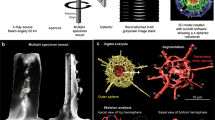
Abstract
The skeletons of long-lived bamboo coral (Family Keratoisididae) are promising archives for deep-water palaeoceanographic reconstructions as they can record environmental variation at sub-decadal resolution in locations where in-situ measurements lack temporal coverage. Yet, detailed three dimensional (3D) characterisations of bamboo coral skeletal architecture are not routinely available and non-destructive investigations into microscale variations in calcification are rare. Here, we provide high-resolution micro-focus computed tomography (µCT) data of skeletal density for two species of bamboo coral (Acanella arbuscula: 5 specimens, voxel size, 15 µm (central branch scans) and 50 µm (complete structure scan); Keratoisis sp.: 4 specimens, voxel size, 15 µm) collected from the Labrador Sea and Baffin Bay deep-water basins, Eastern Canadian Arctic. These data provide reference models useful for developing methods to assess structural integrity and other fine-scale complexities in many biological, geological, and industrial systems. This will be of wider value to those investigating structural composition, arrangement and/or composition of complex architecture within the fields and subdisciplines of biology, ecology, medicine, environmental geology, and structural engineering.
Similar content being viewed by others
Background & Summary
Deep-water bamboo corals form complex structures that, as they grow, archive seasonally resolved oceanographic information1. This information is important for efforts to reconstruct both recent and ancient environmental conditions2. Stands of these corals also play an important role in mediating benthic biodiversity and functioning by enhancing the density of bioturbators and sediment nutrient release3. However, a combination of their extended longevity (>100 years4) and slow growth rates5 mean that populations are vulnerable to physical disturbance6 such that intact specimens have seldom been sampled and are not widely available. Yet, detailed information on coral skeletal architecture is vital for understanding calcification strategies and growth patterns7 in response to changing environmental circumstance, and can be informative for marine planning and conservation measures8.
Techniques used to investigate the microstructure of coral skeletons, such as scanning electron microscopy (SEM) and the grinding of sections, have relied on methods that require high workloads, strict operability and destructive preparation work9. Recent imaging methods, such as high-resolution micro-focus computed tomography (µCT) removes these constraints and, as it allows quantitative analyses of coral skeletal microarchitecture, is emerging as a growing area of scientific focus10,11 for contemporary investigations of reef-building coral skeletons12,13,14,15,16.
This data descriptor presents µCT scans of two species of deep-water bamboo coral (Acanella arbuscula and Keratoisis sp.) obtained from the Eastern Canadian Arctic. These μCT scans can provide reference models which may be of use in the development of novel structural designs, analysis routines and computer models for fields such as ecology17 orthopaedics18, environmental geology and structural engineering19. The data may also be of particular interest to those investigating radial growth patterns and banding20, coral calcification and bioerosion21, impacts of climate change on marine calcifiers22,23, coral skeletal-canal networks24 and coral-to-bone substitute biocompatibility25. The data files are provided as a sequence of stacked tagged image file format (TIFF) images for each scan. These tiff stacks can be opened by a variety of software, including Fiji/ImageJ, which includes instructions for opening in the accompanying user manual26.
