Abstract
Rapid and precise detection of waterborne pathogens is critical for effective public health management and environmental safety. Traditional methods for water quality monitoring, considered the “Gold Standard,” are time-consuming, costly, and rely on centralized laboratories and expertise. These methods are impractical for on-site, real-time monitoring, and requiring further improvements. This review explores various waterborne pathogens and cutting-edge molecular detection techniques. It highlights the growing importance of point-of-care and point-of-application methods to expedite results and improve health risk management. Loop-mediated isothermal amplification emerges as a reliable, rapid, and accessible tool in the realms of on-site diagnostics and surveillance. Moreover, the review emphasizes the crucial role of water sample preparation and in-field nucleic acid isolation in augmenting pathogen detection to enable precise assessments of water quality. The advancement of these techniques will guarantee access to safe water and improve the management of waterborne diseases.
Similar content being viewed by others
Introduction
Understanding the impact of water contamination: risks, causes, and potential solutions
The escalating water stress worldwide presents a critical issue, driven by climate change, population growth, and urbanization1,2 Globally, over two billion people consume unsafe drinking water, posing significant threats to human health, social stability, and economic productivity. Unsafe drinking water contributes to 485,000 deaths annually, predominantly from waterborne diseases like cholera and diarrhea, underscoring the urgent need for enhanced water quality and sanitation efforts1. The broader economic impacts include reduced workforce productivity due to illness and substantial costs to healthcare systems in treating water-related diseases3. Microbial contamination in water is primarily caused by fecal pollution, a result of contamination of recreational or drinking water with wastewater. This pollution introduces a variety of harmful pathogens into water sources, posing significant health risks to humans1.
In 2019, water contamination was a major contributor to global health issues, leading to approximately 1.4 million preventable deaths and 74 million disability-adjusted life years (DALYs) due to inadequate water, sanitation, and hygiene (WASH) services4. These figures represent 2.5% of all deaths and 2.9% of all DALYs worldwide, increasing to 7.6% of all deaths and 7.5% of all DALYs among children under 55. Diarrheal diseases are a major contributor, causing over 1 million deaths and 55 million DALYs, with 69% being preventable. Unsafe hand hygiene also leads to around 356,000 deaths and 17 million DALYs due to acute respiratory infections4,5. These numbers highlight the critical need for global efforts to enhance water quality and sanitation.
Water contamination arises from various sources beyond microbial pollution, encompassing chemical pollutants from industrial runoff, agricultural activities, and improper disposal of pharmaceuticals and personal care products6. Chemical pollutants in water can stem from industrial processes, such as heavy metals, solvents, and toxic chemicals used in manufacturing, which can seep into water sources through stormwater runoff or improper disposal7. Agricultural activities contribute to water pollution through the use of fertilizers, pesticides, and herbicides, which can leach into waterways and contaminate drinking water sources. Improper disposal of pharmaceuticals and personal care products, such as medicines, antibiotics, and cosmetics, can also lead to water contamination, introducing harmful substances into the environment 6.
Prevention of waterborne diseases primarily focuses on reducing or eliminating infectious microorganisms from water supplies. Effective water quality monitoring or surveillance programs are essential to prevent diseases originating from contaminated water (Fig. 1).
Water pollution is caused by various sources, including industrial waste, agricultural runoff, climate change, and wastewater. The POC testing devices can rapidly identify contaminants and pathogens on-site. These portable and user-friendly devices enable timely water quality assessments, allowing for immediate action to mitigate the adverse effects of pollutants. The integration of DNA amplification techniques ensures high sensitivity and specificity, making POC testing a vital tool for monitoring and managing water pollution in diverse environmental settings.
Drinking water may harbor a variety of biological hazards, such as viruses, bacteria, protozoa, and fungi 1. Enteric viruses, such as Noroviruses, Hepatitis A and E, Adenoviruses, Astroviruses, Enteroviruses (Evs), Sapoviruses, Parechoviruses, and Rotaviruses, are the most common viral pathogens found in drinking water sources and can cause mild to severe diseases, including gastroenteritis and even cancers1,8. The transmission of these viruses occurs through the fecal-oral route, with water sources becoming contaminated either through direct human fecal discharge or poor wastewater management9.
There are more than 500 species of bacteria known to be pathogenic to humans10. Among the most frequently encountered bacterial pathogens transmitted through drinking water are Burkholderia pseudomallei, Campylobacter coli and Campylobacter jejuni, Escherichia coli O157, Francisella tularensis, Legionella pneumophila, Mycobacterium avium, Salmonella typhi, Salmonella enterica, Salmonella bongori, Shigella dysenteriae, Vibrio cholerae O1 and O13911,12. Protozoa are another group of waterborne pathogens capable of causing diseases and illnesses in humans when they are either ingested or come into contact with the skin. Common taxa include Cryptosporidium, Giardia, Entamoeba histolytica, and Toxoplasma gondii. These taxa can cause diarrhea and other gastrointestinal symptoms, and in severe cases can be life-threatening1,13. Human health risks associated with these bacterial pathogens increase when either potable or recreational water becomes contaminated12,14.
In contrast to developed nations, many developing countries face challenges with inadequate wastewater infrastructure and the absence of monitoring programs. This leads to increases in unsecured and contaminated water sources and increased human health risks10.
To reduce the incidence of waterborne diseases caused by these pathogens, it is essential to prioritize investments in water treatment infrastructure, water quality monitoring, and wastewater management. Regular monitoring of water quality and effective wastewater management are critical for ensuring the safety of water sources. For example, quantitative microbial risk assessment models indicate that certain levels of microbial source tracking (MST) markers in recreational waters, such as Bacteroides HF183 and human adenovirus (HAdV), correlate with a significant health risk from exposure to untreated or secondary treated sewage15. Routine monitoring of water quality is essential for understanding the true burden of fecal contamination, as evidenced by the variability in E. coli levels at the point of collection and use in different countries and seasons, highlighting the need for more frequent and seasonally adjusted data collection16. The implementation of real-time monitoring systems is effective for the early detection of water contamination. However, achieving safe drinking water for everyone requires routine and systematic monitoring to quickly guide improvements in water safety. This approach guarantees the provision of clean and safe drinking water, as well as maintaining the quality of recreational water. Advancements in water quality monitoring technologies are crucial for effective water management and will ultimately lead to improved global health outcomes.
Challenges and limitations in waterborne pathogen detection
The development of water analysis methods, particularly in pathogen detection, has experienced significant advancements over time. Early microbiological techniques, such as culture-based methods, were developed to identify and quantify microbial pathogens in water samples17,18. In the mid-20th century, the advent of molecular biology revolutionized water analysis with the introduction of polymerase chain reaction (PCR) techniques. PCR allows for the rapid and specific detection of pathogens by amplifying their genetic material and provides high accuracy and precision in detecting target pathogens in most matrixes. PCR and its variants, including quantitative PCR, nested PCR, multiplex PCR, and digital PCR, have broad applications in genotyping, gene expression analysis, pathogen detection, and sequencing due to their ability to amplify target DNAs with high specificity and sensitivity19,20.
Quantitative real-time PCR is considered one of the gold standard assays for the detection of pathogens in water21. However, PCR typically requires a dedicated laboratory for sample preparation and analysis. For instance, the USEPA has approved TaqMan qPCR for detecting Bacteroides as an indicator of human fecal pollution in environmental water22. While this method offers high sensitivity and specificity, its use in the field is limited due to its dependence on centralized laboratory facilities. Additionally, the water sample purification and DNA isolation techniques specified in this guideline are not suited for field deployment23,24.
qPCR techniques are unable to distinguish between viable and non-viable bacterial cells, making them incomparable to standard microbiological methods that detect only viable cells25. Some studies have attempted to develop qPCR methods using DNA intercalating dyes to specifically detect viable bacteria26,27,28,29. However, the practicality of these methods for field use remains unresolved due to the additional steps they require.
qPCR assays are highly sensitive to the presence of inhibitors30, which are frequently found in environmental water samples31,32. While PCR inhibitor removal kits offer a potential solution33, their use necessitates complex sample preparation and access to sophisticated equipment, rendering the process time-consuming and complex, ultimately increasing the overall cost.
Furthermore, the high sensitivity of qPCR assays, when Cq values exceed 35, can increase the risk of false positive results34. Although PCR is faster than traditional culture methods, its efficiency is reduced by the multiple thermal cycling steps required, resulting in a completion time of 2–3 h. These limitations highlight the urgent demand for advanced, rapid technologies that can provide quicker turnaround times for results, paving the way for future developments in water quality monitoring.
Rapid in-field diagnostics
In recent years, the adoption of point-of-care diagnostics (in clinical settings) and in-field diagnostics (in environmental monitoring) has become increasingly prevalent. The field of point-of-care diagnostics has evolved remarkably, from early glucose testing to sophisticated smartphone-based technologies, significantly impacting public health, environmental monitoring, and food safety analysis. The continuous development and evaluation of these technologies are essential to ensure their effectiveness and reliability in various clinical and environmental settings35,36.
To address these constraints, alternative DNA amplification methods that can be quickly executed in non-laboratory settings have been ventured. The aim is to create uncomplicated, budget-friendly techniques that can be conveniently employed on-site with minimal equipment, all while maintaining a high degree of precision. Recent nucleic acid amplification techniques, with simplified procedures and faster results, have gained attention for point-of-care and in-field diagnostics, enhancing public health in various settings. To ensure the effectiveness of tests for use in both developed and developing countries, the World Health Organization (WHO) has recommended that molecular diagnostic tests for diseases adhere to the ASSURED criteria, which stands for affordable, sensitive, specific, user-friendly, robust, and rapid, equipment-free, and deliverable37. By meeting these criteria, point-of-care tests can be cost-effective, precise, user-friendly, durable, quick, require minimal equipment, and easily accessible to those in need.
Efforts to develop new nucleic acid amplification assays have resulted in the development of several isothermal amplification techniques that can be utilized as point-of-application tests, meeting the ASSURED criteria for molecular diagnostics. Examples of assays include Helicase-dependent amplification38, recombinase polymerase amplification (RPA)39, rolling circle amplification (RCA), cross-priming amplification40, nucleic acid sequence-based amplification (NASBA)41, and loop-mediated isothermal amplification (LAMP)42. Isothermal assays operate at a single temperature, which makes them a suitable choice for in-field implementation. However, most of them still require a centralized laboratory to perform.
Rapid diagnostics at the site of application are crucial for the effective management of waterborne diseases, and their importance is only now being recognized (Best et al. 43; Caruso et al. 2023; and Khodaparast et al. 23). Technological advancements in biosensors, molecular diagnostics, and POC technologies have significantly improved the speed and accuracy of pathogen detection. For instance, an in-field LAMP assay has been developed to detect Bacteroides as an indicator of human fecal pollution in water. This assay has been commercialized and is now used by water quality authorities in Australia, demonstrating the practical application and effectiveness of these diagnostics in monitoring environmental health23.
Despite these successes, in-field diagnostics face specific challenges and limitations. These include varying environmental conditions that can affect the performance of assays, the need for thorough operator training to ensure accurate results, and logistical issues in deploying these technologies in remote or resource-poor areas44,45. Ensuring robust sample processing and stability, along with the development of user-friendly, equipment-free technologies, will be crucial for the successful implementation of these diagnostic tools.
In addition to addressing these challenges, the integration of in-field diagnostics with existing health and environmental data systems plays a crucial role in enhancing monitoring and decision-making46. These diagnostics facilitate data sharing, support real-time analytics, and connect with broader health information systems, ensuring that data collected in the field can be quickly analyzed and used to inform public health strategies and environmental interventions.
MST
Advancements in in-field diagnostics are invaluable for enhancing water quality monitoring. These innovations are crucial for identifying contamination sources in various environments, especially in water bodies. The precision and adaptability of in-field diagnostics are particularly vital for detecting waterborne pathogens, where rapid and accurate identification can guide timely interventions to protect public health and environmental integrity. Human and animal feces can contain a variety of microorganisms that can pose a threat to human health if present in water. However, detecting each individual pathogen organism in water can be expensive and technically challenging. An alternative approach is to measure the presence of fecal indicator bacteria (FIB) such as E. coli, as an indicator of fecal pollution in water47. High levels of FIB can indicate the potential presence of other pathogens that are associated with fecal contamination. Measuring FIB concentration in water is a widely used approach for assessing water quality and is used in many countries as a standard method for monitoring water safety48.
While E. coli is frequently used as an indicator for FIB, it has certain limitations in pinpointing the specific host animal and contamination source. This is because E. coli is naturally present in the gastrointestinal tracts of all warm-blooded animals. Furthermore, its ability to survive and proliferate outside an animal’s gut can complicate contamination source identification48,49. Research has indicated that there may not always be a clear correlation between the E. coli levels in water and the risk of human illness, especially when the contamination source is unknown50. Therefore, relying only on E. coli as a FIB may not offer a comprehensive assessment of water quality and the associated health risks50.
Consequently, ongoing scientific investigations are exploring alternative methodologies, such as MST techniques, to accurately discern the origin of fecal contamination and deploy targeted and effective mitigation strategies. Bacteroides exhibit notable proficiency as the marker for source identification due to their exclusive association with warm-blooded animals, their prevalence in fecal matter, and their limited capability for significant re-growth in the environment owing to their anaerobic lifestyle24,48,49,51. The existence of diverse Bacteroides strains that display host-specificity enables precise source identification. Bacteroides has emerged as a promising MST marker, achieved through the amplification of sequence-specific 16S rRNA utilizing DNA amplification techniques49. Unlike E. coli, which is not exclusive to a particular host animal and can survive outside of the host’s body, the use of Bacteroides as a marker provides greater specificity and accuracy in identifying the source of fecal contamination52. Therefore, identifying Bacteroides strains in polluted waters holds the potential to quickly diagnose the contamination source, leading to more effective management. However, to fully comprehend this potential and establish Bacteroides as a widely accepted and reliable tool for MST and water quality evaluation, several challenges must be addressed.
The traditional method of detecting bacteria in water involved cultivating the sample on solid gelatin media and manually counting the resulting colonies after 48 h of incubation53,54. Several studies have attempted to establish culture-based methodologies for investigating microbial contamination in water (Table 1). Although the culture-based method has been the standard for bacterial detection in natural water for almost a century54 and is well-established for detecting most FIB, it has several limitations.
Firstly, it is time-consuming and may take up to 48 h or more to get the results. Secondly, it provides no information on the source of the detected bacteria, which is critical for MST24. Additionally, there is the possibility that the microbe may transition to a viable but non-culturable state in water, which can lead to false-negative results and impact the method’s accuracy53.
