Abstract
Atopic dermatitis (AD) is a chronic, allergic inflammatory skin disorder that lacks a definite cure. Using a mouse DNCB-induced AD-like skin lesions model, this study evaluated the potential therapeutic utility of tHGA as an oral and topical treatment for AD. Male BALB/c mice were sensitised and challenged with 1% and 0.5% DNCB on their shaved dorsal skin. Mice in the treatment group were administered tHGA (20, 40, and 80 mg/kg) orally three times per week for 2 weeks, or tHGA (0.2%, 1%, and 5%) topically once daily for 12 days. On day 34, the mice were euthanized, and blood and dorsal skin samples were obtained for analysis. All doses of orally and topically administered tHGA significantly improved scratching, epidermal thickness, blood eosinophilia and mast cell infiltration. There was a minor discrepancy between the two routes of administration, with orally treated tHGA showing significant reductions in Scoring of Atopic Dermatitis (SCORAD), tissue eosinophil infiltration, serum IgE and skin IL-4 levels with treatment of 40 and 80 mg/kg tHGA, whereas topically applied tHGA showed significant reductions in all dosages. These findings suggest that tHGA exhibited therapeutic potential for AD as both oral and topical treatment ameliorates AD-like symptoms in the murine model.
Similar content being viewed by others
Introduction
Atopic dermatitis (AD) is an allergic, itchy, inflammatory skin condition characterised by erythema, vesicles, and oedema in the acute stage and skin lichenification in the chronic stage. Skin scaling, redness, and itching are among the clinical symptoms1. AD is the most common allergic inflammatory skin disorder in children, and it usually appears in the first year of life. Statistically, AD affects 2.4% of the global population, with prevalence varying by country. Around 25% of children diagnosed with AD will be afflicted by the condition until adulthood, and 75% will recuperate before adolescence2,3. It is one of several allergic illnesses that coexist with asthma and rhinitis4. This is known as the atopic march or triad because it begins with AD in early childhood and progresses to the development of other allergy problems later in life5.
Anti-inflammatory medicines, such as systemic steroids like prednisolone and dexamethasone taken orally, topical corticosteroids and topical calcineurin inhibitors, as well as moisturising and skin barrier-rebuilding agents, are being utilised as oral and topical therapy for AD6. Patients with mild to moderate AD can use moisturiser to minimise transepidermal water loss and avoid skin dryness7. However, moisturiser have limited therapeutic effect by moisturizer as it lacks active ingredients with immunomodulatory effects7. For moderate to severe AD, topical or oral administered corticosteroids and calcineurin inhibitors are required to manage the symptoms8,9. Patients remain concerned and hesitant because it is well-known that long-term usage of corticosteroids and calcineurin inhibitors can induce a variety of undesirable side effects, such as skin striae, atrophy, rashes, and a burning sensation on the skin10. Currently, numerous clinical practice guidelines advise against using oral systemic corticosteroids, owing to an unfavourable risk–benefit ratio, particularly in children8. Furthermore, systemic corticosteroid treatment may increase the risk of a more severe rebound flare once the steroid effect wears off11. As a result, research into novel therapeutic approaches for AD based on natural compounds is actively ongoing.
Previous bioassay-guided identification studies discovered 2,4,6-trihydroxy-3-geranylacetophenone (tHGA) in the medicinal plant Melicope ptelefolia Champ Ex. Benth (Rutaceae), widely known as "tenggek burung" in Southeast Asia12. The compound tHGA was successfully produced synthetically, which further advances the investigation into its pharmacological properties in various experimental models13,14. tHGA has been shown to have mast cell stabilising properties by preserving mast cell structure and suppressing the production of pro-inflammatory mediators in the in vitro model of IgE-mediated mast cell degranulation and the in vivo model of systemic anaphylaxis15,16. Mast cells are implicated in the pathogenesis of AD with increased mast cell infiltration and activation in the skin, disrupting the epidermal differentiation complex leading to skin barrier dysfunction, releasing cytokines like IL-4 and IL-13 resulting in type 2 inflammation, as well as releasing histamine and tryptase which interact with skin peripheral nerves, causing pruritus17. Moreover, tHGA treatment also reduced airway hyperresponsiveness and prevented airway remodelling in a murine model of acute and chronic asthma14,18. Attenuation of IgE levels and mast cell degranulation by tHGA were observed in the murine asthma models. A mechanistic study to determine tHGA’s molecular target demonstrated that tHGA effectively blocked the phosphorylation of the linker for activation of T cells (LAT) protein, while having no effect on the phosphorylation of its upstream protein, spleen tyrosine kinase (Syk). The findings were further validated utilising siRNA to knockdown the expression of LAT in a model of IgE-mediated mast cell degranulation. The results suggested that LAT on mast cells is the specific molecular target of tHGA16.
In this study, instead of delving further into tHGA’s mechanism, we shifted our focus to investigating the potential of tHGA as a treatment for AD. Specifically, we compared two common treatment routes for AD patients: topical and oral administration. This study holds significant importance for enhancing the pharmacological profile of tHGA. It marks the first exploration of tHGA's effectiveness as a topical anti-allergic treatment as previous research on the anti-allergic effects of tHGA predominantly emphasised oral and intraperitoneal routes. An essential aspect of the study of AD medications is the comparison between oral and topical administration methods. Numerous studies have demonstrated that the majority of AD patients have a preference for topical treatment19. Nevertheless, systemic treatment through oral administration remains crucial for managing AD in patients with persistent and severe cases20. With AD being a chronic skin inflammatory disease associated with IgE and one of the atopic triads along with asthma, in this study, we investigated the efficacy of oral and topical administration of tHGA on DNCB-induced AD-like skin lesions in BALB/c mice. Our findings may provide further insights on the efficacy of tHGA in ameliorating AD in support of its development as a new non-steroidal drug lead for AD management.
Materials and methods
tHGA synthesis
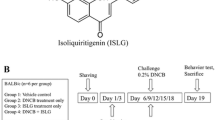
tHGA was synthesised as previously described14,21,22−. A well-stirred mixture of phloracetophenone (1.000 g, 6 mmol), geranyl bromide (0.876 g, 4.80 mmol), and anhydrous potassium carbonate (0.415 g, 3.00 mmol) in dry acetone (3.5 ml) was refluxed for 6 h. The reaction mixture was filtered and evaporated under reduced pressure to give an oily orange residue that was purified by flash column chromatography on Si gel (petroleum ether-EtOAc, 10:1) to afford 2,4,6-trihydroxy-3-geranylacetophenone (tHGA) as a light yellow powder; mp 128–130 °C. 1H NMR (CD3OD) δH 1.58 (3H, s, Me), 1.63 (3H, s, Me), 1.76 (3H, s, Me), 2.64, (3H, s, COMe), 1.96 (2H, q, J = 7.5 Hz), 2.06 (2H, m), 3.21 (2H, d, J = 6.5 Hz), 5.08 (1H, t, J = 7 Hz), 5.20 (1H, t, J = 6.5 Hz), 5.92 (1H, s, ArH); IR (KBr) νmax 3405, 1627 cm–1; EIMS m/z (%) [M] + 304 (38), 289 (3), 261 (9), 235 (25), 181 (100) (Fig. 1a). The purity of tHGA was more than 99%, and it is free of endotoxin.
