Abstract
Sorghum is a self-pollinated crop with multiple economic uses as cereal, forage, and biofuel feedstock. Hybrid breeding is a cornerstone for sorghum improvement strategies that currently relies on cytoplasmic male sterile lines. To engineer genic male sterility, it is imperative to examine the genetic components regulating anther/pollen development in sorghum. To this end, we have performed transcriptomic analysis from three temporal stages of developing anthers that correspond to meiotic, microspore and mature pollen stages. A total of 5286 genes were differentially regulated among the three anther stages with 890 of them exhibiting anther-preferential expression. Differentially expressed genes could be clubbed into seven distinct developmental trajectories using K-means clustering. Pathway mapping revealed that genes involved in cell cycle, DNA repair, regulation of transcription, brassinosteroid and auxin biosynthesis/signalling exhibit peak expression in meiotic anthers, while those regulating abiotic stress, carbohydrate metabolism, and transport were enriched in microspore stage. Conversely, genes associated with protein degradation, post-translational modifications, cell wall biosynthesis/modifications, abscisic acid, ethylene, cytokinin and jasmonic acid biosynthesis/signalling were highly expressed in mature pollen stage. High concurrence in transcriptional dynamics and cis-regulatory elements of differentially expressed genes in rice and sorghum confirmed conserved developmental pathways regulating anther development across species. Comprehensive literature survey in conjunction with orthology analysis and anther-preferential accumulation enabled shortlisting of 21 prospective candidates for in-depth characterization and engineering male fertility in sorghum.
Similar content being viewed by others
Introduction
Sorghum, grown widely for food, feed, and forage, is a gluten-free substitute for staple grains, and a promising feedstock for biofuels1. It exhibits huge phenotypic and morphological diversity in key agronomic traits including photoperiod sensitivity, biomass, grain yield, disease resistance, abiotic stress tolerance etc., with considerable scope for genetic enhancement of cultivated sorghum. However, sorghum is a self-pollinated crop with 75 to 95% rate of self-pollination observed under natural conditions with outcrossing rates varying with panicle type, distance between plants, wind direction, etc2,3. Therefore, overcoming reproductive constraints is a major challenge to utilize sorghum diversity for breeding programs.
Plant breeders have been using cytoplasmic (CMS) and nuclear male sterility (NMS) systems to take advantage of hybrid vigour in many self-pollinated crop species4. While CMS, caused by specific nuclear and mitochondrial genetic interactions, is maternally inherited; NMS results due to defects in nuclear genes usually inherited as a recessive trait5. Studies in rice suggest that hybrids produced through NMS give higher yields and germplasm utilization efficiency with better genetic stability under diverse environmental conditions as compared to those produced through CMS6. However, hybrid production in sorghum exclusively relies on the CMS system where male sterile line is crossed with an identical male fertile line leading to hybrids with sterile seeds. These are then crossed with a restorer line to restore fertility5. This entire process is very time consuming and laborious. Also, repeated use of cytoplasm from male-sterile line is detrimental for plant fitness and adaptability in the long run6. Although NMS is an easier alternative, there is paucity of information about NMS-related genes in sorghum7. So far, only one NMS mutant ms8 has been characterized in sorghum that can be visually identified due to shorter anthers lacking pollen8. Leveraging NMS system in sorghum needs active exploration of anther transcriptome, followed by identification of key genes regulating male fertility.
Christensen9 used light and electron microscopy to examine developmental progression of sporogenous tissue to mature pollen in sorghum anthers. However, transcriptional dynamics in sorghum anthers remain unexplored. In this study, we identified key landmark events and generated a transcriptional landscape from three successive stages of sorghum anther development using RNA sequencing. A total of 1.08 billion high quality reads were generated from nine libraries with an average read length of 100 bp. Out of 31871 genes expressing in sorghum anthers, 5286 genes differentially expressed in different stages of anther development, were categorized into seven distinct transcriptional trajectories based on their expression patterns. Pathway analysis and mapping of orthologs, from model species rice and Arabidopsis, highlighted conserved as well as unique genetic components underlying progression of male gametophyte development in sorghum. The shortlisted candidates provide important targets for engineering male sterility in sorghum.
Results and Discussion
Anther staging and global dynamics of gene activity
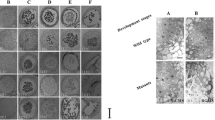
Length of anthers is tightly correlated with the key developmental events in sorghum9. Leveraging this information, we collected sorghum anthers at three temporal stages corresponding to three distinct landmark stages viz., meiotic to tetrad (A1; <1.5 mm), tetrad to microspore (A2; 1.6–2.0 mm), and mature pollen (A3; 2.5–3.0 mm). The position of flag leaf was used as a visual indicator to identify the stage of panicle development (Fig. 1). Concurrently, anthers were sampled from panicles collected from various stages of pre-emergence and post emergence panicle for microscopic analysis. In agreement with the earlier report9, microscopic analysis confirmed meiotic stages and tetrads in stage A1. Stage A2 contained microspores, whereas, anthers at stage A3 harboured mature engorged pollen (Fig. 1).
Staging of sorghum anthers. The position of flag leaf, shown by a white arrow, varying from partial to complete emergence in (a) A1, (b) A2, and (c) A3 stages was used as indicator of stage of development. Sections of florets representing A1 (d,g), A2 (e,h) and A3 (f,i) stages at 100× and 400× magnification, respectively.
Sequencing of nine libraries (three replicates from each stage) yielded >1.12 billion paired-end reads. After filtering at Q30 and adapter trimming, 1.08 billion high-quality reads were obtained. These reads were further filtered to remove rRNA and finally >961 million high-quality reads could be aligned to the sorghum genome (Phytozome V12 Sbicolor 313 v3.0.1; Table S1). A total of 32891 unique genes were determined by using STAR alignment. Fragments per kilobase per million reads (FPKM) were determined for all the unique transcripts. A total of 31871 genes with FPKM >0, in all three replicates of at least one anther stage, were considered as expressed. The expression of these genes was highly correlated among the biological replicates of each stage, with an average Spearman’s correlation coefficient of 0.91 among biological replicates (Table S2). Among the 31871 genes, 26870 genes expressed in all three stages, while 556, 674, and 1050 genes each were unique to A1, A2, and A3 stages, respectively (Fig. 2a). Comparative analysis revealed maximum overlap between A1 and A2 stages with 1172 genes expressing in these two stages, 817 genes common in A2 and A3 stages, while 732 genes were common in A1 and A3 stages (Fig. 2a). Analysis of transcript abundance in all three stages revealed 66.2 to 67.5% genes exhibiting low expression (FPKM >0 to ≤10), 28.9 to 29.7% genes with moderate expression (FPKM >10 to ≤100), whereas, merely 3.5 to 4.1% genes showed high expression with FPKM >100 (Fig. 2b). Evidently, A3 stage containing mature pollen contains largest number of unique transcripts and genes with high abundance.
Pairwise differential expression analysis between all three stages resulted in a set of 5286 differentially expressed genes (DEGs) with ≥2-fold change at q value ≤0.05 (Fig. 2c). Among all comparisons, highest number of DEGs were in the A3 stage with 4448 genes (2585 up and 1863 downregulated) differentially expressed compared to A1 stage and, 4214 (2417 up and 1797 down regulated) genes differentially expressed in comparison to A2 stage. The A1 and A2 stages shared transcriptional repertoire with only 228 genes differentially expressed (195 up and 33 down regulated) in A2 with respect to A1 (Fig. 2c). These results further confirm that mature pollen stage has more complex and distinct expression profile compared to early developmental stages. Further, we used the publicly available data from 47 sorghum RNA-sequencing experiments in Phytozome to identify anther-preferential genes. After filtering genes with FPKM ≥1 in any of the vegetative or seed tissues, 890 of the differentially expressed genes were found to exhibit anther-preferential accumulation. These play role in regulating cell division, cell wall, development, DNA repair & synthesis, transcription, transport, stress, signalling and metabolic processes including amino acid, carbohydrate, lipid and protein metabolism (Table S3A).
Dominant patterns of gene activity and associated genes/pathways
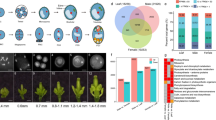
Identification of dominant expression patterns associated with developmental progression is a key step in deciphering co-regulated gene clusters. A total of sixteen clusters were obtained from 5286 DEGs using K-means clustering. Subsequently, gene clusters that only differed in the magnitude of expression were combined together resulting in seven distinct transcriptional trajectories, referred hereafter, as ‘groups 1–7’ (Fig. 3, Table S3). Only four genes (Sobic.001G189300, Sobic.004G189500, Sobic.006G034300 and Sobic.004G033500) showed decrease in expression from A1 to A2 stage with no significant difference in A3 stage. Likely due to small number, these were clubbed with group 1 during clustering.
