Abstract
The ability of 18F-FDG positron emission tomography (PET) to track disease activity and treatment response in patients with Ankylosing Spondylitis (AS) or Psoriatic Arthritis (PsA) remains unclear. Here, we assessed whether 18F-FDG uptake is a marker of disease activity and treatment response in AS or PsA, and explored the ability of 18F-FDG to predict treatment response. Patients with AS (n = 16) or PsA (n = 8) who were scheduled to initiate treatment with biologics were recruited. Participants underwent a clinical evaluation and an 18F-FDG scan prior to therapy initiation. Eleven participants underwent a follow-up 18F-FDG scan 3 months post-treatment. Images were quantified using a composite measure that describes the inflammatory status of the patient. Clinically involved joints/entheses had higher 18F-FDG uptake compared to unaffected areas (median difference > 0.6, p < 0.01). Among patients with AS, pre-treatment 18F-FDG uptake was strongly associated with disease activity (r = 0.65, p = 0.006). Longitudinal 18F-FDG scans demonstrated that decreases in uptake at 3 months were associated to clinical response (βΔgSUVmax > 8.5, p < 0.001). We found no significant association between pre-treatment 18F-FDG uptake and subsequent clinical response. 18F-FDG PET shows potential as a marker of disease activity in AS and PsA, allowing for monitorization of biological treatment efficacy in these patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Seronegative spondyloarthropathies represent a heterogeneous group of chronic inflammatory disorders characterized by axial or peripheral arthritis and enthesitis, typically accompanied by extra-articular features such as uveitis, psoriasis, and inflammatory bowel disease1. Seronegative spondyloarthropathies include ankylosing spondylitis (AS), psoriatic arthritis (PsA), reactive arthritis, inflammatory bowel disease-related arthritis, and undifferentiated seronegative spondyloarthropathies. Among these, AS and PsA are the two most prevalent phenotypes, contributing to approximately 60% to 80% of all seronegative spondyloarthropathies cases2.
One of the fundamental pathological signatures of seronegative spondyloarthropathies is inflammation of the entheses and synovial tissue, though ligaments and bone marrow can also be affected3,4. These inflammatory processes are closely associated with the severity of clinical symptoms and disease activity5,6, thus accurate and objective assessment of inflammation is crucial for early diagnosis and monitoring therapeutic response in patients with seronegative spondyloarthropathies.
To date, standard radiography and magnetic resonance imaging (MRI) are key imaging modalities for the diagnosis and management of patients with seronegative spondyloarthropathies7,8,9. While X-rays can only describe established damages occurring at later stages8, MRI is more sensitive to earlier inflammatory processes such as bone marrow edema10 and is therefore considered the gold standard modality for the assessment of early pathological changes in seronegative spondyloarthropathies9. MRI can also detect structural damage such as fat lesions, erosion, sclerosis, and ankylosis11,12,13. Yet, despite these valuable diagnostic features, MRI has shown to be a poor marker of disease activity in previous randomized controlled trials and longitudinal studies9,14,15, and thus there is a need for more accurate markers of disease activity that can substantially improve monitorization of disease progression in clinical trials and practice.
The ability of positron emission tomography (PET) imaging to detect inflammatory changes at the molecular level before structural changes occur suggests that PET may overcome the current limitations of MRI. PET imaging can accurately reflect the pathological signatures of seronegative spondyloarthropathies, specifically inflammation—with tracers such as 18F-FDG PET16,17,18—and osteoblastic activity—with e.g. 18F-sodium fluoride (NaF) PET19,20. Findings from prior cross-sectional studies demonstrated that PET with 18F-FDG or 18F-NaF depicts active lesions in AS16,20,21, PsA22,23, and other seronegative arthritis24. However, it is still unclear which biological process best reflects disease activity as conflicting findings on the diagnostic performance of PET with 18F-FDG or 18F-NaF have been reported25,26. The field still lacks key studies confirming the utility of both 18F-FDG and 18F-NaF PET for the clinical assessment of seronegative spondyloarthropathies or for treatment trials.
