Abstract
Environmental health research has suggested that fine particulate matter (PM2.5) exposure can lead to high blood pressures, but it is unclear whether the impacts remain the same for systolic and diastolic blood pressures (SBP and DBP). This study aimed to examine whether the effects of PM2.5 exposure on SBP and DBP differ using data from a predominantly non-Hispanic Black cohort collected between 2013 and 2019 in the US. PM2.5 exposure was assessed based on a satellite-derived model across exposure durations from 1 to 36 months. The average PM2.5 exposure level was between 9.5 and 9.8 μg/m3 from 1 through 36 months. Mixed effects models were used to estimate the association of PM2.5 with SBP, DBP, and related hypertension types, adjusted for potential confounders. A total of 6381 participants were included. PM2.5 exposure was positively associated with both SBP and DBP. The association magnitudes depended on exposure durations. The association with SBP was null at the 1-month duration (β = 0.05, 95% CI: − 0.23, 0.33), strengthened as duration increased, and plateaued at the 24-month duration (β = 1.14, 95% CI: 0.54, 1.73). The association with DBP started with β = 0.29 (95% CI: 0.11, 0.47) at the 1-month duration, and plateaued at the 12-month duration (β = 1.61, 95% CI: 1.23, 1.99). PM2.5 was associated with isolated diastolic hypertension (12-month duration: odds ratio = 1.20, 95% CI: 1.07, 1.34) and systolic–diastolic hypertension (12-month duration: odds ratio = 1.18, 95% CI: 1.10, 1.26), but not with isolated systolic hypertension. The findings suggest DBP is more sensitive to PM2.5 exposure and support differing effects of PM2.5 exposure on SBP and DBP. As elevation of SBP and DBP differentially predict CVD outcomes, this finding is relevant for prevention and treatment.
Similar content being viewed by others
Introduction
Air pollution, specifically exposure to fine particulate matter (PM2.5), has been recognized as a significant environmental risk factor affecting human health1. Numerous studies have explored the relationship between air pollution exposure and various health outcomes, including cardiovascular diseases, the leading cause of global mortality and morbidity2. One area of particular interest is the association between PM2.5 exposure and blood pressure (BP), as BP is an essential indicator of general health status and high BP is an important risk factor for various diseases3. Over the past several decades, a large number of human epidemiological studies have examined the relationships of short- and long-term PM2.5 exposure with hypertension and BP levels in the general population, leading to at least four meta-analyses that covered more than 100 studies and more than 700,000 participants4,5,6,7. Evidence from these studies has established a strong relationship of PM2.5 exposure with high BP levels and hypertension in the general population4,5,6,7.
Nonetheless, a close scrutiny of the literature reveals intriguing differences in relationships with systolic and diastolic BP (SBP and DBP, respectively). The most recent meta-analysis reported a robust association between PM2.5 exposure and DBP, regardless of short- or long-term exposures, but the same study concluded that long-term (≥ 30 days) PM2.5 exposure was not associated with elevated SBP4. In contrast, an earlier meta-analysis and several more recent large studies concluded a positive association between long-term PM2.5 exposure (≥ 30 days) and SBP6,8,9,10. The differing findings between SBP and DBP were also noted in studies among children and adolescents. According to a meta-analysis published in 2023 of eight studies that only focused on children and adolescents, long-term PM2.5 exposure was associated with elevated DBP, but not SBP11. In studies looking at the subtypes of heart failure, diastolic heart failure has been reported to be associated with PM2.5 exposure12,13, while the evidence specifically for systolic heart failure was sparse, even though systolic heart failure is generally more common14. These differing observations between SBP and DBP warrant more investigations to understand the biological mechanisms of PM2.5 exposure effects at the population level.
SBP and DBP are two crucial components of cardiovascular health that provide different information about the functioning of the cardiovascular system15. SBP reflects the force with which blood is ejected from the heart and the resistance encountered in the arteries, while DBP reflects the relaxation of the heart and the degree of resistance in the arteries when the heart is at rest between beats15. Although DBP is more closely related to end-organ damage16, observational studies and clinical trials have both demonstrated a greater association of SBP with cardiovascular disease incidence17, and thus clinicians and physicians have primarily focused on SBP in hypertension management18. SBP and DBP reflect distinctive hemodynamics and pathophysiologic features associated with specific BP components19, as DBP tends to decline while SBP continues to increase in later life20. Therefore, investigating the effect of PM2.5 exposure on SBP and DBP separately is important to understand the distinct impacts on cardiovascular health and tailor preventive measures and interventions accordingly, but investigation of the difference has been limited.
