Abstract
Positron emission tomography/computed tomography (PET/CT) is a molecular imaging method commonly used to diagnose and differentiate Parkinson’s disease (PD). This study aimed to evaluate the performance of PET/CT with 11C-2β-Carbomethoxy-3β-(4-fluorophenyl) tropane (11C-CFT) and 18F-fluorodeoxyglucose (18F-FDG) tracers in the differential diagnosis between PD, multiple system atrophy parkinsonian type (MSA-P), progressive supranuclear palsy (PSP) and vascular parkinsonism (VP) using the data of 220 patients with clinical PD-like symptoms. Of the 220 enrolled patients, 166 (PD, n = 80; MSA-P, n = 54; PSP, n = 15; VP, n = 17) completed the motor, cognitive and PET/CT assessment and were included in this study. 11C-CFT and 18F-FDG PET/CT images were analyzed using the SNBPI toolbox and CortexID Suite software. The uptake values of 11C-CFT and 18F-FDG PET/CT were compared among the groups after controlling for covariates using generalized linear models. Receiver operating characteristic (ROC) curves were generated to estimate the diagnostic values. Patients with PSP showed the most significant reduction on 11C-CFT PET/CT, while patients with PD and MSA-P showed similar reductions, and patients with VP did not show any significant reduction in 11C-CFT uptake. The areas under the curve (AUCs) for 11C-CFT PET/CT for distinguishing PD from VP, PSP, and MSA-P were 0.902, 0.830, and 0.580, respectively, and 0.728 for distinguishing advanced-stage PD from PSP. On 18F-FDG PET/CT, the AUCs for distinguishing PD from PSP and MSA-P were 0.968 and 0.963, respectively. These results suggest that 11C-CFT and 18F-FDG PET/CT complement each other in improving the accuracy in differential diagnosis of PD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
The early and accurate diagnosis of Parkinson’s disease (PD) remains challenging because of the lack of any definitive diagnostic biomarker1. Currently, the diagnosis of PD is primarily performed clinically2,3, which has a misdiagnosis rate of 20%4. Approximately 80% of the initially misdiagnosed patients are later diagnosed with multiple system atrophy parkinsonian type (MSA-P) or progressive supranuclear palsy (PSP)1,5. According to the 2015 diagnostic criteria of the International Parkinson and Movement Disorder Society (MDS), normal presynaptic dopamine activity is an exclusionary factor for PD6. 11C-2β-Carbomethoxy-3β-(4-fluorophenyl) tropane (11C-CFT) positron emission tomography/computed tomography (PET/CT) can be used to test this activity7. 11C-CFT is a radioactive tracer that selectively binds to axonal terminals in the striatum8. The level of binding reflects the degree of damage to the dopaminergic neurons in the nigrostriatal system9. Abnormal dopamine function can also help distinguish PD from essential tremor (ET) and vascular parkinsonism (VP)10, but has a limited ability to distinguish PD from MSA-P and PSP11,12, which also present as pathological lesions in the nigrostriatal system.
Neurodegenerative diseases manifest with various degrees of metabolic abnormality, which 18F-fluorodeoxyglucose (18F-FDG) PET/CT can identify at a systemic level13. Both autopsy and 18F-FDG PET/CT studies have indicated that the disease-specific glucose metabolism pattern in the brain can aid in the differential diagnosis of PD and related diseases14,15. PD typically shows hypometabolism in the posterior parietal and frontal cortices, as well as hypermetabolism in the pallidothalamic tracts and cerebellum16. However, the sample sizes of prior autopsy studies have been small. Research data from large, real-world cohorts are required to examine the potential values of these imaging methods for the differential diagnosis of PD-like disorders.
In this study, we performed 11C-CFT and 18F-FDG PET/CT scans in 166 patients with PD-like symptoms to evaluate their performance in the differential diagnosis of PD from MSA-P, PSP, and VP.
Results
Demographic and clinical data
Overall, we observed significant differences between the groups with respect to age (P = 0.006), disease duration (P = 0.005), H-Y (P < 0.001), MDS-UPDRS-III (P < 0.001), and MoCA (P = 0.004). In post-hoc analysis, VP patients were found to be older than MSA-P patients, PD patients had a longer disease duration than MSA-P patients, and PSP patients had higher MDS-UPDRS-III and H-Y scores than others (P < 0.05). In addition, PSP patients had lower MoCA scores than PD and MSA patients (P < 0.05) (Table 1). Detailed comparisons between early- and advanced-stage PD are listed in Supplementary Table 1.