Methods
Five specimens of Acanella arbuscula and four specimens of Keratoisis sp. were collected from two deep-water stations (Davis Strait; 63° 20.7198′ N; 58° 11.7426′ W, 1311 m, 3.5 °C, salinity 34.9, 29th July 2021, Disko Fan; 67° 57.9786′ N, 59° 29.6286′ W, 889 m, 1.1 °C, salinity 33.5, 2nd August 2021) using a remotely operated submersible (Sub-Atlantic® Comanche, Forum Energy TechnologiesTM, USA) during the 2021 Amundsen expedition (15th July 2021 – 12th August 2021, CCGS Amundsen). These stations reside within the historically heavily fished27, and now Marine Conservation Areas (since 201728,29), of the Eastern Canadian Arctic. Permits to Fish for Scientific Purposes were obtained from Fisheries and Oceans Canada (Licence NL-6515-21; Licence S-21/22-1030-NU). A. arbuscula is considered an indicator of Vulnerable Marine Ecosystems30 whilst Keratoisis sp. has not, to date, been found anywhere else in the world31. Where possible, the corals were sampled at or close to the basal internode (near the base of the specimen at the sediment surface). Any external debris and residing fauna were carefully removed from the collected colonies using tweezers before each specimen was sealed in a plastic Ziplock bag and frozen at −20 °C. After 72 hours, the specimens were removed from the freezer and carefully cleaned with jets of re-circulated 0.45 µm membrane-filtered seawater (FSW) at 4 °C using a WaterPikTM before being placed back in −20 °C32. The cleaned skeleton portions were then sealed in new Ziplock plastic sample bags enclosed in Tupperware (Acanella arbuscula) or PVC vinyl tubing (Keratoisis sp.) before being transported to the University of Southampton, UK. Here, the specimens were re-housed within Perspex tubes, sealed with polystyrene bungs (Fig. 1), and brought to the μ-VIS X-ray Imaging Centre (www.muvis.org) for µCT scanning. Specifically, imaging took place at the centre’s 3D X-ray Histology (XRH) facility at the University Hospital Southampton33, which is a dedicated division for biomedical imaging.
Reconstruction of biogenic structures was achieved using a custom designed Nikon XT micro-focus computed tomography housed within the 3D X-ray Histology (XRH) facility. This system is based on the XT H 225 ST (Nikon Tring, UK). As the system used to acquire the scan data requires the corals to be held vertically, specimens were secured upright in custom-made Perspex holding tubes with polystyrene bungs to ensure stability and prevent movement during rotation (360 degrees) and scanning (Fig. 1). The scans (acquisition time: 15 – 83 minutes; total projections: 2001 – 3501) were all performed at 80 KVp using a Molybdenum target with no filtration. The detector in the scanner is 2850 × 2850 pixels and was used un-binned. For the overview scans of A. arbuscula at 50 µm a 12 W power could be used but, for the higher resolution scans (15 µm; A. arbuscula and Keratoisis sp.) power was reduced to 6.9 W to allow for a sharper (smaller) X-ray focal spot (see Table 1 for more scan parameters). Additionally, a tube of water was scanned at the same time as the samples under the same beam conditions to allow it to be used as a density phantom. The work this data was collected to support focuses on studying the phenotype (microanatomy), which does not require densitometric calibration. However, it was recognised that this may be valuable in the future so the raw data required to calibrate the scans was collected at the same time for futureproofing the datasets. As of now, the data-size limitations set by repositories dictate that access to these raw data files can only be obtained from the authors. All reconstructions were performed using CT Pro 3D 6.6 or 6.7 (Nikon Xtek, Tring UK). The reconstructions were performed using Nikon CT Pro/CT Agent with the beam hardening 4 preset. The software performs a linearisation operation of the beam hardening curves using a pre-determined correction profile. Preset 4 uses the following variables: CoefX4 = 0.0, CoefX3 = 0.0, CoefX2 = 0.8, CoefX1 = 0.2, CoefX0 = 0.0, Scale = 4.44. No additional ring filter or noise filter was specified.
