Abstract
Ethnomedicinal plants are thought to have better prospects of harboring endophytes that produce natural products with pharmacological activities. This study aimed to investigate the antiplasmodial and anticancer properties of secondary metabolites of endophytic fungi from three medicinal plants. The endophytic fungi included Lasiodiplodia theobromae isolated from Cola acuminata, Curvularia lunata Bv4 isolated from Bambusa vulgaris, and Curvularia lunata Eg7 isolated from Elaeis guineensis. The identification of the fungi was based on the internal transcribed spacer (ITS-rDNA) sequence. The fungi were subjected to solid-state fermentation and the secondary metabolites were extracted with ethyl acetate. In vitro antiplasmodial screening of extracts was performed using the SYBR green I-based fluorescence assay on the chloroquine-resistant Plasmodium falciparum strain DD2. The cytotoxicity of the extracts on human red blood cells and Jurkat (leukemia) cells was assessed using the tetrazolium-based colorimetric MTT assay. Gas chromatography-mass spectrometry (GC–MS) analysis was used to identify the constituents of the fungal extracts. The extract of L. theobromae showed the best antiplasmodial activity against chloroquine-resistant P. falciparum (IC50 = 5.4 µg/mL) and was not harmful to erythrocytes (CC50 > 100 µg/mL). All three fungal extracts showed a weak cytotoxic effect against Jukart cell lines (CC50 > 100 µg/mL). GC–MS analysis of the three endophytic fungal extracts revealed the presence of forty major bioactive compounds, including: oxalic acid, isobutyl nonyl ester, 2,4-di-tert-butylphenol, and hexadecanoic acid, among others. The endophytic fungi from the medicinal plants in this study were promising sources of bioactive compounds that could be further evaluated as novel drugs for the treatment of malaria caused by P. falciparum-resistant strains.
Similar content being viewed by others
Introduction
Malaria, which is caused by the parasite Plasmodium falciparum in humans, remains a major cause of death and morbidity in the world, with an estimated 627,000 deaths in 2020, which is 69,000 more deaths than the previous year1. The most affected populations are in tropical and subtropical areas, particularly in Sub-Saharan Africa and Southeast Asia, where P. falciparum causes over 80% of malaria cases2. The recent spread of the pandemic drug-resistant P. falciparum necessitates the urgent search for alternatives and new drugs to treat malaria. Due to their high failure rate, chloroquine (CQ) and sulfadoxine-pyrimethamine (SP) are no longer considered first-line treatments for malaria in some parts of Africa, particularly in Nigeria3. On the other hand, cancer prevalence is estimated to have risen to 9.6 million deaths and 18.1 million cases worldwide in 20184. Cancer and its associated diseases are regarded as a global serious healthcare burden 5, with the majority of deaths occurring in low and middle-income African, Asian, Central, and South American countries6.
Natural products continue to be a reliable source for drug development against malaria and cancer, with quinine/artemisinin and taxol being the best examples2,7. Therefore, the exploration of natural resources for drug discovery remains one of the most promising scientific approaches to identify new therapeutic compounds. Microorganisms are enormously diverse compared to other natural sources such as plants, but have not yet been sufficiently explored. Since microorganisms naturally coexist with plants, it is difficult to determine whether the promising metabolites are produced by the plants themselves or by the microorganisms living in plant tissues. Nevertheless, studies suggest that only about 1% of bacteria and 5% of fungi have been characterized, leaving the rest unexplored for their potential to produce novel drugs against a variety of diseases8. One of these understudied groups that is gaining attention due to its potential for drug discovery is endophytic fungi associated with medicinal plants. Endophytic fungi are found in the various tissues of plants, including seeds, flowers, leaves, fruits, and roots. Remarkably, these fungi coexist with the host plants without causing signs of disease9. Increased phytohormone levels and better nutrient uptake by plants are direct benefits of using endophytic fungi. These effects expand the root system, increase biomass production, and increase plant height and plant weight10. Fungal endophytes have recently attracted the interest of scientists due to their ability to synthesize new compounds that can be used as antiplasmodial and anticancer drugs11,12,13. According to Ferreira et al.14, some of these compounds have better activity than most commercially available treatment options. Endophytes are thought to be metabolically more active than free members and have the ability to activate several metabolic pathways necessary for host survival15. In developing countries, more than 80% of the population use medicinal plants to meet their major health needs and treatments16. The study of endophytic fungi associated with these ethnomedicinal plants may therefore lead to the development of new therapeutic approaches.
Cola acuminata trees are characterized by their evergreen nature and can reach a height of up to 20 m17. Their ovoid leaves are glossy and grow up to 30 cm long. C. acuminata is a genus of bitter-tasting trees native to the tropical rainforests of Africa, belonging to the Malvaceae family with about 125 species17. An earlier study by Heroine et al.18 focused on studying the antifungal potential of endophytic fungi found in the tissues of C. acuminata. The endophytic fungi found in C. acuminata demonstrated antifungal effects against Candida krusei ATCC 6258, Candida parapsilosis ATCC 22019, and Candida albicans NR-29450. In another study by Hzounda Fokou et al.19, endophytic fungi: Trichoderma afroharzianum and Trichoderma harzianum from C. acuminata showed antibacterial and antioxidant effects.