In 2012, the USEPA incorporated a TaqMan® quantitative polymerase chain reaction (qPCR) assay as a standard method for detecting Enterococci for water quality assessment55. This molecular detection approach offers several advantages over culture-based techniques, including enhanced specificity, greater output without the need for biochemical confirmation, and significantly quicker results24,56,57. qPCR is a powerful technique that allows for the quantification of a wide range of DNA targets with high sensitivity, capable of detecting as few copies of the target sequence. Compared to traditional detection methods, qPCR is faster, highly automated, and more accurate in detecting and quantifying nucleic acids58.
While qPCRs provide benefits over traditional culture-based methods, they also have limitations. The detection of indicator bacteria can be expensive and still time-consuming due to the required cold-chain logistics, expertise, and equipment. Additionally, meticulous sample preparation and data interpretation are crucial to prevent contamination and guarantee accuracy24,54,56,57.
Advancements and applications of LAMP assay in pathogen detection and MST
LAMP, developed by Notomi et al.42, offers an alternative to PCR with several advantages. A key benefit of LAMP is its shorter runtime, as it eliminates the need for the thermocycling steps required in PCR, making it a faster and more efficient DNA amplification method. Additionally, LAMP’s simplicity enables non-technical personnel to perform the assay, allowing its use in a broader range of environments, including resource-limited areas24,59. LAMP eliminates the need for complex DNA isolation from the sample, thereby streamlining the pathogen detection process. This is because the LAMP DNA polymerase, Bacillus stearothermophilus (Bst), has a higher tolerance to amplification inhibitors that usually inhibit other DNA polymerases used in PCR53. The common biological DNA amplification inhibitors include whole-blood, hemin, blood culture media, N-acetyl cysteine, NaCl, urine, stools, and anticoagulants. Humic acids, tannins, metal ions, and other organic and inorganic compounds are common inhibitors in environmental water60,43. These inhibitors can often interfere with PCR reactions and hinder DNA amplification, but Bst’s ability to tolerate inhibitors can make LAMP a more effective option for amplifying DNA from challenging and dirty samples43,61. Additionally, the use of 4–6 primers in LAMP enables highly specific amplification of the target DNA, comparable to the gold standard assays62. Therefore, LAMP is more likely to provide accurate results in water samples with quality variation61.
The four main primers included in the LAMP reaction are the forward and backward inner primers (FIP and BIP) the forward outer primer (F3) and backward outer primer (B3). The FIP and BIP primers bind to the target region and form loop structures, which help initiate the amplification reaction more efficiently. Additionally, loop forward (LF) and loop backward (LB) primers can be added to target two more regions on the target sequence, further enhancing the reaction speed (Fig. 2)42,63. The specific polymerases used in LAMP reactions have a strand displacement activity that eliminates the need for thermocycling steps42. This allows the LAMP reaction to proceed at a constant temperature, typically around 60–65 °C. This results in faster amplification times and lower power requirements compared to PCR methods, making LAMP more attractive for remote deployments and point-of-application diagnostics.
The LAMP reaction utilizes four main primers designed to recognize six distinct regions of the target DNA. The FIP contains an F2 region at the 3’-end and an F1c region at the 5’-end, while the F3 includes an F3 region complementary to the F3c region of the target sequence. The BIP is composed of a B2 region at the 3’-end and a B1c region at the 5’-end, and the B3 contains a B3 region complementary to the B3c region of the template sequence. The amplification process begins when the F2 region of FIP anneals to the F2c region of the target DNA, allowing Bst DNA polymerase to initiate complementary strand synthesis. Subsequently, the F3 primer anneals to the F3c region of the target and extends, displacing the FIP-linked strand and forming a loop at the 5’-end. This loop serves as a template for BIP, which anneals its B2 region to the B2c region of the template, initiating DNA synthesis and opening the 5’-end loop. Next, the B3 primer anneals to the B3c region of the target DNA and extends, displacing the BIP-linked strand and forming a dumbbell-shaped DNA structure. This dumbbell-shaped DNA is converted into a stem-loop structure, initiating the second stage of the LAMP reaction known as LAMP cycling. The resulting amplicons consist of a mixture of stem-loop and cauliflower-like structures with multiple loops. To further accelerate the LAMP reaction, loop primers (LF and LB) are designed to anneal to the loop regions on the stem-loop structures. The annealing of these loop primers provides additional binding sites for DNA polymerase, enhancing the exponential amplification of the target DNA. This allows multiple rounds of synthesis to occur simultaneously, significantly increasing the reaction speed and yield.
A wide variety of detection methods are available for LAMP products, enabling selection based on the specific application of the assay. These methods include turbidity, agarose gel electrophoresis, calorimetric, and UV light detection, as well as the use of intercalating dyes64,65. The incorporation of intercalating fluorescent dyes in commercially available master mixes has revolutionized the LAMP assay by enabling real-time amplification detection, offering a swift and precise method for measuring DNA amplification. LAMP, known for its high specificity and sensitivity, has gained recognition as a robust alternative to conventional DNA amplification techniques for in-field pathogen detection42,63. The combination of microfluidic chips with LAMP reactions empowers rapid and accurate infectious disease diagnosis, particularly suited for point-of-application diagnostics66. Lateral flow devices are also being developed and used for the detection of LAMP products, offering a simple and easy-to-use method for detection in resource-limited settings65. The inherent simplicity of the LAMP assay makes it feasible to perform even without specialized expertise, enabling its application outside centralized laboratories. This advantage proves crucial in swiftly evaluating and effectively tackling health or environmental issues. These features make LAMP an attractive option for on-site pathogen detection, particularly in resource-limited settings where sophisticated laboratory infrastructure is not readily available. LAMP assays have been shown to be an effective method in MST by detecting various indicator bacteria. One such example is the work by Martzy et al. 53, who developed a LAMP assay for Enterococcus spp., a standard fecal indicator bacterium, in water. The assay was shown to have equal sensitivity and specificity to qPCR, making it suitable for in-field testing. The development of LAMP assay to detect Bacteroides, an indicator bacterium for MST in waterways was first achieved by Jiang et al. 24 and further optimized for in-field detection of human fecal contamination of water by Khodaparast et al. 23. These findings highlight the potential of LAMP assay in MST applications for water quality monitoring.
LAMP assays have demonstrated promising results in detecting several waterborne pathogens such as E. coli O157:H767, Campylobacter spp.68, Salmonella spp.69, Pseudomonas aeruginosa70, Shigella flexneri71, Adenoviruses72, Hepatitis A73, Hepatitis B74, Noroviruses75, Rotaviruses76, or other microscopic parasites that are transmitted through water such as Giardia lamblia77, E. histolytica78, and Cryptosporidium parvum79.
Reverse transcription LAMP (RT-LAMP) integrates reverse transcription with the LAMP amplification technique to identify RNA targets, enabling the detection of RNA-based viruses. Numerous RT-LAMP assays have been specifically developed to identify various RNA viruses. For instance, RT-LAMP assays have been developed to detect viruses such as SARS-CoV-236,80,81,82 and Zika virus83 improving point-of-care diagnostics and facilitating the establishment of more effective control strategies for these devastating pathogens.
Significant advancements in LAMP assay include the development of multiplex versions that detect multiple targets simultaneously, improving diagnostic efficiency and reducing pathogen identification time84,85,86,87. Techniques such as loop-primer endonuclease cleavage (LEC)–LAMP and dual-sample microfluidic chips have been successfully employed to detect multiple targets in a single reaction, demonstrating high sensitivity and specificity87,88. Research on LAMP has evolved significantly since the pivotal studies in 2000 demonstrated its diagnostic potential. Since then, numerous studies have applied LAMP assays for pathogen detection, significantly advancing POC diagnostics. Due to their superior capabilities and continuous advancements, LAMP assays have become an attractive technique for these applications. The increasing number of publications on LAMP assays underscores their growing influence and importance in the field of POC diagnostics (Figs. 3–5).
Significant advancements in LAMP assays have enhanced the detection of pathogens, improving sensitivity, specificity, speed, and practicality for field applications. Key achievements include increased sensitivity and specificity, allowing for the detection of low pathogen concentrations and reducing false positives. LAMP assays offer rapid detection within 30–60 min, making them suitable for timely water quality assessments. Field-friendly protocols and portable devices enable on-site pathogen detection, while modifications like propidium monoazide differentiate between live and dead pathogens. Multiplexing capabilities allow simultaneous detection of multiple pathogens, enhancing efficiency. Integration with biosensors facilitates easy and rapid detection, and robustness improvements ensure consistent performance across varying conditions. Automated systems reduce human error and increase throughput. The cost-effectiveness and commercialization of standardized LAMP kits make these assays accessible and effective for widespread use, ensuring water safety and public health.
This pie chart represents the distribution of published articles on the development of isothermal amplification assays on PubMed. Each segment of the pie chart is presented in a different color, representing a type of isothermal amplification assay. The numbers indicate the number of research articles published for each assay so far.
This figure compares three diagnostic methods—culturing, qPCR, and LAMP—across several criteria relevant to their use in detecting waterborne pathogens. The criteria include water sample preparation, DNA/RNA extraction, laboratory instrument requirements, time required, cost, complexity, and field deployability, highlighting the more resource-intensive nature of qPCR and culturing methods compared to the LAMP assay. Water sample preparation: all three methods require preparation, such as filtration of water samples to enrich the target pathogens. DNA/RNA extraction: required for qPCR but not for culturing; most LAMP assays can be completed without using lab-based DNA extraction. Laboratory instrument needed: required for culturing (growth media and controlled environment) and qPCR (thermocycler), but not for LAMP (can be operated with a simple heat block). Time required: culturing takes 48–72 h, qPCR takes 2–3 h, and LAMP takes 30–60 min. Cost: culturing and LAMP have lower costs (indicated by fewer ‘+‘ signs) compared to qPCR. Complexity: culturing and LAMP are less complex than qPCR. Field deployability: only LAMP is suitable for field deployment. H hours, M minutes.
Common freshwater pathogens
Several human pathogens are present in freshwater environments. Some of the most prevalent ones are listed in Table 2.
Giardia
Giardiasis, a common diarrheal disorder caused by the enteric protozoan parasite Giardia, affects both humans and animals. It is responsible for numerous gastrointestinal outbreaks annually in both developed and developing countries89,90. The impact of giardiasis can be severe, especially for vulnerable populations such as young children, pregnant women, and individuals with weakened immune systems91. In addition, giardia infections are highly prevalent in domestic animals such as cats, dogs, and livestock92, as the cysts of this parasite can spread through surface water that has been contaminated by either human or animal fecal materials89. Contact between agricultural animals, such as cattle, and the contaminated water can lead to diarrhea, resulting in production losses93. The challenge in detecting these infections occurs because infected animals can be asymptomatic, serving as a source of transmission94.
Various diagnostic methods have been developed to detect giardiasis (Table 3). While direct stool examination is commonly used in laboratories, antigen detection methods are suggested as a complementary assay for giardia detection in fecal samples. However, direct stool examination and serologic tests may lack sensitivity and specificity, potentially leading to false-negative or false-positive results95.
Verweij et al.96 developed a qPCR assay to detect the ribosomal RNA (rRNA) gene sequence of G. lamblia, which reported a specificity of 100% and sensitivity of 98%. The assay was found to be more sensitive (98%) than the microscopy analysis (89%) of the samples. This qPCR assay proved useful in detecting patients with giardiasis symptoms but who were negative with microscopy and antigen detection tests96. Later, a multiplex real-time PCR assay was developed for the detection of three parasites responsible for most cases of diarrhea, including E. histolytica, G. lamblia, and C. parvum97.
To enhance the efficiency of Giardia detection, a LAMP assay was developed for detecting G. duodenalis in fecal and environmental water samples (Table 4). This assay has demonstrated superior performance in the presence of DNA amplification inhibitors compared to PCR and qPCR89. When combined with the ARAD microfiber filtration protocol, the LAMP assay enabled the filtration of large volumes of drinking water, reaching up to 1000 L, for the detection of G. duodenalis98. As the LAMP assay can be implemented in settings where laboratory resources are limited, such as in developing countries with a high incidence of giardia infections, it has the potential to significantly improve the detection and control of waterborne diseases in these regions.
Molina-Gonzalez et al.99 improved the accuracy of a previously developed RPA assay for detecting G. duodenalis100 by optimizing sample preparation and adding betaine into the reaction. However, the authors recommended future research to explore the potential of field deployable DNA extraction for in-field application of the assay and further analysis to improve the assay’s specificity.
While DNA-based detection methods offer high sensitivity and specificity for detecting Giardia, the necessity for sample preparation and DNA extraction presents limitations when it comes to conducting the assay in the field. Nevertheless, there is still a lack of detection methods suitable for point-of-application or point-of-care scenarios where rapid and accurate detection is imperative.
Cryptosporidium
Cryptosporidium species are a common cause of diarrhea in children in most developing countries due to poor hygiene and lack of access to safe food and drinking water101, with approximately 30 different species reported to infect both humans and animals through contamination of drinking or environmental water with host feces102,103,104,105. Cryptosporidiosis is responsible for up to half of all deaths in children under the age of 5 due to diarrhea and is the second leading cause of diarrhea-related deaths in children106. Several detection methods have been optimized for the detection of Cryptosporidium in water and food samples, as reviewed in detail by Ahmed and Karanis107, and DNA-based detection techniques utilized to investigate or genotype Cryptosporidium (Table 3).
Molecular detection assays have emerged as powerful tools for detecting cryptosporidium in water due to their high sensitivity and specificity. DNA-based amplification techniques such as PCR enable the differentiation of host species genotypes, aiding in tracking the source of the infection105,108.
In the last decade, PCR-based assays have been improved for detecting Cryptosporidium in water and stool samples. One such improvement is the development of qPCR, which has demonstrated high sensitivity and specificity (100%)109. The qPCR assay reported a limit of detection (LoD) of 1 oocyst for C. hominis and C. parvum and showed a higher sensitivity on water samples tested compared to nested PCR.
Multiplex PCR assays have been developed to simultaneously detect Cryptosporidium along with other pathogens, and some of these assays have become commercially available110. For example, the BD Max parasitic panel (EPP) test can detect Giardia duodenalis, Cryptosporidium hominis, C. parvum, and E. histolytica using multiplex PCR. The test has a high specificity of 99.6% and a sensitivity of 95.5% for C. parvum or C. hominis. The use of multiplex PCR assays for detecting multiple pathogens at once can be advantageous in terms of time and cost efficiency, making them a valuable tool for the surveillance and diagnosis of infectious diseases111.