Dominant patterns exhibited by differentially expressed genes (DEGs), and enriched pathway sub-categories and transcription factor families. Grouping of DEGs was done based on K-means clustering and identification of enriched pathway sub-categories and transcription factor families was carried out using hypergeometric test (p-value ≤ 0.05). Top 10 pathway sub-categories have been shown for the groups containing more than 10 pathways. The asterisks are used to mark the sub-categories for which the p-value for enrichment was significant after multiple correction with Benjamini-Hochberg test (FDR < 0.05).
It is noteworthy that ~80% of the genes belonged to groups 2 and 6, with uniform expression levels in meiotic (A1) to post-meiotic (A2) stages followed by either decline (group 2) or increase (group 6) in the mature pollen (A3) stage. To investigate the functional relevance of these patterns, we used MapMan to map the genes belonging to all seven groups onto biological pathways. Out of 5286 DEGs, 4221 genes mapped to 34 MapMan pathways. Also, 337 of the DEGs mapped to transcription factors. Analysis of over-enrichment of all sub-categories of pathways and transcription factor families was performed using hypergeometric test with a p value cut-off of ≤0.05 and FDR <0.05 (Tables S4 and S5). The detailed functional assessment of each group is elaborated below:
Group 1 genes putatively regulate male meiosis
Group 1 contains 65 genes with peak expression in the meiotic (A1) stage that gradual declines in post-meiotic microspore (A2) and mature pollen (A3) stages (Fig. 3). Pathway mapping revealed several genes mapping to cell cycle, biotic stress, DNA repair, hormone metabolism, phenylpropanoid metabolism, and MAP kinase signalling pathways in this group (Fig. 3, Table S4). Five of the genes in this group exhibit anther-preferential expression (Sobic.002G158300, Sobic.002G263000, Sobic.003G211300, Sobic.003G347100, Sobic.004G204400), however, none of these genes or their orthologs have been experimentally shown to play role in anther development. Hormone-related genes in this group including 1-aminocyclopropane-1-carboxylate oxidase involved in ethylene biosynthesis, and gibberellin 2-oxidase which inactivates endogenous gibberellins highlight the role of ethylene and gibberellins in meiotic progression10. Ethylene production has earlier been associated with degeneration of tapetal cells and middle layers of anther wall in petunia11. Empirical studies will be required to examine if ethylene plays similar role in sorghum anthers as well. Some of the genes relevant to cell cycle such as Sobic.001G462300 and Sobic.008G104000 (orthologous to Arabidopsis Cyclin A3 and Cyclin A2, respectively) also exhibit group 1 pattern. These may also be associated with male meiosis.
Rice orthologs of two genes from this group, Sobic.008G010900 (orthologous to OsDMC1; disrupted meiotic cDNA1) and Sobic.002G003300 (orthologous to Osku70; ATP-dependent DNA helicase 2 subunit KU70) have already been shown to play role in male meiosis (Table 1). OsDMC1 is essential for homologous pairing, whereas, Osku70 maintains chromosomal stability during meiosis in pollen mother cells12,13.
Group 2 genes regulate male meiosis, tapetum degradation and pollen wall formation
Group 2 comprises 1928 genes with high expression in both meiotic and post-meiotic stages and prominent decline in mature pollen stage (Fig. 3). A total of 12 pathway sub-categories were represented in this group with ‘regulation of transcription’ as the most abundant, followed by DNA synthesis/chromatin structure, cell organization, receptor kinases, RNA processing, DNA repair, cell cycle, protein targeting, RNA binding, metabolite transporters, and amino acid activation (Fig. 3, Table S4). Apart from the above categories, several genes associated with brassinosteroid as well as auxin biosynthesis and signalling fall in this group (Table S3b). Brassinosteroid biosynthesis and signalling has earlier been associated with tapetum and microspore development in Arabidopsis14.
Also, several genes encoding transcriptional regulators like Argonautes (AGOs), chromatin remodelling factors, DNA methyltransferases, histone acetyl/deacetyl transferases, chromatin assembly factors, aspartic proteases, etc. were also enriched in this group pointing to the role of RNA silencing machinery and epigenetic modifications in male gametophyte development. Sobic.009G259900 and Sobic.003G129500 are orthologs of rice OsAGO4b and OsAGO4a, respectively, involved in gene silencing through miRNA-mediated cleavage or DNA methylation15. Also, Sobic.001G153600 is orthologous to OsAGO18, which regulates production of phasiRNAs in grass anthers16. While the exact mechanism of action of phasiRNAs has not been deciphered so far, their importance in the male gametophyte development has been emphasised in both rice and maize17. Another important candidate gene Sobic.002G056000, orthologous to Arabidopsis MET1 (Methyltransferase) regulates meiosis during male gametogenesis18. Furthermore, rice orthologs of three genes in this group have been shown to perform important roles in chiasmata formation (Sobic.004G101500) (HEI10; Human enhancer of invasion-1019) and tapetum degeneration (Sobic.003G140100 orthologous to TDR interacting protein 2; TIP220 and Sobic.001G478100 orthologous to Aspartic Protease 25; OsAP2521) (Table 1). Several genes encoding proteins involved in DNA synthesis/chromatin structure including exonucleases, ATP binding proteins, RNA helicase, cell cycle checkpoint proteins, chromosome condensation, and segregation proteins, DNA gyrase, topoisomerases, helicases, etc. are also enriched in group 2.
Overall, rice orthologs of 37 genes of this group have been previously demonstrated to regulate male gametophyte development using transgenic approaches (Table 1). These include sorghum ortholog (Sobic.007G097000) of OsRAD21-3 (Radiation sensitive mutant 21-3) that regulates pollen mitosis22. Transcripts of rice ortholog (LTPL44; Lipid Transfer Family Protein 44) of Sobic.007G180700 are also abundant in tetrads and uninucleate microspores with anticipated role in lipid transport in developing pollen23. Sobic.002G353500 is orthologous to rice OIP30 (OsCPK25/26-interacting protein 30), which codes for a putative ruvB-like 2 protein, detected during early prophase I in meiosis24. DNA repair genes, Sobic.010G243100, Sobic.004G355500, Sobic.006G231800, and Sobic.009G185700, are orthologous to rice DNA repair genes important for meiotic crossovers namely, RPA2c (replication protein 2c), RPA2a (Replication Protein 2a), MRE11(meiotic recombination 11), and OsMSH4 (Mut S Homolog 4), respectively25,26,27.
Cell cycle-related genes encoding cyclins, tetratricopeptide repeat containing proteins, auxin response factors, cyclophilins, and peptidyl-prolyl isomerases are also prominent in this group. Rice ortholog (OsCycB1) of one of the cyclin genes, Sobic.003G328500, is known to exhibit G2/M phase-specific expression in rice28. Sobic.006G101800 is ortholog of ZEP1, a putative synaptonemal complex protein, which regulates the number of crossovers during meiosis29.
Several lipid metabolism-related genes encoding acetyl-CoA carboxylase, acetyl-CoA transacetylase, ACP desaturases, ACP proteins, acyl CoA ligases, acyl-CoA binding protein, beta hydroxyacyl ACP dehydratase, beta ketoacyl-CoA synthases, enoyl ACP reductase, ketoacyl ACP synthases (KCS), and long chain fatty acid CoA ligases are also prominent in this group. These are important candidates for regulating pollen wall formation. In fact, in Arabidopsis, several KCS genes have been already implicated in sporopollenin synthesis and pollen wall formation30.
Receptor kinases or receptor-like kinases (RLKs) also play prominent role in sensing extracellular signals and have also been implicated in pollen development31. Out of 44 receptor kinases present in group 2, 25 are leucine-rich repeat XI types while 8 are leucine-rich repeat III types. Another important gene in this subset is Sobic.007G029900 which is orthologous to rice CYP703A3 (Cytochrome P450 Hydroxylase 703A3), recently shown to be the causal gene for the no-pollen male sterility mutant in indica rice32,33.
Rice orthologs of several other genes in this group are involved in regulating key aspects of anther development including chromosome pairing and synapsis (Completion of meiosis1; OsCOM1, Central region component 1; OsCRC1, OsSGO1 and Homologous Pairing Aberration in Rice Meiosis 3; PAIR3), meiotic crossover (Meiotic chromosome association1; MEICA1, RPA1c, ZEP1 and OsMSH5), pollen wall development (Cytochrome P450 Hydroxylase 704B2; CYP704B2, LTPL44, CYP703A3, ATP Binding Cassette G26; OsABCG26, Polyketide synthase2; OsPKS2 and UDP-arabipyranose mutase3; UAM3), anther wall development (HTH1 (Hothead1)), meiotic regulation (MEL2), and tapetum degeneration (STRL-like2; OsSTRL2, Acyl CoA synthatase12; OsACOS12, and Defective Tapetum Cell Death1; DTC1) (Table 1).