The Arecaceae family includes two species of tropical perennial plants known as the oil palm trees: Elaeis guineensis, which is native to West Africa, and Elaeis oleifera, which is found in tropical Central and South America20. The production of palm oil in commercial agriculture relies on the use of both species20. The E. guineensis tree is highly valued in traditional medicine, as its various parts are used to treat skin infections, relieve headaches, and promote wound healing20. In a previous study by Yurnaliza et al.21, endophytic fungi isolated from E. guineensis were able to secrete exopolysaccharides.
Tabashir, scientifically known as Bambusa vulgaris thrives in tropical and subtropical climates. It belongs to the Poaceae family and has antimicrobial, antioxidant, and anti-inflammatory properties22. To date, the antimicrobial and antioxidant effects of the endophytic fungi isolated from the B. vulgaris plant have not yet been investigated. This study aimed to evaluate the antiplasmodial and anticancer effects of endophytic fungi isolated from the leaves of the medicinal plants C. acuminata, E. guineensis, and B. vulgaris for the first time.
Materials and methods
Ethics statement
This study was approved by the Renaissance University, Enugu, Nigeria. This study did not include any experiments on human participants and animals. All methods were carried out in accordance with relevant guidelines in the method section. The use of E. guineensis, C. acuminata, and B. vulgaris does not violate the local regulations of Nigeria. During the sample collection from a specific plant, permission was taken from the Renaissance University, Enugu, Nigeria. Plant materials were collected according to institutional, national, and international guidelines and legislation.
Collection of plant samples
Leaf samples of C. acuminata and B. vulgaris were collected from mature, healthy plants in Ogbeke Agbani (latitude 6° 19′ 0″ N, longitude 7° 33′ 0″ E), Enugu State, Nigeria during June and October 2021. E. guineensis was related to our previous study23. The botanical specimens were authenticated by a medicinal chemist: Dr. Festus Basden Chiedu Okoye and deposited in the herbarium of the Department of Pharmacognosy and Traditional Medicine, Faculty of Pharmaceutical Sciences, Nnamdi Azikiwe University, Nigeria with the following herbarium numbers: E. guineensis (PCG499/A/040), C. acuminata (PCG499/A/041), and B. vulgaris (PCG499/A/042). E. guineensis (PCG499/A/040) was prepared from our previous study23. Healthy and freshly collected leaves were used for the isolation of endophytic fungi to reduce the risk of contamination.
Isolation and identification of endophytic fungi
Endophytic fungi from plant leaves were isolated aseptically as described by Nwobodo et al.24. Before the plant leaves were cut into small pieces, they were thoroughly washed under running tap water. To ensure surface sterilization, the leaf pieces were immersed in a 2% sodium hypochlorite solution for 2 min, followed by immersion in 70% ethanol for almost 2 min. A final rinse in sterile water for 5 min completed the process. The leaf pieces were cultured on malt extract agar (MEA) medium (Merck, Germany) containing chloramphenicol and placed in an incubator at 27 °C, allowing observation of fungal growth isolated from leaf pieces24. The identification of the isolated endophytic fungi was done by DNA amplification and sequencing of the ITS region25,26. The endophytic fungi were identified using the Basic Local Alignment Search Tool (BLAST) N sequence alignment procedure to compare the amplified sequence with ITS sequence data of strains available in the US National Center for Biotechnology Information (NCBI) database 26.
Fermentation and extraction of secondary metabolites
In this study, the fermentation process was used for the production of secondary metabolites. Secondary compounds are usually formed towards the end of fermentation, specifically in the idiophase, which follows the trophophase where active growth takes place27. Once the microbial growth cycle is complete, secondary metabolites are formed in the fermentation medium27. In a 1000 mL Erlenmeyer flask, approximately 100 g of rice was soaked in 200 mL of sterile water for 10 min. The flask and its contents were then autoclaved at 121 °C for 20 min26. They were then allowed to cool. Each isolated endophytic fungal strain was grown on this sterile solid rice medium at 27 °C for 21 days23. At the end of the incubation period, an approximate volume of 500 ml of ethyl-acetate was added to the culture flask and left undisturbed at room temperature overnight26. The extraction solvent chosen for this study was ethyl acetate, which is known for its medium polar nature and its ability to extract both less polar and slightly polar compounds28,29. Previous studies have proven that ethyl acetate-based extraction yielded high amounts of secondary metabolites, ultimately leading to remarkable antibacterial properties29. Under vacuum conditions by a Buchner funnel, the crude ethyl-acetate fungal mixture was passed through a Whatman filter paper No. 1 for filtration26. To obtain the secondary metabolites, the collected supernatants were concentrated at a reduced temperature of 50℃ using a rotary vacuum evaporator (R000101564, ST15 OSA, UK).