11C-CFT uptake and asymmetry indices
No apparent reduction in 11C-CFT uptake was observed in patients with VP. Patients with PD and MSA-P showed a lower uptake than those with VP, with a similar degree of decline. Patients with PSP had notably lower uptake than other patients (Fig. 1a). After correcting for MDS-UPDRS-III, MoCA, disease duration, age, and H-Y, significant differences were observed in the caudate (P = 0.010), anterior (P = 0.018) and posterior putamen (P < 0.001). A post-hoc analysis indicated substantial differences in the caudate, anterior and posterior putamen between the uptake values of VP patients and those of other patients (P < 0.05; Table 1, Fig. 2a, Supplementary Tables 4–6). Asymmetry indices were further found to be higher in PD patients than in other patients, especially in the anterior putamen (P = 0.003; Table 1, Fig. 2b, Supplementary Tables 7–9).
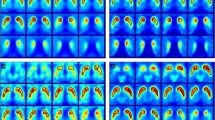
a 11C-CFT uptake images. PD patient shows asymmetrical 11C-CFT uptake reduction; MSA-P patient showed a relatively symmetrical reduction in 11C-CFT uptake that was slightly greater than that observed in PD patient; PSP patient showed significant bilateral uptake reduction; VP patient shows non-significant 11C-CFT uptake reduction. The arrows in Fig. 1a indicate the portion with a reduced uptake. b Schematic diagram of the automatic segmentation of each ROI in 11C-CFT PET/CT. Green represents caudate, yellow represents anterior putamen, red represents posterior putamen, and blue represents occipital lobe. c 18F-FDG Z-score images. PD patient shows hypometabolism in the prefrontal and parieto-occipital cortices, and hypermetabolism in the cerebellum and pons; MSA-P patient shows hypometabolism in the cerebellum and pons; PSP patient shows hypometabolism in the prefrontal and parieto-occipital cortices. The marker at the bottom changed from black to red, indicating a gradual decrease in the Z-score. d 18F-FDG Z-score images of putamen, caudate and thalamus. PD patient shows hypermetabolism in the thalamus and putamen; MSA-P patient shows hypometabolism in the caudate, thalamus and putamen; PSP patient shows hypometabolism in the putamen and thalamus. The marker on the right changed from blue to red, indicating a gradual increase in the Z-score. Z-score >0, hypermetabolism; Z-score <0, hypometabolism. 18F-FDG images of VP patients were not obtained because of the non-specificity of FDG metabolism in VP patients. PD Parkinson’s disease, MSA-P multiple system atrophy parkinsonian type, PSP progressive supranuclear palsy, VP vascular parkinsonism.
a Comparison of 11C-CFT SUVR values among groups. b Comparison of asymmetry index among groups. P values were obtained by generalized linear models, and error bars were generated from the standard deviation. c Box-plots of the Z-score of PD, MSA-P, and PSP. Each box represents the distribution of Z-score in that region of interest (ROI) for that group of patients. The yellow markings in the figure represent brain regions with P < 0.001, while the remaining statistically significant ROIs are detailed in Supplementary Table 2. Centerline, medians; box limits, upper and lower quartiles; whisker, 1.5× interquartile ranges; points, outliers; Z-score >0, hypermetabolism; Z-score <0, hypometabolism. PD Parkinson’s disease, MSA-P multiple system atrophy parkinsonian type, PSP progressive supranuclear palsy, VP vascular parkinsonism, AP anterior putamen, PP posterior putamen, SUVR standardized uptake value ratio, R right, L left; *P < 0.05.
Characteristic patterns of glucose metabolism in 18F-FDG PET/CT
Compared with normal database in the CortexID Suite and hospital physical examination center, PD patients showed notable hypometabolism in the lateral occipital, parietal, precuneus, sensorimotor, and lateral prefrontal cortices, with significant hypermetabolism in the cerebellum, pons, mesial temporal cortex, caudate and putamen. Patients with MSA-P showed significant hypermetabolism in the primary visual cortex and significant hypometabolism in the cerebellum, pons, prefrontal and parietal cortices, caudate, putamen and thalamus. PSP patients showed hypermetabolism in the mesial temporal cortex and hypometabolism in the anterior and posterior cingulate, inferior parietal, prefrontal, precuneus, sensorimotor cortices, thalamus and caudate (P < 0.05; Figs. 1c, d and 2c; Supplementary Table 2).
After correcting for MDS-UPDRS-III, MoCA, age, H-Y, and disease duration, we observed significant differences in Z-score between PD and MSA-P patients in the sensorimotor, primary visual, mesial temporal, precuneus, parietal, lateral occipital cortices, pons, cerebellum, caudate, putamen and thalamus, and between PD and PSP patients in the medial prefrontal, anterior cingulate, inferior parietal, lateral occipital cortices, caudate, putamen and thalamus (P < 0.05; Table 2, Fig. 3b, Supplementary Tables 10–43).