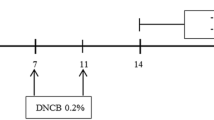
Chemical structure of tHGA and experimental design. (a) The chemical structure of 2,4,6-trigydroxy-3-geranyl acetophenone (tHGA) contains a phloroglucinol structural-core as the bioactive principle. (b) Experimental design: Mice were sensitised with 1% DNCB on day 1. From day 8, mice were challenged with 0.5% DNCB three times per week up to 4 weeks. In the final 2 weeks, orally treated mice were administered different doses of tHGA, drug control or vehicle 1-h prior to DNCB application for 3 days per week, while topical treated mice were applied with different doses of tHGA, drug control or vehicle 4-h prior to DNCB application daily. Mice were observed for scratching behaviour on day 33 and evaluated for SCORAD on day 34 before being sacrificed for blood and skin sample collection.
Animals
Seventy-two specific-pathogen-free (SPF) male BALB/c mice (6 weeks old; 20–25 g) were obtained from the National Institutes of Biotechnology Malaysia (NIBM). The mice were acclimatised for 1 week in accordance with the guideline for animal care and use of laboratory animals23. The mice were housed at 24 °C with a 12-h light–dark cycle, with access to standard chow and distilled water ad libitum. All experiments were carried out at the Comparative Medicine and Technology Unit (COMeT), Universiti Putra Malaysia, in accordance with the protocol approved by the Institutional Animal Care and Use Committee, Universiti Putra Malaysia (UPM/IACUC/AUP-R002/2019) and ARRIVE guidelines24.
Experimental design and sample size calculation
The experimental design is shown in Fig. 1b. BALB/c mice were randomly divided into 12 experimental groups, with 5 groups each for oral and topical tHGA treatment (vehicle control, low, medium and high dose tHGA treatments and drug control) together with normal and DNCB-induced mice25,26,27. The sample size was established using the equation below:
where, DF = degree of freedom (minimal acceptable range = 10; maximal acceptable range 20), k = number of groups to be compared.
In this study, there are two modes of treatment administration: oral and topical. Under each mode of treatment, there are four groups (three treatment groups and one drug control group) to be compared with the negative control group. The minimum n was determined to be 3.5 animals per group while maximum n was 6 animals per group. It is recommended to utilise the maximum required sample size (n = 6 per group) in order to ensure precise statistical analysis28.
Atopic dermatitis induction and tHGA treatment
Mice were sensitised a day after their dorsal hair was removed by applying 1% (w/v) dinitrochlorobenzene (DNCB) in acetone and olive oil (3:1) to their shaved dorsal skin (day 1). After a week (day 8), mice dorsal skin was subjected to DNCB challenge with topical applications of 0.5% (w/v) DNCB three times per week for 4 weeks. On day 22, tHGA, vehicle or positive drug control was given. On day 33, the mice's scratching behaviour and skin condition were assessed, and on day 34, the mice were subjected to anaesthesia with 100 mg/kg ketamine and 10 mg/kg xylazine for blood collection via cardiac puncture. They were subsequently euthanized via cervical dislocation for skin sample collection.
The experimental groups for oral treatment of tHGA were vehicle treatment control, 20 mg/kg tHGA treatment group, 40 mg/kg tHGA treatment group, 80 mg/kg tHGA treatment group and 10 mg/kg ketotifen fumarate (KF) treatment positive control group. The treatment compound was dissolved in a vehicle solution of 5% DMSO and 5% Tween 20 and administered at a volume of 10 ml/kg based on the mouse body weight15. For the final 2 weeks, oral treatment was given 1 h before DNCB application three times per week.
The experimental groups for topical treatment of tHGA included a vehicle treatment control, 0.2% tHGA treatment group, 1% tHGA treatment group, 5% tHGA treatment group and 0.1% dexamethasone (DEX) treatment positive control group. To enhance transdermal delivery, the treatment compound was dissolved in argan oil29. In the final 2 weeks, topical treatment was administered daily for 12 days. If the treatment day coincided with the DNCB challenge day, the treatment was given 4 h before the DNCB application.
Scratching behaviour test
Following the final DNCB challenge on day 33, the mice were housed in the observation chamber for an hour before their scratching behaviour was assessed. Logitech Webcam Software (Logitech, Suzhou, China) was used to record the scratching latency for 20 min. One episode of scratching was defined as the hind paw leaving the floor and scratching incessantly until the paw returned to the floor. The cumulative duration, in seconds, of all episodes of scratching observed within the 20 min period for each mouse was documented. The measurement was performed by a single investigator to limit variation. The mice and the video were coded to avoid observer bias.
Evaluation of clinical skin condition
To determine the severity of dermatitis, erythema, erosion, dryness, and lichenification were examined and graded. The following percentages of the afflicted skin area were used to rate the condition: Score 0 indicates no symptoms were detected; score 1 indicates conditions affecting < 25% of the shaved dorsal area; and scores 2 and 3 indicate conditions affecting 25–50% and > 50% of the shaved dorsal skin, respectively, as detailed earlier30. The scoring of AD (SCORAD) was calculated as the sum of each parameter's scores, with a maximum score of 12. The scoring was done by a pathologist in a single-blinded manner.
Blood eosinophils count
Blood samples were collected in an EDTA blood tube by cardiac puncture. Blood smears were prepared on glass slides, air dried, and stained with Leishman's stain. Briefly, the blood films were immersed in Leishman's stain for 7 min. The stain was then mixed with an equal volume of phosphate buffered saline (PBS) for 5 min before being washed under running water. Eosinophils were observed and counted in nine randomly selected fields using a light microscope at × 400 magnification in a double-blinded manner.
Serum IgE assay
Some of the blood collected from the cardiac puncture was allowed to clot prior to centrifugation at 1000g for 10 min at 4 °C to extract the serum. Serum IgE levels were determined using an ELISA Standard Set Mouse IgE kit (BioLegend, USA) according to the manufacturer’s protocol. Optical densities were measured at 450 nm, with optical correction at 570 nm.
Skin tissue histology
Following euthanasia, the dorsal skin of mice was extracted using a sterile dissection kit. The samples were fixed in 10% buffered formalin and processed in an automated tissue processor (Leica Instrument Gmb, Germany) for 14 h. The skin samples were then embedded in paraffin wax and sectioned into 5 µm sections. Haematoxylin and eosin (H&E) stain was used for epidermal thickness measurement and skin eosinophil counts. A compound microscope and Leica Application Suite Core (LAS) software (Leica Instrument GmbH, Germany) were used to measure epidermal thickness. The epidermal thickness in µm was measured in five fields of the thickest area at × 100 magnification in a double-blind fashion. Eosinophils were counted in ten random high-power fields with a magnification of × 400. Toluidine blue stain was used to examine mast cells. The number of mast cells and degranulated mast cells were counted in ten random high-power fields at a magnification of × 400 and × 1000, respectively.