Droplet digital PCR (ddPCR) was designed for investigating Cryptosporidium oocysts in fecal samples by Yang et al.112. ddPCR was shown to be more robust in the presence of inhibitors compared to qPCR and did not require the generation of a standard curve for the interpretation of results. However, ddPCR is estimated to cost approximately twice as much as qPCR. Another nucleic acid-based detection method, Fluorescence in situ hybridization (FISH), has been optimized for detecting Cryptosporidium oocysts by using fluorescent probes that target the ribosomal rRNA of C. parvum. This method demonstrated the ability to differentiate viable oocysts in water and fecal samples113,114. However, the accuracy of viable determination using FISH is dependent on the decay rate of the rRNA from recently deceased oocysts, leading to the potential for false-positive results for viable oocysts. The susceptibility to environmental factors such as temperature, chlorination, light exposure, and sample preparation should be considered when interpreting FISH results115.
A LAMP assay which was supplemented with stem primers, similar to the previous study by Bakheit et al. 116 achieved a higher sensitivity (10 oocysts/ml) compared to nested PCR in detecting human pathogens C. parvum, C. hominis, and C. meleagridis with a time to positive of about 80 min from reaction preparation101 (Table 4). The assay showed no off-target activity when tested on non-target DNAs and due to the use of lateral flow dipstick (LFD) for detecting the amplified target, this assay may be an alternative for point of care diagnostics especially in the decentralized laboratories, however, the LFD may need improvement to prevent opening the reaction tubes. Similar to Giardia, commercially available DNA-based detection methods for Cryptosporidium remain complex and lack streamlined sample processing and simple field-deployable DNA isolation procedures for point-of-care and point-of-application purposes.
E. histolytica
E. histolytica is a parasitic protozoan that can infect humans and other primates, causing amebiasis. Amongst 40 species of this parasite, eight of them are pathogens to humans117. This protozoan is found in contaminated water and food and can cause a range of symptoms from mild diarrhea to severe abdominal pain and dysentery. In some cases, the parasite can spread beyond the intestines and invade other organs such as the liver, lungs, and brain, causing potentially life-threatening complications118,119. The treatment of E. histolytica infection typically involves the use of antibiotics. It is important to note that asymptomatic carriers of E. histolytica may not require treatment, but they should be monitored for any signs or symptoms of infection. Additionally, prevention measures such as practicing good hygiene, washing hands regularly, and avoiding contaminated food and water sources can help reduce the risk of infection120,121.
E. histolytica can be detected by various methods (Table 3). The microscopy method involves examining stool samples for the presence of E. histolytica cysts or trophozoites. While this approach is common and cost-effective, it cannot distinguish between different species of the parasite118 and suffers from low sensitivity122. On the other hand, culturing methods are highly sensitive but time-consuming, costly, and complex122.
The serology method, which detects antibodies against E. histolytica, is well-established but offers lower sensitivity compared to molecular assays119. Imaging techniques such as ultrasound and computed tomography (CT) scans are useful for detecting liver abscesses potentially caused by E. histolytica infection118,119,123. Antigen detection tests are fast and simple to perform but may have lower sensitivity compared to molecular tests119. ELISAs methods using amebic antigens detection are highly specific however, they are expensive when used as the commercial kit122.
PCR assays were also developed to detect E. histolytica DNA in stool or tissue samples. These tests are highly sensitive and specific for this parasite and can detect very low levels of the parasite124. For example, Ali and Roy117 designed a multiplex real-time PCR assay using hydrolysis probes to detect four human pathogen species of E. histolytica with similar morphology and that are otherwise indistinguishable. The LoD of the assay was 0.1 trophozoite of the parasite. Although multiplex PCR assays offer high sensitivity and specificity, their cost and complexity make them unsuitable for use in resource-limited settings125.
Several studies have evaluated the performance of LAMP assay for E. histolytica detection, finding it to be highly sensitive and specific, which can be performed directly on stool samples without the need for DNA extraction, further simplifying the procedure126,127,128 (Table 4). Foo et al.129 conducted a study to compare the performance of the LAMP assay with three PCR methods (conventional PCR, nested PCR, and qPCR) for detecting E. histolytica DNA in stool samples. Their findings revealed that the LAMP assay demonstrated superior sensitivity and a lower LoD compared to the other PCR methods. Furthermore, the LAMP assay exhibited a shorter turnaround time, suggesting it could serve as a reliable alternative platform for point-of-care E. histolytica DNA detection.
T. gondii
T. gondii is an intracellular protozoan parasite that can infect warm-blooded animals, including humans. It is transmitted through ingestion of oocysts shed by infected cats, ingestion of tissue cysts in undercooked meat from infected animals, or congenital transmission from mother to fetus130,131.
In humans, T. gondii infection, known as toxoplasmosis, can cause a range of clinical manifestations, from asymptomatic infection to severe disease. Acute infection in immunocompetent individuals is usually mild and self-limited, but it can cause flu-like symptoms, swollen lymph nodes, and muscle aches. However, in immunocompromised individuals, T. gondii infection can cause severe disease, including encephalitis, myocarditis, and pneumonitis, and in pregnant women, T. gondii infection can result in congenital infection and fetal abnormalities130,132. Toxoplasmosis is typically treated with antibiotics such as pyrimethamine and sulfadiazine, along with folinic acid133,134. However, with the increasing prevalence of antibiotic-resistant strains, it is crucial to improve diagnostic methods and prevent infections, particularly in high-risk populations such as pregnant women and immunocompromised individuals. Previous studies have highlighted the increasing significance of waterborne transmission of T. gondii to humans through the dispersal of oocysts in surface water. This mode of transmission has significant epidemiological implications and deserves close attention135. Therefore, it is critical to develop effective diagnostic methods and treatment options that can address antibiotic resistance to effectively manage and prevent toxoplasmosis133,134.
Serological tests like ELISA, indirect fluorescent antibody test (IFAT), and immunoblotting were developed to detect the presence of antibodies against T. gondii in serum as the rapid tests, but they cannot distinguish between active and inactive infections131. PCR is a sensitive and specific method for the detection of T. gondii DNA in various samples such as blood, tissue, cerebrospinal fluid, and amniotic fluid136. PCR is also used for the detection of T. gondii in food samples, which can be a potential source of infection137. Microscopic examination of stained smears or tissue sections can be used to detect the presence of T. gondii in various samples such as blood, cerebrospinal fluid, and tissue biopsy specimens138. T. gondii can be cultured in vitro in specialized cell culture systems. This method is not routinely used for diagnostic purposes due to its low sensitivity and the requirement for specialized expertise and equipment131,139. Rapid diagnostic tests such as lateral flow assays are available for the detection of T. gondii-specific antibodies in serum or plasma. These tests are simple to perform and do not require specialized equipment, making them useful for point-of-care and point-of-application testing140.
Commercially available tests for detecting T. gondii in freshwater include PCR141 and immunological assays such as ELISA131. Detecting T. gondii in freshwater can be challenging due to the low abundance of the parasite in water. Additionally, the detection of the parasite may be affected by various factors such as the presence of other microorganisms and the sampling method employed142.
LAMP assay has been developed to detect T. gondii in various biological samples, including blood, cerebrospinal fluid, and tissue samples, and has proven to be a sensitive and specific diagnostic tool143 (Table 4). Several studies demonstrate the utility of the LAMP assay for detecting T. gondii in water samples with high sensitivity and specificity, and provide valuable information for the development of reliable and efficient methods for water quality monitoring144,145, but it’s still unavailable as a commercial test.
Hepatitis A virus (HAV)
HAV is a single-stranded RNA virus and highly contagious and causes acute liver inflammation. The virus is typically spread through contaminated food and water or close contact with an infected individual146,147. Symptoms of HAV infection include fatigue, fever, nausea, vomiting, abdominal pain, dark urine, and yellowing of the skin and eyes. In most cases, the infection resolves on its own within a few weeks, and there is no specific treatment for hepatitis A146. However, in severe cases or for individuals with underlying liver disease, hospitalization, and supportive care may be necessary146.
Detection of HAV infection typically involves serologic testing to detect antibodies to the virus in the blood. In addition to serologic testing, molecular methods such as PCR are used to detect HAV RNA in blood or stool samples. PCR can be useful for diagnosing acute infection or for monitoring treatment response in individuals with chronic HAV infection147. Kozak et al.148 conducted a study to develop an RT-PCR assay for detecting HAV RNA using two different platforms: the Qiagen Rotor-Gene and the BD MAX. The sensitivity of the assay was reported to be 94.8% and 87%, while the specificity was 100% and 94.7%, respectively. The LoD of the assay was 4.3 × 102 copies/ml when tested on a spiked serum with the known concentration of plasmid harboring target region. In the study of Persson et al.149, the 5′ untranslated regions (UTR) of HAV have been identified as the most conserved genomic region. In silico inclusivity, analysis revealed that the assay developed in this study was more accurate than the one recommended by the International Organization for Standardization (ISO) method (ISO 15216-1:2017) and demonstrated increased effectiveness in detecting globally emerging genotype III strains. When it comes to detecting low levels of HAV, a comparison between Reverse transcription PCR (RT-qPCR) and RT-ddPCR revealed that RT-qPCR is preferable for qualitative detection, although RT-ddPCR exhibited higher precision.
HAV can be detected in freshwater sources, such as rivers, lakes, and ponds, where it can be introduced through fecal contamination from infected individuals or animals150. Detection of HAV in fresh water typically involves collecting water samples and testing for the presence of HAV RNA using PCR, which can provide rapid and sensitive detection of HAV151.
RT-LAMP assay has demonstrated high sensitivity and specificity in detecting IA, IB, and IIIB sub-genotypes of HAV in clinical samples (fecal), with detection limits ranging from 0.4 to 0.8 focus forming units (FFU) per reaction73. In addition, other isothermal assays, such as the NASBA assay have been developed to detect HAV152 with a detection limit of 1 PFU of viral RNA. The assay was reported to have high specificity and comparable sensitivity to the real-time RT-PCR assay. However, in some cases, the time to result for NASBA may be longer than other amplification-based assays, and it requires several steps and optimization of reaction conditions for optimal performance153.
Norovirus
Norovirus, a single-stranded RNA virus, induces gastroenteritis (L154.). It has six genogroups, with GI and GII being the most associated with human diseases154. The virus is highly contagious and is a well-known cause of acute gastroenteritis outbreaks in settings where individuals are in close proximity, including hospitals, nursing homes, schools, and cruise ships155. Norovirus transmission occurs via the fecal-oral route, whereby the virus is disseminated through contact with fecal matter or vomit from infected individuals. Additionally, it can be spread via the consumption of contaminated food or water, or through contact with contaminated surfaces, followed by subsequent transfer to one’s nose or mouth155,156. Norovirus infection is characterized by a wide range of symptoms, including nausea, vomiting, diarrhea, abdominal cramps, low-grade fever, headache, and body aches. These symptoms typically manifest within 12–48 h post-exposure to the virus and may persist for a duration of 1–3 days. Norovirus infection is highly transmissible, causing nearly 20% of acute gastroenteritis cases157 and individuals who have been infected can serve as reservoirs of the virus for an extended period. Currently, there is no specific antiviral therapy available for norovirus infection, and the illness is generally self-limited, meaning that it will resolve spontaneously without medical intervention156. However, epidemiological investigations assessing the prevalence of norovirus infections in the United States have demonstrated that this virus imposes a substantial economic burden on society. Specifically, it has been estimated that the total cost associated with norovirus infection in the United States is approximately $10.6 billion158. Given the high degree of contagiousness associated with human noroviruses, timely identification of the virus and its source is of paramount importance during an outbreak. However, the development of rapid and reliable assays for detecting infectious viruses poses significant technical challenges154.
RT-qPCR was developed to detect norovirus, owing to its high sensitivity and specificity. The ISO has recommended the use of RT-qPCR as the preferred method for detecting norovirus in both food and water samples, underscoring its effectiveness and accuracy159,160. Additionally, multiplex real-time RT-PCR has been developed to detect human pathogen genogroups of noroviruses161,162.
Other molecular-based methods that have been developed to detect norovirus include RT-LAMP163 and NASBA164. The colorimetric LAMP assay utilizing hydroxy naphthol blue dye demonstrated a LoD of 103 copies per reaction, comparable to conventional RT-PCR. Additionally, the assay exhibited 100% specificity when tested against other common gastroenteritis viruses163 (Table 4). The NASBA assay exhibited high sensitivity, accurately detecting 100% of positive samples that were confirmed using electron microscopy164. The assay demonstrated remarkable specificity (100%) from fecal samples. Additionally, the LoD for the assay was 5 pg/ml of RNA in fecal samples. A more sensitive RT-LAMP assay was later developed by Jeon et al.165 with a LoD of 101 copies/µl of genogroups GI and GII human norovirus in oyster samples. The assay was reported to be highly specific with time to result of under 60 min.
Moore and Jaykus166 developed an RPA assay to detect human norovirus strain GII.4. Their study found that the assay’s LoD was 3.40 ± 0.20 log10 copies and that it was more resistant to the presence of inhibitors when compared to other RT-qPCR assays. Additionally, the assay demonstrated high specificity and could be completed in under 30 min.
Rotavirus
Rotavirus is a double-stranded RNA virus, highly contagious that can cause severe diarrhea, vomiting, and fever, particularly in infants and young children. It belongs to the Reoviridae family of viruses and is one of the most common causes of gastroenteritis worldwide167,168.
Rotavirus is primarily transmitted through the fecal-oral route and can happen through direct contact with an infected person or through contact with contaminated surfaces. The virus can also be spread through the air by coughing or sneezing and through contaminated food or water167. Due to its high contagiousness, rotavirus outbreaks often occur in crowded settings, such as daycare centers, schools, and nursing homes. Vaccines are also available to protect against rotavirus infection169. The cost of treating rotavirus infection may vary depending on several factors such as the severity of the illness, the location of the treatment facility, and the type of treatment received.
One of the recommended tests to detect rotavirus infection involves identifying rotavirus antigens in the stool sample using an enzyme immunoassay (EIA) or a rapid antigen detection test170. Isothermal DNA amplification techniques were developed to detect rotavirus such as RT-PCR171 and RT-LAMP172. The RT-PCR assay was designed to detect group F adenovirus, rotavirus A, and rotavirus C in stool samples. The PCR results were evaluated by electron microscopy and latex agglutination antigen tests. The RT-PCR assay outperformed in detecting adenovirus F and rotavirus A compared to the other assays, however, rotavirus C was not detected in any samples. The LoD of the PCR assays were ten copies of target DNA/reaction for all assays.
The RT-LAMP assay was reported to be highly specific (100%) and showed higher sensitivity than the PCR assay with a detection limit of 1.26 × 104 copies of standard plasmid compared to PCR with LoD of 1.26 × 105 copies172 (Table 4).
An RT-RPA assay and an RT-RPA assay combined with a lateral flow strip to detect Bovine rotavirus A. The LoD of the assays was 1.4 × 102 copies per reaction and 1.4 × 101 copies per reaction, respectively when tested on the standard RNA of the virus. Both assays demonstrated 100% specificity when compared to RT-PCR and have shown diagnostic sensitivity of 98.39% and 100%, respectively173.
