Abstract
Milvexian, an oral activated Factor XI (FXIa) inhibitor, is in clinical studies where it may be combined with antiplatelet agents, including aspirin and/or clopidogrel, to prevent thromboembolic diseases. This phase I trial assessed safety, pharmacokinetics, and pharmacodynamics of milvexian coadministration with aspirin and/or clopidogrel in healthy participants through 3 drug-drug interaction studies using a 3-period, 3-treatment, crossover design. A total of 113 participants were randomized to receive milvexian (200 mg; twice daily for 5 days) or matched placebo coadministered with once-daily aspirin (325 mg for 5 days) and/or clopidogrel (Day 1: 300 mg; Days 2–5: 75 mg). Milvexian was safe and well tolerated, with and without aspirin and/or clopidogrel. Eight mild bleeding adverse events (AEs) were reported in 5 of 113 participants across various treatment arms. Peak and total exposures of milvexian were similar with or without clopidogrel and/or aspirin. Exposure-dependent prolongation of activated partial thromboplastin time and reduction of FXI clotting activity by milvexian were similar with coadministration of aspirin and/or clopidogrel. Milvexian, with or without coadministration of aspirin and/or clopidogrel, did not affect bleeding time or platelet aggregation. Administration of milvexian alone or with aspirin and/or clopidogrel was safe and well tolerated without increased incidence of AEs, including bleeding. Pharmacokinetic and pharmacodynamic effects of milvexian, including bleeding time, were similar with or without aspirin and/or clopidogrel.
ClinicalTrials.gov Identifier: NCT03698513.
Similar content being viewed by others
Introduction
The clinical benefits of antithrombotic therapy are well established for the prevention of thromboembolic events in patients with a broad range of existing cardiovascular diseases, such as nonvalvular atrial fibrillation, acute coronary syndrome (ACS), coronary artery disease (CAD), and peripheral artery disease (PAD)1,2. Although improvements in the therapeutic index of anticoagulant therapy have been made, dose-dependent bleeding continues to be observed3. Therefore, improving the benefit/risk profiles remains a viable goal for anticoagulant drug discovery.
Blood coagulation involves the activation of plasma proteases, their cofactors, and platelets, with 2 distinct coagulation pathways that converge at Factor X4,5. One coagulation pathway, the intrinsic pathway, is important in pathological conditions (ie, thrombotic and thromboembolic events), but not for hemostasis6. Factor XI (FXI) is a component of the intrinsic pathway and has been proposed to play an important role in maintaining and propagating a formed thrombus7,8. Activated FXI (FXIa) enhances the stability of clots and amplifies thrombin generation when coagulation is initiated by tissue factor or the intrinsic pathway7,8. Clinical, preclinical, and epidemiologic studies have shown that modulation of FXI may provide a novel mechanism for systemic anticoagulation without increasing the risk of clinically relevant bleeding in a variety of conditions associated with a high risk of thrombotic events, though clinical evidence to support the use of FXI modulation is still being generated and assessed9,10,11,12.
Milvexian is an oral, selective, small-molecule inhibitor of FXIa, being developed for the prevention and treatment of thromboembolic events13. Depending on the patient population, milvexian is expected to provide an improved benefit/risk profile as replacement or add-on to current guideline-recommended antithrombotic agents that include aspirin and/or clopidogrel14,15,16,17. In a phase I study of healthy participants, milvexian was safe and well tolerated at doses ≤ 500 mg daily for 14 days with no major bleeding, clinically significant bleeding events, or other serious adverse events (AEs)18. In a phase I study of participants with normal, mildly impaired, and moderately impaired hepatic function, a single 60-mg dose of milvexian was safe and well tolerated19. Additionally, milvexian was safe and well tolerated in a phase I study of participants with normal renal function, and moderate or severe renal impairment who received a single 60-mg dose20.
Preclinical data and clinical drug-drug interaction (DDI) of a spray-dried dispersion formulation studies have indicated that milvexian is predominantly metabolized by cytochrome P450 (CYP)–3A4 with low urine excretion (< 20%)18,19. In comparison, aspirin is primarily hydrolyzed by human carboxylesterase 2 in both intestine and liver to salicylic acid (active metabolite with respect to inflammation, but inactive metabolite with respect to thrombosis or hemostasis), which subsequently is bioconjugated by a number of CYP enzymes, and mainly excreted through the urine21,22,23. Clopidogrel is predominately (~ 85% of absorbed drug) first-passed hydrolyzed by human carboxylesterase 1 in the liver to clopidogrel acid (an inactive metabolite), and the rest is metabolized by CYP2C19, CYP1A2, CYP2B6, CYP2C9, and CYP3A4 to mediate the bioactivation, and mainly excreted through the urine and feces22,24. No pharmacokinetic (PK) interactions between milvexian, aspirin, and clopidogrel were expected18,19,20,25,26, but potential interactions were investigated as part of the drug development process. This study aimed to assess the impact of coadministration of milvexian with aspirin and/or clopidogrel on safety profiles and PK and pharmacodynamic (PD) properties to support the use of these agents in combination.
