Abstract
This paper introduces a hybrid enhanced oil recovery (HEOR) method that combines a low-salinity water flooding (LSWF) and nanoparticles (NPs) stabilized with a green surfactant. We experimentally investigated the use of combinations of silica (SiO2) and gamma alumina (γ-Al2O3) nanohybrids stabilized with Gum Arabic (GA) at different water salinities. Nanofluids (NFs) were prepared by dispersing γ-Al2O3 and SiO2 NPs (0.1 wt%) in deionized water (DW), synthetic seawater (SSW), 2, 5, and 10 times diluted samples of synthetic seawater (in short 2-DSSW, 5-DSSW and 10-DSSW, respectively). The challenge is that NPs become unstable in the presence of cations in saline water. Moreover, an attempt was made to introduce NFs with high stability for a long period of time as the optimal NFs. The effects of temperature on the behaviour of optimal NFs in the presence of different base fluids, distinct mass ratios of γ-Al2O3/SiO2 and various concentrations of surfactant were analysed via interfacial tension (IFT) and viscosity measurements. The results of the viscosity measurement showed that with increasing temperature, the NPs dispersed in DW had lower viscosity than NPs dispersed in various salinities. However, the IFT measurement for NPs dispersed in different base-fluids revealed that with increasing temperature and presence of cations in saline water, IFT values decreases. Although, the minimum IFT for hybrid nanofluid (HNF) γ-Al2O3/SiO2 modified with GA and dispersed in 10-DSSW was reported 0.99 mN/m. Finally, according to the micromodel flooding results, in oil-wet conditions, the highest oil recovery for combination γ-Al2O3/SiO2 modified with GA and dispersed in 2-DSSW was reported 60.34%. It was concluded that NFs modified with GA could enhanced applicability of LSWF via delay in breakthrough time and improving sweep efficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
According to the forecast made until 2040, use of renewable fuels has been noticed worldwide and fossil fuels such as oil, gas and coal are considered essential energy sources worldwide1. In the last few decades, human society has faced impressive growth in population. Therefore, the need for more energy sources is understandable. Limited oil and gas resources have given more attention to enhanced oil recovery (EOR). The LSWF and other EOR methods can be considered new hybrid approaches for improving oil recovery2,3,4. In recent years, to promote oil recovery mechanisms, combining two or more EOR methods, known as hybrid methods, has been explored to address operational challenges, reduce environmental damage and reduce production costs5. The HEOR method could be optimized for different injection scenarios and achieve the highest recovery oil factor6,7,8,9,10.
NPs play an essential role in the EOR process through four main mechanisms: disjoining pressure, wettability alteration, IFT reduction and viscosity control4,11,12,13. NPs could change the wettability toward more water, combine with LSWF and improve oil recovery14,15. The tendency of NPs to aggregate leads to a decrease in their surface energy, which results in the loss of their properties. Therefore, ensuring the stability of NPs in base fluid is the most critical challenge for their application16. It is important to evaluate NFs stability before injecting into complex reservoirs. Therefore, NFs stability improvement in reservoir conditions (high temperature, high salinity and etc.) should be carefully considered. If unstable NFs are injected into the reservoir, NPs tend to aggregate resulting they create deposits that could damage to reservoir permeability, throats structures and pores13.
Surface modification is performed to minimize the surface energy and reduce the tendency of NPs to aggregate. Physical surface modification could be performed with surfactants or adsorbed macromolecules. In this method, surfactant adsorption occurs on the surface of NPs via electrostatic interactions between polar groups. Surface modification is performed through the modification of covalent bonds on the surface of NPs17,18. Adding surfactants to NFs is a cost effective method that may lead to the stability of NFs19,20. The completely different structures of surfactants, which are hydrophilic and hydrophobic, allow them to act as bridges between NPs and base fluids20.
Torsaeter and Hendraningratt21 used Al2O3, SiO2 and TiO2 NPs dispersed in 3 wt% brine to displace residual oil in sandstone core samples. According to their results, the combination of SiO2 dispersed in brine had the highest duration of stability. Moreover, a maximum oil recovery of 67% was achieved by TiO2 NPs with 1 wt% polyvinylpyrrolidone (PVP). Nazari Moghadam et al.22 used different NPs to investigate the wettability change in carbonate reservoirs. The imbibition test results revealed the important role of SiO2 and CaCO3 NPs. The presence of CaCO3 and SiO2 increases the oil recovery factor by 4 and 6% respectively, of the original oil-in-place (OOIP) ratio. Alomair et al.23 used metal oxide NPs including NiO, TiO2, Al2O3 and SiO2 at different concentrations. They observed decrease at viscosity in heavy oil when Al2O3 and SiO2 NPs were used at a concentration of 0.05 wt%. Their results showed that the combination of Al2O3 and SiO2 at 0.05 wt% had the highest oil recovery factor among the other NFs. In another work, Alameri et al.24 investigated the effect of low-salinity water and surfactants on carbonated core samples. They found that adding surfactant to saline water could improve the oil recovery factor. Al-Anssari et al.25 utilized different concentrations of SiO2 dispersed in saline water with concentrations of 3, 8, 10, 15 and 20 wt% to investigate wettability changes. Their results revealed the greatest wettable alterations during the first hour of contact between the NFs and the rock surface. Additionally, they reported an optimal salinity range between 3 and 8 wt%. In a similar study, Kiani et al.26 used γ-Al2O3 NPs at different concentrations dispersed at different salinities. Their results showed that the oil recovery factor increases with decreasing salinity. Hou et al.11 dispersed SiO2 NPs in saline water containing sodium chloride at concentrations of 0.05 and 0.1 wt% to alter the wettability of carbonate rock surfaces. They observed that Na+ ions could reach the oil palmitic acid molecules by approaching the carbonate rock surface. In fact, Na+ ions allow NPs to replace palmitic acid. thus, wettability alterations occur on the carbonate rock surface. Dehaghani and Daneshfar14 applied SiO2 NPs and saline water in their studies. They reported the greatest oil recovery factor related to SiO2 NPs with 0.25 wt% dispersed in 20 times diluted brine, and the lowest oil recovery factor related to formation water. Wang et al.27 reported that the oil recovery factor could be improved to 9% in low-salinity water flooding compared to that in formation water flooding. Their results indicated that the wettability of rock could increase water wetting by using divalent ions such as Mg2+, Ca2+ and SO42−. Additionally, they obtained optimal salinity between 1400 and 3200 mg/L. Moreover, they showed that the relative permeability diagram moved to the right with respect to the initial water saturation during the flooding of low-salinity water and the remaining oil saturation decreased. Bahari et al.28 investigated the effects of different surfactants, cetyl trimethyl ammonium bromide (CTAB), sodium dodecyl sulfate (SDS) and PVP on the stability and electrical conductivity of HNFs Al2O3-SiO2. They found that the HNF with a mass fraction of 30:70 has the highest electrical conductivity. Their results showed that the greatest duration of stability was obtained for HNFs prepared using SDS as a surfactant. Mofard and Saeedi Dehaghani29 studied the effect of smart water, CTAB and SDS by performing a contact angle test. They found that the combination of surfactant and smart water had the highest displacement efficiency compared to that of individual surfactant flooding or smart water flooding. Zallaghi and Khazali10 investigated the effects of CTAB and tridecyl alcohol 30 ethoxylate (TDA 30 EO) surfactants combined with smart water in fractured carbonate reservoirs. They found that oil recovery improved by more than 51% in core floods with strong synergy between smart water and surfactants. Liang et al.30 investigated dynamic stability, IFT and oil recovery using the amphiphilic molybdenum disulfide (MoS2) nanosheets modified with octadecyl amine (ODA) molecules. The results showed improved stability and reduced IFT to 0.1 (mN/m), where core flooding experiments gave the highest oil recovery of 19.1%.