RCA was also developed to detect rotaviruses with a sensitivity of 103 copies using 58 different padlock probes174. The assay showed comparable results to PCR assays when tested on fecal samples, but it required approximately 3.5 h to complete the entire process.
Adenovirus
Adenovirus is a double-stranded DNA virus that can cause a wide range of diseases in humans, including respiratory infections, conjunctivitis, gastroenteritis, and even severe illnesses such as pneumonia, meningitis, and hepatitis175. Adenovirus is highly contagious and is spread through direct contact with infected individuals or their bodily fluids, such as respiratory secretions, saliva, or feces. The virus can also survive on surfaces for extended periods, making it possible for individuals to contract the virus by touching contaminated surfaces175,176.
The pathogenicity of adenoviruses is due to their ability to invade host cells and replicate rapidly, leading to tissue damage and inflammation. Adenovirus infections are often characterized by fever, cough, sore throat, runny nose, diarrhea, and conjunctivitis. In severe cases, adenovirus infections can cause respiratory distress, liver failure, and even death177. Certain populations, such as young children, the elderly, and individuals with weakened immune systems, are at higher risk for developing severe adenovirus infections176,177. Treatment for adenovirus infections is typically supportive and aimed at relieving symptoms. In some cases, antiviral medications may be used to treat severe infections175,176. However, the cost may vary based on the severity of the infection and healthcare resources178.
The choice of detection method depends on factors such as the clinical sample type, method sensitivity, specificity, and available resources. PCR is often the preferred method for detecting adenovirus due to its high sensitivity and specificity. However, it cannot distinguish between infectious and non-infectious viral particles. To address this, a combination of cell culture and PCR is recommended for identifying slow-growing or non-cytopathic adenoviruses179,180.
A TaqMan rtPCR assay was developed to detect six adenovirus species (A-F) with high sensitivity and specificity181. The LoD of the assay was 5 copies of AdV40, 8 copies of AdV41, and 350 copies of AdV3. In this study, a fluorescence resonance energy transfer (FRET) probe was used to differentiate between AdV40 and AdV41 serotypes by analyzing the melting curve181. A highly sensitive and specific multiplex PCR-enzyme hybridization assay was developed to detect all six adenovirus species with a LoD of 102–103 copies/ml of control DNA and 100% specificity against 12 other respiratory pathogens182. However, the assay had a relatively long turnaround time of around 5 h.
A LAMP assay was optimized by Shuryaeva et al.183 to monitor the amplification of a specific region of the hexon gene in HAdV (Table 4). When clinical samples were tested, the assay showed a sensitivity and specificity comparable to that of PCR (LoD of 103 copies/ml). Colorimetric and fluorescent probes LAMP both were successfully optimized to detect the LAMP assay product in the study however, the colorimetric LAMP assay showed a lower sensitivity than the PCR. In another study, Rames and Macdonald184 have developed an RPA assay to detect HAdV as an indicator of viral water quality. Lateral flow detection was combined with the RPA assay with a total time to positive of 20 min. The assay showed a sensitivity of 10–50 copies of the viral plasmid and compared to qPCR the assay was 100% sensitive and specific.
The detection of HAdV was also investigated using a duplex real-time recombinase-aided amplification (RAA) assays by Wang et al. 185. The HAdV 3 and HAdV 7 were targeted by RAA combined with an internal amplification control (IAC) with a LoD of 5.0 and 14.8 copies per reaction, respectively. The use of IAC improved the assay efficacy in reducing the rate of false negative results. The reactions were carried out at 39 °C and completed in 20 min.
Enterovirus
Evs are a group of single-stranded RNA viruses that primarily infect the gastrointestinal tract and can cause a range of illnesses from mild to severe186,187. Evs are spread through contact with fecal matter, respiratory secretions, or contaminated surfaces. In severe cases, enterovirus infection can lead to meningitis, encephalitis, myocarditis, or respiratory failure186,188. Young children, infants, and people with weakened immune systems are at a higher risk of developing severe enterovirus infection188. There is no specific treatment for enterovirus infection, and treatment is usually supportive.
RT-PCR is currently the most used method for Evs detection. Jacques et al.189 have developed an RT-PCR for detecting enterovirus and improved the detection limit of previous cell culture methods enhancing the clinical diagnosis of EV-related meningitis. Using minor groove binder probes and primers modified with complementary locked primer technology, Hong et al.21 have further improved the sensitivity of the previously developed RT-PCR assay190. Compared to the previous TaqMan probe real-time RT-PCR, the assay showed higher sensitivity on clinical samples (62/73 and 29/73).
LAMP assay was optimized to detect Evs in water and stool samples targeting the highly conserved 5′ untranslated region of the human Evs with a sensitivity of ten copies of plasmid191. Evs were detected in all water samples tested 9/9 (100%) and 18/19 (94.7%) in stool samples collected from patients, demonstrating greater sensitivity than conventional PCR. Additionally, several studies developed RT-LAMP assay to detect different species of Evs with high sensitivity and specificity192,193,194.
Yin et al.195 developed an RPA assay to detect enterovirus 71 (EV71) with 100% specificity and 3.767 log10 genomic DNA/reaction sensitivity and performed comparable sensitivity to the commercial RT-qPCR on clinical samples. For reliable molecular detection assays, it’s crucial to employ suitable viral isolation techniques for each sample type, especially in stool samples, to eliminate potential nucleic acid inhibitors. Additionally, careful primer design is essential to ensure the detection of all enterovirus types196.
Blue-green algae
Blue-green algae, or cyanobacteria, are photosynthetic prokaryotes found in various aquatic habitats, including freshwater lakes, rivers, and marine environments. While they are important for maintaining the ecological balance of aquatic systems, they can also generate harmful algal blooms (HABs) under specific environmental conditions. Certain species, such as Planktothrix rubescens, can produce potent toxins called cyanotoxins that pose a significant risk to human and animal health197,198. Exposure to these toxins can occur through ingestion of contaminated water, inhalation of aerosolized toxins, or skin contact with contaminated water. HABs can also deplete oxygen in the water, leading to negative effects on aquatic life. Therefore, it is crucial to monitor and manage blue-green algae populations to prevent harmful blooms and protect both human health and the environment197.
Health issues from blue-green algae are primarily caused by cyanotoxins. In humans, symptoms vary from skin, eye, and respiratory irritation to gastrointestinal issues like nausea, vomiting, or diarrhea, as well as headaches, dizziness, muscle weakness, and potential liver or kidney damage. In rare cases, high cyanotoxin exposure can be fatal. Those at greater risk include children, pets, and individuals with weakened immune systems197,199,200,201.
In animals, exposure to blue-green algae toxins can cause a range of symptoms depending on the species and level of exposure. Some common symptoms include, difficulty breathing or respiratory distress, loss of appetite, vomiting or diarrhea, seizures or convulsions, weakness or lethargy, liver damage or failure197,199,200,202. The cost of exposure to HABs can be significant, including health care expenses, lost tourism revenue, fisheries and aquaculture losses, water treatment costs, and expenditures for monitoring and research199,203.
Monitoring and research are necessary to understand and manage HABs, which can require significant resources. Molecular techniques are utilized to identify specific types of algae, including harmful species, and to track their distribution and abundance over time. PCR methods have been developed for the detection and identification of blue-green algae to assess water quality204,205. Johnson and Mutharasan206 reported the development and validation of a cantilever-based biosensor assay for detecting the presence of a toxin-producing cyanobacteria, Microcystis aeruginosa. Based on the specific hybridization of 16S rRNA probes (27-base DNA sequence) to the target cyanobacterial DNA, the study reported that the biosensor assay could detect M. aeruginosa at concentrations as low as 50 cell/ml, with a detection time of less than 3 h with low false negatives result.
The FISH technique was optimized to detect cyanotoxin-producing species from non-toxin-producing ones in freshwater targeting 16S rRNA and mcyA-mRNA using different DNA probes207. LAMP assay has been developed for detecting microcystin-producing cyanobacterial species. The assay targets the mcyE gene and has a LoD of 8.5 pg/μl DNA. The assay showed no cross-reactivity when tested on four non-toxic cyanobacterial strains208. In another study, the microcystin synthetase B gene (mcyB) was targeted using LAMP assay and demonstrated higher sensitivity than conventional PCR, with a LoD of 1 fg per reaction209.
Li et al.210 demonstrated the efficacy of RPA assay to detect microcystins-producing cyanobacteria in water samples. The researchers utilized RPA combined with lateral flow strips, achieving a sensitivity of 103 copies/ml mcyG without any cross-reactivity with non-toxin-producing cyanobacteria.
The study of Li et al.211 developed an insulated isothermal PCR to detect Raphidiopsis raciborskii—a toxin-producing cyanobacterium- with high sensitivity and specificity compared to qPCR. Using fast DNA isolation, the assay can be completed in 50 min. To enable the quantification of the DNA, the assay was optimized using a spectrometer (Andor spectrometer with Solis spectroscopy software) to measure the fluorescence intensity.
To ensure safe and accessible water for all, water managers require rapid and reliable methods for detecting waterborne pathogens to mitigate health risks. Notably, there is a growing prominence of point-of-care and point-of-application methods driven by the urgency for quicker results to enhance health risk management. Therefore, waterborne pathogen detection methods have evolved to offer speed, sensitivity, and specificity, aligning with their suitability for various applications.
Point-of-care methods using LAMP are known for their high accuracy, rapid analysis time, and cost-effectiveness compared to the gold-standard methods for pathogen detection. The LAMP assay demonstrated superior analytical sensitivity and specificity compared to conventional PCR, nPCR, and qPCR, with a LoD of one E. histolytica trophozoite, making it a highly effective platform for pathogen detection129. Several studies have described the development of LAMP assays for rapid point-of-need testing, with completion times under 30 min23,212. Advancements in LAMP technology have improved both the time and cost efficiency of the method213,214. For example, integrating gold nanoparticles into LAMP assays has been demonstrated to enhance visual detection while keeping costs low, making it ideal for decentralized and resource-constrained laboratories215. LAMP is highlighted as a rapid, sensitive, and specific method that is particularly beneficial in developing and low-income countries due to its affordability and ease of use without the need for sophisticated lab equipment or skilled personnel214. For diseases like the Zika virus, where there is an urgent need for PoC diagnostics in resource-poor settings, LAMP has been recognized as a low-cost molecular system that can be freeze-dried for distribution, offering high specificity and sensitivity213.
Advancements in LAMP assay for detecting eDNA and waterborne pathogens in environmental waters
LAMP simplifies pathogen detection by eliminating the need for complex DNA isolation. It can be applied directly to minimally processed samples, making it suitable for on-site use and streamlining the detection process (Table 4). For instance, an in-field LAMP assay was developed to detect Bacteroides, which serves as an indicator of human fecal contamination in environmental water23. In this study, the bacterial cells from water samples were lysed in the field using KOH, streamlining the process for on-site completion.
Combining the LAMP assay with lateral flow devices or smartphones facilitates simple, cost-effective pathogen detection. This integration underscores the assay’s suitability for point-of-care applications, ultimately contributing to improved public health216. For example, Thio et al.217 developed a smartphone-based lab-on-a-chip system designed for on-site E. coli detection, for assessing fecal contamination in environmental waters. This system incorporated a plasmonic-enhanced optoelectrowetting device, a transparent heater, and a smartphone, all integrated to enable on-chip water quality monitoring utilizing the LAMP technique.
Microfluidic devices can revolutionize water quality monitoring by providing high sensitivity and rapid detection in a portable and cost-effective manner. Its advantages, including miniaturization, in-situ analysis, multiplexing, precise control, and cost-effective fabrication, are propelling microfluidics to the forefront of water monitoring218. This technology facilitates a quick and effective response to water contamination, even in urgent scenarios, making it a promising tool for ensuring water safety. For instance, Jin et al.219 optimized a microfluidic chip that incorporates LAMP (referred to as on-chip LAMP) for simultaneous detection of ten pathogenic bacteria typically found in coastal waters. The limits of detection for this method ranged from 7.92 × 10−3 picograms to 9.54 × 10−1 picograms of pure bacterial genomic DNA per reaction. This dual-sample on-chip LAMP assay demonstrated its effectiveness for analyzing multiple waterborne bacterial pathogens, making it suitable for on-site detection and routine monitoring of aquatic environments.
Drawbacks of using LAMP for point-of-care testing in pathogen detection
While LAMP is known for its simplicity in terms of operation, interpreting the results can sometimes be challenging, especially for inexperienced users. The presence of non-specific amplification or primer–primer annealing may lead to false-positive results, requiring careful analysis220,221. Similar to other nucleic acid amplification techniques, LAMP is susceptible to contamination issues or cross-contamination65,222,223, Contamination can lead to false-positive results, which can be particularly problematic in point-of-care settings where quick and accurate diagnosis is crucial. LAMP is generally less amenable to multiplexing compared to other PCR-based techniques224. This limitation can restrict the ability to detect multiple pathogens simultaneously, which may be necessary for certain diagnostic applications. Another drawback is the perceived complexity of the methodology, which necessitates a sophisticated primer design system. This complexity can limit the selection of target sites and affect the specificity and sensitivity223,225. While LAMP is generally considered simpler to perform than traditional PCR, it still requires a certain level of training and skill to ensure reliable results. This can be a limiting factor in settings where trained personnel are not readily available. For example, users must be cautious to avoid sample contamination and follow aseptic techniques carefully222.
Water sample preparation and in-field nucleic acid isolation for LAMP assay
Water sample preparation is crucial for accurate and timely water quality assessment. Efficient preparation and nucleic acid isolation are key to successful molecular assays. To optimize this process, it is imperative to explore novel and streamlined methodologies for in-field sample preparation and nucleic acid isolation. Emphasis should be placed on enhancing the sensitivity, specificity, and speed of these processes, ensuring their suitability for on-site applications. The development of user-friendly, portable, and cost-effective tools and protocols is essential to empower a broader range of professionals and field operators in conducting real-time environmental monitoring and pathogen detection.
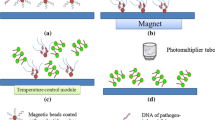
Microbial pathogen and eDNA detection in water samples often require target enrichment due to the typically low quantities of these targets. This process involves filtering large volumes of water to concentrate the target organisms on a membrane filter, which can be achieved using a vacuum pump and a filtration apparatus with replaceable membranes (Fig. 6)60,226,227. In remote and resource-limited settings, a portable pumping system is an accessible option for water filtration228. In scenarios involving smaller water volumes, employing disposable syringe filters is a practical alternative (Fig. 7)23,24.
A backpack pump system is used to filter water samples from a body of water. The inset illustrates the components of the filtration setup, including the filter holder and filter membrane, which are used to trap and collect cells from the water sample. The filter membrane can then be removed from the filter holder for further analysis. This filtration protocol allows for filtering a higher volume of water compared to the syringe filtration protocol.
