Abstract
Schizophyllum commune is a ubiquitous basidiomycetous fungus typically found across the world, which has been detected in indoor and outdoor air. Some studies indicated that sensitization to S. commune is correlated with asthma severity in patients. Patients with chronic severe or acute fatal asthma have neutrophil-dominant airway inflammation. We hypothesized that S. commune can exacerbate asthma. To test this hypothesis, we evaluated the direct immunomodulatory activities of S. commune in allergic airway inflammation induced by non-fungal sensitization. Ovalbumin (OVA)-induced asthma model mice were generated using wild-type (WT) and Il-17a−/−Il-17f−/− mice that were intratracheally exposed to S. commune, then immune responses in the lungs were assessed after 24 h. Intratracheal administration of S. commune in OVA-induced asthma model mice enhanced neutrophilic airway inflammation, increased the mRNA expression of CXCL1 and CXCL2 in the lungs, and provoked IL-17A, and IL-17F production in BAL fluid. In addition, neutrophilic airway inflammation was significantly inhibited in Il-17a−/−Il-17f−/− mice compared with those found in WT mice. We demonstrated that S. commune induces neutrophilic airway inflammation in OVA-induced asthma model mice, and IL-17A and IL-17F had central roles in this activity. As S. commune inhabits the general environment, including indoor and outdoor air, our results suggested that S. commune is a causative agent of asthma exacerbation. This study has provided clues regarding the mechanisms behind fungi and asthma exacerbation.
Similar content being viewed by others
Introduction
The basidiomycetous fungus Schizophyllum commune is typically found in diverse trees and rotting wood across the world1. Recent metagenomic analyses of the mycobiome revealed the presence of Schizophyllum in indoor and outdoor air2,3,4. In particular, Coombs et al. reported that Schizophyllum was the third most abundant genus in indoor air samples collected in Cincinnati, Ohio, USA3.
S. commune causes respiratory allergic diseases, such as allergic bronchopulmonary mycosis5,6 and allergic fungal sinusitis7,8. Some studies indicated that sensitization to S. commune was correlated with asthma severity9, and that this sensitization was identified as a risk factor involved in lung function decline in patients with asthma10. These studies suggest that pulmonary exposures to S. commune is potentially related to asthma exacerbation.
Asthma is a T helper type 2 (Th2) cell-mediated eosinophilic inflammatory disease, and Th2 cytokines IL-4, IL-5, and IL-13 have been implicated in asthma pathology. However, patients with chronic severe or acute fatal asthma have neutrophil-dominant airway inflammation in addition to Th2-associated airway inflammation11,12,13. Recent studies illustrated that IL-17A and IL-17F, which recruit neutrophils into the airway via the release of CXC chemokines from bronchial epithelial cells, were upregulated in patients with asthma14. Elevation of IL-17A and IL-17F levels in the lungs is directly correlated with disease severity15,16,17,18,19.
Fungal allergens contain a wide variety of proteins, including proteases, as well as intracellular and extracellular proteins20,21. Both protein allergens and fungal cell wall polysaccharides, such as β-glucan, α-mannan, and chitin, have immunomodulatory activities. Schizophyllan (SPG), a cell wall β-glucan derived from S. commune, induces the production of proinflammatory cytokines and chemokines regulating neutrophil recruitment22, whereas α-mannan induces Th17 cell differentiation through dectin-223. Additionally, elevation of cell wall chitin content enhances the recruitment of lung eosinophils24,25,26,27. However, the precise mechanism of fungus-induced allergic airway inflammation and its involvement in allergic asthma exacerbation remains unknown.
In the present study, we hypothesized that S. commune is potentially associated with asthma exacerbation. To test this hypothesis, we evaluated the direct immunomodulatory activities of S. commune in a model of allergic airway inflammation induced by non-fungal sensitization.