Gas chromatography-mass spectroscopy (GC–MS) analysis of endophytic fungal extracts
The fungal extracts were subjected to GC–MS analysis as previously described by Nwobodo et al.23. An Agilent 7820A gas chromatograph and an Agilent 5975C inert mass selective detector (MSD) with a triple-axis detector operating in electron impact (EI) mode with an ionization energy of 70 eV were used. The injection temperature of 300 °C was used for the diluted sample (1 μl, diluted 1:100 in dichloromethane), which was injected in splitless mode23. The carrier gas used in this experiment was helium flowing at a rate of 1 mL/min. It had an average velocity of 44.22 cm/sec and an initial nominal pressure of 1.4902 psi23. The run time was 43 min, during which the temperature was maintained at a rate of 5 °C per minute23. Some of the fungal extract's constituents were identified based on their GC retention time (RT) and a comparison of their mass spectra with those of the National Institute of Standards and Technology (NIST) mass spectral database30. The results of GC–MS of the fungal extract of the endophytic fungi isolated from E. guineensis were related to our previous study23.
Antiplasmodial activity of isolated endophytic fungal extracts
Source of Plasmodium parasite
The chloroquine-resistant P. falciparum strain (PfDD2) used for the in vitro study was acquired from the Department of Clinical Pathology, Noguchi Memorial Institute for Medical Research, Legon, Accra, Ghana.
In vitro culture of Plasmodium falciparum
The PfDD2 strain was maintained in continuous culture in fresh O+ve human erythrocytes suspended at 2% (v/v) hematocrit in complete medium consisting of RPMI (Roswell Park Memorial Institute) 1640 (Sigma) supplemented with 1% L-glutamine, 25 mM HEPES, 0.2% sodium bicarbonate, 0.5% Albumax II, 100 μM hypoxanthine, and 1% gentamicin incubated at 37 °C. To proliferate the culture, the used medium was replaced every day with fresh complete medium. Cell cycle transition and parasitemia were monitored by microscopic examination of Giemsa-stained blood smears under oil immersion.
Preparation of endophytic fungal extracts
Stock solutions of fungal extracts were prepared in dimethyl sulfoxide (DMSO) at 25 mg/mL, while artesunate was prepared in 50% ethanol and sterilized through a 0.22 μm filter membrane (Millipore). The required concentrations were achieved by diluting the stocks with incomplete RPMI 1640 medium. The final concentrations for each fungal extract and artesunate were 0.41–100 μg/mL and 0.03–6.3 nM, respectively. The solutions of drugs and extracts were added to 96-well flat-bottom tissue culture plates.
Antiplasmodial screening of extract by SYBR green I-based fluorescence assay
The SYBR green I-based fluorescence assay, as previously described by Ateba et al.2 and Ikem et al.31, was used for the preliminary antiplasmodial screening of the endophytic extracts. The infected red blood cells were incubated in the presence or absence of increasing concentrations of the 100 μl of the fungal extracts (from 3.125 μg/mL to 200 μg/mL) under normal culture conditions. Artesunate served as a positive control, while 0.4% DMSO (v/v), which has been shown to be non-toxic to the parasite, served as a vehicle control. The plates were covered and carefully mixed to ensure even distribution. Using the candle jar method, the microplates were incubated for 72 h at 37 °C in a modular chamber. After 72 h, 100 μl of SYBR Green 1 buffer [0.2 μl of 10,000 × SYBR Green I (Invitrogen) per mL of lysis buffer (Tris (20 mM; pH 7.5), EDTA (5 mM), saponin (0.008%; w/v), and Triton X-100 (0.08%; v/v)] was added to the wells, the contents were slowly mixed and incubated for 3 h in the dark. The Tecan fluorescence (Tecan Infinite M200, Austria) multi-plate reader was used to assess the fluorescence in each well at excitation and emission wavelengths of 485 and 530 nm, respectively. The intensity of the fluorescence signals was plotted against the drug concentrations to obtain a dose–response curve. The curve was analyzed using GraphPad Prism 6.01 (GraphPad Software Inc, San Diego, CA) to determine 50% inhibitory concentrations (IC50) of the drugs. In order to ensure accuracy, the experiments were repeated three times, and the means were calculated along with their standard deviations.
Screening of endophytic fungal extracts for red blood cell (RBC) toxicity
For this study, the 3-(4, 5-dimethylthiazol-2-yl)-5-diphenyltetrazolium bromide (MTT) assay was used as described in a previous study with slight modifications31. Briefly, for the erythrocyte survival assay, 100 μl of washed red blood cells (donated by a member of the research team) containing 2% hematocrit were added to 100 μl volume of serially diluted fungal extracts (five duplicate dilutions were prepared for each extract with concentrations ranging from 6.25 μg/mL to 100 μg/mL). Donated blood was microscopically tested for malaria parasite contamination, and the result was negative. The plates were incubated for 72 h at 37 °C in a modular chamber under low oxygen and carbon dioxide. After 72 h, the colorimetric MTT assay was initiated by adding 20 μl of 7.5 mg/mL MTT and incubated for another 2 h. Formazan formation was stopped by adding 150 μl of DMSO and incubated overnight at room temperature in the dark. The optical densities were then measured at 570 nm using a Tecan fluorescence (Tecan Infinite M200, Austria) multi-plate reader. A survival curve of optical density (OD) versus concentration was plotted to determine the percentage survival of erythrocytes. The 50% cytotoxic concentrations (CC50) were determined by regression analysis. To ensure accuracy, the experiments were repeated three times, and the mean values were calculated together with their standard deviations. The selectivity indices (SI) of the herbal formulation CC50/IC50 values were determined.