Additionally, non-Hispanic Black Americans, an underrepresented population in biomedical research, bear a disproportionate burden of air pollution and hypertension in the US. Non-Hispanic Black Americans have been found to experience higher rates of hypertension and related complications21,22,23, and they often face disproportionate exposure to PM2.5 and other environmental pollutants due to socioeconomic factors, residential segregation, and environmental injustice24,25. Understanding the relationship between PM2.5 exposure and BP in this population can provide valuable insights into the mechanisms underlying health disparities and inform targeted interventions to mitigate the adverse effects of air pollution on cardiovascular health in non-Hispanic Black communities.
Within this context, we conducted this study to investigate the individual associations of ambient PM2.5 exposure with SBP and DBP. We took a duration-specific approach to examine the potential differing sensitive windows of SBP and DBP in relation to PM2.5 exposure, with an exposure duration from 1 to 36 months. Notably, the study leverages data from the Chicago Multiethnic Prevention and Surveillance Study (COMPASS), a predominantly non-Hispanic Black cohort that seeks to uncover the causes of health disparities.
Methods
Study population
COMPASS is a large, longitudinal cohort study. A more detailed description of the study design can be found elsewhere26. Briefly, eligibility for COMPASS includes: (1) age 18 years or older at the time of enrollment; (2) ability to consent and provide survey data in English or Spanish; (3) willingness to provide blood, urine, and saliva samples as well as electronic health record access. Over the past decade of enrollment, COMPASS has employed multiple recruitment modalities, including a population-based approach, a community-based approach, and a hospital/clinic-based approach. During their first visit to the research clinic, participants completed a survey questionnaire. Anthropometry and blood pressure are then measured. The COMPASS questionnaire codebook can be found at https://compass.uchicago.edu/research/self-reported-questionnaire-data/.
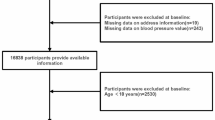
Between 2013 and 2019, 7728 participants were recruited. Their residential addresses were collected at enrollment and have been verified and geocoded by our research staff. Addresses that could not be geocoded, for example, P.O. boxes or missing information on street number, were excluded.
The University of Chicago Institutional Review Board granted approval for this study and written informed consent was obtained from all participants. This study leverages data and BP measures collected during participants’ visit to the research clinic at enrollment. Therefore, the study is cross-sectional in nature.
Blood pressure and hypertension
SBP and DBP were measured by trained staff using an Omron HEM 907XL blood pressure monitor. Staff were trained to allow participants to rest in a sitting position for five minutes before measurement and to ask participants to sit upright with both feet flat on the floor during measurement. Average SBP and DBP were generated from two readings. Pulse pressure was calculated.
We used the 140/90 mmHg criterion to define hypertension in the main analysis27. Based on the measured BP, three types of hypertensions were defined: (a) isolated systolic hypertension (ISH), if SBP ≥ 140 and DBP < 90; (b) isolated diastolic hypertension (IDH), if SBP < 140 and DBP ≥ 90; and (c) systolic–diastolic hypertension (SDH), if SBP ≥ 140 or DBP ≥ 90.
PM2.5 exposure assessment
Ambient PM2.5 exposure data was obtained from the Atmospheric Composition Analysis Group at Washington University at St. Louis. Monthly surface PM2.5 levels for 1998–2021 at a 0.01° × 0.01° scale (i.e., ~ 1.1 km) were estimated by combining Aerosol Optical Depth retrievals from the NASA Moderate Resolution Imaging Spectroradiometer (MODIS), Multi-angle Imaging SpectroRadiometer (MISR), and Sea-viewing Wide Field-of-view Sensor (SeaWiFS) instruments with the GEOS-Chem chemical transport model, and subsequently calibrating to global and North America ground-based observations using a Geographically Weighted Regression. The resultant values showed great cross-validated agreement when all data sources are used (\({R}^{2}=0.99\))28,29,30.
We retrospectively assigned the PM2.5 exposure to each participant according to their residential addresses. Cumulative average PM2.5 levels were generated over different exposure durations, from 1 to 36 months prior to the date when they visited our research clinics.
