Abstract
This study aims to examine whether hypovitaminosis D was associated with cognitive impairment among chronic kidney patients with different level of albuminuria. This population-based cross-sectional study was conducted on elderly (over 60 years old) with urine albumin to creatinine ratio (UACR) ≥ 30 mg/g from 2011 to 2014 in the US National Health and Nutrition Examination Survey (NHANES). Cognitive function was assessed by the Consortium to Establish a Registry for Alzheimer’s Disease Word List Learning (CERAD). Subjects were divided into 2 groups according to the absence or presence of cognitive impairment and a propensity score matching (PSM) was further conducted. The association was assessed with Spearman correlation and logistic regression analysis. The positive association of 25-hydroxyvitamin D3 (25(OH)D3) and cognitive score was presented. PSM analysis revealed that a higher level of 25(OH)D3 correlated to a better cognitive function in CKD patients with albuminuria, especially in patients with 30 mg/g ≤ UACR < 300 mg/g. This study indicated that a low 25(OH)D3 level was associated with poor cognitive performance, especially in patients with microalbuminuria. Thus, early diagnosis of vitamin D insufficiency and an effective intervention might be a useful therapeutic strategy to prevent cognitive decline in patients with the progression of renal dysfunction.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) is a major challenge to public health, associated with an increasing mortality and morbidity1. Cognitive impairment is a common complication in individuals with CKD. Both albuminuria and low estimated glomerular filtration rate (eGFR) are associated with elevated risks for cognitive impairment2,3. Albuminuria is considered as a measure of kidney damage, quantitated by urine albumin to creatinine ratio (UACR)4. Albuminuria (UACR ≥ 30 mg/g) was associated with both worse baseline cognitive function and cognitive decline5,6. Participants having an elevated proteinuria probably showed a neurocognitive dysfunction3,7,8. Multiple risk factors for cognitive dysfunction in CKD patients were demonstrated, including older age, proteinuria, educational level, hypertension, acidosis, anemia, and uremic milieu9,10.
Vitamin D is a kind of fat-soluble seco-steroid for calcium uptake and bone metabolism11. Hypovitaminosis D is highly frequent in older adults12. The functions of vitamin D to regulate mineral homeostasis, adaptive and innate immunity, and cardiovascular disease in patients with CKD have been proposed13. The influence of hypovitaminosis D and cognitive dysfunction was also explored14,15. However, other studies showed nonsignificant associations between lower 25-hydroxyvitamin D (25(OH)D) levels and poor cognitive function in patients with Parkinson’s disease16,17. The effect of 25(OH)D on domain-specific cognitive performance was also unclear in patients with peritoneal dialysis and hemodialysis18,19.
25(OH)D3 is widely applied to assess vitamin D status as a main circulating form of vitamin D. Little is known about the relationship between 25(OH)D3 and the cognitive function in CKD patients. In the present study, we aimed to examine the potential association of serum 25(OH)D3 and the cognitive performance of CKD patients with different level of albuminuria.
Results
Participant characteristics
Table 1 showed the characteristics of the patients before propensity score matching. Among 583 older adults, the median age was 71 years, and 49.4% were male. In comparison to the normal cognition function, patients with cognitive impairment were older and had a lower level of calcium, phosphate, and 25(OH)D3. Characteristics of participants were also summarized in the Supplementary Table 1 stratified by quartile of serum 25(OH)D3. Of these patients, age, education level, serum albumin, BUN, calcium, alkaline phosphatase levels were significantly different among four groups according to the quartile of serum 25(OH)D3. The prevalence of hypertension and diabetes significantly differed among different level of 25(OH)D3. There were significant differences in CERAD scores among the four groups.
Correlation analysis between cognitive function and various parameters
The Spearman correlation test was performed to explore the potential associated factors for cognition function. As shown in Table 2, we found that age was negatively associated with CERAD score, while BMI, BUN, calcium, phosphate and 25(OH) D3 concentrations were positively associated with good cognitive ability in patients with CKD.