Results
Participant characteristics
A total of 304 subjects were screened, and 113 (37.2%) subjects, all of whom were clopidogrel responders, were randomized to Parts 1 (n = 37), 2 (n = 37), and 3 (n = 39). Across groups, participants had similar baseline characteristics, including mean age, weight, and body mass index (BMI; Table 1). The mean age ranged from 33.4 to 35.1 years, 92.3% to 97.3% were male, and mean BMI ranged from 26.1 to 27.2 kg/m2 among participants in Parts 1 to 3 of the study. In Part 1, 35 of 37 (94.6%) randomized participants completed the study; 2 (5.4%) participants requested to discontinue treatment (not due to AEs). In Part 2, 33 of 37 (89.2%) randomized participants completed the study; 4 (10.8%) participants discontinued the study (1 due to an AE of hypotension and 3 due to the participants’ request to discontinue treatment). In Part 3, 35 of 39 (89.7%) randomized participants completed the study; 4 (10.3%) participants discontinued the study (1 due to a bleeding AE of anal fissure hemorrhage and 3 due to poor/noncompliance).
Safety and tolerability
Milvexian 200 mg twice daily (BID) given for 5 days with and without antiplatelet therapy (clopidogrel 300 mg once daily [QD] on Day 1 then 75 mg QD on Days 2–4 and/or aspirin 325 mg QD) was safe and well tolerated. No deaths or serious AEs occurred during the study (Table 2). Mild bleeding AEs assessed as related to various treatment groups were reported in 5 participants, and no numerical imbalance in the incidence of bleeding was observed when milvexian was coadministered with dual antiplatelet therapy or with aspirin or clopidogrel, separately. Specifically, in Part 1, a single participant reported 2 events of contusion after administration of milvexian + aspirin + clopidogrel (Treatment A) and 1 event after placebo + aspirin + clopidogrel (Treatment C), and 1 participant reported 1 event of gingival bleeding and 2 events of vessel puncture–site bruise after administration of placebo + aspirin + clopidogrel (Treatment C). In Part 2, a single participant reported 1 event of contusion after administration of placebo + clopidogrel (Treatment E). In Part 3, a single participant reported 1 event of anal fissure hemorrhage after administration of milvexian alone (Treatment G), and 1 participant reported 1 event of epistaxis after administration of placebo + aspirin (Treatment H). No notable changes in electrocardiogram (ECG), vital signs, or physical examination results were observed during the study (Supplementary Tables 1–3).
Pharmacokinetics
Impact of aspirin and/or clopidogrel on milvexian
After coadministration of aspirin and clopidogrel with milvexian (Treatment A), the maximum observed plasma concentration (Cmax) and area under the plasma concentration–time curve from time 0 to time of the last quantifiable concentration (AUC(TAU)) of milvexian were reduced by 17% (geometric mean ratio [GMR; 90% confidence interval (CI)]: 0.827 [0.750, 0.911]) and 15% (GMR [90% CI]: 0.851 [0.781, 0.927]), respectively, on Day 1, but were not changed on Day 5 compared with milvexian administered alone (Treatment B; Fig. 1). The time of maximum observed concentration (Tmax) and elimination half-life (T1/2) of milvexian coadministered with aspirin and clopidogrel (Treatment A) were similar to those after milvexian was administered alone (Treatment B; Supplementary Table 4). Mean peak concentration of milvexian across Treatments A and B occurred at a median Tmax of 3 to 4 h, and mean (standard deviation [SD]) T1/2 after the final dose of milvexian was 12.2 (2.46) and 11.4 (1.99) h, respectively.
Effect of aspirin and/or clopidogrel on milvexian PK parameters on Days 1 and 5. (a) Mean (± SD) milvexian plasma concentration versus time profile.* (b) GMR (90% CI) of milvexian Cmax and AUC(TAU). AUC(TAU), area under the plasma concentration–time curve from time 0 to time of last quantifiable concentration; BID, twice daily; CI, confidence interval; Cmax, maximum observed concentration; GMR, geometric mean ratio; PK, pharmacokinetics; QD, once daily; SD, standard deviation. *Individual plots of the curves in panel a on Days 1 and 5 can be found in Supplementary Fig. 1. †Milvexian 200 mg BID on Days 1 to 5. ‡Aspirin 325 mg QD on Days 1 to 5. §Clopidogrel 300 mg QD on Day 1 then 75 mg QD on Days 2 to 5.
After coadministration of clopidogrel with milvexian (Treatment F), the Cmax, AUC(TAU), Tmax and T1/2 of milvexian were similar compared with milvexian administered alone (Treatment D; Fig. 1, Supplementary Table 4). After coadministration of aspirin with milvexian (Treatment I), the Cmax, AUC(TAU), Tmax, and T1/2 of milvexian were also similar compared with milvexian administered alone (Treatment G).
Impact of milvexian on clopidogrel
After coadministration of clopidogrel with milvexian (Treatment F), the Cmax of clopidogrel was reduced 9.1% (GMR [90% CI]: 0.909 [0.766, 1.078]) on Day 1 but was similar compared with treatment with clopidogrel alone (Treatment E) on Day 5; AUC(TAU) of clopidogrel with milvexian (Treatment F) on Days 1 and 5 were similar compared with clopidogrel alone (Treatment E; Fig. 2a and b). The Cmax of clopidogrel acid after Treatment F was similar compared with clopidogrel alone (Treatment E) on Day 1, but reduced 14% (GMR [90% CI]: 0.865 [0.788, 0.950]) on Day 5; AUC(TAU) of clopidogrel acid after Treatment F on Days 1 and 5 were similar compared with clopidogrel alone (Treatment E). When clopidogrel was coadministered with milvexian (Treatment F), median Tmax and mean T1/2 of both clopidogrel and clopidogrel acid were generally comparable to the corresponding values observed with clopidogrel alone (Treatment E; Supplementary Table 5). Median Tmax ranged from 1 to 1.5 h on Day 1 and 0.5 to 1 h on Day 5. Mean T1/2 values of approximately 1 to 2 h were observed for clopidogrel administered with (Treatment F) and without (Treatment E) milvexian on Days 1 and 5. Mean T1/2 values for clopidogrel acid were approximately 8 h in both treatments (Treatments F and E) on Days 1 and 5.
