Abstract
Trastuzumab deruxtecan (T-DXd) intracranial activity has been observed in small or retrospective patient cohorts with human epidermal growth factor receptor 2–positive (HER2+) advanced/metastatic breast cancer (mBC) and stable or active (untreated/previously treated and progressing) brain metastases (BMs). The phase 3b/4 DESTINY-Breast12 study investigated T-DXd in patients with HER2+ mBC and is, to our knowledge, the largest prospective study of T-DXd in patients with BMs in this setting. Patients (stable/active BMs (n = 263) and no BMs (n = 241)) treated with one or more prior anti-HER2–based regimens received T-DXd (5.4 mg per kg). Primary endpoints were progression-free survival (PFS; BMs cohort) and objective response rate (ORR) per Response Evaluation Criteria in Solid Tumors version 1.1 (non-BMs cohort). Additional endpoints included central nervous system (CNS) PFS, ORR, time to second progression, CNS ORR (BMs cohort), incidence of new symptomatic CNS metastases (non-BMs cohort), time to progression, duration of response, overall survival and safety (both cohorts). No formal hypothesis testing was conducted for this single-arm, open-label study. In the BMs cohort, 12-month PFS was 61.6% (95% confidence interval (CI): 54.9–67.6), and 12-month CNS PFS was 58.9% (95% CI: 51.9–65.3). In the non-BMs cohort, ORR was 62.7% (95% CI: 56.5–68.8). Grade 3 or higher adverse events occurred in 51% (BMs cohort) and 49% (non-BMs cohort) of patients. Investigator-reported interstitial lung disease/pneumonitis occurred in 16% (grade ≥3: 3%) of patients with BMs and 13% (grade ≥3: 1%) of patients without BMs. These data show substantial and durable overall and intracranial activity for T-DXd, supporting its use in previously treated patients with HER2+ mBC irrespective of stable/active baseline BMs. ClinicalTrials.gov identifier: NCT04739761.
Similar content being viewed by others
Main
Human epidermal growth factor receptor 2–positive (HER2+) breast cancer accounts for between 15% and 20% of all cases of breast cancer1,2. As many as 50% of patients with HER2+ advanced/metastatic breast cancer (mBC) develop brain metastases (BMs), which are associated with a poorer prognosis compared to patients who do not have BMs3,4,5,6.
Local therapy (including surgical resection, stereotactic radiosurgery (SRS), stereotactic radiotherapy and/or whole-brain radiation therapy (WBRT)) is recommended for BMs7; however, central nervous system (CNS) progression typically occurs within 6–12 months of treatment, and no extracranial benefit is conferred8,9,10,11. WBRT, currently recommended for treatment of multiple BMs, is associated with cognitive deterioration7,12; as some patients with HER2+ breast cancer and BMs can survive for several years, this is of particular concern13. As such, additional systemic treatment options for patients with BMs are needed. Trastuzumab-based therapy has long been the mainstay of systemic therapy for patients with HER2+ mBC, and several additional HER2-directed therapies have been investigated for the treatment of HER2+ mBC with BMs, including tucatinib14,15,16,17. Despite this, a large proportion of patients receiving treatment, including those with an initial response, eventually experience disease progression (commonly in the CNS)17.
Trastuzumab deruxtecan (T-DXd) is an antibody–drug conjugate (ADC) composed of a humanized immunoglobulin G1 monoclonal antibody specifically targeting HER2, a tetrapeptide-based cleavable linker and a potent topoisomerase I inhibitor payload18,19. On the basis of results from the randomized phase 3 DESTINY-Breast03 study, T-DXd is approved for the treatment of adult patients with unresectable or metastatic HER2+ breast cancer who have received a prior anti-HER2–based regimen in the metastatic setting or who have received a prior anti-HER2–based regimen in the neoadjuvant or adjuvant setting and developed disease recurrence during or within 6 months of completing therapy20,21.
Promising preliminary evidence of T-DXd intracranial efficacy was reported in a retrospective, exploratory pooled analysis of DESTINY-Breast01, 02 and 03. Patients with HER2+ mBC and stable (n = 104) or active (n = 44) BMs were treated with T-DXd22. The intracranial objective response rate (ORR) was 45.2% in patients with stable BMs and 45.5% in patients with active BMs. Median (95% confidence interval (CI)) CNS progression-free survival (CNS PFS) was 12.3 months (11.1–13.8) in patients with stable BMs and 18.5 months (13.6–23.3) in patients with active BMs22. Encouraging intracranial responses in patients with active (untreated or previously treated and progressing) BMs were also reported in the phase 1b/2 DESTINY-Breast07 study (n = 35); in the ongoing, five-cohort phase 2 DEBBRAH study (n = 13); in ROSET‑BM, a multicenter, retrospective, medical chart review study (n = 67); in the prospective, single-arm, single-center, phase 2 TUXEDO‑1 study (n = 15); and in a retrospective cohort analysis of heavily pretreated patients with BMs (n = 10)23,24,25,26,27.
Here we report results from the phase 3b/4 DESTINY-Breast12 study (NCT04739761), a non-comparative study that evaluated the efficacy and safety of T-DXd in patients with HER2+ mBC from two separate cohorts of patients with and without baseline BMs. DESTINY-Breast12 is, to our knowledge, the largest prospective study of T-DXd in patients with HER2+ mBC with previously treated and stable or active (untreated or previously treated and progressing) BMs.
