Abstract
It remains unclear whether caffeinated beverages could have deleterious renal effects in elderly population with underlying comorbid conditions. We investigated the associations between coffee, tea, or caffeine intake and 1-year changes in glomerular filtration rate (eGFR) in a large Spanish cohort of overweight/obese elderly with metabolic syndrome (MetS). This prospective analysis includes 5851 overweight/obese adults (55–75 years) with MetS from the PREDIMED-Plus study. We assessed coffee, tea, and caffeine consumption from a validated food-frequency questionnaire and creatinine-based eGFR using the Chronic Kidney Disease Epidemiology Collaboration equation. Multivariate-adjusted regression models were applied to test associations between baseline coffee, tea, or caffeine intake and 1-year eGFR changes. Caffeinated coffee (> 2 cups/day) and tea (at least 1 cup/day) drinkers had 0.88 and 0.93 mL/min/1.73 m2 greater eGFR decrease respectively, compared to those with less than 1 cup/day of coffee consumption or non-tea drinkers. Furthermore, caffeinated coffee consumption of > 2 cups/day was associated with 1.19-fold increased risk of rapid eGFR decline > 3 mL/min/1.73 m2 (95% CI 1.01–1.41). Similarly, individuals in the highest (median, 51.2 mg/day) tertile of caffeine intake had a 0.87 mL/min/1.73 m2 greater eGFR decrease. Decaffeinated coffee was not associated with eGFR changes. In conclusion, higher consumption of caffeinated coffee, tea, and caffeine was associated with a greater 1-year eGFR decline in overweight/obese adults with MetS.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) poses a major public health challenge due to its detrimental impact on quality of life, increased morbidity and premature death, specifically in old people1. This heterogeneous condition is characterized by a decline of glomerular filtration rate (eGFR) and/or proteinuria2, which is accelerated when obesity or related cardiovascular risk factors are present3. Therefore, preserving renal function is essential to ensure the well-being and reduce adverse health outcomes in elders.
Many dietary components play a role in preserving renal function and preventing/delaying CKD progression4,5, such as coffee and tea6,7, two caffeine-containing beverages that are widely consumed worldwide. Coffee and tea are rich in bioactive substances, such as phenolic compounds and minerals with antioxidant and anti-inflammatory properties, which can improve blood pressure, oxidative stress, dyslipidemia, and insulin resistance or hyperglycemia8,9, well-established risk factors for renal insufficiency. There is evidence pointing to a protective effect of coffee and tea against type 2 diabetes, hypertension, and cardiovascular disease, conditions in which chronic inflammation plays a critical pathogenic role10,11,12. Nonetheless, there are potential side effects from caffeine contained in these beverages, even at moderate amounts, which may influence CKD-related cardiovascular outcomes and are of concern for the general population and especially for susceptible or at-risk individuals13.
The results of epidemiological studies on coffee and tea consumption and kidney function have been inconsistent14,15,16,17,18,19,20,21,22,23. Two recent meta-analyses, summarizing 7 cross-sectional studies on apparently healthy middle-aged adults, most from Asian countries, have concluded that coffee consumption is not associated with eGFR or CKD risk6,7. Regarding prospective studies; some studies reported a lower incidence of CKD21,24 or end-stage renal disease19 with greater coffee consumption, while others failed to demonstrate this association22. For tea consumption, the few studies examining its association with eGFR or CKD risk reported null findings15,19,20,22. Several limitations of these studies need to be addressed; (1) most previous studies are cross-sectional rather than longitudinal studies, (2) they only reported information on total coffee consumption and not its subtypes (caffeinated and decaffeinated coffee), (3) stratified analyses were often not reported, and (4) they were performed in general healthy adults, and not in vulnerable elderly individuals. In addition, the impact of coffee or tea on eGFR could be different among countries with different lifestyle and tea and coffee-drinking habits. Consequently, it is unclear whether caffeinated beverages have deleterious effects on kidney function in elderly population with underlying comorbid conditions. Therefore, the purpose of this study was to address the associations of caffeinated and decaffeinated coffee, tea, and caffeine consumption with 1-year changes in eGFR in a large Spanish cohort of overweight/obese older individuals with metabolic syndrome (MetS).
Methods
Study design and participants
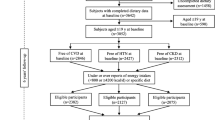
The present data was analysed using an observational prospective design conducted within the frame of the PREDIMED-PLUS study, which included 6874 older adults enrolled between 2013 and 2016 by 23 Spanish centers working in collaboration with 208 National Health System primary care clinics. Briefly, PREDIMED-Plus is an ongoing, 6-year, multicenter, parallel randomized clinical trial (RCT) evaluating the long-term effect of a weight-loss intervention based on an energy-reduced Mediterranean diet (MedDiet), physical activity promotion and behavioral support (intervention), in comparison with usual-care recommending an energy-unreduced MedDiet (control), on primary cardiovascular prevention. Eligible participants were overweight/obese (BMI 27–40 kg/m2) men and women aged 55–75 years harboring the MetS25, but free of cardiovascular disease at enrollment. More specific details of the study cohort and inclusion/exclusion criteria have been reported26,27 and the protocol is available at http://predimedplus.com/. Local Ethics Committee approved the study protocol and all participants signed a written informed consent.