a Histogram of 11C-CFT standardized uptake value ratio (SUVR) in patients with early-stage PD (n = 46), advanced-stage PD (n = 34), and PSP (n = 15), as well as the receiver operating characteristic (ROC) curves for 11C-CFT PET/CT. The distribution of 11C-CFT SUVRs for PD, MSA-P, and VP are shown in Fig. 2a. b Z-score violin plots of ROIs with statistical differences for PD vs. MSA-P and PSP in 18F-FDG PET/CT and the ROC curves for 18F-FDG PET/CT. The comparison results in the violin plot are detailed in Table 2. Centerline, median; bilateral lines, upper and lower quartiles. c Principal components (PC) plots of PD vs. MSA-P and PD vs. PSP and the ROC curves for the combination of the two scans. Principal component analysis was performed on the combination of SUVRs of three ROIs in 11C-CFT PET/CT and Z-score of ROIs shown in Fig. 3b in 18F-FDG PET/CT, and two PCs were obtained. Due to the lack of specificity of 18F-FDG PET/CT in the diagnosis of VP, it was not included in the ROC analysis of 18F-FDG PET/CT and the combination of both scans. The areas under the curve (AUC) and their 95% confidence intervals are shown. PD Parkinson’s disease, MSA-P multiple system atrophy parkinsonian type, PSP progressive supranuclear palsy, VP vascular parkinsonism, Combination, the combination of 11C-CFT and 18F-FDG PET/CT.
Combination of 11C-CFT and 18F-FDG data
Using PCA to analyze the uptake values of the three ROIs in 11C-CFT PET/CT and the Z-scores of ROIs with statistically differences in PD compared with PSP and MSA-P in 18F-FDG PET/CT, which ultimately yielded two principal components (PCs). These two PCs effectively separated PD from MSA-P and PSP (Fig. 3c).
Diagnostic efficacy of 11C-CFT and 18F-FDG PET/CT
The AUCs for 11C-CFT PET/CT in distinguishing PD from MSA-P, PSP, and VP were 0.580 (95% confidence interval, 0.482–0.678), 0.830 (0.701–0.959) and 0.902 (0.842–0.962), respectively, suggesting that 11C-CFT PET/CT alone is sufficient to distinguish PD from VP. This is not surprising as the VP does not have any structural lesions in the nigrostriatal system. For this reason, and because 18F-FDG PET/CT has a low specificity for VP, we did not calculate the diagnostic efficacy of 18F-FDG PET/CT alone or the efficacy of combined scans for differentiating PD from VP. Although the AUC for 11C-CFT PET/CT to differentiate PD from PSP was reasonably high (0.830), the AUCs for differentiating early- and advanced-stage PD from PSP were 0.862 (0.729–0.995) and 0.728 (0.573–0.882), respectively, indicating that it is more difficult to differentiate advanced-stage PD from PSP using this modality alone (Fig. 3a). The AUCs for using 18F-FDG PET/CT alone to differentiate PD from MSA-P and PSP were 0.963 (0.935–0.990) and 0.968 (0.925–1.000), respectively (Fig. 3b), while the AUCs combining the two modalities were 0.969 (0.946–0.993) and 0.978 (0.949–1.000), respectively (Fig. 3c).
When clinical indicators, including MDS-UPDRS-III, MoCA, disease duration, age, and H-Y were combined with 11C-CFT PET/CT, the AUCs for differentiating PD from MSA-P, PSP, and VP improved to 0.745 (0.661–0.829), 0.858 (0.758–0.958), and 0.935 (0.886–0.984), respectively; and the AUCs for discriminating PSP from early- and advanced-stage PD were 0.996 (0.986–1.000) and 0.790 (0.666–0.915), respectively (Fig. 4a). When clinical indicators were combined with 18F-FDG PET/CT, the AUCs for distinguishing PD from MSA-P and PSP were 0.967 (0.941–0.993) and 0.976 (0.947–1.000), respectively (Fig. 4b). When clinical indicators are combined with these two scans, the AUCs for distinguishing PD from MSA-P, PSP increased further to 0.972 (0.949–0.995) and 0.998 (0.992–1.000), respectively (Fig. 4c).
a Receiver operating characteristic (ROC) curves for the combination of clinical indications with 11C-CFT PET/CT. b ROC curves for the combination of clinical indications with 18F-FDG PET/CT. c ROC curves for the combination of clinical indications with these two PET/CT scans. Clinical indicators include MDS-UPDRS-III, MoCA, H-Y, disease duration, and age. The areas under the curve (AUC) and their 95% confidence intervals are shown. PD Parkinson’s disease, MSA-P multiple system atrophy parkinsonian type, PSP progressive supranuclear palsy, VP vascular parkinsonism, Combination, the combination of 11C-CFT and 18F-FDG PET/CT.