Out of 1928 group 2 genes, 231 exhibit anther-preferential expression. Rice orthologs of 10 anther-preferential genes (OsSTRL2, CYP704B2, LTPL45, OsMSH5, TIP2, HEI10, OsACOS12, MEL2, LTPL44, and OsMSH4) have also been shown to play important roles in male gametophyte development (Table 1). Sobic.001G428300 is orthologous to OsSTRL2 that regulates pollen wall formation34. Sobic.002G270400 is ortholog of rice LTPL45 that specifically expresses in the uninucleate microspore stage of rice35. Zhao and co-workers36 reported identification of OsABCG26 as the gene responsible for a male sterile mutant in japonica rice. It helps in the transport of lipids from tapetal cells for anther cuticle development. Another anther-specific gene OsPKS2 has recently been shown to be causal gene for a rice male sterile mutant37. This gene is crucial for pollen development and sporopollenin biosynthesis in both rice and Arabidopsis and therefore, its ortholog Sobic.002G115700 is also a suitable candidate for engineering male sterility in sorghum. Similarly, Sobic.006G079500 is ortholog of ACOS5 (Acetyl-CoA synthetase 5) required for sporopollenin biosynthesis. Another candidate Sobic.002G368600 is orthologous to rice UDP-arabinopyranose mutase 3 (UAM3), which is required for pollen intine development38. Furthermore, rice ortholog of Sobic.008G008100, Defective Tapetum Cell Death1 (DTC1), is a key regulator of tapetum programmed cell death39, while, Sobic.006G185600 corresponds to rice HOTHEAD-Like HTH1 which regulates biosynthesis of cutin monomers in the anther epidermis. It has recently been shown that apart from tapetum, epidermal cutin synthesis is also crucial for pollen fertility40.
Transcription factors (TFs) in this group mainly belong to ARF (Auxin Response Factors), B3, C3H, GRF (Growth Regulating Factor), HD-ZIP (Homeodomain-leucine zipper), SBP (Squamosa Binding Protein), and Whirly families, with the latter two families showing significant enrichment (Fig. 3). Interestingly, sorghum has two putative Whirly TFs with both (Sobic.010G035200 and Sobic.004G047500) showing group 2 expression pattern. One of these, Sobic.004G047500, is orthologous to Arabidopsis Whirly2, crucial for pollen development41. SBP factors are also significantly enriched in this group. In fact, none of the other groups apart from group 2 contain SBP TFs. SBP TF genes, Sobic.010G215700 and Sobic.010G254200, are orthologous to Arabidopsis SPL8 (Squamosa promoter-binding-like 8) and SPL2 (Squamosa promoter-binding-like 2), respectively. Both these genes are known to regulate anther development in a redundant manner42. Besides SBPs, ARFs have also been previously associated with pollen development. Sobic.004G051900 of this group is orthologous to Arabidopsis ARF8 (Auxin response factor 8), involved in anther development43. Sorghum ortholog of ARF17 (Auxin response factor 17) that has been shown to regulate primexine deposition also exhibits group 2 expression pattern44. Overall, large number of candidates from this group have been shown to regulate male development confirming high relevance of this group in engineering male fertility.
Group 3 genes regulate microspore development
Group 3 contains 184 genes with peak expression in A2 stage containing developing microspores (Fig. 3). This group contains many sugar transporters and genes involved in carbohydrate degradation (Fig. 3, Table S4). Analysis of characterized rice orthologs of sorghum genes belonging to group 3 highlighted several important candidates for microspore development (Table 1). For instance, rice ortholog of Sobic.001G320300, OsNP1 (No Pollen 1), regulates tapetum degradation and exine formation; Sobic.004G017500 ortholog, TDR (Tapetum Degeneration Retardation), is a master regulator of anther development in both rice and Arabidopsis45, whereas, Sobic.004G181500 ortholog XRCC3 (X-ray repair cross-complementing group 3), regulates double strand break repair and homologous recombination during meiosis in rice46. Another interesting candidate from this group Sobic.008G016500 encodes for a putative rhamnogalacturonate lyase. Rice ortholog of this gene (LOC_Os12g03790) has been implicated in dissociation of microspores from tetrads through pectin degradation47.
Several rice orthologs of sorghum genes in this group have also been shown to regulate male fertility in response to abiotic stress conditions. For example, Drought-induced lipid transfer protein (OsDIL), orthologous to Sobic.001G266600, affects pollen fertility under drought conditions48, while, rice ortholog of a glycosyl hydrolase gene (Sobic.001G099700) confers cold tolerance to developing pollen49. Overall, 58 genes of this group exhibit anther-preferential expression, among which, rice orthologs of four genes (OsINV4 (Cell wall invertase 4), OsDIL, OsNP1, and TDR) have already been genetically characterized for regulating anther/pollen development. Among transcription factor families, NAC and MYB family transcription factors were particularly enriched in this group (Fig. 3).
Group 4 exhibits preponderance of stress-associated genes
Group 4 contains 168 genes with high expression in both A1 and A3 stages. This characteristic pattern can also be interpreted as dip in expression during microspore stage. Pathway analysis revealed many genes mapping to abiotic stress, calcium signalling pathways, glutathione S transferases, phosphatases, and invertases, and the genes encoding cytochrome P450 enriched in this group (Fig. 3, Table S4). Furthermore, 65 genes of this group exhibited anther-preferential expression. Although, rice orthologs of none of these have been characterized for roles in anther development, some of these genes have been implicated in stress response. Such as, rice ortholog (OsABA8Ox3; ABA 8′-hydroxylase 3) of a cytochrome P450 encoding gene (Sobic.007G156300) is upregulated in response to cold and ABA in anthers50. Also, OsABA8Ox3 exhibits downregulation in cold sensitive genotype, which is associated with cold-induced pollen sterility50. Another gene, Sobic.008G058500, putatively codes for a CYP71E1 protein. CYP71E1 has been previously reported as a stress-related protein in sorghum51.
Glutathione S transferases and calcium signalling genes, prominent in this group, are also important components of abiotic stress-related pathways. Among the calcium signalling genes detected in this group, rice ortholog of Sobic.003G082600, OsCML16 (Calmodulin-like protein 16) has been shown to enhance drought tolerance52. Whereas, rice ortholog of Sobic.003G365600, MID1 (MYB Important for Drought response 1) plays crucial role in anther development in response to drought stress53.
Pollen development is also highly sensitive to heat stress51. Spikelet sterility due to faulty pollen development and premature anther dehiscence in response to heat stress has been well-demonstrated in rice54,55. Frova and co-workers56 observed increased expression of heat shock proteins in early as well as the late stages of pollen development in sorghum. We also observed several genes involved in heat stress tolerance in this group. These include rice ortholog (OsHSP18.2) of one of the heat shock proteins (HSP), Sobic.003G039400, which imparts heat stress tolerance to germinating seeds by restricting ROS accumulation57. Whereas, Sobic.003G039400 ortholog, OsHSP17.0 has been shown to confer salt and drought tolerance in rice58. Additionally, three HSPs in this group are orthologous to Arabidopsis HSP17.6 (AT1G53540), regulated by chemical heat response inducers59. In-depth characterization of these genes will provide candidates to combat with abiotic stress-induced male sterility in sorghum60.
Group 5 genes regulate protein degradation and cell wall modification in the late pollen stages
Group 5 contains 499 genes exhibiting gradual increase in expression from meiotic to post-meiotic stage that peaks in mature pollen stage (Fig. 3). The key pathway categories in this group are associated with protein metabolism and transport including protein degradation, peptides/oligopeptides transport, and major intrinsic proteins. Other prominent categories are lipid metabolism and cell wall modification, with the latter showing significant enrichment (Table S4, Fig. 3). Recently, Moon and co-workers61 also highlighted abundance of the cell wall modification genes in mature pollen of rice.
The protein degradation-related genes include subtilases, ubiquitin-related genes, cysteine proteases, serine proteases, and ATPases. While, four genes in the major intrinsic proteins category in this group code for aquaporins. Conversely, lipid metabolism-related genes code for acetyl-CoA carboxylation, beta ketoacyl-CoA synthases, acyl-CoA binding proteins, and acyl-CoA ligases. Rice ortholog of two of the genes in this group, MID1 (Midline 1) and OsINV1 (Invertase 1) regulate pollen development in response to abiotic stress conditions49,53. Several cell wall modification genes including expansin precursors, a glycosyl hydrolase family 16 gene and four genes that encode for putative pollen allergen proteins are also enriched in this group. Two of the expansin genes, Sobic.001G306200 and Sobic.001G306400, are orthologs of OsIPA, which encode for expansins/pollen allergens with conserved role in both rice and Arabidopsis62.
Furthermore, 99 genes of group 5 exhibit anther-preferential expression. Among these, three are orthologous to rice OSIPA (Oryza sativa indica pollen allergen) and OsPCBP (Pollen specific calmodulin-binding protein) genes involved in pollen development62,63. Several of the group 5 genes encode for cell wall modification-related genes encoding pollen allergens and expansins (Table S3A). Pollen expressed expansins are also known to help in cell wall deposition, pollen germination, pollen tube growth and penetration while some of the expansins belong to the category of pollen allergens64. Group 5 genes, therefore, likely play role in cell wall modifications during pollen development.