In the present study, we contribute to fill the current knowledge gap in seronegative spondyloarthropathies diagnostics by performing an analysis of the ability of 18F-FDG PET to monitor disease progression in patients with AS or PsA who are scheduled to initiate biological treatment27. We chose to study 18F-FDG PET over 18F-NaF PET due to its wider accessibility, more established value as a biomarker in the field of rheumatic diseases, and its ability to reflect inflammatory processes closely associated with disease activity. To this aim, we provide novel data investigating how cross-sectional 18F-FDG PET signal intensity associates with the severity of clinical symptoms in AS and PsA patients. Furthermore, we performed longitudinal 18F-FDG PET scans to assess whether PET signal changes over time do actually reflect clinical improvements related to therapy. Finally, we explored the ability of 18F-FDG PET in predicting treatment response.
Materials and methods
Participants and study design
We prospectively included patients with a prior diagnosis of AS (n = 16) or PsA (n = 8) who were referred to the Rheumatology unit at the Central University Hospital of Asturias to initiate biological treatment (anti-TNF or anti-Il17 inhibitors). Eligible patients must be ≥ 18 years of age, have a life expectancy of more than 12 months, and be willing and able to undergo testing procedures, including musculoskeletal imaging. Participants must not have been treated with biologics before, must meet criteria for prior treatment failure with non-steroidal anti-inflammatory (NSAIDs) or disease-modifying antirheumatic drugs (DMARDs)27, and must not have contraindications to biological therapy. Exclusion criteria include pregnancy/breast-feeding, current treatment with immunosuppressants, other comorbid inflammatory joint disease, presence of clinically significant opportunistic infections, a cancer diagnosis in the past 5 years, history of bleeding diathesis or inherited coagulation disorders, diabetes mellitus with poor glycemic control (> 150), major surgery in the past three months, and have had a non-study related investigational treatment procedure within 28 days prior to screening.
All participants underwent a comprehensive clinical evaluation one week before the scheduled initiation of biological treatment—baseline visit—, as well as at 3- and 6-months post-baseline (except for 1 AS and 1 PsA participants who did not complete the 6-month visit). All participants underwent 18F-FDG PET at baseline, and 6 AS and 5 PsA participants also underwent a longitudinal 18F-FDG PET scan at 3 months post-baseline.
The study protocol was approved by the Central University Hospital of Asturias’ Ethics and Clinical Research Committee. All patients provided written informed consent prior to participation in the study. This study was performed in accordance with the Declaration of Helsinki.
Clinical assessments
Clinical evaluations were performed by a certified rheumatologist at the Rheumatology unit of the HUCA. For AS participants, disease activity was assessed using the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI)28. For PsA participants, disease activity was monitored with the Disease Activity Index for Psoriatic Arthritis (DAPSA)29.
18 F-FDG PET acquisition
Whole-body 18F-FDG PET/CT scans were acquired as per standard institutional protocol at the Nuclear Medicine department of HUCA on a GE Discovery 710 PET/CT scanner. Patients fasted for at least 6 h prior to the injection of 0.1 mCi/Kg of 18F-FDG. Scans were acquired 50–60 min post-injection using a scanning time of 2.5 min per bed position. Three-dimensional PET images were reconstructed using the ordered subset expectation maximization algorithm (3 iterations, 18 subsets) accounting for time-of-flight. Low-dose CT (50–300 mA, 120 kV) was performed previous to the PET acquisition for attenuation correction and localization of PET signal and was not used for identification of structural bone lesions.
Image analysis
An experienced Nuclear Medicine physician (OR-F), blinded to clinical information, analysed the acquired 18F-FDG PET images in a semiquantitative manner. Seventeen peripheral joints (shoulders, elbows, carpal-metacarpophalangeal, hips, knees, tarsal-metatarsophalangeal, spine, and sacroiliac) and 18 entheses (costochondral joints, L5 spinous process, anterior superior iliac spine, iliac crest, posterior iliac spine, pubic symphysis, ischial tuberosities, tibial tuberosities, and Achilles tendons) were evaluated. For each joint/enthesis, a two-dimensional region of interest (ROI) was manually drawn, and the maximum standardized uptake value (SUVmax) within the ROI was measured.