The sensitivity, specificity, and agreement rate for the PD diagnosis were 74%, 87% and 81%, respectively, when 11C-CFT PET/CT was used; 96%, 92% and 94%, respectively, when using 18F-FDG PET/CT; and 84%, 89% and 84%, respectively, when both scans were combined. The sensitivity, specificity, and agreement rate for the MSA-P diagnosis were 74%, 89% and 84%, respectively, when using 11C-CFT PET/CT alone; 93%, 95% and 94%, respectively, when using 18F-FDG PET/CT alone; and 89%, 96% and 93%, respectively, when using both scans. The sensitivity, specificity, and agreement rate for the PSP diagnosis were 73%, 98% and 95%, respectively, when 11C-CFT PET/CT was used; 80%, 100% and 98%, respectively, when using 18F-FDG PET/CT; and 80%, 100% and 98%, respectively, when both scans were used. The sensitivity, specificity, and agreement rate for the VP diagnosis were 94%, 91% and 92%, respectively, when 11C-CFT PET/CT was used (Supplementary Table 3).
The consistency between the clinical and 11C-CFT PET/CT diagnoses was moderate (κ = 0.640, P < 0.001). The consistencies between clinical diagnosis and 18F-FDG PET/CT diagnosis, and between clinical diagnosis and diagnosis of these two scans were good (κ = 0.885, P < 0.001).
Discussion
11C-CFT is a radioactive tracer which specifically binds to dopamine transporters on the plasma membrane of dopaminergic neuron terminals in the striatum17,18. The level of binding reflects the degree of damage to the dopaminergic neurons in the nigrostriatal system19. In the present study, patients with VP showed no obvious 11C-CFT uptake reduction, indicating that the nigrostriatal system was intact. However, 11C-CFT uptake has shown various degrees of dopaminergic neuronal damage in PD, MSA-P, and PSP patients, consistent with their neuropathology20,21, suggesting that 11C-CFT PET/CT could detect changes in central dopaminergic neuron function with a high degree of sensitivity8. Similar reductions in PD and MSA-P patients likely reflect similar degrees of dopaminergic neuron loss in these patients. Additionally, in line with previous findings22, we observed a lower uptake (reflecting more severe dopaminergic system dysfunction) in PSP patients than in PD and MSA-P patients.
Clinically, PD typically initiates with unilateral motor symptoms, maintaining some degree of asymmetry throughout disease progression, whereas most MSA-P and PSP patients display more symmetrical clinical symptoms23. In the present study, the asymmetry indices were found to be higher in PD than in others; however, this difference was mostly limited to the anterior putamen. Similarly, Pirker24 reported that the visual examination of PET/CT images revealed more symmetrical uptake in MSA and PSP patients than in PD patients; however, these differences were not significant. Further studies with more participants are required to clarify these characteristics.
In short, our results showed that 11C-CFT PET/CT is a highly effective method for distinguishing PD from PD-like disorders with an intact nigrostriatal system, such as ET and VP25. However, this modality alone is insufficiently sensitive to differentiate PD from MSA-P or advanced-stage PD from PSP because of a similar reduction in 11C-CFT uptake values, reflecting the overlapping pathological lesions between these diseases. When 11C-CFT PET/CT was combined with clinical indicators, the diagnostic accuracy improved to some extent, but was still not ideal for distinguishing PD from MSA-P (AUC = 0.745) and advanced-staged PD form PSP (AUC = 0.790).
Using 18F-FDG tracer, different diseases have been shown to have disease-specific metabolic patterns linked to functional changes in different brain regions. In contrast to previous studies that used visual image reading techniques or voxel-based spatial covariance methods to diagnose various disorders without considering variations in uptake values throughout the whole brain14,26,27,28, we used CortexID Suite software and the SNBPI toolbox to quantitatively analyze the ROIs of 18F-FDG PET images. When compared with the normal database, we identified several disease-specific glucose metabolic patterns for PD, MSA-P and PSP, consistent with previous studies14,29,30. Furthermore, we observed differences in Z-score among brain regions in various diseases. Overall, PD patients showed more significant hypometabolism in the sensorimotor, precuneus, lateral occipital, and parietal cortices, and hypermetabolism in the caudate, thalamus, putamen, mesial temporal cortex, cerebellum, and pons. MSA-P patients had significant hypometabolism in the caudate, thalamus, putamen, cerebellum and pons, and PSP patients had more obvious hypometabolism in the anterior cingulate, medial prefrontal cortices, caudate, thalamus and putamen. Finally, we evaluated diagnostic efficacy using the statistically significant ROIs listed above and found that the diagnostic efficacy was high with all AUCs exceeded 0.90, indicating that 18F-FDG PET/CT outperformed 11C-CFT PET/CT in distinguishing PD from PSP and MSA-P.
Based on these findings, we recommend performing both 11C-CFT and 18F-FDG PET/CT to achieve an accurate differential diagnosis of PD and related diseases. In clinical practice, we suggest the initial identification of dopaminergic neuron degeneration by 11C-CFT PET/CT to differentiate PD from ET and VP, which present with PD-like symptoms but have no typical pathological lesions in the nigrostriatal system, followed by 18F-FDG PET/CT to identify disease-specific metabolism pattern for further distinction between PD, MSA-P and PSP. Considering financial cost, among patients in whom ET and VP can be ruled out, as well as those with typical extrapyramidal symptoms indicating nigrostriatal pathology, 18F-FDG PET/CT alone is also a good choice.