The field of view for the desired resolution did not allow the full height of the Keratoisis sp. samples (11.1 – 24.5 cm) to be scanned in a single scan, so multiple overlapping vertical scan positions (n = 3) were used which were then concatenated after reconstruction. The chosen overlap was designed specifically to exclude cone-beam under-sampling artifacts that occur at the top and bottom of the reconstructed space from the concatenated volume. The concatenation was performed using a custom written macro for Fiji titled ‘AutomaticConcatenationPlusIntensityEqualisation’ from the XRH toolbox34, which enables the user to manually or automatically select the fusion slice on each volume. If textural information is sufficient and variation from slice to slice significant, the selection can be done automatically. If not, the user can select to bypass the automatic slice selection and select the fusion slice manually. The script then crops the bottom volume between “slice one” and up to the “selected slice”, and top volume from “selected slice” up to “last slice”, and before stitching them into a single volume adjusts the contrast and brightness of the first image of the top volume to match that of the last image of the bottom volume. This ensures a smooth transition from one volume to the other and corrects the intensity variations caused by the heel effect. Intensity calibration is carried out by sampling regions of interest (ROIs) and fitting a straight line using mean intensity values. The parameters obtained from the calibration are applied to the “top” stack to linearly shift the intensity window of the top volume. The two stacks are subsequently concatenated into a single stack and a preview of the concatenated stack is generated by performing a radial reslice to allow the user to evaluate the “smoothness” of the transition. The process can then be repeated to concatenate a third volume onto of the resulted volume-1 + volume-2 volume, etc. Following concatenation on the 32-bit, the resulted volume it was converted to 8-bit in Fiji/ImageJ (v 1.53c26) to reduce the data size making it easier to process. These complete volumes were then exported as tiff stacks to enable upload into the Polar Data Centre35, as such, the macro does not need to be run a second time on the data files.
In the stacked images (Fig. 2) and three-dimensional volumes (Fig. 3), levels of grey scale reflect the level of X-ray attenuation caused by variation in bulk density. In this case, brighter pixels represent denser material (calcium carbonate) with darker pixels representing less dense material (organic tissue). To refine coral visualisations, the three-dimensional image captured of the holding tube can be discarded during image processing to leave the skeletal volume (Fig. 3).
A scaled transverse slice (voxel size, 15 µm) from the (a) Acanella arbuscula 8-bit coral volumes image set and (b) Keratoisis sp. 8-bit coral volumes image set showing rings of low density organic tissue (dark grey) and higher density calcium carbonate (light grey) at the node-internode connection, viewed in Fiji26 (v2.3.0). Each coral volume image set consists of numbered images that are sequentially stacked to create the three-dimensional coral model.
Data Records
All data records (in addition to information regarding data structure, file names, and folder structure) listed in this section are available at the Polar Data Centre35. To override the default maximum number of displayed files (n = 1000) in each sub-directory, add the following string “&max = N” to the end of the repository URL, where “N” is the number of files you would like to access. Computed tomography three-dimensional 8-bit volumes have been converted to stacked tagged image file format (TIFF) images with associated dimension data (image width, image breadth, stack height) and scan information presented in portable document format reports (pdfs) to enable access by multiple processing programs. There are five sets of images for A. arbuscula complete structure (n = 5), five sets of images for A. arbuscula central branch (n = 5) and 4 sets of images for Keratoisis sp. (n = 4).
Technical Validation
µ-CT calibration
Regular quality assurance inspections are carried out on the µ-CT scanner to verify its metrological and geometrical (alignments) accuracy for conducting the scans. The geometry of source to object and source to detector distances are verified whenever there is any significant physical interaction with the source such as re-alignment, change of filament, or source anode change. This calibration process involves scanning a specially designed phantom known as an ‘hourglass’36, which consists of three pairs of high-sphericity spheres. The sphere sizes are as follows: two spheres with a diameter of 3.000 mm, two spheres with a diameter of 6.000 mm, and two spheres with a diameter of 9.525 mm, and each sphere is kept in contact with its size-counterpart. By using this phantom, it becomes possible to accurately determine a known distance, specifically the centre-to-centre distance of the spheres, in a threshold-independent manner. If the measured distance deviates beyond the acceptable limits of metrological accuracy, the system’s calibration parameters are adjusted to ensure agreement between the measured distance and the actual distance.
Usage Notes
The software options suitable for analysing the data files range from open-source suites, such as Fiji/ImageJ26, ITK Snap37 or HOROS® (The Horos Project) to commercial software suites such as VGSTUDIO MAX (Volume Graphics), Avizo® (Thermo Fisher Scientific), Simpleware (Synopsys Inc), OsyriX® (Pixmeo), or Dragonfly (Object Research Systems). For instructions on how to open the files please refer to the user manual of the software chosen. The toolbox containing the custom written macro “AutomaticConcatenationPlusIntensityEqualisation” has a file which summarises the functionality of each script and gives an overview of the options available for each script34.