Group 6 genes regulate cell wall, phytohormone and carbohydrate metabolism in mature pollen
Group 6, exhibiting mature pollen stage preferential/specific expression, contain the maximum number of genes (2291) belonging to 17 pathway categories (Table S4, Fig. 3). Maximum number of genes in this group are implicated in protein degradation and post translational modifications. These include subtilases, ubiquitin-related genes, cysteine proteases, aspartate proteases, serine proteases, ATPases, etc. Other notable categories/pathways enriched in this group include cell wall synthesis, ethylene metabolism, abscisic acid metabolism, metal transporters, and carbohydrate synthesis. In addition, transcription factors belonging to RAV, CO-like, DBB, DOF, ERF, and TALE families are also enriched in group 6.
One of the key events during pollen maturation is pollen wall development. Pollen wall comprises an outer exine made of sporopollenin, and an inner intine which is composed of cellulose, pectin, and hemicellulose. Cellulose synthase (CESA) and synthase like (CSL) genes in Arabidopsis have been shown to play important roles in pollen wall development and tube growth61,65,66. CESA7 ortholog, Sobic.001G224300 and eight cellulose synthase-like genes belonging to CSLD, CSLE, CSLF, and CSLH subfamilies exhibit group 6 pattern. Their increased expression from microspore to mature pollen stage corroborates with potential role in pollen wall synthesis.
Another metabolic checkpoint for pollen maturity is starch accumulation which serves as an energy resource during pollen germination67. Manipulation of genes involved in sugar-starch conversion can lead to starch deficiency, leading to sterility, as exemplified by the expression analysis of these genes in male sterile lines of sorghum and maize67. We identified nine genes involved in sucrose and starch metabolism, in addition to fifteen sugar transporters, seven genes related to trehalose metabolism, and seven genes involved in sugar and nutrient signalling, in this group. These are important candidates for regulating carbohydrate metabolism during pollen maturation. Rice ortholog of Sobic.004G013500 encodes UTP-glucose-1-phosphate uridylyltransferase (OsUGP2) with binucleate and mature pollen stage-preferential expression. OsUGP2 regulates starch accumulation in pollen68. Conversely, rice ortholog (WDA1; Wax-deficient anther 1) of Sobic.001G222700 is involved in fatty acid biosynthesis in tapetum and anther walls69.
In addition, 17 ethylene and 11 abscisic acid metabolism-related genes including aminocyclopropane carboxylate oxidases (ACCs) and aldehyde oxidases are enriched in this group. Ethylene is important for conferring thermotolerance in pollen70. Also, recent findings in rice indicate that enhancement of sucrose metabolism in response to ABA signalling is crucial for pollen protection from heat stress71. Thus, upregulation of ethylene and abscisic acid signalling may be important for the acquisition of abiotic stress tolerance in pollen. Increase in expression of ethylene and ABA genes during pollen development has also been observed in rice72. Furthermore, several key genes involved in jasmonic acid, cytokinin and auxin biosynthesis, and signaling also exhibit group 6 pattern, pointing to coordinated action of different phytohormones in pollen maturation73 (Table S3b).
Metal transporters can be important for maintaining metal ion homeostasis in developing pollen. Li and co-workers74 showed that magnesium homeostasis is important for pollen development. Disruption of magnesium transporter, AtMGT4 (Arabidopsis Magnesium transporter 4) leads to abnormal pollen development. We identified 17 metal transporter genes in group 6 including zinc, cadmium, copper, magnesium, manganese, and iron transporters. Furthermore, 351 genes of group 6 exhibit anther-preferential expression. These include sorghum orthologs of rice OsMADS3 (Sobic.003G027000), OsINV4 (Sobic.006G070032), OsPLIM2b (Sobic.006G159000), and MEL2 (Sobic.008G138200) (Table 1). The male sterility of a recessive male-sterile line RI127S in rice has been attributed to loss-of-function mutation in OsMADS375. Both OsPLIM2b and OsINV4 have been shown to exhibit mature pollen-specific expression and regulate anther development49,76. MEL2 (Meiosis Arrested at Leptotene 2), on the other hand, is involved in translational regulation of several meiotic genes in rice77.
Group 7 genes regulate secondary metabolite biosynthesis in microspore and mature pollen stages
Group 7 contains 151 genes exhibiting increase in expression from meiotic to post-meiotic stage that sustains in mature pollen stage as well (Fig. 3). Genes belonging to NAC family of transcription factors and those encoding for secondary metabolites are particularly enriched in this group (Fig. 3). Eighty-one genes of group 7 are anther-preferential, however, rice orthologs of none of them have been functionally characterized, till date.
Comparative genomic analysis in rice and sorghum emphasize conserved developmental pathways regulating anther development
Though the molecular regulation of anther and pollen development has been well elucidated in several model plants78,79,80,81, little is known about these processes in sorghum. Comparative genomic approaches provide an opportunity to translate this knowledge in agriculturally important crop plants31. However, mere sequence homology is not enough to predict functional conservation among orthologous genes. Comparative expression profiling in analogous stages of development is necessary to identify the functional orthologs with conserved roles31.
Recently, Lin and co-workers82 generated a co-expression network from different developmental stages of anthers, named RiceAntherNet, by compiling data from 57 microarrays. Using hierarchical clustering of DEGs from nine male sterile mutants corresponding to master regulators of anther development viz., MSP1 (Multiple sporocyte 1), TDL1, GAMYB (Gibberellin MYB), TIP2, UDT1 (Undeveloped tapetum 1), TDR, EAT1, PTC1 (Persistent tapetal cell 1), and MADS3, authors identified 29 gene clusters which could be classified into three clades: clade 1 genes specifically expressed in stage 8 and 9 representing tetrads and uninucleate microspore stage, clade 2 genes expressed in stages 2 to 8 harbouring pre-meiotic to tetrad stage anthers, whereas, clade 3 genes expressed in mature pollen (Fig. 4). Out of 764 rice genes comprising clade 1, 2, and 3 of RiceAntherNET, sorghum orthologs of 258 genes were differentially expressed in sorghum anthers as well. Comparison of the gene clusters obtained in our study for sorghum vis-à-vis clades identified in RiceAntherNet revealed interesting parallels (Fig. 4; Table S6). The clade 2 of RiceAntherNet possessed 132 genes. Of these, sorghum orthologs of 48 genes exhibited group 2 pattern. Clade 2 of RiceAntherNet as well as group 2 genes of sorghum in our study exhibited peak expression in premeiotic to tetrad stages of anther development. Similarly, out of 60 clade 1 genes of RiceAntherNet, 22 genes belonged to group 2 and 3 of sorghum, with both these groups exhibiting high expression in tetrad to microspore stages. Furthermore, out of 572 clade 3 genes of RiceAntherNet, sorghum orthologs of 142 genes belonged to group 6, with both clade 3 and group 6 having pollen-specific expression. High concurrence in expression patterns of genes during anther development in rice and sorghum confirm conserved developmental pathways underlying male gametophyte development in grasses.
Comparison of sorghum and rice anther transcriptome. Lin and co-workers82 identified differentially expressed genes (DEGs) in several male sterile mutants of rice and divided them into three clades based on stage specific expression. Clade 2, 1, and 3 represent premeiotic to tetrad, tetrad and microspore, and pollen-related genes, respectively. A comparison with different groups of DEGs in our data highlighted interesting parallels between two studies. Based on orthology, the clade 2 genes of rice showed maximum common genes with group 2 of our data; clade 1 showed common genes with groups 2 and 3; and clade 3 showed maximum common genes with groups 5 and 6.
In fact, Cigan and co-workers7, recently demonstrated conserved role of SbMs26 (Sobic.001G491400), orthologous to rice CYP704B2, in regulating male fertility. Targeted mutagenesis of this gene led to defects in pollen formation. Therefore, collating information about rice orthologs of differentially expressed genes in sorghum anthers is a valid strategy for shortlisting candidates for engineering male fertility in sorghum. Rice orthologs of 63 differentially expressed genes in sorghum anthers have been functionally characterized for their involvement in anther/pollen development using forward and/or reverse genetics approaches (Table 1). Among these, 21 genes exhibit anther-preferential expression and therefore, would serve as important targets for engineering male fertility without off-target effects.