Table 1 presents some mechanisms and improvements in oil recovery using hybrid methods due to synergistic effects.
In recent years, investigation of HNFs and surfactants has been received much attention by different researchers. However, the stability of NFs in reservoir conditions such as high temperature, high salinity and etc., requires more research and a deeper understanding. In addition to sustainability, using friendly environmental materials in HEOR should be one of the goals to play a key role. The development of nanocomposites modified with green surfactants and dispersed in water with salinity similar to seawater and formation water is greatly essential to improve oil recovery, which should be investigated in future studies. This study aims to investigate the potential effect of a novel γ-Al2O3/SiO2 nanocomposite modified with gum arabic surfactant and dispersed in various salinities and viscosities.
Experimental materials and equipment
Experimental materials
SiO2 NPs 99.5% pure and 15–20 nm in diameter and γ-Al2O3 NPs 99% pure and 20 nm in diameter were purchased from US Research Nano-Materials. The mass fraction ratios of γ-Al2O3 to SiO2 NPs were set at 10:90, 30:70 and 50:50. A concentration of 0.1 wt% was used to prepare all the solutions. This number is considered suitable for improving the stability of HNFs and increasing the thermal conductivity28. Crude oil with a density of 54.18 lb/ft3 (31.47° API) and a viscosity of 25.93 CST was used from the Gachsaran oilfield in Iran for the IFT and flooding experiments (Table 2).
Brines were prepared by dissolving different salts (NaCl, NaHCO3, Na2SO4, and CaCl2⋅2H2O and MgCl2⋅6H2O). All the reagents were purchased from Merck (Germany). The brine concentrations were prepared by dissolving salts in water with total dissolved solids (TDS) near 4071, 8142, 20,400 and 40,710 ppm.
Table 3 lists the properties of the brine compositions used in this paper. Hexamethyldisilane, Si2(CH3)6 was utilized to change the wettability of the micromodel from water-wet to oil-wet. Toluene (C7H8) and methanol (CH3OH) were used as wettability-changing agents and washing, respectively, both of which afforded 99% purity.
GA was used as a surfactant to aid in dispersing the NPs and improving the stability of the suspensions; It was purchased from Merck. GA is a natural polysaccharide obtained from the tree Acacia Senegalese. GA is a multicomponent substance, the majority of which consists of approximately 98 wt% polysaccharide and the smallest fraction consists of 2 wt% protein-polysaccharide. Peptides form the hydrophobic part and polysaccharides form the hydrophilic part of GA. Peptides are adsorbed on the oil surface. However, polysaccharides as the most hydrophilic component, stabilize oil droplets with a negative charge on carboxylates41,42. The results of the DLS test conducted by Williams et al.43 showed that magnetic NPs modified with GA accumulated less than unmodified NPs after 30 h.
Experimental equipment
A 2D glass micromodel was used as the porous medium and images were obtained from a CT scan of the core44. The micromodel was a 2D glass sheet with narrow pores etched on its surface. A piece of engraved glass was placed on the other part of the glass. Then, both parts of the glass were placed in the furnace and fused at 730 °C. The glass sheets bond at high temperatures to form a single piece. Thus, only the engraved pattern and drilled holes are open for flow14. The inlet and outlet channels of the micromodel for fluid injection and production are shown in Fig. 1. Additionally, one of the advantages of the glass micromodel is its chemical inertness and its substrate is easily visible4. It consists of a 5 × 5 cm2 matrix and an etch depth of 460 µm corresponding to a porous media with 41.13% porosity and a 0.51 cm3 pore volume (PV).
Schematic of the 2D glass micromodel based on the image obtained from the core CT scan42. The pore bodies are black and the rocks are white.
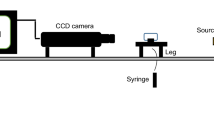
Measured IFT between crude oil and NFs dispersed in aqueous phases (DW, SSW, 2-DSSW, 5-DSSW and 10-DSSW) at 25 °C and 60 °C. The system was equipped with a CCD camera and a computerized macro lens. The formed droplets were analysed by professional drop shape analysis software (Apex DSA) based on pendant drop methods. A photo of the IFT device is shown in Fig. 2. After each test, the IFT measurement chambers for crude oil and NFs were washed with distilled water and toluene.
A Brookfield DV3T digital viscometer was used at different temperatures to measure the viscosity of the NFs. The temperature was continuously measured and controlled by a sensor. The spindle ULA was utilized for measuring low viscosity. After selecting the optimal NFs, 16 ml of the 0.1 wt% solution was added to the container with a thermal jacket connected to the rheometer at a constant shear rate of 73 (1⁄S). Before the viscosity of the NFs was measured, distilled water was used to calibrate the device and reduce error.
Methodology
Designing experiments
The Taguchi method was used to determine the stability of the nanosolutions in response to low-salinity water (ppm), the mass fraction of γ-Al2O3/SiO2 (wt%) and the surfactant concentration (ppm) as operating variables. The Taguchi method is based on three factors and five levels and was used to determine the optimal process characteristics that minimize the sensitivity45. In this research, the first factor was the salinity of the water, the second was the mass fraction of γ-Al2O3/SiO2 and the third was the concentration of GA. According to the factors and their levels in Table 4, an L-25 orthogonal arrangement was used to design the experiments. Since most of the stability was used in the response, a larger qualitative characteristic was chosen as the better criterion. The average values of the two generated responses were considered the original response.
