Abstract
Lymphatic filariasis (LF) is a crippling and disfiguring parasitic condition. India accounts for 55% of the world’s LF burden. The filarial parasite Wuchereria bancrofti is known to cause 99.4% of the cases while, Brugia malayi accounts for 0.6% of the issue occurring mainly in some pockets of Odisha and Kerala states. The Balasore (Baleswar) district of Odisha has been a known focus of B. malayi transmission. We employed molecular xenomonitoring to detect filarial parasite DNA in vectors. In six selected villages, Gravid traps were used to collect Culex mosquitoes and hand catch method using aspirators was followed for collection of mansonioides. A total of 2903 mosquitoes comprising of Cx. quinquefasciatus (n = 2611; 89.94%), Cx. tritaeniorhynchus (n = 100; 3.44%), Mansonia annuliferea (n = 139; 4.78%) and Mansonia uniformis (n = 53; 1.82%) were collected from six endemic villages. The species wise mosquitoes were made into 118 pools, each with a maximum of 25 mosquitoes, dried and transported to the laboratory at VCRC, Puducherry. The mosquito pools were subjected to parasite DNA extraction, followed by Real-time PCR using LDR and HhaI probes to detect W. bancrofti and B. malayi infections, respectively. Seven pools (6.66%) of Cx. quinquefasciatus, showed infection with only W. bancrofti while none of the pools of other mosquito species showed infection with either W. bancrofti or B. malayi. Although the study area is endemic to B. malayi, none of the vectors of B. malayi was found with parasite infection. This study highlights the ongoing transmission of bancroftian filariasis in the study villages of Balasore district of Odisha and its implications for evaluating LF elimination programme.
Similar content being viewed by others
Introduction
The three primary nematode parasite species that cause lymphatic filariasis (LF), namely Wuchereria bancrofti, Brugia malayi, and Brugia timori, are responsible for the disease's transmission by mosquito species of the Culex, Anopheles, and Mansonia1. According to estimates by the World Health Organization (WHO), over 120 million people are already afflicted with the disease, and roughly 1.3 billion individuals pose a risk of infection2. The W. bancrofti is the most common and extensively dispersed parasite species and responsible for 90% of LF, in Africa, Southeast Asia, and the Western Pacific while, B. malayi is prevalent in Southeast Asia and B. timori is found only in Indonesia3 and Timor Leste4. In India, W. bancrofti is known to cause 99.4% of the cases while, B. malayi is responsible for 0.6% of the problem5. Both brugian and bancroftian infections are prevalent throughout southern parts of India, Odisha, Madhya Pradesh, Assam, and West Bengal6. Currently, 339 districts are endemic for LF in India7.
In India, filariasis elimination programme was launched in 2004 and expanded to cover all the then 256 endemic districts. The main strategy of the elimination programme is the annual Mass Drug Administration (MDA) with diethylcarbmazine and later albendazole in 2007. Currently the programme is covering 339 districts and stopped in 138 districts following the successful demonstration of interruption as evidenced by transmission assessment survey (TAS). Ivermectin is added to two drugs in 2019 and initially covered 5 districts and then expanded to 63 districts in 20235. Now the programme is conducted in two phases, one in February and another in August (Ministry of Health and Family Welfare, 2022; https://pib.gov.in/PressReleasePage.aspx?PRID=2004769).
