Abstract
Trichomonas vaginalis infection is one of the most widespread sexually transmitted infections in the world. There are approximately 276 million cases worldwide. Most men remain undiagnosed and untreated because they are asymptomatic. The chronic inflammation induced by persistent infection may increase the risk of developing genitourinary cancers. In this study, we aimed to investigate the association between trichomoniasis and benign prostate hyperplasia (BPH), prostate cancer (PCa), and bladder cancer (BC) in Taiwan. We designed a case–control study by using the database of the National Health Insurance program in Taiwan. We used the International Classification of Diseases, 9th Revision classifications to classify all the medical conditions in the case and control groups. All odds ratios (ORs) and 95% confidence intervals (CIs) were analyzed using multivariable logistic regression to adjust for all comorbidities and variables. From 2000 to 2015, we enrolled a total of 62,544 individuals as the case group and 187,632 as the control group. Trichomoniasis exposure had a significant association with BPH and PCa (adjusted OR: BPH = 2.685, 95% CI = 1.233–4.286, P = 0.013; PCa = 5.801, 95% CI = 1.296–26.035, P = 0.016). The relative risk was much higher if patients had both trichomoniasis and depression (adjusted OR = 7.682, 95% CI = 5.730–9.451, P < 0.001). Men with trichomoniasis had a significantly higher risk of developing BPH and PCa than those without. Healthcare professionals should not only pay more attention to disease treatment, but also to public health education.
Similar content being viewed by others
Introduction
Benign prostate hyperplasia (BPH), prostate cancer (PCa), and bladder cancer (BC) are common diseases in the elderly male population. The pathological mechanism of these diseases is not yet fully understood. Inflammation of the prostate, which can cause proliferation of epithelium and stroma, is considered to be related to both BPH and PCa1,2. In addition, urinary tract infection (UTI) is significantly associated with genitourinary cancers (GUC), including kidney, prostate, and bladder cancers3. Trichomonas vaginalis infection is one of the most common sexually transmitted infections (STIs), accounting for approximately 276.4 million new cases annually4. Because most male patients are asymptomatic and remain undiagnosed and untreated, persistent infection may cause chronic inflammation, which may increase the risk of GUC. There is a lack of research into the relationship between T. vaginalis infection and BC; however, some studies have mentioned that T. vaginalis infection may induce proliferation of prostatic epithelial cells and stromal cells5,6. Some in vitro studies showed that PCa may be associated with the up-regulation of the expression of genes that can control cell apoptosis or be overexpressed as a proto-oncogene7,8. The study from Vienna General Hospital discovered that 29/86 (33.7%) patients with BPH were positive for T. vaginalis on polymerase chain reaction (PCR) testing9. The Health Professionals Follow-up Study (HPFS) demonstrated that T. vaginalis seropositivity had a positive correlation with PCa risk10. However, conflicting results have also been reported. Miguelle et al. demonstrated that there was no significant association between T. vaginalis infection and PCa in Caucasian or African-American groups11. Another multicenter study in the USA revealed that patients with a history of STIs and positive STI serologies demonstrated no association with BPH12. In addition, there is still a lack of related literature regarding BC and Asian male populations. Thus, this study aimed to examine the association between T. vaginalis infection and BPH, BC, or PCa.
Materials and methods
Data source
We designed a population-based nationwide nested case–control study and obtained inpatient and outpatient files from Taiwan’s National Health Insurance Research Database (NHIRD). The data were collected from the Longitudinal Health Insurance Database 2005 (LHID2005), a part of NHIRD. We randomly selected approximately 2,000,000 people among the total population. All personal information was encrypted by National Health Research Institutes before released.
Ethical approval
Our study was approved by the Institutional Review Board of Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan (TSGHIRB No.: B-109-31). All stages of the study were carried out in accordance with relevant guidelines and regulations. Because the patient identifiers were encrypted before their data were used for research purposes to protect confidentiality, the requirement for written informed consent from patients for data linkage was waived by Institutional Review Board of Tri-Service General Hospital, National Defense Medical Center, Taipei.