The stability test results were analysed with a signal-to-noise diagram. In this way, if the quality characteristic is large or better, the signal-to-noise ratio (S/N) is calculated as follows: The signal-to-noise ratio is calculated from the mean square of the standard deviation (MSD), where Yi is the result of N repeated experiments46,47.
Preparation of NFs and selection of optimal NFs (screening)
The preparation of hybrid nanofluids (HNFs) involves different steps. Each step has different results. There has been no consensus among researchers about predicting HNFs18. In the first step, the solutions were prepared by adding NPs and GA to base fluids with various salinities. Next, the solutions were homogenized by a magnetic stirrer at 500 rpm for 15 min. Finally, to ensure the complete dispersion of the NPs in the base fluids, the mixture was sonicated for 1 h. Due to the instability of NFs in saline water and rapid aggregation, screening was performed. On an operational scale, the aggregation of NPs causes leakage around the injection well, which ultimately causes damage to the formation in the areas around the injection well48.
Micromodel test
In the first step, the micromodel was washed with sodium hydroxide solution and saturated for 30 min. Next, the vacuum pump evacuated the micromodel from any trapped fluid or air. To ensure that no fluid was trapped in the micromodel, the micromodel was washed with DW and dried in an oven at 200 °C. The micromodel was saturated with 2% hexamethyldisilane and 98% toluene for 30 min, as shown in Fig. 3, after which the glass surfaces became completely oil wet. Toluene and methanol were used to clean the micromodel. Finally, the micromodel was dried in an oven at 100 °C. All flooding tests were performed at atmospheric pressure and ambient temperature. The micromodel was placed horizontally to remove the effects of gravity14.
Flooding of the micromodel took place in three steps:
-
1.
The micromodel was saturated with formation water.
-
2.
The micromodel was saturated with oil.
-
3.
The micromodel was flooded with injected fluids29.
All analyses performed on the micromodel were based on photographs taken. Micromodel photographs were taken every 0.2 PV for all the tests. Figure 4 shows that a syringe pump was used to injection the fluids. A digital microscope camera (digimicro1/3) that located above the micromodel was used for imaging. Finally, the images were analysed using image processing software to calculate the oil recovery factor. The oil recovery factor is calculated by Eq. 3, where the initial oil saturation (Soi) and the residual oil saturation (Sor).
Results and discussion
Stability evaluation of the nanosolutions
Among the 25 NFs prepared, 15 had longer stability times. These nanosolutions with longer stability times were selected as the optimal NFs. In Table 5, the optimal NFs are shown with (*). The stability test was performed visually, similar to the methods of other researchers21,28,48. Sediment formation was observed at intervals of time on day one (1) and day sixty-third (63). In Fig. 5, photos of the stabilities of the different NFs are shown. (a) Two hours after preparation (b) 48 h after preparation. The NFs were considered stable when their dispersity remained constant in various base fluids, indicating that no sedimentation had formed. The interval time when sedimentation occurred at the end of the test tube and phase separation occurred at the top of the laboratory tube was considered the instability of the NFs.
In rows 10, 14, 18, and 22 of Table 5, NFs for which GA was not used as a surfactant are shown. The duration of their stability was reported to be less than 2 h. However, the addition of different surfactants, such as ionic sodium dodecyl benzene sulfonate (SDBS) and CTAB, nonionic surfactants (Span 80, Tween 20) and polymers polyvinyl alcohol (PVA) and GA during NF preparation can also control particle aggregation49. This finding could indicate that the increase in stability with surfactant coating on NPs leads to the dominance of electrostatic repulsion over van der Waals attraction and thus prevents the accumulation of NPs.
According to the Derjaguin–Landau–Verwey–Overbeek (DLVO) theory, two forces cause the dispersion of particles: the van der Waals force of attraction and the electrostatic repulsion force of the double layer. The aggregation of NPs leads to their instability and in the case of nonaggregation their stability is maintained.
Salinity causes the instability of nanosuspensions50. In the rows 21–25 of Table 5, the NFs dispersed in SSW had the lowest stability. However, the NFs dispersed in DW had the highest stability compared to that of all the prepared nanosolutions. The first row of Table 5 shows that the SiO2 NPs were stable in DW without surfactant for 63 days. Additionally, according to the results of Zulkeflee and Mamat51 the addition of GA surfactant to silica NPs dispersed in DW improves the stability time compared to dodecylbenzene sulfonic acid (DBSA) and Chinese ink (CI) surfactants. In the 21st row of Table 5, silica NPs were dispersed in SSW with 1000 ppm GA and were stable for 65 h. According to the results of Hamdi et al.52 the functional groups of GA modify the surface of NPs and reduce the effect of van der Waals forces between nanosheets. As a result, more stable NFs were created even in high-salinity environments for a longer time. In row 5 of Table 5, the maximum stability time was 16 h for γ-Al2O3 NPs dispersed in DW with 1000 ppm GA.
Divalent cations such as Ca2+ can effectively neutralize the negative charge of SiO2 NPs. Therefore, when the van der Waals force of attraction between particles is greater than the electrostatic repulsion force, the accumulation and instability of the solution occur53. HNFs exhibit different behaviours when dispersed in DWs and brines. They are completely hydrophilic when dispersed in DW and have good stability, while they tend to cause sedimentation in brine. However, adding surfactants to HNFs results in a smaller aggregation size than in the absence of a surfactant54. The presence of monovalent cations such as Na+ in brine reduces the negative surface charge on silica NPs. Because silica NPs absorb monovalent cations such as Na+ present in brine, the stability of the solution decreases17. The dispersion of divalent ions such as Ca2+ and Mg2+ in brine results in amphoteric properties in HNFs6.