Code availability
The code used for the concatenation of scans is available as part of the XRH toolbox at https://doi.org/10.5281/zenodo.1114875234.
Concatenation code description
A high-level overview of the custom concatenation code is given below. This can be used as template to reproduce the code in any language the reader is more familiar with.
Start
1. Prompt user to select the “BOTTOM” stack and store its title and bit depth.
2. Prompt user to select the “TOP” stack and store its title and bit depth.
3. Set measurements for analysis.
4. Create a dialog box to configure options.
5. Retrieve selected options from the dialog box.
6. If bit depths are different, display error message and exit.
7. If manual XY translation option is selected:
a. Set the measurement tool to a point.
b. Prompt the user to select a point of alignment in the “btm” stack and measure its coordinates.
c. Prompt the user to select a point of alignment in the “top” stack and measure its coordinates.
d. Calculate the translation values and convert them to pixel units.
e. Translate the “top” stack using the calculated translation values.
8. If automatic slice selection option is selected:
a. Prompt user to navigate to fusion point in “btm” stack.
b. Create reference image from selected slice.
c. Normalize reference image.
d. Normalize each slice in “top” stack.
e. Subtract reference image from “top” stack.
f. Calculate standard deviation for each slice.
g. Find slice with minimum standard deviation.
9. If manual slice selection option is selected:
a. Prompt user to navigate to fusion point on both “top” and “bottom” volumes and retrieve the slice numbers.
10. Create duplicates of “btm” and “top” stacks by cropping btm volume between “slice 1” up to the “selected slice”, and “top” volume from “selected slice” up to “last slice”
11. Perform intensity calibration by sampling ROIs and fitting a straight line.
12. Apply intensity calibration parameters to “top” stack.
13. Concatenate cropped “btm” and cropped & calibrated “top” stacks into single stack.
14. Perform preview concatenation by creating radial reslice.
End
References
Robinson, L. F. et al. The geochemistry of deep-sea coral skeletons: A review of vital effects and applications for palaeoceanography. Deep Sea Res. Part I Oceanogr. Res. 99, 184–198 (2014).
Thresher, R. E., Fallon, S. J. & Townsend, A. T. A. “core-top” screen for trace element proxies of environmental conditions and growth rates in the calcite skeletons of bamboo corals (Isididae). Geochim. Cosmochim. Acta 193, 75–99 (2016).
Pierrejean, M. et al. Influence of Deep-Water Corals and Sponge Gardens on Infaunal Community Composition and Ecosystem Functioning in the Eastern Canadian Arctic. Front. Mar. Sci. 7 (2020).
Hill, T. M. et al. Temperature and vital effect controls on bamboo coral (Isididae) isotope geochemistry: A test of the “lines method”. Geochem. Geophys. Geosystems 12, Q04008 (2011).
Farmer, J. R., Robinson, L. F. & Honisch, B. Growth rate determinations from radiocarbon in bamboo corals (genus Keratoisis). Deep-Sea Res. Part I-Oceanogr. Res. Pap. 105, 26–40 (2015).
Tambutté, E. et al. Morphological plasticity of the coral skeleton under CO2-driven seawater acidification. Nat. Commun. 6, 7368 (2015).
Li, Y. et al. The 3D Reconstruction of Pocillopora Colony Sheds Light on the Growth Pattern of This Reef-Building Coral. iScience 23, 101069 (2020).
Alvarez-Filip, L., Côté, I. M., Gill, J. A., Watkinson, A. R. & Dulvy, N. K. Region-wide temporal and spatial variation in Caribbean reef architecture: is coral cover the whole story? Glob. Change Biol 17, 2470–2477 (2011).
Odum, H. T. & Odum, E. P. Trophic structure and productivity of a windward coral reef community on Eniwetok Atoll. Ecol Monogr. 25, 291–320 (1995).
