Abstract
In 2003, the pest species Bactrocera dorsalis (Hendel) was reported for the first time in Kenya, Africa, and subsequently on many other African countries. In this work, 20 locations along the Rusitu Valley (Zimbabwe) were sampled in 2014 during the sweet oranges fruiting seasons, to verify the fruit fly taxonomy, invasion source, population dynamics, and fruit damage. The trapped fruit flies were identified using morphological traits and molecular techniques, as B. dorsalis. The haplotype network analysis revealed that Zimbabwe COI sequences were identical to other African B. dorsalis sequences. Fruit fly trappings per day varied during the year, although it remained always high. The same applies to fruit damage, most likely due to the permanent availability of cultivated and wild fruit varieties during the year. Rusitu Valley was invaded by B. dorsalis, most likely from neighbouring countries. Ten years after the first report in Kenya, the complete or near complete invasion of Africa has been achieved by B. dorsalis. In northern Africa the distribution is clearly limited by the Sahara desert. The large population size, the polyphagous nature of the species, and the continuous availability of suitable host fruit species during the year complicates the eradication of this species.
Similar content being viewed by others
Introduction
Fruit and vegetable production is one of the fast-growing agricultural sectors in Zimbabwe, providing income to farmers. One of the major fruit crops which strengthen the livelihood of many farmers in Zimbabwe is sweet orange (Citrus sinensis). Oranges are mainly grown in areas within or surrounding Rusitu, Limpopo, Save, and Mazowe Valleys in Zimbabwe. The production of oranges in Zimbabwe is mainly for consumption as fresh fruit and/or juice in domestic and export markets. Orange production in Zimbabwe has been increasing since 1980, reaching a peak of 116078 tonnes in 20031. Nevertheless, orange production declined to 97512 tonnes in 20111, mainly because of challenges related to poor pre-and post harvest management2. The decline was further exacerbated by the recent invasion of Zimbabwe by alien fruit flies (Diptera: Tephritidae), known as African invader fruit flies (B. dorsalis (Hendel)) in 20103. Although the presence of B. dorsalis (Hendel) in Zimbabwe was first recorded in 20103, it was only recognized in 2012 by the government’s Plant Quarantine Services Institute [Chikwenhere, G., personal communication]. The B. dorsalis species was first reported in Kenya in 20033,4, but, it has already become a pest of major concern to fruit growers in many parts of Africa.
The African invader fruit fly species belongs to B. dorsalis complex and is devastating to crops causing millions in lost production each year5,6,7,8,9. The Bactrocera genus harbours over 75 species with broad but, essentially allopatric distributions with regions of transition occurring around the south east of Asia10,11,12,13,14. Studies have revealed that amongst these species, the most damaging one is B. dorsalis15,16,17 and there are some morphological differences in their populations7,8,9,10.
Since its arrival in Africa, B. dorsalis is believed to have rapidly expanded its range at an alarming rate. It is now reported to have spread throughout the equatorial, tropical and subtropical regions of Africa but, not yet reported in Malawi, Somalia, and Lesotho3. This rapid expansion is likely due to its polyphagous nature and high reproductive potential. Indeed, B. dorsalis is known to attack at least 46 host plants, including many commercially grown fruit crops such as mango, oranges, guava, cucurbit, papaya, and avocado, as well as many other species indigenous to Africa15,17,18,19. In some African regions, such as Tanzania, Kenya, Benin, and Cameroon, B. dorsalis is now the major pest on host species such as mangoes, having displaced the native Ceratitis species as the main pest16,17,20. Reports indicate that horticultural yield losses averaging 15–50% are caused by B. dorsalis in several African countries21. The damaging activity of B. dorsalis is mainly due to female oviposition since they use their ovipositor to lay eggs in clutches under the skin of the fruit22. By this process, spoilage microbes are introduced into the fruit causing the fruit to breakdown and rot. Once in the fruit, the eggs hatch into larvae or maggots. It is the decaying flesh that provides food for the larvae or maggots. When fully grown, the larvae escape from the fruit, burrow into the soil or organic matter and transform into pupae. Twenty days after eggs are laid the adult fruit fly emerges from the puparium22,23, and the cycle restarts.
The details of the African invasion are currently being worked out but, so far, two clear main outbreaks have been recognized, with an east African origin, likely in Kenya and Tanzania24. There is an evident absence of geographical structure across Africa, with the exception of Nigeria that could represent a third independent outbreak24. It should be noted that, according to other studies13,19, there are likely suitable habitats for B. dorsalis in other regions of the world such as the neo-tropics, Europe and Australia, and outbreaks have been reported in California, USA, in 2006 and 201225. Understanding how Africa has been invaded and how this species has become established in a large geographic region may help in the formulation of strategies to eradicate this species from Africa and help prevent future invasions of other geographic regions.