To globally describe the inflammatory status of the patient, we defined a composite index based on the SUVmax measured on the clinically affected joints/entheses at baseline. For this, we first averaged the SUVmax measured on the spine, hips, and knee joints with the SUVmax measured on their corresponding entheses (L5, greater trochanter, and patellar tendons, respectively), as it was challenging to reliably determine whether there was joint or entheseal involvement in these regions. Then, we derived a global SUVmax (gSUVmax) index by averaging the SUVmax (with the aforementioned modification) of the clinically affected regions at baseline. Note that, for longitudinal 18F-FDG PET scans, gSUVmax is derived using the longitudinal SUVmax measured on the clinically involved regions at baseline.
Statistical analysis
Sign tests were used to compare the medians of the distributions of average SUVmax in clinically uninvolved vs clinically involved regions, as well as to compare the medians of the distributions of baseline gSUVmax vs post-baseline gSUVmax. Cross-sectional relationships between gSUVmax and baseline disease activity (BASDAI or DAPSA) were assessed using linear regression and the corresponding correlation coefficient (r). The association between gSUVmax change at 3 months (ΔgSUVmax = gSUVmax,3 m − gSUVmax,bl) with change in disease activity at 3- and 6-months follow-up was assessed using linear mixed effects (LME) models. The LME model included change from baseline in the respective disease activity score (BASDAI or DAPSA) as dependent variable. Model’s fixed effects included a study visit term (categorical variable, 3- and 6-months), baseline score, and ΔgSUVmax, as well as visit-by-baseline score and visit-by-ΔgSUVmax interactions. A subject-specific intercept was included as random effects. Given the relevance of sacroiliac involvement in AS, we repeated the aforementioned cross-sectional and longitudinal analyses using SUVmax measured at the sacroiliac joints, instead of gSUVmax. Similar models as for the longitudinal analyses were run for the assessment of the association between baseline gSUVmax and disease activity change, by replacing ΔgSUVmax by baseline gSUVmax.
Results
Baseline and follow-up cohort characteristics
Demographics and baseline clinical characteristics of the study participants are summarized in Table 1. Overall, AS and PsA participants had similar age and disease duration distributions. The distribution of previous, non-biologic treatments was similar across AS and PsA patients, except for disease-modifying therapies, which were more commonly used in PsA patients. AS participants had more entheseal involvement, while PsA participants had more painful/swollen/tender joints. Seven out of the 16 AS participants were clinically affected at the sacroiliac joints. Both AS and PsA participants had moderate/high disease activity (BASDAI > 4 and DAPSA > 18). After the baseline clinical evaluation, 2 PsA patients did not initiate treatment with biologics as per indication of their managing rheumatologist.
At the 3-month follow-up visit, 2 AS participants changed their biologic treatment due to lack of efficacy (Adalimumab); the remaining participants continued with their prescribed biologic at baseline, and the 2 PsA participants that did not initiate treatment at baseline continued without biologic therapy. Both AS and PsA participants undergoing biologic therapy showed statistically significant improvements in their clinical symptoms at both 3- and 6-month follow-up (Table 1, last 4 rows).
Cross-sectional associations
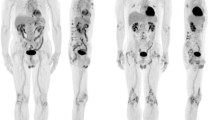
First, we investigated whether active disease associated with changes in 18F-FDG uptake. Clinically involved—painful/swollen/tender—joints and/or entheses had significantly higher average SUVmax (gSUVmax) compared to the average SUVmax of pain- and inflammation-free joints/entheses (Fig. 1A–C, AS: median SUV difference = 0.61, p < 0.001; PsA: median SUV difference = 0.72, p = 0.008). Furthermore, we found that baseline gSUVmax was strongly associated with baseline disease activity in AS (r = 0.65, p = 0.006, Fig. 1D); no statistically significant association was found for PsA participants (r = − 0.37, p = 0.37) (Fig. 1E).