Potential confounders
Potential confounders were selected based on prior publications in this cohort31,32. Accordingly, potential confounders adjusted in the final analysis included age in categories (≤ 35, 36–45, 46–55, 56–65, > 65), race/ethnicity (non-Hispanic White, non-Hispanic Black, other), gender (male, female), education (less than high school, high school, some college, college or more), household income (< $15,000, $15,000–$34,999, $35,000–$69,999 and > $69,999), neighborhood area deprivation index (ADI), body mass index (BMI; < 25, 25–29.9, 30–39.9, > 39.9), heart attack history (yes, no), type 2 diabetes (yes, no), smoking status (non-smoker, former smoker, current smoker), insurance status (not insured, Medicare, Medicaid, private, other), and season (spring, summer, autumn, winter). Age, race/ethnicity, gender, education, household income, heart attack history, and season were collected through questionnaires. ADI is a composite score to measure neighborhood disadvantages. The ADI score was created based on 17 neighborhood socioeconomic variables, such as poverty level, unemployment rate, and home value, at the census tract level using principal component analysis33. Therefore, ADI is able to reflect the overall neighborhood socioeconomic status (SES) and has been widely used in health research. As neighborhood SES can affect both air pollution level and residents’ health, it is considered as a confounder in this study. Therefore, we adjusted ADI to represent the neighborhood SES in our model. BMI and type 2 diabetes status were ascertained when participants visited our research clinic.
Statistical analysis
We used mixed effects models to assess the association of PM2.5 exposure with BP levels and different hypertension types. Linear regression was used to estimate the average change (β) in the BP levels and the corresponding 95% confidence interval (CI). Logistic regression was used to estimate the odds ratio (OR) for hypertension types (ISH, IDH, SDH, and any hypertension) compared to the non-hypertensive group and the corresponding 95% CI. The average PM2.5 exposure concentration level was treated as a continuous variable in the model and results should be interpreted for each 1 µg/m3 increase in PM2.5 level. The average PM2.5 concentration levels of different exposure durations were put in the model as separate variables. To further eliminate potential confounding arising from spatial variation, we included a random intercept for the residential zip code in the model. A total of 130 unique zip codes were identified, among which 20 zip codes had over 100 participants and 47 zip codes only had 1 participant. The median number of the number of participants in the zip code was 2. We then described the trend of effect estimates across different exposure durations using a smoothing line generated from LOESS34. To examine the non-linear association between PM2.5 exposure and BP, we included spline terms for PM2.5 exposure of the duration that demonstrated the most sensitive association with SBP and DBP, respectively.
To understand how the impacts of PM2.5 exposure on SBP and DBP interact with each other, we stratified the population according to hypertension status (ISH, IDH, SDH, and any), and then ran the regression for SBP and DBP again in these stratified populations.
The models were adjusted for all aforementioned potential confounders. Missing values were noted in some covariates, particularly for household income and smoking status. The overall prevalence of missing values were less than 15% in the study population. Missing values in potential confounders were assumed to be missing at random and then imputed using random forest imputation algorithm35. All variables were used to generate the imputed data. Ten complete datasets were generated via imputation and combined using Reiter’s partially synthetic data pooling rules36.
We conducted several sensitivity analyses. First, given that hypertension medications are usually used to control BP, we also ran analyses after excluding participants who self-reported using hypertension medications. Second, we classified hypertension status using 130/80 mmHg for SBP/DBP, as suggested by the guideline updated in 201737.
All analyses were performed in accordance with the IRB guidelines and regulations. All statistical analysis in this study was performed using R 4.3.0 (R Core Team, Vienna, Austria).
Results
A total of 6381 participants with valid residential addresses and blood pressure measures were analyzed in this study. The distributions of selected sociodemographic and health characteristics are presented in Table 1. The overall study population was comprised of predominantly non-Hispanic Black individuals (82.1%), more than 45% were above 55 years of age, and over half reported an annual household income less than $15,000 and received high school education or less. A third of the study population was obese (BMI ≥ 30). SDH was the most common hypertension type, followed by ISH, while IDH was noted as the least common type in this study population. Individuals with SDH had higher average SBP and DBP than individuals with ISH or IDH (a more detailed distribution of BP can be found in Table S1). No substantial difference in the demographic and socioeconomic characteristics was observed between different hypertension groups, but it should be noted that IDH group had the lowest prevalence of type 2 diabetes and heart attack history.
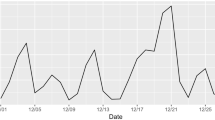
Figure 1 presents the distribution of PM2.5 exposure levels of different exposure durations in this study (original values in Table S2). The average PM2.5 exposure level was close to 9 µg/m3 and remained similar across these exposure durations.
