Abstract
Serum globulin, which is composed mainly of immunoglobulins and acute phase proteins, can be considered as reflecting the inflammatory state. We conducted the present study to investigate the role of globulin in mortality risk in patients undergoing peritoneal dialysis (PD). The study participants were categorized by the median globulin value (2.8 g/dL) as the high globulin group (≥ 2.8 g/dL), and low globulin group (< 2.8 g/dL). Serum globulin is calculated by the equation: (serum total protein-serum albumin). The area under the curve (AUC) by the receiver operating characteristics curve analysis was calculated to compare the mortality prediction capacity of globulin with that of ferritin, and WBC counts. Among the 554 patients, 265 (47.83%) were men, the mean age was 52.91 ± 15.54 years and the body mass index was 23.44 ± 3.88 kg/m2. Multivariate Cox models showed the high globulin group had higher mortality risks of all-cause and cardiovascular disease (CVD), compared with the low globulin group with adjusted HRs of 2.06 (95% CI 1.39–3.05) and 1.94 (95% CI 1.18–3.16), respectively. The AUC of univariate and multivariate models for all-cause mortality resulted in higher AUC values for globulin than for ferritin and white blood cell (WBC) counts. In patients undergoing PD, the serum globulin can serve as a novel and independent determinant of predicting overall and CVD- associated mortality.
Similar content being viewed by others
Introduction
Approximately 11.5% of the general population had chronic kidney disease (CKD), which also occurred in 40% of diabetic patients in the United States1,2. Peritoneal dialysis (PD) is one of the accepted renal replacement therapies for patients with end-stage renal disease (ESRD) worldwide. The mortality rate of those patients remains much higher than the general population despite the modern improvement in medical science. CKD is an equivalent of cardiovascular disease, which was the leading cause of mortality among patients with CKD and accounted for about 40% of patient deaths3,4. Persistent low-grade inflammation, observed in the clinical setting of CKD, particularly ESRD, plays a pivotal role in the pathogenesis of high cardiovascular morbidity and mortality in this population5,6.
Inflammation had been reportedly modifying and catalyzing the vicious cycle of risk factors in ESRD, including atherosclerosis, vascular calcification and protein-energy wasting (PEW), leading to the tremendous adverse outcomes6. Therefore, inflammation has emerged as a therapeutic target through various nutritional and pharmacological interventions with the goal of improving the frustrating prognosis. C-reactive protein (CRP), a member of the pentraxin protein family produced by liver cells, is the most studied inflammatory marker because of its wide availability and low cost7. A post hoc analysis of a randomized controlled trial involving 4038 diabetic patients with anemia and CKD reported that baseline CRP levels are associated with the future development of ESRD and the composite of death or ESRD8.
Aside from CRP, several biochemical inflammatory markers, such as interleukin-6, interleukin-18, endoxin and gelsoin, had been shown to predict cardiovascular risk and mortality in patients with CKD5,9,10,11. Serum globulin consisting primarily of immunoglobulins and acute phase proteins can be considered to reflect an inflammatory state. However, there is a lack of research on the relationship between serum globulin and mortality in patients undergoing PD. Therefore, we conducted the present study to test the hypothesis that higher serum globulin levels predicted higher risk of death.
Results
Patients’ baseline characteristics
A total of 554 patients on PD were left for the final analysis with 257 patients in the low globulin group (< 2.8 g/dL) and 297 in the high globulin group (≧2.8 g/dL). The mean follow-up time was 3.87 ± 3.15 years. Among the study participants, 265 (47.83%) were men, the mean age was 52.91 ± 15.54 years and the BMI was 23.44 ± 3.88 kg/m2 (Table 1.). The vast majority of them never smoked (n = 456, 82.31%) and had primary school (n = 176, 31.77%) as the educational level. Chronic glomerulonephritis, diabetes mellitus and hypertension were the three major causes of CKD. The low globulin group had higher proportions of using ACE inhibitors/ARB, ESA, and calcium channel blocker, and having hypertension compared with the high globulin group. In terms of laboratory data, serum creatinine and phosphorus levels were higher in the low globulin group, and ALP, triglyceride, hemoglobin, and WBC count levels were lower than in the high globulin group. In addition, the low globulin group also had higher daily nPNA.