Results
Patients
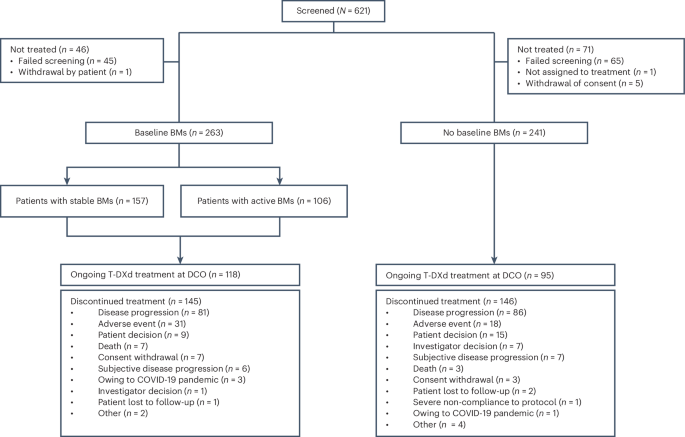
A total of 504 patients were treated across 78 sites between June 2021 and February 2024; 263 patients had baseline BMs, and 241 patients had no baseline BMs (Fig. 1). Of patients with baseline BMs, 157 had previously treated and stable BMs, and 106 had active BMs (39 had untreated BMs; 67 had previously treated BMs that were progressive at study entry (hereafter termed previously treated/progressing BMs), with no clinical indication for immediate retreatment with local therapy). Demographics and baseline disease characteristics for both cohorts are summarized in Table 1. Patients with baseline BMs received a median of 1.0 regimen (range, 0–4) of previous anti-cancer therapy in the metastatic setting, and 158 patients (60.1%) received prior intracranial radiotherapy (including 40 patients (15.2%) who had WBRT and 15 (5.7%) who had SRS; the type of intracranial radiotherapy was not always recorded by investigators, and only WBRT and SRS intracranial radiotherapy were reported). Median time from last intracranial radiotherapy to treatment initiation in patients with prior intracranial radiotherapy was 164 d (range, 9–2,115) overall and 116.5 d (range, 9–1,798) and 214.5 d (range, 15–2,115) in the stable (n = 90) and active (n = 68) BMs subgroups, respectively. The median follow-up duration in this cohort was 15.4 months (range, 0.1–30.0), and 118 patients (44.9%) were continuing to receive treatment at final data cutoff (8 February 2024). The most common reasons for discontinuation of study treatment included progressive disease (PD; 30.8%) and adverse events (AEs; 11.8%) (Fig. 1). Patients with no baseline BMs received a median of 1.0 regimen (range, 0–4) of previous anti-cancer therapy in the metastatic setting and had a median follow-up duration of 16.1 months (range, 0.8–28.4); 95 patients (39.4%) were continuing to receive treatment at final data cutoff. Primary reasons for discontinuation of study treatment included PD (35.7%) and AEs (7.5%) (Fig. 1).
Response and progression in both cohorts were assessed by independent central review (ICR) per Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1).
Overall efficacy in the baseline BMs cohort
Overall PFS at 12 months was 61.6% (95% CI: 54.9–67.6) in all patients with BMs (Fig. 2a and Table 2) and 62.9% (95% CI: 54.0–70.5) and 59.6% (95% CI: 49.0–68.7) in patients with stable and active BMs, respectively (Table 2). Within the active BMs subgroup, PFS at 12 months was 47.0% (95% CI: 29.6–62.7) and 66.7% (95% CI: 53.4–76.9) in patients with untreated and previously treated/progressing BMs, respectively (post hoc analysis). Overall, 89 patients (33.8%) were free of progression at the time of the analysis, and median PFS (post hoc analysis) was 17.3 months (95% CI: 13.7–22.1). Time to progression (time from first dose until documented disease progression) data were immature, and the median was not calculated. Time to second progression (PFS2; time from first dose to second progression or death) data were immature, and the median was not reached. PFS2 at 12 months was 83.1% (95% CI: 77.5–87.4). Overall survival (OS) data were immature at the time of analysis (16.3% maturity); 12-month OS was 90.3% (95% CI: 85.9–93.4) (Fig. 2b and Table 2). In the baseline BMs full analysis set, confirmed ORR was 51.7% (95% CI: 45.7–57.8) (Table 2). A total of 11 patients (4.2%) had a complete response, and 125 (47.5%) patients had a partial response. Most responses (121/136) were reported by 6 months; at the time of analysis, response was ongoing in more than 50% of patients (n = 134 (51.0%)), and, therefore, median duration of response (DOR) was not calculated (Extended Data Fig. 1a). The ORR in patients with stable and active BMs was 49.7% (95% CI: 41.9–57.5) and 54.7% (95% CI: 45.2–64.2), respectively (Table 2). In a post hoc analysis restricted to patients with measurable disease at baseline (n = 198), confirmed ORR was 64.1% (95% CI: 57.5–70.8) overall and 67.0% (95% CI: 58.1–75.8) and 60.7% (95% CI: 50.5–70.8) in patients with stable (n = 109) and active (n = 89) BMs, respectively (Table 2). The best percentage change in target lesion size is shown in Fig. 3a.
a, Best percentage change from baseline in target lesion size in patients with baseline BMs and measurable disease at baseline (full analysis set). b, Best percentage change from baseline in CNS target lesion size in patients with baseline BMs and measurable CNS disease at baseline. c, Best percentage change from baseline in target lesion size, in patients with no baseline BMs and measurable disease at baseline (full analysis set). All patients had at least one post-baseline scan. Responses were assessed per RECIST 1.1 by ICR. A value of +20% was imputed as best percentage change from baseline if best percentage change could not be calculated because of missing data in the following situations: a patient had a new lesion or progression of non-target lesions or target lesions or a patient had withdrawn because of PD and had no evaluable target lesion data before or at PD. The dashed line indicates a 30% decrease in tumor size (partial response). Asterisks indicate imputed values.
CNS efficacy in the baseline BMs cohort
Overall, CNS (including intracranial) progression was reported in 101 (38.4%) patients treated with T-DXd; CNS PFS at 12 months was 58.9% (95% CI: 51.9–65.3) (Fig. 2c and Table 2) and 57.8% (95% CI: 48.2–66.1) and 60.1% (95% CI: 49.2–69.4) in patients with stable and active BMs, respectively (Table 2). In total, 138 (52.5%) patients had measurable CNS disease at baseline (stable BMs: n = 77; active BMs: n = 61). The proportion of these patients with confirmed CNS ORR overall was 71.7% (95% CI: 64.2–79.3) and 79.2% (95% CI: 70.2–88.3) and 62.3% (95% CI: 50.1–74.5) in patients with stable and active BMs, respectively (Table 2). Within the active BMs subgroup, CNS ORR was reported in 19 out of 23 patients (82.6% (95% CI: 67.1–98.1)) and in 19 out of 38 patients (50.0% (95% CI, 34.1–65.9)) with untreated and previously treated/progressing BMs, respectively (post hoc analysis). The best percentage change in CNS target lesion size is shown in Fig. 3b.
Overall efficacy in the no baseline BMs cohort
The proportion of patients in the no baseline BMs full analysis set with confirmed ORR was 62.7% (95% CI: 56.5–68.8) (Table 2). A total of 23 patients (9.5%) had a complete response, and 128 (53.1%) patients had a partial response (Table 2). Most responses (140/151) were reported by 6 months; at the time of analysis, response was ongoing in more than 50% of patients (n = 151 (62.7%)), and, therefore, median DOR was not calculated (Extended Data Fig. 1b). In a post hoc analysis of patients with measurable disease at baseline (n = 215), confirmed ORR was 68.4% (95% CI: 62.2–74.6) (Table 2). The best percentage change in target lesion size is shown in Fig. 3c. OS data were immature at the time of analysis (17.0% maturity); 12-month OS was 90.6% (95% CI: 86.0–93.8) (Table 2). At 12 months, 72.1% (95% CI: 65.4–77.8) of patients had not experienced progression; time to progression data were immature, and the median was not calculated (Extended Data Fig. 2). Only four patients developed new symptomatic CNS metastases (incidence rate 0.017%; 95% CI: 0.00452–0.04250) (Table 2).