Three factors, the base-fluid salt concentration, mass fraction of nanosolutions and GA concentration, were studied to investigate the duration of stability, as shown in Fig. 6. The slope of the salinity graph could indicate an increase in the concentration of polyvalent ions. The results showed that the stability of HNFs with different mass fractions decreased with increasing amounts of alumina NPs, which could be due to the tendency of alumina to aggregate. Therefore, it is necessary to use stabilizers for the dispersion of alumina NPs12. Throughout the stability evaluation of the nanosolutions dispersed in the base fluid types, the highest stabilities were as follows: SiO2 NPs > combination of γ-Al2O3/SiO2 with a mass fraction of 10:90 > combination of γ-Al2O3/SiO2 with a mass fraction of 30:70 > γ-Al2O3 NPs > combination of γ-Al2O3/SiO2 with a mass fraction of 50:50. According to the results shown in Fig. 6, the most suitable concentration for GA was reported 250 ppm. At low surfactant concentrations, due to the Gibbs adsorption effect, surfactant molecules are absorbed at the fluid‒solid interface, which causes a decrease in the surface energy between the fluid and NPs, ultimately leading to a decrease in the aggregation of NPs54.
IFT measurement
Any change in water composition (ionic or nonionic) could change the IFT. All IFT tests were performed at constant pressure. To ensure the reproducibility of the results and to determine the standard error, IFT measurements were performed at least seven times for the optimized NFs. According to the results of Afzalitabar et al.55 adding HNFs reduces the IFT. Figure 7 shows the IFT of NFs at 25 °C. HNFs γ-Al2O3/SiO2 with different mass fractions have lower IFT than SiO2 NPs; however, their IFT is greater than that of γ-Al2O3 NPs. Figure 7 shows that the IFT of NFs decreases as the temperature increases from 25 to 60 °C. At high temperatures, the IFT decreases, which could be due to an increase in the brownian motion of NPs or the weak interaction between molecules, ultimately leading to improved oil recovery56. Figure 7 shows that with increasing temperature from 25 to 60 °C, the IFT for SiO2 NPs decreased from 9.97 (mN/m) to 6.66 (mN/m) and that for γ-Al2O3 decreased to 1.57 (mN/m) and 1.07 (mN/m), respectively. In another attempt, Nowerouzi et al.57 reported that among the NPs of TiO2 and MgO and γ-Al2O3, the most successful at reducing the IFT was related to γ-Al2O3. Figure 7 shows the IFT with increasing temperature from 25 to 60 °C for HNFs with mass fractions of 10:90, 30:70 and 50:50 and GA concentrations of 250, 500 and 750 ppm, from 5.71, 2.44 and 1.85 (mN/m) to 2.32, 1.7 and 1.47 (mN/m), respectively. The presence of NPs modified with GA could reduce the IFT between crude oil and displacing nanofluid. Adsorption of modified NPs with GA at the interface between oil and brine occurs due to the hydrophilic and hydrophobic functional groups of the modified NPs, which cause the modified NPs to act as amphiphilic surfactants. Finally, the functional groups of the modified NPs create an additional layer at the interface between brine and oil to reduce the IFT52.
The effect of salt on IFT values in oil-salt water-containing systems is complex. A water-in-oil emulsion is formed in the presence of surfactants at low salt concentrations. In this system, increasing the amount of salt causes a further decrease in IFT. An oil-in-water emulsion is formed at high salt concentrations and the IFT increases with increasing salt concentration58,59. Adsorption of cations at the interface occurs due to the interaction between cations and the hydrocarbon phase. The interface concentration increases or becomes positive due to the placement of cations at the interface. As a result, the IFT decreases with decreasing salinity. However, at high brine concentrations, the cations are surrounded by water molecules, so the cations are less inclined to transfer to the interface. Separation of cations from the interface could reduce the positive charge of the interface. As a result, the IFT increases at high brine concentrations59.
Figure 8a shows the IFT of the NFs dispersed in 10-DSSW at ambient temperature and 60 °C. As shown, the NFs dispersed in 10-DSSW at 25 °C had a lower IFT than did the NFs dispersed in DW. This could be due to the reduction in IFT at low salt concentrations. The combination of HNF γ-Al2O3/SiO2 with a mass fraction of 50:50 modified with GA dispersed in 10-DSSW had the minimum IFT of 0.99 (mN/m) in 60 °C compared to that of the other NFs. However, Khaksar et al.60 reported the lowest IFT for silica-bentonite nanocomposite treated with anethum graveolens surfactant dispersed in 10,000 ppm NaCl at 1.73 (mN/m).
By increasing temperature, SiO2 NPs and HNFs with mass ratios of 10:90 and 30:70 were modified with GA (250, 500 and 750 ppm) respectively, their IFT decreased 1.57, 1.7 and 1.08 (mN/m). However, the results showed that the NFs dispersed in 10-DSSW had the lowest IFT.
Figure 8b shows the IFT of the NFs dispersed in 5-DSSW at ambient temperature and 60 °C. SiO2 NPs and HNFs with mass ratios of 10:90 and 30:70 were modified with GA (500, 750 and 1,000 ppm) and their IFT at 25 °C decreased from 2.25, 4.24 and 2.18 (mN/m) to 1.78, 2.28 and 1.59 (mN/m) respectively, at 60 °C.
Figure 8c shows that with increasing salinity of the base fluid, the IFT of the SiO2 NPs and HNF γ-Al2O3/SiO2 dispersed in 2-DSSW increased at ambient temperature compared to that of the NPs dispersed in 5-DSSW. This increase in the IFT of the NFs dispersed in 2-DSSW could be due to the high concentration of salt. As mentioned, increasing the salt concentration could reduce the separation of cations from the interface. Therefore, the silica NPs and HNF γ-Al2O3/SiO2 dispersed in 2-DSSW at ambient temperature had an IFT of 2.7 and 4.7 (mN/m) respectively. On the other hand, with increasing temperature, the IFT of the NFs dispersed in 2-DSSW decreased. However, in Fig. 8d, the IFT for SiO2 NPs dispersed in SSW modified with 1,000 ppm GA at ambient temperature and 60°C was reported 2.1 and 1.52 (mN/m), respectively.
Viscosity measurement
The dynamic viscosities of the optimal nanosolutions were measured at temperatures of 25 °C, 35 °C, 45 °C and 55 °C. As shown in Fig. 9, the NFs dispersed in DW had lower viscosities than the NFs dispersed in various salinities. The results of Hashemzadeh and Hormozi34showed that γ-Al2O3 and SiO2 NPs dispersed in DW have relatively the same viscosity in the temperature range 10–55 °C. However, Fig. 9 shows that γ-Al2O3 NPs have a higher viscosity than SiO2 NPs. However, with increasing temperature, the viscosity of γ-Al2O3 decreases significantly compared to that of silica. The reason for this difference could be the presence of GA, which was applied together with the γ-Al2O3 NPs dispersed in DW at a mass ratio of 1:1. However, no surfactant was used with SiO2 NPs. As the temperature increases, the viscosity of the NFs decreases61,62. As inferred by Ma et al.54 adding surfactant improves nanofluid viscosity. According to the results of Buckley and Leverett63, decreasing mobility of the displacing fluid delays the breakthrough time. Additionally, the brownian motion of NPs induces interactions with surface-active compounds at the water–oil interface by attracting natural surfactants at the interface, thus reducing the IFT and improving oil recovery14. The reduction in nanocolloidal viscosity largely depends on the NPs and base fluids used. HNFs have a higher viscosity than do normal fluids and nanofluids. However, the viscosity of HNFs depends on the selected NPs and their composition62.