For the current study, participants who did not complete the semiquantitative food-frequency questionnaire (SFFQ) at baseline (n = 53) or those whose total energy intake was outside pre-defined limits (women < 500 and > 3500 kcal/day, and men < 800 and > 4000 kcal/day) (n = 188) were excluded. We also excluded subjects who died (n = 11) or were lost to follow-up (n = 16) within first year of follow-up, and who had missing data on eGFR at baseline or at the 1-year assessment. The remaining 5851 participants comprised the final sample.
Assessment of coffee, tea, and caffeine
The exposures of interest were baseline consumption of coffee (caffeinated and decaffeinated coffee), tea, and total caffeine intake. Trained dietitians collected participant information about dietary intake through face-to-face interviews using a Spanish version of the validated 143-item SFFQ28. Participants reported their average frequency of consumption over the preceding year for a specified serving size of each item. The nine possible answers ranged from “never or less than once per month” to “six or more per day”, which were transformed into grams or milliliters per day using the standard portion size of each food and beverage. Two and one items on the FFQ were specifically related to coffee consumption (one for caffeinated coffee and one for decaffeinated coffee) and tea consumption, respectively. The standard serving size of 1 cup of coffee or tea was assigned as 50 mL in the questionnaire. Total coffee consumption was considered as the sum of caffeinated and decaffeinated coffee. Spanish food composition tables were used to estimate daily energy and nutrient intakes29.
We modeled three categories of coffee consumption (0 to < 1cup/day, 1–2 cups/day, and > 2 cups/day) and tea consumption (0 cup/day, < 1 cup/day, and ≥ 1 cups/day) with the lowest category as the referent group. Total caffeine intake was computed from the SFFQ using the caffeine contained in caffeinated coffee (400 mg/L), decaffeinated coffee (10.7 mg/L), tea (100 mg/L), regular sodas (79.2 mg/L), artificially sweetened soda (128 mg/L) and chocolate (180 mg/kg). Reference values from the European Food Safety Authority30 were used to calculate caffeine intake. For data analyses, caffeine intake (mg/day) was categorized into tertiles. We did not collect any information on the types of tea, soda, and chocolate.
Assessment of the outcome and covariates
The main outcome of interest for the present study was 1-year changes in kidney function, assessed by changes in eGFR (1-year data minus baseline data) estimated indirectly from serum creatinine (SCr) using the Chronic Kidney Disease Epidemiology Collaboration equation for Caucasian individuals31. SCr levels were determined by the enzymatic creatinine assay method (coefficient of variation < 4.0%). The secondary outcome was a rapid decline of kidney function, defined as 1-year eGFR decline rate > 3 mL/min/1.73 m2. This threshold represents a magnitude of change that is 3 times the decline rate imposed by natural ageing.
At baseline, trained staff collected participant’s information through questionnaires regarding sociodemographic characteristics (sex, age, education, and employment status), lifestyles factors (smoking, physical activity32,33), history of illnesses and medication use. Additionally, we assessed adherence to the MedDiet using a 17-item questionnaire, adapted from a previously validated questionnaire34. This tool was used to evaluate compliance with the intervention and as a key element to guide the motivational interviews during the follow-up. Compliance with each of the 17 items relating to characteristic food habits was scored with 1 or 0 points, when the criterion was met or not met, respectively. Therefore, the total MedDiet score range was 0–17, with 0 meaning no adherence and 17 meaning maximum adherence to MedDiet. Weight, height and waist circumference were determined in duplicate by trained personnel following a pre-established protocol. BMI was calculated by dividing the weight (kg) by the height squared (m2). Blood pressure was measured at rest in triplicate by an automated digital device (Omron-HEM297705C). At baseline and 1-year, blood samples were collected after an overnight fast, and routine biochemical analyses including fasting glucose, lipid profile, and SCr was performed.
Statistical analyses
All analyses were performed by STATA software, v.15.0 (StataCorp LP, Tx. USA) using the PREDIMED-Plus database updated in March 2019.
Descriptive data are presented as mean ± SD for quantitative variables or percentages for qualitative variables. Baseline characteristics of the study population across categories of total coffee and tea consumption were compared using ANOVA and chi-square tests, as appropriate.
Multivariate linear regression models were applied to test associations between categories (lowest category as reference) of coffee, tea, and caffeine intake and changes in eGFR at 1 year. For these associations, coffee, tea, (both for each cup/day increase) and caffeine (for each 50 mg/day increase) were also analyzed as continuous variables. The results are presented as β-coefficients and 95% confidence intervals (CIs). Furthermore, ORs were calculated for the association between categories of coffee and tea consumption and rapid eGFR decline (1-year changes in eGFR > 3 mL/min/1.73 m2). Three models with additional adjustment for potential confounders were fitted: model 1, adjusted for sex (men/women), age (years, continuous), and baseline eGFR (mL/min/1.73 m2, continuous); model 2, additionally adjusted for BMI (kg/m2, continuous), smoking (never, current or former smoker), education (primary, secondary education or academic/graduate), physical activity (MET-min/day, continuous), type 2 diabetes (yes/no), dyslipidemia (yes/no), hypertension (yes/no), center (four categories by number of recruited participants: < 200, 200–250, 250–300, or ≥ 300), treatment group (intervention/control), MedDiet score (17-points, continuous), and total energy intake (kcal/day, quartiles); to minimize confounding by other dietary factors, a third model (model 3) was additionally adjusted for dietary intakes of total protein, saturated fat, alcohol, fiber, sodium, magnesium, potassium (each categorized at energy-adjusted quartiles by using the residual method35), 1-year changes in systolic blood pressure (mmHg, continuous), and 1-year changes body weight (kg, continuous). Model 3 was further adjusted (cup/day, continuous) for coffee (for tea), tea (for each coffee), caffeinated coffee (for decaffeinated coffee), or decaffeinated coffee (for caffeinated coffee). All models were conducted with robust variance estimators to account for intra-cluster correlations, considering as clusters the members of the same household (n = 354 couples). Tests for linear trends across categories were conducted by modeling the median value for coffee, tea, or caffeine intake categories as continuous variables.