Determination of anticancer activity using Jukart cell lines
The ability of the samples to inhibit cancer cell proliferation was evaluated using the MTT method, as previously described by Appiah-Opong et al.32. Human leukemia (Jurkat) cell lines obtained from ATCC (Manassas, VA, USA), were seeded in 96-well plates at a cell density of 1 × 105 cells per well. Cells were treated with 100 μl of different concentrations of reconstituted fungal extract (0–1000 µg/mL), curcumin as positive control or media only (negative control) and incubated at 37 °C, 5% CO2 for 72 h. After incubation, 20 µl of MTT solution (2.5 mg/ml) was added to each well, and the plates were incubated at 37 °C for 4 h. The supernatant was removed and 150 µL DMSO (quenching agent) was added to each well to dissolve any formazan crystals. Formazan formation was measured at a wavelength of 570 nm using a microplate reader (Tecan Infinite M200 PRO, Switzerland). All experiments were performed in triplicate, and the percentage viability of cells at each concentration of extract was calculated as follows:
Statistical analysis
The results were presented as the Mean ± SD (standard deviation) of 3 replicates. All data were analyzed at a 95% confidence interval using GraphPad Prism 6.01 (GraphPad Software, Inc, San Diego, CA). P-values less than 0.05 were considered statistically significant. The IC50 and CC50 values were obtained from the log-linear regression analysis of log-dose response curves using the GraphPad Prism 6.01.
Ethical statement
This study was approved by the Renaissance University, Enugu, Nigeria. This study did not include any experiments on human participants and animals. All methods were carried out in accordance with relevant guidelines in the method section. The use of E. guineensis, C. acuminata, and B. vulgaris does not violate the local regulations of Nigeria. During the sample collection from a specific plant, permission was taken from the Renaissance University, Enugu, Nigeria. Plant materials were collected according to institutional, national, and international guidelines and legislation.
Result and discussion
Isolation and identification of endophytic fungi
Three endophytic fungi molecularly characterized as Lasiodiplodia theobromae, Curvularia lunata Bv4, and Curvularia lunata Eg7 were isolated from three different medicinal plants: C. acuminata, B. vulgaris, and E. guineensis, respectively. The isolates were identified using molecular techniques (ITS region), with all three fungi showing over 98% similarity to the reference strain in the NCBI nucleotide database. The DNA sequence data were also deposited in the NCBI database (GenBank) at https://www.ncbi.nlm.nih.gov/nucleotide/ with accession numbers OL342232 (L. theobromae), OL347861 (C. lunata Bv4), and OL347929 (C. lunata Eg7). According to Maadon et al.33, a homology of more than 98% with the referenced culture is required to confirm the preliminary identification of the test sequence.
GC–MS analysis of the fungal secondary metabolites
GC–MS analysis of the crude extracts of the three endophytic fungi showed the presence of a variety of important compounds with considerable percentage compositions and quality matches between 70 and 99%. Table 1 shows the identified compounds, retention time (RT), peak area (%), molecular weight, and type of compound. The peak area is directly proportional to the concentration of the compound present in the solvent. In total, 16, 17, and 27 bioactive compounds were detected in ethyl acetate extracts from the endophytic fungi L. theobromae, C. lunata Bv4, and C. lunata Eg7, respectively. Of these, oxalic acid, isobutyl nonyl ester (10.44%), and 2,4-di-tert-butylphenol (7.44%) were the most abundant compounds produced by L. theobromae. C. lunata Bv4 extracts showed hexadecanoic acid, ethyl ester (5.54%), linoleic acid ethyl ester (4.05%), and 2,4-di-tert-butylphenol (4.04%) as the most abundant compounds. Also, heptadecane, 2,6,10,14-tetramethyl (14.57%), 2,4-di-tert-butylphenol (9.22%) and 1-octadecene (7.00%) were the most abundant compounds in the extract of C. lunata23. A comparative analysis of the chemical composition of the extracts produced by the three fungi investigated showed that 2,4-di-tert-butylphenol, decane, 2,4-dimethyl-, and tridecane were produced in different concentrations (for the GS-MS chromatograms of the crude extracts of L. theobromae, C. lunata Bv4, and C. lunata Eg7 see supplementary files 1, 2, and 3).
Most of the listed compounds detected in the endophytic fungal extracts in this study have been produced by other species of endophytic fungi in other studies and observed with different biological properties23,34,35. In this study, the majority of the active compounds identified by GC–MS are essential oils, which may contribute to the antiplasmodial properties shown. According to Osuntokun and Cristina36, the importance of essential oils in the discovery of new drugs cannot be overestimated, especially in an era of antimicrobial resistance.
Overall, the identified compounds produced by the three fungi studied represented the phenolic and terpenoid classes of secondary metabolites as well as fatty acids, alkanes, alkenes, alcohol derivatives, and others (alkaloid, benzothiazole, ether, and aromatic hydrocarbons). Alkane compounds were the most abundant (30%), followed by terpenoids and fatty acids, with 17.5% each (Fig. 1). The percentage composition of each group of bioactive compounds produced by each fungus is shown in Fig. 2. The most frequently identified groups of compounds in the fungal extracts were as follows: L. theobromae [alkanes (38%) and phenols (13%)]; C. lunata Bv4 [terpenoids (29%) and fatty acids (24%)], and C. lunata Eg7 [alkanes (41%), terpenoids (15%), and alkenes (15%)]. The majority of these chemical constituents have been shown to exhibit impressive biological activity via diverse mechanisms37,38,39,40,41,42.