Immunoassay of IL-4 in skin tissue and serum
Using mechanical cryogenic grinding, liquid nitrogen snap-frozen skin tissue samples were crushed with a pestle into small, fragmented bits. The shattered tissue samples were lysed in RIPA buffer and proteinase inhibitor overnight at 4 °C. The supernatants were collected after centrifuging the lysed samples at 10,000g for 20 min. The serum was collected as described in section “Blood eosinophils count”. IL-4 was quantified by using the IL-4 DuoSet ELISA kit (R&D Systems, USA) according to the manufacturer’s protocol. Optical densities were measured at 450 nm, with optical correction at 570 nm.
Statistical analysis
Data were analysed with one-way ANOVA using SPSS software version 26 (IBM, USA). Significant differences between experimental groups were tested using Tukey's post hoc test. All data were expressed as the mean ± SEM of at least six mice per group. When the treatment groups were compared to their respective vehicle groups, differences were considered significant when P < 0.05.
Results
Oral and topical administration of tHGA attenuate the development of clinical symptoms of DNCB-induced AD-like skin lesions
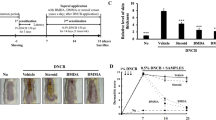
Repeated DNCB challenges resulted in the formation of skin lesions (Fig. 2a) with a high SCORAD score in BALB/c mice (Fig. 2c). When compared to the vehicle group, oral treatment of 40 mg/kg and 80 mg/kg tHGA significantly lowered the SCORAD scores by 35.1% (P < 0.01) and 43.4% (P < 0.001), respectively. These two doses were statistically equivalent to KF. However, the lowest dose of oral tHGA, 20 mg/kg, showed a slight reduction of SCORAD at 20.9% but without statistical significance. On the other hand, topical treatment with 0.2%, 1% and 5% tHGA, as well as 0.1% DEX, significantly reduced SCORAD by 29.3% (P < 0.05), 56.1% (P < 0.001), 51.2% (P < 0.001) and 56.1% (P < 0.001), respectively. No statistical difference was observed between the topical tHGA treatment groups and the drug control group.
tHGA improves the clinical symptoms of AD-like skin lesions in BALB/c mice. (a) Representative images of the dorsal skin conditions of mice on day 34 before euthanasia. (b) On day 33, mice were observed for scratching behaviour and the scratching latency was recorded. Data were represented as the mean of the cumulative duration, measured in seconds, of all episodes of scratching observed within the 20 min period for 6 mice in each group. (c) On day 34, mice SCORAD scores were evaluated based on the severity of erythema, erosion, dryness, and lichenification. Data are presented as means ± S.E.M. Significant differences were compared to DNCB-sensitised respective vehicle-treated control mice. *P < 0.05, **P < 0.01 and ***P < 0.001. KF Ketotifen fumarate, DEX Dexamethasone.
Oral and topical administration of tHGA reduce the scratching latency of DNCB-induced AD-like skin lesions in mice
Scratching behaviour in DNCB-challenged mice was evaluated, and both orally and topically administered tHGA significantly attenuated scratching behaviour (Fig. 2b). DNCB-induced vehicle-treated mice scratched 46-fold and 56-fold more than normal mice in oral treatment and topical treatment experiments, respectively. Orally administered tHGA at 20, 40, and 80 mg/kg were able to reduce the scratching latency by 46.3% (P < 0.05), 55.9% (P < 0.01) and 56.3% (P < 0.01), respectively compared to the vehicle control group, while topically applied tHGA at 0.2%, 1% and 5% reduced scratching latency by 57.6% (P < 0.01), 50.4% (P < 0.01) and 46.3% (P < 0.05), respectively when compared to their vehicle group. Scratching latency was significantly reduced in the drug control group for the orally administered KF group (52.2%) and the topically applied DEX group (44.2%). There were no significant differences between the tHGA treatment groups and their respective drug control groups.
Oral and topical administration of tHGA suppress the epidermal thickness of DNCB-induced AD-like skin lesions
The negative and vehicle control groups exhibited the thickest epidermal layer as compared to the normal group, as shown in Fig. 3a,c. Mice administered with tHGA orally and topically at all three doses, as well as drug controls, demonstrated a significant reduction in epidermal thickness. In comparison to the vehicle group, 20 mg/kg, 40 mg/kg, 80 mg/kg tHGA and 10 mg/kg KF oral treatments significantly (P < 0.001) suppressed epidermal thickness by 30.1%, 37.7%, 36.8% and 41.4% respectively; while 0.2%, 1.0%, 5.0% tHGA and 0.1% DEX topical treatments recorded epidermal thickness suppression of 24.1% (P < 0.01), 21.7% (P < 0.05), 34.3% (P < 0.001) and 47.5% (P < 0.001), respectively. All tHGA treatment groups were statistically comparable to their respective drug control groups.
The effects of tHGA on epidermis thickness and eosinophilia. (a) Histological images of mouse dorsal skin for epidermal thickness measurement. (b) Histological images of eosinophilia in mouse skin tissue. (c) The epidermal thickness was determined in five areas with the thickest skin. (d) The number of eosinophils in the skin tissues was counted in ten random high-power fields of histology sections, whereas (e) eosinophils in the blood were counted in nine random high-power fields of blood film. Data are presented as means ± S.E.M. Significant differences were compared to DNCB-sensitised respective vehicle-treated control mice. *P < 0.05, **P < 0.01 and ***P < 0.001. KF Ketotifen fumarate, DEX Dexamethasone.
Oral and topical administration of tHGA inhibit the blood and tissue eosinophils count of DNCB-induced AD-like skin lesions
We examined the eosinophil infiltration in the skin tissue, which is the pathological site of AD. As shown in Fig. 3b, H&E staining revealed that orally administering tHGA at 40 and 80 mg/kg and 10 mg/kg KF significantly attenuated eosinophil infiltration in the tissue by 28.9% (P < 0.05), 53.8% (P < 0.001) and 48.2% (P < 0.001), respectively. However, whereas 20 mg/kg tHGA did demonstrate some effect in inhibiting eosinophil infiltration (27.6%), it was not statistically significant when compared to the vehicle group. Eosinophil infiltration in tissue (Fig. 3d) was also significantly (P < 0.001) reduced by applying 0.2%, 1%, 5% tHGA and DEX topically by 62.8%, 56.5%, 69.8% and 68.3%, respectively, which corresponded to the findings of the blood eosinophil count. The inhibitory effects of 40 mg/kg, 80 mg/kg oral and all doses of topical tHGA treatment were statistically comparable to their respective drug control groups.
As shown in Fig. 3e, oral administration of tHGA at all doses (20, 40, and 80 mg/kg) as well as KF significantly (P < 0.001) reduced blood eosinophil levels by 54.7%, 65.6%, 87.4% and 76.3%, respectively compared to the vehicle group. Although the effect on blood eosinophil count was not as substantial as with oral treatment, topical application of tHGA at all concentrations (0.2%, 1%, and 5%) and DEX resulted in a significant reduction by 44.6% (P < 0.001), 42.5% (P < 0.01), 75.4% (P < 0.001) and 75.4% (P < 0.001), respectively in blood eosinophil count. The effects of all tHGA oral and topical treatments showed no statistical difference from their respective drug control groups.