Association of globulin with all-cause mortality
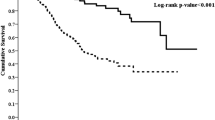
During the study period, the high globulin group had a significantly higher all-cause mortality rate than the low globulin group (n = 106, 35.69% vs. n = 45, 17.51%; p < 0.001). Kaplan–Meier survival curve showed the high globulin group had lower survival rate than the low globulin group (Fig. 1; log-rank test, p < 0.001). Both univariate and multivariate Cox models showed the high globulin group had higher all-cause mortality risk compared with the low globulin group with an adjusted HR of 2.06 (95% CI 1.39–3.05) in the fully adjusted model (Table 2). The hazard ratios for all variables included in the model 5 were provided as supplementary materials (Supplementary Table 1). The sensitivity tests yielded similar results, which corroborated the primary results showing globulin was an independent covariate for all-cause mortality.
Association of globulin with cardiovascular disease mortality
During the study period, the high globulin group had a significantly higher cardiovascular disease mortality rate than the low globulin group (n = 65, 21.89% vs. n = 30, 11.67%; p < 0.001). Kaplan–Meier survival curve showed the high globulin group had lower cardiovascular survival rate than the low globulin group (Fig. 2; log-rank test, p < 0.001). Both univariate and multivariate Cox models showed the high globulin group had higher CVD mortality risk compared with the low globulin group with an adjusted HR of 1.94 (95% CI 1.18–3.16) in the fully adjusted model (Table 2). Consistent results were produced by the sensitivity tests, which corroborated the primary results showing globulin was an independent covariate for CVD mortality.
Association of globulin with mortality after excluding patients with malignancy or autoimmune disease
After excluding those patients who had malignancy or autoimmune disease, globulin remained a significantly independent predictor of mortality of all causes and CVD whether it was evaluated by the median value or as a continuous variable (Table 2).
Correlation between globulin and clinical parameters
The direction and strength of associations between globulin and various clinical parameters were shown in Table 3. Variables that had negative correlations with globulin included creatinine and nPNA while the levels of WBC counts, intact PTH, triglyceride and ALP showed positive associations with globulin.
Predictive value of globulin for mortality compared with ferritin and WBC counts
The AUC of ROC curve for all-cause mortality was plotted and calculated to compare the predictive capacity between globulin, ferritin and WBC counts (Figs. 3, 4, 5 and 6). The AUCs of globulin, ferritin, and WBC in multivariable models were close to each other. The predictive power of globulin for 3-year mortality might be not inferior or slightly higher than that of ferritin and WBC counts (AUC 0.649, 0.607 and 0.636 for globulin, ferritin and WBC, respectively). The AUC of globulin for the overall mortality was also slightly higher than that of ferritin and WBC counts (AUC 0.646, 0.540 and 0.618 for globulin, ferritin and WBC, respectively). In the multivariate AUC calculation for the prediction of 3-year and overall mortality, similar results were produced by adding globulin to the variables in Model 4 to produce the highest AUC.
Discussion
In the developing and developed countries, the prevalence of PD patients is on the rise. Despite significant improvements in technology and modernity, the mortality rate of dialysis patients, with CVD as the leading cause, was still estimated at almost sevenfold higher than that of the general population2. Because implementation of strategic treatments aimed at traditional Framingham risk factors failed to improve the clinical prognosis in ESRD patients, this realization has focused attention on nontraditional risk factors, including anemia, microalbuminuria, inflammation, oxidative stress and deranged mineral metabolism12. Among those novel risk factors, inflammation has been linked with adverse impact on nutritional, metabolic and cardiovascular systems. Inflammation was also reportedly to accelerate the processes of atherosclerosis in ESRD patients, and a lot of existing evidence has addressed the issue of inflammation and tried to develop anti-inflammation treatments13. For the first time, we found in our study that serum globulin is a new determinant of predicting overall and CVD mortality in patients undergoing PD and the association was independent of other established risk factors.