Terpenoids have a wide range of medicinal applications, the most notable of which is antiplasmodial activity, as their mechanism of action is similar to that of the widely used antimalarial drug chloroquine37. Lee et al.38 also reported that terpenoids have good anticancer activity. Studies have demonstrated the pharmacological properties of the monoterpenes p-cymene, including antiparasitic and antitumor activities39,40. Similarly, myrcene (β-myrcene), a monoterpene41, and the sesquiterpene humulene, also known as α-humulene or α-caryophyllene42, have both been reported to exhibit anticancer properties.
The phenolic compound 2,4-di-tert-butylphenol (2,4-DTBP), has been identified as one of the abundant compounds produced by all three fungi and is known for a variety of biological activities, such as in vitro antimalarial properties43,44 and cytotoxicity45.
Naphthalenes are bioactive compounds that have been reported from plants, liverworts, fungi, and insects, and have been shown to be effective against P. falciparum46. Similarly, Abdissa et al.47 and Wongsa et al.48 demonstrated that naphthalene derivatives exhibited antiplasmodial activity against chloroquine-resistant and chloroquine- susceptible strains of P. falciparum, as well as cytotoxic activity against cancer cell lines. Since the extract of L. theobromae contained the highest concentration of naphthalene (1.51%), this may have played an important role in the very good antiplasmodial activity of the fungus.
The results of this study suggest that these compounds identified in the fungal extracts of L. theobromae from C. acuminata, C. lunata Bv4 from B. vulgaris, and C. lunata Eg7 isolated from E. guineensis could be responsible for the biological activities demonstrated by these endophytes. Thus, these endophytic fungi in this study were found to have high bioactive potential and can be utilized for the development of new, effective antiplasmodial agents for the treatment of malaria.
Antiplasmodial activity
The antiplasmodial activity of the isolated endophytic fungal extracts against chloroquine-resistant P. falciparum (PfDD2), the effects on uninfected human erythrocytes, and the selectivity index (SI) are presented in Table 2. Of the three (3) fungi studied, only the extract of L. theobromae showed potent antiplasmodial activity with an IC50 value of 5.4 μg/mL. According to GC–MS analysis, the most frequently identified groups of compounds in the fungal extract of L. theobromae were alkanes (38%) and phenols (13%). The antiplasmodial activity of L. theobromae may be due to the presence of these compounds. A previous study from Cameroon49 reported the antiplasmodial properties of some phenolic compounds isolated from Cameroonians Allanblackia plant. However, proving this claim and identifying the mechanisms behind the curtain still require more detailed investigations.
The extracts of C. lunata Bv4 and C. lunata Eg7 had IC50 values of over 100 μg/mL. According to Toghueoa et al.4, the antiplasmodial activity of extracts is classified as strongly active (IC50 < 2 μg/mL), very active (IC50 2–5 μg/mL), and active (IC50 > 5–10 μg/mL) based upon standard activity ranking. Based on this ranking, the extract of L. theobromae was considered very active against chloroquine-resistant strains of P. falciparum. This is very interesting as the rapid spread of resistant P. falciparum poses a major challenge to available antimalarial drugs and leads to varying degrees of failure3,50. However, it was found to be less potent than the positive control, artesunate, which had an IC50 value of 0.093 ng/mL.
Before this study, the genus Lasidioplodia has not yet been investigated for its antiplasmodial activity. However, other endophytes such as the genera Fusarium, Trichoderma, Aspergillus, and Penicillium have previously been reported to demonstrate antiplasmodial activities4,51. Ateba et al.2 reported the antiplasmodial activity of extracts prepared from three endophytic fungi isolated from Symphonia globulifera, specifically against PfDD2. These endophytes, including Paecilomyces lilacinus (IC50 = 0.44 μg/mL), Penicillium janthinellum (IC50 = 0.2 μg/mL), and Paecilomyces sp. (IC50 = 0.55 μg/mL), showed even better activity than that of L. theobromae (5.4 μg/mL) in this study. Other reports have shown that the endophyte Diaporthe miriciae produces secondary metabolites with strong antimalarial activity against a chloroquine-resistant strain of P. falciparum14. The observed activities could be due to the fact that L. theobromae produces similar bioactive secondary metabolites as the host plant. According to Zailani et al.52, several Cola spp. varieties, including Cola acuminata, have been shown to have antimalarial properties. The results of this study suggest that the endophytic fungus L. theobromae from C. acuminata is a promising source of active compounds that could be further investigated as new drugs against the malaria parasite.
The endophytic fungal extract is expected to have a promising antiplasmodial effect but should not be harmful to uninfected human erythrocytes. The effect of the fungal extracts on uninfected human erythrocytes is also shown in Table 2. All three fungal extracts had CC50 values greater than 100 μg/mL, demonstrating the safety of these fungal extracts to the erythrocytes. With a CC50 value of 102.75 μg/mL, the crude extract of the most effective endophytic fungus, L. theobromae, did not inhibit the viability of untreated erythrocytes. Furthermore, a selectivity index of 18.97 was found for the fungal extract, indicating its limited cytotoxicity towards uninfected erythrocytes. This means that the fungal extract produced by L. theobromae was not harmful to the erythrocytes but had a very active antiplasmodial activity.