Odisha state is known to be endemic for both brugian and bancroftian filarial infections. In 1989, a new focus of B. malayi was detected in Chudamani, in Balasore (Baleswar) district of Orissa for the first time8. Recently, Balasore district has been reported as a focus of B. malayi transmission9. Similarly, high prevalence of W. bancrofti infection has been reported from five districts i.e., Bargarh, Balangir, Kandhamal, Kendujhar, and Sambalpur in western Odisha. These districts were earlier considered to be non-endemic10. Mass Drug Administration (MDA) programme was implemented in Odisha in 2004 to achieve the objective of LF elimination as a public health problem by 2027. The initial assessment of impact of the MDA programme depended on night blood smear which is cumbersome, time-consuming and lacks the desired sensitivity. For final assessment, the Transmission Assessment Survey (TAS) is carried out to verify absence of transmission. Filariasis Test strips (FTS) are recommended for TAS which is not only cost prohibitive, but also with short-expiry period. Hence, molecular xenomonitoring in vectors as an alternative tool to evaluate transmission disruption was taken into account11,12,13,14,15,16. Collection of Mansonia mosquitoes, presents notable challenge due to their distinct behavior, making the process of collecting and testing Mansonia mosquitoes a labor-intensive and resource-demanding technique. Alternatively, Culex quinquefasciatus mosquitoes can be used as proxy to check for parasite infection. In the current study we carried out molecular xenomonitoring of W. bancrofti and B. malayi in Culex and Mansonia spp., respectively collected from LF endemic villages of Balasore district, Odisha.
Methods
Study area
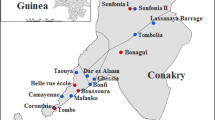
This research study was conducted from September, 2022 to April, 2023 in Balasore district, Odisha, India. Balasore, a coastal district of Odisha, is known to be the old focus of lymphatic filariasis. Both Wuchereria bancrofti and Brugia malayi parasites are prevalent in this district9. According to the endemicity of the district, six villages namely, Aladiha, Dwarika, Kalasimuli, Katramahal, Nuagaon and Panchupalli were selected for the study (Fig. 1). We followed standard protocol of molecular xenomonitoring to know the filarial infection in the mosquito vectors. These details are represented in Fig. 2.
Outline of molecular xenomonitoring activities. Mosquitoes were collected from the study villages using the Gravid traps or mechanical aspirators. The mosquitoes were identified, pooled and transferred to the laboratory. Real-time PCR amplification was carried out using species-specific probes after extraction of DNA form the mosquito pools. B.m – Brugia malayi; W.b – Wuchereria bancrofti.
Mosquito collection
Mosquitoes were collected during the months of September and October 2022 following a standardized procedure of ICMR-Vector Control Research Centre (VCRC), viz. Gravid trap method of Culex mosquito collection16. In brief, mosquitoes were collected by modified CDC Gravid trap using hay infusion as oviposition attractant. The Gravid traps were placed near the selected houses around 6 PM. The traps were left overnight and the head of the family was informed about the survey. Adult mosquitoes trapped were collected, the following day by 6 AM and transferred to the field-laboratory at the Filariasis Control Unit of Balasore. The mosquitoes were carefully transferred to test tube using mechanical aspirator, identified using the key of Barraud17, pooled (25 mosquitoes/pool), dried and transferred to VCRC, Puducherry to check for filarial infection by RT-PCR assay. Since the gravid traps were less productive for the vectors of B. malayi, sweep-net collections were made for outdoor resting mosquitoes (06:00 AM to 07:00 AM) and hand catch collections using aspirators were made for indoor resting mosquitoes (07:00 AM to 09:00 AM).
DNA extraction from mosquito pools
Tris–EDTA (TE) buffer-based boiling method was followed for DNA extraction from the dried mosquito pools18. Briefly, the working table surface was cleaned with 70% ethanol to avoid surface contamination. A 1X TE buffer (pH 8.0) was prepared from 10X stock solution (Medox Biotech India Pvt. Ltd.). From this, 100 µl buffer was added to the mosquito pools and homogenized in TissueLyser II (Qiagen) for 15 min at 25 frequency/sec. The homogenate was gently vortexed, boiled for 10 min and centrifuged. The supernatant containing the DNA sample was used for further processing.