Association between the level of 25(OH) D3 and cognitive function
After propensity score matching, 133 patients were matched in each group. As shown in Table 3, no significant differences were observed between the two groups in age, sex, education level, hemoglobin, serum albumin, calcium, phosphate, creatinine, eGFR, and UACR levels. Multiple logistic regression analysis was used to investigate the relationship between cognitive test and 25(OH) D3 concentration. Table 4 showed that a lower level of 25(OH) D3 was independently associated with a risk of cognitive impairment. The interaction of sex and albuminuria might modify the association between 25(OH)D3 and cognition function (p for interaction = 0.005 and 0.008, respectively), and subgroup analysis was performed in Table 4. In the 30 mg/g ≤ UACR < 300 mg/g level, we found serum 25(OH)D3 was negatively associated with the risk for cognitive impairment. However, a higher level of 25(OH)D3 might not correlate to a better cognitive performance in participants with UACR ≥ 300 mg/g. Our results inferred that a higher level of 25(OH) D3 specifically benefited the cognitive function of patients with microalbuminuria. In addition, an elevated 25(OH) D3 level was associated with a better cognition performance in old male patients with CKD, but not in female patients.
Discussion
In this study, we investigated the association between serum 25(OH)D3 and cognitive function in CKD patients with albuminuria. The main finding of this study is that serum levels of 25(OH)D3 positively correlated with cognitive function in patients with CKD, especially in patients with microalbuminuria (30 mg/g ≤ UACR < 300 mg/g).
CKD is classified on the basic of category of eGFR and albuminuria20. Cognitive impairment is a deficit in one or more key brain functions, such as attention, memory, visuospatial ability, language skills, and execution skills21. The prevalence of cognitive impairment is high among patients with albuminuria, when screened with both California Verbal Learning Test and Symbol Digit Modalities Test22. A decline of cognitive function is associated with a prolonged hospitalization, and high mortality23,24. Accordingly, itis critical to prevent progression of cognitive impairment.
The mechanism for cognitive dysfunction in patients with kidney damage is complex. It is well known that an older age is a risk factor for cognitive frailty and dysfunction25. The severity of renal dysfunction is independently correlated with cognitive impairment26, probably owing to an accumulation of uric toxins. Vascular injury and various uremic toxins are suspected to induce cognitive impairment in CKD, including uric acid, indoxyl sulfate, and p-cresyl sulfate27,28. Several other risk factors have also been linked to cognitive impairment, such as hyperhomocysteinemia, hypercoagulable states, neuroinflammation, oxidative stress, and impaired cerebral blood flow autoregulation29,30.
Vitamin D is a fat-soluble steroid that exerts its effects by binding to the vitamin D receptor. Activation of vitamin D takes place in the liver and kidney by enzymatic hydroxylation with generation of 1.25-dihydroxycholecalciferol or 1.25-dihydroxyvitamin D331. In addition to regulating bone metabolism, vitamin D exerts multiple biological actions, such as obesity, cardiovascular, and neurodegenerative diseases32,33. A meta-analysis showed an inverse association of 25(OH)D and cardiovascular and all-cause mortality34. However, no associations were found between serum 25(OH)D and risk of dementia35 or Alzheimer’s disease36 . A study by Parveen et al.37 even reported an inverse association of 25(OH)D and cognitive function in patients with type 2 diabetes. 25(OH)D3 is regarded as a marker for vitamin D reserve, and its deficiency is prevalent in patients with CKD38. However, the exact association between serum 25(OH)D3 and cognitive function in CKD patients remains inconclusive.