Figure 2 shows the association between PM2.5 exposure and changes in SBP across different exposure durations (original values in Table S3). In the overall population, the association steadily strengthened from null to significantly positive when the exposure duration increased (Fig. 2A). Within a duration of 10 months, PM2.5 exposure was not associated with SBP. However, beyond 10 months, we observed associations between PM2.5 exposure and SBP, and the magnitude of this association plateaued when the duration reached 24 months. Specifically, the association was β = 0.66 (95% CI: 0.07, 1.25) for 12-month duration, strengthened to β = 0.85 (95% CI: 0.26, 1.43) for 18-month duration, and further to β = 1.14 (95% CI: 0.54, 1.73). Beyond 24 months, the association magnitudes lingered within a range roughly between 1.10 and 1.20.
Average change in systolic blood pressure associated with PM2.5 exposure of different exposure durations in linear regression. The grey area indicates 95% confidence interval. The blue curve is the smoothing line generated from LOESS that describes the trend of effect estimates over these exposure durations. The mixed effect models were adjusted for age (35–39, 40–44, 45–49, 50–64, 65+), race (non-Hispanic White, non-Hispanic Black, Hispanic, other), gender (male, female), education (less than high school, high school, some college, college or more), household income (< $15,000, $15,000–$24,999, $25,000–$34,999 and > $34,999), body mass index (BMI; < 25, 25–29.9, 30–39.9, > 39.9), smoking status (never, former, current), health insurance status (uninsured, Medicaid, Medicare, private, other), type 2 diabetes (yes, no), and heart attack history (yes, no), and neighborhood area deprivation index (ADI, in quartiles).
When looking at the association with SBP by hypertension status, we observed completely different patterns. PM2.5 exposure was not associated SBP in non-hypertensive participants across these exposure durations (Fig. 2B); however, a positive association with SBP was observed in hypertensive participants (Fig. 2C). The positive association in hypertensive participants was even observed within short durations and then strengthened when exposure duration increased. For example, in hypertensive participants, association was β = 0.48 (95% CI: 0.09, 0.87) for 1-month duration, and strengthened to β = 1.79 (95% CI: 0.96, 2.62) for 12-month duration. This association appeared to plateau beyond a duration of 24 months. The positive association was not observed in participants with ISH or SDH, among whom the association was close to null (Fig. 2D,F, respectively). In contrast, a positive association was observed in participants with IDH (Fig. 2E), and demonstrated a pattern similar to that in the overall hypertensive participant.
Figure 3 shows the results for DBP (original values in Table S4), which reveal a differing pattern from SBP, especially in populations with different hypertension status. In the overall population, PM2.5 exposure was positively associated with higher DBP across all exposure durations (Fig. 3A). The association strengthened as duration increased. Beyond 12 months, the association magnitude plateaued, remaining within a range roughly between 1.60 and 1.80.
Average change in diastolic blood pressure associated with PM2.5 exposure of different exposure durations in linear regression. The grey area indicates 95% confidence interval. The blue curve is the smoothing line generated from LOESS that describes the trend of effect estimates over these exposure durations. The mixed effect models were adjusted for age (35–39, 40–44, 45–49, 50–64, 65+), race (non-Hispanic White, non-Hispanic Black, Hispanic, other), gender (male, female), education (less than high school, high school, some college, college or more), household income (< $15,000, $15,000–$24,999, $25,000–$34,999 and > $34,999), body mass index (BMI; < 25, 25–29.9, 30–39.9, > 39.9), smoking status (never, former, current), health insurance status (uninsured, Medicaid, Medicare, private, other), type 2 diabetes (yes, no), and heart attack history (yes, no), and neighborhood area deprivation index (ADI, in quartiles).
We also observed positive associations with DBP across different exposure durations in both hypertensive and non-hypertensive populations (Fig. 3B,C). In both populations by hypertensive status, the associations strengthened when duration increased and plateaued roughly beyond 12 months, though a slight declining trend was noted beyond 24 months in the hypertensive population. The association was stronger in the hypertensive population. By hypertension subtypes, the positive association with DBP was observed in participants with ISH and SDH (Fig. 3D,F), but not in participants with IDH (Fig. 3E). In participants with ISH, the association exhibited a declining trend beyond 12 months, while in participants with SDH, the association remained steady beyond 18 months. However, the association with DBP was null and remained unchanged across these durations in participants with IDH.