Factors contributing to chronic inflammation in ESRD can be classified as decreased renal function, dialysis-related factors, co-morbidities and intestinal dysbiosis14. Persistent low-grade inflammation emerges with the gradual decline in kidney function owing to accumulation of uremic toxins, increased level of endotoxin and decreased clearance of pro-inflammatory cytokines. A negative association between residual renal function and inflammatory burden was reported among dialysis patients in numerous studies15,16,17,18. DM caused the majority of CKD and many CKD patients were also complicated with coronary heart disease. These comorbidities can further result in higher inflammation in CKD. Unique contributors in PD include continuous exposure to biologically incompatible PD fluids, peritonitis, tunnel tract infection and exit site infection. Furthermore, the alteration of intestinal microbiota caused by disruption of normal intestinal barrier with altered permeability and the increased absorption of toxic substances produced by proteolytic bacterial species in uremia milieu leads to high inflammation19,20. In addition, the adipose tissue has been recognized as an endocrine organ and can exert pleiotropic effects on inflammation through its ability of secreting numerous proinflammatory cytokines. The serum levels of leptin, a protein predominantly secreted by adipocytes and excreted by the kidney, were significantly higher in PD patients than in HD patients and ESRD without dialysis, where the constant glucose load can result in the increase in fat mass over time in PD patients21. The underlying causes of inflammation in PD do not work independently, but rather interact with each other to amplify the degree of inflammation through a vicious circle. Moreover, the bidirectional associations between the causes and consequences of inflammation are also observed in a positive manner. Consequently, the high prevalence of inflammation in PD leaded to high cardiovascular morbidities and mortality.
The predictive role of inflammation has been thoroughly investigated in PD patients. The surrogate markers included C-reactive protein (CRP), interleukin (IL), myeloperoxidase, and tumor necrosis factor (TNF), with CRP and IL-6 being the most studied22. A systemic review and meta-analysis of 109 CRP studies and 22 IL-6 studies showed elevated levels of CRP or IL-6 were significantly associated with higher overall and CVD mortality in dialysis patients23. The disproportionately high mortality in the presence of traditional cardiovascular burden in CKD patients has been explained by protein-energy wasting (PEW) syndrome, where inflammation also plays a key role. PEW, a progressive depletion of protein and energy, is often observed in patients with CKD, particularly ESRD, and accounts for higher mortality and worsened quality of life24. The coexistence of inflammation makes it distinguishable from other forms of malnutrition. Inflammation not only causes an increase in nutritional requirements but also results in loss of appetite from an imbalance between orexigenic and anorexigenic mechanisms in CKD25,26. In the current study, we found that the protein equivalent of nitrogen appearance (PNA), the indirect marker of protein intake, was lower in patients with higher globulin levels, supporting the notion of inhibitory effect of inflammation on appetite. The existence of significant association between higher globulin and higher mortality after adjustment of nutritional indicators, such as BMI, serum albumin, and nPNA, excludes the possibility of globulin-mortality relation caused by malnutrition.
Serum globulins, one of the major constituents of total serum proteins, are believed as a good biomarker reflecting the degree and severity of inflammation and immunity because they are synthesized and secreted by the mononuclear phagocytes and mainly composed of inflammatory cytokines and antibodies. Thus, the rise in serum globulin concentrations results from the accumulation of immunoglobulins and acute inflammatory proteins. These changes are indicated of an inflammatory state as evidenced by our findings that globulin is positively associated with WBC counts in the Pearson correlation tests. Research on globulin has attracted much less interests. Li et al. in 2015 reported high preoperative serum globulin as an unfavorable survival factor in 293 locally advanced rectal cancer patients receiving neoadjunctive chemotherapy followed by radical surgery27. Later, a retrospective cohort study of 186 gastric cancer patients undergoing radical surgery in China evaluated the prognostic role of albumin and globulin in cancer-specific mortality, demonstrating that a high globulin level was a significant risk factor for poor survival in univariate analysis although not included in multivariate analysis28. The prognostic value of pretreatment serum globulin level was also shown in patients with nasopharyngeal carcinoma with a high globulin associating with a poor progression-free survival29. We also found serum globulin concentration as a negative survival determinant amongst patients undergoing PD.
Systemic inflammation is estimated at a range of between 12 and 65% in PD patients, depending on the cut-off value of the selected inflammatory markers30. Since convincing evidence confirms the detrimental impact of inflammation on clinical outcomes of PD patients, researches have been devoted to suppress the severity of inflammation both systemically and intraperitoneally. Potential therapeutic options showing promising results in preliminary studies include preserving residual kidney function, using biocompatible PD fluids, maintaining intestinal commensalism, optimizing fluid status and avoiding catheter-related infections31. Serum globulin level emerged as a novel biomarker for predicting mortality amongst our PD cohort. Whether the timely variation of serum globulin levels after instituting potential therapeutic interventions could reflect the changes of mortality risk requires future large-scale, prospective clinical trials.
