142 (2023).
Zafar, S. & Yaddanapudi, S. S. Parkinson Disease, in StatPearls (Treasure Island (FL), 2024).
DeMaagd, G. & Philip, A. Parkinson’s disease and its management: part 1: disease entity, risk factors. Pathophysiol. Clin. Present. Diagnosis. P t 40, 504–532 (2015).
Bloem, B. R., Okun, M. S. & Klein, C. Parkinson’s disease. Lancet 397, 2284–2303 (2021).
Hauser, R. A. et al. A home diary to assess functional status in patients with parkinson’s disease with motor fluctuations and dyskinesia. Clin. Neuropharmacol. 23, 75–81 (2000).
Erb, M. K. et al.Health and wearable technology should replace motor diaries to track motor fluctuations in Parkinson’s disease. npj Digital Med. 3, 6 (2020).
Sigcha, L. et al. Deep learning and wearable sensors for the diagnosis and monitoring of Parkinson’s disease: a systematic review. Expert Syst. Appl. 229, 120541 (2023).
Antonini, A. et al. Toward objective monitoring of Parkinson’s disease motor symptoms using a wearable device: wearability and performance evaluation of PDMonitor(®). Front. Neurol. 14, 1080752 (2023).
Asci, F. et al. Wearable electrochemical sensors in Parkinson’s disease. Sens. (Basel) 22, 951 (2022).
Luis-Martínez, R., Monje, M. H. G., Antonini, A., Sánchez-Ferro, Á. & Mestre, T. A. Technology-enabled care: integrating multidisciplinary care in Parkinson’s disease through digital technology. Front. Neurol. 11, 575975 (2020).
Moreau, C. et al. Overview on wearable sensors for the management of Parkinson’s disease. NJP Parkinson’s Dis. 9, 153 (2023).
Xu, S., Kim, J., Walter, J. R., Ghaffari, R. & Rogers, J. A. Translational gaps and opportunities for medical wearables in digital health. Sci. Transl. Med. 14, eabn6036 (2022).
Fryback, D. G. & Thornbury, J. R. The efficacy of diagnostic imaging. Med Decis. Mak. 11, 88–94 (1991).
Goldsack, J. C. et al. Verification, analytical validation, and clinical validation (V3): the foundation of determining fit-for-purpose for biometric monitoring technologies (BioMeTs). NJP Digit. Med. 3, 55 (2020).
Page, M. J. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj 372, n71 (2021).
Botros, A. et al. Long-term home-monitoring sensor technology in patients with Parkinson’s disease-acceptance and adherence. Sensors (Basel) 19, 5169 (2019).
Cohen, S. et al. Characterizing patient compliance over six months in remote digital trials of Parkinson’s and Huntington disease. BMC Med Inf. Decis. Mak. 18, 138 (2018).
Silva de Lima, A. L. et al. Feasibility of large-scale deployment of multiple wearable sensors in Parkinson’s disease. PLoS One 12, e0189161 (2017).
Abrami, A. et al. Using an unbiased symbolic movement representation to characterize Parkinson’s disease states. Sci. Rep. 10, 7377 (2020).
Ravichandran, V. et al. iTex gloves: design and in-home evaluation of an e-textile glove system for tele-assessment of Parkinson’s disease. Sensors (Basel) 23, 2877 (2023).
Raykov, Y. P. et al. Probabilistic modelling of gait for robust passive monitoring in daily life. IEEE J. Biomed. Health Inf. 25, 2293–2304 (2021).
Nouriani, A. et al. Real world validation of activity recognition algorithm and development of novel behavioral biomarkers of falls in aged control and movement disorder patients. Front. Aging Neurosci. 15, 1117802 (2023).
Powers, R. et al. Smartwatch inertial sensors continuously monitor real-world motor fluctuations in Parkinson’s disease. Sci Transl. Med. 13, eabd7865 (2021).
Adams, J. L. et al. Multiple wearable sensors in Parkinson and Huntington disease individuals: a pilot study in clinic and at home. Digit Biomark. 1, 52–63 (2017).
Boroojerdi, B. et al. Clinical feasibility of a wearable, conformable sensor patch to monitor motor symptoms in Parkinson’s disease. Parkinsonism Relat. Disord. 61, 70–76 (2019).
Ullrich, M. et al. Detection of unsupervised standardized gait tests from real-world inertial sensor data in Parkinson’s disease. IEEE Trans. Neural Syst. Rehabil. Eng. 29, 2103–2111 (2021).
Brand, Y. E. et al. Gait detection from a wrist-worn sensor using machine learning methods: a daily living study in older adults and people with Parkinson disease. Sensors 22, 7094 (2022).