Effect of milvexian on the PK parameters of clopidogrel and its metabolite (clopidogrel acid) on Days 1 and 5. (a) Mean (± SD) clopidogrel and clopidogrel acid plasma concentration versus time profiles. (b) GMR (90% CI) of clopidogrel and clopidogrel acid Cmax and AUC(TAU). AUC (TAU), area under the plasma concentration–time curve from time 0 to time of last quantifiable concentration; BID, twice daily; CI, confidence interval; Cmax, maximum observed concentration; GMR, geometric mean ratio; PK, pharmacokinetics; QD, once daily; SD, standard deviation. *Clopidogrel 300 mg QD on Day 1 then 75 mg QD on Days 2 to 5. †Aspirin 325 mg QD on Days 1 to 5. ‡Milvexian 200 mg BID on Days 1 to 5.
Under additional influence by aspirin (coadministration of aspirin and clopidogrel with milvexian; Treatment A), the Cmax of clopidogrel was reduced 14% (GMR [90% CI]: 0.861 [0.745, 0.995]) and 10% (GMR [90% CI]: 0.904 [0.794, 1.031]) on Days 1 and 5, respectively, while AUC(TAU) was similar on Day 1, but increased 8.7% (GMR [90% CI]: 1.087 [0.894, 1.321]) on Day 5 compared with aspirin and clopidogrel without milvexian (Treatment C; Fig. 2); the Cmax and AUC(TAU) of clopidogrel acid after Treatment A were similar on Days 1 and 5 compared with aspirin and clopidogrel without milvexian (Treatment C). When clopidogrel was coadministered with aspirin and milvexian (Treatment A), median Tmax and mean T1/2 of both clopidogrel and clopidogrel acid were generally comparable to the corresponding values observed with aspirin and clopidogrel without milvexian (Treatment C; Supplementary Table 5).
Impact of milvexian on aspirin
After coadministration of aspirin with milvexian (Treatment I), Cmax and AUC(TAU) of acetylsalicylic acid increased 40% (GMR [90% CI]: 1.404 [1.226, 1.607]) and 24% (GMR [90% CI]: 1.240 [1.176, 1.308]), respectively, on Day 1 and increased 33% (GMR [90% CI]: 1.332 [1.138, 1.560]) and 19% (GMR [90% CI]: 1.194 [1.144, 1.247]), respectively, on Day 5 compared with administration of aspirin alone (Treatment H; Fig. 3). When aspirin was coadministered with milvexian (Treatment I), the Cmax and AUC(TAU) of salicylic acid were similar on both Days 1 and 5 compared with aspirin alone (Treatment H); median Tmax and mean T1/2 of both acetylsalicylic acid and salicylic acid (after Treatment I) were generally comparable to the corresponding values observed with aspirin alone (Treatment H; Supplementary Table 6).
Effect of milvexian on the PK parameters of aspirin (acetylsalicylic acid) and its metabolite (salicylic acid) on Days 1 and 5. (a) Mean (± SD) acetylsalicylic acid and salicylic acid plasma concentration versus time profiles. (b) GMR (90% CI) of acetylsalicylic acid and salicylic acid Cmax and AUC(TAU). AUC (TAU), area under the plasma concentration–time curve from time 0 to time of last quantifiable concentration; BID, twice daily; CI, confidence interval; Cmax, maximum observed concentration; GMR, geometric mean ratio; PK, pharmacokinetics; QD, once daily; SD, standard deviation. *Aspirin 325 mg QD on Days 1 to 5. †Clopidogrel 300 mg QD on Day 1 then 75 mg QD on Days 2 to 5. ‡Milvexian 200 mg BID on Days 1 to 5.
Under additional influence by clopidogrel (coadministration of aspirin and clopidogrel with milvexian; Treatment A), the Cmax, AUC(TAU), Tmax, and mean T1/2 of acetylsalicylic acid on Day 1 were similar compared with aspirin and clopidogrel without milvexian (Treatment C; Fig. 3); the Cmax and AUC(TAU) of acetylsalicylic acid increased 12% (GMR [90% CI]: 1.118 [0.994, 1.258]) and 22% (GMR [90% CI]: 1.218 [1.159, 1.280]), respectively, on Day 5. When aspirin and clopidogrel were coadministered with milvexian (Treatment A), the Cmax, AUC(TAU), Tmax, and mean T1/2 of salicylic acid were similar on both Days 1 and 5 compared with aspirin and clopidogrel without milvexian (Treatment C; Supplementary Table 6).
Pharmacodynamics
Impact of aspirin and/or clopidogrel on milvexian aPTT and FXIc
Exposure-dependent prolongation of activated partial thromboplastin time (aPTT) and reduction of Factor XI clotting activity (FXIc) were observed with milvexian administration, and effects were similar when milvexian was administered alone (Treatments B, D, and G) or in combination with aspirin and/or clopidogrel (Treatments A, F, and I; Fig. 4a, b). The maximum mean aPTT value ranged from 68.3 to 72.2 s at 16 h following milvexian administration alone (Treatments B, D, and G) or in combination with aspirin and/or clopidogrel (Treatments A, F, and I) on Day 1 and returned to baseline by 72 h postdose on Day 5 (Fig. 4a). Similarly, the minimum mean FXIc ranged from 26.7% to 32.5% at 16 h following milvexian administration alone (Treatments B, D, and G) or with aspirin and/or clopidogrel (Treatments A, F, and I) on Day 1 and returned to baseline by 72 h postdose on Day 5 (Fig. 4b).