Evidence for introgression between B. dorsalis and B. kandiensis has been previously reported8. Introgression between the Australian species B. tryoni and B. neohumeralis has been proposed as a potential adaptive mechanism allowing the expansion of B. tryoni into new climatic regions26, and thus the same could be true for B. dorsalis. B. kandiensis is recorded, so far, from Sri Lanka only, but, it may occur sympatrically with B. dorsalis in India and Myanmar8. The source of the two clear east African outbreaks is very likely Asian countries east of Sri Lanka, India and Myanmar, since only a very small percentage of African flies, show evidence of introgression. Verification of the taxonomy, population dynamics, fruit damage, and invasion source of B. dorsalis populations that have invaded Zimbabwean territories is critical for development of management, control and/or eradication measures. Understanding the fruit fly population dynamics and invasion pathway will also assist in drawing the population structure of B. dorsalis complex. In turn, this will help in the implementation of global policies which reduce the expansion of this species. Therefore, in this work we identified the fruit fly species in Zimbabwe’s Rusitu Valley, determined possible sources of the invasion, and established the population dynamics and fruit damage in this region.
Results and Discussion
Fruit fly species identification
The male fruit fly specimens from Rusitu Valley possessed a very narrow coastal band and anal streak, scutum which is dark orange-brown with a black lanceolate pattern, narrow lateral post-sutural vittae and abdominal tergites III–V with a dark ‘T’ pattern and narrow dark lateral markings on all three terga, as shown in Fig. 1. These morphological features are congruent with the identification key for B. dorsalis4.
The right-hand wings of the trapped fruit flies were also analysed and found to be compatible with the 15 wing landmarks identification key for B. dorsalis7 (Table 1; Supplementary Fig. S1). A mean wing length of 4.3 mm (±0.2) was observed for Zimbabwean populations of B. dorsalis, slightly lower than 6 mm and 7.3 mm observed, respectively for B. dorsalis of Asian and American origin27,28. This also supports the idea of morphological differences across B. dorsalis complex as recently observed by several authors5,7,8,9,29. It should be noted that about 16% of the sampled individuals have a broken dorsal stripe thus, resembling B. kandiensis.
Confirmation of fruit fly identification and invasion pathway
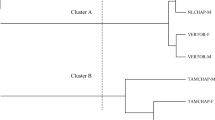
In order to have a molecular confirmation of the species identification and infer the source of Zimbabwe population as an independent invasion or from neighbouring countries, the mtDNA COI region was amplified, cloned and sequenced from 11 randomly chosen individuals (assigned as B in Fig. 2a). Additional sequences were retrieved from GenBank to perform a Haplotype Network analysis (since mtDNA is non-recombining) with 533 sequences for which the origin of the sample is declared in GenBank nucleotide records (the alignment is 658 bp long; it also includes a non-random sample of six individuals from Zimbabwe (assigned as Bbs in Fig. 2a; Supplementary Fig. S2). All 11 sequences from Zimbabwe flies cluster into two large groups, namely, six sequences are identical to 101 B. dorsalis sequences available in GenBank, four are identical to 129 B. dorsalis sequences available in GenBank and one sequence shows three nucleotide differences from the latter group. Therefore, the collected flies are clearly B. dorsalis. Moreover, all Zimbabwean B. dorsalis samples cluster into the two clades that likely represent the two original main outbreaks with an east African origin, probably from Kenya and Tanzania24. Therefore, the source of the Zimbabwean invasion is likely from neighbouring countries and not from an independent invasion. Kenya and Tanzania are the most probable source of Zimbabwean invasion since several fruit fly outbreaks were recorded earlier in these two countries. In addition, no control and/or management programs were implemented in these countries to combat the African invader fruit flies3,15. It should be noted that there is an evident absence of geographical structure in Africa, with the exception of Nigeria that could represent a third independent outbreak24. Our analyses support this hypothesis since 10 out of 11 B. dorsalis sequences from Zimbabwe are identical to B. dorsalis sequences already reported from other African regions. As expected, given that the two original invasions likely involved a small number of individuals, for the Zimbabwe population, polymorphism levels are low (π = 0.0038; Θ = 0.0031; 11 sequences analysed presenting 6 segregating sites). Similar values have been reported using other molecular markers for African populations30. Invasive populations in Africa and also in Hawaii are those presenting lower genetic diversity. In our sample Tajima’s D is, however, non-significant (D = 0.96038; P > 0.05) showing that there is no excess of rare polymorphisms as expected under a scenario of population growth. Similar results are reported for another African sample for different loci30. The low polymorphism level implies that most sequences are identical to those already obtained or differ by a few mutations, thus, a very large sample would not be more informative.