A Drawing a water sample into a 60 ml syringe. B Passing the water sample through a syringe filter to trap cells on the membrane. C Back-flushing the filter with ~0.2 ml elution buffer using the syringe to collect any retained cells and eDNA. D Collect the eluted sample in a collection tube for further analysis.
It’s worth highlighting that the maximum water filtration volume is contingent on factors such as water turbidity and the filter membrane’s pore size. For instance, a filter membrane with a 0.2 μm pore size may become clogged even when filtering a small volume of water227. An eDNA analysis study demonstrated that filtering larger water volumes enhances the accuracy of eDNA recovery but can lead to filter clogging60. To overcome this issue, the study recommended combining multiple filters during the isolation process, significantly improving eDNA yield. Notably, the CTAB-PCI method proved effective in increasing eDNA yield and removing inhibitors. However, these steps introduced complexity and time consumption, making on-site isolation less practical.
The eDNA recovers from filtration is also depends on filtration pressure and water volume filtered. Thomas et al.228 found a complex relationship between filtration pressure, water volume, and eDNA retention on the filter surface. Higher pressure led to a 39% decrease in eDNA concentration per liter of filtered water. While the impact of pressure was somewhat weak in their small sample, it hinted that higher pressures might reduce eDNA retention, possibly due to cell lysis. However, the decrease in eDNA retention was offset by an increased water volume that could be filtered at higher pressures, explaining the lack of a straightforward relationship between filtration pressure and total eDNA per filter. Additionally, the filtration efficiency peaked at a flow rate of 1.0 l/m, and the 5 μm filters captured significantly more target eDNA than the 1 μm filters.
Various pathogenic microorganisms necessitate distinct filtration methods. Francy et al.229 conducted a study to identify efficient filtration methods for the simultaneous sampling of various classes of pathogens (bacteria, viruses, and protozoa) in recreational lake water studies. Among the different filtration techniques tested, automatic ultrafiltration (UF) showed the highest median recoveries for E. coli, Enterococci, and protozoa. Glass wool filtration performed consistently well in recovering enteric viruses from lake water samples, particularly for adenovirus. The automatic UF method proved efficient for sampling large volumes of lake water, even in the presence of high turbidity or algae, and could be used for regional public health risk studies at beaches.
In-field implementation of isothermal nucleic acid assays faces challenges due to equipment and expertise requirements, limiting their use for real-time monitoring in resource-limited settings. To overcome this, studies have improved in-field DNA isolation methods. Lee et al.230 research enhanced DNA extraction using magnetic beads to detect FIB (i.e., E. coli and E. faecalis), improving purity, reducing hands-on work, and reagent use. This method offers high throughput, low contamination risk, and ease of use. Potential PCR inhibition was addressed using bovine serum albumin (BSA) and DNA dilution, enabling efficient FIB detection through LAMP assays. BSA is used in DNA amplification sample preparation to stabilize and protect DNA from degradation, reduce interference by inhibitors, and prevent non-specific binding231.
He et al.232 introduced an integrated colorimetric RT-LAMP assay for detecting SARS-CoV-2 by combining viral RNA purification, amplification, and detection within a single tube using a colorimetric RT-LAMP. Silicon hydroxyl magnetic beads (Si–OH MBs) were used for nucleic acid extraction, with 200-nm MBs showing the highest RNA extraction efficiency. The assay was optimized with a 5-min incubation time for efficient RNA capture and improved sensitivity was enhanced by increasing the initial sample volume. Furthermore, the RT-LAMP reaction exhibited robustness in the presence of Si–OH MBs, hinting at potential improvements.
A KOH-based method was employed by Hulse et al.233 to lyse Chlamydia pecorum cells from swab samples, releasing genomic DNA into the solution for direct use in the LAMP reaction. Similarly, KOH was used to lyse Bacteroides from syringe filters, with the lysate directly employed in the LAMP reaction for MST in environmental waters23.
Alkaline polyethylene glycol (alkaline-PEG) serves as an effective and rapid field-deployable lysis buffer for efficient sample processing that is compatible with LAMP assays. Successful utilization of alkaline-PEG was demonstrated for detecting Dichelobacter nodosus in sheep swab samples using LAMP assay43. This method was observed to effectively adjust the pH to a more suitable range for LAMP DNA amplification while also mitigating the presence of inhibitors (due to sample dilution). However, Alkaline-PEG may not be as effective when targeting low-concentration DNA, such as eDNA detection in water samples.
Heat treatment at 95 °C has proven effective in releasing viral DNA without the necessity of prior extraction, facilitating successful DNA amplification techniques234,235. Although this method is suitable for direct point-of-care diagnostics, it’s primarily effective for detecting viruses and some bacteria. Therefore, careful consideration is essential when opting for heat treatment as a lysis step, depending on the specific diagnostic needs.
In eDNA monitoring, effectively communicating the sensitivity of sampling protocols is vital for resource managers. Schultz and Lance236 explored a simulation model to estimate sensitivity and assessed the impact of protocol changes on detection limits. Low target species density can result in significant false negatives in eDNA surveys. The study considered factors affecting sensitivity, such as target marker distribution, and suggested various strategies to reduce false negatives, including increasing replicates, sample volume, and sample numbers. It underscores the need for a balanced sampling protocol that considers sensitivity, feasibility, and cost to enhance survey accuracy, particularly when targeting low ambient target marker concentrations.
In general, ensuring precise and timely water quality assessment and pathogen detection relies on effective water sample preparation. Simplifying sample preparation is essential for streamlined in-field diagnostics, reducing time and complexity, and increasing the feasibility of real-time monitoring, especially in resource-limited settings.
Future applications of LAMP in analyzing waterborne pathogens
Molecular diagnostic tools may be further advanced by integrating them with other technologies, such as biosensors and artificial intelligence (AI), which are expected to enhance detection accuracy and enable real-time analysis, particularly beneficial for monitoring water quality17. Molecular diagnostic methods, including LAMP, are increasingly utilized for rapid and robust microbial water quality assessment, with ongoing technological advancements expanding their applications237. LAMP has demonstrated high efficiency in detecting Vibrio parahaemolyticus in various infection models, suggesting its potential as a primary method for rapid pathogen detection in clinical and environmental settings238. The future use of LAMP technology is likely to focus on field employability, integration with sample preparation, and automatic result analysis using smartphones, facilitated by technological innovations and integration with other advanced methods such as biosensors and AI. This trend indicates a shift towards more rapid, accurate, and on-site pathogen detection, which could significantly impact public health and water safety monitoring. Biosensors can provide rapid, on-site analysis, reducing the need for centralized laboratory facilities. For example, biosensors have combined LAMP with electrochemical detection for the rapid and sensitive detection of digestive bacterial pathogens such as E. coli and Salmonella239.
AI can improve the accuracy and efficiency of LAMP-based pathogen detection by analyzing complex data patterns. AI algorithms can interpret LAMP results, identify specific pathogens, and even predict potential outbreaks240. For instance, AI can be used to analyze the results of LAMP assays for waterborne pathogens and provide real-time alerts to water management authorities in case of contamination. Expanding beyond diagnostics at the individual level, PoC devices can leverage integrated blockchain technology241. This integration allows PoC devices to function as decentralized and interoperable systems, ensuring data security. Consequently, PoC devices with blockchain integration can quickly map outbreaks and support informed public health responses221.
The evolution of LAMP-on-a-chip technology, combining the LAMP technique with microfluidic chips, presents a significant stride forward in pathogen detection. Classical microfluidic chips, such as those made from polymethyl methacrylate81 and poly-dimethylsiloxane82, provide a protected environment for testing, reducing the risk of cross-contamination. Paper-based and paper-polymer hybrid microfluidic devices offer cost-effective alternatives with high biocompatibility and easy fabrication. Fully integrated chips combine nucleic acid extraction, purification, and LAMP amplification in a single device, enabling on-site detection, especially in resource-limited settings242.
Conclusion
Waterborne pathogens present a substantial concern for both public health and environmental well-being. Ensuring the timely and precise detection of these pathogens is of paramount importance for effective risk management. In response to the pressing need for expedited results and enhanced water quality monitoring, the prominence of point-of-care and point-of-application methods is on the rise. Current approaches, including culture-based methods, offer invaluable insights into microorganism types and quantities, yet are hampered by the laborious nature of their processes and their dependency on specialized equipment and expertise. Molecular techniques, exemplified by PCR, excel in sensitivity and speed but are often confined to centralized laboratory facilities, incurring associated expenses. In contrast, the integration of modern isothermal techniques such as LAMP with portable or microfluidic devices, along with the development of in-field sample preparation and DNA isolation methods, offers a promising trajectory for water monitoring and pathogen detection. This progress is particularly vital in resource-limited settings, where conventional methods may not be practical. Efficient water sample preparation emerges as essential for accurate water quality assessments. The process of target enrichment through filtration techniques is indispensable, with various filtration methods tailored to distinct pathogen categories. Advancements in waterborne pathogen detection, extending from streamlined sample preparation to on-site nucleic acid isolation, are pivotal in enhancing sensitivity, specificity, cost-effectiveness, simplicity, and rapidity. These innovations ultimately underpin the assurance of safe and accessible water for all.
References
WHO. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda. Report No. ISBN: 978-92-4-004506-4 (WHO, 2022).
Dudgeon, D. et al. Freshwater biodiversity: importance, threats, status and conservation challenges. Biol. Rev. 81, 163–182 (2006).
Li, P. & Wu, J. Drinking water quality and public health. Expo. Health 11, 73–79 (2019).
Wolf, J. et al. Burden of disease attributable to unsafe drinking water, sanitation, and hygiene in domestic settings: a global analysis for selected adverse health outcomes. Lancet 401, 2060–2071 (2023).
Wolf, J. et al. Effectiveness of interventions to improve drinking water, sanitation, and handwashing with soap on risk of diarrhoeal disease in children in low-income and middle-income settings: a systematic review and meta-analysis. Lancet 400, 48–59 (2022).
Lin, L., Yang, H. & Xu, X. Effects of water pollution on human health and disease heterogeneity: a review. Front. Environ. Sci. https://doi.org/10.3389/fenvs.2022.880246 (2022).
Chowdhary, P., Bharagava, R. N., Mishra, S. & Khan, N. in Environmental Concerns and Sustainable Development: Volume 1: Air, Water and Energy Resources (eds Vertika Shukla & Narendra Kumar) 235–256 (Springer Singapore, 2020).
Gall, A. M., Mariñas, B. J., Lu, Y. & Shisler, J. L. Waterborne viruses: a barrier to safe drinking water. PLoS Pathog. 11, e1004867 (2015).
Upfold, N. S., Luke, G. A. & Knox, C. Occurrence of human enteric viruses in water sources and shellfish: a focus on Africa. Food Environ. Virol. 13, 1–31 (2021).
Ramírez-Castillo, F. Y. et al. Waterborne pathogens: detection methods and challenges. Pathogens 4, 307–334 (2015).
Ashbolt, N. J. Microbial contamination of drinking water and human health from community water systems. Curr. Environ. Health Rep. 2, 95–106 (2015).
WHO. Drinking-Water. Report No. 978-92-4-004506-4 (World Health Organisation, 2022).
CDC. Waterborne Pathogens (Centers for Disease Control and Prevention, 2022).
WHO. Guidelines for Drinking-water Quality. Report No. 978-92-4-004506-4 (World Health Organisation, 2022).
Ahmed, W. et al. Quantitative microbial risk assessment of microbial source tracking markers in recreational water contaminated with fresh untreated and secondary treated sewage. Environ. Int. 117, 243–249 (2018).
Charles, K. J. & Greggio, E. Invited perspective: beyond national water quality surveys: improving water quality surveillance to achieve safe drinking water for all (sustainable development goal 6.1). Environ. Health Perspect. 129, 091301 (2021).
Oon, Y. L. et al. Waterborne pathogens detection technologies: advances, challenges, and future perspectives. Front. Microbiol. 14, 1286923 (2023).
Aw, T. G. & Rose, J. B. Detection of pathogens in water: from phylochips to qPCR to pyrosequencing. Curr. Opin. Biotechnol. 23, 422–430 (2012).
Oliveira, B. B., Veigas, B. & Baptista, P. V. Isothermal amplification of nucleic acids: the race for the next “Gold Standard”. Front. Sens. https://doi.org/10.3389/fsens.2021.752600 (2021).
Baldi, P. & La Porta, N. Molecular approaches for low-cost point-of-care pathogen detection in agriculture and forestry. Front. Plant Sci. https://doi.org/10.3389/fpls.2020.570862 (2020).
Hong, J. et al. Development of a highly sensitive real-time one step RT-PCR combined complementary locked primer technology and conjugated minor groove binder probe. Virol. J. 8, 330 (2011).
USEPA. 821R19002 56 (United States Environmental Protection Agency, 2019).
Khodaparast, M., Sharley, D., Best, N., Marshall, S. & Beddoe, T. In-field LAMP assay for rapid detection of human faecal contamination in environmental water. Environ. Sci. Water Res. Technol. 8, 2641–2651 (2022).
Jiang, Y. S. et al. Portable platform for rapid in-field identification of human fecal pollution in water. Water Res. 131, 186–195 (2018).
Gensberger, E. T. et al. Evaluation of quantitative PCR combined with PMA treatment for molecular assessment of microbial water quality. Water Res. 67, 367–376 (2014).
Takahashi, H., Gao, Y., Miya, S., Kuda, T. & Kimura, B. Discrimination of live and dead cells of Escherichia coli using propidium monoazide after sodium dodecyl sulfate treatment. Food Control 71, 79–82 (2017).
Nocker, A. & Camper, A. K. Selective removal of DNA from dead cells of mixed bacterial communities by use of ethidium monoazide. Appl. Environ. Microbiol. 72, 1997–2004 (2006).
Li, H., Xin, H. & Li, S. F. Y. Multiplex PMA–qPCR assay with internal amplification control for simultaneous detection of viable Legionella pneumophila, Salmonella typhimurium, and Staphylococcus aureus in environmental waters. Environ. Sci. Technol. 49, 14249–14256 (2015).
Bae, S. & Wuertz, S. Discrimination of viable and dead fecal bacteroidales bacteria by quantitative PCR with propidium monoazide. Appl. Environ. Microbiol. 75, 2940–2944 (2009).
Sidstedt, M., Rådström, P. & Hedman, J. PCR inhibition in qPCR, dPCR and MPS—mechanisms and solutions. Anal. Bioanal. Chem. 412, 2009–2023 (2020).
Scott, G., Evens, N., Porter, J. & Walker, D. I. The inhibition and variability of two different RT-qPCR assays used for quantifying SARS-CoV-2 RNA in wastewater. Food Environ. Virol. 15, 71–81 (2023).