Several limitations of this study are worth mentioning. First, routine measurements of CRP and inflammatory cytokines were not done in such a retrospective study. Although the independent role of globulin in predicting mortality among PD patients was robust in light of sensitivity tests, it remains unclear whether this relationship remains after adding some more inflammatory markers into the adjustment. Second, PD patients treated with biocompatible dialysates had a slower rate of decline of residual renal function and a higher achieved Kt/V compared with those treated with conventional solutions32. It is plausible that the use of bio-incompatible PD may lead to a higher risk of inflammation and death. Due to the retrospective nature of study design and technical limitation, we cannot address this important issue. Third, single measurement of serum globulin may under- or over-estimate its relations with mortality in the long term. Statistics approaches with time-varying covariates may be more optimal to clarify the relationship between globulin and mortality. Fourth, instead of being directly measured, serum globulin is calculated by the equation: (serum total protein-serum albumin). Nonetheless, our study provided the evidence that globulin is an independent variable for mortality after adjusting clinical confounders. Our method of calculating serum globulin is inexpensive and practical in clinical medical care.
Thousands of articles had been published on inflammation and dialysis because of its pivotal role in triggering the vicious cycle of cardiovascular burden, such as atherosclerosis, malnutrition, and muscle wasting, where inflammation also is magnified by positive feedback of its consequences. The independent role of serum globulin for the prediction of mortality in our study suggested that serum globulin could be used as a sensitive potential therapeutic target for various anti-inflammatory and immunomodulatory interventions. Further research is needed to elucidate whether the biological mechanism of globulin as an independent predictor of mortality is mediated by specific immunoglobulins or inflammatory proteins.
Materials and methods
Participants and measurements
We conducted a retrospective longitudinal study in patients with ESRD undergoing PD at a single dialysis unit of a medical center in Taiwan with the aim to evaluate the prognostic value of serum globulin level in mortality risk. Patients were considered to be enrolled in the study if they started receiving PD treatment between 2001 and 2016. Exclusion criteria included age < 18 years (n = 12) or the time on PD < 3 months (n = 12). A total of 554 patients who matched the selection criteria were eligible for study finally. The causes of deaths were collected and recorded for the analysis of cause-specific mortality. We conducted this study as per the ethnical regulations of declaration of Helsinki with the approval and surveillance of Institutional Review Board of Changhua Christian Hospital. The informed consent document from each participant was waived for a retrospective study in Taiwan.
Patients’ covariates regarding the socio-demographic characteristics, medications use, PD related data, comorbidities and laboratory variables were collected and recorded at study entry from the hospital’s database using established electronic medical records. Smoking status was classified as never, ever or current smoker, while body mass index (BMI) was derived from body weight in kilograms divided by the square of body height in meters. Clinical medical conditions consisted of diabetes mellitus (DM), hypertension, hyperlipidemia, cancer, autoimmune disease and cardiovascular disease (CVD), which was defined as the presence of coronary artery disease, cerebrovascular disease or peripheral artery disease. PD related data included normalized protein nitrogen appearance (nPNA), adequacy of dialysis (weekly Kt/V urea), 24 h urine output, and dialysate-to-plasma creatinine ratio at 4 h (D/P (creatinine) at 4 h), and residual glomerular filtration rate, which was calculated as the average of 24 h urine clearance of urea and creatinine.
Laboratory covariates used for baseline characteristics included blood levels of albumin, globulin, creatinine, glutamic-pyruvic transaminase (GPT), white blood cell (WBC) count, alkaline phosphate (ALP), hemoglobin, ferritin, cholesterol, triglyceride, intact parathyroid hormone (PTH), calcium, and phosphate. The information on pharmacotherapy included angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARB), diuretics, erythropoiesis stimulating agents (ESA), vitamin D and calcium channel blockers. The study participants were categorized by the median globulin value (2.8 g/dL) as the high globulin group (≥ 2.8 g/dL), and low globulin group (< 2.8 g/dL). Globulin is derived from the difference between total protein and albumin (serum total protein-serum albumin). All patients were followed up from the date of commencing PD until death, or the end of study on 31 July 2017. Cardiovascular disease is the leading cause of death our PD cohort. The primary clinical outcome studied was mortality from all causes and CVD mortality as the secondary outcomes.