In our study, we divided our CKD patients with UACR ≥ 30 mg/g into 2 groups according to the cutoff value of CERAD. The 25(OH)D3 levels were significantly different between patients with and without cognitive impairment. In addition,, 25(OH)D3 was positively associated with cognitive score in patients with CKD. A propensity-score matching approach was applied to further reduce the bias. In the matched group, a lower level of 25(OH)D3 appeared to be an independent associated factor for cognitive dysfunction in patients with albuminuria. Vitamin D receptors are found in the central nervous system involving in a neuroprotective effect39. There is robust evidence that vitamin D3 and 1,25(OH)2D3 could regulate the toxicity of reactive oxygen species40,41, and neurotrophic factors such as nerve growth factor42. The hydroxylated vitamin D3 could induce cell differentiation of embryonic hippocampal cells43. In Alzheimer’s disease, 1,25(OH)2D3 strongly stimulated amyloid-beta clearance while ameliorating inflammation and apoptosis44,45. Meanwhile, the association between 25(OH)D3 and neuroprotection was also explored via improving neuroinflammation and endothelial integrity of the blood–brain barrier46. Additionally, evidence demonstrated a protective role of active metabolite of vitamin D3 on renin-angiotensin system and vascular endothelial function47. Recent studies showed that a low 25(OH)D was associated with reduced volumes of hippocampal subfields, connection deficits48 and a large lateral ventricle volume49 in elderly people. However, mores studies are needed to uncover the definite relationship between 25(OH)D3 and hippocampal structure to validate our results. .
By subgroup analysis, we found the association of 25(OH)D3 and cognitive impairment was significant in patients with 30 mg/g ≤ UACR < 300 mg/g, but not in patients with UACR ≥ 300 mg/g. Patients with macroalbuminuria are generally older and have more comorbidities in comparison to those with normal urinalysis50. Besides, macroalbuminuria represents a poor residual renal function. Patients with macroalbuminuria displayed higher levels of biomarkers for kidney injury, including neutrophil gelatinase-associated lipocalin51. Progression of renal dysfunction is accompanied by the steady accumulation of various uremic toxins. Uremia can cause progressive loss of tissue 25(OH)D3 receptor, probably leading to a tissue vitamin D resistance52. Namely, an early and comprehensive evaluation of 25(OH)D3 levels should be emphasized in patients with preserved residual renal function to improve cognition function. Interestingly, our research showed that 25(OH)D3 correlated to a better cognition performance only in male patients, but not in female patients. A supplementing with 25(OH)D3 might increase serum 25(OH)D3 and testosterone level in obese mice53. Bioavailable testosterone presented a positive association of cognitive function in older men54, while prolactin, progesterone and estradiol might have deleterious effects on cognitive function in older females after menopause55,56. However, the number of patients in our subgroup is relatively limited, and the insignificance may be due to the small sample size. Other larger longitudinal studies are needed to confirm our results.
Our study explored a beneficial association of higher levels of 25(OH)D3 and cognitive impairment in CKD patients. Although the enrolled patients were matched with the propensity scores to reduce potential selection bias, there were still certain limitations in the study. This study was a cross-sectional study design which cannot identify causal causation between two factors. In addition, only CERAD test was included in this study, and our results might lead to a bias for other domains of cognitive function. Although this study has adjusted for multiple covariates and a propensity matching was used, more other potential unmeasured confounders should be included in future studies with larger samples.
Conclusion
Our results suggest that a higher level of serum 25(OH)D3 correlate to a better cognitive performance in CKD patients. With the progression of renal function impairment, a routinely screen and correcting 25(OH)D3 deficiency should be emphasized in the early-stage of CKD. More prospective studies are necessary to confirm the protective effects of 25(OH)D3 supplements on cognition in CKD patients.
Methods
Study population
For the present study, we analyzed secondary data from the 2011 to 2012 and 2013 to 2014 NHANES. The National Health and Nutrition Examination Survey (NHANES) is a large, complex, survey of US population conducted by National Center for Health Statistics to provide nationally representative estimates on the health and nutritional status. The NHANES protocol was approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board (http://www.cdc.gov/nchs/nhanes). The study was conducted in accordance with the relevant guidelines and regulations of the Declaration of Helsinki. All the NHANES participants provided with informed consent.