Effect of aspirin and/or clopidogrel on the PD parameters of milvexian. Mean (± SD) (a) aPTT and (b) FXIc versus time profiles of milvexian with or without aspirin and/or clopidogrel. aPTT, activated partial thromboplastin time; BID, twice daily; FXIc, Factor XI clotting activity; PD, pharmacodynamic; QD, once daily; SD, standard deviation. *Milvexian 200 mg BID on Days 1 to 5. †Aspirin 325 mg QD on Days 1 to 5. ‡Clopidogrel 300 mg QD on Day 1 then 75 mg QD on Days 2 to 5.
Impact of milvexian on aspirin and/or clopidogrel on bleeding time and platelet aggregation
Mean bleeding time at baseline ranged from 3.78 to 4.56 min across treatment groups. Administration of milvexian alone (Treatments B, D, and G) did not increase bleeding times; mean bleeding times ranged from 4.16 to 5.08 min at 4 h following milvexian administration on Days 1 and 5 (Fig. 5a). Coadministration of aspirin and/or clopidogrel with milvexian (Treatments A, F, and I) increased bleeding time to a similar value as administration of aspirin and/or clopidogrel without milvexian (Treatments C, E, and H), respectively. Mean bleeding times ranged from 4.97 to 6.13 min at 4 h following administration of aspirin alone (Treatment H) or with milvexian (Treatment I) on Days 1 and 5, whereas mean bleeding times ranged from 9.1 to 12.13 min at 4 h following administration of clopidogrel alone (Treatment E) or with milvexian (Treatment F) on Days 1 and 5.
Effect of milvexian on the PD parameters of aspirin and/or clopidogrel.* (a) Mean (± SD) bleeding time versus time profiles. (b–d) mean (± SE) plasma platelet aggregation induced by (b) ADP, (c) collagen, and (d) AA versus time profiles. AA, arachidonic acid; ADP, adenosine diphosphate; BID, twice daily; PD, pharmacodynamic; QD, once daily; SD, standard deviation; SE, standard of error. *Milvexian 200 mg BID on Days 1 to 5. †Aspirin 325 mg QD on Days 1 to 5. ‡Clopidogrel 300 mg QD on Day 1 then 75 mg QD on Days 2 to 5.
Platelet aggregation induced by adenosine diphosphate (ADP) or collagen was changed by < 10% compared with baseline following administration of milvexian alone (Treatment B, D, and G) on Days 1 and 5 (Fig. 5b, c). Although a decrease in arachidonic acid (AA)-induced platelet aggregation was observed following administration of milvexian alone (Treatment B, D, and G), this was not consistent and did not correlate with milvexian concentration (Fig. 5d). Coadministration of aspirin and clopidogrel with or without milvexian (Treatments A and C, respectively) resulted in ~ 50%, 60%, and 95% inhibition of ADP-, collagen-, and AA-induced aggregation, respectively, compared with baseline. Administration of clopidogrel alone (Treatment E) resulted in ~ 50% inhibition of ADP-induced aggregation and ~ 60% inhibition of AA-induced aggregation with little effect (< 10%) on collagen-induced aggregation compared with baseline. Administration of aspirin alone (Treatment H) resulted in ~ 15% inhibition of ADP-induced aggregation, ~ 25% inhibition of collagen-induced platelet aggregation, and ~ 95% inhibition of AA-induced platelet aggregation compared with baseline.
Discussion
Despite clear evidence that the combination of anticoagulant and antiplatelet therapy reduces the incidence of thrombotic events in patients with cardiovascular disease (eg, ACS, CAD, or PAD), it is also associated with an increase in the risk of bleeding-related AEs27,28,29,30. Therefore, it is important to fully characterize the potential DDI of new anticoagulant agents with the current guideline-recommended antiplatelet drugs in these patient populations. Aspirin and clopidogrel are some of the most commonly recommended antiplatelet therapies for treatment of and to reduce the risk of thrombotic events in patients with coronary disease and non-cardioembolic stroke14,15,16,17. This phase I study evaluated the potential DDI between milvexian and aspirin and/or clopidogrel in healthy participants. The findings demonstrate that there is no evidence of increased risk of serious or non-serious AEs, including serious bleeding events, associated with the administration of multiple doses of milvexian with dual antiplatelet therapy (aspirin and clopidogrel) or single antiplatelet therapy (aspirin or clopidogrel) or significant changes in PK parameters of milvexian in healthy participants.
The percentage of participants reporting AEs was generally consistent across treatments in each part of the study with a slightly higher number of AEs reported after coadministration of placebo and aspirin in Part 3 (23.7% versus 13.5% to 19.4% across all other treatments). Further, the number of participants reporting bleeding AEs was similar across treatments; there was no numerical imbalance in the incidence of bleeding AEs and all bleeding AEs were mild. The results showed no evidence of an increased risk of AEs overall, including bleeding, associated with the administration of multiple doses of milvexian, with or without dual antiplatelet therapy.