Identification of the case and control groups
We selected patients from 2000 to 2015 who had been diagnosed with BPH, PCa, or BC based on the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes as the case group (Table S1). We defined the date of the first disease diagnosis as the index date. We also used ICD-9-CM codes to identify patients with T. vaginalis infection (Table S1). In contrast, the control groups were patients without BPH, PCa, or BC. Among all patients in the case and control groups, we not only selected patients in a 1:3 case:control ratio, matching based on age and index date, but also excluded (1) women and patients of unknown sex, (2) patient’s aged less than 18 years, and (3) those last diagnosed with trichomoniasis within 1 year before the index date (Fig. 1). The matching method was taken propensity score matching, wherein match tolerance was set at 0.15. The propensity score matching was set as using logistic regression in estimation algorithm and nearest neighbor matching in matching algorithm. The options for nearest neighbor were random in matching order, non-replacement, 1 to 3 matching, and no caliper. The comorbidities in our study included hypertension, myocardial infarction, congestive heart failure, cerebral or peripheral vascular disease, dementia, chronic obstructive pulmonary disease (COPD), type 2 diabetes, renal disease, and malignant disease except PCa and BC. We also evaluated depression as one of the comorbidities in our study because it may be associated with some cancers13.
Covariates for analysis
The covariates in our study included age group (18–44, 45–64, ≥ 65 years), four seasons (spring, summer, autumn and winter), with or without diagnosis of depression, geographical area of residence (north, center, south, east and outlying islands of Taiwan), urbanization level of residence (levels 1 to 4), levels of hospitals as medical centers, regional and local hospitals, and monthly income (in New Taiwan Dollars [NT$]; < 18,000, 18,000–34,999, ≥ 35,000). The urbanization level of residence was defined according to the population, along with various indicators of the level of political, economic, cultural, and metropolitan development. Level 1 was defined as a population of > 1,250,000, and a specific designation as political, economic, cultural, and metropolitan development. Level 2 was defined as a population between 500,000 and 1,249,999, and as playing an important role in the political system, economy, and culture. Urbanization levels 3 and 4 were defined as a population between 149,999 and 499,999, and < 149,999, respectively.
Statistical analysis
The statistical analyses were performed using SPSS version 22.0 (IBM Corp, Armonk, NY, USA). A P-value < 0.05 was considered significant. The Chi-squared or Fisher exact test was used to evaluate distributions between the case and control groups. Continuous variables were evaluated using the t test. Unconditional multiple logistic regression analyses were performed to evaluate the risks of BPH, PCa, and BC associated with trichomoniasis after adjusting for age, insurance premium, comorbidities, season, urbanization, and level of care. Adjusted models with significant covariates were constructed using background selection with the likelihood ratio test.
Results
Demographic characteristics of the study population
Table 1 demonstrates the population distribution of different characteristics for 62,544 cases and 187,632 controls from 2000 to 2015. There were no significant differences in age between groups after matching. The proportion with trichomoniasis in the case group was 0.02% (14/62,544), while it was 0.01% (14/187,632) in the control group (P < 0.001).
Variable evaluation in the multiple logistic regression
We present the results of the multivariable logistic regression analyses in Table 2. Patients with trichomoniasis had a significantly higher risk of BPH, PCa, or BC (adjusted odds ratio [AOR] = 2.999, 95% confidence interval [CI] = 1.426–5.301, P = 0.002). There was also a significantly higher risk for patients with depression (AOR = 3.124, 95% CI = 1.808–4.838, P < 0.001). The opposite result was noted in patients with middle or high insurance premiums (insurance premium NT$18,000–34,999: AOR = 0.745, 95% CI = 0.688–0.799, P < 0.001; insurance premium > NT$35,000: AOR = 0.836, 95% CI = 0.701–0.979, P = 0.019). Patients diagnosed in summer, autumn, or winter also had significantly lower risk than the control group (summer: AOR = 0.938, 95% CI = 0.902–0.953, P < 0.001; autumn: AOR = 0.790, 95% CI = 0.758–0.805, P < 0.001; winter: AOR = 0.862, 95% CI = 0.824–0.878, P < 0.001). Patients who lived in areas with a higher urbanization level had a significantly higher risk of BPH, PCa, or BC (urbanization level 1: AOR = 1.160, 95% CI = 1.124–1.189, P < 0.001; urbanization level 2: AOR = 1.211, 95% CI = 1.179–1.235, P < 0.001) but had significantly lower risk when diagnosed at a higher level of care (hospital center: AOR = 0.819, 95% CI = 0.796–0.902, P < 0.001; regional hospital: AOR = 0.745, 95% CI = 0.724–0.808, P < 0.001) instead.