Pre-treatment relationships between 18F-FDG uptake in clinically affected joints/entheses and disease activity. (A) Example of an AS patient displaying elevated 18F-FDG uptake at the knees. (B) Example of a PsA patient displaying 18F-FDG uptake at the right knee and left trochanter areas. (C) Comparison of 18F-FDG uptake in clinically involved vs clinically uninvolved joints/entheses. Reported p-values are from sign tests comparing the median 18F-FDG uptake between the involved/uninvolved regions. (D,E) represent the associations between 18F-FDG uptake, as measured using the composite score gSUVmax, and disease activity in AS (D) and PsA (E). Correlation coefficients (r), together with respective p-values, are reported in the boxes.
We obtained similar results when assessing SUVmax at the sacroiliac joints in patients with AS, namely higher SUVmax in clinically involved sacroiliac joints vs clinically uninvolved joints/entheses (median SUV difference: 1.30, p = 0.016, Fig. 2A) and a positive association with disease activity (r = 0.55, p = 0.027, Fig. 2B).
(A) Comparison of 18F-FDG uptake in clinically involved sacroiliac joints vs clinically uninvolved joints/entheses. Reported p-values are from sign tests comparing the median 18F-FDG uptake between the involved/uninvolved regions. (B) Associations between 18F-FDG uptake in the sacroiliac joints and disease activity in AS (BASDAI). Correlation coefficients (r), together with respective p-values, are reported in the box.
Longitudinal associations
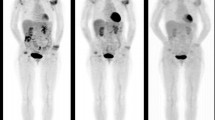
Next, we analysed the longitudinal changes in 18F-FDG uptake at 3-months and its relationship with changes in disease activity at 3- and 6-months after the initiation of biologic therapy. We found that gSUVmax decreased in both AS (median change = − 0.41) and PsA (median change = − 0.26) participants at 3-months follow-up, though these changes reached statistical significance only in AS patients (Fig. 3A–C). In addition, more pronounced gSUVmax decreases (i.e. more negative ΔgSUVmax) at 3 months were significantly associated with stronger clinical improvements at 3-months for both AS and PsA patients (AS: βΔgSUVmax = 8.5, p < 0.001; PsA: βΔgSUVmax = 20.0, p < 0.001), and these improvements were sustained at 6 months follow-up (Fig. 3D,E).
Longitudinal relationships between 18F-FDG uptake in clinically affected joints/entheses and disease activity. (A,B) Example of pre-treatment vs 3-month post-treatment 18F-FDG PET scans of an AS (A) and PsA (B) patient. For the AS patient (A), the pre-treatment scan shows elevated 18F-FDG uptake at the clinically involved areas (shoulders, knees, and hips). After 3 months of biologic treatment, the follow-up 18F-FDG PET scan shows clear reductions in 18F-FDG uptake in the aforementioned regions. For the PsA patient (B), the pre-treatment scan showed elevated 18F-FDG uptake at the right knee and left trochanter areas. After 3 months of treatment with biologics, the follow-up scan revealed significantly lower 18F-FDG uptake in the knee and slightly lower uptake in the trochanter area. (C) Comparison of 18F-FDG uptake, as measured using gSUVmax, before and 3 months after treatment. Reported p-values are from sign tests comparing the median gSUVmax between the pre- and post-treatment scans. (D,E) Results from linear mixed effects models showing the adjusted mean change from baseline in BASDAI for AS (D) and in DAPSA for PsA (E) at 3 and 6 months after biological treatment initiation. Results are shown for the first (Q1) and third quartile (Q3) of the 3-month change in gSUVmax. Stronger reductions in gSUVmax (Q3) were associated with greater clinical improvements at follow-up (blue lines).
Only 2 AS patients with clinical involvement at the sacroiliac joints had available follow-up PET scans. Both of these patients showed reductions in SUVmax at the sacroiliac joints at the 3-month visit (1.44 and 0.55 SUV units lower than the baseline visit, see Fig. 4A). When analysing the entire group of AS patients, we observed a reduction in SUVmax at the sacroiliac joints at 3 months (median SUV difference: 0.44 SUV units), although this reduction was not statistically significant (Fig. 4B). Similarly, no statistically significant association between SUVmax change and BASDAI change was found (Fig. 4C).