Regarding PK, coadministration of aspirin and/or clopidogrel with milvexian did not affect the median Tmax and mean T1/2 of milvexian, aspirin (acetylsalicylic acid) and its metabolite (salicylic acid), and clopidogrel and its metabolite (clopidogrel acid). Coadministration of aspirin and clopidogrel with milvexian did not alter the Cmax and AUC(TAU) of milvexian on Day 5, although slight reductions were observed on Day 1 (Cmax, 17%; AUC[TAU], 15%; both were not statistically significant), compared with milvexian administered alone. Coadministration of milvexian with clopidogrel did not alter the Cmax and AUC(TAU) of clopidogrel and its metabolite. However, coadministration of milvexian with aspirin increased the Cmax and AUC(TAU) of acetylsalicylic acid on Day 5, compared with treatment with aspirin alone, whereas coadministration of milvexian with aspirin had no effect on the salicylic acid metabolite. Potential explanations for increased exposure in acetylsalicylic acid include: an increase in bioavailability due to disintegration of a tablet formulation of aspirin31, period effect, and gastric emptying rates32,33. Nevertheless, it should be noted that this increase in acetylsalicylic acid exposure was not expected to be clinically relevant by investigators given the small magnitude of increase.
Prolongation of aPTT and reduction of FXIc were observed after administration of milvexian with greater effects observed at higher concentrations. These observations are consistent with milvexian’s mechanism of action and previous observations in phase I studies18,19,20 and the in vitro studies and in vivo evaluation in experimental thrombosis in rabbits34. No additional prolongation of aPTT or reduction in FXIc were observed when milvexian was coadministered with aspirin or clopidogrel compared with administration of milvexian alone; this lack of effect on coagulation tests is expected based on the mechanism of action of clopidogrel and aspirin observed in other studies2,21,35. Additionally, these results are similar to previous observation from a phase I study of SHR2285 (ClinicalTrials.gov Identifier: NCT04945616), a small-molecule FXIa inhibitor, which showed that when combined with aspirin, clopidogrel, or ticagrelor, SHR2285 was safe and well tolerated with significant effects on aPTT and FXI activity and no evidence of an increased risk of bleeding36. Furthermore, the current study found no effects on platelet aggregation beyond the effects of aspirin and/or clopidogrel alone with coadministration of milvexian. Platelet aggregation was not affected by administration of milvexian alone. These observations are consistent with the mechanism of action of milvexian, which selectively inhibits FXIa and acts as an anticoagulant13,18,19,34.
Milvexian has been investigated in 2 phase II studies. In the AXIOMATIC-TKR study of patients who underwent knee arthroplasty, postoperative treatment with milvexian reduced the incidence of venous thromboembolism in a dose-dependent manner (ranging from 25 mg QD to 200 mg BID) and was associated with a low risk of bleeding37. The safety and efficacy of milvexian were also investigated in the phase II AXIOMATIC-SSP study of patients with acute ischemic stroke or high risk transient ischemic attack and high risk of recurrent stroke on a background of aspirin and clopidogrel38; milvexian (25 mg QD or 25, 50, 100, 200 mg BID) did not produce a statistically significant dose response for the composite outcome of symptomatic ischemic stroke or covert brain infarction and did not increase symptomatic intracranial or fatal bleeding compared with placebo39.
The current study showed no evidence of a safety impact with no increased risk of bleeding and limited impact on PK and PD profiles. A key strength of this study is that overall it is a relatively large, comprehensive phase I study design controlling for intra-individual variability across all potential interactions among the aforementioned drugs. A potential limitation of the study is the small sample sizes in each part of the study. Additional measurements of the concentrations of aspirin and/or clopidogrel and their metabolites are warranted for future studies to confirm potential DDIs of milvexian with aspirin and/or clopidogrel as well as to confirm the safety findings of the current study. Another potential limitation is the generalizability of the study findings as the study population included healthy individuals who were relatively young, thus, these results may not fully extend to patients with cardiovascular diseases or individuals of older age.
Conclusions
This phase I DDI study in healthy adults demonstrated that administration of milvexian alone or in combination with aspirin and/or clopidogrel was safe and well tolerated and was not associated with an increased incidence of AEs, including bleeding. PK parameters and PD effects of milvexian were similar when milvexian was administered alone or in combination with aspirin and/or clopidogrel. Likewise, PK parameters and PD effects of aspirin and/or clopidogrel were generally similar when aspirin and/or clopidogrel were administered alone or in combination with milvexian. Lack of effect of milvexian on bleeding time may have promising safety implications for its potential use as an add-on to aspirin and/or clopidogrel. The results obtained in this study will help to inform the future clinical development of milvexian.
Methods
Participants
Healthy participants aged 18 to 55 years with a BMI of 18.0 to 32.0 kg/m2 were eligible for inclusion in the study. Women who were of childbearing potential or breastfeeding, or participants who had any significant acute or chronic medical illness, any other condition listed as a contraindication in the aspirin package insert, evidence of coagulopathy, or a history of bleeding were excluded from the study.