Oral and topical administration of tHGA inhibit mast cell and degranulated mast cell counts in DNCB-induced AD-like skin lesions
Toluidine blue staining of skin tissue sections revealed that both the DNCB-induced negative and vehicle control groups had high mast cell infiltration in the skin (Fig. 4a,c). When compared to the vehicle group, orally treated tHGA at doses of 20 mg/kg, 40 mg/kg, and 80 mg/kg significantly reduced mast cell count by 59.6% (P < 0.001), 51.6% (P < 0.01), and 56.8% (P < 0.001), respectively. There was no statistical difference between these results and KF, which showed a 38.0% (P < 0.05) reduction in comparison to the vehicle group. Meanwhile, the mast cell count was reduced by 36.2% (P < 0.05), 36.9% (P < 0.05), and 41.6% (P < 0.01), respectively, when tHGA was applied topically at doses of 0.2%, 1.0%, and 5.0%. These three figures were statistically comparable to DEX, which reduced mast cell count by 45.0% (P < 0.01) relative to the vehicle group.
The effects of tHGA on mast cell count and degranulation. (a) Histological images of mast cells in mice dorsal skin. (b) Histological images of degranulated mast cells in mouse skin tissue. (c) The mast cell count and (d) the degranulated mast cell count were determined in ten random fields of toluidine blue-stained histology sections. Data are presented as means ± S.E.M. Significant differences were compared to DNCB-sensitised respective vehicle-treated control mice. *P < 0.05, **P < 0.01 and ***P < 0.001. KF Ketotifen fumarate, DEX Dexamethasone.
We further enumerated the number of degranulated mast cells on the stained tissue sections (Fig. 4b,d). In DNCB-induced BALB/c mice, all three dosages of oral tHGA treatment and higher doses of tHGA topical treatment (5% and 1%) significantly reduced the number of degranulated mast cells when compared to the vehicle control groups. Oral tHGA treatment at 20, 40, and 80 mg/kg reduced the number of degranulated mast cells by 46.2% (P < 0.01), 52.0% (P < 0.001) and 49.1% (P < 0.01), respectively. Treatment with KF resulted in a 40.0% reduction (P < 0.05), compared to the vehicle control group. Topical application of tHGA at dosages of 1.0% and 5.0% significantly (P < 0.001) reduced the number of degranulated mast cells by 46.9% and 42.5%, respectively. These two doses were statistically comparable to DEX, which reduced the number of degranulated mast cells by 61.6% (P < 0.001) in comparison to the vehicle group. These treatment groups were statistically comparable to the drug control groups. The lowest topical tHGA dose, 0.2%, reduced the number of degranulated mast cells marginally by 19.0% but was not statistically significant.
Oral and topical administration of tHGA suppress serum IgE level in DNCB-induced AD mice
DNCB-induced mice exhibited a marked increase in serum IgE levels of more than 12-fold (Fig. 5a). In comparison to the vehicle group, oral treatment with tHGA at 40 mg/kg, and 80 mg/kg, as well as topical treatment with tHGA at 0.2%, 1% and 5%, significantly lowered serum IgE concentrations at a quantum of 40.2% (P < 0.05), 66.6% (P < 0.001), 54.2% (P < 0.001), 77.5% (P < 0.001) and 87.1% (P < 0.001) respectively. However, oral treatment of tHGA at 20 mg/kg did not show a statistically significant reduction in serum IgE compared to the vehicle group. The inhibition of tHGA treatments was statistically comparable to their respective KF and DEX control groups.
The effects of tHGA on (a) serum IgE and (b) skin IL-4. Further evaluation of the (c) serum IL-4 levels in orally tHGA-treated mice was performed. Data are presented as means ± S.E.M. Significant differences were compared to DNCB-sensitised respective vehicle-treated control mice. *P < 0.05, **P < 0.01 and ***P < 0.001. KF Ketotifen fumarate, DEX Dexamethasone.
Oral and topical administration of tHGA reduce IL-4 levels in the skin tissue and serum of DNCB-induced AD mice
The levels of IL-4 in skin tissue were significantly elevated after the inductive challenge by DNCB (Fig. 5b). Orally administered tHGA at 80 mg/kg significantly suppressed IL-4 levels in skin tissue by 50.9% (P < 0.01), but no significant inhibition was observed for 20 mg/kg or 40 mg/kg. It is worth mentioning that even at 80 mg/kg, the inhibitory effect of orally administered tHGA was not as effective as the 91.6% (P < 0.001) IL-4 inhibition achieved by 10 mg/kg KF. On the other hand, all three dosages of topically applied tHGA (0.2%, 1%, and 5%) significantly (P < 0.001) reduced IL-4 levels in skin tissue by 66.8%, 102.7% and 101.1% and were statistically comparable to the DEX group with an inhibition of 96.9%.
We further evaluated serum IL-4 levels in orally treated tHGA mice since orally administered tHGA that enters the systemic circulation significantly attenuated and ameliorated other AD-related parameters but not IL-4 in skin tissue. Orally administered tHGA was able to significantly (P < 0.01) suppress serum IL-4 levels by 92.4% for 20 mg/kg dosage, 100.7% for 40 mg/kg dosage and 98.2% for 80 mg/kg dosage. Even at the lowest dose of 20 mg/kg, oral tHGA treatment was statistically comparable to the drug control KF group which reduced serum IL-4 levels by 89.6% (Fig. 5c).
Discussion
AD is a chronic inflammatory skin illness characterised by acute pruritus and eczema that affects people all over the world. Skin barrier protein degradation is a critical phase in the aetiology of AD as a result of inflammatory reactions that lead to erythema, lichenification, pain and itch31,32. Skin lichenification induces epidermal hyperplasia, which is characterised by the thickening of the epidermis' spinous and cornified layers33. Pathological findings in AD patients as well as AD animal models include epidermal hyperplasia, substantial mast cell infiltration, and eosinophils at the lesion site34. tHGA has been shown to exert various pharmacological properties, including anti-allergic activity14,18. tHGA can decrease passive systemic anaphylaxis via inhibition of IgE-mediated mast cell degranulation. tHGA pretreatment retained mast cell shape and inhibited degranulation, resulting in lower levels of serum preformed and de novo mediators15. AD, along with asthma, is one of the atopic triads, with IgE-mediated mast cell activity being strongly implicated in the pathogenesis1. In this investigation, oral treatment of tHGA at doses of 20, 40, and 80 mg/kg, as well as topical application of tHGA at doses of 0.2, 1, and 5%, showed that it can alleviate AD-like skin lesions as well as other AD characteristics and metrics.