Furthermore, stratified analyses were conducted by intervention group (intervention/control), sex, age (≤ 65/ > 65 years), baseline eGFR (≥ 90 mL/min/1.73 m2, 60–89 mL/min/1.73 m2, < 60 mL/min/1.73 m2), obesity (yes/no), type 2 diabetes (yes/no), hypertension (yes/no), dyslipidemia (yes/no), smoking (non-smoker/smoker or ex-smoker), and MedDiet score (≤ 10/ > 10-points [P75]); The potential effect modification for eGFR by these stratification variables was explored by likelihood-ratio tests comparing the models with and without cross-product interaction terms, after adjusting for model 3.
Finally, we performed a sensitivity analysis, in which we repeated the primary analysis but using percentage of eGFR change ((1-year eGFR − baseline eGFR)/(baseline eGFR) * 100) instead of eGFR change, adjusting for baseline eGFR. Statistical significance was set at p < 0.05.
Ethics approval and consent to participate
The study protocol and procedures were approved according to the ethical standards of the Declaration of Helsinki by the Institutional Review Boards (IRBs) of all the participating institutions: CEI Provincial de Málaga, CEI de los Hospitales Universitarios Virgen Macarena y Virgen del Rocío, CEI de la Universidad de Navarra, CEI de las Illes Balears, CEIC del Hospital Clínic de Barcelona, CEIC del Parc de Salut Mar, CEIC del Hospital Universitari Sant Joan de Reus, CEI del Hospital Universitario San Cecilio, CEIC de la Fundación Jiménez Díaz, CEIC Euskadi, CEI en Humanos de la Universidad de Valencia, CEIC del Hospital Universitario de Gran Canaria Doctor Negrín, CEIC del Hospital Universitario de Bellvitge, CEIC de IMDEA Alimentación, CEIC del Hospital Clínico San Carlos, CEI Provincial de Málaga, CCEIBA de la Investigación Biomédica de Andalucía, CEIC del Hospital General Universitario de Elche, Comité de Ética del Hospital Universitario Reina Sofía and CEIC de León. All participants provided informed written consent.
Results
At baseline, the mean age of participants was 65 years, the mean BMI 32.5 kg/m2, and 48% were women. The mean baseline eGFR was 84.2 mL/min/1.73 m2 and mean change at 1 year was − 0.96 mL/min/1.73 m2. Mean daily consumption of caffeinated coffee, decaffeinated coffee, and tea were 42 mL/day (0.8 cup/day), 36 mL/day (0.7 cup/day), and 11 mL/day (0.2 cup/day) respectively. The mean intake of caffeine was 24.8 ± 25.7 mg/day. Table 1 shows baseline characteristics of study population by categories of coffee and tea consumption. Overall, 91.7% of participants were daily coffee consumers, and 36.4% drank > 2 cups of coffee/day. In contrast, only 32.5% were tea consumers and 11.3% drank at least 1 cup of tea/day. Compared with participants who consumed coffee less than daily (< 1 cup/day), those who consumed > 2 cups/day were more likely to be men, whereas tea consumers were more frequently women. Individuals who drank > 2 cups/day of coffee were younger and had higher BMI and baseline eGFR. They were more likely to have obesity, diabetes, and dyslipidemia and more frequently used oral hypoglycemic drugs, smoked, had higher waist circumference, fasting glucose and educational levels, and lower MedDiet adherence. For tea consumption, the associations were mostly in the opposite direction, except for education. High coffee consumption was also associated with a high intake of saturated fat, protein, sodium, magnesium, and potassium and with low fiber intake. Similar trends were observed for tea, except for saturated fat, fiber and alcohol intake, which showed an opposite pattern.
Total coffee consumption was not significantly associated with eGFR changes: β: − 0.51; 95% CI − 1.17, 0.14 mL/min/1.73 m2 for > 2 cups/day compared with none to < 1 cup/day (p-trend = 0.07) in the fully adjusted model 3 (Table 2). Regarding coffee subtypes, caffeinated coffee consumption was associated with a 1-year eGFR decline in the model adjusted for age, sex, and baseline eGFR, with a significant β value of − 1.00 (95% CI − 1.52, − 0.45) mL/min/1.73 m2 for participants drinking > 2 cups/day compared with those in the lowest category (p-trend = 0.001). This association was not substantially affected after adjustment for other potential confounders in model 3, with an eGFR change of − 0.88 (95% CI − 1.52, − 0.23) mL/min/1.73 m2 lower in participants drinking > 2 cups/day compared to those with none to < 1 cup/day (p-trend = 0.008). When caffeinated coffee was analyzed as continuous, eGFR decreased by − 0.17 (95% CI − 0.40, 0.05) mL/min/1.73 m2 per 1 cup/day increment in the fully adjusted model, however, this result was not significant (p = 0.15). Conversely, the frequency of decaffeinated coffee consumption was not associated with changes in eGFR (Table 2).