Evers, L. J. et al. Real-life gait performance as a digital biomarker for motor fluctuations: the Parkinson@home validation study. J. Med. Internet Res. 22, e19068 (2020).
Shah, V. V. et al. Laboratory versus daily life gait characteristics in patients with multiple sclerosis, Parkinson’s disease, and matched controls. J. Neuroeng. Rehabil. 17, 159 (2020).
Shah, V. V. et al. Digital biomarkers of mobility in Parkinson’s disease during daily living. J. Parkinsons Dis. 10, 1099–1111 (2020).
Silva de Lima, A. L. et al. Home-based monitoring of falls using wearable sensors in Parkinson’s disease. Mov. Disord. 35, 109–115 (2020).
Srulijes, K. et al. Fall risk in relation to individual physical activity exposure in patients with different neurodegenerative diseases: a pilot study. Cerebellum 18, 340–348 (2019).
Corrà, M. F. et al. Comparison of laboratory and daily-life gait speed assessment during on and off states in Parkinson’s disease. Sensors (Basel) 21, 3974 (2021).
Silva de Lima, A. L. et al. Impact of motor fluctuations on real-life gait in Parkinson’s patients. Gait Posture 62, 388–394 (2018).
Lipsmeier, F. et al. Reliability and validity of the Roche PD mobile application for remote monitoring of early Parkinson’s disease. Sci. Rep. 12, 12081 (2022).
Rodríguez-Molinero, A. et al. Analysis of correlation between an accelerometer-based algorithm for detecting Parkinsonian gait and UPDRS subscales. Front Neurol. 8, 431 (2017).
Safarpour, D. et al. Surrogates for rigidity and PIGD MDS-UPDRS subscores using wearable sensors. Gait Posture 91, 186–191 (2022).
Burq, M. et al. Virtual exam for Parkinson’s disease enables frequent and reliable remote measurements of motor function. NJP Digit. Med., 2022 5, 65 (2022).
Gaßner, H. et al. Clinical relevance of standardized mobile gait tests. Reliability analysis between gait recordings at hospital and home in Parkinson’s disease: a pilot study. J. Parkinsons Dis. 10, 1763–1773 (2020).
Oyama, G. et al. Analytical and clinical validity of wearable, multi-sensor technology for assessment of motor function in patients with Parkinson’s disease in Japan. Sci. Rep. 13, 3600 (2023).
Marano, M. et al. Remote smartphone gait monitoring and fall prediction in Parkinson’s disease during the COVID-19 lockdown. Neurol. Sci. 42, 3089–3092 (2021).
Atrsaei, A. et al. Effect of fear of falling on mobility measured during lab and daily activity assessments in Parkinson’s disease. Front Aging Neurosci. 13, 722830 (2021).
Mancini, M. et al. Measuring freezing of gait during daily-life: an open-source, wearable sensors approach. J. Neuroeng. Rehabil. 18, 1 (2021).
Mancini, M., Weiss, A., Herman, T. & Hausdorff, J. M. Turn around freezing: community-living turning behavior in people with Parkinson’s disease. Front. Neurol. 9, 18 (2018).
Rodríguez-Molinero, A. et al. A kinematic sensor and algorithm to detect motor fluctuations in Parkinson disease: validation swtudy under real conditions of use. JMIR Rehabil. Assist Technol. 5, e8 (2018).
Zhu, L. et al. Comparing GPS-based community mobility measures with self-report assessments in older adults with Parkinson’s disease. J. Gerontol. A Biol. Sci. Med Sci. 75, 2361–2370 (2020).
Tsakanikas, V. et al. Evaluating gait impairment in Parkinson’s disease from instrumented insole and IMU sensor data. Sensors (Basel) 23, 3902 (2023).
Adams, J. L. et al. Using a smartwatch and smartphone to assess early Parkinson’s disease in the WATCH-PD study. NPJ Parkinsons Dis. 9, 64 (2023).
Kanellos, F. S. et al. Clinical evaluation in Parkinson’s disease: is the golden standard shiny enough? Sensors (Basel) 23, 3807 (2023).
Heldman, D. A. et al. Telehealth management of Parkinson’s disease using wearable sensors: an exploratory study. Digit Biomark. 1, 43–51 (2017).
Hadley, A. J., Riley, D. E. & Heldman, D. A. Real-world evidence for a smartwatch-based Parkinson’s motor assessment app for patients undergoing therapy changes. Digit Biomark. 5, 206–215 (2021).
Isaacson, S. H. et al. Effect of using a wearable device on clinical decision-making and motor symptoms in patients with Parkinson’s disease starting transdermal rotigotine patch: a pilot study. Parkinsonism Relat. Disord. 64, 132–137 (2019).