Partial view of the TCS haplotype network of 533 Bactrocera sequences. (a) The Zimbabwean sample (B–random sample; Bbs–sample resembling B. kandiensis). (b) The B. kandiensis sample. The full image is available as Supplementary Fig. S2.
In our haplotype network analysis, Nigerian B. dorsalis samples cluster with B. kandiensis samples and with B. dorsalis samples from Sri Lanka, India, and Myanmar (Fig. 2b; Supplementary Fig. S2). This observation is compatible with the evidence for introgression that has been reported between B. dorsalis and B. kandiensis8, but also shows that the Nigerian population is the result of an independent invasion from Sri Lanka, India or Myanmar. This could also explain why there is an evident absence of geographical structure across Africa, with the exception of Nigeria24. The limited distribution of the individuals showing evidence for introgression suggests that in contrast to what happens with B. tryoni in Australia26, it is unlikely that these individuals have any advantage that would allow their expansion.
While performing the phenotypic characterization, we noted that about 16% of the sampled individuals have a broken dorsal stripe thus resembling B. kandiensis. Since these individuals are not present at a very high frequency, it was possible that such individuals were not represented in the random sample of 11 individuals that we analyzed molecularly. Therefore, we also characterized six individuals with a broken dorsal stripe using molecular methods. Nevertheless, the network analysis that we performed clearly shows that this biased sample is not very different from the random sample that was originally characterized (Fig. 2a; Supplementary Fig. S2). Therefore, there is no evidence for introgression in the Zimbabwean sample.
The positive morphological and molecular identification of Zimbabwe fruit flies as B. dorsalis allows us to join one more piece into the puzzle of the African invasion by this species. Figure 3 shows where and when, in Africa, B. dorsalis has been reported. With the exception of Lesotho, Malawi, and Somalia, B. dorsalis has now been reported in every African country where suitable habitat has been predicted for this species19. It should be noted that it is unclear whether in Lesotho, Malawi, and Somalia there was an attempt to look for B. dorsalis. Therefore, 10 years have been enough for the complete or near complete invasion of Africa by this species, since in northern Africa the distribution is clearly limited by the Sahara desert. In some regions, such as Tanzania, B. dorsalis is now the major pest species in hosts such as mangoes, having displaced the native Ceratitis species as the main pest16,17. Since B. dorsalis African invasion appears to be the result of a single recent invasion, with little or no subsequent gene flow with source populations in South Asia9,24,30, it is unexpected to see marked morphological and biological differences between these geographic populations.
Fruit fly population dynamics and fruit damage
Zimbabwean farmers from the Rusitu Valley also perceive that fruit flies are causing havoc in post harvest management of their fruits. The livelihood of most rural district population in Rusitu Valley depends on fruit crop production. One of the major fruit crops which strengthen their livelihood is sweet orange. Reports indicate that farmers are facing orange postharvest losses estimated to be more than 30% and most of these losses are being caused by fruit flies2. Therefore, in this work, we also looked at the population dynamics of B. dorsalis and fruit damage during the time of sweet orange ripening. There are differences in the number of fruit flies trapped per day (FTD) during the four months in which farmers harvest sweet oranges (Fig. 4a). December had the highest FTD in all the trap positions (Fig. 4b) and this could be justified by prevailing weather conditions of relatively high humidity and temperature. Indeed, in 2014, April and June recorded a mean temperature of 14 °C, September 16 °C, and December was humid with a mean temperature of 19 °C (Fig. 4b). A statistically significant positive correlation (r = 0.685; P < 0.05) existed between FTD and temperature. The weather conditions prevailing in December coupled with the fruiting of mango fruits can be linked to higher FTD. Previous studies by other researchers supported evidence that fluctuations in temperature in horticultural agro-ecosystems help to explain shifts in dominance of B. dorsalis in many parts of Africa31. The locations 16, 17, 18, 19, and 20 positioned to the South-western side of the valley recorded low FTD values compared to other trapping locations and this part of the valley is not always evergreen compared to the North-eastern sides (Fig. 4a). In this part of the valley, fruit production is generally low and farmers grow tea and coffee as their main source of livelihood32,33. It is also important to note that the month of April recorded very low FTD values, lower than June, September, and December (Fig. 4b). This could be linked to decreasing temperature and humidity since previous studies have shown that fruit flies change behaviour in preparation for late summer34,35. Another factor that could have contributed to low FTDs in April is a shift in the availability of quality food for the fruit flies. Although B. dorsalis is a polyphagous species, they do prefer mango fruits more than oranges17,20. In April, in Rusitu Valley, only local varieties of oranges will be ripening unlike in other studied months. Spatial variation existed in FTD (Kruskal-Wallis Test; P < 0.05, DF = 3), as well as temporal variation (Friedman ANOVA; F = 573.812, P < 0.05, DF = 3). Likewise, the Mann-Whitney and Wilcoxon pair wise comparison tests confirmed significant subtle spatio-temporal differences (P < 0.05) in FTD for April versus June, April versus September, and April versus December. However, there were no pair wise statistical differences in FTD for December versus June, and December versus September.