Safety: baseline BMs cohort
Median total treatment duration was 11.5 months (range, 0.1–26.9; Extended Data Table 1). The most common AEs included nausea, fatigue and constipation (Table 3). Grade 3 or higher AEs occurred in 134 (51.0%) patients, and the most common grade 3 or higher AEs were neutropenia (n = 43 (16.3%)), fatigue (n = 23 (8.7%)) and anemia (n = 19 (7.2%)). AEs led to treatment discontinuation in 40 (15.2%) patients (Extended Data Table 1); the most common AE leading to discontinuation was interstitial lung disease (ILD)/pneumonitis (n = 27 (10.3%)). Investigator-reported ILD/pneumonitis occurred in 42 patients (16.0%) with baseline BMs; most events were grade 1 (n = 26 (9.9%)), and there were six (2.3%) grade 5 events (Extended Data Tables 2 and 3). The median time to first onset of ILD/pneumonitis was 168.0 d (range, 35–646). Seven patients (2.7%) had a reported opportunistic infection (no systematic testing for infection was done); five patients had opportunistic infection reported as co-occurring with ILD/pneumonitis (ILD/pneumonitis events were grade 4 (n = 1) or grade 5 (n = 4); opportunistic infections were aspergillus (n = 1) and Pneumocystis jirovecii pneumonia (PJP)/infection (n = 4)). Two patients had opportunistic infections that were not reported to co-occur with ILD/pneumonitis (cytomegalovirus infection (n = 1) and PJP (n = 1)). Left ventricular ejection fraction decrease from baseline occurred in 31 patients (11.8%); no grade 4 or higher events were reported (Extended Data Table 2).
Safety: no baseline BMs cohort
Median total treatment duration was 12.0 months (range, 0.7–28.4; Extended Data Table 1). The most common AEs included fatigue, nausea and constipation (Table 3). Grade 3 or higher AEs occurred in 118 (49.0%) patients, and the most common grade 3 or higher AEs were neutropenia (n = 44 (18.3%)), fatigue (n = 24 (10.0%)) and anemia (n = 12 (5.0%)). AEs led to treatment discontinuation in 23 (9.5%) patients (Extended Data Table 1); the most common AE leading to discontinuation was ILD/pneumonitis (n = 13 (5.4%)). Investigator-reported ILD/pneumonitis occurred in 31 (12.9%) patients with no baseline BMs; most events were grade 1 (n = 22 (9.1%)), and there were three (1.2%) grade 5 events (Extended Data Tables 2 and 3). The median time to first onset of ILD/pneumonitis was 169.0 d (range, 24–484). Left ventricular ejection fraction decrease occurred in 26 (10.8%) patients; there were no grade 3 or higher events (Extended Data Table 2).
Discussion
DESTINY-Breast12 is, to our knowledge, the largest prospective study reporting intracranial activity of T-DXd in patients with HER2+ mBC and baseline BMs. This phase 3b/4 study was designed to collect data from settings that resemble real-world clinical practice, to provide a detailed understanding of T-DXd outcomes in patients previously treated with HER2-targeted agents.
PFS was selected as the primary endpoint in the BMs cohort because it was anticipated that a large proportion of this patient population may have no measurable disease at baseline and to minimize any potential confounding effect from prior locally directed therapy28. The 12-month overall PFS rate was 61.6% (95% CI: 54.9–67.6) in patients with baseline BMs. Overall ORR (including patients with no measurable disease at baseline) was lower in patients with stable BMs (49.7%) compared to patients with active BMs (54.7%); however, in line with clinical expectations, a post hoc analysis of ORR in patients with measurable disease at baseline revealed a higher ORR in the stable BMs subgroup (67.0%) versus the active BMs subgroup (60.7%). The different trends observed are likely explained by the imbalance of patients with measurable disease between the two subgroups.
Currently, tucatinib in combination with trastuzumab and capecitabine is the preferred systemic therapy for previously treated patients with HER2+ mBC and active BMs7,29,30. The phase 2 HER2CLIMB study investigated trastuzumab and capecitabine with either placebo or tucatinib in patients with previously treated HER2+ mBC. Patients in the tucatinib arm of the total population (N = 612) were heavily pretreated at baseline (median of three (range, 1–14) previous therapy regimens in the metastatic setting31,32). In an updated exploratory subanalysis of patients with measurable baseline BMs (n = 198), confirmed intracranial ORR was 47.3% (95% CI: 33.7–61.2) in patients with active BMs receiving tucatinib, capecitabine and trastuzumab. Median CNS PFS by investigator per RECIST 1.1 was 9.9 months (95% CI: 8.4–11.7) overall and 9.6 months (95% CI: 7.6–11.1) and 13.9 months (95% CI: 9.7–24.9) in patients with active and stable BMs, respectively32. In DESTINY-Breast12, tucatinib as a previous regimen was exclusionary, to avoid any confounding effect from a drug known to be active on CNS lesions.
Because of the decreased quality of life and poor prognosis observed in patients with BMs3,4,5,6, additional treatment options for this patient population are needed, particularly for later lines of therapy. CNS activity with tucatinib (a small-molecule tyrosine kinase inhibitor) is well established; however, questions remain regarding the intracranial efficacy of ADCs, including T-DXd, in patients with active BMs. Despite its large molecular size, promising CNS activity for T-DXd was previously reported. The 12-month PFS for the overall BMs population in DESTINY-Breast12 was similar to that observed in an exploratory analysis of patients with stable and active BMs enrolled in DESTINY-Breast03 (72.0% (95% CI: 55.0–83.5); n = 43), a phase 3, randomized, open-label study that investigated T-DXd versus trastuzumab emtansine (T-DM1) in patients with HER2+ mBC previously treated with trastuzumab and a taxane33.