HNFs with mass ratios of 10:90 and 30:70 had lower viscosities than SiO2 and γ-Al2O3 NPs at low temperatures. However, with increasing temperature, the viscosity of these NFs increased compared to that of mono NFs. However, for HNFs a mass ratio of 50:50 was reported to result in a lower viscosity than that of mono nanofluids and this trend was observed at all the measured temperatures. In general, the viscosity of HNFs is greater than that of base fluids and single NPs34,62.
Figure 10a shows the viscosity as a function of temperature for SiO2 NPs and HNFs with mass ratios of 10:90, 30:70 and 50:50 modified with 250, 500, 750 and 1,000 ppm GA dispersed in 10-DSSW. The results show that HNFs with different mass ratios at different temperatures (25, 35, 45 and 55 °C) have higher viscosities than silica NPs and the lowest viscosity is obtained for SiO2 NPs at 55 °C. However, the combination of γ-Al2O3/SiO2 dispersed in 10-DSSW with a mass fraction of 50:50 has a higher viscosity than other nanosolutions at 25 °C. Although, by increasing temperature the combination of γ-Al2O3/SiO2 with a mass fraction of 50:50 the measured viscosity difference between 25 and 55 °C was reported 0.29 cp, which was observed greater than that of another HNFs with mass ratios of 10:90 and 30:70.
Figure 10b shows that the silica NPs dispersed in the 5-DSSW modified with 500 ppm GA at 25 °C had a greater viscosity than did the HNFs with mass ratios of 10:90 and 30:70 that, modified with 750 and 1000 ppm GA. However, with increasing temperature to 35 °C, the viscosities of the silica NPs and HNFs are somewhat equal. At high temperatures, the viscosity of HNFs was greater than the viscosity of silica NPs. The results showed that silica NPs and HNFs with a mass ratio of 10:90 exhibited a decreasing trend with increasing temperature, while the lowest viscosity was reported for the combination of HNF γ-Al2O3/SiO2 with a mass fraction of 30:70 at 45 °C.
Figure 10c shows that the HNF with a mass ratio of 10:90 and 1,000 ppm GA dispersed in 2-DSSW, compared to the viscosity of the SSW at 25 °C and 55 °C, has a lower viscosity than the silica NPs with 750 ppm GA. At 45 °C, the viscosities of the combinations of HNF γ-Al2O3/SiO2 with a mass fraction of 10:90 and silica NPs were equal. Moreover, the viscosity of the optimal NFs dispersed in 2-DSSW decreased. As shown in Fig. 10d, the viscosity of the silica NPs dispersed in SSW decreased with increasing temperature.
Flooding tests
Figure 11 shows that when NFs were dispersed in DW, breakthrough occurred after 0.4 PV. According to the results of the micromodel flooding, the minimum oil recovery was 34.4% when SiO2 NPs were injected into DW. Additionally, oil recovery was reported during the injection of γ-Al2O3 NPs (35.82%). According to the results of Nowrouzi et al.57 γ-Al2O3 NPs improve oil recovery by reducing the IFT and viscosity. However, during the injection of HNFs γ-Al2O3/SiO2 with mass fractions of 10:90, 30:70 and 50:50 and concentrations of 250, 500 and 750 ppm GA, oil recoveries of 38.92%, 43.2% and 45.85%, respectively, were obtained. In all the flooding tests, the synergistic effect of injecting HNFs was evident, and the oil recovery was greater than that of mono NFs. In similar studies, Khaksar et al.60 showed that SiO2/bentonite nanocomposites modified dill and hop surfactants can improve the oil recovery. The modified nanocomposite with 4000ppm dill extract can improve oil recovery by 14% of original oil in place. Their results confirmed the effectiveness of the formulated green nano-solution as a sustainable and consistent method with the environment in the EOR process.
HNFs increase oil recovery by transporting more residual oil. However, compared with water flooding, GA flooding improved the oil recovery factor on average by 4.6%. GA can reduce the IFT between brine and oil through its migration to the oil–water interface, where the hydrophobic polypeptide chain of GA reacts with oil and the hydrophilic arabinogalactan unit of GA reacts with water. Additionally, adding GA to silica and alumina NPs improved the oil recovery factor by 14.4% and 20.9%, respectively64.
Figure 12 shows that breakthrough occurs at 0.5 PV when the NFs are dispersed in 10-DSSW. The oil recoveries during the injection of SiO2 NPs and HNFs with mass fractions of 10:90, 30:70 and 50:50 along with different concentrations of GA were reported as 45, 45.75, 46.28 and 57.75%, respectively. We observed that, among the optimized NFs that were stably dispersed in 10-DSSW, HNF γ-Al2O3/SiO2, with a mass fraction of 50:50 and modified with 1,000 ppm GA, initially had lower oil recovery than the other NFs. However, after 0.8 PV, the oil recovery of the HNF γ-Al2O3/SiO2 composite increased significantly.
As evident in Fig. 13, a delayed breakthrough occurred when the NFs dispersed in 5-DSSW were injected in comparison to the NFs dispersed in 10-DSSW, and the breakthrough time occurred at 0.6 PV. The oil recovery during the injection of SiO2 NPs and HNFs with mass fraction 10:90 and 30:70 and modified with 500, 750 and 1000 ppm GA were reported 43.8, 46.77 and 49.68%, respectively. We observed in micromodel flooding tests that increasing the concentration of brine can improve oil recovery. According to the results of Rostami et al.4, NPs dispersed in brines have a better performance in improving the oil recovery compared to NPs dispersed in deionized water. NPs flooding modified with GA compared with water flooding, has a greater ability to sweep out oil. The ability of the modified NPs to reduce the IFT between the crude oil and nanofluid solutions ultimately reduced the capillary pressure inside the pores. Additionally, the modified nanofluids alter the wettability of the pores surface to strong water wetting52.