Results
S. commune enhances neutrophilic airway inflammation in OVA-induced asthma model mice
To investigate the direct immunomodulatory activities of S. commune on non-fungus-induced allergic airway inflammation, OVA-induced asthma model mice were intratracheally administered S. commune and BAL fluid was collected 24 h after administration (Fig. 1). Intratracheal administration of S. commune to OVA-induced asthma model mice (OVA/Sc group) increased the number of neutrophils in BAL fluid (Fig. 2A). On the contrary, the numbers of eosinophils and lymphocytes decreased in the OVA/Sc group (Fig. 2A). We next evaluated the mRNA expression levels of neutrophil (CXCL1 and CXCL2) and eosinophil chemotactic factors (eotaxin-1 and eotaxin-2) in the lungs. The mRNA expression of CXCL1 and CXCL2 was increased in the OVA/Sc group (Fig. 2B). On the contrary, eotaxin-1 and eotaxin-2 expression was comparable between OVA/Sc and OVA/PBS group (Fig. 2B). Histological examinations revealed that OVA/Sc mice presented with higher lung inflammation scores compared with the PBS/Sc and OVA/PBS groups (Fig. 2C,D). Lung permeability and cellular damage were assessed by evaluating total protein (TP) and lactate dehydrogenase (LDH) activity in BAL fluid, and levels of TP and LDH activity in BAL fluid were elevated in the OVA/Sc group compared with the PBS/Sc and OVA/PBS groups (Fig. 2F). PAS scores reflecting goblet cell hyperplasia in airway epithelium did not vary between the OVA/Sc and OVA/PBS groups (Fig. 2C,E).
Experimental protocol for OVA-induced asthma model mice and intratracheal administration of Schizophyllum commune. Mice were divided into four groups: OVA-sensitized/challenged and intratracheally administered S. commune (OVA/Sc), OVA-sensitized/challenged and intratracheally administered PBS (OVA/PBS), non-sensitized and intratracheally administered S. commune (PBS/Sc), and non-sensitized and intratracheally administered PBS (PBS/PBS). Mice were intraperitoneally sensitized with OVA on day −15 and day −10, then challenged via exposure to aerosolized OVA on days −3 and −2. Mice in the OVA/Sc and PBS/Sc groups were intratracheally administered a S. commune mycelial suspension on day 0. All animals were euthanized 24 h after the intratracheal administration of S. commune.
Schizophyllum commune enhances neutrophilic airway inflammation in OVA-induced asthma model mice. (A) The numbers of total cells, neutrophils, eosinophils, lymphocytes, and alveolar macrophages (AM) in BAL fluid were counted. (B) The mRNA expression of CXCL1, CXCL2, eotaxin-1 and eotaxin-2 in the lungs was measured via quantitative real-time PCR. The mRNA levels were normalized to β-actin mRNA levels, then presented as fold differences relative to those in the PBS/PBS group. (C) Histological examination of lung tissues was performed via staining with hematoxylin and eosin (HE), and periodic acid-Schiff (PAS) stain. Scale bar, 100 μm. (D) Lung inflammation scores. (E) PAS scores of airway epithelium graded for goblet cell hyperplasia. (F) Lactate dehydrogenase (LDH) activity, and total protein (TP) levels in BAL fluids. All results are expressed as mean ± SEM (n = 7 mice/group). Each symbol represents an individual sample. *P < 0.05, **P < 0.01, ***P < 0.001. NS, not significant.
S. commune induces Th17-related cytokine production in OVA-induced asthma model mice
To determine Th1, Th2, and Th17 immune responses in the lungs after S. commune administration in OVA-induced asthma model mice, Th1-, Th2- and Th17-related cytokine levels in BAL fluid were measured using ELISA. Intratracheal administration of S. commune to the OVA-induced asthma model mice induced the production of the Th17-related cytokines, IL-17A and IL-17F, as well as Th1-related cytokine INF-γ in the lungs (Fig. 3). On the contrary, levels of the Th2-related cytokines IL-4 and IL-13 were comparable between the OVA/Sc and OVA/PBS groups (Fig. 3).
S. commune induces Th17-related cytokine production in OVA-induced asthma model mice. IFN-γ, IL-4, IL-13, IL-17A, and IL-17F levels in BAL fluid were measured by ELISA. All results are expressed as mean ± SEM (n = 7 mice/group). Each symbol represents an individual sample. *P < 0.05, **P < 0.01, ***P < 0.001, NS, not significant.
IL-17A and IL-17F have central roles in neutrophilic airway inflammation induced by S. commune
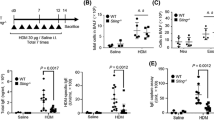
IL-17A and IL-17F levels in BAL fluid were clearly increased in the OVA/Sc group (Fig. 3). We next hypothesized that IL-17A and IL-17F were involved in neutrophilic airway inflammation induced by S. commune in OVA-induced asthma model mice. To demonstrate this hypothesis, we investigated the roles of IL-17A and IL-17F using OVA-induced asthma model mice, generated by knocking out IL-17a and IL-17f (Il-17a−/−Il-17f−/−), and WT mice. Neutrophilic infiltration in BAL fluid after the intratracheal administration of S. commune was reduced in Il-17a−/−Il-17f−/− mice compared with that in WT mice (Fig. 4A). Similarly, the mRNA expression of CXCL1 and CXCL2 in the lungs after S. commune administration was concomitantly suppressed in these mice (Fig. 4B). Moreover, LDH activity and TP levels in BAL fluid were reduced in Il-17a−/−Il-17f−/− mice (Fig. 4C). Histological examinations revealed that Il-17a−/−Il-17f−/− mice presented with lower lung inflammation and PAS scores than WT mice (Fig. 4D–F).