Fruit flies per trap per day (FTD) and weather data. (a) FTD data for all locations (assigned as L1 to L20) for the months of April (black line with x), June (grey line with squares), September (black dotted line with triangles), and December (grey dotted line with dots). (b)Monthly averages of FTD (in black), mean temperature (°C; in white), mean rainfall (cm; in grey) for April, June, September, and December.
When looking to fly infestation, a picture similar to that obtained for the FTD analysis is obtained (Fig. 5). The means for infestations were 80.50% (N = 20, standard error of 1.02) and 73.75% (N = 20, standard error of 2.51) for June and December respectively. Therefore, although December had a higher density of fruit flies than June, the percentage of fruits showing ovipositor marks was higher in June than in December (Mann-Whitney test; P < 0.05; Fig. 5). The high infestation rates imply a loss in orange production since most fruit damage is caused during oviposition by the introduction of spoilage microbes into the fruit. The ovipositor marks also reduce the aesthetic value of oranges at markets.
Despite the high infestation rates, a low number of adults emerged per fruit were observed (Fig. 6). The mean number of emerged adults/fruit was 0.445 ± 0.118 and 0.258 ± 0.116 adults/fruit, respectively for the months of June and December. These values are significantly different (T-test; P < 0.0001 at α = 0.05, t = 4.955 with 38 degrees of freedom). The assumption that the data were sampled from populations that follow Gaussian distribution was tested using the Kolmogorov and Smirnov (KS) method (P > 0.10) for both June and December. The results support the idea that sometimes fruit flies make pseudo-punctures (punctures without eggs) which increases post harvest losses through a reduction in the market value of fruits36. These results also support the suggestion that oranges are possible hosts for B. dorsalis though they are not the most preferred17. More studies are needed to determine the most preferred hosts of B. dorsalis in the valley.
Conclusions
In conclusion, Rusitu Valley has been colonised by B. dorsalis, with high numbers of individuals observed during the year that cause a high degree of damage in fruits. The high numbers of individuals are likely maintained by the permanent availability of cultivated and wild fruit varieties along the year. In light of study findings, the invasion of Zimbabwe’s Rusitu Valley was not an independent invasion. The source of the invasion in Zimbabwe is likely from neighbouring countries. Zimbabwe’s invasion pathway is linked to the two reported clear east African outbreaks whose source was revealed to be Asian countries east of Sri Lanka, India, and Myanmar. It can be noted that, ten years after the first report in Kenya of B. dorsalis, the complete or near complete invasion of Africa has been achieved since in northern Africa the distribution is clearly limited by the Sahara desert. The large population size, the polyphagous nature of the species, and the availability of suitable fruits along the year make it difficult to fight this species.
Materials and Methods
Description of the study area
Zimbabwe’s Rusitu Valley (20°S 032°E), on an altitude of about 460 m above sea level, receives moderately high rainfall (>1000 mm) and temperatures (>19 °C) almost throughout the year, thus the valley is characterised by a warm and humid climate32,37. The soil particles are well graded and consolidated making them less vulnerable to erosion, enabling farmers to plough and grow fruit crops on slopes and hilly places38. These conditions make Rusitu Valley suitable for the production of a wide variety of fruits which include bananas, oranges, mandarins, and mangoes.
Study approach
The study addressed three important issues in Rusitu Valley’s sweet orange production chain. These are: (1) identification of fruit fly species, (2) determination of possible sources of the invasion, and (3) establishing the population dynamics and fruit damage in Rusitu Valley, Zimbabwe. Permission for field studies in Rusitu Valley was sought from communal traditional leaders.
Data sources
The fruit fly infestation, emergence, and trapping experiments were all carried out from the four sweet orange producing wards in Rusitu Valley to constitute a sampling block (Fig. 7). From the sampling block, twenty locations were selected using snowball sampling technique because of the steep and hilly terrain in the Rusitu Valley that made access to a random sample extremely difficult39. These locations were backyard orchards less than half of an acre (>2023.5 m2) and each location had on average 25 trees. At these locations, trapping measurements were done once per month for four months. Infestation and emergence measurements were done once per month for two months. The assumption was that weather conditions were uniform across the sampling block and that they were varying according to month of the year. Weather data used in the study were drawn from Chisengu Weather Station records available online; https://www.wunderground.com/zw/chisengu.