Analysis of cis-regulatory elements
The spatial and temporal specificity of gene expression is mediated by specific binding of transcription factors on cis-regulatory elements. Therefore, analysis of cis-regulatory elements is essential to elucidate mechanistic basis of gene regulation. Several cis-regulatory elements have been earlier associated with genes exhibiting anther and/or pollen-specific expression in model species, rice and Arabidopsis83,84,85,86,87. Based on the published literature, we compiled a list of 65 such elements and queried them separately in 1 kb regulatory regions of differentaily expressed genes. The enrichment analysis was performed separately for each group using regulatory regions of all the genes annotated in sorghum as reference. Notably, 41 out of 65 elements analysed exhibited significant enrichment in the regulatory regions of differentially expressed genes in sorghum anthers (Supplementary Table S7). Most of them have been identified from regulatory regions of tapetum and/or male gamete-specific genes in rice (Supplementary Table S7). Among unique motifs enriched in regulatory regions of group 1 genes is MSACRCYM motif which has earlier been reported from promoter of cell cycle-related genes in Catharanthus84. This is particularly interesting because cell cycle-related genes are also enriched in group 1 (Fig. 3). An ABA-responsive motif ABRE3HVA22 is specifically enriched in regulatory regions of group 6 genes which are also enriched in ABA biosynthesis/signalling-related genes84 (Supplementary Table S7 and Fig. 3). Interestingly, group 7 genes exhibit enrichment of 5659BOXLELAT5659 element which has been shown to be responsible for pollen specific expression of pollen expressed genes, LAT56 and 59, of tomato84. These findings hint to a conserved regulatory mechanism for cell/tissue/developmental stage-specific expression of genes regulating male gametophyte development in plants.
Validation of RNA-seq data by qRT-PCR analysis
To validate the expression patterns of differentially expressed genes in the three anther stages, we selected ten representative genes exhibiting varied expression patterns (Supplementary Table S8). Rice orthologs of five of these genes have been earlier shown to perform crucial functions during anther development in rice (Table 1). Except one gene which exhibited Pearson’s correlation coefficient of 0.49 with the RNA-seq data, expression patterns of all other genes showed good correlation ranging from 0.80 to 0.99 between two techniques, indicating high reproducibility of the transcriptomic data (Fig. 5).
Relative expression of ten genes in anther stages measured by qRT-PCR. Expression of ten genes exhibiting varied expression patterns in RNA sequencing data was analyzed using qRT-PCR. The locus IDs of genes are given on the top with corresponding rice orthologs (if characterized). The Pearson’s correlation coefficient (R) value for each gene indicating correlation between qRT-PCR and RNA-seq data is given on the top right corner.
Overall, transcriptional trajectories collated with enriched pathways and functions of rice orthologs depict conserved molecular cascade underlying progression of anther development in sorghum (Fig. 6). The genes belonging to these cascades will serve as valuable resource for engineering male sterility in sorghum with potential applications in hybrid breeding, eliminating pollen allergens and avoiding gene flow from genetically modified plants. The expression of ribonuclease barnase genes driven by anther/pollen-specific promoter has been extensively used for engineering male sterility in other crop plants88,89,90. Therefore, the regulatory elements of shortlisted 21 anther-preferential genes would be important candidates for driving tissue-specific expression. To facilitate this, anther expressed genes can be experimentally validated for their tissue specificity using reporter genes. Another strategy would be to engineer male sterility by targeted mutagenesis of anther-specific genes using CRISPR/cas9 or gene silencing strategies. The genetically engineered male sterile lines would then be assessed for genetic stability, heterosis and, potential linkage with unwanted traits using empirical approaches91,92. Recently, we have reported small RNA profiles of meiotic and post-meiotic anthers in sorghum. Interestingly, several of the differentially expressed genes in anthers are also miRNA targets providing additional opportunity to engineer male sterility by targeting non-coding RNAs93,94.
Overview of dominant patterns and associated functions. Four different types of patterns representing premeiotic to tetrad stage preferential expression, microspore-preferential, early and late pollen-preferential and mature pollen preferential patterns are shown in the middle. The key pathways associated with each category are shown on the left, while right panel indicating key functions exhibited by rice orthologs of sorghum genes identified in these categories.
Methods
Staging and harvesting of anther samples
Sorghum bicolor variety M35-1 plants were grown at the ICAR-Indian Agricultural Research Institute, New Delhi, India. The panicles were collected at different stages of development based on the position of flag leaf ranging from pre-emergence to fully emerged panicles, so as to harvest the anthers involving meiotic to mature pollen stages. The length of anthers, dissected from panicles at different stages of development, were measured and recorded. Freshly extracted anthers, based on their length, were categorized into three stages and stored in separate tubes containing TRIzolTM reagent with stage A1 containing anthers of lengths smaller than 1.5 mm, stage A2 containing 1.6 to 2.0 mm anthers and, stage A3 containing 2.5 to 3.0 mm anthers. Three replicates of anthers collected for each stage were snap frozen in liquid nitrogen and stored at −80 °C. About 15–20 anthers from each stage were simultaneously stored in a fixative (ethanol and glacial acetic acid in 3:1 ratio) at 4 °C for microscopic analysis.
Microscopic analysis
To confirm the staging of anther development, microscopic analysis was performed with the anthers sampled from each stage (A1-A3) using a published protocol with minor modifications95. Briefly, anthers were dehydrated using ethanol gradient (50%, 80%, 90% and 100%) for 1 hour each followed by two washes of 100% and 90% acetone for one hour each. Anthers were cleared in 100% and 90% xylene for one hour each and, passed through two changes of paraffin wax at 75 °C for 1.5 hours each. Further, 5 µm sections were obtained from the embedded tissue using a Leica RM2235 microtome (Leica, Germany). Samples were deparaffinized in xylene and again passed through graded ethanol series for 2–3 minutes each. Finally, the sections were washed in running tap water and stained with toluidine blue O for 15–20 seconds. Excess stain was removed by rinsing with tap water. Sections were dried, mounted using resinous mounting medium and viewed using bright field microscope (Nikon, Eclipse 80i).
RNA isolation, library preparation and sequencing
Total RNA was extracted from anther tissue using TRIzolTM reagent as per manufacturer’s instructions (Invitrogen) with minor modifications. Briefly, anthers suspended in 200 µl of TRIzolTM were crushed finely using epi-pestles. To the grinded sample, 800 µl of TRIzolTM was added and mixed by inverting the tubes. After 5 min incubation at room temperature (RT), tubes were centrifuged at 10,000 rpm for 10 min at RT to remove the debris. To the supernatant, 200 µl of chloroform was added followed by centrifugation at 13000 rpm for 10 min at RT. To the supernatant, half volume of 7.5 M ammonium acetate was added and mixed gently. Samples were centrifuged at 12000 rpm for 10 min. To the supernatant collected in fresh tube, 3 volumes of chilled ethanol was added, and tubes were stored at −20 °C overnight for RNA precipitation. Next day, samples were centrifuged at 10,000 rpm for 5 min at 4 °C. The pellet was washed twice with 75% chilled ethanol, dried at RT and dissolved in RNAse free water. The quantity and quality of RNA samples were determined using agarose gel electrophoresis, NanodropTM 2000 and Agilent 2100 Bioanalyzer. Only RNA samples with RIN value >8 were used for library preparation. The sequencing libraries were prepared using TruSeq® Stranded Total RNA kit using manufacturer’s instructions and sequenced using Illumina HiSeq 2500 paired-end sequencing with an average read length of 100 bp. The RNA-seq data has been submitted to NCBI Gene Expression Omnibus (GEO) and can be accessed using its assigned accession number, GSE141035.
Data analysis
Low-quality reads with Phred score <30 were filtered and adapters were trimmed using AdapterRemoval version 2.2.096. From the pre-processed reads, ribosomal RNA sequences were removed by aligning the reads with SILVA database97 using Bowtie version 2.2.9 with default parameters. Reference genome sequence and annotations of sorghum were downloaded from Phytozome (URL: https://genome.jgi.doe.gov/portal/pages/dynamicOrganismDownload.jsf?organism=Sbicolor). High quality reads were aligned to the sorghum genome using STAR aligner98 using default parameters. The aligned reads were used for estimating expression of the genes and transcripts using StringTie version 1.3.3b99. The correlation between biological replicates was calculated using Spearman’s correlation coefficient using R.
Pairwise differential expression analyses between different developmental stages was performed using cuffdiff program of cufflinks package100. The transcripts with log2 fold change ≥1 (up-regulated genes) or ≤−1 (down-regulated genes) and q-value cut off ≤0.05 were considered as differentially expressed. FPKM values of differentially expressed transcripts were used for clustering the data using K-means clustering in MultiExperiment Viewer (MeV)101. Pathway analysis was carried out using MapMan102 with Sbicolor_79 1.0 mapping file (https://mapman.gabipd.org/mapmanstore), and enrichment of pathway sub-categories was determined using hypergeometric test (p-value ≤0.05). Further, p-values were corrected using Benjamini-Hochberg multiple-testing correction (false discovery rate (FDR) < 0.05). The information about transcription factors in sorghum was extracted from ‘Plant Transcription Factor Database’103 and used for enrichment analysis using hypergeometric test (p value ≤0.05). The p-values were corrected using Benjamini-Hochberg test (FDR < 0.05). The information of the rice as well as Arabidopsis orthologs was obtained from Phytozome v3.1.1 (https://phytozome.jgi.doe.gov/pz/portal.html). Rice genes, previously characterized for their role in anther development, were extracted from FunRiceGenes database104 (URL: https://funricegenes.github.io/). The phytohormone-related genes from rice were obtained from published literature28 and the information about their sorghum orthologs was extracted from Phytozome v3.1.1 (https://phytozome.jgi.doe.gov/pz/portal.html). To identify anther-preferential genes, publicly available expression data from 47 RNA sequencing experiments corresponding to different stages of root, shoot, leaf, panicle and seed development as well as stress treatments, was downloaded using “PhytoMine” tool available at Phytozome database. The genes exhibiting FPKM > 1 in any of the vegetative or seed stages with or without treatment were filtered from differential expressed gene set to extract anther-preferential genes.