Risk of BPH/PCa and BC in the trichomoniasis group stratified by covariates
The risk of BPH, PCa, or BC stratified based on variables using multivariable logistic regression is shown in Table 3. Patients with trichomoniasis had a 2.999 times higher risk of BPH, PCa, or BC than the control group (AOR = 2.999, 95% CI = 1.426–5.301). In the case of trichomoniasis, there were significantly higher risks of BPH, PCa, or BC in patients aged > 65 years old, with lower insurance premiums, with/without depression, first diagnosed in winter, urbanization level 2, and first diagnosed in a local hospital (age > 65 years: AOR = 3.685, 95% CI = 1.704–8.015; insurance premium < NT$18,000: AOR = 2.999, 95% CI = 1.326–5.301; with depression: AOR = 3.104, 95% CI = 1.706–5.972; without depression: AOR = 2.545, 95% CI = 1.138–4.289; first diagnosed in winter: AOR = 4.806, 95% CI = 1.104–19.675; urbanization level 2: AOR = 3.284, 95% CI = 1.057–10.978; first diagnosed in local hospital: AOR = 15.121, 95% CI = 1.762–118.976).
Risk of BPH/PCa and BC in subgroup with T. vaginalis exposure and the joint effect
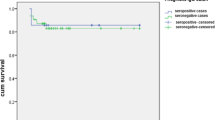
Table 4 presents the T. vaginalis exposure ratio in each subgroup of BPH/PCa and BC. T. vaginalis exposure is significantly associated with a higher risk of BPH and PCa (BPH: AOR = 2.685, 95% CI = 1.233–4.286, P = 0.013; PCa: AOR = 5.801, 95% CI = 1.296–26.035, P = 0.016), but has no significant association with BC (AOR = 4.012, 95% CI = 0.524–31.145, P = 0.151). In addition, patients with both depression and T. vaginalis exposure had a significantly higher risk of developing BPH, PCa, or BC in comparison with other groups with only one condition or without them (AOR = 7.682, 95% CI = 5.730–9.451, P < 0.001) (Table 5, Fig. 2).
Discussion
We designed this case–control study based on nationwide data from Taiwan NHIRD. We found that T. vaginalis infection was significantly associated with BPH and PCa in a male population. Therefore, T. vaginalis could be a pathogen that induces BPH and PCa. However, there was no significant association between trichomoniasis and BC. Furthermore, patients with both trichomoniasis and depression had 7.682 times higher risk of developing BPH, PCa, or BC. This result suggests that the joint effect of trichomoniasis and depression could increase the risk of BPH, PCa, or BC.
The mechanism of T. vaginalis inducing BPH and PCa still remains unclear. Several studies have demonstrated different possible mechanisms. In women, T. vaginalis induces pro-inflammatory cytokine production, including interleukin-6 (IL-6), interleukin-8 (IL-8), and chemokine ligand 2 (CCL2), while attaching to vaginal epithelial cells14. A similar inflammatory reaction was also noted in T. vaginalis-infected prostatic epithelial cells in some in vitro studies5,6. Repeated cell damage and repair in chronic inflammation is likely to play an important role in inducing BPH15. Furthermore, the alteration in cytokine expression during chronic inflammation may have effects on cell growth and proliferation of the prostate epithelium and stroma in BPH15. The activated mast cells stimulated by T. vaginalis-infected prostatic epithelial cells can initiate IL-8 and CCL2 expression5. IL-8 could be a predictive marker for BPH16. Some in vitro studies demonstrated that IL-8 can stimulate fibroblast growth factor 2 (FGF-2), which causes the mitosis of prostate stromal cells17. IL-8 could also cause cyclin D1 expression to promote stromal cells proliferation18. In addition, CCL2, secreted by the prostatic stroma fibroblast, could promote both BPH and PCa progression5.