This study had some limitations. First, the number of VP and PSP patients was relatively small. Future research with more participants is required to examine the relationships between the uptake values and clinical indices in these groups with higher statistical power. Second, this study was a single-center study. In the future, further multi-center research is required to confirm the findings.
In conclusion, we found that 11C-CFT PET/CT accurately detects dopaminergic axonal loss in the striatum, thereby enabling the differentiation of PD from VP, but had a limited ability to differentiate PD from PSP and MSA-P. 18F-FDG PET/CT is highly sensitive for differentiating PD from PSP and MSA-P by revealing disease-specific metabolic patterns. The combination of these two imaging modalities will help improve the diagnostic accuracy and guide treatment strategies.
Methods
Participants and design
In this study, we enrolled 220 patients with PD-like symptoms between 2019 and 2022. Of these, 54 were excluded due to their inability to complete PET/CT scans or neuropsychological measurements; finally, 166 patients were ultimately included in our study. Of the study cohort, 80 patients were diagnosed PD according to the 2015 MDS PD diagnostic criteria6 (46 with early-stage PD; 34 with advanced-stage PD; early-stage, H-Y ≤ 2; advanced-stage, H-Y > 231), while 54 were diagnosed with MSA-P32, 15 with PSP33, and 17 with VP34. To determine the final clinical diagnosis, specialists followed each patient for at least two years following the initial diagnosis. Patients with clearly signs of mental illness (e.g., depression), those on long-term antipsychotic drugs (as some antipsychotic drugs may cause patients to develop PD-like symptoms that can confound study results, such as fluoxetine), those who underwent deep brain stimulation surgery, those with severe organ (heart, liver, or kidney) damage, and those with structural imaging suggestive of lesions in the basal ganglia region were all excluded. Demographic and clinical data were collected, and all patients underwent 11C-CFT and 18F-FDG PET/CT. Subsequently, receiver operating characteristic (ROC) curves were plotted to estimate the value of PET/CT in the differential diagnosis of PD. A flowchart of the study is presented in Fig. 5.
The Jilin University First Hospital of Bethune Ethical Review Board approved this study (NO. 2024-441), and all patients signed informed consent forms.
Motor and neuropsychological assessment
One neurologist performed the neuropsychological assessments. In the “off” state, we used the part III of MDS Unified Parkinson Disease Rating Scale (MDS-UPDRS-III) and Hoehn-Yahr scale (H-Y) to assess the severity of motor symptoms. Further, we used the Montreal Cognitive Assessment (MoCA) to assess the cognitive function of patients in the “on” state.
PET/CT imaging process
11C-CFT and 18F-FDG with ≥95% radiochemical purity were produced using an HM-12 cyclotron (Sumitomo Heavy Industries, Ltd., Kyoto, Japan). Brain PET/CT examinations were performed using a Siemens Discovery 16HR PET/CT (Siemens, Berlin, Germany). All patients fasted for at least 6 h, and discontinued dopaminergic drugs for at least 12 h prior to the scan. An 11C-CFT PET scan lasting 10 min was started 60 min35 following the intravenous injection of 370 MBq36 of 11C-CFT; and an 18F-FDG PET scan of 10 min was started 60 min37 after the intravenous injection of 3.7 MBq/Kg28 of 18F-FDG. All participants were asked to remain in a quiet, dark environment before the scan. We used a head rest to hold the patient’s head motionless during the scanning process to prevent head movements from affecting the results. Two PET examinations were completed within one week. A CT scan of the brain was initially conducted using a fixed tube voltage of 120 kV (tube current 100 mA) for attenuation correction and anatomical localization, followed by a PET scan in the 3D mode. The attenuation of PET images was finally corrected using CT data, followed by iterative image reconstruction to obtain cross-sectional, coronal, sagittal CT, PET, and PET/CT images. Using the 3D ordered-subset expectation maximization (OSEM) algorithm (4 iterations, 24 subsets; Gaussian filter: 2 mm; zoom: 3; thickness: 3 mm; FOV: 585 mm*162 mm), PET images were reconstructed using the manufacturer’s scatter, and random correction in addition to CT-based attenuation correction was performed. The reconstructed images measured 0.68 × 0.68 × 1.5 mm in voxel size and 400 × 400 × 148 in matrix size.