Trapping of B. dorsalis male fruit flies
Unlike banana cultivars which are available throughout the year, orange fruiting seasons in Rusitu Valley of Zimbabwe is in April, June, September, and December depending on the orange cultivars. Thus in this study, trapping was performed in 2014, on orange trees in these four months. Mango fruits are only available from December up to early February also depending on the cultivars40, and thus flies collected in April, June and September are likely to represent a good sample of fruit flies infesting mainly oranges. Nevertheless, B. dorsalis species are highly polyphagous, and thus we cannot exclude the possibility that these flies are completing their life cycle on other Zimbabwean native plant species as well. In order to trap flies, methyl-eugenol (here after ME) which is a known para-pheromone that has a powerful attraction to the adult male fruit flies of the B. dorsalis complex was used. Twenty traps were prepared using locally available empty 2 litre polyethylene (PET) bottle containers. Each bottle was cleaned thoroughly with water and cut at the circular/curved position which signified the starting point of the cone so that the cone is inverted and placed into the container with the lid opening pointing towards the base of the container. The flashing cut edges were sealed using a strong adhesive cellulose tape. Two holes (~3 cm × 2 cm) were cut on the sides of the container at an orientation directly opposite to each other. A solution of ME and Malathion 50% EC was prepared (5 ml ME + 5 ml Malathion 50% EC + 990 ml water) and a cotton swab dipped into the solution for one minute. The cotton swab was then placed into the container to constitute a trap, which was then hung onto the canopy of the host orange fruit tree (Supplementary Fig. S3). One trap was placed per study location. Trapped flies were collected and counted after 30 days of setting the trap. The counted flies were pooled and kept in a dry state at room temperature in the Post harvest Laboratory, Department of Crop Science and Postharvest Technology at Chinhoyi University of Technology, Zimbabwe for use in morphological and molecular identification experiments.
Morphological description of fruit flies
A total of 100 fruit fly specimens were randomly selected from the pooled population of fruit flies. The specimens were examined using stereomicroscope (Nikon SMZ1000) for the three major B. dorsalis discriminating morphological features which are: clear wings with a continuous dark coastal band, two yellow stripes on the thorax, and a dark T-shaped marking on abdomen4,7. The right-hand wings of the 100 specimens were also analysed for wing length40 and 15 wing landmarks identification key7 (Supplementary Fig. S1), using Nikon NIS-Elements version 3.2 (Nikon Instruments, Japan).
Confirmation of fruit fly identification and invasion sources
DNA extraction and PCR amplification
A sample of 11 individual fruit flies was randomly selected from the pooled population41. Six individuals having a broken dorsal stripe, thus resembling B. kandiensis, were also analysed, to address introgression in Zimbabwe. The genomic DNA of each selected individual was extracted using the QIAamp DNA Mini Kit (250) as per manufacturer’s instructions (Quiagen, Germany). The genomic DNA was stored at −20 °C.
The PCR experiments were carried out using the universal primers developed by Folmer et al.42; primers LCO (5′-1490GGTCAACAAATCATAAAGATATTGG-3′) and HCO (5′-2198TAAACTTCAGGGTGACCAAAAAATCA-3′) to amplify an approximately 700 base pair fragments of the COI gene. The reaction mixture of 20 µl for each sample consisted of 9.2 µl H2O, 2 µl Buffer (10X), 2 µl of dNTP mix (100 mM), 1 µl of Forward primer (100 mM), 1 µl of Reverse primer (100 mM), 2.4 µl MgCl2 (25 mM), 0.4 µl Taq DNA polymerase (5 U/µl) and 1 µg of genomic DNA. Standard cycling conditions were 96 °C for 2 minutes for initial denaturation of DNA, followed by 40 cycles of denaturation at 95 °C for 30 seconds; 50 °C for 45 seconds and 72 °C for 2 minutes respectively, and strand extension at 72 °C for 5 minutes. PCR products were separated by electrophoresis on a 1.5% agarose gel in 1 × TBE buffer (pH 8.0) and the DNA amplification products were visualised using GreenSafe Premium (Nzytech) staining, and a transilluminator. DNA was extracted from the gel using the QIAEX II Gel Extraction kit as per manufacturer’s instructions (Quiagen, Germany), and kept at −20 °C for cloning. The TOPO TA Cloning Kit for Sequencing (Invitrogen, California, USA) was used to clone the DNA fragments according to the manufacturer’s instructions. Plasmid DNA of three colonies was extracted using NZY Mini prep kit according to the manufacturer’s instructions (NZY tech, Portugal), and sequenced at StabVida (Lisbon) using the M13F and M13R universal primers, and the ABI Big Dye v1.1 chemistry (Applied Biosystems Europe, Spain) chemistry, as recommended by the manufacturers. Chromatograms were analysed using PROcessor of SEQuences version 2.9143, and for each individual, a consensus sequence was obtained in order to correct for possible errors that may occur during the PCR amplification step. COI gene sequences were submitted to GenBank.