DNCB and substances with a structural resemblance, like 2,4-dinitrofluorobenzene and picryl chloride, can combine with different skin proteins to produce covalent conjugates, which serve as immunogens. Local antigen presenting cells, such as cutaneous Langerhans cells, dermal dendritic cells, and macrophages, internalise DNCB-modified macromolecules, after which they are presented to T cell, which stimulate differentiation into Th2 cells35,36. Th2 cells secrete a variety of type 2 cytokines, such as IL-4, IL-13, IL-5, and IL-3137. IL-4, a type 2 cytokine, is essential for the aetiology of AD and is capable of inducing all histopathological features of the disease38. In AD, IL-4 induces IgE production and enhances inflammation by driving Th2 cell differentiation, as well as mediating pruritus by acting on tissue cells39,40. Eosinophil recruitment and activation by IL-5 can lead to eosinophilia41. They can be prevalent in the blood and at the inflammatory sites of allergic illnesses, where they function as pro-inflammatory cells by secreting a variety of pro-inflammatory mediators42. Eosinophils are well known to be present in the inflammatory infiltrate of AD and to be associated with disease activity43. The IL-4 and IL-13 cytokines also induce B cells to produce IgE which causes IgE-mediated immediate hypersensitivity by activating the high-affinity receptor for IgE (FcεRI) on mast cells44,45. A high IgE level in the blood is one of the hallmarks of AD. IgE activates mast cells and basophils, causing them to produce pro-inflammatory mediators and cytokines that aggravate AD, leading to erythema and lichenification of the skin46,47. All topically and orally administered tHGA doses significantly reduced both blood and tissue eosinophilia. Furthermore, tHGA was demonstrated to attenuate mast cell infiltration and degranulation. Both topical and oral treatment of tHGA also suppressed the levels of IgE and Th2 cytokines. It's worth mentioning that the positive drug control for oral treatment, ketotifen fumarate, was unable to block mast cell degranulation when compared to the positive drug control for topical treatment, dexamethasone. Our findings were partly compatible with Finn and Walsh48, who reported that ketotifen fumarate inhibited mast cell degranulation in the lung and tonsillar tissues but not in the skin. Ketotifen fumarate is less effective as an antihistamine and mast cell stabiliser than other drugs with dual characteristics49. These accounted for the non-statistically significant effect of ketotifen fumarate on mast cell degranulation. Dexamethasone, on the other hand, has been shown to lower the level of FcεRI on mast cells50,51. FcεRI suppression reduces mast cell activation and degranulation, which may explain the significant decrease in degranulated mast cell count by dexamethasone in the data.
Topically applied tHGA and orally administered tHGA at all doses successfully attenuate most of the AD-related parameters following DNCB stimulation. Nevertheless, oral treatment of tHGA at a lower dose of 20 mg/kg did not result in a substantial reduction in SCORAD, tissue eosinophil infiltration and serum IgE levels. Moreover, only the highest dose of orally administered tHGA, 80 mg/kg, reduced IL-4 levels in the skin. The current findings were consistent with previous observations on tHGA oral treatment in a DNP-induced passive systemic anaphylaxis model in rats, where the lowest dose of 20 mg/kg tHGA demonstrated limited effects on IL-4 release and mast cell degranulation15. Similar results were reported in a murine model of chronic asthma, where the lowest dose of 20 mg/kg tHGA was unable to significantly attenuate serum OVA-specific IgE levels as well as serum IL-4 levels18. On the other hand, topically applied tHGA at all tested doses, even the lowest at 0.2%, significantly reduced the SCORAD, eosinophil infiltration and level of IL-4 in the skin. We examined blood eosinophil count and serum IL-4 levels to validate tHGA's effectiveness when taken orally. All tested doses (20, 40, and 80 mg/kg) reduced blood eosinophils and IL-4 levels in the serum in this murine AD model. The inability of the modest dose of oral tHGA to lower SCORAD, tissue eosinophil infiltration and IL-4 levels in the skin could be explained by its limited therapeutic effects based on previous studies, as well as the skin being one of the poorest perfused organs15,18,52. The epidermis was well-known for its lack of blood vessels. The dermal network of interconnected arterioles and venules called the superficial vascular plexus runs beneath the epidermis in the dermis53,54. The rate of systemic transfer into different organs and tissues is determined by the rate of blood flow to the tissue, tissue mass, and blood-tissue partitioning characteristics, and it follows the perfusion-rate of the diffusion process55. Organs and tissues that are heavily perfused with blood are more likely to distribute drugs quickly. We hypothesise that tHGA taken orally requires a greater dose to improve the drug distribution to the skin and to have a significant effect on SCORAD, tissue eosinophil infiltration and IL-4 levels in the skin.
The bioactive principle of tHGA is the phloroglucinol structural core56. Both synthetic and natural phloroglucinols have shown a wide variety of biological actions. 5-methoxypsoralen, a phloroglucinol-derived compound, has been patented for the oral and topical treatment of psoriasis and other skin disorders57. The tHGA structure includes an acetophenone moiety in addition to the phloroglucinol functional group58. An acetophenone found in another plant called Helichrysum italicum, possesses wound healing and skin protecting characteristics when supplied orally and topically due to its anti-inflammatory action, which was demonstrated in a chronic inflammation model59. Based on the structure of tHGA, we can confidently say that our findings are consistent with prior research that used acetophenone and phloroglucinol to treat skin conditions. However, because different researchers use different experimental methodologies, comparing the results of different investigations can be difficult.
IgE, mast cells, and cytokines, particularly IL-4, all play roles in the pathogenesis of AD. High-affinity IgE receptors, FcɛRI, are expressed on mast cells and increased in the presence of IL-4 and IgE. Furthermore, the presence of IL-4 and IgE promotes mast cell activation60,61. Mast cells release mediators and cytokines such as IL-4 and IL-13, which mediate pro-inflammatory responses and may contribute to the progression of AD60,62. In both oral and topical tests, results from all research parameters suggest that tHGA decreased mast cell-related parameters such as mast cell count, degranulated mast cell count, IL-4, and IgE. We hypothesise that it is related to tHGA's mast cell stabilising effect. Previous research discovered that tHGA reduces IgE-mediated systemic anaphylaxis by blocking mast cell degranulation15,16. It has been observed that tHGA inhibits numerous key proteins in the signalling cascade of IgE-mediated mast cell degranulation, including 5-LOX, COX-2, LAT, PLCγ1, p38, JNK, ERK, cPLA2, PI3K, NF-κB, IκBα, and IKKα/β16. tHGA was unable to block Syk phosphorylation and attenuate its kinase. Further experiments employing the siRNA knockdown assay showed that the compound was unable to prevent mast cell degranulation in LAT-deficient RBL-2H3 cells. These findings suggested that LAT protein might be the molecular target of tHGA in IgE-mediated mast cell activation16. Analysis and assessment of the mechanism of action of tHGA in a number of disease models, such as cancer, allergies, and asthma, have been conducted and thoroughly reviewed13. In this study, instead of delving further into this mechanism, we shifted our focus to investigating the potential of tHGA as a treatment for AD. Specifically, we compared two common treatment routes for AD patients: topical and oral administration. This study holds significant importance for enhancing the pharmacological profile of tHGA. It marks the first exploration of tHGA's effectiveness as a topical anti-allergic treatment, as previous research on the anti-allergic effects of tHGA predominantly emphasised oral and intraperitoneal routes13.
The efficacy of orally and topically treated tHGA in suppressing mast cell-related parameters is potentially associated with reducing both sensitivity and potency of mast cells due to relief of IgE, IL-4, and a significant reduction in activated and degranulated mast cell counts in the current study. Future research should investigate the impact of tHGA on other AD-related biomarkers. For instance, Th1 cytokines (IFN-γ and TNF-α) and other Th2 cytokines (IL-5 and IL-13) are significant in the pathophysiology of AD because they influence the severity of the disease as well as the recruitment of inflammatory cells63,64. Furthermore, skin barrier dysfunction in AD has been linked to decreased expression of molecules associated with epidermal differentiation such as FLG, LOR, and involucrin, as well as decreased total ceramide levels and ceramide composition alterations65.