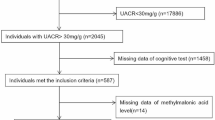
A total of 2045 participants with albuminuria (UACR ≥ 30 mg/g) were included. Serum creatinine was used to calculate eGFR with the Modification of Diet in Renal Disease (MDRD) equation. The CKD stages were defined with eGFR and/or evidence of kidney damage according to the KDIGO guidelines: stage 1, eGFR ≥ 90 mL/min/1.73 m2 with UACR ≥ 30 mg/g; stage 2, eGFR 60–89 mL/min/1.73 m2 with UACR ≥ 30 mg/g; stage 3, eGFR 30–59 mL/min/1.73 m2; stage 4, eGFR 15–29 mL/min/1.73 m2; stage 5, eGFR < 15 mL/min/1.73 m257. Among them, 587 individuals ≥ 60 years old underwent cognitive assessment. Participants were further excluded due to missing data for 25(OH)D3 levels. Finally, 583 participants were recruited for the subsequent analysis (shown in Fig. 1).
Measurement of serum 25(OH)D3
In the present study, we extracted serum 25(OH)D3 from the database. In the 2011–2014 cycle of the NHANES, serum 25(OH)D3 concentrations were measured by a standardized ultra-high performance liquid chromatography–tandem mass spectrometry method to quantitatively detect 25(OH)D3.
Cognitive function assessment
The Consortium to Establish a Registry for Alzheimer’s Disease Word Learning subtest (CERAD) was used to assess participants’ cognitive function. CERAD assesses immediate and delayed learning ability for new verbal information (memory sub-domain)58. The test consists of three consecutive learning trials and a delayed recall trial. For each trial, participants were asked to read ten random words and recall as many words as they could immediately. According to the previous papers, we used the 25th percentile of the scores as the cutoff points59. Thus, participants were divided into low and normal cognition performance groups.
Covariates
The sociodemographic variables and health condition information were obtained, including age, sex, education level, smoking (yes/no), alcohol consumption (yes/no), hypertension (yes/no), and diabetes (yes/no). Body mass index (BMI) is calculated by dividing weight in kilograms by the square of height in meters. Serum albumin, calcium, phosphate, alkaline phosphatase, creatinine, blood urea nitrogen (BUN), cholesterol, triglyceride, and uric acid were also collected.
Statistical analysis
All the data were analyzed by SPSS 23.0 statistical software. Baseline characteristics of patients were reported using percentages for categorical variables, and medians (interquartile range, IQR) for continuous data. Continuous variables and categorical variables were compared among groups using Kruskal–Wallis and χ2 test, separately. The relationship between cognitive score and serum 25(OH)D3 level were assessed using the Spearman’s correlation analysis for non-normally distributed. To identify a more accurate relationship between cognition and 25(OH)D3, propensity matching analysis was performed. Patients in the cognition impairment group were matched at a ratio of 1:1 with those in the non-cognition impairment group by propensity scores. Variables age, sex, education level, race, smoke, drink, hypertension, diabetes, hemoglobin, albumin, BUN, eGFR, UACR, calcium, and phosphate were selected for the propensity matching model. Logistic regressions were performed to assess the association between cognitive function (dependent variable) and serum 25(OH)D3 (independent variable). Confounding variables were selected based on clinical relevance and a p < 0.05 in the correlation analysis. Subgroup analysis was performed according to the UACR levels and sex. In detail, populations were grouped into 30 mg/g ≤ UACR < 300 mg/g (microalbuminuria) and UACR ≥ 300 mg/g (macroalbuminuria) groups60. Statistical hypotheses were tested using a two-tailed P ≤ 0.05 level of significance.