Santiago, A. et al. Qualitative evaluation of the personal KinetiGraphTM movement recording system in a Parkinson’s clinic. J. Parkinsons Dis. 9, 207–219 (2019).
Farzanehfar, P., Woodrow, H. & Horne, M. Assessment of wearing off in Parkinson’s disease using objective measurement. J. Neurol. 268, 914–922 (2021).
Cochen De Cock, V. et al. BeatWalk: Personalized music-based gait rehabilitation in Parkinson’s disease. Front Psychol. 12, 655121 (2021).
Chomiak, T., Watts, A., Meyer, N., Pereira, F. V. & Hu, B. A. A training approach to improve stepping automaticity while dual-tasking in Parkinson’s disease: a prospective pilot study. Medicine 96, e5934 (2017).
Gaßner, H. et al. The effects of an individualized smartphone-based exercise program on self-defined motor tasks in Parkinson disease: pilot interventional study. JMIR Rehabil. Assist Technol. 9, e38994 (2022).
Gatsios, D. et al. Feasibility and utility of mhealth for the remote monitoring of Parkinson disease: ancillary study of the PD_manager randomized controlled trial. JMIR Mhealth Uhealth 8, e16414 (2020).
Heijmans, M. et al. Monitoring Parkinson’s disease symptoms during daily life: a feasibility study. NPJ Parkinsons Dis., 2019 5, 21 (2019).
Bouça-Machado, R. et al. Feasibility of a mobile-based system for unsupervised monitoring in Parkinson’s disease. Sensors (Basel) 21, 4972 (2021).
Flynn, A., Allen, N. E., Dennis, S., Canning, C. G. & Preston, E. Home-based prescribed exercise improves balance-related activities in people with Parkinson’s disease and has benefits similar to centre-based exercise: a systematic review. J. Physiother. 65, 189–199 (2019).
Aarsland, D. et al. Parkinson disease-associated cognitive impairment. Nat. Rev. Dis. Prim. 7, 47 (2021).
Klucken, J., Krüger, R., Schmidt, P. & Bloem, B. R. Management of Parkinson’s disease 20 years from now: towards digital health pathways. J. Parkinsons Dis. 8, S85–s94 (2018).
Sharma, Y., Cheung, L., Patterson, K. K. & Iaboni, A. Factors influencing the clinical adoption of quantitative gait analysis technologies for adult patient populations with a focus on clinical efficacy and clinician perspectives: protocol for a scoping review. JMIR Res. Protoc. 12, e39767 (2023).
Rodríguez-Molinero, A. et al. Estimating dyskinesia severity in Parkinson’s disease by using a waist-worn sensor: concurrent validity study. Sci. Rep. 9, 13434 (2019).
Hssayeni, M. D., Jimenez-Shahed, J., Burack, M. A. & Ghoraani, B. Ensemble deep model for continuous estimation of unified Parkinson’s disease rating scale III. Biomed. Eng. Online 20, 32 (2021).
Keogh, A., Argent, R., Anderson, A. & Johnston, W. Assessing the usability of wearable devices to measure gait and physical activity in chronic conditions: a systematic review. J. Neuroeng. Rehabil. 18, 138 (2021).
Huhn, S. et al. The impact of wearable technologies in health research: scoping review. JMIR Mhealth Uhealth 10, e34384 (2022).
Nomeikaite, A. et al. Exploring reasons for usage discontinuation in an internet-delivered stress recovery intervention: a qualitative study. Internet Inter. 34, 100686 (2023).
Mumtaz, H. et al. Current challenges and potential solutions to the use of digital health technologies in evidence generation: a narrative review. Front. Digit Health 5, 1203945 (2023).
Madanian, S., Nakarada-Kordic, I., Reay, S. & Chetty, T. Patients’ perspectives on digital health tools. PEC Innov. 2, 100171 (2023).
Bally, E. L. S. et al. Value-based methodology for person-centred, integrated care supported by information and communication technologies’ (ValueCare) for older people in Europe: study protocol for a pre-post controlled trial. BMC Geriatr. 22, 680 (2022).
Bombard, Y. et al. Engaging patients to improve quality of care: a systematic review. Implement. Sci. 13, 98 (2018).
Byrne, A. L., Baldwin, A. & Harvey, C. Whose centre is it anyway? defining person-centred care in nursing: an integrative review. PLoS One 15, e0229923 (2020).
Schwaninger, I., Carros, F., Weiss, A., Wulf, V. & Fitzpatrick, G. Video connecting families and social robots: from ideas to practices putting technology to work. Univ. Access Inf. Soc. 22, 931–943 (2023).