Promising CNS activity in patients with active BMs treated with T-DXd was observed in small prospective studies24,26. In the phase 2 DEBBRAH (n = 13) and TUXEDO-1 (n = 15) studies, intracranial ORR per Response Assessment in Neuro-Oncology (RANO)-BM criteria was 46.2% (95% CI: 19.2–74.9) and 73.3% (95% CI: 48.1–89.1), respectively24,26. In an interim analysis of the dose-expansion phase of the ongoing DESTINY-Breast07 study (n = 35), which assessed T-DXd monotherapy in patients with HER2+ mBC and active BMs in the first-line or second-line setting, PFS at 12 months was 75.0% (80% CI: 63.5–83.4), and median PFS was 19.5 months (80% CI: 19.4–24.3) (Anders et al.23). Results from DESTINY-Breast12 extend these observations to a larger group of patients with active BMs (12-month PFS: 59.6% (95% CI: 49.0–68.7)), including those with untreated BMs (47.0% (95% CI, 29.6–62.7)) and previously treated/progressing BMs (66.7% (95% CI, 53.4–76.9)).
CNS ORR in our study was 71.7% overall and 79.2% and 62.3% in patients with stable and active BMs, respectively. Within the active BMs subgroup, CNS ORR was 82.6% in patients with untreated BMs and 50.0% in patients with previously treated/progressing BMs. These results are numerically higher than those observed in the pooled DESTINY-Breast01, 02 and 03 analysis of patients with treated/stable (45.2%; n = 104) or active (45.5%; n = 44) BMs22. This may be reflective of the heavily pretreated population included in the pooled analysis (median 3.0 prior treatment regimens in the metastatic setting versus 1.0 for DESTINY-Breast12). In DESTINY-Breast12, responses in patients with baseline BMs were durable, despite a relatively short follow-up duration (15.4 months).
Results from clinical studies have also been corroborated by real-world evidence. In the retrospective ROSET-BM study, 12-month PFS was 62.0% (95% CI: 47.8–73.4) in patients with active BMs (n = 67) and 71.4% (95% CI: 33.7–90.1) in patients with stable BMs (n = 12), in line with the results of the current study25. Patients with leptomeningeal metastases (LM) were excluded from DESTINY-Breast12. However, T-DXd showed sustained activity in two small retrospective studies in patients with mBC and LM: ROSET-BM (n = 19) and a small case series (n = 8)25,34. Further investigation is needed to confirm the efficacy of T-DXd in this patient population.
ORR was chosen as the primary endpoint for the cohort of patients without baseline BMs as it is an early indicator of treatment effect and allowed for early assessment of T-DXd benefit. In this cohort, overall efficacy was in line with prior reports; however, the proportion of patients with complete responses (9.5%) was lower than that reported in DESTINY-Breast03 (21%)35. CNS as a site of symptomatic progression was very uncommon in the non-BMs cohort of DESTINY-Breast12.
Overall, the safety profile of T-DXd was consistent with previous reports22,23, with no new safety signals identified. Discontinuation rates due to AEs were low (15.2% and 9.5% for patients with and without BMs, respectively). Regarding the rates of decreased left ventricular ejection fraction (11.8% and 10.8% for patients with and without BMs, respectively), most cases were grade 1 or grade 2, with only two grade 3 or higher events reported in the BMs cohort.
Lack of an adjudication committee in DESTINY-Breast12 limits direct comparison of ILD/pneumonitis rates with those from previous clinical studies that included an adjudication committee within the protocol. However, rates of ILD/pneumonitis events observed in both cohorts (16% (grade 5: 2%) and 13% (grade 5: 1%) of patients with and without baseline BMs, respectively) were consistent with T-DXd data for patients with HER2+ mBC in the DESTINY-Breast01 (16%; grade 5: 3%), DESTINY-Breast02 (10%; grade 5: <1%) and DESTINY-Breast03 (15%; grade 5: 0) studies35,36,37. Most cases of ILD/pneumonitis were mild or moderate; however, six deaths in the baseline BMs cohort and three deaths in the no baseline BMs cohort were judged by investigators to be caused by ILD/pneumonitis. Although opportunistic infections were not systematically tested, five cases of opportunistic infection were reported as co-occurring with ILD/pneumonitis (one grade 4 event and four grade 5 events) in the baseline BMs cohort. Clinical and radiologic features of drug-induced ILD/pneumonitis can resemble infectious etiology38; in patients with co-occurring opportunistic infection and ILD/pneumonitis, differentiating the underlying cause of pulmonary toxicity can be challenging, and drug-induced ILD is a diagnosis of exclusion39. These results highlight the need to consider PJP prophylaxis in patients taking chronic corticosteroids. Prompt initiation of steroidal treatment in patients with suspected ILD/pneumonitis is required in accordance with current guidelines, and T-DXd should be interrupted as a precaution until the etiology is confirmed39,40. Delays in providing this treatment (for example, waiting for results of blood culture tests) should be avoided where possible to minimize worsening of ILD/pneumonitis and associated fatalities in this patient population. Where ILD/pneumonitis is suspected, the possibility of infectious etiology should be explored subsequent to immediate treatment to inform future treatment decisions.
Ongoing studies are further defining the potential CNS efficacy of T-DXd in settings beyond HER2+ mBC, including HER2-low breast cancer in the phase 2 TUXEDO-4 study41. After the pan-tumor approval of T-DXd in HER2+ solid tumors42, exploring CNS efficacy of T-DXd outside of breast cancer may be informative. Other ADCs are being tested in prospective clinical studies, including datopotamab deruxtecan in patients with breast cancer and BMs or LM (TUXEDO-2 and DATO-BASE) and patritumab deruxtecan in patients with breast cancer and BMs, non-small cell lung cancer and BMs or solid tumors and LM (TUXEDO-3)43,44,45.
Limitations of DESTINY-Breast12 include the open-label, single-arm study design and exclusion of patients with LM. Few relevant historical cohorts for comparison were available at the time of study protocol development in 2019. Efficacy conclusions relied on single-arm time-to-event efficacy analyses. The immaturity of the final dataset makes cross-trial comparisons challenging, and no long-term follow-up is planned. For the non-BMs cohort, ORR was the primary endpoint despite including patients with no measurable disease at baseline, and PFS was not investigated. A proportion of patients with stable BMs and those with active BMs who were previously treated and progressing had prior intracranial radiotherapy, which may have impaired assessment of target lesions. Patients without baseline BMs did not undergo regular brain imaging; therefore, only incidence of symptomatic CNS metastases could be investigated in that cohort. Patients with Black and Asian ethnicities were underrepresented in the treated population. Patient-reported and neurocognitive outcomes were recorded as part of the study, and these analyses will be reported in future reports.
The results of the DESTINY-Breast12 study indicate the CNS efficacy of T-DXd in a large, prospective patient cohort. Without a direct comparison between T-DXd and the tucatinib, trastuzumab and capecitabine regimen, treatment selection for previously treated patients with HER2+ mBC and BMs should be balanced between efficacy and toxicity considerations on an individual basis.