Concerning tea, the age-, sex, and baseline eGFR-adjusted revealed a non-significant higher decrease in eGFR, with β: − 0.61; 95% CI − 1.33, 0.11 mL/min/1.73 m2) in participants drinking ≥ 1cup tea/day compared to non-drinkers (p-trend = 0.10). This association was strengthened after adjustment for confounders (model 3), resulting in a significant β of − 0.93 (95% CI − 1.67, − 0.18) mL/min/1.73 m2 1-year decrease in eGFR for participants drinking ≥ 1cup/day compared with non-drinkers (p-trend = 0.02). Similarly, for each 1 cup/day increase in tea consumption a decrease of − 0.55 (95% CI − 0.93, − 0.16) mL/min/1.73 m2 in eGFR (model 3) was observed (Table 2).
Similar results were observed when all of these analyses were repeated using percentage of change of eGFR instead of eGFR change, adjusting for baseline eGFR values (data not shown). Overall, we found no evidence that the observed associations varied across subgroups of sex, age, eGFR, obesity, diabetes, hypertension, dyslipidemia, smoking, MedDiet score at baseline, and intervention group (Fig. 1 and Supplementary Fig. S1 online).
Mean multivariable-adjusted differences (95% CI) for 1-year changes in eGFR between the two highest and the lowest categories of caffeinated coffee consumption at baseline stratified by group. eGFR, estimated glomerular filtration rate; MedDiet, Mediterranean diet. Model adjusted (except the stratification factor itself) for sex (men or women), age (years, continuous), baseline eGFR (mL/min/1.73 m2, continuous), BMI (kg/m2, continuous), smoking (never, current, or former smoker), educational level (primary, secondary education or academic/graduate), physical activity (MET-min/day, continuous), type 2 diabetes prevalence (yes or no), dyslipidemia (yes or no), hypertension (yes or no), center (four categories by number of recruited participants: < 200, 200–250, 250–300, or ≥ 300), intervention group (intervention or control group), MedDiet score (17-points, continuous), total energy intake (kcal/day, quartiles), dietary intakes of total protein, saturated fat, alcohol, fiber, sodium, magnesium, potassium (each categorized at energy-adjusted quartiles), 1-year changes in systolic blood pressure (mmHg, continuous), and 1-year changes in body weight (kg, continuous), decaffeinated coffee, and tea consumption (cup/day, continuous). All analyses were conducted with robust estimates of the variance to correct for intra-cluster correlation. aP < 0.05 (compared to the reference group). The standard serving size of 1 cup was defined as 50 mL in the questionnaire. (StataCorp. 2015. Stata Statistical Software: Release 15. College Station, TX: StataCorp LP. https://www.stata.com).
In multivariable logistic regression models, the caffeinated coffee consumption remained significantly associated with an increased risk of a rapid eGFR decline > 3 mL/min/1.73 m2 when comparing > 2 versus none to < 1 cups/day (full adjusted OR, 1.19; 95% CI 1.01–1.41) (Table 3).
Compared to participants in the lowest tertile of caffeine intake, in the fully adjusted model, those in the top tertile showed a significantly higher eGFR decline, with a mean difference of − 0.94 (95% CI − 1.51, − 0.38) mL/min/1.73 m2 (p-trend = 0.001) (Table 4). For each additional 50 mg/day of caffeine intake, eGFR decreased by − 0.60 (95% CI − 1.04, − 0.17) mL/min/1.73 m2 (model 3). When analyzed as estimated caffeine intake from coffee and tea, a similar pattern of eGFR decline was observed (Table 4). No significant association between caffeine intake from soft drinks and kidney function was observed, (tertile 3 vs. tertile 1, β: − 0.23; 95% CI − 0.82, 0.37; p-trend = 0.47).
Discussion
In this study, we examined the association between coffee, tea, and caffeine intake and 1-year changes in eGFR in older Mediterranean individuals with overweight/obesity and MetS. We found no association between total coffee consumption and eGFR changes. However, when we explored the association by coffee subtypes, individuals who consumed > 2 cups/day of caffeinated coffee disclosed a greater eGFR decline and higher risk of rapid kidney function decline compared to those who consumed none to < 1 cup/day. Similar results were observed for drinkers of one or more cups of tea/day compared to non-consumers. Besides, participants in the highest tertile of total dietary caffeine intake showed a higher decline in eGFR than those in the reference tertile. No association of decaffeinated coffee consumption with eGFR changes was observed.
Coffee is a seed that contains a complex matrix of vitamins, minerals, bioactive phytochemicals, caffeine, diterpenes, melanoidins, and trigonelline with potential beneficial effects on human health8,10,36. Therefore, the association of coffee with health outcomes, especially non-communicable chronic diseases such as CKD has been of research interest in the last decade10,11.