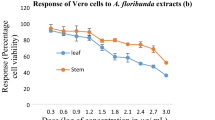
Anticancer cytotoxicity activity against leukemia (Jurkat) cell lines
The cytotoxic activity of the fungal extracts against leukemia (Jurkat) cell lines is shown in Fig. 3. All endophytic fungal extracts showed only weak cytotoxic effects in vitro against the cancer cell lines used in this study. The CC50 values obtained were well below the activity threshold of < 30 μg/mL defined by the US National Cancer Institute for a crude extract53. The ethyl acetate crude extracts of all isolated endophytic fungi were not cytotoxic to the cancer cell lines tested (CC50 > 100 μg/mL) (Fig. 3). The extract of L. theobromae had the best potential with a CC50 value of 191.03 μg/mL, which was significantly higher than that of the positive control, curcumin (2.93 μg/mL). Similar to the antiplasmodial activity of L. theobromae, this may be due to the presence of phenolic or fatty acid compounds. Previous studies have confirmed the anticancer properties of phenolic compounds and fatty acids of microorganismic origin54,55. This needs to be further investigated to uncover the exact mechanisms involved. Nevertheless, the result suggests that an effective anticancer agent could be developed from the extract of L. theobromae after further fractionation and purification, as Pandi et al.7 already reported the production of taxol (a very effective antitumor agent) by L. theobromae isolated from Morinda citrifolia. Similarly, other species of the genus Lasiodiplodia such as L. pseudotheobromae have also shown very strong anticancer activity56.
Conclusion
It is assumed that endophytes with the ability to produce pharmacologically interesting natural compounds are often associated with plants of ethnomedicinal importance. Three endophytic fungi, L. theobromae, C. lunata Bv4, and C. lunata Eg7, were isolated from three medicinal plants, C. acuminata, B. vulgaris, and E. guineensis respectively, and their extracts were screened for their antiplasmodial and anticancer activities. L. theobromae showed the best antiplasmodial activity against chloroquine-resistant P. falciparum and at the same time was non-toxic to erythrocytes. All three fungal extracts showed weak cytotoxic activity against Jukart cell lines. GC–MS analysis of the fungal extracts revealed the presence of a variety of bioactive secondary metabolites that could be responsible for the observed biological activity. They should therefore be studied in more detail for their bioactivity to find out how they can best be used pharmaceutically for the benefit of mankind. They could also be improved to obtain more effective agents for the treatment of malaria caused by resistant strains of P. falciparum.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary material. The DNA sequence data were deposited in the NCBI database (GenBank) available from https://www.ncbi.nlm.nih.gov/nucleotide/ under the accession numbers: OL342232 (Lasiodiplodia theobromae), OL347861 (Curvularia lunata Bv4), and OL347929 (Curvularia lunata Eg7).
References
World Health Organization. Malaria. WHO fact sheet (2022). Available at: https://www.who.int/news-room/fact-sheets/detail/malaria. Accessed on 22 may 2022.
Ateba, J. E. T. et al. Antiplasmodial properties and cytotoxicity of endophytic fungi from Symphonia globulifera (Clusiaceae). J. Fungi 4, 1–9 (2018).
Ikem, C. J. et al. In vitro and in vivo antiplasmodial assays of selected Nigerian commercial herbal formulations. J. Herbmed. Pharmacol. 9, 374–381 (2020).
Toghueoa, R. M. K. et al. Antiplasmodial potential and GC-MS fingerprint of endophytic fungal extracts derived from Cameroonian Annona muricata. J. Ethnopharmacol. 235, 111–121 (2019).
Singh, M., Kumar, A., Singh, R. & Pandey, K. D. Endophytic bacteria: A new source of bioactive compounds. 3Biotech 7, 315 (2017).
World Health Organization. Fact Sheets. Cancer (2020). Retrieved from https://www.who.int/news-room/fact-sheets/detail/cancer, Accessed on 24 Jun 2021.
Pandi, M. et al. Isolation and detection of taxol, an anticancer drug produced from Lasiodiplodia theobromae, an endophytic fungus of the medicinal plant Morinda citrifolia. Afr. J. Biotechnol. 10, 1428–1435 (2011).
Pham, J. V. et al. A review of the microbial production of bioactive natural products and biologics. Front. Microbiol. 10, 1404 (2019).
Dos Reis, J. B. A., Lorenzi, A. S. & do Vale, H. M. M. Methods used for the study of endophytic fungi: A review on methodologies and challenges, and associated tips. Arch. Microbiol. 204, 675 (2022).
Baron, N. C. & Rigobelo, E. C. Endophytic fungi: A tool for plant growth promotion and sustainable agriculture. Mycology 13, 39–55 (2022).
Manganyi, M. C. & Ateba, C. N. Untapped potentials of endophytic fungi: A review of novel bioactive compounds with biological applications. Microorganisms 8, 1934 (2020).