In conclusion, T-DXd showed substantial and durable overall and intracranial clinical activity in patients with HER2+ mBC, including a large cohort with stable and active BMs. No new safety signals were identified. ILD/pneumonitis remains an important identified safety risk of T-DXd. These results support the use of T-DXd for previously treated patients with HER2+ mBC, including those with stable and active BMs.
Methods
Inclusion and ethics
This study was approved by the institutional review board or ethics committee at each investigational site before initiation (Supplementary Information). This study was performed in accordance with International Council for Harmonisation Good Clinical Practice guidelines, the Declaration of Helsinki and local regulations on the conduct of clinical research. An independent data monitoring committee was responsible for monitoring patient safety during the study. Patients provided written informed consent before participating in the study. Patients were eligible for inclusion regardless of sex or gender.
Study design and treatment
We conducted a prospective, open-label, single-arm, multicenter, international phase 3b/4 study involving patients with pathologically documented HER2+ advanced or metastatic breast cancer with or without baseline BMs. HER2+ expression was locally confirmed as determined by American Society of Clinical Oncology–College of American Pathologists guidelines46.
Patients were eligible if they were aged 18 years or older, had disease progression on one or more prior anti-HER2–based regimens, received no more than two prior therapy regimens in the metastatic setting (had to be tucatinib naive) and had an Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0 or 1. Patients with known or suspected LM were excluded.
Patients were allocated to one of two cohorts: those with baseline BMs (previously treated stable BMs and active (untreated or previously treated and progressing) BMs) and those with no evidence of BMs at baseline. Patients with no measurable disease at baseline were permitted to enroll. Stable BMs were defined as BMs radiographically stable for ≥4 weeks since completion of treatment; active BMs were defined as untreated BMs with lesions ≤2 cm or BMs that had progressed since local CNS therapy, with no clinical indication for immediate retreatment with local therapy. Washout periods before the first day of dosing were ≥7 d and ≥21 d for SRS or gamma knife and WBRT, respectively. Patients who received local therapy for isolated CNS progression in either cohort could continue study treatment until a second progression (brain or body) was observed, upon which study treatment was discontinued. Concomitant use of ≤3 mg of dexamethasone daily or equivalent was permitted in patients with baseline BMs. T-DXd was administered intravenously every 3 weeks (21-d cycle) at a dose of 5.4 mg per kg of body weight until RECIST 1.1-defined47 disease progression outside the CNS, unless there was unacceptable toxicity or withdrawal of consent or another criterion for discontinuation was met.
Tumor assessments of the chest, abdomen (including the entire liver and both adrenal glands) and pelvis used images from computed tomography (CT) or magnetic resonance imaging (MRI; with intravenous (IV) contrast) collected at screening/baseline and every 6 weeks for the first 48 weeks and 9 weeks thereafter during study intervention. For patients with baseline BMs, MRI (with and without IV contrast) or contrast-enhanced CT images of the brain were collected for all patients at baseline and every 6 weeks for the first 48 weeks and 9 weeks thereafter during study intervention. Patients with active and measurable BMs had intracranial lesions included as target lesion(s) for RECIST 1.1 CNS assessments.
Endpoints
The primary endpoint for patients with baseline BMs was PFS (time from first dose to disease progression or death (by any cause in absence of progression)). Secondary endpoints included CNS PFS (time from first dose to CNS progression or death); OS (time from first dose to death by any cause); ORR (proportion of patients with confirmed complete or partial response); PFS2 (time from first dose to second progression or death); time to progression (time from first dose until documented disease progression); CNS ORR (proportion of patients with measurable BMs at baseline with confirmed complete or partial response of brain lesions); DOR (time from first documented confirmed response until documented progression or death by any cause); and safety. The primary endpoint for patients with no baseline BMs was ORR. Secondary endpoints included DOR, OS, time to progression, incidence of new symptomatic CNS metastases (number of new symptomatic CNS metastasis during study intervention period / total number of patients without symptomatic CNS metastasis at baseline) and safety. Response and progression in both cohorts were assessed by ICR per RECIST 1.1. Additional prespecified secondary endpoints not reported in this analysis are site of next progression (CNS versus extracranial versus both), duration of treatment on subsequent lines of therapy, patient-reported outcomes (European Organization for the Research and Treatment of Cancer 30-item core quality of life questionnaire (EORTC QLQ-C30), Neurologic Assessment in Neuro-Oncology (NANO) scale, cognitive tests and St. George’s Respiratory Questionnaire–idiopathic pulmonary fibrosis version (SGRQ-I; patients with ILD/pneumonitis only)) in both cohorts and time to new CNS lesions, CNS DOR and MD Anderson Symptom Inventory (MDASI) Symptom Diary (brain tumor-specific outcomes) in the BMs cohort only.
Safety
AEs were coded using Medical Dictionary for Regulatory Activities version 26.1 preferred terms and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. For potential cases of ILD or pneumonitis, study intervention was interrupted and a full investigation was carried out, based on the investigator’s judgment and sponsor review by medical monitor and study safety physician. Adjudication of reported ILD/pneumonitis cases by a separate committee was not conducted in this study; an ILD advisory committee reviewed the diagnosis and management of ILD/pneumonitis cases (outside the parameters of the study). Pulmonary toxicity management guidelines were described previously48.
Statistical analysis
Data analyses were completed using SAS software version 9.4. This single-arm study was not designed to test any prespecified hypothesis; therefore, no formal sample size calculation was performed. The sample size was chosen based on precision estimates for the primary endpoint in each cohort. Assuming an underlying PFS in the BMs cohort and an underlying ORR in the non-BMs cohort in line with available data at the time of study design, a sample of 250 participants in each cohort ensured that the one-sided width of a two-sided 95% CI for each endpoint would not exceed 6.3%. Efficacy analyses were conducted in the full analysis set (defined as all patients who were enrolled in the study and received at least one treatment dose), and no data were excluded. Safety data are reported for the safety analysis set (identical to the full analysis set). Analyses were performed separately by cohort, and no comparison of results between the two cohorts was planned. Safety analyses were descriptive only. PFS, OS, CNS PFS (all 12-month rates), PFS2 and DOR were analyzed by the Kaplan–Meier method. CIs for median PFS were derived based on the Brookmeyer–Crowley method. ORR was assessed using data obtained from first dose until progression, or the last evaluable assessment in the absence of progression, regardless of whether patients withdrew from therapy. CNS ORR was assessed using data obtained from first dose until brain progression, or the last evaluable assessment in the absence of brain progression, regardless of whether patients withdrew from therapy. For PFS, patients who had not progressed or had died by the time of analysis were censored at the time of the latest date of assessment from their last evaluable RECIST 1.1 assessment. Patients who progressed or died immediately after two or more consecutive missed visits were censored at the time of the latest evaluable RECIST 1.1 assessment before the two missed visits. For CNS PFS, patients who had systemic progression, but no CNS progression, were censored at the time of the progression assessment; the analysis did not account for systemic progression as a competing event. For OS, patients not known to have died at the time of analysis were censored on the last recorded date on which the patient was known to be alive. For ORR and CNS ORR, patients who stopped treatment without a response or progression, received a subsequent therapy and then responded were not included as responders. Prespecified subgroup analyses of the full analysis set were conducted for patients with active and stable BMs in the baseline BMs cohort, and descriptive statistics are provided.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data underlying the findings described in this paper may be obtained in accordance with AstraZeneca’s data-sharing policy, described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at https://vivli.org/. Data for studies not listed on Vivli can be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. The AstraZeneca Vivli member page is also available outlining further details: https://vivli.org/ourmember/astrazeneca.