Unlike our results, epidemiological studies suggest that regular coffee consumption is either unassociated with kidney function6,7,20 or has a protective effects against CKD in healthy middle-aged adults21,24. Cross-sectionally, the association between coffee and kidney function has been mainly explored in Asian populations. In two studies conducted in healthy Japanese adults14,15, higher eGFR was observed in usual coffee drinkers (≥ 1 cups/day) compared to non-drinkers. Similarly, in Korean women18, habitual coffee consumption (≥ 2 cups/day) was associated with a lower risk of CKD only in diabetic women. However, other studies in Japanese middle-aged adults reported no association between coffee and eGFR16,17. Notwithstanding, higher cross-sectional eGFRs values do not necessarily reflect better kidney function; therefore, these results should be taken with caution since certain kidney diseases may also manifest hyperfiltration in early stages37. Finally, the results of a recent meta-analysis of cross-sectional studies conducted by Wijarnpreecha et al.7 reported no significant association between coffee consumption and CKD risk in males, and a trend to a lower risk of CKD in women. Contrary, in our study, the associations between caffeinated coffee or tea consumption and eGFR were most apparently among men, despite interactions were not significant. Further research is warranted to clarify the differences observed by sex, and if they are proven to understand the plausible mechanisms involved.
Recently, prospective studies have reported inconsistent results. Three studies, one conducted in US adults21 and two in Asian populations19,24, reported a decreased risk of incident CKD in healthy individuals with greater coffee consumption. In contrast, a study conducted in an Iranian population revealed no association between coffee and CKD incidence after 6 years of follow-up22. The only epidemiological study conducted in an European population reported a cross-sectional positive association between coffee consumption and eGFR at baseline, but no prospective association with eGFR changes after 15 year follow-up20. Until now, the only RCT in 19 young Japanese revealed a beneficial effect of coffee consumption for 2 weeks, but not green tea, on cystatin-C-based eGFR23.
The main discrepancies between previous studies and our observations might be attributable to the populations examined, follow-up time, and the assessment tools used. Most studies were conducted in apparently healthy middle-aged populations, and none of them included overweight/obese older adults at high cardiovascular risk. Additionally, previous studies have focused on exploring the association between total coffee and kidney function, but none of them have explored differences by coffee subtypes. Further, we also determined whether the observed detrimental effects of caffeinated coffee and tea consumption on kidney function differed across subgroups according to various baseline factors and similar trends were seen; this type of analysis was seldom performed in previous studies.
The association between tea and kidney dysfunction has been less explored and the few studies in the field had reported no significant associations15,19,20,22. We observed that the consumption of ≥ 1cup tea/day was associated with higher eGFR decline. Despite, in our study tea consumption was lower than caffeinated coffee consumption, our findings regarding caffeine intake suggest that this nutrient could be the causal for the deleterious renal effect observed.
Additionally, we found no association between decaffeinated coffee and kidney function, that might reinforce the potentially harmful effect of caffeine13. However, the mechanism that confers this negative effect of caffeine intake is uncertain36. A possible explanation could be related with the structural similarity of caffeine and adenosine acting as a nonselective antagonist of A1 adenosine receptors, which may be a potential mechanism for the impairment of renal function11. On distal afferent arterioles, A1 adenosine receptor activation causes vasoconstriction and decreases eGFR38. By contrast, adenosine antagonism by caffeine may prevent afferent arteriolar constriction or induce vasodilation, increasing renal plasma flow and acute elevation of eGFR11. Probably this increase in eGFR could lead to acute compensatory hyperfiltration and subsequent accelerated decline in GFR37. This hypothesis may be partly supported by the observed relationship in our study between caffeinated coffee and a slightly higher baseline eGFR and, subsequently, a higher 1-year eGFR decline. In this context, it has been reported that, compared to decaffeinated coffee, caffeinated coffee could cause a short-lasting increase in blood pressure in healthy young subjects39. Also, in young-to-middle-age hypertensive adults, caffeinated coffee drinking has been linked with glomerular hyperfiltration40, which may be exaggerated in older individuals with pre-existing comorbidities13 (e.g., hypertension) as our studied population, but warrants further studies. In fact, in experimental studies conducted in obese diabetic rats, administration of caffeine for 2 weeks induced early renal injury characterized by proteinuria, increased renal vascular resistance, and increased heart rate41. Moreover, the same authors reported that long-term caffeine consumption exacerbated renal failure and induced more severe tubule-interstitial and glomerular damage42. Nevertheless, this has not been explored in humans and RCTs are needed.
Our study has some strengths. First, the relatively large sample size, its prospective design, and adjustment for many potential confounding factors. Secondly, we examined the association between eGFR and coffee subtypes, which gives greater insight into the potential effect of coffee and caffeine on kidney function. Finally, we conducted sub-group analyses to examine whether kidney function decline was accelerated by unhealthy behaviors (e.g., smoking or a poor diet) or pre-existing comorbid conditions. Nevertheless, we also acknowledge some limitations. It should be underlined that the caffeine content in coffee, tea, and other beverages (e.g., soft drinks) and foods varies greatly, which may lead to misclassification, and also that coffee composition can depend on the type of coffee bean and the brewing process used, which may influence the biological effects it has on the human organism. Also, SCr-based eGFR, a common biomarker of kidney function used in almost all epidemiologic studies, may overestimate true GFR values. Finally, our study was conducted in aged Mediterranean individuals with MetS, therefore our findings cannot be extrapolated to other populations.
In summary, caffeinated coffee and tea consumption and caffeine intake were associated with a greater 1-year decline of renal function in older individuals at high cardiovascular risk. Further studies are needed to clarify these associations; if proven, advice on coffee and tea consumption along with caffeine intake should be included in nutritional strategies for kidney disease prevention, particularly in individuals at high risk.