Ethics approval
All participants submitted written informed consent and the study was approved by the NCHS Research Ethics Review Board (Continuation of Protocol #2011–17). The studies were conducted according to the guidelines of the Declaration of Helsinki throughout.
Data availability
The datasets generated and analyzed in the present study are available on the website of NHANES datasets 2011–2014 (https://wwwn.cdc.gov/nchs/nhanes).
References
Xie, Y. et al. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 94(3), 567–581 (2018).
Paterson, E. N. et al. Association of renal impairment with cognitive dysfunction in the Northern ireland cohort for the longitudinal study of ageing (NICOLA). Nephrol. Dial. Transplant. 36(8), 1492–1499 (2021).
Kurella Tamura, M. et al. Albuminuria, kidney function, and the incidence of cognitive impairment among adults in the United States. Am. J. Kidney Dis. 58(5), 756–763 (2011).
Stevens, P. E. & Levin, A. Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 158(11), 825–830 (2013).
Murray, A. M. et al. CKD biomarkers, cognitive impairment, and incident dementia in an older healthy cohort. Kidney 3(3), 435–445 (2022).
Barzilay, J. I. et al. Albuminuria and dementia in the elderly: A community study. Am. J. Kidney Dis. 52(2), 216–226 (2008).
Hooper, S. R. et al. Neurocognitive functioning of children and adolescents with mild-to-moderate chronic kidney disease. Clin. J. Am. Soc. Nephrol. 6(8), 1824–1830 (2011).
Verhagen, C. et al. Chronic kidney disease and cognitive decline in patients with type 2 diabetes at elevated cardiovascular risk. J. Diabetes Complicat. 36(10), 108303 (2022).
Gela, Y. Y. et al. Cognitive impairment and associated factors among chronic kidney disease patients: A comparative cross-sectional study. Neuropsychiatr. Dis. Treat. 17, 1483–1492 (2021).
Steinbach, E. J. & Harshman, L. A. Impact of chronic kidney disease on brain structure and function. Front. Neurol. 13, 797503 (2022).
Turner, A. G. et al. The local production of 1,25(OH)2D3 promotes osteoblast and osteocyte maturation. J. Steroid Biochem. Mol. Biol. 144, 114–118 (2014).
Annweiler, C. et al. Fall prevention and vitamin D in the elderly: An overview of the key role of the non-bone effects. J. Neuroeng. Rehabil. 7, 50 (2010).
Mozos, I. & Marginean, O. Links between vitamin D deficiency and cardiovascular diseases. Biomed. .Res Int. 2015, 109275 (2015).
Annweiler, C. et al. “Vitamin D and cognition in older adults”: Updated international recommendations. J. Intern. Med. 277(1), 45–57 (2015).
Annweiler, C. et al. Vitamin D and cognitive performance in adults: A systematic review. Eur. J. Neurol. 16(10), 1083–1089 (2009).
Petersen, M. S. et al. The role of vitamin D levels and vitamin D receptor polymorphism on Parkinson’s disease in the Faroe Islands. Neurosci. Lett. 561, 74–79 (2014).
Fullard, M. E. & Duda, J. E. A review of the relationship between Vitamin D and parkinson disease symptoms. Front. Neurol. 11, 454 (2020).
Liu, G. L. et al. Vitamin D status is an independent risk factor for global cognitive impairment in peritoneal dialysis patients. PLoS One. 10(12), e0143782 (2015).
Shaffi, K. et al. Low 25-hydroxyvitamin D levels and cognitive impairment in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 8(6), 979–986 (2013).
Ketteler, M. et al. Diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder: Synopsis of the kidney disease: Improving global outcomes 2017 clinical practice guideline update. Ann. Intern. Med. 168(6), 422–430 (2018).
Drew, D. A., Weiner, D. E. & Sarnak, M. J. Cognitive impairment in CKD: Pathophysiology, management, and prevention. Am. J. Kidney Dis. 74(6), 782–790 (2019).