Schrader, C., Schielke, A., Ellerbroek, L. & Johne, R. PCR inhibitors—occurrence, properties and removal. J. Appl. Microbiol. 113, 1014–1026 (2012).
Hu, Q., Liu, Y., Yi, S. & Huang, D. A comparison of four methods for PCR inhibitor removal. Forensic Sci. Int. Genet. 16, 94–97 (2015).
Ahmed, W. et al. Minimizing errors in RT-PCR detection and quantification of SARS-CoV-2 RNA for wastewater surveillance. Sci. Total Environ. 805, 149877 (2022).
Kumar, S. et al. Aspects of point-of-care diagnostics for personalized health wellness. Int J. Nanomed. 16, 383–402 (2021).
Mozsary, C. et al. A rapid, isothermal, and point-of-care system for COVID-19 diagnostics. J. Biomol. Tech. 32, 221–227 (2021).
Land, K. J., Boeras, D. I., Chen, X.-S., Ramsay, A. R. & Peeling, R. W. REASSURED diagnostics to inform disease control strategies, strengthen health systems and improve patient outcomes. Nat. Microbiol. 4, 46–54 (2019).
Vincent, M., Xu, Y. & Kong, H. Helicase-dependent isothermal DNA amplification. EMBO Rep. 5, 795–800 (2004).
Piepenburg, O., Williams, C. H., Stemple, D. L. & Armes, N. A. DNA detection using recombination proteins. PLoS Biol. 4, e204 (2006).
Xu, G. et al. Cross priming amplification: mechanism and optimization for isothermal DNA amplification. Sci. Rep. 2, 246 (2012).
Compton, J. Nucleic acid sequence-based amplification. Nature 350, 91–92 (1991).
Notomi, T. et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 28, E63 (2000).
Best, N., Rawlin, G., Suter, R., Rodoni, B. & Beddoe, T. Optimization of a loop mediated isothermal amplification (LAMP) assay for in-field detection of dichelobacter nodosus with aprV2 (VDN LAMP) in Victorian sheep flocks. Front. Vet. Sci. https://doi.org/10.3389/fvets.2019.00067 (2019).
Malekjahani, A., Sindhwani, S., Syed, A. M. & Chan, W. C. W. Engineering steps for mobile point-of-care diagnostic devices. Acc. Chem. Res. 52, 2406–2414 (2019).
Ghouneimy, A., Mahas, A., Marsic, T., Aman, R. & Mahfouz, M. CRISPR-based diagnostics: challenges and potential solutions toward point-of-care applications. ACS Synth. Biol. 12, 1–16 (2023).
Mao, K. et al. Paper-based microfluidics for rapid diagnostics and drug delivery. J. Controlled Release 322, 187–199 (2020).
USEPA. 17 (United States Environmental Protection Agency, U.S. Environmental Protection Agency Office of Water (4303T) 1200 Pennsylvania Avenue, NW Washington, 2003).
Converse, R. R., Blackwood, A. D., Kirs, M., Griffith, J. F. & Noble, R. T. Rapid QPCR-based assay for fecal Bacteroides spp. as a tool for assessing fecal contamination in recreational waters. Water Res. 43, 4828–4837 (2009).
Layton, A. et al. Development of Bacteroides 16S rRNA gene TaqMan-based real-time PCR assays for estimation of total, human, and bovine faecal pollution in water. Appl. Environ. Microbiol. 72, 4214 (2006).
Harwood, V. J., Staley, C., Badgley, B. D., Borges, K. & Korajkic, A. Microbial source tracking markers for detection of fecal contamination in environmental waters: relationships between pathogens and human health outcomes. FEMS Microbiol. Rev. 38, 1–40 (2014).
Silkie, S. S. & Nelson, K. L. Concentrations of host-specific and generic fecal markers measured by quantitative PCR in raw sewage and fresh animal feces. Water Res. 43, 4860–4871 (2009).
Bree Tillett, V. P. Bacteroidales: revolutionising microbial source tracking. Water e-J. https://doi.org/10.21139/wej.2017.006 (2017).
Martzy, R. et al. A loop-mediated isothermal amplification (LAMP) assay for the rapid detection of Enterococcus spp. in water. Water Res. 122, 62–69 (2017).
James Chapman, A. A., Aoife Power, Chandra, S., Voss, L., Rajapaksha, P. & Cosford, S. Detection methods for faecal contamination events: the gap for Australia. Water e-J. https://doi.org/10.21139/wej.2016.041 (2016).
USEPA. Vol. EPA-821-R-12-008 (United States Environmental Protection Agency, 2012).
Srinivasan, S., Aslan, A., Xagoraraki, I., Alocilja, E. & Rose, J. B. Escherichia coli, Enterococci, and Bacteroides thetaiotaomicron qPCR signals through wastewater and septage treatment. Water Res. 45, 2561–2572 (2011).
Matthews, B., Stratton, H., Schreoder, S. & Toze, S. Pathogen Detection Methodologies for Wastewater and Reservoirs. Urban Water Security Research Alliance Technical Report No. 32 (Urban Water Security Research Alliance, 2010).
Valasek, M. A. & Repa, J. J. The power of real-time PCR. Adv. Physiol. Educ. 29, 151–159 (2005).
Hayashida, K., Kajino, K., Hachaambwa, L., Namangala, B. & Sugimoto, C. Direct blood dry LAMP: a rapid, stable, and easy diagnostic tool for human African Trypanosomiasis. PLOS Neglected Tropical Dis. 9, e0003578 (2015).
Hunter, M. E., Ferrante, J. A., Meigs-Friend, G. & Ulmer, A. Improving eDNA yield and inhibitor reduction through increased water volumes and multi-filter isolation techniques. Sci. Rep. 9, 5259 (2019).
Francois, P. et al. Robustness of a loop-mediated isothermal amplification reaction for diagnostic applications. FEMS Immunol. Med. Microbiol. 62, 41–48 (2011).
Martzy, R. et al. Challenges and perspectives in the application of isothermal DNA amplification methods for food and water analysis. Anal. Bioanal. Chem. 411, 1695–1702 (2019).
Best, N., Rodoni, B., Rawlin, G. & Beddoe, T. The development and deployment of a field-based loop mediated isothermal amplification assay for virulent Dichelobacter nodosus detection on Australian sheep. PLoS One 13, e0204310 (2018).
Gadkar, V. J., Goldfarb, D. M., Gantt, S. & Tilley, P. A. G. Real-time detection and monitoring of loop mediated amplification (LAMP) reaction using self-quenching and de-quenching fluorogenic probes. Sci. Rep. 8, 5548 (2018).
Wong, Y. P., Othman, S., Lau, Y. L., Radu, S. & Chee, H. Y. Loop‐mediated isothermal amplification (LAMP): a versatile technique for detection of micro‐organisms. J. Appl. Microbiol. 124, 626–643 (2018).
Safavieh, M. et al. Emerging loop-mediated isothermal amplification-based microchip and microdevice technologies for nucleic acid detection. ACS Biomater. Sci. Eng. 2, 278–294 (2016).
Ravan, H., Amandadi, M. & Sanadgol, N. A highly specific and sensitive loop-mediated isothermal amplification method for the detection of Escherichia coli O157:H7. Microb. Pathogenesis 91, 161–165 (2016).
Kreitlow, A. et al. Combined loop-mediated isothermal amplification assays for rapid detection and one-step differentiation of Campylobacter jejuni and Campylobacter coli in meat products. Front. Microbiol. https://doi.org/10.3389/fmicb.2021.668824 (2021).
Yang, Q., Domesle, K. J. & Ge, B. Loop-mediated isothermal amplification for salmonella detection in food and feed: current applications and future directions. Foodborne Pathog. Dis. 15, 309–331 (2018).
Chen, Y. et al. Point-of-care and visual detection of P. aeruginosa and its toxin genes by multiple LAMP and lateral flow nucleic acid biosensor. Biosens. Bioelectron. 81, 317–323 (2016).
Li, S., Ji, S., Zhu, X., Chen, H. & Jin, D. Development of a loop-mediated isothermal amplification assay for molecular serotyping of Shigella flexneri serotypes 2 and Xv. J. Appl. Microbiol. 132, 2980–2989 (2022).
Ziros, P. G., Kokkinos, P. A., Allard, A. & Vantarakis, A. Development and evaluation of a loop-mediated isothermal amplification assay for the detection of adenovirus 40 and 41. Food Environ. Virol. 7, 276–285 (2015).
Yoneyama, T. et al. Rapid and real-time detection of hepatitis A virus by reverse transcription loop-mediated isothermal amplification assay. J. Virological Methods 145, 162–168 (2007).
Cai, T., Lou, G., Yang, J., Xu, D. & Meng, Z. Development and evaluation of real-time loop-mediated isothermal amplification for hepatitis B virus DNA quantification: a new tool for HBV management. J. Clin. Virol. 41, 270–276 (2008).
Fukuda, S., Takao, S., Kuwayama, M., Shimazu, Y. & Miyazaki, K. Rapid detection of norovirus from fecal specimens by real-time reverse transcription-loop-mediated isothermal amplification assay. J. Clin. Microbiol. 44, 1376–1381 (2006).
Yang, B.-Y. et al. Rapid and sensitive detection of human astrovirus in water samples by loop-mediated isothermal amplification with hydroxynaphthol blue dye. BMC Microbiol. 14, 38 (2014).
Li, J. et al. Sensitive and rapid detection of Giardia lamblia infection in pet dogs using loop-mediated isothermal amplification. Korean J. Parasitol. 51, 237–241 (2013).
Singh, P., Mirdha, B. R., Ahuja, V. & Singh, S. Loop-mediated isothermal amplification (LAMP) assay for rapid detection of Entamoeba histolytica in amoebic liver abscess. World J. Microbiol. Biotechnol. 29, 27–32 (2013).
Karanis, P. et al. Development and preliminary evaluation of a loop-mediated isothermal amplification procedure for sensitive detection of Cryptosporidium oocysts in fecal and water samples. Appl. Environ. Microbiol. 73, 5660–5662 (2007).
Yang, W. et al. Rapid Detection of SARS-CoV-2 Using Reverse transcription RT-LAMP method. medRxiv 2020.03.02.20030130; https://doi.org/10.1101/2020.03.02.20030130 (2020).
Rodriguez-Mateos, P. et al. A lab-on-a-chip platform for integrated extraction and detection of SARS-CoV-2 RNA in resource-limited settings. Anal. Chim. Acta. 1177, 338758 (2021).
Jhou, Y-R., Wang, C-H., Tsai, H-P., Shan, Y-S., & Lee, G-B. An integrated microfluidic platform featuring real-time reverse transcription loop-mediated isothermal amplification for detection of COVID-19. Sens. Actuators B: Chem. 358, 131447 (2022).
da Silva, S. J. R., Pardee, K., Balasuriya, U. B. R. & Pena, L. Development and validation of a one-step reverse transcription loop-mediated isothermal amplification (RT-LAMP) for rapid detection of ZIKV in patient samples from Brazil. Sci. Rep. 11, 4111 (2021).
Dong, Y. et al. Multiplex, real-time, point-of-care RT-LAMP for SARS-CoV-2 detection using the HFman probe. ACS Sens. 7, 730–739 (2022).
Kim, J. et al. Development and evaluation of a multiplex loop-mediated isothermal amplification (LAMP) assay for differentiation of Mycobacterium tuberculosis and non-tuberculosis Mycobacterium in clinical samples. PLoS One 16, e0244753 (2021).
Kumar, S. et al. Magnetic multiplex loop mediated isothermal amplification (MM-LAMP) technique for simultaneous detection of dengue and chikungunya virus. J. Virol. Methods 300, 114407 (2022).
Higgins, O. & Smith, T. J. Loop-primer endonuclease cleavage–loop-mediated isothermal amplification technology for multiplex pathogen detection and single-nucleotide polymorphism identification. J. Mol. Diagn. 22, 640–651 (2020).
Zhou, Q.-J. et al. Simultaneous detection of multiple bacterial and viral aquatic pathogens using a fluorogenic loop-mediated isothermal amplification-based dual-sample microfluidic chip. J. Fish. Dis. 44, 401–413 (2021).
Plutzer, J. & Karanis, P. Rapid identification of Giardia duodenalis by loop-mediated isothermal amplification (LAMP) from faecal and environmental samples and comparative findings by PCR and real-time PCR methods. Parasitol. Res. 104, 1527–1533 (2009).
Zahedi, A. et al. Longitudinal analysis of Giardia duodenalis assemblages in animals inhabiting drinking water catchments in New South Wales and Queensland—Australia (2013–2015). Sci. Total Environ. 718, 137433 (2020).
Capewell, P., Krumrie, S., Katzer, F., Alexander, C. L. & Weir, W. Molecular epidemiology of Giardia infections in the genomic era. Trends Parasitol. 37, 142–153 (2021).
Thompson, R. C. A., Palmer, C. S. & O’Handley, R. The public health and clinical significance of Giardia and Cryptosporidium in domestic animals. Vet. J. 177, 18–25 (2008).
Olson, M. E., O’Handley, R. M., Ralston, B. J., McAllister, T. A. & Andrew Thompson, R. C. Update on Cryptosporidium and Giardia infections in cattle. Trends Parasitol. 20, 185–191 (2004).
Kuthyar, S., Kowalewski, M. M., Seabolt, M., Roellig, D. M. & Gillespie, T. R. Molecular characterization of Giardia duodenalis and evidence for cross-species transmission in Northern Argentina. Transbound. Emerg. Dis. 69, 2209–2218 (2022).
Krolewiecki, A. J. et al. Transrenal DNA-based diagnosis of Strongyloides stercoralis (Grassi, 1879) infection: Bayesian latent class modeling of test accuracy. PLoS Negl. Trop. Dis. 12, e0006550 (2018).
Verweij, J. J. et al. Real-time PCR for the detection of Giardia lamblia. Mol. Cell. Probes 17, 223–225 (2003).
Verweij, J. J. et al. Simultaneous detection of Entamoeba histolytica, Giardia lamblia, and Cryptosporidium parvum in fecal samples by using multiplex real-time PCR. J. Clin. Microbiol. 42, 1220–1223 (2004).
Plutzer, J., Törökné, A. & Karanis, P. Combination of ARAD microfibre filtration and LAMP methodology for simple, rapid and cost-effective detection of human pathogenic Giardia duodenalis and Cryptosporidium spp. in drinking water. Lett. Appl. Microbiol. 50, 82–88 (2010).
Molina-Gonzalez, S. J. et al. Application of a recombinase polymerase amplification (RPA) assay and pilot field testing for Giardia duodenalis at Lake Albert, Uganda. Parasites Vectors 13, 289 (2020).