Data availability
The dataset (including data dictionaries) of PREDIMED-Plus is available to external investigators in order to make possible the replication of the main analyses used for the published article. However, due to the restrictions imposed by the Informed Consent and the Institutional Review Boards (IRB), bona fide investigators interested in analyzing the PREDIMED-Plus dataset may submit a brief proposal and statistical analysis plan to the corresponding author (JS-S) at jordi.salas@urv.cat. Upon approval from the Steering Committee and IRBs, the data will be made available to them using an onsite secure access data enclave. The study protocol is available at http://predimedplus.com/.
Abbreviations
- ARBs:
-
Angiotensin-type 2 receptor blockers
- ACEis:
-
Angiotensin converting enzyme inhibitors
- CKD:
-
Chronic Kidney Disease
- CKD-EPI:
-
Chronic Kidney Disease Epidemiology Collaboration
- CIs:
-
Confidence intervals
- DBP:
-
Diastolic blood pressure
- eGFR:
-
Estimated glomerular filtration rate
- HDL-c:
-
High-density lipoprotein-cholesterol
- MedDiet:
-
Mediterranean diet
- MetS:
-
Metabolic syndrome
- BMI:
-
Body mass index
- PREDIMED:
-
PREvención con DIeta MEDiterranea
- SFFQ:
-
Semiquantitative food-frequency questionnaire
- SBP:
-
Systolic blood pressure
References
Xie, Y. et al. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 94, 567–581 (2018).
Mallappallil, M., Friedman, E. A., Delano, B. G., McFarlane, S. I. & Salifu, M. O. Chronic kidney disease in the elderly: evaluation and management. Clin Pract. 11, 525–535 (2014).
Gansevoort, R. T. et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 382, 339–352 (2013).
Campbell, K. L. & Carrero, J. J. Diet for the management of patients with chronic kidney disease; it is not the quantity, but the quality that matters. J. Ren. Nutr. 26, 279–281 (2016).
Goraya, N. & Wesson, D. E. Dietary interventions to improve outcomes in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 24, 505–510 (2015).
Kennedy, O. J., Roderick, P., Poole, R. & Parkes, J. Coffee and kidney disease. Int. J. Clin. Pract. 71, e12980 (2017).
Wijarnpreecha, K., Thongprayoon, C., Thamcharoen, N., Panjawatanan, P. & Cheungpasitporn, W. Association of coffee consumption and chronic kidney disease: a meta-analysis. Int. J. Clin. Pract. 71, e12919 (2017).
Ludwig, I. A., Clifford, M. N., Lean, M. E. J., Ashihara, H. & Crozier, A. Coffee: biochemistry and potential impact on health. Food Funct. 5, 1695–1717 (2014).
McKay, D. L. & Blumberg, J. B. The role of tea in human health: an update. J. Am. Coll. Nutr. 21, 1–13 (2002).
Poole, R. et al. Coffee consumption and health: umbrella review of meta-analyses of multiple health outcomes. BMJ 359, j5024 (2017).
Higdon, J. V. & Frei, B. Coffee and health: a review of recent human research. Crit. Rev. Food Sci. Nutr. 46, 101–123 (2006).
Di Lorenzo, A., Curti, V., Tenore, G. C., Nabavi, S. M. & Daglia, M. Effects of tea and coffee consumption on cardiovascular diseases and relative risk factors: an update. Curr. Pharm. Des. 23, 2474–2487 (2017).
Lim, D., Chang, J., Ahn, J. & Kim, J. Conflicting effects of coffee consumption on cardiovascular diseases: does coffee consumption aggravate pre-existing risk factors?. Processes 8, 438 (2020).
Kotani, K., Sakane, N., Yamada, T. & Taniguchi, N. Association between coffee consumption and the estimated glomerular filtration rate in the general Japanese population: preliminary data regarding C-reactive protein concentrations. Clin. Chem. Lab. Med. 48, 1773–1776 (2010).
Nakajima, K., Hirose, K., Ebata, M., Morita, K. & Munakata, H. Association between habitual coffee consumption and normal or increased estimated glomerular filtration rate in apparently healthy adults. Br. J. Nutr. 103, 149–152 (2010).
Pham, N. M. et al. The relation of coffee consumption to serum uric acid in Japanese men and women aged 49–76 years. J. Nutr. Metab. 2010, 930757 (2010).
Miyatake, N. et al. The relation between estimated glomerular filtration rate (eGFR) and coffee consumption in the Japanese. Health (Irvine, CA) 03, 549–552 (2011).
Kim, B. H., Park, Y. S., Noh, H. M., Sung, J. S. & Lee, J. K. Association between coffee consumption and renal impairment in Korean women with and without diabetes: analysis of the Fourth Korea National Health and Nutrition Examination Survey in 2008. Korean J. Fam. Med. 34, 265–271 (2013).
Lew, Q.-L.J., Jafar, T. H., Jin, A., Yuan, J.-M. & Koh, W.-P. Consumption of coffee but not of other caffeine-containing beverages reduces the risk of end-stage renal disease in the Singapore Chinese Health Study. J. Nutr. 148, 1315–1322 (2018).
Herber-Gast, G.-C.M. et al. Coffee and tea consumption in relation to estimated glomerular filtration rate: results from the population-based longitudinal Doetinchem Cohort Study. Am. J. Clin. Nutr. 103, 1370–1377 (2016).