PET/CT analysis
The 11C-CFT PET/CT brain data were processed with toolbox Spatial Normalization of Brain PET Images (SNBPI; https://github.com/IHEP-Brain-Imaging/Spatial-Normalization-of-Brain-PET-Images; Chinese Academy of Sciences, Beijing, China)38 running on MATLAB 2022a (MathWorks Inc., Sherborn, MA). Using the SNBPI toolbox, which is based on the DARTEL method, each patient’s scan was spatially normalized into the Montreal Neurological Institute (MNI) brain space, and subsequently resliced to 2-2-2 mm, after which SPM12 (Statistical Parametric Mapping; Wellcome Department of Imaging Neuroscience, London, UK) using an isotropic 3D Gaussian kernel (FWHM = 8-8-8 mm) was applied to smooth. Anatomical automatic labeling (AAL) atlas was used to extract the region of interest (ROI) of each patient’s bilateral caudate, anterior and posterior putamen, and occipital lobe using the SNBPI toolbox (Fig. 1b). In the AAL atlas, the putamen is automatically separated into two equally sized segments. The first half was identified as the anterior putamen, and the second as the posterior putamen. The mean standardized uptake value ratio (SUVR) values were then automatically calculated for the ROIs. The occipital lobe was used as a background reference. Each ROI’s SUVR was computed using this formula: (striatum-occipital)/occipital count. The average SUVR values were then obtained from the values calculated for both sides of the caudate, and the anterior and posterior putamen. The asymmetry of the SUVR values in the bilateral striatum was expressed using the asymmetry index39: (high-side value – low-side value)/high-side value × 100%.
18F-FDG PET/CT images were analyzed using CortexID Suite (GE Healthcare, Marlborough, MA, USA). Using this software, a 3D stereotactic surface projection (3D-SSP) method (Seattle, Washington, USA) are used, which automatically normalizes the brain anatomy by combining rotational corrections and stereotactic transformations to extract peaks of cortical activity from a predefined set of pixels on the brain surface40. This activity was compared with that of an age-matched database of normal subjects (Z-score = 0) using Z-score subtraction, which yield hypometabolic (Z-score) maps in eight standard directions. The ROI was defined automatically in 26 regions, including the bilateral lateral and medial prefrontal and temporal, sensorimotor, precuneus, inferior and superior parietal, anterior and posterior cingulate, occipital, primary visual cortices, whole cerebellum and pons. The SUVR was further calculated with the whole-brain mean values serving as the reference41. The Z-scores denoting the deviation from the normal data were computed as follows: (SUVRsubj − SUVRnormal)/SDnormal, where SUVRsubj is the mean SUVR of the study subject, SUVRnormal is the mean SUVR from the normal subject database, and SDnormal is the standard deviation of the SUVR from the normal subject database42. We subsequently obtain the average value of Z-score on both sides of each ROI.
The metabolism of the caudate, putamen and thalamus on 18F-FDG PET/CT was analyzed using toolbox SNBPI38 running on MATLAB 2022a. Each image was spatially normalized to MNI space based on DARTEL algorithm and resliced to 2-2-2 mm. Then utilizing SPM12 to smooth using an isotropic 3D Gaussian kernel (FWHM = 8-8-8 mm). The ROIs of the bilateral putamen, caudate and thalamus for each patient were retrieved from the AAL atlas. The whole brain was selected as the reference area to automatically calculate the SUVR. The mean SUVR value was automatically calculated for each ROI. In addition, 43 age-matched healthy individuals from the Physical Examination Center of the First Hospital of Jilin University were selected as the normal control group and their SUVRs for each ROI were calculated. The Z-scores were calculated using the Z-score formula described above.
Statistical methods
SPSS 26 (IBM Corporation, Armonk, NY, USA) was used to analyze the data. The Shapiro–Wilk test was used to ascertain the normality of quantitative data. Demographic and clinical characteristics were compared using analysis of variance (ANOVA), followed by Bonferroni post-hoc analysis. Generalized linear models were further employed to compare 11C-CFT uptake values among the groups and 18F-FDG Z-scores for PD versus MSA-P and PD versus PSP, correcting for the effects of MDS-UPDRS-III, MoCA, age, disease duration, and H-Y. One-sample t-test was used to assess the distribution of Z-score for different diseases relative to the normal database using CortexID Suite software and the Physical Examination Center of the First Hospital of Jilin University (Z-score = 0). Principal component analysis (PCA) was performed on the combination of uptake values of the three ROIs in 11C-CFT PET/CT and the Z-scores of the ROIs with statistically differences in PD compared to MSA-P and PSP obtained from the generalized linear models in 18F-FDG PET/CT. Evaluation of the diagnostic value of PET/CT was further performed to facilitate the differential diagnosis of PD using ROC curves and aera under the curve (AUC). Using binary logistic regression, disease groupings were used as dependent variables, and imaging metrics to be combined (uptake values of the caudate, anterior and posterior putamen in 11C-CFT and Z-scores of the ROIs that differed between the two groups obtained in the generalized linear model in 18F-FDG) were used as covariates to compute the predictive probability, while ROC curves were plotted using the predictive probability. Using the identified metabolic patterns of the disease as PET/CT diagnostic criteria, we calculated the sensitivity, specificity, and agreement rate. The Kappa test was used to evaluate the coincidence between PET/CT findings and clinical diagnoses. Statistical significance was set at P < 0.05.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request. The data were not publicly available due to privacy or ethical restrictions.