(A) Example of pre-treatment (left) vs 3-month post-treatment (right) 18F-FDG PET scans of an AS patient with clinical involvement at the sacroiliac level. The follow-up scan reveals significantly lower 18F-FDG PET signal at the sacroiliac joints. (B) Comparison of 18F-FDG uptake at the sacroiliac joints before and 3 months after treatment. Reported p-values are from sign tests comparing the median SUVmax between the pre- and post-treatment scans. (C) Results from linear mixed effects models showing the adjusted mean change from baseline in BASDAI for AS at 3 and 6 months after biological treatment initiation. Results are shown for the first (Q1) and third quartile (Q3) of the 3-month change in SUVmax at the sacroiliac joints.
Prediction of clinical progression
Finally, we explored whether baseline gSUVmax was associated with subsequent changes in disease activity at 3- and 6-months follow-up after the initiation of biologics. Among AS participants, lower baseline gSUVmax were nominally associated with stronger reductions in disease activity during follow-up, although the associations were not statistically significant (Fig. 5A). No statistically significant associations between baseline gSUVmax and changes in disease activity was found for PsA participants (Fig. 5B).
Results from linear mixed effects models showing the adjusted mean change from baseline in BASDAI for AS (A) and in DAPSA for PsA (B) at 3 and 6 months after biological treatment initiation. Results are shown for the first (Q1) and third quartile (Q3) of the pre-treatment gSUVmax. No statistically significant associations between pre-treatment gSUVmax and clinical improvements at follow-up were found.
Discussion
The present study investigated the ability of 18F-FDG PET to track disease activity, both cross-sectionally and longitudinally, in AS and PsA patients who were candidates for initiation of biologic therapy. We found that 18F-FDG uptake in clinically involved joints/entheses was strongly correlated with pre-treatment disease activity in patients with AS. This result was supported by our longitudinal analysis, in which we confirmed that more pronounced decreases in 18F-FDG uptake were significantly associated with better responses to biologic treatment, both in AS and PsA participants. Together, our findings indicate that 18F-FDG PET may be a useful tool for objective monitorisation of disease activity and assessment of treatment response in AS and PsA patients undergoing therapy with biologics.
A number of prior cross-sectional studies investigated the ability of 18F-FDG PET for the detection of lesions in patients with AS16,17,18,25,30. However, the potential of 18F-FDG PET as an objective marker of disease activity in AS remains largely unexplored, with only two case reports (n ≤ 3 participants31,32) suggesting potential of longitudinal 18F-FDG PET to monitor treatment effects in patients with AS. Here, we provide the largest analysis to date supporting this notion. By performing quantification of 18F-FDG uptake, we first demonstrated that clinically involved joints/entheses showed a statistically significant increase of 30% in 18F-FDG uptake compared to uninvolved regions. Although this result aligns well with findings from previous reports16,17,18, a previous study showed no elevations in 18F-FDG uptake in 10 out of 12 AS patients25. These conflicting results are, however, likely explained by differences in the characteristics of the AS population studied in25, which includes patients with low disease activity (n = 5) and an overall milder inflammatory state (only 3 of these patients showed bone edema on MRI). Despite this controversy, our study provides novel evidence supporting the ability of 18F-FDG PET to capture disease-related inflammatory processes. We found that pre-treatment 18F-FDG uptake in the clinically involved joints/entheses, as measured with the composite measure gSUVmax, was strongly correlated with pre-treatment disease activity, as reflected by the BASDAI score. Furthermore, we confirmed this cross-sectional result in the longitudinal analysis, which showed that larger clinical benefits were accompanied by larger decreases in 18F-FDG uptake of the clinically involved joints/entheses. Our results suggest that 18F-FDG PET may be a useful marker of disease activity in AS patients with clinically active disease. 18F-FDG PET may also have a relevant application for tracking treatment effects in therapeutic trials or clinical settings.
We also conducted a complementary analysis in AS patients focusing on 18F-FDG PET signal in a key anatomical region in this condition, the sacroiliac joints. In line with our main results, we found elevated 18F-FDG PET signal in clinically involved sacroiliac joints, and this signal was strongly associated with disease activity as measured with the BASDAI score. Longitudinal findings suggest that 18F-FDG PET signal in this region can also track treatment effects, however, the number of AS participants with clinical involvement at the sacroiliac level and available follow-up scans was very limited. Further longitudinal studies focusing on AS patients with clinically affected sacroiliac joints are warranted to finally elucidate the ability of 18F-FDG PET to monitor treatment response in patients with an axial profile of clinical symptoms.