Rai, N. et al. Plant associated fungal endophytes as a source of natural bioactive compounds. Mycology 12, 139–159 (2021).
Ujam, N. T. et al. Assessment of antiplasmodial and immunomodulatory activities of endophytic fungal metabolites from Azadirachta indica A Juss. Afr. J. Microbiol. Res. 16, 121–131 (2022).
Ferreira, M. C. et al. Antimycobacterial and antimalarial activities of endophytic fungi associated with the ancient and narrowly endemic neotropical plant Vellozia gigantea from Brazil. Mem. Inst. Oswaldo Cruz 112, 692–697 (2017).
Riyaz-Ul-Hassan, S. et al. Modulation of volatile organic compound formation in the Mycodiesel producing endophyte-Hypoxylon sp. C1–4. Microbiology 158, 464–473 (2012).
World Health Organization. WHO global report on traditional and complementary medicine 2019. Geneva: World Health Organization. Licence: CC BY-NC-SA 3.0 IGO (2019). Available at: https://iris.who.int/handle/10665/312342. Accessed 19 Sept 2021.
Ugwuowo, B. O., Ahmed, A., Oluwasola, H. O. & Ukoha, P. O. Comparative assessment of phytochemicals, antioxidant activity and antimicrobial activity of Cola acuminata, Garcinia kola and Vernonia amygdalina. J. Chem. Soc. Nigeria 46, 0698–0710 (2021).
Heroine, M. M. et al. Isolation of endophytics fungi from Cola acuminata Schott & Endl, and antifungal activity against Candida Sp. Eur. J. Biol. Biotechnol. 1, 1–7 (2022).
Hzounda Fokou, J. B. et al. Antibacterial and antioxidant properties of endophytic fungi extracts from Cola acuminata (Sterculiaceae). Austin J. Pharmacol. Ther. 10, 1159–1167 (2022).
Tow, W. K. et al. Flavonoid composition and pharmacological properties of Elaeis guineensis Jacq leaf extracts: A systematic review. Pharmaceuticals 14, 961 (2021).
Yurnaliza, Y., Jamilah, I., Hartanto, A. & Lutfia, A. Screening of endophytic fungi from oil palm (Elaeis guineensis) in producing exopolysaccharides. Biodiversitas 22, 1467–1473 (2021).
Ghanbarinasab, Z. et al. Topical Bambusa vulgaris extract enhances wound healing in cutaneous Leishmaniasis. J. Pathog. 2021, 7860474 (2021).
Nwobodo, D. C. et al. Bioactive compounds characterization and antimicrobial potentials of crude extract of Curvularia lunata, a fungal endophyte from Elaeis guineensis. Trop. J. Nat. Prod. Res. 6, 395–402 (2022).
Nwobodo, D. C., Ihekwereme, C. P. & Okoye, F. B. C. Screening of endophytic fungal metabolites from Cola nitida leaves for antimicrobial activities against clinical isolates of Pseudomonas aeruginosa. EuroBiotech J. 4, 161–166 (2020).
Eze, P. M. et al. Secondary metabolites of a marine-derived Penicillium ochrochloron. Not. Sci. Biol. 13, 11020 (2021).
Ibrahim, M. et al. Extracts of endophytic fungi from leaves of selected Nigerian ethnomedicinal plants exhibited antioxidant activity. BMC Complem. Med. Ther. 21, 98 (2021).
Nigam, P. S. & Singh, A. Metabolic pathways: Production of secondary metabolites—fungi. In Encyclopedia of Food Microbiology 570–578 (Elsevier, 2014).
Nyalo, P. O., Omwenga, G. I. & Ngugi, M. P. Antibacterial properties and GC-MS analysis of ethyl acetate extracts of Xerophyta spekei (Baker) and Grewia tembensis (Fresen). Heliyon 9, e14461 (2023).
Jibril, S. et al. Phytochemical and antibacterial screening of leaf extracts from Cassia singueana Del. (Fabaceae). Bayero J. Pure Appl. Sci. 13, 108–112 (2021).
Shahdadi, F. et al. GC-MS profiling of Pistachio vera L., and effect of antioxidant and antimicrobial compounds of it’s essential oil compared to chemical counterparts. Sci. Rep. 13, 21694 (2023).
Ikem, C. J. et al. Screening of five herbal formulations sold in south-east Nigeria for their phytochemical properties, in vitro antioxidant, antiplasmodial and cytotoxic activities. Trop. J. Nat. Prod. Res. 6, 150–155 (2022).
Appiah-Opong, R. et al. Cytotoxic effects of Albizia zygia (Dc) J.F. Macbr, a Ghanaian medicinal plant, against human T-Lymphoblast-like leukemia, prostate and breast cancer cell lines. Int. J. Pharm. Pharm. Sci. 8, 392–396 (2016).
Maadon, S. N. et al. Isolation and identification of endophytic fungi from UiTM Reserve Forest Negeri Sembilan. Sains Malays 47, 3025–3030 (2018).
Jayaram, H., Marigowda, V. & Thara Saraswathi, K. J. Secondary metabolite production and terpenoid biosynthesis in endophytic fungi Cladosporium cladosporioides isolated from wild Cymbopogon martini (Roxb.) wats. Microbiol. Res. 12, 812–828 (2021).