References
Tolosa, E., Garrido, A., Scholz, S. W. & Poewe, W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 20, 385–397 (2021).
Seiffert, A. P. et al. Differences in Striatal Metabolism in [18F] FDG PET in Parkinson’s Disease and Atypical Parkinsonism. Diagnostics 13, 6 (2022).
Armstrong, M. J. & Okun, M. S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 323, 548–560 (2020).
Golan, H., Volkov, O. & Shalom, E. Nuclear imaging in Parkinson’s disease: The past, the present, and the future. J. Neurol. Sci. 436, 120220 (2022).
Peralta, C., Biafore, F., Depetris, T. S. & Bastianello, M. Recent Advancement and Clinical Implications of 18FDG-PET in Parkinson’s Disease, Atypical Parkinsonisms, and Other Movement Disorders. Curr. Neurol. Neurosci. Rep. 19, 56 (2019).
Postuma, R. B. et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 30, 1591–1601 (2015).
Sun, X. L. et al. (11)C-CFT PET brain imaging in Parkinson’s disease using a total-body PET/CT scanner. EJNMMI Phys. 11, 40 (2024).
Xin, M. et al. Exploring the nigrostriatal and digestive interplays in Parkinson’s disease using dynamic total-body [(11)C] CFT PET/CT. Eur. J. Nucl. Med. Mol. Imaging 51, 2271–2282 (2024).
Xie, L. J., Zhao, J. H., Li, Y. & Bai, J. PET brain imaging in neurological disorders. Phys. Life Rev. 49, 100–111 (2024).
Ko, J. H., Lee, C. S. & Eidelberg, D. Metabolic network expression in parkinsonism: Clinical and dopaminergic correlations. J. Cereb. Blood Flow. Metab. 37, 683–693 (2017).
Liu, F. T. et al. Clinical, Dopaminergic, and Metabolic Correlations in Parkinson Disease: A Dual-Tracer PET Study. Clin. Nucl. Med. 43, 562–571 (2018).
Chen, Q. S. et al. Dopamine transporter imaging in progressive supranuclear palsy: Severe but nonspecific to subtypes. Acta Neurol. Scand. 146, 237–245 (2022).
Ruppert, M. C. et al. Network degeneration in Parkinson’s disease: multimodal imaging of nigro-striato-cortical dysfunction. Brain 143, 944–959 (2020).
Tang, C. C. et al. Differential diagnosis of parkinsonism: a metabolic imaging study using pattern analysis. Lancet Neurol. 9, 149–158 (2010).
Niethammer, M. et al. A disease-specific metabolic brain network associated with corticobasal degeneration. Brain 137, 3036–3046 (2014).
Meles, S. K., Oertel, W. H. & Leenders, K. L. Circuit imaging biomarkers in preclinical and prodromal Parkinson’s disease. Mol. Med. 27, 111 (2021).
Xie, L. L. & Hu, L. D. Research progress in the early diagnosis of Parkinson’s disease. Neurol. Sci. 43, 6225–6231 (2022).
Xu, J. H. et al. Computer-Aided Classification Framework of Parkinsonian Disorders Using (11)C-CFT PET Imaging. Front. Aging Neurosci. 13, 792951 (2021).
Kangli, F. et al. Characteristics and influencing factors of 11C-CFT PET imaging in patients with early and late onset Parkinson’s disease. Front. Neurol. 14, 1195577 (2023).
Dickson, D. W. Neuropathology of Parkinson disease. Parkinsonism Relat. D. 46, S30–S33 (2018).
Jellinger, K. A. Neuropathology of multiple system atrophy: New thoughts about pathogenesis. Mov. Disord. 29, 1720–1741 (2014).
Chen, M. J. et al. Striatal dopaminergic lesions contributed to the disease severity in progressive supranuclear palsy. Front. Aging Neurosci. 14, 998255 (2022).
Fearnley, J. M. & Lees, A. J. Striatonigral degeneration. A clinicopathological. Brain 113, 1823–1842 (1990).
Pirker, W. et al. 123I] beta-CIT SPECT in multiple system atrophy, progressive supranuclear palsy, and corticobasal degeneration. Mov. Disord. 15, 1158–1167 (2000).
Bidesi, N. S. R., Vang Andersen, I., Windhorst, A. D., Shalgunov, V. & Herth, M. M. The role of neuroimaging in Parkinson’s disease. J. Neurochem. 159, 660–689 (2021).
Gu, S., Ye, Q. & Yuan, C. Metabolic pattern analysis of 18F-FDG PET as a marker for Parkinson’s disease: a systematic review and meta-analysis. Rev. Neurosci. 30, 743–756 (2019).