Analysis of cis-regulatory regions
To assess the enrichment of known motif elements in the regulatory regions of differentially expressed genes in anthers, sequences corresponding to 1 kb regulatory regions of all the differentially expressed genes were downloaded using BioMart tool of Phytozome (https://phytozome.jgi.doe.gov/biomart/martview/f7080098cbb5de1a01647b16a4422139). The list of 65 known motifs implicated in anther/pollen development was compiled from literature83,84,85,86,87 and separately queried in the regulatory regions of genes belonging to each cluster of DEGs using CentriMo tool under MEME suite (http://meme-suite.org/tools/centrimo) with default parameters. For enrichment analysis, dataset of 1 kb promoter regions of all sorghum genes was used as reference.
qRT-PCR analysis
RNA was extracted from three biological replicates of each anther stage as described above. cDNA was prepared using iScript™ cDNA Synthesis Kit (Biorad) and qPCR was carried out using EvaGreen dye (G-Biosciences) on a CFX96 Real Time System (BioRad) following manufacturer’s instructions. Briefly, a 10 μL reaction was prepared using 5 μL EvaGreen dye, 1 μL primer (each primer), 1 μL of diluted cDNA template and 2 μL of nuclease free water. Reaction conditions were as follows: 95 °C for 2 mins, followed by 40 cycles of 15 s at 95◦C and 30 s at 60 °C. After amplification, melt curves were analysed to ensure primer specificity in each reaction. Relative transcript expression levels were obtained using the double delta CT (ΔΔCT) method. Eukaryotic Initiation Factor 4α (eIF4α) was used as an internal control105,106. The expression values were expressed as mean ± SD. The primers used for the qRT-PCR are listed in Supplementary Table S7. Pearson’s correlation coefficient (R) was calculated between the expression values obtained using RNA sequencing and qRT-PCR analysis for each gene.
References
Mathur, S., Umakanth, A., Tonapi, V., Sharma, R. & Sharma, M. K. Sweet sorghum as biofuel feedstock: recent advances and available resources. Biotechnol. Biofuels 10, 146 (2017).
Ellstrand, N. & Foster, K. Impact of population structure on the apparent outcrossing rate of grain sorghum (Sorghum bicolor). Theor. Appl. Genet. 66, 323–327 (1983).
Schertz, K. & Dalton, L. Sorghum. Hybridization of Crop Plants, 577–588 (1980).
Kim, Y.-J. & Zhang, D. Molecular control of male fertility for crop hybrid breeding. Trends Plant Sci. (2017).
Budar, F. & Pelletier, G. Male sterility in plants: occurrence, determinism, significance and use. Comptes Rendus de l’Académie des Sciences-Series III-Sciences de la Vie 324, 543–550 (2001).
Chang, Z. et al. Construction of a male sterility system for hybrid rice breeding and seed production using a nuclear male sterility gene. Proc. Natl. Acad. Sci. USA 113, 14145–14150 (2016).
Cigan, A. M. et al. Targeted mutagenesis of a conserved anther‐expressed P450 gene confers male sterility in monocots. Plant Biotechnol. J. 15, 379–389 (2017).
Xin, Z. et al. Morphological characterization of a new and easily recognizable nuclear male sterile mutant of sorghum (Sorghum bicolor). PLoS One 12, e0165195 (2017).
Christensen, J. E. Developmental aspects of microsporogenesis in Sorghum bicolor (1972).
Lo, S. F. et al. Ectopic expression of specific GA 2 oxidase mutants promotes yield and stress tolerance in rice. Plant Biotechnol. J. 15, 850–864 (2017).
Kovaleva, L., Timofeeva, G., Rodionova, G., Zakharova, E. & Rakitin, V. Y. Role of ethylene in the control of gametophyte-sporophyte interactions in the course of the progamic phase of fertilization. Russian J. Dev. Biol. 44, 69–77 (2013).
Deng, Z.-Y. & Wang, T. OsDMC1 is required for homologous pairing in Oryza sativa. Plant Mol. Biol. 65, 31–42 (2007).
Hong, J.-P. et al. OsKu70 is associated with developmental growth and genome stability in rice. Plant Physiol. 152, 374–387 (2010).
Ye, Q. et al. Brassinosteroids control male fertility by regulating the expression of key genes involved in Arabidopsis anther and pollen development. Proc. Natl. Acad. Sci. USA 107, 6100–6105 (2010).
Zhang, H., Xia, R., Meyers, B. C. & Walbot, V. Evolution, functions, and mysteries of plant ARGONAUTE proteins. Curr. Opin. Plant Biol. 27, 84–90 (2015).
Fei, Q., Yang, L., Liang, W., Zhang, D. & Meyers, B. C. Dynamic changes of small RNAs in rice spikelet development reveal specialized reproductive phasiRNA pathways. J. Exp. Bot. 67, 6037–6049 (2016).
Zhai, J. et al. Spatiotemporally dynamic, cell-type–dependent premeiotic and meiotic phasiRNAs in maize anthers. Proc. Natl. Acad. Sci. USA 112, 3146–3151 (2015).
Lorenzo, J. L., Hobza, R. & Vyskot, B. Epigenetic control of reproductive development. Plant Syst. Evol. 303, 991–1000 (2017).
Wang, K. et al. The role of rice HEI10 in the formation of meiotic crossovers. PLoS Genetics 8, e1002809 (2012).
Fu, Z. et al. The rice basic helix-loop-helix transcription factor TDR INTERACTING PROTEIN2 is a central switch in early anther development. Plant Cell, tpc. 114.123745 (2014).
Niu, N. et al. EAT1 promotes tapetal cell death by regulating aspartic proteases during male reproductive development in rice. Nat. Commut. 4, 1445 (2013).
Tao, J., Zhang, L., Chong, K. & Wang, T. OsRAD21‐3, an orthologue of yeast RAD21, is required for pollen development in Oryza sativa. Plant J. 51, 919–930 (2007).
Zhang, D.-S. et al. Tapetum degeneration retardation is critical for aliphatic metabolism and gene regulation during rice pollen development. Mol. Plant 1, 599–610 (2008).
Wang, C.-W. et al. OIP30, a RuvB-like DNA helicase 2, is a potential substrate for the pollen-predominant OsCPK25/26 in rice. Plant Cell Physiol. 52, 1641–1656 (2011).
Ji, J. et al. MRE11 is required for homologous synapsis and DSB processing in rice meiosis. Chromosoma 122, 363–376 (2013).
Li, X. et al. Replication protein A2c coupled with replication protein A1c regulates crossover formation during meiosis in rice. Plant Cell, tpc. 113.118042 (2013).
Zhang, L. et al. Crossover formation during rice meiosis relies on interaction of OsMSH4 and OsMSH5. Genetics, genetics. 114.168732 (2014).
Ma, Q. et al. Enhanced tolerance to chilling stress in OsMYB3R-2 transgenic rice is mediated by alteration in cell cycle and ectopic expression of stress genes. Plant Physiol. 150, 244–256 (2009).
Wang, M. et al. The central element protein ZEP1 of the synaptonemal complex regulates the number of crossovers during meiosis in rice. Plant Cell 22, 417–430 (2010).
Zhang, D., Shi, J. & Yang, X. In Lipids in plant and algae development 315–337 (Springer, 2016).
Tang, X. et al. Global gene profiling of laser-captured pollen mother cells indicates molecular pathways and gene subfamilies involved in rice meiosis. Plant Physiol., pp. 110.161661 (2010).
Yang, X. et al. Rice CYP703A3, a cytochrome P450 hydroxylase, is essential for development of anther cuticle and pollen exine. J. Integr. Plant Biol. 56, 979–994 (2014).
Yang, Z. et al. Identification of cyp703a3-3 and analysis of regulatory role of CYP703A3 in rice anther cuticle and pollen exine development. Gene 649, 63–73 (2018).
Zou, T. et al. An atypical strictosidine synthase, OsSTRL2, plays key roles in anther development and pollen wall formation in rice. Sci. Rep. 7, 6863 (2017).
Hihara, Y., Hara, C. & Uchimiya, H. Isolation and characterization of two cDNA clones for mRNAs that are abundantly expressed in immature anthers of rice (Oryza sativa L.). Plant Mol. Biol. 30, 1181–1193 (1996).
Zhao, G. et al. Two ATP Binding Cassette G (ABCG) transporters, OsABCG26 and OsABCG15, collaboratively regulate rice male reproduction. Plant Physiol., pp. 00262.02015 (2015).