When using 24-month and 12-month PM2.5 exposure for SBP and DBP in non-linear analysis, respectively, we observed slight non-linear trends for the PM2.5 impacts (Fig. S1). However, these non-linear associations were monotonic and we did not observe extreme changes in the PM2.5 impacts.
PM2.5 was inversely associated with pulse pressure across different exposure durations, in the overall population and in populations stratified by hypertension status as well (Fig. S2). The associations with pulse pressure demonstrated similar trend to DBP. The association strengthened as duration increased and then plateaued beyond 12 months.
PM2.5 exposure was inversely associated with ISH across different exposure durations (Fig. 4A). The inverse association exhibited a U-shaped trend across these exposure durations, as it started with OR = 0.91 (95% CI: 0.87, 0.95) for 1-month duration, strengthened to OR = 0.76 (95% CI: 0.70, 0.83) for 15-month duration, and ultimately weakened to OR = 0.82 (95% CI: 0.76, 0.89) for 36-month duration. In contrast, positive associations were observed for IDH and SDH (Fig. 4B,C). These positive associations strengthened when exposure duration increased, but plateaued at different time points, with IDH showing a shorter plateauing duration. The association with IDH started with OR = 1.06 (95% CI: 1.00, 1.12) for 1-month duration, strengthened to OR = 1.20 (95% CI: 1.07, 1.34) for 12-month duration, and then stayed roughly unchanged beyond. In comparison, the association with SDH started with OR = 1.02 (95% CI: 0.99, 1.05) for 1-month duration, and strengthened to OR = 1.18 (95% CI: 1.10, 1.26) and further to OR = 1.22 (95% CI: 1.13, 1.31) for 24-month duration. We observed a positive association with any hypertension types that was close to null within short durations (Fig. 4D).
Odds ratio for hypertension according to PM2.5 exposure of different exposure durations in logistic regression. The grey area indicates 95% confidence interval. The blue curve is the smoothing line generated from LOESS that describes the trend of effect estimates over these exposure durations. The mixed effect models were adjusted for age (35–39, 40–44, 45–49, 50–64, 65+), race (non-Hispanic White, non-Hispanic Black, Hispanic, other), gender (male, female), education (less than high school, high school, some college, college or more), household income (< $15,000, $15,000–$24,999, $25,000–$34,999 and > $34,999), body mass index (BMI; < 25, 25–29.9, 30–39.9, > 39.9), smoking status (never, former, current), health insurance status (uninsured, Medicaid, Medicare, private, other), type 2 diabetes (yes, no), and heart attack history (yes, no), and neighborhood area deprivation index (ADI, in quartiles).
In sensitivity analysis that excluded participants using hypertension medication, we observed consistent results with our main analysis, though the confidence intervals were wider, possibly due to smaller sample size (Figs. S3 and S4). Moreover, when using the 2017 hypertension guidelines, we also observed consistent results with our analysis (Figs. S5–S7). Briefly, we observed a positive association between PM2.5 exposure only in patients with IDH, and positive associations of PM2.5 exposure with IDH, but not with ISH.
Discussion
In this predominantly non-Hispanic Black cohort that represents a population historically underrepresented in biomedical research, we employed a duration-specific approach to analyze the association of PM2.5 exposure with SBP, DBP, and different hypertension types. The results suggested that DBP is more sensitive to PM2.5 exposure compared to SBP, as the effect of PM2.5 exposure reached the plateau in a shorter duration for DBP. Moreover, we observed that the associations with SBP and DBP were evident in different populations. Furthermore, PM2.5 demonstrated positive associations with IDH and SDH, not ISH. Overall, findings from this study support differing effects of PM2.5 exposure on SBP and DBP.
One highlight of our findings in this study is the different exposure durations for PM2.5 exposure effect to reach the plateau for SBP and DBP. Results from an early study actually imply the presence of this difference. In a population-based study in India with exposure assessed using a satellite-based model, Prabhakaran et al. took a similar duration-specific approach and presented the associations of PM2.5 exposure with SBP and DBP within a 1-year exposure window, but they only included five different durations9. Their results clearly showed that the association with SBP strengthened as the duration increased, while the association with DBP remained steady despite a slight increase at the beginning of the duration period9. Therefore, exposure duration appears to be key in understanding the differing effects of PM2.5 exposure on SBP and DBP. However, the differing effects were not the primary focus of their study and thus they did not provide more analysis regarding the different exposure durations for specific effect plateaus. Additionally, although many studies incorporated both short- and long-term exposures in their research and reported different effects of these exposures6,8,9,10, the differing effects on SBP and DBP were generally overlooked due to a lack of duration-specific analysis at finer resolution similar to ours. Our study overcomes limitations of these prior studies by systematically examining the duration-specific effects, adding evidence to the differing effects of PM2.5 exposure on SBP and DBP.