Houssein, N. J., Henriksen, A. C., Hejl, A.-M. & Marner, L. Diagnostic accuracy of cerebral [18F] FDG PET in atypical parkinsonism. EJNMMI Res 13, 74 (2023).
Hu, X. H. et al. Multivariate radiomics models based on (18)F-FDG hybrid PET/MRI for distinguishing between Parkinson’s disease and multiple system atrophy. Eur. J. Nucl. Med. Mol. Imaging 48, 3469–3481 (2021).
Saeed, U., Lang, A. E. & Masellis, M. Neuroimaging Advances in Parkinson’s Disease and Atypical Parkinsonian Syndromes. Front. Neurol. 11, 572976 (2020).
Meles, S. K., Teune, L. K., Jong, B. M. D., Dierckx, R. A. & Leenders, K. L. Metabolic Imaging in Parkinson Disease. J. Nucl. Med. 58, 23–28 (2017).
Xiong, M., Qian, Q., Liang, X. & Wei, Y. D. Serum levels of lipocalin-2 in patients with Parkinson’s disease. Neurol. Sci. 43, 1755–1759 (2022).
Wenning, G. K. et al. The Movement Disorder Society Criteria for the Diagnosis of Multiple System Atrophy. Mov. Disord. 37, 1131–1148 (2022).
Höglinger, G. U. et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov. Disord. 32, 853–864 (2017).
Wang, L. J. et al. Chinese experts’ consensus of diagnosis and treatment of vascular parkinsonism. Chin. J. Neurol. 50, 326–331 (2017).
Yang, Y. J. et al. Preserved caudate function in young-onset patients with Parkinson’s disease: a dual-tracer PET imaging study. Ther. Adv. Neurol. Disord. 12, 1756286419851400 (2019).
Zhao, Y. et al. Decoding the dopamine transporter imaging for the differential diagnosis of parkinsonism using deep learning. Eur. J. Nucl. Med. Mol. Imaging 49, 2798–2811 (2022).
Huang, Z. M. et al. Correlations between dopaminergic dysfunction and abnormal metabolic network activity in REM sleep behavior disorder. J. Cereb. Blood Flow. Metab. 40, 552–562 (2020).
Zhang, T., Nie, B., Liu, H., Shan, B. & Alzheimer’s Disease Neuroimaging, I. Unified spatial normalization method of brain PET images using adaptive probabilistic brain atlas. Eur. J. Nucl. Med. Mol. Imaging 49, 3073–3085 (2022).
Perju-Dumbrava, L. D. et al. Dopamine transporter imaging in autopsy-confirmed Parkinson’s disease and multiple system atrophy. Mov. Disord. 27, 65–71 (2012).
Black, J. A. et al. Frontal hypometabolism in the diagnosis of progressive supranuclear palsy clinical variants. J. Neurol. Sci. 271, 4267–4280 (2024).
Lindstrom, E., Danfors, T., Lindsjo, L. & Lubberink, M. Brain-PET image reconstruction methods affect software-aided diagnosis in patients with neurodegenerative diseases. J. Nucl. Med. 59, 1780 (2018).
Lindström, E. et al. Image reconstruction methods affect software-aided assessment of pathologies of [18F] flutemetamol and [18F] FDG brain-PET examinations in patients with neurodegenerative diseases. Neuroimage Clin. 28, 102386 (2020).
Acknowledgements
The work was supported by the First Hospital of Jilin University. We thank Professor Yanhua Wu for her help in statistical analysis and Professor Binbin Nie for her help in automated analysis of PET/CT images. The work was supported by grants from the National Natural Science Foundation of China (No. 81974194), the Natural Science Foundation of Jilin Province (No. YDZJ202201ZYTS116), Jilin Province Department of Finance Project (No. JLSWSRCZX2020-0011) and Jilin Province Health and Technology Innovation Project (No. 2018J045) to Y.Z.
Author information
Authors and Affiliations
Contributions
X.X.D.: conception, methodology, data acquisition, analysis, interpretation of the results, writing of the original draft; H.G.Z.: methodology, interpretation of the results, revision of the manuscript for intellectual content; Y.H.L.: methodology, interpretation of the results; Y.Y.D.: methodology, interpretation of the results; Y.L.: data acquisition, analysis; K.L.F.: data acquisition, analysis; Z.H.S.: data acquisition, analysis; Y.Z.: conception, methodology, interpretation of the results, supervision, revision of the manuscript for intellectual content. All authors read and approved the final manuscript. X.X.D. and H.G.Z. are considered co-first author.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Du, X., Zhao, H., Li, Y. et al. The value of PET/CT in the diagnosis and differential diagnosis of Parkinson’s disease: a dual-tracer study. npj Parkinsons Dis. 10, 171 (2024). https://doi.org/10.1038/s41531-024-00786-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-024-00786-z
- Springer Nature Limited