IL-17A/F have central roles in Schizophyllum commune-induced neutrophilic airway inflammation in OVA-induced asthma model mice. Il-17a−/−Il-17f−/− and wild-type (WT) mice were sensitized and challenged with OVA, then administered a S. commune mycelial suspension as described in Fig. 1. All animals were euthanized 24 h after the administration of S. commune. (A) The numbers of total cells, neutrophils, eosinophils, lymphocytes, and alveolar macrophages (AM) in BAL fluids were counted. (B) The mRNA expression of CXCL1, CXCL2, eotaxin-1 and eotaxin-2 in the lungs was measured via quantitative real-time PCR. The mRNA levels were normalized to β-actin mRNA levels and presented as fold differences relative to those in the WT group. (C) Lactate dehydrogenase (LDH) and total protein (TP) levels in BAL fluid. (D) Histological examination of lung tissues after staining with hematoxylin and eosin (HE), and periodic acid-Schiff (PAS) stain. Scale bar, 100 μm. (E) Lung inflammation scores. (F) PAS scores of airway epithelium graded for goblet cell hyperplasia. All results are expressed as mean ± SEM (n = 5 mice/group). Each symbol represents an individual sample. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
We found that S. commune, a ubiquitous basidiomycetous fungus in the environment, induces neutrophilic airway inflammation in non-fungus-induced asthma model mice and that IL-17A and IL-17F have central roles in the neutrophilic airway inflammation induced by S. commune.
Recently, Coombs et al. reported that Schizophyllum was the third most abundant genus in indoor air samples in Cincinnati, Ohio, USA3. Ogawa et al. reported that S. commune sensitization is correlated with asthma severity9 and that it is one of the risk factors involved in lung function decline in patients with asthma10.
It is not clear which component of S. commune (mycelium, fruiting body, basidiospore) is inhaled. This study used lyophilized mycelium because it is easy to culture and quantify. For intratracheal administration, 100 µg of S. commune mycelia (total protein, 80 µg) was used. In previous reports with inhalation mouse models of allergic fungal asthma28,29, 20–100 μg of Aspergillus fumigatus extract was used to sensitize and challenge the mice. We chose the 100-µg dose of mycelial suspension with reference to these reports.
Meanwhile, IL-17A and IL-17F are highly homologous members of the IL-17 cytokine family, meaning they may bind the same receptor complexes consisting of IL-17RA and IL-17RC30,31. IL-17A and IL-17F induce the production of pro-inflammatory cytokines (IL-1, IL-6, TNF-α) and chemokines (CXCL1, CXCL2), thus enhancing neutrophil recruitment31,32. Recent studies illustrated the upregulation of IL-17A and IL-17F in asthma and reported that elevation of IL-17A and IL-17F levels in the lungs is directly correlated with disease severity15,16,17,18,19. Some studies observed neutrophil-dominant airway inflammation in certain patients with chronic severe or acute fatal asthma11,12,13. In line with our findings, these data suggest that S. commune can exacerbate asthma by inducing IL-17A– and IL-17F–mediated neutrophilic airway inflammation.
Some fungal components can trigger IgE-mediated allergies, whereas others are immunomodulators with effects on asthma independent of their potential antigenic activity33. Fungal cell wall polysaccharides, such as β-glucan, α-mannan, and chitin, have immunomodulatory activities. β-glucan is recognized by the innate immune receptor dectin-1, while signaling through dectin-1 promotes fungal immunity by stimulating dendritic cells to polarize T cells toward Th17 cells34,35. SPG, a cell wall β-glucan derived from S. commune, also has immunomodulating potential and antitumor activity22,36,37,38, and it induces the production of IL-6, IL-8, and TNF-α, which regulate neutrophil recruitment22. Fungal mannose residues are recognized by dectin-2, macrophage mannose receptor, and DC-SIGN, which are expressed on the surface of macrophages or dendritic cells23,39,40. Signaling through dectin-2 promotes the polarization of T cells toward Th17 cells23. Chitin induces eosinophilic infiltration in the lungs41,42 by inducing the release of epithelial cell-derived cytokines, namely TSLP, IL-25, and IL-33, which activate innate lymphoid type 2 cells43,44,45. In our study, S. commune provoked Th17-related cytokine production in OVA-induced asthma model mice. It was presumed that the fungal components inducing Th17 immune responses in OVA-induced asthma model mice might be β-glucan or mannan. However, the precise mechanism of these responses still remains unclear.