Another intriguing observation is that the association with SBP was evident only in participants with ISH, while the association with DBP was evident in participants with IDH or SDH. Considering the distinct contributions of SBP and DBP to the cardiovascular system15, one plausible explanation for these findings is that the effects of PM2.5 exposure on SBP and DBP are independent of each other, and the effect on DBP is more enduring. Elevated SBP is influenced by factors such as arterial stiffness, cardiac contractility, and the ejection of blood from the heart38. PM2.5 exposure may contribute to increased arterial stiffness and impaired vascular function39, which can lead to higher SBP. DBP, on the other hand, depends on factors such as peripheral vascular resistance, arterial compliance, and the relaxation of the heart40. PM2.5 exposure can impact these factors differently than those affecting SBP. For example, PM2.5-induced inflammation and oxidative stress may impair the relaxation of blood vessels41, leading to elevated DBP. Understanding the differing effects and underlying biological mechanisms of PM2.5 exposure on SBP and DBP is important for accurately assessing the cardiovascular risks associated with air pollution exposure.
Additionally, we observed that PM2.5 exposure was only associated with hypertension types characterized by high DBP (i.e., IDH and SDH), not with ISH. This observation reflects the different mechanisms underlying these conditions, and, again, suggests that DBP is more sensitive to PM2.5 exposure. It also should be noted that we observed an inverse association with ISH. This inverse association may not imply protective effects of PM2.5 exposure. One possible explanation is that, as DBP is more sensitive, PM2.5 exposure would initially lead to IDH that constitutes a competing event against ISH in regression models. As a result, we observed an inverse association with ISH.
This study stands out as a large-scale study that utilize a duration-specific approach to investigate the health impact of PM2.5 exposure among a diver population. The air pollution exposure is also based on a high-resolution, well-characterized approach. Moreover, the blood pressure was measured objectively, overcoming potential recall bias. However, there are several limitations to consider in this study. First, the study population may not be nationally representative, nor was it designed to be geographically representative. A focus on historically underrepresented populations can be a strength, but also leads to limited generalizability. Since non-Hispanic Black Americans are more vulnerable to the adverse effect of PM2.5 exposure as suggested by prior studies, the strong effects observed in this study may not apply to other cohorts. Second, the air pollution exposure level was satellite-derived, which cannot accurately reflect the true exposure. Measurement error should be considered when interpreting the results. However, we expect bias from the measurement error was non-differential. Moreover, the air pollution level and variation are low in our study, as most exposure levels fell between 8 and 12 µg/m3. Therefore, results in this study may not be applied to more polluted areas. Third, we did not investigate the non-linear effects of PM2.5 exposure for all durations, but the most sensitive durations for SBP and DBP. Based on our non-linear analysis for the most sensitive durations, we do not expect the non-linear effect of PM2.5 exposure would substantially alter our results. Fourth, this study is cross-sectional in nature. BP was only measured at a single time point. We are unable to investigate how PM2.5 exposure affects changes in blood pressure over time and how these changes reciprocally influence PM2.5 exposure effects. However, the exposure was assigned prior to participants’ enrollment, and thus reverse causation was not possible. Last, the study did not account for changes in residential address. An ideal approach is to collect accurate data on individuals’ mobility and adjust for mobility in analysis. However, this approach requires substantial resources and may breach individual privacy. Environmental epidemiology studies have demonstrated the validity of exposure assessment based on current residential address42,43,44. Therefore, though misclassification cannot be ruled out, we are confident that our estimates of the joint impact of neighborhood context are valid.
Overall, the study took a duration-specific approach to examine the association of PM2.5 exposure on SBP and DBP, and provides many interesting findings, most of which are reported in the literature for the first time. The magnitudes of associations with SBP and DBP were dependent on exposure durations and the association with DBP plateaued within a shorter duration. We also observed that PM2.5 exposure was more likely to increase the risk for hypertension types characterized by high DBP. The findings from this study support differing effect of PM2.5 exposure on SBP and DBP.
Data availability
The datasets generated and analyzed during the current study are not publicly available due to its inclusion of private medical and residential data, but are available from the corresponding author on reasonable request.