Khaled, J. M. et al. Biochemical profile by GC–MS of fungal biomass produced from the Ascospores of Tirmania nivea as a natural renewable resource. J. Fungi 7, 1083 (2021).
Osuntokun, O. T. & Cristina, G. M. Bio isolation, chemical purification, identification, antimicrobial and synergistic efficacy of extracted essential oils from stem bark extract of Spondias mombin (Linn). Int. J. Mol. Biol. 4, 135–143 (2019).
Cox-Georgian, D., Ramadoss, N., Dona, C. & Basu, C. Therapeutic and medicinal uses of terpenes. In Medicinal Plants: From Farm to Pharmacy (eds Joshee, N. et al.) 333–359 (Springer, 2019).
Lee, T. K. et al. Pinecone of Pinus koraiensis inducing apoptosis in human lung cancer cells by activating Caspase-3 and its chemical constituents. Chem. Biodivers. 14, 1612–1880 (2017).
Balahbib, A. et al. Health beneficial and pharmacological properties of p-cymene. Food Chem. Toxicol. 153, 112259 (2021).
Marchese, A. et al. Update on monoterpenes as antimicrobial agents: A particular focus on p-cymene. Materials 10, 947 (2017).
Bai, X. & Tang, J. Myrcene exhibits antitumor activity against lung cancer cells by inducing oxidative stress and apoptosis mechanisms. Nat. Prod. Commun. 15, 1–7 (2020).
Ali, N. et al. Antimicrobial, antioxidant, and cytotoxic activities of Ocimum forskolei and Teucrium yemense (Lamiaceae) essential oils. Medicines 4, 17 (2017).
Kusch, P. et al. In vitro and in vivo antimalarial activity assays of seeds from Balanites aegyptiaca: compounds of the extract show growth inhibition and activity against plasmodial aminopeptidase. J. Parasitol. Res. 2011, 368692 (2011).
Varsha, K. K. et al. 2,4-Di-tert-butyl phenol as the antifungal, antioxidant bioactive purified from a newly isolated Lactococcus sp. Int. J. Food Microbiol. 211, 44–50 (2015).
Zhao, F. et al. Natural sources and bioactivities of 2,4-Di-Tert-butylphenol and its analogs. Toxins 12, 35 (2020).
Abraham, A. W. et al. Antimalarial compounds from Kniphofia foliosa roots. Phytother. Res. 19, 472–476 (2005).
Abdissa, N. et al. Knipholone cyclooxanthrone and an anthraquinone dimer with antiplasmodial activities from the roots of Kniphofia foliosa. Phytochem. Lett. 6, 241–245 (2013).
Wongsa, N. et al. Parviflorals A-F, trinorcadalenes and bis-trinorcadalenes from the roots of Decaschistia parviflora. Phytochemistry 95, 368–374 (2013).
Azebaze, A. G. et al. Antiplasmodial activity of some phenolic compounds from Cameroonians Allanblackia. Afr. Health Sci. 15, 835–840 (2015).
Ariey, F. et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 505, 50–55 (2014).
Kaushik, N. K., Murali, T. S., Sahal, D. & Suryanarayanan, T. A search for antiplasmodial metabolites among fungal endophytes of terrestrial and marine plants of southern India. Acta Parasitol. 59, 745–757 (2014).
Zailani, H. A., Banyawa, M. & Muhammad, A. A. Effects of aqueous leaf extract of Cola acuminata on parasitaemia, haematological and liver function parameters in Plasmodium berghei infected mice. Direct. Res. J. 4, 14–20 (2016).
Fadeyi, S. A. et al. In vitro anticancer screening of 24 locally used Nigerian medicinal plants. BMC Complem. Altern. Med. 13, 79 (2013).
Tanvir, R., Javeed, A. & Rehman, Y. Fatty acids and their amide derivatives from endophytes: New therapeutic possibilities from a hidden source. FEMS Microbiol. Lett. 365, fny314 (2018).
Matulja, D. et al. Anticancer activities of marine-derived phenolic compounds and their derivatives. Molecules 27, 1449 (2022).
Xiaojing, L. et al. Palmarumycins from the endophytic fungus Lasiodiplodia pseudotheobromae XSZ-3. Cheminform 46, 1269–1294 (2014).
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
D.C.N. conceptualization; formal analysis; investigation; writing-original draft; writing-review and editing. N.N.O. data curation; formal analysis; writing-original draft. M.S.M. data curation; formal analysis; writing-original draft. J.C.I. conceptualization and methodology. P.M.E. project administration; writing-original draft (supporting); writing-reviewing and editing. F.B.C.O. conceptualization; data curation, investigation; methodology; project administration; validation; writing-original draft; writing-review and editing. M.S. investigation; writing-original draft; writing-reviewing and editing. C.O.E. conceptualization; data curation, investigation; methodology; project administration; writing-original draft. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nwobodo, D.C., Okoye, N.N., Sifir Mudkhur, M. et al. In vitro antiplasmodial and anticancer analyses of endophytic fungal extracts isolated from selected Nigerian medicinal plants. Sci Rep 14, 19765 (2024). https://doi.org/10.1038/s41598-024-66456-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-66456-5
- Springer Nature Limited