Hu, E. A. et al. Coffee consumption and incident kidney disease: results from the atherosclerosis risk in communities (ARIC) study. Am. J. Kidney Dis. 72, 214–222 (2018).
Gaeini, Z., Bahadoran, Z., Mirmiran, P. & Azizi, F. Tea, coffee, caffeine intake and the risk of cardio-metabolic outcomes: findings from a population with low coffee and high tea consumption. Nutr. Metab. (Lond.) 16, 28 (2019).
Saito, M. et al. Coffee consumption and cystatin-C-based estimated glomerular filtration rates in healthy young adults: results of a clinical trial. J. Nutr. Metab. 2011, 146865 (2011).
Jhee, J. H. et al. Effects of coffee intake on incident chronic kidney disease: a community-based prospective cohort study. Am. J. Med. 131, 1482-1490.e3 (2018).
Alberti, K. G. M. M. et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International. Circulation 120, 1640–1645 (2009).
Martínez-González, M. A. et al. Cohort profile: design and methods of the PREDIMED-Plus randomized trial. Int. J. Epidemiol. 48, 387–388o (2019).
Salas-Salvadó, J. et al. Effect of a lifestyle intervention program with energy-restricted Mediterranean diet and exercise on weight loss and cardiovascular risk factors: one-year results of the PREDIMED-Plus trial. Diabetes Care 42, 777–788 (2019).
Fernández-Ballart, J. D. et al. Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br. J. Nutr. 103, 1808–1816 (2010).
Moreiras, O., Carbajal, A., Cabrera, L. & Cuadrado, C. Tablas de composición de alimentos: guía de prácticas [food composition tables: practical guides] 19th edn. (Ediciones Piramide, 2018).
Zucconi, S. et al. Gathering consumption data on specific consumer groups of energy drinks. EFSA Support Publ. 10, 190 (2013).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150, 604–612 (2009).
Molina, L. et al. Validation of the Regicor short physical activity questionnaire for the adult population. Lucía A, editor. PLoS ONE 12, e0168148 (2017).
Martínez-González, M. A., López-Fontana, C., Varo, J. J., Sánchez-Villegas, A. & Martinez, J. A. Validation of the Spanish version of the physical activity questionnaire used in the Nurses’ Health Study and the Health Professionals’ Follow-up Study. Public Health Nutr. 8, 920–927 (2005).
Schröder, H. et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 141, 1140–1145 (2011).
Willett, W. Nutritional Epidemiology 2nd edn. (Oxford University Press, 1998).
Godos, J. et al. Coffee components and cardiovascular risk: beneficial and detrimental effects. Int. J. Food Sci. Nutr. 65, 925–936 (2014).
Tuttle, K. R. Back to the future: glomerular hyperfiltration and the diabetic kidney. Diabetes 66, 14–16 (2017).
Vallon, V. & Osswald, H. Adenosine receptors and the kidney. Handb. Exp. Pharmacol. 193, 443–470 (2009).
Mahmud, A. & Feely, J. Acute effect of caffeine on arterial stiffness and aortic pressure waveform. Hypertens (Dallas, Tex 1979) 38, 227–231 (2001).
Palatini, P. et al. Factors associated with glomerular hyperfiltration in the early stage of hypertension. Am. J. Hypertens. 25, 1011–1016 (2012).
Tofovic, S. P., Salah, E. M., Jackson, E. K. & Melhem, M. Early renal injury induced by caffeine consumption in obese, diabetic ZSF1 rats. Ren. Fail. 29, 891–902 (2007).
Tofovic, S. P., Kost, C. K., Jackson, E. K. & Bastacky, S. I. Long-term caffeine consumption exacerbates renal failure in obese, diabetic, ZSF1 (fa-fa(cp)) rats. Kidney Int. 61, 1433–1444 (2002).