Study design
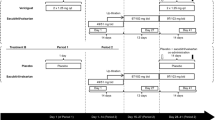
The study (ClinicalTrials.gov Identifier: NCT03698513) was conducted at 1 clinical research center in the United States. The study planned to enroll 108 healthy participants and consisted of 3 parts; each part was a single DDI study using a randomized, 3-period, 3-treatment, crossover design (Fig. 6a and b). The following DDIs were analyzed in each part: Part 1: aspirin, clopidogrel, and milvexian; Part 2: clopidogrel and milvexian; and Part 3: aspirin and milvexian. Participants underwent screening evaluations to determine eligibility within 35 days of study drug administration, and eligible participants were randomly assigned 1:1:1 to a single part of the study. Participants randomized to Parts 1 and 2 underwent prerandomization screening to confirm that they were clopidogrel responders (ie, a decrease in platelet aggregation of ≥ 30% after a single 600-mg dose of clopidogrel compared with baseline40). In Part 1, randomized participants received either coadministration of milvexian 200 mg BID on Days 1 to 5, aspirin 325 mg QD on Days 1 to 5, and clopidogrel 300 mg QD on Day 1 then 75 mg QD on Days 2 to 5 (Treatment A); milvexian 200 mg BID alone on Days 1 to 5 (Treatment B); or coadministration of matched placebo BID on Days 1 to 5, aspirin 325 mg QD on Days 1 to 5, and clopidogrel 300 mg QD on Day 1 then 75 mg QD on Days 2 to 5 (Treatment C; Fig. 6b). In Part 2, randomized participants received either milvexian 200 mg BID alone on Days 1 to 5 (Treatment D); coadministration of matched placebo on Days 1 to 5 and clopidogrel 300 mg QD on Day 1 then 75 mg QD on Days 2 to 5 (Treatment E); or coadministration of milvexian 200 mg BID on Days 1 to 5 and clopidogrel 300 mg QD on Day 1 then 75 mg QD on Days 2 to 5 (Treatment F). In Part 3, randomized participants received either milvexian 200 mg BID alone on Days 1 to 5 (Treatment G); coadministration of matched placebo BID and aspirin 325 mg QD on Days 1 to 5 (Treatment H); or coadministration of milvexian 200 mg BID and aspirin 325 mg QD on Days 1 to 5 (Treatment I).
The 200-mg BID dose regimen of milvexian was selected for this study as it was shown to be generally safe and well tolerated18,41, and the steady-state plasma concentrations of milvexian produced by this dose were within the therapeutic ranges observed in preclinical studies34. Milvexian 200 mg BID was also the highest dose of milvexian planned for investigation in phase II studies. Aspirin 325-mg tablets were investigator-sourced in commercial packaging (batch Nos. NAA6C2B and NAA6NT3), clopidogrel 75-mg and 300-mg tablets were investigator-sourced in commercial packaging (batch Nos. T-01758 and T01924D [300 mg] and 3088070 [75 mg]), and milvexian and matching placebo were provided as 100-mg size 0 gray capsules in 100-count bottles (batch Nos. ABA1625 [milvexian] and ABA2214 [placebo]).
Ethics
These studies were conducted in accordance with Good Clinical Practice, as defined by the International Council for Harmonisation and in accordance with the ethical principles underlying European Union Directive 2001/20/EC and the United States Code of Federal Regulations and was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki. The protocols, amendments, and participant-informed consents received appropriate approval by the Institutional Review Board/Independent Ethics Committee prior to the initiation of the study at the site. Prior to beginning the studies, all participants provided written informed consent.
Assessments
Safety assessments were performed at selected times throughout the dosing interval (Supplementary Table 7). The assessments were based on medical review of AE and bleeding-related AE reports, and the results of vital sign measurements, ECG measurements, physical examinations, and clinical laboratory tests. PK assessments of milvexian were performed on Days 1 (≤ 24 h) and 5 (≤ 72 h) of each period (Supplementary Table 8); assessments included Cmax, AUC[(TAU) Tmax, and T1/2. Individual PK parameters were derived by noncompartmental methods using Phoenix® WinNonlin® PK analysis program (Certara USA, Inc., Princeton, New Jersey; version 8.0). The plasma samples for milvexian, aspirin (acetylsalicylic acid) and its metabolite (salicylic acid), and clopidogrel and its metabolite (clopidogrel acid) were analyzed by validated high-performance liquid chromatography (LC)–tandem mass spectrometry/mass spectrometry (MS/MS) assays18. Quantification of milvexian in plasma samples was performed by LC-tandem MS/MS with the quantification range of 1.00 to 1000 ng/mL. Quantitation of clopidogrel and clopidogrel acid in plasma samples were performed by high-performance liquid chromatography (HPLC) coupling MS/MS with the quantification range of 0.200 to 200 ng/mL, and 10.0 to 10,000 ng/mL, respectively. Quantitation of acetylsalicylic acid and salicylic acid in plasma samples were also performed via HPLC coupling MS/MS with the quantification range of 20.0 to 10,000 ng/mL, and 100 to 50,000 ng/mL, respectively.
PD assessments were performed on Days 1 (≤ 24 h) and 5 (≤ 72 h) of each period (Supplementary Table 8); assessments included aPTT18, FXIc18, and bleeding time (assessed according to the PPD® Laboratories [Austin, TX] procedure where a blood pressure cuff, situated above the incision site, provided pressure at 40 mmHg and blood from the incision was wicked with filter paper every 30 s until cessation to record bleeding time). In addition, AA (1 mM), ADP) (20 µM)-, and collagen (5 μg/ml)-induced platelet aggregation assays were performed using light transmission aggregometry (PPD® Laboratories) and performed on Days 1 and 5 (≤ 24 h) for only Period 1.