T. vaginalis possibly induces carcinogenesis of the prostate. The infected prostatic epithelial cells produce IL-6 in chronic inflammation19. In early studies, an elevated serum IL-6 level was noted in patients with advanced PCa20. The positive correlation between IL-6 receptor expression and cell proliferation has been reported21. IL-6 also induces epithelial–mesenchymal transition (EMT) in breast cancer growth and metastasis22, and the same reaction may also occur in prostatic epithelial cells23. In addition, more than one study has demonstrated that IL-6 could enhance androgen receptor (AR) activity and AR gene expression24, which is also related to prostate cancer growth. Twu et al. demonstrated that T. vaginalis macrophage migration inhibitory factor (TvMIF) plays an important role in inducing PCa7. There are already studies that have proven that higher human macrophage migration inhibitory factor (HuMIF) levels are present in several cancers, including PCa25. The structure of TvMIF is similar to that of HuMIF, which might explain why TvMIF also has the ability to promote cell proliferation, sustain inflammation, and stimulate the growth of prostate cancer cells7.
In previous studies, T. vaginalis could play an important role as a carcinogen of female cervical cancer26,27. However, there is no consensus regarding the relationship between trichomoniasis and cervical cancer28. Likewise, the role of T. vaginalis in the development of PCa is still controversial. Zhu et al. demonstrated that there was a negative association between PCa and trichomoniasis29. Instead, they discovered culture supernatant of T. vaginalis not only inhibited growth but also induced apoptosis of prostate cancer cell. T. vaginalis could enhance anti-proliferative molecules and decrease the expression of anti-apoptotic molecule29. The T. vaginalis adhesion protein could induce T helper 2 cell cytokines reaction to stimulate the productions of specific antibody30. This enhancement of the immune response might suppress the cancer cell activity31. Moreover, another further study also showed that T. vaginalis seropositivity does not raise mortality risk in men with PCa32. The inflammatory response caused by T. vaginalis might not have influence in the development and progression of PCa32. However, the detail mechanism of immune response between T. vaginalis and prostate epithelial cell still remained unclear, further investigations are necessary.
There were still a lack of studies to prove that trichomoniasis is associated with BC. We still included BC patients in our study because the inflammatory cytokines found in trichomoniasis, including IL-6 and IL-8, are also associated with a higher risk of developing BC33,34 and some parasites, such as Schistosoma haematobium, can induce BC. However, our study shows no significant association between T. vaginalis infection and BC probably because of limited sample.
We added depression as one of the comorbidities in our study due to another previous nationwide population-based cohort study in Taiwan which showed that patients with trichomoniasis had higher risks for developing an individual psychiatric disorder, including depression, anxiety, bipolar disorder, schizophrenia and substance abuse35. Our study results demonstrate that except for depression, no comorbidities had a significant association with BPH, PCa, or BC. The joint effect of trichomoniasis and depression increased the risk by 7.682 times that of the control group. A recent study showed that depression is associated with decreased immunity36. Moreover, depression can also cause cytokine dysregulation and increased serum IL-6 concentration36, which might enhance carcinogenesis after T. vaginalis infection.