In conclusion, we demonstrated that the basidiomycetous fungus S. commune induces neutrophilic airway inflammation in OVA-induced asthma model mice, while IL-17A and IL-17F play central roles in neutrophilic airway inflammation induced by S. commune. Considering the ubiquitous nature of S. commune in the general environment, our results suggested that S. commune is a causative agent of asthma exacerbation. These findings provide clues regarding the mechanism behind fungi and asthma exacerbation.
Materials and Methods
Preparation of mycelial suspension
This study used a dikaryotic strain of S. commune (IFM 47009; Medical Mycology Research Center, Chiba University, Chiba, Japan) isolated from a patient with allergic bronchopulmonary mycosis46. After 7 days of culture on potato dextrose agar (PDA) (Becton, Dickinson and Company, New Jersey, USA) at 25 °C, mycelia were inoculated into yeast nitrogen base broth (Becton, Dickinson and Company, New Jersey, USA), supplemented with 1% glucose, and cultured for 5 days at 37 °C with agitation at 200 rpm. The mycelia were collected using Miracloth (Merck Millipore Limited, Massachusetts, USA) and lyophilized after twice washing with phosphate-buffered saline (PBS). Lyophilized mycelia were resuspended in PBS at a final concentration of 2 mg/ml, disrupted by beads beating using a Multibeads Shocker® (Yasui Kikai Co., Osaka, Japan). The inactivation of mycelia was confirmed by culturing the mycelial suspension on PDA plates at 25 °C for 7 days. The total protein concentration of the mycelial suspension was 1.6 mg/ml. The mycelial suspension was subsequently stored at −80 °C until use. A photomicrograph of the mycelial suspension is shown in Fig. S1.
Animals
Specific pathogen-free female C57BL/6 mice, aged 8 weeks, were purchased from Charles River Laboratories Japan (Yokohama, Japan). Female Il-17a−/−Il-17f−/− mice (C57BL/6 background)47, aged 8 weeks, were kindly gifted by Prof. Y. Iwakura (Research Institute for Biomedical Sciences, Tokyo University of Science, Japan). Genotyping of Il-17a−/−Il-17f−/− mice prior to experimentation was performed as described previously47,48. All mice were housed under specific pathogen-free conditions with food and water ad libitum. All animal experiments were approved by the Committee on Animal Experiments of Chiba University and carried out according to the Chiba University Animal Experimentation Regulations.
OVA-induced asthma model mice and intratracheal administration of S. commune
Mice were divided into four groups as follows: ovalbumin (OVA)-sensitized/challenged and intratracheally administered S. commune (OVA/Sc group), OVA-sensitized/challenged and intratracheally administered PBS (OVA/PBS group), non-sensitized and intratracheally administered S. commune (PBS/Sc group), and non-sensitized and intratracheally administered PBS (PBS/PBS group). Mice in the OVA/PBS and OVA/Sc groups were intraperitoneally sensitized with 20 µg of OVA (Grade III; Sigma-Aldrich, Missouri, USA) and 2 mg of alum (Thermo Fisher Scientific, Massachusetts, USA) in 0.2 ml of PBS on days −15 and −10, then challenged via exposure to aerosolized 1% OVA (w/v) for 40 min using a nebulizer (PARI Boy N, PARI, Starnberg, Germany) on days −3 and −2. Under anesthesia with ketamine and xylazine, mice in the PBS/Sc and OVA/Sc groups were intratracheally administered 100 µg of S. commune mycelia suspended in 50 µl of PBS on day 0. All animals were euthanized 24 h after the intratracheal administration of S. commune (Fig. 1).
Total and differential leukocyte counts in BAL fluid
Airway contents were recovered via the instillation and retrieval of 2 ml of sterile PBS. The lavage fluid was centrifuged, and the cell pellet was resuspended in PBS. Total cell numbers were quantified with a hemocytometer under a light microscope. Cells were centrifuged onto glass slides using Cytospin™ (Thermo Fisher Scientific) and stained with Diff-Quick (Wako Chemicals, Osaka, Japan) for differential counts of leukocytes. A total of 300 cells were counted on each slide.