Statistical analyses
All statistical analyses and calculations were performed using SAS® software (SAS Institute, Inc., Cary, North Carolina; version 9.3). All safety and plasma PK/PD data were summarized using descriptive statistics. Descriptive summaries were presented for continuous variables using number of subjects (N), mean, SD; median, minimum, and maximum. GMRs with 90% CIs were calculated to compare effects on plasma PK parameters (Cmax and AUC[TAU]). CIs were constructed using a linear mixed-effects model with treatment as a fixed effect and measurements within participants as repeated measures fitted to the log-transformed PK parameters. These calculations assumed that log transformed PK parameters were normally distributed with intrasubject SD of difference (log scale) ≤ 0.338 and 0.182 for Cmax and AUC(TAU), respectively. These SDs of difference were estimated from corresponding 90% CI of adjusted GMRs with (1.197, 1.652) and (1.134, 1.348) for acetylsalicylic acid Cmax and AUC(TAU) observed from a previous study (ClinicalTrials.gov Identifier: NCT03341390) and selected due to higher variabilities compared with the same PK parameters of milvexian and clopidogrel in literature.
Data availability
BMS policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
References
Chen, A., Stecker, E. & Warden, A. Direct oral anticoagulant use: a practical guide to common clinical challenges. J. Am. Heart Assoc. 9, e017559 (2020).
Eikelboom, J. W., Hirsh, J., Spencer, F. A., Baglin, T. P. & Weitz, J. I. Antiplatelet drugs: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 141, e89S-e119S (2012).
Ezekowitz, M. D. et al. Dabigatran with or without concomitant aspirin compared with warfarin alone in patients with nonvalvular atrial fibrillation (PETRO Study). Am. J. Cardiol. 100, 1419–1426 (2007).
Cate, H. T., Hackeng, T. M. & Garcia de Frutos, P. Coagulation factor and protease pathways in thrombosis and cardiovascular disease. Thromb. Haemost. 117, 1265–1271 (2017).
Quan, M. L. et al. Factor XIa inhibitors as new anticoagulants. J. Med. Chem. 61, 7425–7447 (2018).
Fredenburgh, J. C. & Weitz, J. I. Factor XI as a target for new anticoagulants. Hamostaseologie. 41, 104–110 (2021).
Gailani, D., Bane, C. E. & Gruber, A. Factor XI and contact activation as targets for antithrombotic therapy. J. Thromb. Haemost. 13, 1383–1395 (2015).
Puy, C., Rigg, R. A. & McCarty, O. J. The hemostatic role of factor XI. Thromb Res. 141(Suppl 2), S8–S11 (2016).
Salomon, O., Steinberg, D. M., Koren-Morag, N., Tanne, D. & Seligsohn, U. Reduced incidence of ischemic stroke in patients with severe factor XI deficiency. Blood 111, 4113–4117 (2008).
Yang, D. T., Flanders, M. M., Kim, H. & Rodgers, G. M. Elevated factor XI activity levels are associated with an increased odds ratio for cerebrovascular events. Am. J. Clin. Pathol. 126, 411–415 (2006).
Gailani, D. & Gruber, A. Factor XI as a therapeutic target. Arterioscler. Thromb. Vasc. Biol. 36, 1316–1322 (2016).
Salomon, O. et al. Patients with severe factor XI deficiency have a reduced incidence of deep-vein thrombosis. Thromb. Haemost. 105, 269–273 (2011).
Dilger, A. K. et al. Discovery of milvexian, a high-affinity, orally bioavailable inhibitor of factor XIa in clinical studies for antithrombotic therapy. J. Med. Chem. 65, 1770–1785 (2022).
Powers, W. J. et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the Early Management of Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 50, e344–e418 (2019).
European Stroke Organisation Executive Committee & ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc. Dis. 25, 457–507 (2008).
Amsterdam, E. A. et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 130, e344-426 (2014).
Collet, J. P. et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 42, 1289–1367 (2021).
Perera, V. et al. First-in-human study of milvexian, an oral, direct, small molecule factor XIa inhibitor. Clin. Transl. Sci. 15, 330–342 (2022).
Perera, V. et al. Single-dose pharmacokinetics of milvexian in participants with mild or moderate hepatic impairment compared with healthy participants. Clin. Pharmacokinet. 61, 857–867 (2022).
Perera, V. et al. Single-dose pharmacokinetics of milvexian in participants with normal renal function and participants with moderate or severe renal impairment. Clin. Pharmacokinet. 61, 1405–1416 (2022).
Kapil, N. et al. Antiplatelet and anticoagulant therapies for prevention of ischemic stroke. Clin. Appl. Thromb. Hemost. 23, 301–318 (2017).
Tang, M. et al. Antiplatelet agents aspirin and clopidogrel are hydrolyzed by distinct carboxylesterases, and clopidogrel is transesterificated in the presence of ethyl alcohol. J. Pharmacol. Exp. Ther. 319, 1467–1476 (2006).
Bojic, M., Sedgeman, C. A., Nagy, L. D. & Guengerich, F. P. Aromatic hydroxylation of salicylic acid and aspirin by human cytochromes P450. Eur. J. Pharm. Sci. 73, 49–56 (2015).
Jiang, X. L., Samant, S., Lesko, L. J. & Schmidt, S. Clinical pharmacokinetics and pharmacodynamics of clopidogrel. Clin. Pharmacokinet. 54, 147–166 (2015).
Perera, V. et al. Effects of itraconazole and diltiazem on the pharmacokinetics and pharmacodynamics of milvexian, a factor XIa inhibitor. Cardiol. Therapy 11, 407–419 (2022).
Perera, V. et al. Pharmacokinetic and pharmacodynamic effects of coadministration of rifampin and BMS-986177/JNJ-70033093, an oral, direct, reversible, small molecule factor XIa inhibitor. Clin. Pharmacol. Ther. 109, S73 (2021).