According to our findings, both orally and topically given tHGA can greatly alleviate AD symptoms, with neither route being superior. In silico prediction studies on the pharmacokinetics and pharmacodynamics of tHGA revealed that tHGA potentially has good intestinal absorption, aqueous solubility, moderate blood–brain barrier penetration, cytochrome P4502D6 (CYP2D6) inhibition, and no hepatotoxicity, making it a promising medication for the oral treatment of tHGA66. Previous studies on tHGA administered orally demonstrated that it reduced airway hyperresponsiveness, prevented airway remodelling, and prevented IgE-mediated passive systemic anaphylaxis and morphological changes in peritoneal mast cells related to mast cell degranulation, indicating that tHGA has adequate oral bioavailability for therapeutic efficacy15,18. Based on allometric scaling for dosage conversion from mice to humans, the pharmacologically active human equivalent dose for a 60 kg human adult was estimated to be 194.4 to 388.8 mg/kg for a mouse dose of 40 to 80 mg/kg67. On the other hand, the tHGA molecular weight is equivalent to 304.08 Da58. To facilitate skin penetration, the majority of topical drugs used in dermatotherapy have molecular weights less than 500 Da, which may explain the efficiency of tHGA when applied topically68. It was expected to have a local effect when applied directly to the diseased skin69,70. Furthermore, data from Toxicity Prediction by Komputer Assisted Technology (TOPKAT) showed that tHGA is biodegradable, non-mutagenic, non-carcinogenic, and non-irritant to the eyes and skin66. Additional comprehensive pharmacokinetic studies of oral and topical administered tHGA are warranted to provide further understanding of the disparity in bioavailability between the two methods of administration.
The utilization of mice as a model has been extensively employed to conduct in-depth research on AD and to facilitate the expedited evaluation of potential treatments for AD71,72. While there have been new developments in rat models of AD, canine and mouse models are already well-established73. Mice AD models are most common utilized due to their cost-effectiveness, ease of handling, rapid maturation, and the ability to manipulate their genetic makeup with ease74. There has been a significant number of literature supporting the preference for using male mice over female mice72. This preference is because male mice are easier to handle, show less variation in results and are more cost-effective. Female mice have a 4-day oestrous cycle, which would require a large number of animals to maintain synchronisation for experiments75. This results in reduced cost-effectiveness. In addition, the hormonal changes that occur throughout the oestrous cycle of female mice lead to variations in their behaviour. There is a minor female predominance in adult AD, according to survey-based epidemiological research76. While sex differences have been reported in general immune system response, there is very limited highlighting the differences in the pathophysiology of AD and responsiveness to therapy between male and female subjects77. Males experience more baseline transepidermal water loss than females in healthy subjects; however, no sex differences in basal transepidermal water loss were observed in a study of children with AD78. The production of oestrogen has been found to have a deleterious effect on the healing process of skin lesions similar to those found in AD79. On the other hand, male mouse castration or androgen receptor antagonistic therapy improved epidermal barrier repair following tape peeling in a male mouse model of AD80. Female mice models were generally excluded due to the assumption that they exhibit greater inherent variation compared to males, which may potentially affect drug discovery and development for improving AD. However, it is imperative to conduct additional trials that evaluate both male and female animals and human subjects to comprehensively examine the influence of sex on treatment outcomes.
In conclusion, orally administered and topically applied tHGA improved systemic inflammation (serum IgE, IL-4) and local barrier disruption (SCORAD, epidermal thickness, inflammatory cell histology), demonstrating that tHGA has systemic and topical inhibitory effects in AD, which may be promising in managing AD in both mild and severe patients. tHGA has the potential to be a viable therapeutic lead for the treatment of AD. The current findings extend prior studies on the pharmacological efficacy and therapeutic potential of tHGA.
Data availability
Data will be made available on request.
References
Ständer, S. Atopic dermatitis. N. Engl. J. Med. 384, 1136–1143 (2021).
Hadi, H. A. et al. The epidemiology and global burden of atopic dermatitis: A narrative review. Life Basel Switz. 11, 936 (2021).
Urban, K. et al. The global, regional, and national burden of atopic dermatitis in 195 countries and territories: An ecological study from the Global Burden of Disease Study 2017. JAAD Int. 2, 12–18 (2021).
Pinart, M. et al. Comorbidity of eczema, rhinitis, and asthma in IgE-sensitised and non-IgE-sensitised children in MeDALL: A population-based cohort study. Lancet Respir. Med. 2, 131–140 (2014).
Bantz, S. K., Zhu, Z. & Zheng, T. The atopic march: Progression from atopic dermatitis to allergic rhinitis and asthma. J. Clin. Cell Immunol. 5, 202 (2014).
Chow, S. et al. A clinician’s reference guide for the management of atopic dermatitis in Asians. Asia Pac. Allergy 8, e41. https://doi.org/10.5415/apallergy.2018.8.e41 (2018).
Zuuren, E. J., Fedorowicz, Z., Christensen, R., Lavrijsen, A. P. & Arents, B. W. Emollients and moisturisers for eczema. Cochrane Database Syst. Rev. 2, CD12119. https://doi.org/10.1002/14651858.cd012119.pub2 (2017).
Drucker, A. M. et al. Use of systemic corticosteroids for atopic dermatitis: International Eczema Council consensus statement. Br. J. Dermatol. 178, 768 (2018).
Griffiths, C. E. M., van de Kerkhof, P. & Czarnecka-Operacz, M. Psoriasis and atopic dermatitis. Dermatol. Ther. 7, 31–41 (2017).
Choi, H. et al. Manifestation of atopic dermatitis-like skin in TNCB-induced NC/Nga mice is ameliorated by topical treatment of substance P, possibly through blockade of allergic inflammation. Exp. Dermatol. 27, 396–402 (2018).
Arkwright, P. D. et al. Management of difficult-to-treat atopic dermatitis. J. Allergy Clin. Immunol. Pract. 1, 142–151 (2013).
Shaari, K. et al. Bioassay-guided identification of an anti-inflammatory prenylated acylphloroglucinol from Melicope ptelefolia and molecular insights into its interaction with 5-lipoxygenase. Bioorg. Med. Chem. 19, 6340–6347 (2011).
Chan, Y. H. et al. Pharmacological properties of 2,4,6-trihydroxy-3-geranyl acetophenone and the underlying signaling pathways: Progress and prospects. Front. Pharmacol. 12, 736339. https://doi.org/10.3389/fphar.2021.736339 (2021).
Ismail, N. et al. A geranyl acetophenone targeting cysteinyl leukotriene synthesis prevents allergic airway inflammation in ovalbumin-sensitized mice. Toxicol. Appl. Pharmacol. 259, 257–262 (2012).
Tan, J. W. et al. Anti-allergic activity of 2,4,6-trihydroxy-3-geranylacetophenone (tHGA) via attenuation of IgE-mediated mast cell activation and inhibition of passive systemic anaphylaxis. Toxicol. Appl. Pharmacol. 319, 47–58 (2017).
Tan, J. W. et al. LAT is essential for the mast cell stabilising effect of tHGA in IgE-mediated mast cell activation. Biochem. Pharmacol. 144, 132–148 (2017).