Sequence analysis and inference of invasion sources
The 17 Zimbabwe sequences and 533 GenBank sequences from all over the world were aligned with Clustal Omega44. The haplotype network technique was used to infer the invasion sources of fruit flies in Zimbabwe. The haplotype network was obtained using the TCS method as implemented in PopArt (http://popart.otago.ac.nz)45. Summary statistics were calculated using DNAsp31.
Fruit fly population dynamics and fruit damage
Fruit fly per trap per day
The number of flies per trap per day (hereafter FTD) was calculated using the following formula46:
Infestation experiments
The infestation experiments were carried out in all the 20 locations during the months of June and December. A total of 20 mature oranges were randomly selected from orange trees at each study location avoiding old fallen fruits to reduce the possibility of picking fruit damaged by other ground-dwelling insects. Therefore, a total of 400 fruits were used for infestation experiments and they were not discarded but, kept for adult emergence experiments. Each selected fruit was visually examined with the aid of a hand lens to detect fresh oviposition marks that are not visible to the naked eye. The number of fruits showing oviposition marks was determined and the percentage infestation was calculated using the following formula46:
Adult fly emergence experiments
Sweet orange fruits were weighed to determine the average unit weight before being placed in rearing boxes. For each location, 20 fruits were used and to minimise difficulties in managing the experiments, four fruits were placed in one rearing box prepared using transparent plastic cube box containers and measuring 20 × 10 × 10 cm in length, width, and height respectively. Five rearing boxes were allocated per location. Each rearing container was lined with approximately 8 cm3 of insecticide-free dry sieved sand. Four orange fruits (from the same location) were placed in a rearing container and 1 mm mesh-size rayon cloth was used to tightly cover the container. Elastic rubber bands were used to tighten the rayon cover to prevent flies from entering or escaping the rearing container. These containers were placed in an airy room protected from ants for a period of four weeks and monitored on weekly intervals. At the end of the fourth week, the rearing boxes were carefully examined and all pupae and/or flies were collected. The average number of adult fruit flies that emerged per orange fruit per location was calculated as:
Statistical analysis
Stata1147 was used to determine spatial and temporal heterogeneity (variation) in FTD using non-parametric tests (Kruskal-Wallis and Friedman ANOVA) at 5% level of significance. GraphPad In Stat version 3.10 was used to analyse for normality, correlation and descriptive statistics on FTD, adult emergence, infestation, and weather data48.
Data Availability
The data sets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Faostat. Value of agricultural production; orange production, 2011. Agriculture Organization of the United Nations (2015).
Musasa, S. T., Musundire, R., Mashingaidze, A. B. & Makuza, S. M. A preliminary study of the orange (Citrus sinensis) fruitvalue-chain in Chimanimani Rural District, Zimbabwe. African J. Agric. Res. 10, 3507–3516 (2015).
EPPO. PQR-EPPO data base on quarantine pests. (2016). Available at, http://www.eppo.int. (Accessed: 3rd April 2016).
Drew, R., Tsuruta, K. & White, I. A new species of pest fruit fly (Diptera: Tephritidae: Dacinae) from Sri Lanka and Africa. African Entomol. 13, 149–154 (2005).
Frey, J. E. et al. Developing diagnostic SNP panels for the identification of true fruit flies (Diptera: Tephritidae) within the limits of COI-based species delimitation. BMC Evol. Biol. 13, 1 (2013).
Goergen, G., Vayssières, J.-F., Gnanvossou, D. & Tindo, M. Bactrocera invadens (Diptera: Tephritidae), a new invasive fruit fly pest for the Afro tropical region: host plant range and distribution in West and Central Africa. Environ. Entomol. 40, 844–854 (2011).
Schutze, M. K. et al. Population structure of Bactrocera dorsalis ss, B. papayae and B. philippinensis (Diptera: Tephritidae) in southeast Asia: evidence for a single species hypothesis using mitochondrial DNA and wing-shape data. BMC Evol. Biol. 12, 1 (2012).
Schutze, M. K. et al. Synonymization of key pest species within the Bactrocera dorsalis species complex (Diptera: Tephritidae): Taxonomic changes based on a review of 20 years of integrative morphological, molecular, cytogenetic, behavioural and chemoecological data. Syst. Entomol. 40, 456–471 (2015).
Schutze, M. K. et al. One and the same: integrative taxonomic evidence that Bactrocera invadens (Diptera: Tephritidae) is the same species as the Oriental fruit fly Bactrocera dorsalis. Syst. Entomol. 40, 472–486 (2015).
Adsavakulchai, A., Baimai, V., Prachyabrued, W., Grote, P. J. & Lertlum, S. Morphometric study for identification of the Bactrocera dorsalis complex (Diptera: Tephritidae) using wing image analysis. Biotropia (Bogor). 13, 37–48 (1999).