Mega, J. L. et al. Rivaroxaban in patients with a recent acute coronary syndrome. N. Engl. J. Med. 366, 9–19 (2012).
Eikelboom, J. W. et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N. Engl. J. Med. 377, 1319–1330 (2017).
Bonaca, M. P. et al. Rivaroxaban in peripheral artery disease after revascularization. N. Engl. J. Med. 382, 1994–2004 (2020).
Qu, J. et al. Dual antiplatelet therapy with clopidogrel and aspirin versus aspirin monotherapy in patients undergoing coronary artery bypass graft surgery. J. Am. Heart Assoc. 10, e020413 (2021).
Markl, D. & Zeitler, J. A. A review of disintegration mechanisms and measurement techniques. Pharm. Res. 34, 890–917 (2017).
Fleisher, D., Li, C., Zhou, Y., Pao, L. H. & Karim, A. Drug, meal and formulation interactions influencing drug absorption after oral administration clinical implications. Clin. Pharmacokinet. 36, 233–254 (1999).
Palleria, C. et al. Pharmacokinetic drug-drug interaction and their implication in clinical management. J. Res. Med. Sci. 18, 601–610 (2013).
Wong, P. C. et al. Milvexian, an orally bioavailable, small-molecule, reversible, direct inhibitor of factor XIa: In vitro studies and in vivo evaluation in experimental thrombosis in rabbits. J. Thromb. Haemost. 20, 399–408 (2022).
Karabulut, H. et al. Clopidogrel does not increase bleeding and allogenic blood transfusion in coronary artery surgery. Eur. J. Cardiothorac. Surg. 25, 419–423 (2004).
Ma, T. et al. SHR2285, the first selectively oral FXIa inhibitor in China: Safety, tolerability, pharmacokinetics and pharmacodynamics combined with aspirin, clopidogrel or ticagrelor. Front. Pharmacol. 13, 1027627 (2022).
Weitz, J. I. et al. Milvexian for the prevention of venous thromboembolism. N. Engl. J. Med. 385, 2161–2172 (2021).
Sharma, M. et al. Rationale and design of the AXIOMATIC-SSP phase II trial: antithrombotic treatment with factor XIa inhibition to optimize management of acute thromboembolic events for secondary stroke prevention. J. Stroke Cerebrovasc. Dis. 31, 106742 (2022).
Sharma, M. et al. Safety and efficacy of factor XIa inhibition with milvexian for secondary stroke prevention (AXIOMATIC-SSP): A phase 2, international, randomised, double-blind, placebo-controlled, dose-finding trial. Lancet Neurol. 23, 46–59 (2024).
Kubitza, D., Becka, M., Muck, W. & Schwers, S. Effect of co-administration of rivaroxaban and clopidogrel on bleeding time, pharmacodynamics and pharmacokinetics: a phase I study. Pharmaceuticals (Basel). 5, 279–296 (2012).
Perera, V. et al. Safety, pharmacokinetics, and pharmacodynamics of milvexian in healthy Japanese participants. Sci. Rep. 12, 5165 (2022).
Acknowledgements
This study was sponsored by Bristol Myers Squibb and Janssen Research & Development, LLC. Medical writing support was provided by Panita Maturavongsadit, PhD, of Lumanity Communications Inc., and was funded by Bristol Myers Squibb and Janssen Global Services, LLC.
Author information
Authors and Affiliations
Contributions
V.P. contributed to the conception and design of the study, acquisition of data, and analysis and interpretation of data; reviewed the article and revised it critically for important intellectual content; and approved of the final version to be submitted. G.A. contributed to the analysis and interpretation of data, reviewed the article and revised it critically for important intellectual content, and approved of the final version to be submitted. J.L. contributed to the conception and design of the study, and analysis and interpretation of data; reviewed the article and revised it critically for important intellectual content; and approved of the final submitted version. R.A. contributed to the conception and design of the study, and interpretation of data; reviewed the article and revised it critically for important intellectual content; and approved of the final submitted version. D.L. contributed to the conception and design of the study, and interpretation of data; reviewed the article and revised it critically for important intellectual content; and approved of the final version to be submitted. Z.W. contributed to the conception and design of the study, acquisition of data, and analysis and interpretation of data; reviewed the article and revised it critically for important intellectual content; and approved of the final version to be submitted. L.Z. contributed to the conception and design of the study, acquisition of data, or analysis and interpretation of data; reviewed the article and revised it critically for important intellectual content; and approved of the final version to be submitted. S.L. contributed to the conception and design of the study, and analysis and interpretation of data; reviewed the article and revised it critically for important intellectual content; and approved of the final version to be submitted. S.M. contributed to the conception and design of the study, and interpretation of data; reviewed the article and revised it critically for important intellectual content; and approved of the final version to be submitted. BM contributed to the conception and design of the study, and analysis and interpretation of data; reviewed the article and revised it critically for important intellectual content; and approved of the final version to be submitted.
Corresponding author
Ethics declarations
Competing interests
G.A., J.L., R.A., D.L., Z.W., S.L., S.M., and B.M. are employees of Bristol Myers Squibb. V.P. was an employee of Bristol Myers Squibb at the time of the study. L.Z. is an employee of Janssen Research & Development, LLC
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Perera, V., Abelian, G., Luettgen, J. et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of milvexian with aspirin and/or clopidogrel in healthy participants. Sci Rep 14, 16591 (2024). https://doi.org/10.1038/s41598-024-67182-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-67182-8
- Springer Nature Limited