Statistical analysis
Frequency and percentage were used to display the distribution of categorical data while means ± standard deviation (SD) or median (interquartile range, IQR) were used for continuous data depending on whether there is normal distribution, which was determined by the Kolmogorov–Smirnov test. For the comparisons of baseline patients’ characteristics between the high and low globulin groups, Student’s test or Mann–Whitney test was used for continuous data and Chi-square test or Fisher’s exact test for categorical data. Survival plot was depicted by the Kaplan–Meier estimates and the differences in survival status between groups were compared using a log-rank test. Cox proportional hazards models were conducted for multivariate adjustments to evaluate the significance between the clinical outcomes and covariates and the results were shown as hazard ratio (HR) with 95% confidence interval (CI).
We performed a hierarchical regression technique in Cox proportional hazards models to determine whether the significance between globulin and clinical outcomes was independent. The unadjusted model was constructed to determine the association between globulin and mortality risk in the univariate Cox regression model. In hierarchical framework, we built five adjustments models as follows: model 1, globulin plus age, sex, BMI, smoking status, the cause of CKD, the status ahead of PD, and educational level; model 2, variables in model 1 plus medications use; model 3, variables in model 2 plus comorbidities; model 4, variables in model 3 plus PD related data; model 5, variables in model 4 plus laboratory parameters. In this study, variables selection was non-parsimonious manner and all variables in Table 1 were considered.
The normal range of globulin was not available and the appropriate cut-off value for globulin was also undetermined. Thus, we performed three sensitivity analyses to corroborate our results. First, we re-ran the Cox analysis by treating globulin as a continuous variable. Second, the study cohort was re-grouped to three tertiles based on the globulin levels. Third, the optimal cut-off value for globulin was determined by the Receiver Operating Characteristics (ROC) curve analysis and used in the Cox models. Patients with malignancy and autoimmune disease may have increased levels of serum globulin, thus confounding our results. We repeated our analyses after excluding those with cancers or autoimmune diseases to test the robustness of our results.
The significance and strength of association between globulin and laboratory and PD related parameters were assessed by Pearson rank correlation test and multiple linear regression analyses. We calculated the area under the curve (AUC) by the ROC curve analysis to compare the mortality prediction capacity of globulin with that of ferritin, and WBC counts, both of which indicated the degree of inflammation. In addition to the comparison of AUC for individual variable, we also conducted multivariate AUC after adding the selected variable (globulin, ferritin and WBC counts) into the variables in model 4. All the statistical analyses were performed using IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY). A two-sided p value of < 0.05 was considered statistically significant.
Data availability
The data sets generated or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150(9), 604–612 (2009).
Saran, R. et al. US renal data system 2014 annual data report: epidemiology of kidney disease in the United States. Am. J. Kidney Dis. 65(6), S1–S305 (2015).
Foley, R. N., Parfrey, P. S. & Sarnak, M. J. Epidemiology of cardiovascular disease in chronic renal disease. J. Am. Soc. Nephrol. 9, S16–S23 (1998).
Hage, F. G. et al. The scope of coronary heart disease in patients with chronic kidney disease. J. Am. Coll. Cardiol. 53(23), 2129–2140 (2009).
Stenvinkel, P. et al. Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: How do new pieces fit into the uremic puzzle?. Clin. J. Am. Soc. Nephrol. 3, 505–521 (2008).
Carrero, J. J. & Stenvinkel, P. Persistent inflammation as a catalyst for other risk factors in chronic kidney disease: A hypothesis proposal. Clin. J. Am. Soc. Nephrol. 4(Suppl 1), S49–S55 (2009).
Carrero, J. J. & Stenvinkel, P. Inflammation in end-stage renal disease: What have we learned in 10 years?. Semin Dial 23, 498–509 (2010).
Mc Causland, F. R. et al. C-reactive protein and risk of ESRD: Results from the trial to reduce cardiovascular events with aranesp therapy (TREAT). Am. J. Kidney Dis. 68(6), 873–881 (2016).
Witasp, A. et al. Increased expression of proinflammatory genes in abdominal subcutaneous fat in advanced chronic kidney disease patients. J. Intern. Med. 77, 550–556 (2010).
McIntyre, C. W. et al. Circulating endotoxemia: a novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 6, 133–141 (2011).
Lee, P. S. et al. Plasma gelsolin and circulating actin correlate with hemodialysis mortality. J. Am. Soc. Nephrol. 20, 1140–1148 (2009).
Kendrick, J. & Chonchol, M. B. Nontraditional risk factors for cardiovascular disease in patients with chronic kidney disease. Nat. Clin. Pract. Nephol. 4, 672–681 (2008).
Velloso, M. S. et al. Peritoneal dialysis and inflammation. Clin. Chim. Acta. 430, 109–114 (2014).