The pattern of findings in our PsA cohort is less clear, though still consistent with our findings in AS patients. We found that clinically involved joints/entheses displayed ~ 25% higher 18F-FDG uptake compared to unaffected regions; however, we did not find a statistically significant association between pre-treatment 18F-FDG uptake and disease activity. This result may be explained by the lower sample size of the PsA group (n = 8) together with the relatively homogeneous distribution of DAPSA scores (range: [18–34]), which may result in a limitation of the power to detect a statistical association. Yet, in line with our findings in AS patients, we did find a statistically significant association between stronger clinical improvements and longitudinal decreases in 18F-FDG uptake in the affected regions. Together, these findings extend previous cross-sectional results indicating that 18F-FDG PET can capture inflammatory processes in PsA22, and support the notion that this imaging modality may also represent a useful marker of disease activity and treatment effectiveness. However, additional studies with larger sample sizes and wider range of baseline disease activity are needed to confirm these results.
Interestingly, we did not find a statistically significant association between pre-treatment 18F-FDG uptake and clinical response to biological treatment at follow-up. This result resonates with findings from previous studies using 18F-NaF PET, in which pre-treatment uptake was not predictive of clinical response to TNFα antagonist therapy26. These results suggest that the factors that influence the likelihood of clinical response are not fully captured by PET imaging with 18F-FDG or 18F-NaF. Nevertheless, it is still possible that pre-treatment 18F-FDG uptake could be predictive of clinical response in different patient populations, in particular in those at a more severe disease stage. Given that 18F-FDG uptake correlates well with disease activity, it is likely that higher pre-treatment 18F-FDG uptake may be associated with better clinical response, as patients with high baseline disease activity tend to respond better to biological treatment33. Further studies are warranted to clarify the power of 18F-FDG PET to predict clinical response in AS and PsA patients undergoing therapy with biologics.
Another important question is the prediction of relevant long-term outcomes in patients with seronegative spondyloarthropathies. To date, no previous study has investigated the ability of PET imaging (either with 18F-FDG or 18F-NaF) to anticipate bone formation and subsequent loss of function. Given that 18F-NaF reflects osteoblastic activity, this PET modality appears to be more appropriate than 18F-FDG PET (a marker of inflammation) for the prediction of long-term outcomes. Yet, further studies are needed to confirm this hypothesis and to evaluate the strengths and weakness of 18F-FDG and 18F-NaF PET in both the short- and long-term assessment of patients with seronegative spondyloarthropathies.
The present study had a number of limitations. First, although relatively large for a PET imaging study on AS and PsA patients, the sample size of our study was modest, particularly for the longitudinal analysis. Second, our inclusion criteria led to relatively homogenous clinical characteristics for our study participants. This may have limited our statistical power to detect associations between 18F-FDG PET and disease severity scores. The performance of 18F-FDG PET may also vary for more diverse patient populations. Third, study participants underwent different treatment regimes, which might have influenced our longitudinal findings. Fourth, due to low the sample size in, we were underpowered to analyse how 18F-FDG uptake in specific joints/entheses influenced clinical outcomes. Finally, PET imaging is inevitably associated with an increased radiation dose, which may limit the implementation of this imaging technique in clinical practice. Future studies are needed to investigate whether the benefits of using this technique outweigh the risks associated with radiation exposure.
In summary, we provided first-time evidence suggesting that 18F-FDG PET is an accurate marker of disease activity in patients with AS and PsA. Our findings suggest that 18F-FDG PET may be a useful surrogate marker of treatment effectiveness in therapeutic trials for AS or PsA, and may provide valuable information to clinicians evaluating clinical response to biologic treatments.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- AS:
-
Ankylosing spondylitis
- BASDAI:
-
Bath Ankylosing Spondylitis Disease Activity Index
- DAPSA:
-
Disease Activity Index for Psoriatic Arthritis
- DMARDs:
-
Disease-modifying antirheumatic drugs
- gSUVmax :
-
Global SUVmax
- LME:
-
Linear mixed-effects
- MRI:
-
Magnetic resonance imaging
- NaF:
-
18F-sodium fluoride
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- PET:
-
Positron emission tomography
- PsA:
-
Psoriatic arthritis
- r:
-
Correlation coefficient
- ROI:
-
Region of interest
- SUVmax :
-
Maximum standardized uptake value
References
Dougados, M. & Baeten, D. Spondyloarthritis. Lancet 377, 2127–2137 (2011).