Clarke, A. R. et al. Invasive phytophagous pests arising through a recent tropical evolutionary radiation: the Bactrocera dorsalis complex of fruit flies. Annu. Rev. Entomol. 50, 293–319 (2005).
Krosch, M. N. et al. Piecing together an integrative taxonomic puzzle: microsatellite, wing shape and aedeagus length analyses of Bactrocera dorsalis (Diptera: Tephritidae) find no evidence of multiple lineages in a proposed contact zone along the Thai/Malay Peninsula. Syst. Entomol. 38, 2–13 (2013).
Stephens, A., Kriticos, D. & Leriche, A. The current and future potential geographical distribution of the oriental fruitfly, Bactrocera dorsalis (Diptera: Tephritidae). Bull. Entomol. Res. 97, 369–378 (2007).
Tan, K. H., Tokushima, I., Ono, H. & Nishida, R. Comparison of phenylpropanoid volatiles in male rectal pheromone gland after methyleugenol consumption, and molecular phylogenetic relationship of four global pest fruit fly species: Bactrocera invadens, B. dorsalis, B. correcta and B. zonata. Chemoecology 21, 25–33 (2011).
José, L., Cugala, D. & Santos, L. Assessment of invasive fruit fly fruit infestation and damage in Cabo Delgado Province, Northern Mozambique. African CropSci. J. 21, 21–28 (2013).
Mwatawala, M. W., DeMeyer, M., Makundi, R. H. & Maerere, A. Biodiversity of fruit flies (Diptera,Tephritidae) in orchards in different agro-ecological zones of the Morogoro region, Tanzania. Fruits 61, 321–332 (2006).
Mwatawala, M., DeMeyer, M., Makundi, R. & Maerere, A. Seasonality and host utilization of the invasive fruit fly, Bactrocera invadens (Dipt.,Tephritidae) in central Tanzania. J. Appl. Entomol. 130, 449–457 (2006).
Cugala, D., Ekesi, S., Ambasse, D., Adamu, R. & Mohamed, S. Assessment of ripening stages of Cavendish dwarf bananas as host or non‐host to Bactrocera invadens. J. Appl. Entomol. 138, 449–457 (2014).
DeMeyer, M. et al. Ecological niche and potential geographic distribution of the invasive fruit fly Bactrocera invadens (Diptera, Tephritidae). Bull. Entomol. Res. 100, 35–48 (2010).
Ekesi, S., Billah, M. K., Nderitu, P. W., Lux, S. A. & Rwomushana, I. Evidence for competitive displacement of Ceratitis cosyra by the invasive fruit fly Bactrocera invadens (Diptera: Tephritidae) on mango and mechanisms contributing to the displacement. J. Econ. Entomol. 102, 981–991 (2009).
Vayssières, J.-F., Goergen, G., Lokossou, O., Dossa, P. & Akponon, C. A new Bactrocera species in Benin among mango fruit fly (Diptera: Tephritidae) species. Fruits 60, 371–377 (2005).
Ekesi, S., Nderitu, P. & Rwomushana, I. Field infestation, life history and demographic parameters of the fruit fly Bactrocera invadens (Diptera: Tephritidae) in Africa. Bull. Entomol. Res. 96, 379–386 (2006).
Allwood, A., Leblanc, L., Tora, V. E. & Bull, R. Fruit fly control methods for Pacific Island countries and territories. Pest Advisory Leaflet 40 (2001).
Khamis, F. et al. Uncovering the tracks of a recent and rapid invasion: the case of the fruit fly pest Bactrocera invadens (Diptera: Tephritidae) in Africa. Mol. Ecol. 18, 4798–4810 (2009).
Barr, N. B. et al. Genetic diversity of Bactrocera dorsalis (Diptera: Tephritidae) on the Hawaiian Islands: Implications for an introduction pathway into California. J. Econ. Entomol. 107, 1949–1958 (2014).
Lewontin, R. & Birch, L. Hybridization as a source of variation for adaptation to new environments. Evolution (N.Y). 20, 315–336 (1966).
Drew, R. A., Raghu, S. & Halcoop, P. Bridging the morphological and biological species concepts: studies on the Bactrocera dorsalis (Hendel) complex (Diptera: Tephritidae: Dacinae) in south‐east Asia. Biol. J. Linn. Soc. 93, 217–226 (2008).
Weems, H., Heppner, J., Nation, J. & Fasulo, T. R. Oriental Fruit Fly, Bactrocera dorsalis (Hendel) (Insecta: Diptera:T ephritida). Featured Creatures: Entomology and Nematology Entomology Circulars 21 (2012).