TP and LDH levels in BAL fluid
The TP level in BAL fluid was measured using a BCA Protein Assay Reagent Kit (Thermo Fisher Scientific). LDH activity in BAL fluid was measured using the LDH-Cytotoxic Test (Wako Chemicals).
Cytokine levels in BAL fluid
IFN-γ, IL-17A, IL-17F, IL-13, and IL-4 levels in BAL fluid were measured using enzyme-linked immunoassay (ELISA) kits (R&D Systems, Minnesota, USA), according to the manufacturer’s instructions.
Lung histopathology
Mouse lungs were fixed in 4% formaldehyde, routinely embedded in paraffin, and sectioned at a thickness of 4 µm. Sections were stained separately with hematoxylin and eosin and periodic acid-Schiff (PAS) stain. Inflammation was graded using a 0–4 grade scoring system: 0, no inflammation; 1, mild inflammation; 2, moderate inflammation; 3, severe inflammation; and 4, extreme inflammation, as described previously49. Hyperplasia of goblet cells in the epithelial samples was assessed via PAS staining using a 0–4 grade scoring system. Ten bronchi in the lungs were examined, and average scores were calculated. Each bronchus was graded as follows: 0, no PAS-positive cells; 1, 1%–25% PAS-positive cells; 2, 26%–50% PAS-positive cells; 3, 51%–75% PAS-positive cells; and 4, 76%–100% PAS-positive cells, as described previously50.
mRNA extraction and quantitative real-time PCR
Mouse lungs were fixed in RNAlater® (Thermo Fisher Scientific) and stored at −20 °C. Fixed lungs were homogenized using a Multibeads Shocker® and total RNA was isolated using RNAiso plus (Takara Bio, Shiga, Japan) and Zymo-Spin II (Zymo Research, California, USA), according to the manufacturers’ instructions. The purity of total RNA was checked using a NanoDrop 1000 (Thermo Fisher Scientific) and agarose gel electrophoresis. cDNA was generated via reverse transcription using PrimeScript RT Master Mix (Takara Bio). Quantitative real-time PCR was performed using SYBR® Premix Ex Taq™ II (Takara Bio) and the following primer pairs: CXCL1 (Fwd, 5′-TGGCTGGGATTCACCTCAAG-3′, Rev, 5′-CAGACAGGTGCCATCAGAGC-3′); CXCL2 (Fwd, 5′-AGACAGAAGTCATAGCCACTCTCAAG-3′, Rev, 5′-CCTCCTTTCCAGGTCAGTTAGC-3′); eotaxin-1 (Fwd, 5′-TCCACAGCGCTTCTATTCCT-3′, Rev, 5′-CTATGGCTTTCAGGGTGCAT-3′); eotaxin-2 (Fwd, 5′- GCTGCACGTCCTTTATTTCC-3′, Rev, 5′- TCTTATGGCCCTTCTTGGTG-3′); and β-actin (Fwd, 5′-GCTGTATTCCCCTCCATCGTG-3′, Rev, 5′-CACGGTTGGCCTTAGGGTTCAG-3′). Real-time PCR amplification was performed using an Applied Biosystems 7300 Real-Time PCR System (Thermo Fisher Scientific) under the following conditions: one cycle at 95 °C for 30 s, followed by 40 cycles at 95 °C for 5 s and 60 °C for 31 s. mRNA levels were normalized to those of β-actin and presented as fold changes, relative to the PBS/PBS or WT group.
Statistical analysis
Statistical analysis was performed using GraphPad InStat 3 and JMP®7. Unpaired, two-tailed Student’s t-tests and one-way ANOVA with post-hoc Tukey–Kramer tests were used to assess statistical significance. P < 0.05 was considered to indicate a significant difference.
Data availability
The datasets during and/or analysed during the current study available from the corresponding author on reasonable request.
References
Takemoto, S., Nakamura, H., Erwin, Imamura, Y. & Shimane, T. Schizophyllum commune as a Ubiquitous Plant Parasite. Jarq-Japan Agricultural Research Quarterly 44, 357–364, https://doi.org/10.6090/jarq.44.357 (2010).
Konya, T. & Scott, J. A. Recent Advances in the Microbiology of the Built Environment. Current Sustainable/Renewable Energy Reports 1, 35–42, https://doi.org/10.1007/s40518-014-0007-4 (2014).