Stolwijk, C. et al. Epidemiology of spondyloarthritis. Rheum. Dis. Clin. N. Am. 38, 441–476 (2012).
Tam, L. S., Gu, J. & Yu, D. Pathogenesis of ankylosing spondylitis. Nat. Rev. Rheumatol. 6, 399–405 (2010).
Muche, B. et al. Anatomic structures involved in early- and late-stage sacroiliitis in spondylarthritis: A detailed analysis by contrast-enhanced magnetic resonance imaging. Arthritis Rheum. 48, 1374–1384 (2003).
Schett, G. et al. Enthesitis: From pathophysiology to treatment. Nat. Rev. Rheumatol. 13, 731–741 (2017).
van der Heijde, D. et al. Selection of instruments in the core set for DC-ART, SMARD, physical therapy, and clinical record keeping in ankylosing spondylitis. Progress report of the ASAS Working Group. Assessments in Ankylosing Spondylitis. J. Rheumatol. 26, 951–954 (1999).
Ostergaard, M. et al. Magnetic resonance imaging in spondyloarthritis—How to quantify findings and measure response. Best Pract. Res. Clin. Rheumatol. 24, 637–657 (2010).
Maksymowych, W. P. Progress in spondylarthritis. Spondyloarthritis: Lessons from imaging. Arthritis Res. Ther. 11, 222 (2009).
Mandl, P. et al. EULAR recommendations for the use of imaging in the diagnosis and management of spondyloarthritis in clinical practice. Ann. Rheum. Dis. 74, 1327–1339 (2015).
Braun, J. et al. Use of dynamic magnetic resonance imaging with fast imaging in the detection of early and advanced sacroiliitis in spondylarthropathy patients. Arthritis Rheum. 37, 1039–1045 (1994).
Baraliakos, X. et al. The relationship between inflammation and new bone formation in patients with ankylosing spondylitis. Arthritis Res. Ther. 10, R104 (2008).
Krohn, M. et al. Erosions and fatty lesions of sacroiliac joints in patients with axial spondyloarthritis: Evaluation of different MRI techniques and two scoring methods. J. Rheumatol. 41, 473–480 (2014).
Maksymowych, W. P. et al. Inflammatory lesions of the spine on magnetic resonance imaging predict the development of new syndesmophytes in ankylosing spondylitis: Evidence of a relationship between inflammation and new bone formation. Arthritis Rheum. 60, 93–102 (2009).
MacKay, J. W., Aboelmagd, S. & Gaffney, J. K. Correlation between clinical and MRI disease activity scores in axial spondyloarthritis. Clin. Rheumatol. 34, 1633–1638 (2015).
Goh, L. et al. Disease activity in longstanding ankylosing spondylitis: A correlation of clinical and magnetic resonance imaging findings. Clin. Rheumatol. 27, 449–455 (2008).
Taniguchi, Y. et al. Positron emission tomography/computed tomography: A clinical tool for evaluation of enthesitis in patients with spondyloarthritides. Rheumatology (Oxford). 49, 348–354 (2010).
Yamashita, H. et al. Similarities and differences in fluorodeoxyglucose positron emission tomography/computed tomography findings in spondyloarthropathy, polymyalgia rheumatica and rheumatoid arthritis. Jt. Bone Spine. 80, 171–177 (2013).
Vijayant, V. et al. Potential of (18)F-FDG-PET as a valuable adjunct to clinical and response assessment in rheumatoid arthritis and seronegative spondyloarthropathies. World J. Radiol. 4, 462–468 (2012).
de Jongh, J., Verweij, N. J. F., Yaqub, M. et al. [(18)F]Fluoride PET provides distinct information on disease activity in ankylosing spondylitis as compared to MRI and conventional radiography. Eur. J. Nucl. Med. Mol. Imaging. (2022).