Abbreviations
- BP:
-
Blood pressure
- CI:
-
Confidence interval
- COMPASS:
-
Chicago Multiethnic Prevention and Surveillance Study
- DBP:
-
Diastolic blood pressure
- IDH:
-
Isolated diastolic hypertension
- ISH:
-
Isolated systolic hypertension
- OR:
-
Odds ratio
- SBP:
-
Systolic blood pressure
- SDH:
-
Systolic–diastolic hypertension
- SES:
-
Socioeconomic status
References
Landrigan, P. J. et al. The Lancet Commission on pollution and health. Lancet 391(10119), 462–512 (2018).
Rajagopalan, S., Al-Kindi, S. G. & Brook, R. D. Air pollution and cardiovascular disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 72(17), 2054–2070 (2018).
Danaei, G. et al. Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardiometabolic risk factors from 1980 to 2010: A comparative risk assessment. Lancet Diabetes Endocrinol. 2, 634–647 (2014).
Yang, B.-Y. et al. Global association between ambient air pollution and blood pressure: A systematic review and meta-analysis. Environ. Pollut. 235, 576–588 (2018).
Cai, Y. et al. Associations of short-term and long-term exposure to ambient air pollutants with hypertension: A systematic review and meta-analysis. Hypertension 68(1), 62–70 (2016).
Liang, R. et al. Effect of exposure to PM2.5 on blood pressure: A systematic review and meta-analysis. J. Hypertens. 32(11), 2130–2141 (2014).
Zhang, H. et al. A study of the association between atmospheric particulate matter and blood pressure in the population. Blood Pressure 25(3), 169–176 (2016).
Song, J. et al. Association of long-term exposure to PM2.5 with hypertension prevalence and blood pressure in China: A cross-sectional study. BMJ Open 11(12), e050159 (2021).
Prabhakaran, D. et al. Exposure to particulate matter is associated with elevated blood pressure and incident hypertension in urban India. Hypertension 76(4), 1289–1298 (2020).
Lv, S. et al. Long-term effects of PM2.5 components on hypertension: A national analysis in China. Environ. Res. 222, 115323 (2023).
Tandon, S. et al. Association of ambient air pollution with blood pressure in adolescence: A systematic-review and meta-analysis. Curr. Probl. Cardiol. 48(2), 101460 (2023).
Zheng, C. et al. Left ventricular diastolic dysfunction and cardiovascular disease in different ambient air pollution conditions: A prospective cohort study. Sci. Total Environ. 831, 154872 (2022).
Ohlwein, S. et al. Air pollution and diastolic function in elderly women—Results from the SALIA study cohort. Int. J. Hygiene Environ. Health 219(4–5), 356–363 (2016).
Borlaug, B. A. & Redfield, M. M. Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum. Circulation 123(18), 2006–2014 (2011).
Flint, A. C. et al. Effect of systolic and diastolic blood pressure on cardiovascular outcomes. N. Engl. J. Med. 381(3), 243–251 (2019).
Rutan, G. H., McDonald, R. H. & Kuller, L. H. A historical perspective of elevated systolic vs diastolic blood pressure from an epidemiological and clinical trial viewpoint. J. Clin. Epidemiol. 42(7), 663–673 (1989).
Yano, Y. et al. Isolated diastolic hypertension and risk of cardiovascular disease: Controversies in hypertension-pro side of the argument. Hypertension 79(8), 1563–1570 (2022).
Izzo, J. L. Jr., Levy, D. & Black, H. R. Importance of systolic blood pressure in older Americans. Hypertension 35(5), 1021–1024 (2000).
Yano, Y. et al. Hemodynamic and mechanical properties of the proximal aorta in young and middle-aged adults with isolated systolic hypertension: The Dallas Heart Study. Hypertension 70(1), 158–165 (2017).
Mitchell, G. F. et al. Hemodynamic correlates of blood pressure across the adult age spectrum: Noninvasive evaluation in the Framingham Heart Study. Circulation 122(14), 1379–1386 (2010).
Graham, G. Disparities in cardiovascular disease risk in the United States. Curr. Cardiol. Rev. 11(3), 238–245 (2015).
Benjamin, E. J. et al. Heart disease and stroke statistics—2019 update: A report from the American Heart Association. Circulation 139(10), e56–e528 (2019).
Cefalu, W. T. & Golden, S. H. Innovative approaches to understanding and addressing health disparities in diabetes care and research. Diabetes Care 38(2), 186 (2015).