Sacre, J. W. et al. Associations of chronic kidney disease markers with cognitive function: A 12-year follow-up study. J. Alzheimers Dis. 70(s1), S19-s30 (2019).
Griva, K. et al. Cognitive impairment and 7-year mortality in dialysis patients. Am. J. Kidney Dis. 56(4), 693–703 (2010).
Raphael, K. L. et al. Cognitive function and the risk of death in chronic kidney disease. Am. J Nephrol. 35(1), 49–57 (2012).
Fabrício, D. M., Chagas, M. H. N. & Diniz, B. S. Frailty and cognitive decline. Transl. Res. 221, 58–64 (2020).
Coppolino, G. et al. Kidney function and cognitive decline in frail elderly: Two faces of the same coin?. Int. Urol. Nephrol. 50(8), 1505–1510 (2018).
Bugnicourt, J. M. et al. Cognitive disorders and dementia in CKD: The neglected kidney-brain axis. J. Am. Soc. Nephrol. 24(3), 353–363 (2013).
Watanabe, K., Watanabe, T. & Nakayama, M. Cerebro-renal interactions: Impact of uremic toxins on cognitive function. Neurotoxicology 44, 184–193 (2014).
Madero, M., Gul, A. & Sarnak, M. J. Cognitive function in chronic kidney disease. Semin. Dial. 21(1), 29–37 (2008).
Viggiano, D. et al. Mechanisms of cognitive dysfunction in CKD. Nat. Rev. Nephrol. 16(8), 452–469 (2020).
Müller, T. et al. Vitamin D rise enhances blood perfusion in patients with multiple sclerosis. J. Neural Transm. (Vienna) 126(12), 1631–1636 (2019).
Cekic, M. et al. Vitamin D deficiency reduces the benefits of progesterone treatment after brain injury in aged rats. Neurobiol. Aging 32(5), 864–874 (2011).
Rajakumar, K. et al. Vitamin D status and response to Vitamin D(3) in obese vs. non-obese African American children. Obesity 16(1), 90–95 (2008).
Chowdhury, R. et al. Vitamin D and risk of cause specific death: Systematic review and meta-analysis of observational cohort and randomised intervention studies. Bmj 348, g1903 (2014).
Karakis, I. et al. Association of serum vitamin D with the risk of incident dementia and subclinical indices of brain aging: The framingham heart study. J. Alzheimers Dis. 51(2), 451–461 (2016).
Olsson, E. et al. Vitamin D is not associated with incident dementia or cognitive impairment: An 18-y follow-up study in community-living old men. Am. J. Clin. Nutr. 105(4), 936–943 (2017).
Parveen, R. et al. Attenuated serum 25-hydroxyvitamin D and vitamin D binding protein associated with cognitive impairment in patients with type 2 diabetes. Diabetes Metab. Syndr. Obes. 12, 1763–1772 (2019).
Jones, G. Interpreting vitamin D assay results: proceed with caution. Clin. J. Am. Soc. Nephrol. 10(2), 331–334 (2015).
McCann, J. C. & Ames, B. N. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction?. Faseb. J. 22(4), 982–1001 (2008).
Uthaiah, C. A. et al. Comparative assessment of cognitive impairment and oxidative stress markers among vitamin D insufficient elderly patients with and without type 2 diabetes mellitus (T2DM). PLoS One 17(6), e0269394 (2022).
Cetinkalp, S. et al. The effect of 1alpha,25(OH)2D3 vitamin over oxidative stress and biochemical parameters in rats where Type 1 diabetes is formed by streptozotocin. J. Diabetes Complicat. 23(6), 401–408 (2009).
Schlögl, M. & Holick, M. F. Vitamin D and neurocognitive function. Clin. Interv. Aging 9, 559–568 (2014).
Marini, F. et al. Effect of 1alpha,25-dihydroxyvitamin D3 in embryonic hippocampal cells. Hippocampus 20(6), 696–705 (2010).