Keith, Y. H., Egawa, G., Honda, T. & Kabashima, K. Mast cells in type 2 skin inflammation: Maintenance and function. Eur. J. Immunol. 53, 2250359. https://doi.org/10.1002/eji.202250359 (2023).
Lee, Y. Z. et al. An orally active geranyl acetophenone attenuates airway remodeling in a murine model of chronic asthma. Eur. J. Pharmacol. 797, 53–64 (2017).
Myers, K. et al. Treatment preferences among patients with mild-to-moderate atopic dermatitis. J. Dermatol. Treat. 34, 2215356. https://doi.org/10.1080/09546634.2023.2215356 (2023).
Megna, M. et al. Systemic treatment of adult atopic dermatitis: A review. Dermatol. Ther. 7, 1–23 (2016).
Lee, Y. Z. et al. Blockade of eosinophil-induced bronchial epithelial–mesenchymal transition with a geranyl acetophenone in a coculture model. Front. Pharmacol. 8, 837 (2017).
Yap, H. M. et al. The geranyl acetophenone tHGA attenuates human bronchial smooth muscle proliferation via inhibition of AKT phosphorylation. Sci. Rep. 8, 16640. https://doi.org/10.1038/s41598-018-34847-0 (2018).
National Research Council. Guide for the Care and Use of Laboratory Animals, 8th edn. http://www.ncbi.nlm.nih.gov/books/NBK54050/ (National Academies Press, 2011).
Sert, N. P. et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. BMC Vet. Res. 16, 242 (2020).
Jung, M. et al. Inhibitory effect of 5,6-dihydroergosteol-glucoside on atopic dermatitis-like skin lesions via suppression of NF-κB and STAT activation. J. Dermatol. Sci. 79, 252–261 (2015).
Choi, Y. Y., Kim, M. H., Lee, H., Jo, S. Y. & Yang, W. M. (R)-(+)-pulegone suppresses allergic and inflammation responses on 2,4-dinitrochlorobenzene-induced atopic dermatitis in mice model. J. Dermatol. Sci. 91, 292–300 (2018).
Ku, J. M. et al. Effects of Angelicae dahuricae radix on 2, 4-dinitrochlorobenzene-induced atopic dermatitis-like skin lesions in mice model. BMC Complement. Altern. Med. 17, 98 (2017).
Arifin, W. N. & Zahiruddin, W. M. Sample size calculation in animal studies using resource equation approach. Malays. J. Med. Sci. 24, 101–105 (2017).
Lococo, D., Mora-Huertas, C. E., Fessi, H., Zaanoun, I. & Elaissari, A. Argan oil nanoemulsions as new hydrophobic drug-loaded delivery system for transdermal application. J. Biomed. Nanotechnol. 8, 843–848 (2012).
Hikita, I. et al. Characterization of dermatitis arising spontaneously in DS-Nh mice maintained under conventional conditions: Another possible model for atopic dermatitis. J. Dermatol. Sci. 30, 142–153 (2002).
Weidinger, S., Beck, L. A., Bieber, T., Kabashima, K. & Irvine, A. D. Atopic dermatitis. Nat. Rev. Dis. Primer 84, 1–20 (2018).
Silverberg, J. I. et al. What are the best endpoints for Eczema Area and Severity Index and Scoring Atopic Dermatitis in clinical practice? A prospective observational study. Br. J. Dermatol. 184, 888–895 (2021).
De Vuyst, E., Salmon, M., Evrard, C., Lambert de Rouvroit, C. & Poumay, Y. Atopic dermatitis studies through in vitro models. Front. Med. 4, 119. https://doi.org/10.3389/fmed.2017.00119 (2017).
Jin, H., He, R., Oyoshi, M. & Geha, R. S. Animal models of atopic dermatitis. J. Investig. Dermatol. 129, 31–40 (2009).
Grabbe, S. & Schwarz, T. Immunoregulatory mechanisms involved in elicitation of allergic contact hypersensitivity. Immunol. Today 19, 37–44 (1998).
Watanabe, H., Unger, M., Tuvel, B., Wang, B. & Sauder, D. N. Contact hypersensitivity: The mechanism of immune responses and T cell balance. J. Interferon Cytokine Res. Off J. Int. Soc. Interferon Cytokine Res. 22, 407–412 (2002).
Brandt, E. B. & Sivaprasad, U. Th2 cytokines and atopic dermatitis. J. Clin. Cell Immunol. 2, 110 (2011).
Chiricozzi, A., Maurelli, M., Peris, K. & Girolomoni, G. Targeting IL-4 for the treatment of atopic dermatitis. ImmunoTargets Ther. 9, 151–156 (2020).
Lee, G. R. & Flavell, R. A. Transgenic mice which overproduce Th2 cytokines develop spontaneous atopic dermatitis and asthma. Int. Immunol. 16, 1155–1160 (2004).
Spergel, J. M., Mizoguchi, E., Oettgen, H., Bhan, A. K. & Geha, R. S. Roles of TH1 and TH2 cytokines in a murine model of allergic dermatitis. J. Clin. Investig. 103, 1103–1111 (1999).
Radonjic-Hoesli, S., Brüggen, M.-C., Feldmeyer, L., Simon, H.-U. & Simon, D. Eosinophils in skin diseases. Semin. Immunopathol. 43, 393–409 (2021).
Wu, T., Tang, L., Feng, Y., Jia, Y. & Li, F. Eosinophils and associated parameters in different types of skin diseases related to elevated eosinophil levels. Ann. Transl. Med. 10, 73 (2022).
Simon, D., Braathen, L. R. & Simon, H.-U. Eosinophils and atopic dermatitis. Allergy 59, 561–570 (2004).
Galli, S. J., Maurer, M. & Lantz, C. S. Mast cells as sentinels of innate immunity. Curr. Opin. Immunol. 11, 53–59 (1999).
Kawakami, T., Ando, T., Kimura, M., Wilson, B. S. & Kawakami, Y. Mast cells in atopic dermatitis. Curr. Opin. Immunol. 21, 666–678 (2009).
Vaneckova, J. & Bukač, J. The severity of atopic dermatitis and the relation to the level of total IgE, onset of atopic dermatitis and family history about atopy. Food Agric. Immunol. 27, 734–741 (2016).
Vigo, P. G. et al. Efficacy of anti-IgE therapy in patients with atopic dermatitis. J. Am. Acad. Dermatol. 55, 168–170 (2006).
Finn, D. F. & Walsh, J. J. Twenty-first century mast cell stabilizers. Br. J. Pharmacol. 170, 23–37 (2013).
McGill, J. I. A review of the use of olopatadine in allergic conjunctivitis. Int. Ophthalmol. 25, 171–179 (2004).
Robin, J. L., Seldin, D. C., Austen, K. F. & Lewis, R. A. Regulation of mediator release from mouse bone marrow-derived mast cells by glucocorticoids. J. Immunol. 135, 2719–2726 (1985).
Yamaguchi, M. et al. Regulation of mouse mast cell surface Fc epsilon RI expression by dexamethasone. Int. Immunol. 13, 843–851 (2001).
Bruley, D. F. & Bicher, H. I. Oxygen Transport to Tissue: Pharmacology, Mathematical Studies, and Neonatology (Springer, 1973). https://doi.org/10.1007/978-1-4684-5089-7.