Although this study was a large-scale population-based nationwide design with long-term monitoring from 2000 to 2015, there are still several limitations. First, the NHIRD does not contain detailed information regarding the symptom severity of BPH, the histological and TNM classification of PCa and BC, serum sex hormone concentrations, Prostate-Specific Antigen (PSA) levels, T. vaginalis antibody test, family history, or personal history such as sexual exposure, physical activity, alcohol consumption or tobacco smoking. Second, we did not include body mass index (BMI) as one of our variables. Obesity is one of the risk factors for BPH and PCa37, which might affect their association with trichomoniasis. Third, our study might underestimate the exact number of patients with trichomoniasis. Most male patients would not seek treatment due to being asymptomatic, and ineffective screening protocols because of the lack of public health awareness could also lead to possible T. vaginalis infection being neglected38. Another reason that caused underestimation of our case group is that the antibody tests of T. vaginalis were not performed popularly during diagnosis and mostly were female patients39. It is possible that T. vaginalis was substantially undercoded and underrepresented in the study population. Fourth, the number of cases of BC might be too small to be significant and the tracking time might not be sufficient for disease monitoring. Trichomoniasis can be a chronic infection. The outcomes in the study might present later in life, so in some men trichomonas exposure may happen a few years before these outcomes appear or many decades prior to diagnosis. Fifth, the outcome of each case was defined as the first code for BPH, PCa, or BC. This assumes that there is a common pathway between trichomoniasis and these 3 separate diseases. However, this approach method could also ignore these outcomes as comorbidities. For example, patients with PCa or BC could also have BPH or other urinary symptoms. It is possible that many PCa or BC outcomes were ignored if BPH was coded first. This might be another reason that our study samples were underestimated. Sixth, our study was designed as an observational case–control study, so the causation cannot be detected. We hope that in the future more research will support our thesis.
Conclusion
Male patients with T. vaginalis infection have an increased risk of developing BPH and PCa, especially in trichomoniasis patients with comorbid depression. Due to the lack of awareness of this pathogen, clinicians should not only treat patients who are already diagnosed but should also pay more attention to groups with higher trichomoniasis exposure risk.
Data availability
Data supporting the conclusions of this article are included within the article and its additional files. The datasets used and/or analyzed during the present study will be made available by the corresponding author upon reasonable request.
Abbreviations
- AOR:
-
Adjusted odds ratio
- AR:
-
Androgen receptor
- BC:
-
Bladder cancer
- BMI:
-
Body mass index
- BPH:
-
Benign prostate hyperplasia
- CCL2:
-
Chemokine ligand 2
- CI:
-
Confidence interval
- COPD:
-
Chronic obstructive pulmonary disease
- EMT:
-
Epithelial–mesenchymal transition
- FGF-2:
-
Fibroblast growth factor 2
- GUC:
-
Genitourinary cancers
- HPFS:
-
Health Professionals Follow-up Study
- HuMIF:
-
Human macrophage migration inhibitory factor
- IL:
-
Interleukin
- LHID2005:
-
Longitudinal Health Insurance Database 2005
- NHI:
-
National Health Insurance
- NHIRD:
-
National Health Insurance Research Database
- NT$:
-
New Taiwan Dollars
- OR:
-
Odds ratio
- PCa:
-
Prostate cancer
- PSA:
-
Prostate-specific antigen
- STI:
-
Sexually transmitted infection
- T. vaginalis :
-
Trichomonas vaginalis
- TvMIF:
-
Trichomonas vaginalis Macrophage migration inhibitory factor
- UTI:
-
Urinary tract infection
References
St Sauver, J. L. & Jacobsen, S. J. Inflammatory mechanisms associated with prostatic inflammation and lower urinary tract symptoms. Curr. Prostate Rep. 6, 67–73 (2008).
Orsted, D. D. & Bojesen, S. E. The link between benign prostatic hyperplasia and prostate cancer. Nat. Rev. Urol. 10, 49–54 (2013).
Huang, C. H. et al. Risk of cancer after lower urinary tract infection: A population-based cohort study. Int. J. Environ. Res. Public Health 16, 390 (2019).
World Health Organization. Global Incidence and Prevalence of Selected Curable Sexually Transmitted Infections—2008 (WHO, 2012).
Kim, J. H. et al. Proliferation of prostate stromal cell induced by benign prostatic hyperplasia epithelial cell stimulated with Trichomonas vaginalis via crosstalk with mast cell. Prostate 76, 1431–1444 (2016).
Kim, S. S., Kim, J. H., Han, I. H., Ahn, M. H. & Ryu, J. S. Inflammatory responses in a benign prostatic hyperplasia epithelial cell line (BPH-1) infected with Trichomonas vaginalis. Korean J. Parasitol. 54, 123–132 (2016).