Figure 14a shows that, for NFs dispersed in 2-DSSW breakthrough occurs delayed at 0.8 PV compared to NFs dispersed in 5-DSSW. We observed a maximum oil recovery of 60.34% when the combination of γ-Al2O3/SiO2 was injected with a mass fraction of 10:90 dispersed in 2-DSSW. On the other hand, Khaksar et al. were achieved the maximum oil recovery factor for KCl–SiO2–xanthan nanocomposite treated with the aloe vera biopolymer surfactant at 73.35%. They also attributed the increase in oil recovery to the reduction of IFT, wettability alteration and mobility improvement mechanisms65. Figure 14b shows that by injecting 0.1 wt% SiO2 nanofluid dispersed in SSW into the micromodel, 51.53% oil recovery was obtained.
Figure 15 shows micromodel images used for sweep efficiency calculations. The images show the injection of some NFs after the injection of 1 PV into the treated to oil-wet micromodel. From these images, it could be concluded that the injection of HNF γ-Al2O3/SiO2 in combination with a mass fraction of 10:90 dispersed in 2-DSSW with 1,000 ppm GA into the micromodel had the highest areal sweep efficiency. Figure 15e shows the synergistic effect of HMF γ-Al2O3/SiO2 modified with GA dispersed in 2-DSSW, which increased the displacement efficiency of the oil mixture among the other injection scenarios. Figure 15a also shows that the silica NPs dispersed in DW without GA had the lowest oil recovery among the other optimal NFs.
Oil recovery for different NFs after 1 PV the injection. (a) SiO2 NPs dispersed in DW without GA. (b) γ-Al2O3 NPs dispersed in DW modified with GA. (c) HNF γ-Al2O3/SiO2 with a mass fraction of 30:70 dispersed in 5-DSSW modified with GA. (d) HNF γ-Al2O3/SiO2 with a mass fraction of 50:50 dispersed in 10-DSSW modified with GA. (e) HNF γ-Al2O3/SiO2 with a mass fraction of 10:90 dispersed in 2-DSSW modified with GA.
Conclusions
In this experimental study, the effects of mono/hybrid γ-Al2O3/SiO2 nanofluids dispersed at various salinities and modified with GA surfactant on the stability, viscosity, IFT and oil recovery were investigated. The results showed that the NFs became highly unstable with increasing salt concentration to 40,710 ppm and quickly accumulated at the end of the test tube. However, the NFs that were not modified with GA became unstable after approximately 2 h. The stability of NPs in base fluid is essential for the long-term utilization of NFs, particularly for EOR applications.
In addition, the effects of temperature, various salinities of base fluid and mass fractions of γ-Al2O3/SiO2 on the viscosity and IFT of long-term stable NFs were evaluated. The results of the IFT evaluation showed that increasing temperature had a greater effect on the dispersal of NFs at various salinities than on the dispersion of NFs in DW. However, the results clearly showed that with increasing salinity to 20,400 ppm the IFT increased.
Also, the evaluation of viscosity results shows that with increasing temperature, NFs dispersed in DW have lower viscosity than NFs dispersed in various salinities. According to results of experimental viscosity data nanohybrids with different mass fractions by increasing temperature show different action in the presence of cations in salt water and deionized water. The lowest viscosity was observed at 55 °C for the nanohybrid γ-Al2O3/SiO2 with a mass fraction of 50:50 dispersed in deionized water.
The results of the micromodel flooding test showed that increasing the salinity of the base fluid leads to a delay in breakthrough. In this way, NFs dispersed in DW were observed at 0.4 PV breakthrough time; however, NFs dispersed in 2-DSSW were reported at 0.8 PV breakthrough time.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
US Energy Information Adminstration. http://www.eai.gov/todayinenergy/detail.php?id=26212-MAY.2016-12 (2016).
Olayiwola, S. O. & Dejam, M. A comprehensive review on interaction of nanoparticles with low salinity water and surfactant for enhanced oil recovery in sandstone and carbonate reservoirs. Fuel. 241, 1045–1057 (2018).
Ding, Y., Zheng, S., Meng, X. & Yang, D. Low salinity hot water injection with addition of nanoparticles for enhancing heavy oil recovery. J. Energy Resour. Technol. 141(7), 072904 (2019).
Rostami, P., Sharifi, M., Aminshahidy, B. & Fahimpour, J. Enhanced oil recovery using silica nanoparticles in the presence of salts for wettability alteration. J. Dispers. Sci. Technol. 41(3), 402–413 (2019).
Moradpour, N. & Pourafshary, P. Hybrid EOR methods utilizing low-salinity water. Enhanc. Oil Recov. Process. New Technol. 2019(8), 25 (2019).
Habibi, S., Jafari, A. & Fakhroueian, Z. Wettability alteration analysis of smart water/novel functionalized nanocomposites for enhanced oil recovery. Pet. Sci. 17(5), 1318–1328 (2020).
Ahmadi, S., Hosseini, M., Tangestani, E., Mousavi, S. E. & Niazi, M. Wettability alteration and oil recovery by spontaneous imbibition of smart water and surfactants into carbonates. Pet. Sci. 17(3), 712–721 (2020).
Hu, Y., Zhao, Z., Dong, H., Vladimirovna Mikhailova, M. & Davarpanah, A. Hybrid application of nanoparticles and polymer in enhanced oil recovery processes. Polymers 13(9), 1414. https://doi.org/10.3390/polym13091414 (2021).
Mahmoudpour, M. & Pourafshary, P. Investigation of the effect of engineered water/nanofluid hybrid injection on enhanced oil recovery mechanisms in carbonate reservoirs. J. Pet. Sci. Eng. 196, 107662 (2021).
Zallaghi, M. & Khazali, A. Experimental and modelling study of enhanced oil recovery from carbonate reservoirs with smart water and surfactant injection. Fuel. 304, 121516 (2021).
Hou, B. et al. Wettability alteration of oil-wet carbonate surface induced by self-dispersing silica nanoparticles: Mechanism and monovalent metal ion’s effect. J. Mol. Liq. 294, 111601 (2019).
Arain, Z. U. A. Influence of Silica Nanoparticles on the Surface Properties of Carbonate Reservoirs (Curtin University, 2020).
Liang, T., Hou, J. R., Qu, M., Xi, J. X. & Raj, I. Application of nanomaterial for enhanced oil recovery. Pet. Sci. 19(2), 882–899 (2022).