Sabatino, A. et al. Protein-energy wasting and nutritional supplementation in patients with end-stage renal disease on hemodialysis. Clin. Nutr. 36(3), 663–671 (2017).
Akchurin, O. M. & Kaskel, F. Update on inflammation in chronic kidney disease. Blood Purif. 39(1–3), 84–92 (2015).
Lee, B. T. et al. Association of C-reactive protein, tumcor necrosis factor-alpha, and interleukin- 6 with chronic kidney disease. BMC Nephrol. 16, 77 (2015).
Rossi, M. et al. Protein-bound uremic toxins, inflammation and oxidative stress: A cross- sectional study in stage 3–4 chronic kidney disease. Arch. Med. Res. 45, 309–317 (2014).
Demirci, M. S. et al. Relations between malnutrition- inflammation-atherosclerosis and volume status. The usefulness of bioimpedance analysis in peritoneal dialysis patients. Nephrol. Dial. Transplant. 26, 1708–1716 (2011).
Sabatino, A. et al. Alterations of intestinal barrier and microbiota in chronic kidney disease. Nephrol. Dial. Transpl. 30, 924–933 (2015).
Ramezani, A. et al. Role of the gut microbiome in uremia: A potential therapeutic target. Am. J. Kidney Dis. 67, 483–498 (2016).
Fontán, M. P. et al. Hyperleptinemia in uremic patients undergoing conservative management, peritoneal dialysis, and hemodialysis: a comparative analysis. Am. J. Kidney Dis. 34, 824–831 (1999).
Miyamoto, T., Carrero, J. J. & Stenvinkel, P. Inflammation as a risk factor and target for therapy in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 20(6), 662–668 (2011).
Zhang, W. et al. Prognostic role of C-reactive protein and interleukin-6 in dialysis patients: A systematic review and meta-analysis. J. Nephrol. 26, 243–253 (2013).
Ikizler, T. A. et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: a consensus statement by the International society of renal nutrition and metabolism. Kidney Int. 84, 1096–1107 (2013).
Obi, Y. et al. Latest consensus and update on protein-energy wasting in chronic kidney disease. Curr. Opin. Clin. Nutr. Metab. Care 18, 254–262 (2015).
Wang, X. H. & Mitch, W. E. Mechanisms of muscle wasting in chronic kidney disease. Nat. Rev. Nephrol. 10, 504–516 (2014).
Li, Q. et al. High preoperative serum globulin in rectal cancer treated with neoadjunctive chemoradiation therapy is a risk factor for poor outcome. Am. J. Cancer Res. 5(9), 2856–2864 (2015).
Chen, J. et al. Low pretreatment serum globulin may predict favorable prognosis for gastric cancer patients. Tumour Biol. 37(3), 3905–3911 (2016).
Zhong, L. T. et al. An elevated pretreatment serum globulin level predicts a poor prognosis of nasopharyngeal carcinoma. Nan Fang Yi Ke Da Xue Xue Bao 36(2), 151–156 (2016).
Cho, Y., Hawley, C. M. & Johnson, D. W. Clinical causes of inflammation in peritoneal dialysis patients. Int. J. Nephrol. 2014, 909373 (2014).
Li, P. K., Ng, J. K. & Mcintyre, C. W. Inflammation and peritoneal dialysis. Semin. Nephrol. 37(1), 54–65 (2017).
Wang, J., Zhu, N. & Yuan, W. Effect of neutral pH and low-glucose degradation product-containing peritoneal dialysis solution on residual renal function in peritoneal dialysis patients: a meta-analysis. Nephron 129(3), 155–163 (2015).
Funding
This study was funded by grants 106-CCH-IRP-029, 107-CCH-HCR-033, 108-CCH-IRP-092, 109-CCH-IRP-020 and 110-CCH-IRP-027 from the Changhua Christian Hospital Research Foundation.
Author information
Authors and Affiliations
Contributions
S.-M.T. collected and analyzed the data. P.-F.C. conceived the study and revised the paper. C.-T.K. analyzed the data. Y.-P.H. conceived and designed the experiments and wrote the paper. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hsieh, YP., Tsai, SM., Kor, CT. et al. Serum globulin is a novel predictor of mortality in patients undergoing peritoneal dialysis. Sci Rep 13, 1139 (2023). https://doi.org/10.1038/s41598-023-27688-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-27688-z
- Springer Nature Limited