Hendrichs, J., Vera, M. T., DeMeyer, M. & Clarke, A. R. Resolving cryptic species complexes of major tephritid pests. Zookeys 540, 5–39 (2015).
Qin, Y. et al. Population structure of a global agricultural invasive pest, Bactrocera dorsalis (Diptera: Tephritidae). Evol. Appl. 11, 1990–2003 (2018).
Librado, P. & Rozas, J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25, 1451–1452 (2009).
Rukuni, M. & Eicher, C. Zimbabwe’s agricultural revolution. (University of Zimbabwe Publications Office, 2006).
Roth, M. & Gonese, F. Delivering land and securing rural livelihoods: Post-independence land reform and resettlement in Zimbabwe. (Centre for Applied Social Sciences, University of Zimbabwe and Land Tenure Center of the University of Wisconsin-Madison, 2003).
Rwomushana, I., Ekesi, S., Ogol, C. K. & Gordon, I. Mechanisms contributing to the competitive success of the invasive fruit fly Bactrocera invadens over the indigenous mango fruit fly, Ceratitis cosyra: the role of temperature and resource pre‐emption. Entomol. Exp. Appl. 133, 27–37 (2009).
Neilsen, D. Fundamentals of temperate zone tree fruit production. In Tree Physiology (ed. Tromp, J., Wertheim, S. J. & Webster, A)1571–1572 Backhuys (2005).
Ganie, S. et al. Population dynamics, distribution, and species diversity of fruit flies on cucurbits in Kashmir Valley, India. J. Insect Sci. 13, 65 (2013).
Vincent, V. & Thomas, R. Anagricultural survey of Southern Rhodesia. Part1. Agro-ecological survey (1960).
Nyamapfene, K. W. The soils of Zimbabwe. (Nehanda Publishers, 1991).
Goodman, L. A. Snowball sampling. Ann. Math. Stat. 32, 148–170 (1961).
Manzou, C. Communal Fruit Production and Processing: the Case of the Rusitu Valley in Chimanimani, 1980–1999. (University of Zimbabwe, 2000).
Trask, J. A. S. et al. The effect of SNP discovery method and sample size on estimation of population genetic data for Chinese and Indian rhesus macaques(Macaca mulatta). Primates 52, 129–138 (2011).
Folmer, O., Black, M., Hoeh, W., Lutz, R. & Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 3, 294–299 (1994).
Filatov, D. A. ProSeq: a software for preparation and evolutionary analysis of DNA sequence data sets. Mol. Ecol. Notes 2, 621–624 (2002).
Remmert, M. et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539–539 (2011).
Clement, M. J., Snell, Q., Walker, P., Posada, D. & Crandall, K. A. TCS: estimating gene genealogies. ipdps (2002).
Prabhat, K. et al. The 1-2-3 of Fruit Fly Population Monitoring (Agroecosystem analysis) Guidelines for IPM farmers and trainers:- Area-Wide Integrated Pest Management of Fruit flies in South and Southeast Asian Countries. (Asian Fruit Fly IPM Project Bangkok, Thailand, 2011).
Statacorp. Stata statistics/data analysis. (StataCorp LP, 2009).
Motulsky, H. GraphPadInStat 3.0 User’s Guide. (GraphPad Software Inc, 1995).
Acknowledgements
The authors would like to thank the project Norte-01-0145-FEDER-000008-Porto Neurosciences and Neurologic Disease Research Initiative at I3S, supported by Norte Portugal Regional Operational Programme (NORTE2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (FEDER) for financing this work. Fruit fly trapping and data collection was financed by the Chinhoyi University of Technology under the Postgraduate Grant PG4001. Travel and stay at the University of Porto was financed by Erasmus Mundus Dream project, Action 2 - STRAND 1, Lot 18, ACP Countries, Master Mobility Scholarship.
Author information
Authors and Affiliations
Contributions
Fruit fly collections were performed by S.T.M. and R.M. The design of the experimental work was performed by S.T.M., C.P.V., A.A.R.M.A. and J.V. All authors contributed in the conceptualization of the manuscript. C.P.V., J.V., A.A.R.M.A., A.B.M., S.T.M. and R.M. contributed in funding acquisition. The formal analyses were done by S.T.M., C.P.V., J.V. and A.B.M. All authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41598_2019_50001_MOESM1_ESM.pdf
Fruit fly identification, population dynamics and fruit damage during fruiting seasons of sweet oranges in Rusitu Valley, Zimbabwe
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Musasa, S.T., Mashingaidze, A.B., Musundire, R. et al. Fruit fly identification, population dynamics and fruit damage during fruiting seasons of sweet oranges in Rusitu Valley, Zimbabwe. Sci Rep 9, 13578 (2019). https://doi.org/10.1038/s41598-019-50001-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-50001-w
- Springer Nature Limited