Li, Y. et al. Micro‐CT reconstruction reveals the colony pattern regulations of four dominant reef‐building corals. Ecol. Evol. 11, 16266–16279 (2021).
Urushihara, Y., Hasegawa, H. & Iwasaki, N. X-ray micro-CT observation of the apical skeleton of Japanese white coral Corallium konojoi. J. Exp. Mar. Biol. 475, 124–128 (2016).
Gutiérrez-Heredia, L., D’Helft, C. & Reynaud, E. G. Simple methods for interactive 3D modeling, measurements, and digital databases of coral skeletons. Limnol. Oceanogr. Meth. 13, 178–193 (2015).
Crook, E. D., Cohen, A. L., Rebolledo-Vieyra, M., Hernandez, L. & Paytan, A. Reduced calcification and lack of acclimatization by coral colonies growing in areas of persistent natural acidification. Proc. Natl. Acad. Sci. USA 110, 11044–11049 (2013).
Corbera, G. et al. Glacio-eustatic variations and sapropel events as main controls on the Middle Pleistocene-Holocene evolution of the Cabliers Coral Mound Province (W Mediterranean). Quat Sci Rev. 253, 106783 (2021).
Morales Pinzon, A. et al. A semi-automatic method to extract canal pathways in 3D micro-CT images of Octocorals. PLoS One 9, e85557 (2014).
Mollica, N. R. et al. Skeletal records of bleaching reveal different thermal thresholds of Pacific coral reef assemblages. Coral Reefs 38, 743–757 (2019).
Reznikov, N., Buss, D. J., Provencher, B., McKee, M. D. & Piche, N. Deep learning for 3D imaging and image analysis in biomineralization research. J. Struct. Biol. 212, 107598 (2020).
Lu, X. Z., Lai, C. P. & Chan, L. C. Novel design of a coral-like open-cell porous degradable magnesium implant for orthopaedic application. Mater. Des. 188, 108474 (2020).
Chen, D. A., Ross, B. E. & Klotz, L. E. Lessons from a Coral Reef: Biomimicry for Structural Engineers. J. Struct. Eng. 141 (2015).
DeCarlo, T. M. & Cohen, A. L. Dissepiments, density bands and signatures of thermal stress in Porites skeletons. Coral Reefs 36, 749–761 (2017).
DeCarlo, T. M. & Cohen, A. L. coralCT: software tool to analyze computerized tomography (CT) scans of coral skeletal cores for calcification and bioerosion rates. Zenodo https://doi.org/10.5281/zenodo.3402343 (2019).
Fordyce, A. J. et al. Understanding decay in marine calcifiers: Micro‐CT analysis of skeletal structures provides insight into the impacts of a changing climate in marine ecosystems. Methods Ecol. 11, 1021–1041 (2020).
Williams, T. J. et al. Geochemical proxies for deep-sea temperature and nutrient content in cold-water bamboo corals. Chem. Geol. 654, 122053 (2024).
Li, Y., Liao, X., He, C. & Lu, Z. Calcium Transport along the Axial Canal in Acropora. Diversity 13, 407 (2021).
Geiger, F. et al. VEGF producing bone marrow stromal cells (BMSC) enhance vascularization and resorption of a natural coral bone substitute. Bone 41, 516–522 (2007).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Gilkinson, K., & Edinger, E. (Eds.) The ecology of deep-sea corals of Newfoundland and Labrador waters: biogeography, life history, biogeochemistry, and relation to fishes. Can. Tech. Rep. Fish. Aquat. Sci. 2830. vi + 136 (2009).
Hiltz, E., Fuller, S. D. & Mitchell, J. Disko Fan Conservation Area: a Canadian case study. Parks 24, 17–30 (2018).
Roff, J. C. et al. Marine Ecological Conservation for the Canadian Eastern Arctic (MECCEA) – a Systematic Planning Approach for Identifying Priority Areas for Conservation. 281 + xxii (2020).