Coombs, K. et al. Variability of indoor fungal microbiome of green and non-green low-income homes in Cincinnati, Ohio. Science of the Total Environment 610, 212–218, https://doi.org/10.1016/j.scitotenv.2017.07.274 (2018).
Yamamoto, N. et al. Particle-size distributions and seasonal diversity of allergenic and pathogenic fungi in outdoor air. Isme Journal 6, 1801–1811, https://doi.org/10.1038/ismej.2012.30 (2012).
Kamei, K. et al. Allergic bronchopulmonary mycosis caused by the basidiomycetous fungus Schizophyllum commune. Clinical Infectious Diseases 18, 305–309, https://doi.org/10.1093/clinids/18.3.305 (1994).
Chowdhary, A. et al. Allergic bronchopulmonary mycosis due to fungi other than Aspergillus: a global overview. Critical Reviews in Microbiology 40, 30–48, https://doi.org/10.3109/1040841x.2012.754401 (2014).
Clark, S., Campbell, C. K., Sandison, A. & Choa, D. I. Schizophyllum commune: An unusual isolate from a patient with allergic fungal sinusitis. Journal of Infection 32, 147–150, https://doi.org/10.1016/s0163-4453(96)91436-x (1996).
Taguchi, K. et al. Allergic fungal sinusitis caused by Bipolaris spicifera and Schizophyllum commune. Medical Mycology 45, 559–564, https://doi.org/10.1080/13693780701487813 (2007).
Ogawa, H., Fujimura, M., Takeuchi, Y. & Makimura, K. The Influence of Schizophyllum commune on Asthma Severity. Lung 189, 485–492, https://doi.org/10.1007/s00408-011-9320-5 (2011).
Ogawa, H., Fujimura, M., Takeuchi, Y. & Makimura, K. Impact of Schizophyllum sensitization on decline of lung function in asthma. Journal of Asthma 50, 764–768, https://doi.org/10.3109/02770903.2013.803573 (2013).
Kikuchi, S., Nagata, M., Kikuchi, L., Hagiwara, K. & Kanazawa, M. Association between neutrophilic and eosinophilic inflammation in patients with severe persistent asthma. International Archives of Allergy and Immunology 137, 7–11, https://doi.org/10.1159/000085425 (2005).
Nakagome, K., Matsushita, S. & Nagata, M. Neutrophilic Inflammation in Severe Asthma. International Archives of Allergy and Immunology 158, 96–102, https://doi.org/10.1159/000337801 (2012).
Jatakanon, A. et al. Neutrophilic inflammation in severe persistent asthma. American Journal of Respiratory and Critical Care Medicine 160, 1532–1539, https://doi.org/10.1164/ajrccm.160.5.9806170 (1999).
Laan, M. et al. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. Journal of Immunology 162, 2347-2352 (1999).
Doe, C. et al. Expression of the T Helper 17-Associated Cytokines IL-17A and IL-17F in Asthma and COPD. Chest 138, 1140–1147, https://doi.org/10.1378/chest.09-3058 (2010).
Al-Ramli, W. et al. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. Journal of Allergy and Clinical Immunology 123, 1185–1187, https://doi.org/10.1016/j.jaci.2009.02.024 (2009).
Barczyk, A., Pierzchala, W. & Sozanska, E. Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine. Respiratory Medicine 97, 726–733, https://doi.org/10.1053/rmed.2003.1507 (2003).
Chakir, J. et al. Airway remodeling-associated mediators in moderate to severe asthma: Effect of steroids on TGF-beta, IL-11, IL-17, and type I and type III collagen expression. Journal of Allergy and Clinical Immunology 111, 1293–1298, https://doi.org/10.1067/mai.2003.1557 (2003).
Hashimoto, T., Akiyama, K., Kobayashi, N. & Mori, A. Comparison of IL-17 production by helper T cells among atopic and nonatopic asthmatics and control subjects. International Archives of Allergy and Immunology 137, 51–54, https://doi.org/10.1159/000085432 (2005).
Chaudhary, N., Staab, J. F. & Marr, K. A. Healthy Human T-Cell Responses to Aspergillus fumigatus Antigens. Plos One 5, https://doi.org/10.1371/journal.pone.0009036 (2010).
Horner, W. E., Helbling, A., Salvaggio, J. E. & Lehrer, S. B. FUNGAL ALLERGENS. Clinical Microbiology Reviews 8, 161–179 (1995).