Zou, T. et al. OsPKS2 is required for rice male fertility by participating in pollen wall formation. Plant Cell Rep. 37, 759–773 (2018).
Sumiyoshi, M. et al. UDP-arabinopyranose mutase 3 is required for pollen wall morphogenesis in rice (Oryza sativa). Plant Cell Physiol. 56, 232–241 (2014).
Yi, J. et al. Defective Tapetum Cell Death 1 (DTC1) regulates ROS levels by binding to metallothionein during tapetum degeneration. Plant Physiol., pp. 01561.02015 (2016).
Xu, Y. et al. HOTHEAD-Like HTH1 is Involved in Anther Cutin Biosynthesis and is Required for Pollen Fertility in Rice. Plant Cell Physiol. 58, 1238–1248 (2017).
Cai, Q. et al. Elevation of pollen mitochondrial DNA (mtDNA) copy number by WHIRLY2: altered respiration and pollen tube growth in Arabidopsis. Plant Physiol., pp. 00437.02015 (2015).
Xing, S., Salinas, M., Höhmann, S., Berndtgen, R. & Huijser, P. miR156-targeted and nontargeted SBP-box transcription factors act in concert to secure male fertility in Arabidopsis. Plant Cell, tpc. 110.079343 (2010).
Wu, M.-F., Tian, Q. & Reed, J. W. Arabidopsis microRNA167 controls patterns of ARF6 and ARF8 expression, and regulates both female and male reproduction. Development 133, 4211–4218 (2006).
Yang, J. et al. AUXIN RESPONSE FACTOR17 is essential for pollen wall pattern formation in Arabidopsis. Plant Physiol., pp. 113.214940 (2013).
Li, N. et al. The rice tapetum degeneration retardation gene is required for tapetum degradation and anther development. Plant Cell 18, 2999–3014 (2006).
Zhang, B. et al. XRCC3 is essential for proper double-strand break repair and homologous recombination in rice meiosis. J. Exp. Bot. 66, 5713–5725 (2015).
Fujita, M. et al. Rice expression atlas in reproductive development. Plant Cell Physiol. 51, 2060–2081 (2010).
Guo, C., Ge, X. & Ma, H. The rice OsDIL gene plays a role in drought tolerance at vegetative and reproductive stages. Plant Mol. Biol. 82, 239–253 (2013).
Oliver, S. N. et al. Cold‐induced repression of the rice anther‐specific cell wall invertase gene OSINV4 is correlated with sucrose accumulation and pollen sterility. Plant Cell Environ. 28, 1534–1551 (2005).
Oliver, S. N., Dennis, E. S. & Dolferus, R. ABA regulates apoplastic sugar transport and is a potential signal for cold-induced pollen sterility in rice. Plant Cell Physiol. 48, 1319–1330 (2007).
Roy, S. K. et al. Proteome characterization of copper stress responses in the roots of sorghum. BioMetals 30, 765–785 (2017).
Jung, H. et al. Overexpression of Os ERF 48 causes regulation of Os CML 16, a calmodulin‐like protein gene that enhances root growth and drought tolerance. Plant Biotechnol. J. 15, 1295–1308 (2017).
Guo, C. et al. MID1 plays an important role in response to drought stress during reproductive development. Plant J. 88, 280–293 (2016).
Rieu, I., Twell, D. & Firon, N. Pollen development at high temperature: from acclimation to collapse. Plant Physiol. 173, 1967–1976 (2017).
Jagadish, S., Craufurd, P. & Wheeler, T. High temperature stress and spikelet fertility in rice (Oryza sativa L.). J. Exp. Bot. 58, 1627–1635 (2007).
Frova, C., Taramino, G. & Ottaviano, E. Sporophytic and gametophytic heat shock protein synthesis in Sorghum bicolor. Plant Sci. 73, 35–44 (1991).
Kaur, H. et al. Differentially expressed seed aging responsive heat shock protein OsHSP18. 2 implicates in seed vigor, longevity and improves germination and seedling establishment under abiotic stress. Front Plant Sci. 6, 713 (2015).
Wang, A. et al. Overexpression of a small heat‐shock‐protein gene enhances tolerance to abiotic stresses in rice. Plant Breed. 134, 384–393 (2015).
Hara, M., Yamauchi, N. & Sumita, Y. Monoterpenes induce the heat shock response in Arabidopsis. Zeitschrift für Naturforschung C 73, 177–184 (2018).
De Storme, N. & Geelen, D. The impact of environmental stress on male reproductive development in plants: biological processes and molecular mechanisms. Plant Cell Environ. 37, 1–18 (2014).
Moon, S. et al. Genome-wide analyses of late pollen-preferred genes conserved in various rice cultivars and functional identification of a gene involved in the key processes of late pollen development. Rice 11, 1–14 (2018).
Swapna, L., Khurana, R., Kumar, S. V., Tyagi, A. & Rao, K. Pollen-specific expression of Oryza sativa indica pollen allergen gene (OSIPA) promoter in rice and Arabidopsis transgenic systems. Mol. Biotechnol. 48, 49–59 (2011).
Zhang, Q. et al. Characterisation and immunolocalisation of a pollen-specific calmodulin-binding protein from rice. Funct. Plant Biol. 33, 555–562 (2006).
Chen, M. et al. Origin and functional prediction of pollen allergens in plants. Plant Physiol., pp. 00625.02016 (2016).
Goubet, F. et al. AtCSLA7, a cellulose synthase-like putative glycosyltransferase, is important for pollen tube growth and embryogenesis in Arabidopsis. Plant Physiol. 131, 547–557 (2003).
Persson, S. et al. Genetic evidence for three unique components in primary cell-wall cellulose synthase complexes in Arabidopsis. Proc. Natl. Acad. Sci. USA 104, 15566–15571 (2007).
Datta, R., Chourey, P. S., Pring, D. R. & Tang, H. V. Gene-expression analysis of sucrose-starch metabolism during pollen maturation in cytoplasmic male-sterile and fertile lines of sorghum. Sex. Plant Reprod. 14, 127–134 (2001).
Mu, H., Ke, J., Liu, W., Zhuang, C. & Yip, W. UDP-glucose pyrophosphorylase2 (OsUgp2), a pollen-preferential gene in rice, plays a critical role in starch accumulation during pollen maturation. Chin. Sci. Bull. 54, 234 (2009).
Jung, K.-H. et al. Wax-deficient anther1 is involved in cuticle and wax production in rice anther walls and is required for pollen development. Plant Cell 18, 3015–3032 (2006).
Jegadeesan, S. et al. Ethylene production and signaling in tomato (Solanum lycopersicum) pollen grains is responsive to heat stress conditions. Plant Reprod., 1–17 (2018).
Rezaul, I. M. et al. Abscisic acid prevents pollen abortion under high‐temperature stress by mediating sugar metabolism in rice spikelets. Physiol. Plant (2018).
Hirano, K. et al. Comprehensive transcriptome analysis of phytohormone biosynthesis and signaling genes in microspore/pollen and tapetum of rice. Plant Cell Physiol. 49, 1429–1450 (2008).
Song, S., Qi, T., Huang, H. & Xie, D. Regulation of stamen development by coordinated actions of jasmonate, auxin, and gibberellin in Arabidopsis. Mol. Plant 6, 1065–1073 (2013).
Li, J. et al. An endoplasmic reticulum magnesium transporter is essential for pollen development in Arabidopsis. Plant Sci. 231, 212–220 (2015).
Zhang, L. et al. Loss of function of OsMADS3 via the insertion of a novel retrotransposon leads to recessive male sterility in rice (Oryza sativa). Plant Sci. 238, 188–197 (2015).
Ye, J., Zhou, L. & Xu, M. Arabidopsis LIM proteins PLIM2a and PLIM2b regulate actin configuration during pollen tube growth. Biologia plantarum 57, 433–441 (2013).
Miyazaki, S., Sato, Y., Asano, T., Nagamura, Y. & Nonomura, K.-I. Rice MEL2, the RNA recognition motif (RRM) protein, binds in vitro to meiosis-expressed genes containing U-rich RNA consensus sequences in the 3′-UTR. Plant Mol. Biol. 89, 293–307 (2015).
Deveshwar, P., Bovill, W. D., Sharma, R., Able, J. A. & Kapoor, S. Analysis of anther transcriptomes to identify genes contributing to meiosis and male gametophyte development in rice. BMC Plant Biol. 11, 78 (2011).
Gómez, J. F., Talle, B. & Wilson, Z. A. Anther and pollen development: a conserved developmental pathway. J. Integr. Plant Biol. 57, 876–891 (2015).
Honys, D. & Twell, D. Comparative analysis of the Arabidopsis pollen transcriptome. Plant Physiol. 132, 640–652 (2003).
Rutley, N. & Twell, D. A decade of pollen transcriptomics. Plant Reprod. 28, 73–89 (2015).