Crannell, Z. A. et al. Recombinase polymerase amplification-based assay to diagnose Giardia in stool samples. Am. Soc. Tropical Med. Hyg. 92, 583–587 (2015).
Mamba, T. S. et al. Lateral flow loop-mediated isothermal amplification test with stem primers: detection of Cryptosporidium Species in Kenyan children presenting with diarrhea. J. Trop. Med. 2018, 7659730 (2018).
Fayer, R. Cryptosporidium: a water-borne zoonotic parasite. Vet. Parasitol. 126, 37–56 (2004).
Schets, F. M., Engels, G. B., During, M. & Husman, A. Md. R. Detection of infectious Cryptosporidium oocysts by cell culture immunofluorescence assay: applicability to environmental samples. Appl. Environ. Microbiol. 71, 6793–6798 (2005).
Power, M. L., Holley, M., Ryan, U. M., Worden, P. & Gillings, M. R. Identification and differentiation of Cryptosporidium species by capillary electrophoresis single-strand conformation polymorphism. FEMS Microbiol. Lett. 314, 34–41 (2011).
Hassan, E. M. et al. A review of Cryptosporidium spp. and their detection in water. Water Sci. Technol. 83, 1–25 (2020).
Wang, T. et al. Molecular detection and genetic characterization of Cryptosporidium in kindergarten children in southern Xinjiang, China. Infect., Genet. Evolution 103, 105339 (2022).
Ahmed, S. A. & Karanis, P. An overview of methods/techniques for the detection of Cryptosporidium in food samples. Parasitol. Res. 117, 629–653 (2018).
Hallier-Soulier, S. & Guillot, E. Detection of cryptosporidia and Cryptosporidium parvum oocysts in environmental water samples by immunomagnetic separation–polymerase chain reaction. J. Appl. Microbiol. 89, 5–10 (2000).
Yang, R. et al. Specific and quantitative detection and identification of Cryptosporidium hominis andC. parvum in clinical and environmental samples. Exp. Parasitol. 135, 142–147 (2013).
Ryan, U., Paparini, A. & Oskam, C. New technologies for detection of enteric parasites. Trends Parasitol. 33, 532–546 (2017).
Madison-Antenucci, S. et al. Multicenter evaluation of BD max enteric parasite real-time PCR assay for detection of Giardia duodenalis, Cryptosporidium hominis, Cryptosporidium parvum, and Entamoeba histolytica. J. Clin. Microbiol. 54, 2681–2688 (2016).
Yang, R., Paparini, A., Monis, P. & Ryan, U. Comparison of next-generation droplet digital PCR (ddPCR) with quantitative PCR (qPCR) for enumeration of Cryptosporidium oocysts in faecal samples. Int. J. Parasitol. 44, 1105–1113 (2014).
Vesey, G. et al. The use of a ribosomal RNA targeted oligonucleotide probe for fluorescent labelling of viable Cryptosporidiumparvum oocysts. J. Appl. Microbiol. 85, 429–440 (1998).
Lemos, V. et al. Identification and determination of the viability of Giardia lamblia cysts and Cryptosporidium parvum and Cryptosporidium hominis oocysts in human fecal and water supply samples by fluorescent in situ hybridization (FISH) and monoclonal antibodies. Parasitol. Res. 98, 48 (2005).
Smith, J. J., Gunasekera, T. S., Barardi, C. R. M., Veal, D. & Vesey, G. Determination of Cryptosporidium parvum oocyst viability by fluorescence in situ hybridization using a ribosomal RNA-directed probe. J. Appl. Microbiol. 96, 409–417 (2004).
Bakheit, M. A. et al. Sensitive and specific detection of Cryptosporidium species in PCR-negative samples by loop-mediated isothermal DNA amplification and confirmation of generated LAMP products by sequencing. Vet. Parasitol. 158, 11–22 (2008).
Ali, I. K. M. & Roy, S. A real-time PCR assay for simultaneous detection and differentiation of four common Entamoeba Species that infect humans. J. Clin. Microbiol. 59, e01986–01920 (2020).
Stanley, S. L. Amoebiasis. Lancet 361, 1025–1034 (2003).
Parija, S. C., Mandal, J. & Ponnambath, D. K. Laboratory methods of identification of Entamoeba histolytica and its differentiation from look-alike Entamoeba spp. Trop. Parasitol. 4, 90–95 (2014).
Haque, R., Huston, C. D., Hughes, M., Houpt, E. & Petri, W. A. Jr Amebiasis. N. Engl. J. Med. 348, 1565–1573 (2003).
Petri, W. A. & Haque, R. in Handbook of Clinical Neurology, Vol. 114 (eds Garcia, H. H., Tanowitz, H. B. & Del Brutto, O. H.) (Elsevier, 2013).
Carrero, J. C. et al. Intestinal amoebiasis: 160 years of its first detection and still remains as a health problem in developing countries. Int. J. Med. Microbiol. 310, 151358 (2020).
Watanabe, K., Yanagawa, Y., Gatanaga, H., Kikuchi, Y. & Oka, S. Performance of an enzyme-linked immunosorbent-based serological assay for Entamoeba histolytica: comparison with an indirect immunofluorescence assay using stored frozen samples. J. Infect. Chemother. 27, 736–739 (2021).
Roy, S. et al. Real-time-PCR assay for diagnosis of Entamoeba histolytica infection. J. Clin. Microbiol. 43, 2168–2172 (2005).
Morio, F. et al. Evaluation of a new multiplex PCR assay (ParaGENIE G-amoeba real-time PCR kit) targeting Giardia intestinalis, Entamoeba histolytica and Entamoeba dispar/Entamoeba moshkovskii from stool specimens: evidence for the limited performances of microscopy-based approach for amoeba species identification. Clin. Microbiol. Infect. 24, 1205–1209 (2018).
Foo, P. C. et al. Development of a thermostabilised triplex LAMP assay with dry-reagent four target lateral flow dipstick for detection of Entamoeba histolytica and non-pathogenic Entamoeba spp. Analytica Chim. Acta 966, 71–80 (2017).
Rivera, W. L. & Ong, V. A. Development of loop-mediated isothermal amplification for rapid detection of Entamoeba histolytica. Asian Pac. J. Tropical Med. 6, 457–461 (2013).
Liang, S.-Y. et al. Development of loop-mediated isothermal amplification assay for detection of Entamoeba histolytica. J. Clin. Microbiol. 47, 1892–1895 (2009).
Foo, P. C. et al. Loop-mediated isothermal amplification (LAMP) reaction as viable PCR substitute for diagnostic applications: a comparative analysis study of LAMP, conventional PCR, nested PCR (nPCR) and real-time PCR (qPCR) based on Entamoeba histolytica DNA derived from faecal sample. BMC Biotechnol. 20, 34 (2020).
Tenter, A. M., Heckeroth, A. R. & Weiss, L. M. Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 30, 1217–1258 (2000).
Liu, Q., Wang, Z.-D., Huang, S.-Y. & Zhu, X.-Q. Diagnosis of toxoplasmosis and typing of Toxoplasma gondii. Parasites Vectors 8, 292 (2015).
Robert-Gangneux, F. & Dardé, M.-L. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin. Microbiol. Rev. 25, 264–296 (2012).
Montoya, J. G. & Liesenfeld, O. Toxoplasmosis. Lancet 363, 1965–1976 (2004).
CDC. Parasites—Toxoplasmosis (Toxoplasma infection) (Centers for Disease Control and Prevention, 2018).
Karanis, P., Aldeyarbi, H. M., Mirhashemi, M. E. & Khalil, K. M. The impact of the waterborne transmission of Toxoplasma gondii and analysis efforts for water detection: an overview and update. Environ. Sci. Pollut. Res. 20, 86–99 (2013).
Bou, G., Figueroa, M. S., Martí-Belda, P., Navas, E. & Guerrero, A. Value of PCR for detection of Toxoplasma gondii in aqueous humor and blood samples from immunocompetent patients with ocular toxoplasmosis. J. Clin. Microbiol. 37, 3465–3468 (1999).
Sroka, J. et al. Detection and molecular characteristics of Toxoplasma gondii DNA in retail raw meat products in Poland. Foodborne Pathog. Dis. 16, 195–204 (2019).
Li, S. et al. Transfer learning for Toxoplasma gondii recognition. mSystems 5, e00445–00419 (2020).
Danielson, J. J., Perez, N., Romano, J. D. & Coppens, I. Modelling Toxoplasma gondii infection in a 3D cell culture system In Vitro: comparison with infection in 2D cell monolayers. PLoS One 13, e0208558 (2018).
Khan, A. H. & Noordin, R. Serological and molecular rapid diagnostic tests for Toxoplasma infection in humans and animals. Eur. J. Clin. Microbiol. Infect. Dis. 39, 19–30 (2020).
Yang, W. et al. Detection of Toxoplasma gondii Oocysts in Water Sample Concentrates by Real-Time PCR. Appl. Environ. Microbiol. 75, 3477–3483 (2009).
Shapiro, K. et al. Environmental transmission of Toxoplasma gondii: Oocysts in water, soil and food. Food Waterborne Parasitol. 15, e00049 (2019).
Hegazy, M. K., Awad, S. I., Saleh, N. E. & Hegazy, M. M. Loop mediated isothermal amplification (LAMP) of Toxoplasma DNA from dried blood spots. Exp. Parasitol. 211, 107869 (2020).
Sotiriadou, I. & Karanis, P. Evaluation of loop-mediated isothermal amplification for detection of Toxoplasma gondii in water samples and comparative findings by polymerase chain reaction and immunofluorescence test (IFT). Diagn. Microbiol. Infect. Dis. 62, 357–365 (2008).
Gallas-Lindemann, C., Sotiriadou, I., Mahmoodi, M. R. & Karanis, P. Detection of Toxoplasma gondii oocysts in different water resources by loop mediated isothermal amplification (LAMP). Acta Tropica 125, 231–236 (2013).
WHO. Hepatitis A (WHO, 2022).
Sánchez, G., Bosch, A. & Pintó, R. M. Hepatitis A virus detection in food: current and future prospects. Lett. Appl. Microbiol. 45, 1–5 (2007).
Kozak, R. A. et al. Development and evaluation of a molecular hepatitis A virus assay for serum and stool specimens. Viruses 14, 159 (2022).
Persson, S. et al. A new assay for quantitative detection of hepatitis A virus. J. Virological Methods 288, 114010 (2021).
Girones, R. et al. Molecular detection of pathogens in water—the pros and cons of molecular techniques. Water Res. 44, 4325–4339 (2010).
Villar, L. M. et al. Molecular detection of hepatitis A virus in urban sewage in Rio de Janeiro, Brazil. Lett. Appl. Microbiol. 45, 168–173 (2007).
Abd el-Galil, K. H. et al. Real-time nucleic acid sequence-based amplification assay for detection of hepatitis A virus. Appl. Environ. Microbiol. 71, 7113–7116 (2005).
Gabrielle, M. E. et al. Nucleic acid sequence-based amplification (NASBA) for the identification of mycobacteria. Microbiology 139, 2423–2429 (1993).
Liu, L. & Moore, M. D. A survey of analytical techniques for Noroviruses. Foods 9, 318 (2020).
Patel, M. M. et al. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg. Infect. Dis. 14, 1224–1231 (2008).
Atmar, R. L. Noroviruses—state of the art. Food Environ. Virol. 2, 117–126 (2010).
Ahmed, S. M. et al. Global prevalence of norovirus in cases of gastroenteritis: a systematic review and meta-analysis. Lancet Infect. Dis. 14, 725–730 (2014).
Bartsch, S. M., O’Shea, K. J. & Lee, B. Y. The clinical and economic burden of norovirus gastroenteritis in the United States. J. Infect. Dis. 222, 1910–1919 (2020).
ISO 15216-2:2019. Microbiology of the Food Chain—Horizontal Method For Determination of Hepatitis A Virus and Norovirus Using Real-Time RT-PCR—Part 2: Method for Detection (International Organization for Standardization, 2019).
ISO 15216‐1 (ISO Geneva, 2017).
Farkas, T. et al. Multiplex real-time RT-PCR for the simultaneous detection and quantification of GI, GII and GIV noroviruses. J. Virol. Methods 223, 109–114 (2015).
Miura, T. et al. Environmental detection of genogroup I, II, and IV Noroviruses by using a generic real-time reverse transcription-PCR assay. Appl. Environ. Microbiol. 79, 6585–6592 (2013).
Luo, J. et al. Visual detection of Norovirus genogroup II by reverse transcription loop-mediated isothermal amplification with hydroxynaphthol blue dye. Food Environ. Virol. 6, 196–201 (2014).
Kou, X., Wu, Q., Zhang, J. & Fan, H. Rapid detection of noroviruses in fecal samples and shellfish by nucleic acid sequence-based amplification. J. Microbiol 44, 403–408 (2006).
Jeon, S. B., Seo, D. J., Oh, H., Kingsley, D. H. & Choi, C. Development of one-step reverse transcription loop-mediated isothermal amplification for norovirus detection in oysters. Food Control 73, 1002–1009 (2017).
Moore, M. D. & Jaykus, L.-A. Development of a recombinase polymerase amplification assay for detection of epidemic human Noroviruses. Sci. Rep. 7, 40244 (2017).
CDC. Rotavirus (Centers for Disease Control and Prevention, 2021).
Hasegawa, M. et al. Detection of rotavirus in clinical specimens using an immunosensor prototype based on the photon burst counting technique. Biomed. Opt. Express 8, 3383–3394 (2017).
Parashar, U. D., Hummelman, E. G., Bresee, J. S., Miller, M. A. & Glass, R. I. Global illness and deaths caused by rotavirus disease in children. Emerg. Infect. Dis. 9, 565–572 (2003).
WHO. Manual of Rotavirus Detection and Characterization Methods (WHO, 2009).
Logan, C., O’Leary, J. J. & O’Sullivan, N. Real-time reverse transcription-PCR for detection of Rotavirus and Adenovirus as causative agents of acute viral gastroenteritis in children. J. Clin. Microbiol. 44, 3189–3195 (2006).
Malik, Y. S. et al. Rapid detection of human rotavirus using NSP4 gene specific reverse transcription loop-mediated isothermal amplification assay. Indian J. Virol. 24, 265–271 (2013).
Liu, Y. et al. Rapid detection of bovine rotavirus a by isothermal reverse transcription recombinase polymerase amplification assays. BMC Vet. Res. 18, 339 (2022).
Mezger, A., Öhrmalm, C., Herthnek, D., Blomberg, J. & Nilsson, M. Detection of Rotavirus using padlock probes and rolling circle amplification. PLoS One 9, e111874 (2014).
Lynch, J. P. 3rd & Kajon, A. E. Adenovirus: epidemiology, global spread of novel serotypes, and advances in treatment and prevention. Semin Respir. Crit. Care Med. 37, 586–602 (2016).