Twu, O. et al. Trichomonas vaginalis homolog of macrophage migration inhibitory factor induces prostate cell growth, invasiveness, and inflammatory responses. Proc. Natl. Acad. Sci. U.S.A. 111, 8179–8184 (2014).
Sutcliffe, S., Neace, C., Magnuson, N. S., Reeves, R. & Alderete, J. F. Trichomonosis, a common curable STI, and prostate carcinogenesis—A proposed molecular mechanism. PLoS Pathog. 8, e1002801 (2012).
Mitteregger, D. et al. High detection rate of Trichomonas vaginalis in benign hyperplastic prostatic tissue. Med. Microbiol. Immunol. 201, 113–116 (2012).
Sutcliffe, S. et al. Plasma antibodies against Trichomonas vaginalis and subsequent risk of prostate cancer. Cancer Epidemiol. Biomark. Prev. 15, 939–945 (2006).
Marous, M. et al. Trichomonas vaginalis infection and risk of prostate cancer: Associations by disease aggressiveness and race/ethnicity in the PLCO trial. Cancer Causes Control 28, 889–898 (2017).
Breyer, B. N. et al. Sexually transmitted infections, benign prostatic hyperplasia and lower urinary tract symptom-related outcomes: Results from the prostate, lung, colorectal and ovarian cancer screening trial. BJU Int. 117, 145–154 (2016).
Lin, C. L., Liu, T. C., Wang, Y. N., Chung, C. H. & Chien, W. C. The association between sleep disorders and the risk of colorectal cancer in patients: A population-based nested case–control study. In Vivo 33, 573–579 (2019).
Han, I. H., Park, S. J., Ahn, M. H. & Ryu, J. S. Involvement of mast cells in inflammation induced by Trichomonas vaginalis via crosstalk with vaginal epithelial cells. Parasite Immunol. 34, 8–14 (2012).
Schauer, I. G. & Rowley, D. R. The functional role of reactive stroma in benign prostatic hyperplasia. Differentiation 82, 200–210 (2011).
Penna, G. et al. Seminal plasma cytokines and chemokines in prostate inflammation: Interleukin 8 as a predictive biomarker in chronic prostatitis/chronic pelvic pain syndrome and benign prostatic hyperplasia. Eur. Urol. 51, 524–533 (2007) (discussion 533).
Giri, D. & Ittmann, M. Interleukin-8 is a paracrine inducer of fibroblast growth factor 2, a stromal and epithelial growth factor in benign prostatic hyperplasia. Am. J. Pathol. 159, 139–147 (2001).
MacManus, C. F. et al. Interleukin-8 signaling promotes translational regulation of cyclin D in androgen-independent prostate cancer cells. Mol. Cancer Res. 5, 737–748 (2007).
Han, I. H., Kim, J. H., Kim, S. S., Ahn, M. H. & Ryu, J. S. Signalling pathways associated with IL-6 production and epithelial–mesenchymal transition induction in prostate epithelial cells stimulated with Trichomonas vaginalis. Parasite Immunol. 38, 678–687 (2016).
Adler, H. L. et al. Elevated levels of circulating interleukin-6 and transforming growth factor-beta1 in patients with metastatic prostatic carcinoma. J. Urol. 161, 182–187 (1999).
Giri, D., Ozen, M. & Ittmann, M. Interleukin-6 is an autocrine growth factor in human prostate cancer. Am. J. Pathol. 159, 2159–2165 (2001).
Sullivan, N. J. et al. Interleukin-6 induces an epithelial–mesenchymal transition phenotype in human breast cancer cells. Oncogene 28, 2940–2947 (2009).
Rojas, A. et al. IL-6 promotes prostate tumorigenesis and progression through autocrine cross-activation of IGF-IR. Oncogene 30, 2345–2355 (2011).
Lee, S. O. et al. Interleukin-6 promotes androgen-independent growth in LNCaP human prostate cancer cells. Clin. Cancer Res. 9, 370–376 (2003).