Dehaghani, A. H. S. & Daneshfar, R. How much would silica nanoparticles enhance the performance of low-salinity water flooding?. Pet. Sci. 16(3), 591–605 (2019).
Ebrahim, T., Mohsen, V. S., Mahdi, S. M., Esmaeel, K. T. & Saeb, A. Performance of low-salinity water flooding for enhanced oil recovery improved by SiO2 nanoparticles. Pet. Sci. 16(2), 357–365 (2019).
Sun, Y. et al. Properties of nanofluids and their applications in enhanced oil recovery: A comprehensive review. Energy Fuels. 34(2), 1202–1218 (2020).
Mahmoudi, S., Jafari, A. & Javadian, S. Temperature effect on performance of nanoparticle/surfactant flooding in enhanced heavy oil recovery. Pet. Sci. 16(6), 1387–1402 (2019).
Kshirsagar, D. P. & Venkatesh, M. A review on hybrid nanofluids for engineering applications. Mater. Today Proc. 44, 744–755 (2021).
Yu, W. & Xie, H. A. Review on nanofluids: Preparation, stability mechanisms, and applications. J. Nanomaterials. 2012, 1–17 (2012).
Mukherjee, S. & Paria, S. Preparation and stability of nanofluids—A review. IOSR J. Mech. Civil Eng. 9(2), 63–69 (2013).
Torsaeter, L. & Hendraningrat, A. O. Unlocking the Potential of Metal Oxides Nanoparticles to Enhance the Oil Recovery (One Petro, 2014).
Nazari, M. R., Bahramian, A., Fakhroueian, Z., Karimi, A. & Arya, S. Comparative study of using nanoparticles for enhanced oil recovery: Wettability alteration of carbonate rocks. Energy Fuels. 29(4), 2111–2119 (2015).
Alomair, O. A., Matar, K. M. & Alsaeed, Y. H. Experimental study of enhanced-heavy-oil recovery in Berea sandstone cores by use of nanofluids applications. SPE Reserv. Eval. Eng. 18, 387–399 (2015).
Alameri, W., Teklu, T., Graves, R., Kazemi, H. & AlSumaiti, A. Low-salinity water-alternate-surfactant in low permeability carbonate reservoirs. In IOR 2015–18th European Symposium on Improved Oil Recovery.European Association of Geoscientists & Engineers (2015).
Al-Anssari, S., Barifcani, A., Wang, S., Maxim, L. & Iglauer, S. Wettability alteration of oil-wet carbonate by silica nanofluid. J. Colloid Interface Sci. 461, 435–442 (2016).
Kiani, S., Mansouri, Z. M., Khodabakhshi, S., Rashidi, A. & Moghadasi, J. Newly prepared nano gamma alumina and its application in enhanced oil recovery: An approach to low-salinity waterflooding. Energy Fuels. 30(5), 3791–3797 (2016).
Wang, J., Song, H. & Wang, Y. Investigation on the microflow mechanism of enhanced oil recovery by low-salinity water flooding in carbonate reservoir. Fuel 266, 117156 (2020).
Bahari, N. M., Che Mohamed Hussein, S. N. & Othman, N. H. Synthesis of Al2O3–SiO2/water hybrid nanofluids and effects of surfactant toward dispersion and stability. Part. Sci. Technol. 39(7), 844–858 (2020).
Mofrad, S. & Saeedi Dehaghani, A. H. An experimental investigation into enhancing oil recovery using smart water combined with anionic and cationic surfactants in carbonate reservoir. Energy Rep. 6, 543–549 (2020).
Liang, T., Wang, H. & Yang, C. H. Mechanisms and effects of amphiphilic lamellar nanofluid for enhanced oil recovery in low permeability reservoirs. J. Mol. Liq. 397, 124043 (2024).
Afrand, M., Nazari Najafabadi, K. & Akbari, M. Effects of temperature and solid volume fraction on viscosity of SiO2-MWCNTs/SAE40 hybrid nanofluid as a coolant and lubricant in heat engines. Appl. Therm. Eng. 102, 45–54 (2016).
Dardan, E., Afrand, M. & Meghdadi Isfahani, A. H. Effect of suspending hybrid nanoadditives on rheological behavior of engine oil and pumping power. Appl. Therm. Eng. 109, 524–534 (2016).
Orodu, O. D., Orodu, K. B., Alfolabi, R. O. & Dafe, E. A. Dataset on experimental investigation of gum Arabic coated alumina nanoparticles for enhanced recovery of Nigerian medium crude oil. Data Brief 19, 475–480 (2018).
Hashemzadeh, S. & Hormozi, F. An experimental study on hydraulic and thermal performances of hybrid nanofluids in mini-channel. J. Therm. Anal. Calorim. 140(3), 891–903 (2019).
Betancur, S. et al. Importance of the nanofluid preparation for ultra-low interfacial tension in enhanced oil recovery based on surfactant-nanoparticle-brine system interaction. ACS Omega. 4(14), 16171–16180 (2019).
Sadatshojaei, E., Jamialahmadi, M., Esmaeilzadeh, F., Wood, D. A. & Ghazanfari, M. H. The impacts of silica nanoparticles coupled with low-salinity water on wettability and interfacial tension: Experiments on a carbonate core. J. Dispers. Sci. Technol. 41(8), 1159–1173 (2019).
Rezvani, H. et al. A novel foam formulation by Al2O3/SiO2 nanoparticles for EOR applications: A mechanistic study. J. Mol. Liq. 2020(304), 112730 (2020).
Tajikmansori, A., Hosseini, M. & Dehaghani, A. H. Mechanistic study to investigate the injection of surfactant assisted smart water in carbonate rocks for enhanced oil recovery: An experimental approach. J. Mol. Liq. 325, 114648 (2021).
Hosseini-Nasab, S. M., Rezaee, M., Fahimpour, J. & Mohammad, S. M. New Insight on improving foam stability and foam flooding using fly ash in the presence of crude oil. J. Pet. Sci. Eng. 214, 110534 (2022).
Maleki, A. et al. Effect of alumina and silica nanocomposite based on polyacrylamide on light and heavy oil recovery in presence of formation water using micromodel. Petroleum. https://doi.org/10.1016/j.petlm.2023.03.001 (2023).
Dror, Y., Cohen, Y. & Yerushalmi-Rozen, R. Structure of gum arabic in aqueous solution. J. Polym. Sci. Part B Polym. Phys. 44(22), 3265–3271 (2006).