Idolazzi, L. et al. 18F-fluoride PET/CT for detection of axial involvement in ankylosing spondylitis: Correlation with disease activity. Ann. Nucl. Med. 30, 430–434 (2016).
Strobel, K. et al. 18F-fluoride PET/CT for detection of sacroiliitis in ankylosing spondylitis. Eur. J. Nucl. Med. Mol. Imaging. 37, 1760–1765 (2010).
Chaudhari, A. J. et al. High-resolution (18)F-FDG PET/CT for assessing disease activity in rheumatoid and psoriatic arthritis: Findings of a prospective pilot study. Br. J. Radiol. 89, 20160138 (2016).
de Jongh, J. et al. (18)F-sodium fluoride PET-CT visualizes both axial and peripheral new bone formation in psoriatic arthritis patients. Eur. J. Nucl. Med. Mol. Imaging. 50, 756–764 (2023).
Taniguchi, Y. et al. F-18 FDG PET/CT provides the earliest findings of enthesitis in reactive arthritis. Clin. Nucl. Med. 36, 121–123 (2011).
Bruijnen, S. T. et al. Bone formation rather than inflammation reflects ankylosing spondylitis activity on PET-CT: A pilot study. Arthritis Res. Ther. 14, R71 (2012).
Darrieutort-Laffite, C. et al. Sodium (18)F-sodium fluoride PET failed to predict responses to TNFalpha antagonist therapy in 31 patients with possible spondyloarthritis not meeting ASAS criteria. Jt. Bone Spine. 82, 411–416 (2015).
Bruner, V. et al. Biological therapies for spondyloarthritis. Ther. Adv. Musculoskelet. Dis. 6, 92–101 (2014).
Garrett, S. et al. A new approach to defining disease status in ankylosing spondylitis: The Bath Ankylosing Spondylitis Disease Activity Index. J. Rheumatol. 21, 2286–2291 (1994).
Schoels, M. M. et al. Disease activity in psoriatic arthritis (PsA): defining remission and treatment success using the DAPSA score. Ann. Rheum. Dis. 75, 811–818 (2016).
Manabe, Y. et al. [18F] FDG uptake in patients with spondyloarthritis: Correlation with serum inflammatory biomarker levels. EJNMMI Res. 13, 15 (2023).
Wendling, D. et al. FDG-PET/CT scan of inflammatory spondylodiscitis lesions in ankylosing spondylitis, and short term evolution during anti-tumour necrosis factor treatment. Ann. Rheum. Dis. 64, 1663–1665 (2005).
Taniguchi, Y. et al. Clinical implication of 18F-fluorodeoxyglucose PET/CT in monitoring disease activity in spondyloarthritis. Rheumatology (Oxford). 49, 829 (2010).
Lord, P. A. et al. Predictors of response to anti-TNF therapy in ankylosing spondylitis: Results from the British Society for Rheumatology Biologics Register. Rheumatology (Oxford). 49, 563–570 (2010).
Author information
Authors and Affiliations
Contributions
O.R-F wrote the main manuscript and conducted the analyses. P.A. revised the manuscript and provided intellectual content. F.G.G. revised the manuscript and provided intellectual content. B.F.L. revised the manuscript and provided intellectual content. C.V.D. revised the manuscript and provided intellectual content. M.D.G. revised the manuscript and provided intellectual content. R.Q.S. revised the manuscript and provided intellectual content. A.B.-G. revised the manuscript and provided intellectual content. S.A.C. revised the manuscript and provided intellectual content. J.C.H. revised the manuscript and provided intellectual content.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Rodríguez-Fonseca, O.D., Aguiar, P., García, F.M.G. et al. 18F-FDG positron emission tomography as a marker of disease activity and treatment response in ankylosing spondylitis and psoriatic arthritis. Sci Rep 14, 19907 (2024). https://doi.org/10.1038/s41598-024-60669-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-60669-4
- Springer Nature Limited