Lion, T. Adenovirus infections in immunocompetent and immunocompromised patients. Clin. Microbiol. Rev. 27, 441–462 (2014).
Zhang, D. et al. Severe pneumonia caused by human adenovirus type 55 in children. Front Pediatr. 10, 1002052 (2022).
CDC. Adenoviruses (Centers for Disease Control and Prevention, 2023).
Fongaro, G. et al. Evaluation and molecular characterization of human adenovirus in drinking water supplies: viral integrity and viability assays. Virol. J. 10, 166 (2013).
Marti, E. & Barardi, C. R. M. Detection of human adenoviruses in organic fresh produce using molecular and cell culture-based methods. Int. J. Food Microbiol. 230, 40–44 (2016).
Jothikumar, N. et al. Quantitative real-time PCR assays for detection of human Adenoviruses and identification of serotypes 40 and 41. Appl. Environ. Microbiol. 71, 3131–3136 (2005).
Pehler-Harrington, K., Khanna, M., Waters, C. R. & Henrickson, K. J. Rapid detection and identification of human adenovirus species by adenoplex, a multiplex PCR-enzyme hybridization assay. J. Clin. Microbiol. 42, 4072–4076 (2004).
Shuryaeva, A. K. et al. Development and application of LAMP assays for the detection of enteric adenoviruses in feces. Microbiol. Spectr. 10, e00516–00522 (2022).
Rames, E. K. & Macdonald, J. Rapid assessment of viral water quality using a novel recombinase polymerase amplification test for human adenovirus. Appl. Microbiol. Biotechnol. 103, 8115–8125 (2019).
Wang, R.-H. et al. Development and evaluation of recombinase-aided amplification assays incorporating competitive internal controls for detection of human adenovirus serotypes 3 and 7. Virol. J. 16, 86 (2019).
CDC. Entroviruses (Centers for Disease Control and Prevention, 2022).
Rmadi, Y. et al. Molecular characterization of enterovirus detected in cerebrospinal fluid and wastewater samples in Monastir, Tunisia, 2014–2017. Virol. J. 19, 45 (2022).
Zhang, M. et al. Clinical characteristics of severe neonatal enterovirus infection: a systematic review. BMC Pediatr. 21, 127 (2021).
Jacques, J. et al. New reverse transcription-PCR assay for rapid and sensitive detection of Enterovirus genomes in cerebrospinal fluid specimens of patients with aseptic meningitis. J. Clin. Microbiol. 41, 5726–5728 (2003).
Verstrepen, W. A., Kuhn, S., Kockx, M. M., Vyvere, M. E. V. D. & Mertens, A. H. Rapid detection of Enterovirus RNA in cerebrospinal fluid specimens with a novel single-tube real-time reverse transcription-PCR assay. J. Clin. Microbiol. 39, 4093–4096 (2001).
Zhao, H.-B. et al. Development of loop-mediated isothermal amplification (LAMP) for universal detection of Enteroviruses. Indian J. Microbiol. 54, 80–86 (2014).
Daskou, M. et al. WarmStart colorimetric RT-LAMP for the rapid, sensitive and specific detection of Enteroviruses A-D targeting the 5’UTR region. J. Appl. Microbiol. 130, 292–301 (2021).
Yaqing, H. et al. Detection of human Enterovirus 71 reverse transcription loop-mediated isothermal amplification (RT-LAMP). Lett. Appl. Microbiol. 54, 233–239 (2012).
Yan, G. et al. Rapid and visual detection of human enterovirus coxsackievirus A16 by reverse transcription loop‐mediated isothermal amplification combined with lateral flow device. Lett. Appl. Microbiol. 61, 531–537 (2015).
Yin, D. et al. Development and evaluation of a rapid recombinase polymerase amplification assay for the detection of human enterovirus 71. Arch. Virol. 163, 2459–2463 (2018).
Harvala, H. et al. Recommendations for enterovirus diagnostics and characterisation within and beyond Europe. J. Clin. Virol. 101, 11–17 (2018).
WHO. Cyanobacterial toxins: microcystins. (2020).
Weller, M. G. Immunoassays and biosensors for the detection of cyanobacterial toxins in water. Sensors 13, 15085–15112 (2013).
USEPA. Harmful Algal Blooms (USEPA, 2022).
Huisman, J. et al. Cyanobacterial blooms. Nat. Rev. Microbiol. 16, 471–483 (2018).
CDC. Harmful Algal Bloom (HAB)-Associated Illness (Centers for Disease Control and Prevention, 2022).
Rastogi, R. P., Madamwar, D. & Incharoensakdi, A. Bloom dynamics of Cyanobacteria and their toxins: environmental health impacts and mitigation strategies. Front. Microbiol. https://doi.org/10.3389/fmicb.2015.01254 (2015).
Hoagland, P., Anderson, D. M., Kaoru, Y. & White, A. W. The economic effects of harmful algal blooms in the United States: estimates, assessment issues, and information needs. Estuaries 25, 819–837 (2002).
Al-Tebrineh, J., Gehringer, M. M., Akcaalan, R. & Neilan, B. A. A new quantitative PCR assay for the detection of hepatotoxigenic cyanobacteria. Toxicon 57, 546–554 (2011).
Sipari, H., Rantala-Ylinen, A., Jokela, J., Oksanen, I. & Sivonen, K. Development of a chip assay and quantitative PCR for detecting microcystin synthetase E gene expression. Appl. Environ. Microbiol. 76, 3797–3805 (2010).
Johnson, B. N. & Mutharasan, R. A cantilever biosensor-based assay for toxin-producing Cyanobacteria Microcystis aeruginosa using 16S rRNA. Environ. Sci. Technol. 47, 12333–12341 (2013).
Brient, L. et al. Rapid characterization of microcystin-producing Cyanobacteria in freshwater lakes by TSA-FISH (tyramid signal amplification-fluorescent in situ hybridization). Front. Environ. Sci. https://doi.org/10.3389/fenvs.2017.00043 (2017).
Zhu, P. et al. Sensitive and rapid detection of microcystin synthetase E gene (mcyE) by loop-mediated isothermal amplification: a new assay for detecting the potential microcystin-producing microcystis in the aquatic ecosystem. Harmful Algae 37, 8–16 (2014).
Ramya, M., Kayalvizhi, M., Haripriya, G. & Rathinasabapathi, P. Detection of microcystin-producing cyanobacteria in water samples using loop-mediated isothermal amplification targeting mcyB gene. 3 Biotech 8, 378 (2018).
Li, J., Feng, M. & Yu, X. Rapid detection of mcyG gene of microcystins producing cyanobacteria in water samples by recombinase polymerase amplification combined with lateral flow strips. J. Water Health 19, 907–917 (2021).
Li, H. et al. Rapid detection methods and modelling simulations provide new insights into cyanobacteria detection and bloom management in a tropical reservoir. J. Environ. Manag. 326, 116730 (2023).
Gärtner, K. et al. A fast extraction-free isothermal LAMP assay for detection of SARS-CoV-2 with potential use in resource-limited settings. Virol. J. 19, 77 (2022).
Silva, S. J. Rd, Pardee, K. & Pena, L. Loop-mediated isothermal amplification (LAMP) for the diagnosis of zika virus: a review. Viruses 12, 19 (2020).
Augustine, R. et al. Loop-mediated isothermal amplification (LAMP): a rapid, sensitive, specific, and cost-effective point-of-care test for coronaviruses in the context of COVID-19 pandemic. Biology 9, 182 (2020).
Wong, J. K. F., Yip, S. P. & Lee, T. M. H. Ultrasensitive and closed-tube colorimetric loop-mediated isothermal amplification assay using carboxyl-modified gold nanoparticles. Small 10, 1495–1499 (2014).
Kim, S. et al. Loop-mediated isothermal amplification-based nucleic acid lateral flow assay for the specific and multiplex detection of genetic markers. Analytica Chim. Acta 1205, 339781 (2022).
Thio, S. K., Bae, S. W. & Park, S.-Y. Lab on a smartphone (LOS): a smartphone-integrated, plasmonic-enhanced optoelectrowetting (OEW) platform for on-chip water quality monitoring through LAMP assays. Sens. Actuators B Chem. 358, 131543 (2022).
Saez, J., Catalan-Carrio, R., Owens, R. M., Basabe-Desmonts, L. & Benito-Lopez, F. Microfluidics and materials for smart water monitoring: a review. Analytica Chim. Acta 1186, 338392 (2021).
Jin, J. et al. A real-time LAMP-based dual-sample microfluidic chip for rapid and simultaneous detection of multiple waterborne pathogenic bacteria from coastal waters. Anal. Methods 13, 2710–2721 (2021).
Garg, N., Ahmad, F. J. & Kar, S. Recent advances in loop-mediated isothermal amplification (LAMP) for rapid and efficient detection of pathogens. Curr. Res. Microb. Sci. 3, 100120 (2022).
Yigci, D., Atçeken, N., Yetisen, A. K. & Tasoglu, S. Loop-mediated isothermal amplification-integrated CRISPR methods for infectious disease diagnosis at point of care. ACS Omega 8, 43357–43373 (2023).
Soroka, M., Wasowicz, B. & Rymaszewska, A. Loop-mediated isothermal amplification (LAMP): the better sibling of PCR? Cells. https://doi.org/10.3390/cells10081931 (2021).
Moehling, T. J., Choi, G., Dugan, L. C., Salit, M. & Meagher, R. J. LAMP diagnostics at the point-of-care: emerging trends and perspectives for the developer community. Expert Rev. Mol. Diagn. 21, 43–61 (2021).
Zhang, Y. & Tanner, N. A. Efficient multiplexing and variant discrimination in reverse-transcription loop-mediated isothermal amplification with sequence-specific hybridization probes. Biotechniques 73, 235–243 (2022).
Kashir, J. & Yaqinuddin, A. Loop mediated isothermal amplification (LAMP) assays as a rapid diagnostic for COVID-19. Med. Hypotheses 141, 109786 (2020).
Acharya, K. et al. Metagenomic water quality monitoring with a portable laboratory. Water Res. 184, 116112 (2020).
Hinlo, R., Gleeson, D., Lintermans, M. & Furlan, E. Methods to maximise recovery of environmental DNA from water samples. PLoS One 12, e0179251 (2017).
Thomas, A. C., Howard, J., Nguyen, P. L., Seimon, T. A. & Goldberg, C. S. eDNA sampler: a fully integrated environmental DNA sampling system. Methods Ecol. Evolut. 9, 1379–1385 (2018).
Francy, D. S. et al. Comparison of filters for concentrating microbial indicators and pathogens in lake water samples. Appl. Environ. Microbiol. 79, 1342–1352 (2013).
Lee, S. et al. Rapid and in-situ detection of fecal indicator bacteria in water using simple DNA extraction and portable loop-mediated isothermal amplification (LAMP) PCR methods. Water Res. 160, 371–379 (2019).
Al-Soud, W. A. & Rådström, P. Purification and characterization of PCR-inhibitory components in blood cells. J. Clin. Microbiol. 39, 485–493 (2001).
He, Y., Xie, T. & Tong, Y. Rapid and highly sensitive one-tube colorimetric RT-LAMP assay for visual detection of SARS-CoV-2 RNA. Biosens. Bioelectron. 187, 113330 (2021).
Hulse, L. S., McDonald, S., Johnston, S. D. & Beagley, K. W. Rapid point-of-care diagnostics for the detection of Chlamydia pecorum in koalas (Phascolarctos cinereus) using loop-mediated isothermal amplification without nucleic acid purification. Microbiologyopen 8, e916 (2019).
Dudley, D. M. et al. Optimizing direct RT-LAMP to detect transmissible SARS-CoV-2 from primary nasopharyngeal swab samples. PLoS One 15, e0244882 (2021).
Pavšič, J., Žel, J. & Milavec, M. Digital PCR for direct quantification of viruses without DNA extraction. Anal. Bioanal. Chem. 408, 67–75 (2016).
Schultz, M. T. & Lance, R. F. Modeling the sensitivity of field surveys for detection of environmental DNA (eDNA). PLoS One 10, e0141503 (2015).
Lisa, P. Molecular diagnostic tools applied for assessing microbial water quality. Int. J. Environ. Res. Public Health. https://doi.org/10.3390/ijerph19095128 (2022).
Jun, L. et al. Rapid identification and detection of Vibrio parahaemolyticus via different types of modus operandi with LAMP method in vivo. Ann. Microbiol. https://doi.org/10.1186/s13213-020-01585-6 (2020).
Xia, Y. et al. Identifying multiple bacterial pathogens by loop-mediated isothermal amplification on a rotate & react slipchip. Sens. Actuators B Chem. 228, 491–499 (2016).
Maroju, R. G., Choudhari, S. G., Shaikh, M. K., Borkar, S. K. & Mendhe, H. Application of artificial intelligence in the management of drinking water: a narrative review. Cureus 15, e49344 (2023).
Altay, A., Learney, R., Güder, F. & Dincer, C. Sensors in blockchain. Trends Biotechnol. 40, 141–144 (2022).
Dhrubajyoti, D. D., Cheng-Wen, L. & Chuang, H. LAMP-based point-of-care biosensors for rapid pathogen detection. Biosensors. https://doi.org/10.3390/bios12121068 (2022).
Odonkor, S. E. coli as an indicator of bacteriological quality of water: an overview. Microbiol. Res. https://doi.org/10.4081/mr.2013.e2 (2013).
Rompré, A., Servais, P., Baudart, J., de-Roubin, M.-R. & Laurent, P. Detection and enumeration of coliforms in drinking water: current methods and emerging approaches. J. Microbiol. Methods 49, 31–54 (2002).
Fu, J., Chiang, E. L. C., Medriano, C. A. D., Li, L. & Bae, S. Rapid quantification of fecal indicator bacteria in water using the most probable number-loop-mediated isothermal amplification (MPN-LAMP) approach on a polymethyl methacrylate (PMMA) microchip. Water Res. 199, 117172 (2021).
Ye, X. et al. Equipment-free nucleic acid extraction and amplification on a simple paper disc for point-of-care diagnosis of Rotavirus A. Anal. Chim. Acta 1018, 78–85 (2018).
Acknowledgements
We like to thank members of the Beddoe Lab for their comments on drafting the manuscript. This research was supported by the Cooperative Research Centres Project (CRC-P) awarded to GeneWorks and La Trobe University.
Author information
Authors and Affiliations
Contributions
M.K. wrote the manuscript. D.S., S.M., and T.B. revised the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Khodaparast, M., Sharley, D., Marshall, S. et al. Advances in point-of-care and molecular techniques to detect waterborne pathogens. npj Clean Water 7, 74 (2024). https://doi.org/10.1038/s41545-024-00368-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41545-024-00368-9
- Springer Nature Limited