Bloem, B. R. et al. ParkinsonNet: A low-cost health care innovation with a systems approach from the Netherlands. Health Aff. (Millwood) 36, 1987–1996 (2017).
van Leeuwen, K. G., Schalekamp, S., Rutten, M. J. C. M., van Ginneken, B. & de Rooij, M. Artificial intelligence in radiology: 100 commercially available products and their scientific evidence. Eur. Radiol. 31, 3797–3804 (2021).
Downes, M. J., Brennan, M. L., Williams, H. C. & Dean, R. S. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open 6, e011458 (2016).
Sica, M. et al. Continuous home monitoring of Parkinson’s disease using inertial sensors: a systematic review. PLoS One 16, e0246528 (2021).
Burkhart, P. V. & Sabaté, E. Adherence to long-term therapies: evidence for action. J. Nurs. Scholarsh. 35, 207 (2003).
Handbook of Research on Digital Libraries. Design, development and impact. Program 43, 342–343 (2009).
Bhidayasiri, R. et al. Rotigotine for nocturnal hypokinesia in Parkinson’s disease: quantitative analysis of efficacy from a randomized, placebo-controlled trial using an axial inertial sensor. Parkinsonism Relat. Disord. 44, 124–128 (2017).
Iijima, M., Mitoma, H., Uchiyama, S. & Kitagawa, K. Long-term monitoring gait analysis using a wearable device in daily lives of patients with Parkinson’s disease: the efficacy of selegiline hydrochloride for gait disturbance. Front. Neurol. 8, 542 (2017).
Khodakarami, H., et al. Prediction of the levodopa challenge test in Parkinson’s disease using data from a wrist-worn sensor. Sensors (Basel) 19, 5153 (2019).
Bouça-Machado, R. et al. Kinematic and clinical outcomes to evaluate the efficacy of a multidisciplinary intervention on functional mobility in Parkinson’s disease. Front Neurol. 12, 637620 (2021).
Caballol, N., Bayés, À., Prats, A., Martín-Baranera, M. & Quispe, P. Feasibility of a wearable inertial sensor to assess motor complications and treatment in Parkinson’s disease. PLoS One https://doi.org/10.1371/journal.pone.0279910 (2023).
Thomas, I. et al. Sensor-based algorithmic dosing suggestions for oral administration of levodopa/carbidopa microtablets for Parkinson’s disease: a first experience. J. Neurol. 266, 651–658 (2019).
Haertner, L. et al. Effect of fear of falling on turning performance in Parkinson’s disease in the lab and at home. Front. Aging Neurosci. 10, 78 (2018).
Kyritsis, K. et al. Assessment of real life eating difficulties in Parkinson’s disease patients by measuring plate to mouth movement elongation with inertial sensors. Sci. Rep. 11, 1632 (2021).
Mirelman, A. et al. Tossing and turning in bed: nocturnal movements in Parkinson’s disease. Mov. Disord. 35, 959–968 (2020).
Knudson, M., Thomsen, T. H. & Kjaer, T. W. Comparing objective and subjective measures of Parkinson’s disease using the Parkinson’s KinetiGraph. Front Neurol. 11, 570833 (2020).
Papadopoulos, A. et al. Detecting parkinsonian tremor from IMU data collected in-the-wild using deep multiple-instance learning. IEEE J. Biomed. Health Inf. 24, 2559–2569 (2020).
San-Segundo, R., et al. Parkinson’s disease tremor detection in the wild using wearable accelerometers. Sensors 20, 5817 (2020).
Habets, J. G. V., et al. Rapid dynamic naturalistic monitoring of bradykinesia in Parkinson’s disease using a wrist-worn accelerometer. Sensors (Basel) 21, 7876 (2021).
Acknowledgements
This work was supported by the Luxembourg National Research Fund, Project 14146272 and 17981757 for the conceptualization, the writing, and the interpretation of the data; and ERA PerMed, Project 01KU2110 for the interpretation of the data.
Author information
Authors and Affiliations
Contributions
J.K. conceptualized the manuscript. S.S., O.T., M.G., A.C.M., G.Z. screened the papers to include in the review. S.S. had a major role in writing and generating the figures. O.T. and A.C.M. contributed in drafting the clinical sections of the manuscript. M.G. and G.Z. created the tables. IS supported the evaluation of user experience results.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sapienza, S., Tsurkalenko, O., Giraitis, M. et al. Assessing the clinical utility of inertial sensors for home monitoring in Parkinson’s disease: a comprehensive review. npj Parkinsons Dis. 10, 161 (2024). https://doi.org/10.1038/s41531-024-00755-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-024-00755-6
- Springer Nature Limited