Al-kaf, A. G. A. & Othman, A. M. A review on needle free injections. Univers. J. Pharm. Res. 2, 1–5 (2017).
Mohamed, S. A. & Hargest, R. Surgical anatomy of the skin. Surg. Oxf. 40, 1–7 (2022).
Kok-Yong, S. & Lawrence, L. Drug distribution and drug elimination in basic pharmacokinetic concepts and some clinical applications. IntechOpen https://doi.org/10.5772/59929 (2015).
Ng, C. H. et al. Synthesis and docking studies of 2,4,6-trihydroxy-3-geranylacetophenone analogs as potential lipoxygenase inhibitor. Molecules 19, 11645–11659 (2014).
Singh, I. P., Sidana, J., Bansal, P. & Foley, W. J. Phloroglucinol compounds of therapeutic interest: Global patent and technology status. Expert Opin. Ther. Pat. 19, 847–866 (2009).
Shaari, K., Safri, S., Abas, F., Lajis, N. H. & Israf, D. A. A geranylacetophenone from the leaves of Melicope ptelefolia. Nat. Prod. Res. 20, 415–419 (2006).
Antunes Viegas, D., Palmeira-de-Oliveira, A., Salgueiro, L., Martinez-de-Oliveira, J. & Palmeira-de-Oliveira, R. Helichrysum italicum: From traditional use to scientific data. J. Ethnopharmacol. 151, 54–65 (2014).
Sehra, S., Serezani, A. P. M., Ocaña, J. A., Travers, J. B. & Kaplan, M. H. Mast cells regulate epidermal barrier function and the development of allergic skin inflammation. J. Investig. Dermatol. 136, 1429–1437 (2016).
Stone, K. D., Prussin, C. & Metcalfe, D. D. IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol. 125, S73–S80. https://doi.org/10.1016/j.jaci.2009.11.017 (2010).
Oh, M., Zhu, Z., Yu, J. & Zheng, T. Role of mast cells in the development of atopic dermatitis induced by IL-13. J. Allergy Clin. Immunol. 129, AB36. https://doi.org/10.1016/j.jaci.2011.12.796 (2012).
Furue, M. et al. Pathogenesis of atopic dermatitis: Current paradigm. Iran. J. Immunol. 16, 97–107 (2019).
Renert-Yuval, Y. et al. Biomarkers in atopic dermatitis—A review on behalf of the International Eczema Council. J. Allergy Clin. Immunol. 147, 1174-1190.e1. https://doi.org/10.1016/j.jaci.2021.01.013 (2021).
Nakahara, T., Kido-Nakahara, M., Tsuji, G. & Furue, M. Basics and recent advances in the pathophysiology of atopic dermatitis. J. Dermatol. 48, 130–139 (2021).
Ng, C. H. et al. Hits-to-lead optimization of the natural compound 2,4,6-trihydroxy-3-geranyl-acetophenone (tHGA) as a potent LOX inhibitor: Synthesis, structure-activity relationship (SAR) study, and computational assignment. Mol. J. Synth. Chem. Nat. Prod. Chem. 23, 2509 (2018).
Nair, A. B. & Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 7, 27–31 (2016).
Bos, J. D. & Meinardi, M. M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. 9, 165–169 (2000).
Herkenne, C. et al. In vivo methods for the assessment of topical drug bioavailability. Pharm. Res. 25, 87–103 (2008).
Price, G. & Patel, D. A. Drug Bioavailability. http://www.ncbi.nlm.nih.gov/books/NBK557852/ (StatPearls Publishing, 2023).
Inagaki, N. & Nagai, H. Analysis of the mechanism for the development of allergic skin inflammation and the application for its treatment: Mouse models for the development of remedies for human allergic dermatitis. J. Pharmacol. Sci. 110, 251–259 (2009).
Mohd Kasim, V. N. K. et al. Management of atopic dermatitis via oral and topical administration of herbs in murine model: A systematic review. Front. Pharmacol. 13, 785782. https://doi.org/10.3389/fphar.2022.785782 (2022).
Martel, B. C., Lovato, P., Bäumer, W. & Olivry, T. Translational animal models of atopic dermatitis for preclinical studies. Yale J. Biol. Med. 90, 389–402 (2017).
Löwa, A., Jevtić, M., Gorreja, F. & Hedtrich, S. Alternatives to animal testing in basic and preclinical research of atopic dermatitis. Exp. Dermatol. 27, 476–483 (2018).
Wald, C. & Wu, C. Biomedical research. Of mice and women: The bias in animal models. Science 327, 1571–1572 (2010).
Barbarot, S. et al. Epidemiology of atopic dermatitis in adults: Results from an international survey. Allergy 73, 1284–1293 (2018).
Tuttle, K. L., Forman, J. & Beck, L. A. Novel systemic treatments in atopic dermatitis: Are there sex differences?. Int. J. Womens Dermatol. 7, 606–614 (2021).
Hon, K. L. et al. Age, sex, and disease status as determinants of skin hydration and transepidermal water loss among children with and without eczema. Hong Kong Med. J. 26, 19–26 (2020).
Mukai, K., Urai, T., Asano, K., Nakajima, Y. & Nakatani, T. Evaluation of effects of topical estradiol benzoate application on cutaneous wound healing in ovariectomized female mice. PLoS One 11, e0163560. https://doi.org/10.1371/journal.pone.0163560 (2016).
Kao, J. S. et al. Testosterone perturbs epidermal permeability barrier homeostasis. J. Investig. Dermatol. 116, 443–451 (2001).
Acknowledgements
The authors would also like to convey their profound appreciation to Chan Yee Han and Liew Kong Yen for their help, counsel, and unwavering support during all phases of this study.
Funding
This work was supported by Universiti Putra Malaysia under Geran Putra Berimpak (UPM/800-3/3/1/GPB/9657600). Vivi Nur Khalieda Mohd Kasim was a recipient of Universiti Putra Malaysia Graduate Research Assistantship (GRA). The authors would like to thank Research Management Centre, Universiti Putra Malaysia and Center of Excellence for Research, Value Innovation and Entrepreneurship (CERVIE), UCSI University for their financial support in publishing this work.
Author information
Authors and Affiliations
Contributions
Vivi Nur Khalieda Mohd Kasim: Conceptualization, Investigation, Data curation, Formal analysis, Writing—original draft. Yu Zhao Lee: Formal analysis, Visualization, Writing—original draft. Ikmal Hisyam Bakrin: Methodology, Validation, Resources. Mohd Khairi Hussain: Methodology, Validation, Resources. Daud Ahmad Israf: Supervision, Methodology, Validation, Resources. Khozirah Shaari: Supervision, Methodology, Validation, Resources. Ji Wei Tan: Methodology, Writing—review and editing. Ming Tatt Lee: Methodology, Validation, Resources. Chau Ling Tham: Conceptualization, Funding acquisition, Supervision, Writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mohd Kasim, V.N.K., Lee, Y.Z., Bakrin, I.H. et al. Oral and topical administration of a geranyl acetophenone attenuates DNCB-induced atopic dermatitis-like skin lesions in BALB/c mice. Sci Rep 14, 17623 (2024). https://doi.org/10.1038/s41598-024-66601-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-66601-0
- Springer Nature Limited