Acknowledgements
We thank all the volunteers for the participation and personnel for the contribution in the PREDIMED-Plus trial. CIBEROBN, CIBERESP and CIBERDEM are initiatives of ISCIII, Madrid, Spain. The authors also thank the PREDIMED-Plus Biobank Network as a part of the National Biobank Platform of the ISCIII for storing and managing the PREDIMED-Plus biological samples. This work was supported by the official Spanish Institutions for funding scientific biomedical research, CIBER Fisiopatología de la Obesidad y Nutrición (CIBEROBN) and Instituto de Salud Carlos III (ISCIII), through the Fondo de Investigación para la Salud (FIS), which is co-funded by the European Regional Development Fund (five coordinated FIS projects leaded by JS-S and JVi, including the following projects: PI13/00673, PI13/00492, PI13/00272, PI13/01123, PI13/00462, PI13/00233, PI13/02184, PI13/00728, PI13/01090, PI13/01056, PI14/01722, PI14/00636, PI14/00618, PI14/00696, PI14/01206, PI14/01919, PI14/00853, PI14/01374, PI14/00972, PI14/00728, PI14/01471, PI16/00473, PI16/00662, PI16/01873, PI16/01094, PI16/00501, PI16/00533, PI16/00381, PI16/00366, PI16/01522, PI16/01120, PI17/00764, PI17/01183, PI17/00855, PI17/01347, PI17/00525, PI17/01827, PI17/00532, PI17/00215, PI17/01441, PI17/00508, PI17/01732, PI17/00926, PI19/00957, PI19/00386, PI19/00309, PI19/01032, PI19/00576, PI19/00017, PI19/01226, PI19/00781, PI19/01560, PI19/01332, PI20/01802, PI20/00138, PI20/01532, PI20/00456, PI20/00339, PI20/00557, PI20/00886, PI20/01158); the Especial Action Project entitled: Implementación y evaluación de una intervención intensiva sobre la actividad física Cohorte PREDIMED-Plus Grant to JS-S; the European Research Council (Advanced Research Grant 2014–2019; Agreement #340918) granted to MÁM-G.; the Recercaixa (Number 2013ACUP00194) grant to JS-S; grants from the Consejería de Salud de la Junta de Andalucía (PI0458/2013, PS0358/2016, PI0137/2018); the PROMETEO/2017/017 grant from the Generalitat Valenciana; the SEMERGEN grant; funds from the European Regional Development Fund (CB06/03); International Nut & Dried Fruit Council – FESNAD (Long-term effects of an energy-restricted Mediterranean diet on mortality and cardiovascular disease 2014 –2015, No. 201302) (PI: MÁM-G); the AstraZeneca Young Investigators Award in Category of Obesity and T2D 2017 (PI: DR); grant of support to research groups no. 35/2011 (Balearic Islands Gov.; FEDER funds) (JAT and CB); the JR17/00022 (ISCIII) grant to OC; the Boosting young talent call grant program for the development of IISPV research projects 2019–2021 (Ref.: 2019/IISPV/03 grant to AD-L); the Societat Catalana d'Endocrinologia i Nutrició (SCEN) Clinical-Research Grant 2019 (IPs: JS-S and AD-L). Collaborative Nutrition and/or Obesity Project for Young Researchers 2019 supported by CIBEROBN entitled: Lifestyle Interventions and Chronic Kidney Disease: Inflammation, Oxidative Stress and Metabolomic Profile (LIKIDI study) grant to AD-L. IP-G receives a grant from the Spanish Ministry of Education, Culture and Sports (FPU17/01925). Jordi Salas-Salvadó, the senior author of this work, gratefully acknowledges the financial support by ICREA under the ICREA Academia programme. None of the funding sources took part in the design, collection, analysis, interpretation of the data, or writing the report, or in the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
A.D.-L., I.P.-G., V.R., E.T., N.B.-T., D.C., O.C., J.A.M., A.M.A.-G., J.W., J.V., D.R., J.L.M., R.E., F.J.T., J.L., L.S.-M., A.B.-C., J.A.T., V.M.S., X.P., M.D.-R., P.M.-M., J.V., C.V., L.D., T.F.V., E.R., S.E., N.B., J.V.S., A.G., I.A., L.T.S., F.J.B.-L., L.T.-C., M.M., A.G.-R., R.C., M.R.B.-L., J.M.S.-L., A.N., J.I.G., M.D.Z., M.A.Z., J.V.L., R.R., M.F., and J.S.-S. designed and conducted the research. A.D.-L. and J.S.-S., analysed the data. A.D.-L., I.P.-G., and J.S.-S., wrote the article. All authors revised the manuscript for important intellectual content and read and approved the final manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. A.D.-L., and J.S.-S., are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding authors
Ethics declarations
Competing interests
JS-S reported receiving research support from the Instituto de Salud Carlos III (ISCIII), Ministerio de Educación y Ciencia, Departament de Salut Pública de la Generalitat de Catalunya, the European Commission, the California Walnut Commission, Patrimonio Comunal Olivarero, La Morella Nuts, and Borges S.A; receiving consulting fees or travel expenses from Danone, California Walnut Commission, Eroski Foundation, Instituto Danone Spain, Nestle, and Abbott Laboratories, receiving nonfinancial support from Hojiblanca, Patrimonio Comunal Olivarero, and Almond Board of California; serving on the board of and receiving grant support through his institution from the International Nut and Dried Foundation and the Eroski Foundation; and grants and personal fees from Instituto Danone Spain. ER reported receiving grants, personal fees, and nonfinancial support from the California Walnut Commission during the conduct of the study and grants, personal fees, nonfinancial support from Alexion; grants and personal fees from Sanofi Aventis; personal fees and nonfinancial support from Ferrer International Danone, and Merck Sharp & Dohme; personal fees from Amarin; and nonfinancial support from the International Nut Council outside the submitted work. RE reported receiving grants from Instituto de Salud Carlos III and olive oil for the trial from Fundacion Patrimonio Comunal Olivarero\during the conduct of the study and personal fees from Brewers of Europe, Fundación Cerveza y Salud, Interprofesional del Aceite de Oliva, Instituto Cervantes, Pernaud Richar, Fundación Dieta Mediterránea, Wine and Culinary International Forum; nonfinancial support from Sociedad Española de Nutrición and Fundación Bosch y Gimpera; and grants from Uriach Laboratories outside the submitted work. XP reported receiving grants from ISCIII during the conduct of the study; receiving consulting fees from Sanofi Aventis, Amgen, and Abbott laboratories; receiving lecture personal fees from Esteve, Lacer and Rubio laboratories. All other authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Díaz-López, A., Paz-Graniel, I., Ruiz, V. et al. Consumption of caffeinated beverages and kidney function decline in an elderly Mediterranean population with metabolic syndrome. Sci Rep 11, 8719 (2021). https://doi.org/10.1038/s41598-021-88028-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-88028-7
- Springer Nature Limited