References
Morales, S., Gasol, A. & Sanchez, D. R. HER2-positive cancers and antibody-based treatment: state of the art and future developments. Cancers 13, 5771 (2021).
Wolff, A. C. et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J. Clin. Oncol. 31, 3997–4013 (2013).
Freedman, R. A. et al. TBCRC 022: a phase II trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2–positive breast cancer and brain metastases. J. Clin. Oncol. 37, 1081–1089 (2019).
Hackshaw, M. D. et al. Prognostic factors of brain metastasis and survival among HER2-positive metastatic breast cancer patients: a systematic literature review. BMC Cancer 21, 967 (2021).
DeBusk, K. et al. Real-world outcomes among patients with HER2+ metastatic breast cancer with brain metastases. J. Manag. Care Spec. Pharm. 28, 657–666 (2022).
Mounsey, L. A. et al. Changing natural history of HER2-positive breast cancer metastatic to the brain in the era of new targeted therapies. Clin. Breast Cancer 18, 29–37 (2018).
Ramakrishna, N. et al. Management of advanced human epidermal growth factor receptor 2–positive breast cancer and brain metastases: ASCO guideline update. J. Clin. Oncol. 40, 2636–2655 (2022).
Mahajan, A. et al. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. 18, 1040–1048 (2017).
Kocher, M. et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J. Clin. Oncol. 29, 134–141 (2011).
Tsao, M. N. et al. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst. Rev. 1, CD003869 (2018).
Patchell, R. A. et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA 280, 1485–1489 (1998).
van Grinsven, E. E., Nagtegaal, S. H. J., Verhoeff, J. J. C. & van Zandvoort, M. J. E. The impact of stereotactic or whole brain radiotherapy on neurocognitive functioning in adult patients with brain metastases: a systematic review and meta-analysis. Oncol. Res. Treat. 44, 622–636 (2021).
Riecke, K. et al. Long-term survival of breast cancer patients with brain metastases: subanalysis of the BMBC registry. ESMO Open 8, 101213 (2023).
Lin, N. U. et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J. Clin. Oncol. 38, 2610–2619 (2020).
Montemurro, F. et al. Trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer and brain metastases: exploratory final analysis of cohort 1 from KAMILLA, a single-arm phase IIIb clinical trial. Ann. Oncol. 31, 1350–1358 (2020).
Krop, I. E. et al. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Ann. Oncol. 26, 113–119 (2015).
Hurvitz, S. A. et al. Central nervous system metastasis in patients with HER2-positive metastatic breast cancer: patient characteristics, treatment, and survival from SystHERs. Clin. Cancer Res. 25, 2433–2441 (2019).
Ogitani, Y. et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin. Cancer Res. 22, 5097–5108 (2016).
Nakada, T., Sugihara, K., Jikoh, T., Abe, Y. & Agatsuma, T. The latest research and development into the antibody–drug conjugate, [fam-] trastuzumab deruxtecan (DS-8201a), for HER2 cancer therapy. Chem. Pharm. Bull. (Tokyo) 67, 173–185 (2019).
Cortés, J. et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N. Engl. J. Med. 386, 1143–1154 (2022).
US Food and Drug Administration. ENHERTU (fam-trastuzumab deruxtecan-nxki): highlights of prescribing information https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761139s028lbl.pdf (2024).
Hurvitz, S. A. et al. A pooled analysis of trastuzumab deruxtecan (T-DXd) in patients (pts) with HER2-positive (HER2+) metastatic breast cancer (mBC) with brain metastases (BMs) from DESTINY-Breast(DB) -01, -02, and -03. Ann. Oncol. 34, S335–S336 (2023).
Anders, C. et al. Safety, tolerability, and antitumor activity of trastuzumab deruxtecan (T-DXd) in patients (pts) with HER2+ metastatic breast cancer (mBC) and active brain metastases (BM) in DESTINY Breast07 (DB-07). ESMO Open 9, 103207 (2024).
Pérez-García, J. M. et al. Trastuzumab deruxtecan in patients with central nervous system involvement from HER2-positive breast cancer: the DEBBRAH trial. Neuro Oncol. 25, 157–166 (2023).
Niikura, N. et al. Treatment with trastuzumab deruxtecan in patients with HER2-positive breast cancer and brain metastases and/or leptomeningeal disease (ROSET-BM). NPJ Breast Cancer 9, 82 (2023).
Bartsch, R. et al. Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: a single-arm, phase 2 trial. Nat. Med. 28, 1840–1847 (2022).
Kabraji, S. et al. Preclinical and clinical efficacy of trastuzumab deruxtecan in breast cancer brain metastases. Clin. Cancer Res. 29, 174–182 (2023).
Lin, N. U. et al. Challenges relating to solid tumour brain metastases in clinical trials, part 1: patient population, response, and progression. A report from the RANO group. Lancet Oncol. 14, e396–e406 (2013).
Gennari, A. et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 32, 1475–1495 (2021).
Vogelbaum, M. A. et al. Treatment for brain metastases: ASCO-SNO-ASTRO guideline. J. Clin. Oncol. 40, 492–516 (2022).
Murthy, R. K. et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N. Engl. J. Med. 382, 597–609 (2020).
Lin, N. U. et al. Tucatinib vs placebo, both in combination with trastuzumab and capecitabine, for previously treated ERBB2 (HER2)-positive metastatic breast cancer in patients with brain metastases: updated exploratory analysis of the HER2CLIMB randomized clinical trial. JAMA Oncol. 9, 197–205 (2023).
Hurvitz, S. A. et al. Trastuzumab deruxtecan versus trastuzumab emtansine in HER2-positive metastatic breast cancer patients with brain metastases from the randomized DESTINY-Breast03 trial. ESMO Open 9, 102924 (2024).