Kubala, L. et al. The effect of (1–>3)-beta-D-glucans carboxymethylglucan and schizophyllan on human leukocytes in vitro. Carbohydrate Research 338, 2835–2840, https://doi.org/10.1016/j.carres.2003.09.007 (2003).
Saijo, S. et al. Dectin-2 Recognition of alpha-Mannans and Induction of Th17 Cell Differentiation Is Essential for Host Defense against Candida albicans. Immunity 32, 681–691, https://doi.org/10.1016/j.immuni.2010.05.001 (2010).
Amarsaikhan, N. et al. Caspofungin Increases Fungal Chitin and Eosinophil and gamma delta T Cell-Dependent Pathology in Invasive Aspergillosis. Journal of Immunology 199, 624–632, https://doi.org/10.4049/jimmunol.1700078 (2017).
Walker, L. A., Lee, K. K., Munro, C. A. & Gow, N. A. R. Caspofungin Treatment of Aspergillus fumigatus Results in ChsG-Dependent Upregulation of Chitin Synthesis and the Formation of Chitin-Rich Microcolonies. Antimicrobial Agents and Chemotherapy 59, 5932–5941, https://doi.org/10.1128/aac.00862-15 (2015).
Amarsaikhan, N., O’Dea, E. M., Tsoggerel, A. & Templeton, S. P. Lung eosinophil recruitment in response to Aspergillus fumigatus is correlated with fungal cell wall composition and requires gamma delta T cells. Microbes and Infection 19, 422–431, https://doi.org/10.1016/j.micinf.2017.05.001 (2017).
O’Dea, E. M. et al. Eosinophils Are Recruited in Response to Chitin Exposure and Enhance Th2-Mediated Immune Pathology in Aspergillus fumigatus Infection. Infection and Immunity 82, 3199–3205, https://doi.org/10.1128/iai.01990-14 (2014).
Zimmermann, N. et al. Dissection of experimental asthma with DNA microarray analysis identifies arginase in asthma pathogenesis. Journal of Clinical Investigation 111, 1863–1874, https://doi.org/10.1172/jci200317912 (2003).
Schuh, J. M. & Hoselton, S. A. An inhalation model of allergic fungal asthma: Aspergillus fumigatus-induced inflammation and remodeling in allergic airway disease. Methods Mol Biol 1032, 173–184, https://doi.org/10.1007/978-1-62703-496-8_14 (2013).
Toy, D. et al. Cutting edge: Interleukin 17 signals through a heteromeric receptor complex. Journal of Immunology 177, 36–39, https://doi.org/10.4049/jimmunol.177.1.36 (2006).
Kawaguchi, M., Adachi, M., Oda, N., Kokubu, F. & Huang, S. K. IL-17 cytokine family. Journal of Allergy and Clinical Immunology 114, 1265–1274, https://doi.org/10.1016/j.jaci.2004.10.019 (2004).
Kolls, J. K. & Linden, A. Interleukin-17 family members and inflammation. Immunity 21, 467–476, https://doi.org/10.1016/j.immuni.2004.08.018 (2004).
Zhang, Z. H., Reponen, T. & Hershey, G. K. K. Fungal Exposure and Asthma: IgE and Non-IgE-Mediated Mechanisms. Current Allergy and Asthma Reports 16, https://doi.org/10.1007/s11882-016-0667-9 (2016).
Osorio, F. et al. DC activated via dectin-1 convert Treg into IL-17 producers. European Journal of Immunology 38, 3274–3281, https://doi.org/10.1002/eji.200838950 (2008).
Tassi, I. et al. Requirement of phospholipase C-gamma 2 (PLC gamma 2) for Dectin-1-induced antigen presentation and induction of T(H)1/T(H)17 polarization. European Journal of Immunology 39, 1369–1378, https://doi.org/10.1002/eji.200839313 (2009).
Sugawara, I., Lee, K. C. & Wong, M. Schizophyllan (SPG)-treated macrophages and anti-tumor activities against syngeneic and allogeneic tumor cells. I. Characteristics of SPG-treated macrophages. Cancer Immunology Immunotherapy 16, 137–144 (1984).
Arika, T., Amemiya, K. & Nomoto, K. Combination therapy of radiation and Sizofiran (SPG) on the tumor growth and metastasis on squamous-cell carcinoma NR-S1 in syngeneic C3H/He mice. Biotherapy 4, 165–170, https://doi.org/10.1007/bf02171761 (1992).