Masoumi, A. et al. 1alpha,25-dihydroxyvitamin D3 interacts with curcuminoids to stimulate amyloid-beta clearance by macrophages of Alzheimer’s disease patients. J. Alzheimers Dis. 17(3), 703–717 (2009).
Mizwicki, M. T. et al. 1α,25-dihydroxyvitamin D3 and resolvin D1 retune the balance between amyloid-β phagocytosis and inflammation in Alzheimer’s disease patients. J. Alzheimers Dis. 34(1), 155–170 (2013).
Gotelli, E. et al. Understanding the immune-endocrine effects of vitamin D in SARS-CoV-2 Infection: A role in protecting against neurodamage. Neuroimmunomodulation 30(1), 185–195 (2023).
Targher, G., Pichiri, I., Lippi, G. & Vitamin, D. thrombosis, and hemostasis: More than skin deep. Semin. Thromb. Hemost. 38(1), 114–124 (2012).
Al-Amin, M. et al. Vitamin D deficiency is associated with reduced hippocampal volume and disrupted structural connectivity in patients with mild cognitive impairment. Hum. Brain Mapp. 40(2), 394–406 (2019).
Annweiler, C. et al. Vitamin D concentration and lateral cerebral ventricle volume in older adults. Mol. Nutr. Food Res. 57(2), 267–276 (2013).
Leitão, L. et al. Intensive blood pressure treatment reduced stroke risk in patients with albuminuria in the SPRINT trial. Stroke 50(12), 3639–3642 (2019).
Baldimtsi, E., Whiss, P. A. & Wahlberg, J. Systemic biomarkers of microvascular alterations in type 1 diabetes associated neuropathy and nephropathy—A prospective long-term follow-up study. J. Diabetes Complicat. 37(12), 108635 (2023).
Fukuda, N. et al. Decreased 1,25-dihydroxyvitamin D3 receptor density is associated with a more severe form of parathyroid hyperplasia in chronic uremic patients. J. Clin. Invest. 92(3), 1436–1443 (1993).
Feng, M. et al. Serum 25OHD3 of obese mice is affected by liver injury and correlates with testosterone levels and sperm motility. Obes. Facts. 14(5), 559–567 (2021).
Giannos, P. et al. Associations of bioavailable serum testosterone with cognitive function in older men: Results from the national health and nutrition examination survey. J. Gerontol. A Biol. Sci. Med. Sci. 78(1), 151–157 (2023).
Henderson, V. W. Progesterone and human cognition. Climacteric. 21(4), 333–340 (2018).
Feng, L. et al. Positive associations between sex hormones, bone metabolism and cognitive impairment in Chinese oldest-old females. BMC Psychiatry. 23(1), 562 (2023).
Sun, D. Q. et al. MAFLD and risk of CKD. Metabolism 115, 154433 (2021).
Morris, J. C. et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 39(9), 1159–1165 (1989).
Chen, S. P., Bhattacharya, J. & Pershing, S. Association of vision loss with cognition in older adults. JAMA Ophthalmol. 135(9), 963–970 (2017).
Levey, A. S. et al. The definition, classification, and prognosis of chronic kidney disease: A KDIGO controversies conference report. Kidney Int. 80(1), 17–28 (2011).
Funding
This study was supported by the Xuanwu Hospital Huizhi talent leader training program to Aihua Zhang.
Author information
Authors and Affiliations
Contributions
A.H.Z contributed to the study concept and design; J.L.Z contributed to data collection; J.L.Z, and A.H.Z contributed to the statistical analysis; J.L.Z contributed to the original draft; A.H.Z contributed to the review draft. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, J., Zhang, A. Association between serum 25-hydroxyvitamin D3 level and cognitive impairment in older chronic kidney disease patients. Sci Rep 14, 12403 (2024). https://doi.org/10.1038/s41598-024-63350-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-63350-y
- Springer Nature Limited