Alder, L. et al. Durable responses in patients with HER2+ breast cancer and leptomeningeal metastases treated with trastuzumab deruxtecan. NPJ Breast Cancer 9, 19 (2023).
Hurvitz, S. A. et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet 401, 105–117 (2023).
Saura, C. et al. Trastuzumab deruxtecan in previously treated patients with HER2-positive metastatic breast cancer: updated survival results from a phase II trial (DESTINY-Breast01). Ann. Oncol. 35, 302–307 (2024).
André, F. et al. Trastuzumab deruxtecan versus treatment of physician’s choice in patients with HER2-positive metastatic breast cancer (DESTINY-Breast02): a randomised, open-label, multicentre, phase 3 trial. Lancet 401, 1773–1785 (2023).
Schwaiblmair, M. et al. Drug induced interstitial lung disease. Open Respir. Med. J. 6, 63–74 (2012).
Swain, S. M. et al. Multidisciplinary clinical guidance on trastuzumab deruxtecan (T-DXd)-related interstitial lung disease/pneumonitis—focus on proactive monitoring, diagnosis, and management. Cancer Treat. Rev. 106, 102378 (2022).
Classen, A. Y. et al. Primary prophylaxis of bacterial infections and Pneumocystis jirovecii pneumonia in patients with hematologic malignancies and solid tumors: 2020 updated guidelines of the Infectious Diseases Working Party of the German Society of Hematology and Medical Oncology (AGIHO/DGHO). Ann. Hematol. 100, 1603–1620 (2021).
ClinicalTrials.gov. T-DXd therapy for HER2-low breast cancer patients with brain metastases https://clinicaltrials.gov/study/NCT06048718 (2024).
AstraZeneca. Enhertu approved in the US as first tumour-agnostic HER2-directed therapy for previously treated patients with metastatic HER2-positive solid tumours https://www.astrazeneca.com/media-centre/press-releases/2024/enhertu-approved-in-the-us-as-first-tumour-agnostic-her2-directed-therapy-for-previously-treated-patients-with-metastatic-her2-positive-solid-tumours.html (2024).
ClinicalTrials.gov. Phase II study of Dato-DXd in triple-negative breast cancer patients with newly diagnosed or progressing brain metastases (TUXEDO-2) https://clinicaltrials.gov/study/NCT05866432 (2023).
ClinicalTrials.gov. DATO-BASE: DATOpotamab-deruxtecan for Breast Cancer Brain metAstaSEs https://clinicaltrials.gov/study/NCT06176261 (2024).
ClinicalTrials.gov. HER3-DXd in breast cancer and NSCLC brain metastases and solid tumor leptomeningeal disease (TUXEDO-3) https://clinicaltrials.gov/study/NCT05865990 (2024).
Wolff, A. C. et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J. Clin. Oncol. 36, 2105–2122 (2018).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Modi, S. et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N. Engl. J. Med. 387, 9–20 (2022).
Acknowledgements
This study was sponsored by AstraZeneca and Daiichi Sankyo. In March 2019, AstraZeneca entered into a global development and commercialization collaboration agreement with Daiichi Sankyo for T-DXd (DS-8201). This study was designed by AstraZeneca and Daiichi Sankyo. The authors prepared and approved the paper and made the decision to submit the paper for publication.
Medical writing support, under the direction of the authors, was provided by K. Rimmer of Helios Medical Communications, part of Helios Global Group, and was funded by AstraZeneca in accordance with Good Publication Practice guidelines (http://www.ismpp.org/gpp-2022).
We thank the patients who participated in this study as well as their families and caregivers, the staff and investigators at all the study sites and members of the ILD advisory committee. We also thank the following AstraZeneca employees: M. Haughton for publication leadership, interpretation of the data and scientific review of the paper; R. Antony for clinical leadership of the study, interpretation of the data and scientific review of the paper; M. Leroux for overseeing the operational functions of the study; and S. Anand for statistical analysis. We would like to acknowledge G. James of Medical Statistics Consultancy, Ltd. for assistance with statistical analysis.
Author information
Authors and Affiliations
Consortia
Contributions
N.H. and N.U.L. served as steering committee co-chairs. N.H., N.U.L., M.P., N.S., and S.V. contributed to the conception and/or design of the study and development of the study protocol. N.H., N.U.L., E.C., G.J., V.M., N.N., G.V., R.B. and C.K. were members of the steering committee. M.P., H.B. and N.K.-C. were involved in data acquisition and analysis. All authors participated in the interpretation of data. All authors were involved in drafting the paper and reviewing it for publication. All authors approved the final version of the paper and accept responsibility to submit for publication.