Adachi, Y. et al. Characterization of beta-glucan recognition site on C-type lectin, dectin 1. Infection and Immunity 72, 4159–4171, https://doi.org/10.1128/iai.72.7.4159-4171.2004 (2004).
van de Veerdonk, F. L. et al. The Macrophage Mannose Receptor Induces IL-17 in Response to Candida albicans. Cell Host & Microbe 5, 329–340, https://doi.org/10.1016/j.chom.2009.02.006 (2009).
Cambi, A. et al. Dendritic cell interaction with Candida albicans critically depends on N-linked mannan. Journal of Biological Chemistry 283, 20590–20599, https://doi.org/10.1074/jbc.M709334200 (2008).
Reese, T. A. et al. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature 447, 92–97, https://doi.org/10.1038/nature05746 (2007).
Kogiso, M. et al. Chitin particles induce size-dependent but carbohydrate-independent innate eosinophilia. Journal of Leukocyte Biology 90, 167–176, https://doi.org/10.1189/jlb.1110624 (2011).
Kim, L. K. et al. AMCase is a crucial regulator of type 2 immune responses to inhaled house dust mites. Proceedings of the National Academy of Sciences of the United States of America 112, E2891–E2899, https://doi.org/10.1073/pnas.1507393112 (2015).
Yasuda, K. et al. Contribution of IL-33-activated type II innate lymphoid cells to pulmonary eosinophilia in intestinal nematode-infected mice. Proceedings of the National Academy of Sciences of the United States of America 109, 3451–3456, https://doi.org/10.1073/pnas.1201042109 (2012).
Van Dyken, S. J. et al. Chitin Activates Parallel Immune Modules that Direct Distinct Inflammatory Responses via Innate Lymphoid Type 2 and gamma delta T Cells. Immunity 40, 414–424, https://doi.org/10.1016/j.immuni.2014.02.003 (2014).
Unno, H., Honda, A., Kuriyama, T., Kamei, K. & Nishimura, K. A murine model of pulmonary basidiomycosis by Schizophyllum commune. Journal of Infection and Chemotherapy 11, 136–140, https://doi.org/10.1007/s10156-005-0382-2 (2005).
Ishigame, H. et al. Differential Roles of Interleukin-17A and-17F in Host Defense against Mucoepithelial Bacterial Infection and Allergic Responses. Immunity 30, 108–119, https://doi.org/10.1016/j.immuni.2008.11.009 (2009).
Nakae, S. et al. Antigen-specific T cell Sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 17, 375–387, https://doi.org/10.1016/s1074-7613(02)00391-6 (2002).
Li, Y. C. et al. Silencing IL-23 expression by a small hairpin RNA protects against asthma in mice. Experimental and Molecular Medicine 43, 197–204, https://doi.org/10.3858/emm.2011.43.4.024 (2011).
Kagawa, S. et al. Role of Prostaglandin D-2 Receptor CRTH2 in Sustained Eosinophil Accumulation in the Airways of Mice with Chronic Asthma. International Archives of Allergy and Immunology 155, 6–11, https://doi.org/10.1159/000327257 (2011).
Acknowledgements
This work was partially supported by the Japan Agency for Medical Research and Development (AMED) under Grant Number JP18ek0410026. We thank Prof. Y. Iwakura (Research Institute for Biomedical Sciences, Tokyo University of Science, Japan) and Dr. S. Saijo (The Medical Mycology Research Center, Chiba University, Japan) for gifting Il-17a−/−Il-17f−/− mice. We also thank Ms. R. Seki (The Medical Mycology Research Center, Chiba University, Japan) for technical assistance with histopathology analyses.
Author information
Authors and Affiliations
Contributions
J.H., Y.M. and K.K. conceived the study. T.T., A.W. and K.H. participated in the study design and gave technical support and conceptual advice. J.H. and Y.M. performed the experiments and analyzed the data. J.H. and Y.M. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hanashiro, J., Muraosa, Y., Toyotome, T. et al. Schizophyllum commune induces IL-17-mediated neutrophilic airway inflammation in OVA-induced asthma model mice. Sci Rep 9, 19321 (2019). https://doi.org/10.1038/s41598-019-55836-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-55836-x
- Springer Nature Limited
This article is cited by
-
Influence of the early-life gut microbiota on the immune responses to an inhaled allergen
Mucosal Immunology (2022)
-
Blockade of protease-activated receptor 2 attenuates allergen-mediated acute lung inflammation and leukocyte recruitment in mice
Journal of Biosciences (2022)
-
Dynamic Changes of NCR− Type 3 Innate Lymphoid Cells and Their Role in Mice with Bronchopulmonary Dysplasia
Inflammation (2022)