Woo, B. et al. Residential segregation and racial/ethnic disparities in ambient air pollution. Race Soc. Probl. 11, 60–67 (2019).
Tessum, C. W. et al. PM2.5 polluters disproportionately and systemically affect people of color in the United States. Sci. Adv. 7(18), eabf4491 (2021).
Aschebrook-Kilfoy, B. et al. Cohort profile: The ChicagO Multiethnic Prevention and Surveillance Study (COMPASS). BMJ Open 10(9), e038481 (2020).
Zhou, B. et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 398(10304), 957–980 (2021).
Van Donkelaar, A. et al. Monthly global estimates of fine particulate matter and their uncertainty. Environ. Sci. Technol. 55(22), 15287–15300 (2021).
Hammer, M. S. et al. Global estimates and long-term trends of fine particulate matter concentrations (1998–2018). Environ. Sci. Technol. 54(13), 7879–7890 (2020).
Van Donkelaar, A. et al. Regional estimates of chemical composition of fine particulate matter using a combined geoscience-statistical method with information from satellites, models, and monitors. Environ. Sci. Technol. 53(5), 2595–2611 (2019).
Luo, J. et al. Urban spatial accessibility of primary care and hypertension control and awareness on Chicago’s south side: A study from the COMPASS cohort. Circulation 15(9), e008845 (2022).
Luo, J. et al. The impact of neighborhood disadvantage on asthma prevalence in a predominantly African American Chicago-based cohort. Am. J. Epidemiol. 192, 549–559 (2023).
Kind, A. J. & Buckingham, W. R. Making neighborhood-disadvantage metrics accessible—The neighborhood atlas. N. Engl. J. Med. 378(26), 2456 (2018).
Cleveland, W. S. Robust locally weighted regression and smoothing scatterplots. J. Am. Stat. Assoc. 74(368), 829–836 (1979).
Shah, A. D. et al. Comparison of random forest and parametric imputation models for imputing missing data using MICE: A CALIBER study. Am. J. Epidemiol. 179(6), 764–774 (2014).
Reiter, J. P. Inference for partially synthetic, public use microdata sets. Surv. Methodol. 29(2), 181–188 (2003).
Carey, R. M., Whelton, P. K., AAHGW Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: Synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension Guideline. Ann. Intern. Med. 168(5), 351–358 (2018).
Franklin, S. S. Beyond blood pressure: Arterial stiffness as a new biomarker of cardiovascular disease. J. Am. Soc. Hypertens. 2(3), 140–151 (2008).
Sun, D. et al. Long-term effects of fine particulate matter exposure on the progression of arterial stiffness. Environ. Health 20(1), 1–7 (2021).
McEvoy, J. W. et al. Diastolic blood pressure, subclinical myocardial damage, and cardiac events: Implications for blood pressure control. J. Am. Coll. Cardiol. 68(16), 1713–1722 (2016).
Xing, Y.-F. et al. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 8(1), E69 (2016).
Gehring, U. et al. Air pollution exposure and lung function in children: The ESCAPE project. Environ. Health Perspect. 121(11–12), 1357–1364 (2013).
Liu, Y. et al. Short-term exposure to ambient air pollution and asthma mortality. Am. J. Respir. Crit. Care Med. 200(1), 24–32 (2019).
Héritier, H. et al. A systematic analysis of mutual effects of transportation noise and air pollution exposure on myocardial infarction mortality: A nationwide cohort study in Switzerland. Eur. Heart J. 40(7), 598–603 (2019).
Acknowledgements
We thank the dedicated COMPASS field staff and community partners for their support of this work.
Funding
This research was supported by funding from NIH grants P30ES027792 and U2RTW010122, and the University of Chicago Medicine Comprehensive Cancer Center.
Author information
Authors and Affiliations
Contributions
J.L., R.R.J, and B.A conceived the research concept. J.L. and Z.J performed the data analysis and created the figures. T.P, K.K., C.O.O., and J.P. provided clinic support for this study. H.A. and B.A. supervised the study and acquired research funding. J.L. and B.A. drafted the study. All authors read the manuscript and provided critical reviews for the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Luo, J., Jones, R.R., Jin, Z. et al. Differing associations of PM2.5 exposure with systolic and diastolic blood pressures across exposure durations in a predominantly non-Hispanic Black cohort. Sci Rep 14, 20256 (2024). https://doi.org/10.1038/s41598-024-64851-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-64851-6
- Springer Nature Limited