Corresponding author
Ethics declarations
Competing interests
N.H. has received lecture honoraria from Art Tempi, AstraZeneca, Daiichi Sankyo, Eli Lilly, Gilead Sciences, Medscape, Merck Sharp & Dohme, Novartis, Onkowissen, Pierre Fabre, Roche, Sanofi, Seagen, Viatris and Zuellig Pharma; has received consulting or advisory honoraria from Aptitude Health, Gileadn Sciences, Pfizer, Sandox-Hexal, Sanofi and Seagen; has received research funding from AstraZeneca, Bristol Myers Squibb, Daiichi Sankyo, Gilead Sciences, Merck Sharp & Dohme, Roche, Seagen, TRIO and WSG; has participated on independent data monitoring committees/steering committees for Eli Lilly, Pierre Fabre and Roche; and has ownership interest in the West German Study Group. E.C. has received lecture, consulting or advisory honoraria from AstraZeneca, Daiichi Sankyo, Eli lilly, Gilead Sciences, Menarini, Merck Sharp & Dohme, Novartis, Pfizer, Reveal Genomics and Roche; has received support for attending meetings and/or travel from AstraZeneca, Pfizer and Roche; has received research funding from Daiichi Sankyo, Pfizer and Roche; and has participated on steering committees for AstraZeneca, Daiichi Sankyo, Gilead Sciences, Novartis, Reveal Genomics and Roche. G.J. has received consulting honoraria from AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Daiichi Sankyo, Diaccurate/Evexta Bio, Eli Lilly, Novartis, Pfizer, Roche and Seagen; has received honoraria from AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Daiichi Sankyo, Eli Lilly, Novartis, Pfizer, Roche and Seagen; has received support for attending meetings and/or travel from Amgen, AstraZeneca, Bristol Myers Squibb, Eli Lilly, Gilead Sciences, Novartis, Pfizer and Roche; and has received medical writing support from Amgen, AstraZeneca, Bristol Myers Squibb, Eli Lilly, Novartis and Roche. V.M. has received lecture honoraria from AstraZeneca, Daiichi Sankyo, Eisai, Gilead Sciences, high5 Oncology, iMED Institut, Eli Lilly, Medac, Medscape, Merck Sharp & Dohme, Novartis, Onkowissen, Pfizer, Pierre Fabre, Roche and Seagen; has received consulting or advisory honoraria from ClinSol, Daiichi Sankyo, Eisai, Eli Lilly, Gilead Sciences, Menarini Stemline, Merck Sharp & Dohme, Novartis, Pierre Fabre, PINK, Roche and Menarini Stemline; and has received research funding from AstraZeneca, Genentech, Novartis, Roche and Seagen. N.N. has received consulting or advisory honoraria from AstraZeneca, Chugai Pharmaceutical and Daiichi Sankyo; has received lecture honoraria from AstraZeneca, Chugai Pharmaceutical, Eisai, Daiichi Sankyo, Eli Lilly Japan, Nippon Kayaku and Pfizer Japan; and has received research funding from Chugai Pharmaceutical, Daiichi Sankyo, Eli Lilly Japan, Nippon Kayaku and Pfizer Japan. G.V. has received research grants from Dako/Agilent Technologies, Roche/Genentech and Ventana Medical Systems and has received honoraria from AstraZeneca, Daiichi Sankyo, Dako/Agilent Technologies, Gilead Sciences, Merck Sharp & Dohme Oncology, Pfizer, Roche and Ventana Medical Systems. R.B. has held advisory roles at AstraZeneca, Daiichi Sankyo, Eisai, Eli Lilly, Gilead Sciences, Grünenthal, Merck Sharp & Dohme, Novartis, Pfizer, Pierre Fabre, Roche, Seagen and Menarini Stemline; has received lecture honoraria from AstraZeneca, Bristol Myers Squibb, Daiichi Sankyo, Eisai, Eli Lilly, Gilead Sciences, Grünenthal, Menarini Stemline, Merck Sharp & Dohme, Novartis, Pfizer, Pierre Fabre, Roche and Seagen; and has received research support from Daiichi Sankyo, Merck Sharp & Dohme, Novartis and Roche. C.K. has received lecture honoraria from AstraZeneca, Eli Lilly, Genomic Health/Exact Sciences, Gilead Sciences, GlaxoSmithKline, Novartis, PharmaMar, Pfizer and Roche; has received consulting or advisory honoraria from AstraZeneca, Eli Lilly, Genomic Health/Exact Sciences, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, Novartis, PharmaMar and Roche; and has participated on steering committees for AstraZeneca. M.J.H. has received lecture honoraria from AstraZeneca. R.M.C. received an unrestricted educational grant from Pfizer; has received research support from AstraZeneca, Daiichi Sankyo, MSD Ireland and Pfizer; has received honoraria from AstraZeneca, Daiichi Sankyo, Eli Lilly, Gilead Sciences and Seagen; and has received support for attending meetings and/or travel from Gilead Sciences, Novartis and Roche. M.G. has received support for attending meetings and/or travel from AstraZeneca, Gilead Sciences, Roche and Pfizer and has received honoraria from AstraZeneca, Gilead Sciences and Pfizer. V.G. has received honoraria from AstraZeneca, Daiichi Sankyo, Eli Lilly, Exact Sciences, Gilead Sciences, Menarini Stemline, Merck Sharp & Dohme, Novartis, Pfizer, Olema Oncology, Pierre Fabre and Roche; has received lecture honoraria from AstraZeneca, Daiichi Sankyo, Eli Lilly, Exact Sciences, Gilead Sciences, GlaxoSmithKline, Menarini Stemline, Novartis, Roche and Zentiva; and has received expert testimony honoraria from Eli Lilly. G.B. has received honoraria from Agendia, Amgen, AstraZeneca, Chugai Pharmaceutical/Roche, Daiichi Sankyo, Eisai, Exact Sciences, Gilead Sciences, Helsinn Lilly, Menarini Stemline, Merck Sharp & Dohme, Novartis, Pfizer, Roche, Sanofi and Seagen and received a research grant from Gilead Sciences. H.W. has received lecture honoraria from Seagen; has received consulting or advisory honoraria from AstraZeneca, Augustine Therapeutics, Daiichi Sankyo, E Squared Communications, Eli Lilly, Gilead Sciences, Immutep, MediMix, Menarini Stemline, Novartis, NV Hict, Pfizer, PSI CRO and Roche; has received support for attending meetings and/or travel from Daiichi Sankyo and Pfizer; has received research funding from Novartis, Roche and Syneos Health; and has received subscription fees from Gilead Sciences. S.E.-d.-R. has received honoraria from AstraZeneca, COR2ED, Daiichi Sankyo, Jazz Pharmaceuticals, Medistream, Pierre Fabre, Roche and Seagen; has received research funding from AstraZeneca, Byondis, Daiichi Sankyo, Jazz Pharmaceuticals, MEDSIR, Roche, SOLTI and Zymeworks; and has received support for attending meetings and/or travel from AstraZeneca, Daiichi Sankyo, Kern Pharma, Pfizer, Seagen and SOLTI. M.P., H.B., N.K.-C., N.S. and S.V. are employees of AstraZeneca. N.K.-C. holds stocks in AstraZeneca and AbbVie. N.U.L. has received honoraria from AstraZeneca and has received research funding from AstraZeneca. The other authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Alexandra Zimmer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Ulrike Harjes, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Kaplan-Meier analysis of duration of response.
Duration of response per RECIST 1.1 by ICR in all patients with a confirmed complete or partial response. a, Patients with baseline BMs. b, Patients with no baseline BMs. Tick marks indicate censored data. BM, brain metastasis; ICR, independent central review; RECIST 1.1, Response Evaluation Criteria in Solid Tumors version 1.1.
Extended Data Fig. 2 Kaplan-Meier analysis of time to progression in patients with no baseline BMs.
Time to progression per RECIST 1.1 as assessed by ICR in patients from the full analysis set. Tick marks indicate censored data. BM, brain metastasis; ICR, independent central review; RECIST 1.1, Response Evaluation Criteria in Solid Tumors version 1.1.
Supplementary information
Supplementary Information
List of investigators and list of independent ethics committees/institutional review boards consulted.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Harbeck, N., Ciruelos, E., Jerusalem, G. et al. Trastuzumab deruxtecan in HER2-positive advanced breast cancer with or without brain metastases: a phase 3b/4 trial. Nat Med (2024). https://doi.org/10.1038/s41591-024-03261-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41591-024-03261-7
- Springer Nature America, Inc.