Lin, H., Yu, J., Pearce, S. P., Zhang, D. & Wilson, Z. A. RiceAntherNet: a gene co‐expression network for identifying anther and pollen development genes. Plant J. 92, 1076–1091 (2017).
Alvarado, V. Y., Tag, A. & Thomas, T. L. A cis regulatory element in the TAPNAC promoter directs tapetal gene expression. Plant Mol. Biol. 75, 129–139 (2011).
Hobo, T. et al. Various spatiotemporal expression profiles of anther-expressed genes in rice. Plant Cell Physiol. 49, 1417–1428 (2008).
Li, J., Yuan, J. & Li, M. Characterization of putative cis-regulatory elements in genes preferentially expressed in Arabidopsis male meiocytes. BioMed research international 2014 (2014).
Sharma, N., Russell, S. D., Bhalla, P. L. & Singh, M. B. Putative cis-regulatory elements in genes highly expressed in rice sperm cells. BMC research notes 4, 319 (2011).
Khurana, R., Kapoor, S. & Tyagi, A. K. Anthology of anther/pollen-specific promoters and transcription factors. Crit. Rev. Plant Sci. 31, 359–390 (2012).
Mishra, S. & Kumari, V. A review on male sterility-concepts and utilization in vegetable crops. Int. J. Curr. Microbiol. App. Sci. 7, 3016–3034 (2018).
Dutt, M., Dhekney, S. A., Soriano, L., Kandel, R. & Grosser, J. W. Temporal and spatial control of gene expression in horticultural crops. Horticulture research 1, 14047 (2014).
Roque, E. M., Gómez-Mena, C., Hamza, R., Beltrán, J. P. & Cañas, L. A. Engineered male sterility by early anther ablation using the pea anther-specific promoter PsEND1. Front Plant Sci. 10, 819 (2019).
Wan, X. & Wu, S. In Molecular Cloning-Methods and Applications (IntechOpen, 2019).
Wan, X. et al. Maize genic male-sterility genes and their applications in hybrid breeding: Progress and perspectives. Mol. Plant (2019).
Dhaka, N. et al. Small RNA profiling from meiotic and post-meiotic anthers reveals prospective miRNA-target modules for engineering male fertility in sorghum. Genomics (2019).
Mishra, A. & Bohra, A. Non-coding RNAs and plant male sterility: current knowledge and future prospects. Plant Cell Rep. 37, 177–191 (2018).
Kim, M.-J. & Kim, J. Semi-thin Sectioning, Light and Fluorescence Microscopy of Floral Bud to Study Microspore Development in Arabidopsis. Plant J. (2015).
Schubert, M., Lindgreen, S. & Orlando, L. AdapterRemoval v2: rapid adapter trimming, identification, and read merging. BMC Research Notes 9(1) (2016)
Quast, C. et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596 (2012).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, (15–21 (2013).
Pertea, M. et al. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 33, 290 (2015).
Trapnell, C. et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 7, 562 (2012).
Howe, E. et al. In Biomedical informatics for cancer research 267–277 (Springer, 2010).
Usadel, B. et al. A guide to using MapMan to visualize and compare Omics data in plants: a case study in the crop species, Maize. Plant Cell Environ. 32, 1211–1229 (2009).
Pérez-Rodríguez, P. et al. PlnTFDB: updated content and new features of the plant transcription factor database. Nucleic Acids Res. 38, D822–D827 (2009).
Yao, W., Li, G., Yu, Y. & Ouyang, Y. funRiceGenes dataset for comprehensive understanding and application of rice functional genes. GigaScience 7, gix119 (2017).
Sudhakar Reddy, P. et al. Evaluation of sorghum [Sorghum bicolor (L.)] reference genes in various tissues and under abiotic stress conditions for quantitative real-time PCR data normalization. Front Plant Sci. 7, 529 (2016).
Ji, Y. et al. Defining reference genes for quantitative real-time PCR analysis of anther development in rice. Acta Biochim. Biophys. Sin. 46, 305–312 (2014).
Luo, Q. et al. The role of OsMSH5 in crossover formation during rice meiosis. Mol. Plant 6, 729–742 (2013).
Saito, K. et al. Identification of two closely linked quantitative trait loci for cold tolerance on chromosome 4 of rice and their association with anther length. Theor. Appl. Genet. 103, 862–868 (2001).
Ji, J. et al. The role of OsCOM1 in homologous chromosome synapsis and recombination in rice meiosis. Plant J. 72, 18–30 (2012).
Miao, C. et al. Central region component1, a novel synaptonemal complex component, is essential for meiotic recombination initiation in rice. Plant Cell 25, 2998–3009 (2013).
Wang, M. et al. OsSGO1 maintains synaptonemal complex stabilization in addition to protecting centromeric cohesion during rice meiosis. Plant J. 67, 583–594 (2011).
Yuan, W. et al. Mutation of the rice gene PAIR3 results in lack of bivalent formation in meiosis. Plant J. 59, 303–315 (2009).
Li, H. et al. Cytochrome P450 family member CYP704B2 catalyzes the ω-hydroxylation of fatty acids and is required for anther cutin biosynthesis and pollen exine formation in rice. Plant Cell 22, 173–190 (2010).
Zhu, X. et al. The polyketide synthase OsPKS2 is essential for pollen exine and Ubisch body patterning in rice. J. Integr. Plant Biol. 59, 612–628 (2017).
Hu, Q. et al. MEICA 1 (meiotic chromosome association 1) interacts with TOP3α and regulates meiotic recombination in rice. Plant Cell, tpc. 00241.02017 (2017).
Zhang, L., Tao, J., Wang, S., Chong, K. & Wang, T. The rice OsRad21-4, an orthologue of yeast Rec8 protein, is required for efficient meiosis. Plant Mol. Biol. 60, 533–554 (2006).
Nonomura, K.-I. et al. A novel RNA-recognition-motif protein is required for premeiotic G1/S-phase transition in rice (Oryza sativa L.). PLoS Genetics 7, e1001265 (2011).
Li, Y. et al. OsACOS12, an orthologue of Arabidopsis acyl-CoA synthetase5, plays an important role in pollen exine formation and anther development in rice. BMC Plant Biol. 16, 256 (2016).
Unte, U. S. et al. SPL8, an SBP-box gene that affects pollen sac development in Arabidopsis. Plant Cell 15, 1009–1019 (2003).
Cecchetti, V., Altamura, M. M., Falasca, G., Costantino, P. & Cardarelli, M. Auxin regulates Arabidopsis anther dehiscence, pollen maturation, and filament elongation. Plant Cell 20, 1760–1774 (2008).
Zhao, Z. et al. A role for a dioxygenase in auxin metabolism and reproductive development in rice. Dev. Cell 27, 113–122 (2013).
Hu, L. et al. Rice MADS3 regulates ROS homeostasis during late anther development. Plant Cell, tpc. 110.074369 (2011).
Chueasiri, C. et al. Rice ORMDL controls sphingolipid homeostasis affecting fertility resulting from abnormal pollen development. PLoS One 9, e106386 (2014).
Acknowledgements
This study was carried out with the research grant from Science and Engineering Research Board (SERB), Department of Science and Technology (DST), Government of India (ECR/2016/001581) to RS and MKS. RS and MKS acknowledge Department of Biotechnology, Government of India for Ramalingaswami fellowships. ND acknowledges DST INSPIRE Faculty Fellowship [DST/INSPIRE/04/2017/002597] and KK thanks University Grants Commission (UGC), Government of India for Rajiv Gandhi National Fellowship. MK thanks JNU and UGC for non-NET fellowship. We also acknowledge AIRF (JNU) and DBT grant (no. BT/PR3130/INF/22/139/2011) for microscopy facility.
Author information
Authors and Affiliations
Contributions
R.S. conceived and designed the study. N.D., K.K., I.V. and M.K. performed the experiments and data analysis; N.D., K.K. and R.S. drafted the manuscript; M.P. and M.K.S. participated in experiment design and implementation. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dhaka, N., Krishnan, K., Kandpal, M. et al. Transcriptional trajectories of anther development provide candidates for engineering male fertility in sorghum. Sci Rep 10, 897 (2020). https://doi.org/10.1038/s41598-020-57717-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-57717-0
- Springer Nature Limited
This article is cited by
-
Comparative Transcriptomics Identifies Different Gene Expression Networks Regulating Gametic Development in Arabidopsis
Journal of Plant Biology (2023)
-
New Data and New Features of the FunRiceGenes (Functionally Characterized Rice Genes) Database: 2021 Update
Rice (2022)
-
Aldehyde dehydrogenase superfamily in sorghum: genome-wide identification, evolution, and transcript profiling during development stages and stress conditions
BMC Plant Biology (2022)
-
Transcriptome analysis reveals cell cycle-related transcripts as key determinants of varietal differences in seed size of Brassica juncea
Scientific Reports (2022)
-
Characterization of BRASSINOSTEROID F-BOX Proteins BRFPs that Regulate BRASSINOSTEROID-INSENSITIVE 2 Kinase
Journal of Plant Biology (2022)