Fuller, S. D., Perez, F. J. M., Wareham, V. & Kenchington, E. Vulnerable Marine Ecosystems Dominated by Deep-Water Corals and Sponges in the NAFO Convention Area. Report No. N5524, (Northwest Atlantic Fisheries Organization, 2008).
Neves, Bd. M. et al. Deep-water bamboo coral forests in a muddy Arctic environment. Mar. Biodivers. 45, 867–871 (2014).
Johannes, R. E. & Wiebe, W. J. Method for determination of coral tissue biomass and composition. Limnol. Oceanogr. 15, 822–824 (1970).
Katsamenis, O. L. et al. A high-throughput 3D X-ray histology facility for biomedical research and preclinical applications. Wellcome Open Res. 8, 366 (2023).
Katsamenis, O. L., Basford, P. J. & Robinson, S. K. muvis-tomography/XRH-processing-toolbox (v0.2). Zenodo https://doi.org/10.5281/zenodo.11148752 (2023).
Williams, T. J. et al. High-resolution three-dimensional images of deep-sea bamboo corals Acanella arbuscula and Keratoisis sp. collected in the Baffin Bay and Davis Strait during the CCGS Amundsen expedition in 2021 (Version 1.0) NERC EDS UK Polar Data Centre. https://doi.org/10.5285/76534D05-BA4E-47B7-9685-0A8C88404EF2 (2023).
Carmignato, S., Pierobon, A., Rampazzo, P., Parisatto, M. & Savio, E. CT for Industrial Metrology – Accuracy and Structural Resolution of CT Dimensional Measurements. Nondestruct. Test. Evaluation 17(12) (2012).
Yushkevich, P. A. & Gerig, G. ITK-SNAP: An Intractive Medical Image Segmentation Tool to Meet the Need for Expert-Guided Segmentation of Complex Medical Images. IEEE Pulse 8(4), 54–57 (2017).
Acknowledgements
Supported by a National Environmental Research Council Funded (INSPIRE) PhD [grant number NE/S007210/1, 2019–2027, awarded to T.J.W], the National Research Facility for Lab X-ray CT (NXCT) [EPSRC grant number EP/T02593X/1], the European Research Council Advanced Grant Microns2Reefs [grant agreement ID 884650, awarded to G.L.F.] and a grant from NVIDIA [utilized an NVIDIA RTX A6000]. For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission. We acknowledge the Université Laval for additional support that allowed participation in the 2021 Amundsen expedition onboard the Canadian research icebreaker CCGS Amundsen. We thank Keith Tamburri (CSSF-ROPOS), Peter Lockhart (CSSF-ROPOS), Barry Brake (CSSF-ROPOS), Dr. Evan Edinger (Memorial University of Newfoundland), Dr. Bárbara de Moura Neves (Fisheries and Oceans Canada) and Guillaume Blais (Université Laval) for assisting in the collection and processing of the coral specimens. We thank the scientific personnel and crew of the 2021 Amundsen expedition, and the Amundsen Science Inc. team, which is supported by the Canada Foundation for Innovation, for their assistance and logistical support leading up to, during and after the expedition. We appreciated the feedback and insightful comments received from the editor and reviewers which improved the clarity of our manuscript. The views expressed in this publication do not necessarily represent the views of Amundsen Science or that of its partners.
Author information
Authors and Affiliations
Contributions
T.J.W. participated in the 2021 Amundsen expedition and collected, processed and transported the corals to the University of Southampton for μCT. P.J.B. and O.L.K. developed the imaging protocol and acquired the three-dimensional coral volumes using µCT. T.J.W. processed the coral volumes with assistance from O.L.K. and P.J.B. T.J.W. and M.S. prepared the manuscript. P.J.B. and O.L.K. provided details on methods and technical validation. All authors provided input on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Williams, T.J., Basford, P.J., Katsamenis, O.L. et al. Three-dimensional reconstruction of high latitude bamboo coral via X-ray microfocus Computed Tomography. Sci Data 11, 602 (2024). https://doi.org/10.1038/s41597-024-03396-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41597-024-03396-9
- Springer Nature Limited