Hussain, F. et al. Human anti-macrophage migration inhibitory factor antibodies inhibit growth of human prostate cancer cells in vitro and in vivo. Mol. Cancer Ther. 12, 1223–1234 (2013).
Zhang, Z. F. et al. Trichomonas vaginalis and cervical cancer. A prospective study in China. Ann. Epidemiol. 5(4), 325–332 (1995).
Feng, R. M. et al. Risk of high-risk human papillomavirus infection and cervical precancerous lesions with past or current trichomonas infection: A pooled analysis of 25,054 women in rural China. J. Clin. Virol. 99–100, 84–90 (2018).
Zhu, Z. et al. Trichomonas Vaginalis inhibits HeLa cell growth through modulation of critical molecules for cell proliferation and apoptosis. Anticancer Res. 38(9), 5079–5086 (2018).
Zhu, Z. et al. Trichomonas vaginalis: A possible foe to prostate cancer. Med. Oncol. 33, 115 (2016).
Zhang, Z. et al. The molecular characterization and immunity identification of trichomonas vaginalis adhesion protein 33 (AP33). Front. Microbiol. 11, 1433 (2020).
Zhao, K. S., Mancini, C. & Doria, G. Enhancement of the immune response in mice by Astragalus membranaceus extracts. Immunopharmacology 20(3), 225–233 (1990).
Tsang, S. H. et al. Association between Trichomonas vaginalis and prostate cancer mortality. Int. J. Cancer 144(10), 2377–2380 (2019).
Chen, M. F., Lin, P. Y., Wu, C. F., Chen, W. C. & Wu, C. T. IL-6 expression regulates tumorigenicity and correlates with prognosis in bladder cancer. PLoS One 8, e61901 (2013).
Inoue, K. et al. Interleukin 8 expression regulates tumorigenicity and metastasis in human bladder cancer. Cancer Res. 60, 2290–2299 (2000).
Lin, H. C. et al. Infection with Trichomonas vaginalis increases the risk of psychiatric disorders in women: A nationwide population-based cohort study. Parasit. Vectors 12(1), 88 (2019).
Glaser, R. et al. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch. Gen. Psychiatry 60, 1009–1014 (2003).
Allott, E. H., Masko, E. M. & Freedland, S. J. Obesity and prostate cancer: Weighing the evidence. Eur. Urol. 63, 800–809 (2013).
Roth, A. M. et al. Changing sexually transmitted infection screening protocol will result in improved case finding for Trichomonas vaginalis among high-risk female populations. Sex. Transm. Dis. 38, 398–400 (2011).
Petrin, D., Delgaty, K., Bhatt, R. & Garber, G. Clinical and microbiological aspects of Trichomonas vaginalis. Clin. Microbiol. Rev. 11, 300–317 (1998).
Acknowledgements
We would like to thank the National Defense Medical Center team for support.
Funding
This work was supported by Tri-Service General Hospital Songshan Branch, Taiwan (TSGH-SS_E_111006) to JYW, (TSGH-SS_A_111001) to HAL, (TSGH-SS_E_111005) to CCC, Ministry of National Defense-Medical Affairs Bureau, Taiwan (MND-MAB-D-111140) to HCL, and Tri-Service General Hospital, Taiwan (TSGH-E-111224) to HCL, (TSGH-B-111018) to WCC.
Author information
Authors and Affiliations
Contributions
H.C.L., H.Y.Y. and C.C.C. conceived the idea and wrote the first draft manuscript. R.Y.S. and K.Y.H. contributed to the manuscript. W.C.C., H.A.L., J.Y.W., and C.H.C. research data collection and statistical analyses. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, HY., Su, RY., Chung, CH. et al. Association between trichomoniasis and prostate and bladder diseases: a population-based case–control study. Sci Rep 12, 15358 (2022). https://doi.org/10.1038/s41598-022-19561-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-19561-2
- Springer Nature Limited
This article is cited by
-
Transcriptional profile of Trichomonas vaginalis in response to metronidazole
BMC Genomics (2023)