Isobe, N. et al. Primary structure of gum arabic and its dynamics at oil/water interface. Carbohydr. Polym. 249, 116843 (2020).
Williams, D. N., Gold, K. A., Holoman, P., Ehrman, S. H. & Wilson, O. C. Surface modification of magnetic nanoparticles using gum Arabic. J. Nanoparticle Res. 8(5), 749–753 (2006).
Yu, F. et al. New insights into flow physics in the EOR process based on 2.5D reservoir micromodels. J. Pet. Sci. Eng. 181, 106214 (2019).
Mousavi, S. M., Yaghmaei, S., Jafari, A., Vossoughi, M. & Ghobadi, Z. Optimization of ferrous biooxidation rate in a packed bed bioreactor using Taguchi approach. Chem. Eng. Process. Process Intensif. 46(10), 935–940 (2007).
Pignatiello, J. J. An overview of the strategy and tactics of Taguchi. IIE Trans. 20(3), 247–254 (1988).
Daneshvar, N., Khataee, A. R., Rasoulifard, M. H. & Pourhassan, M. Biodegradation of dye solution containing Malachite green: Optimization of effective parameters using Taguchi method. J. Hazard Mater. 143(1–2), 214–219 (2007).
Mahpishanian, A. M., Simjoo, M. & Zaeri, M. Experimental investigation of nano silica on wettability alteration and enhanced oil recovery from carbonate reservoir using low salinity water. Pet. Res. 30(99–6), 3–20 (2021).
KumarSingh S. Review on the Stability of the Nanofluids (2022).
Yousefvand, H. A. & Jafari, A. Stability and flooding analysis of nanosilica/NaCl/HPAM/SDS solution for enhanced heavy oil recovery. J. Pet. Sci. Eng. 162, 283–291 (2018).
Zulkeflee, R. & Mamat, H. Stability and thermal performance of silica nanofluid in water block heat sink. J. Phys. Sci. 30(Supp. 2), 1–11 (2019).
Hamdi, S. S., Al-Kayiem, H. H. & Muhsan, A. S. Natural polymer noncovalently grafted graphene nanoplatelets for improved oil recovery process: A micromodel evaluation. J. Mol. Liq. https://doi.org/10.1016/j.molliq.2020.113076 (2020).
Ahangaran, F. & Navarchian, A. H. Recent advances in chemical surface modification of metal oxide nanoparticles with silane coupling agents: A review. Adv. Colloid Interface Sci. 286, 102298 (2020).
Ma, M., Zhai, Y., Yao, P., Li, Y. & Wang, H. Effect of surfactant on the rheological behavior and thermophysical properties of hybrid nanofluids. Powder Technol. 379, 373–383 (2021).
AfzaliTabar, M., Alaei, M., Ranjineh, K. R., Motiee, F. & Rashidi, A. M. Preference of multiwalled carbon nanotube (MWCNT) to single-walled carbon nanotube (SWCNT) and activated carbon for preparing silica nanohybrid pickering emulsion for chemical enhanced oil recovery (C-EOR). J. SolidState Chem. 245, 164–173 (2017).
Lashari, N. & Ganat, T. Emerging applications of nanomaterials in chemical enhanced oil recovery: Progress and perspective. Chin. J. Chem. Eng. 28(8), 1995–2009 (2020).
Nowrouzi, I., Khaksar Manshad, A. & Mohammadi, A. H. Effects of TiO2, MgO, and γ-Al2O3 nanoparticles in carbonated water on water-oil interfacial tension (IFT) reduction in chemical enhanced oil recovery (CEOR) process. J. Mol. Liq. https://doi.org/10.1016/j.molliq.2019.111348 (2019).
Al-Sahhaf, T., Elkamel, A., Suttar Ahmed, A. & Khan, A. R. The influence of temperature, pressure, salinity, and surfactant concentration on the interfacial tension of the N-octane-water system. Chem. Eng. Commun. 192(5), 667–684 (2005).
Divandari, H., Hemmati-Sarapardeh, A., Schaffie, M. & Ranjbar, M. Integrating functionalized magnetite nanoparticles with low salinity water and surfactant solution: Interfacial tension study. Fuel 281, 118641 (2020).
Khaksar, M. A. et al. Performance evaluation of the green surfactant-treated nanofluid in enhanced oil recovery: Dill-hop extracts and SiO2/bentonite nanocomposites. Energy Fuels. 38, 1799–1812 (2024).
Moldoveanu, G. M., Ibanescu, C., Danu, M. & Minea, A. A. Viscosity estimation of Al2O3, SiO2 nanofluids and their hybrid: An experimental study. J. Mol. Liq. 253, 188–196 (2018).
Babar, H., Sajid, M. U. & Ali, M. Viscosity of hybrid nanofluids: A critical review. Therm. Sci. 23(3), 1713–1754 (2019).
Buckley, S. E. & Leverett, M. Mechanism of fluid displacement in sands. Trans. AIME. 146(01), 107–116. https://doi.org/10.2118/942107-G (1942).
Sowunmi, A. O., Efeovbokhan, V. E., Orodu, O. D. & Oni, B. A. Polyelectrolyte–nanocomposite for enhanced oil recovery: Influence of nanoparticle on rheology, oil recovery and formation damage. J. Pet. Explor. Prod. Technol. 12(2), 493–506. https://doi.org/10.1007/s13202-021-01358-0 (2021).
Khaksar, M. A. et al. Application of a novel green nano polymer for chemical EOR purposes in sandstone reservoirs: Synergetic effects of different fluid/fluid and rock/fluid interacting mechanisms. ACS Omega. 8, 43930–43954 (2023).
Acknowledgements
We sincerely thank Dr. Mohammad Nader Lotfollahi from the Semnan University Faculty of Chemical, Petroleum and Gas Engineering. In addition, we appreciate the time and effort of the editor and reviewers in significantly improving the quality of this manuscript.
Author information
Authors and Affiliations
Contributions
A..K.K performed experimental laboratory study and wrote the main manuscript of this paper. S.M.H.N, and F. H supervised the research and experimental laboratory works and then assisted in writing and reviewing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khajeh Kulaki, A., Hosseini-Nasab, S.M. & Hormozi, F. Low-salinity water flooding by a novel hybrid of nano γ-Al2O3/SiO2 modified with a green surfactant for enhanced oil recovery. Sci Rep 14, 14033 (2024). https://doi.org/10.1038/s41598-024-64171-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-64171-9
- Springer Nature Limited