Real-time PCR assay
Real-Time quantitative PCR (qPCR) assay was carried out using specific primers and probes to check for the infection of B. malayi as well as W. bancrofti in mosquito pools, separately19,20. To detect the B. malayi infection, forward primer, HhaI- For- 5’GCGCATAAATTCATCAGC-3’, reverse primer, HhaI- Rev- 5’GCGCAAAACTTAATTACAAAAGC-3’ and TaqMan HhaI-probe- FAM-ACGTGAATTGTACCAGTGCTGGTCG-TAMRA were used. The PCR mixture contained 12.5 µl of TaqMan master mix, primers (1.0 µl each), probe (10 pM; 0.312 µl), and 1.0 µl of the DNA template and nuclease-free water (9.188 µl) in a final volume of 25 µl reaction mixture. For positive controls, the genomic DNA of B. malayi was used. The cycling conditions were 50 °C for 2 min, 95 °C for 10 min, followed by 40 cycles of 95 °C for 15s and 600 C for 1 min.
To check for the W. bancrofti infection, DNA from the same pools of mosquitoes was subjected to qPCR assay using long DNA repeat (LDR) forward primer, LDR1-For-5’ATTTTGATCATCTGGGAACGTTAATA-3’, reverse primer, LDR2-Rev-5’CGACTGTCTAATCCATTCAGAGTGA-3’ and Probe-FAM-ATCTGCCCATAGAAATAAACTACGGTGGATCTG- TAMRA. The cycling parameters for qPCR remained same as mentioned for detection of B. malayi infection. For positive controls, the genomic DNA of W. bancrofti was used and for negative control, nuclease free water was used.
Data analysis
A pool was considered ‘positive’ if the Cycle threshold (Ct) values of samples ranged from 1.0 to 39.0. Indeterminate pool (a mosquito pool was unable to attain the required level of fluorescence above 39) was repeated for confirming the pool as negative or positive20. The percentage of pool positivity was calculated by dividing the total number of pools where filarial parasite DNA is positive over total number of pools screened16,21.
Results
Distribution of mosquito species and composition of pools
A total of 2903 female mosquitoes were collected from six villages of Balasore district during the months of September and October 2022. Among the Culex spp., highest percentage of mosquitoes belonged to Cx. quinquefasciatus (89.94%), followed by Cx. tritaeniorhynchus (3.44%), but only 4.78% and 1.82% of the mosquitoes belonged to Ma. annulifera and Ma. uniformis respectively (Table 1). The Cx. quinquefasciatus mosquitoes were collected from all the six villages while, Cx. tritaeniorhynchus was collected only from Panchupalli village. Similarly, Ma. annulifera was collected from KalasimuIi and Panchupalli villages while Ma. uniformis was collected only from Kalasimuli. Highest number (1050; 36.16%) of mosquitoes were collected from Panchupalli village that included all the species of mosquito except Ma. uniformis. A total of 118 pools were prepared that comprised 105 pools of Cx. quinquefasciatus mosquitoes, followed by 4 pools of Cx. tritaeniorhynchus, 6 pools of Ma. annulifera and 3 pools of Ma. uniformis. These pools were subjected to qPCR for W. bancrofti and B. malayi infection.
Pool positivity in LF endemic villages
Initially, 9 pools of Mansonia spp. mosquitoes were subjected to B. malayi real-time qPCR. Since there was no infection found in these pools, 109 non-vector pools of Culex mosquito spp. were checked for B. malayi infection. None of the pools showed B. malayi infection. However, when they were tested for W. bancrofti, 7 out of 105 pools of Cx. quinquefasciatus showed positivity (6.7%) while, other non-vector mosquitoes didn’t show any infection (Table 2). Among the 7 positive pools, 4 pools from Panchupalli village followed by 2 positive pools from Aladiha and one pool from Kalasimuli.
Discussion
Lymphatic filariasis is a debilitating parasitic illness that affects people all over tropical and subtropical areas of the world. With an intention of elimination of LF as a public health problem by 2020, the global program to eliminate lymphatic filariasis (GPELF) was launched in 2000, and has advanced remarkably. Balasore district of Odisha, India has been covered under annual MDA with DEC and albendazole since 2004 onwards. With over 65% coverage of MDA, the preTAS showed persistence of infection with Mf rate above 1%5. During a coverage and compliance survey in 2002, Balasore district has reported the highest coverage (78.1%) but low (54.4%) consumption22. Since 2004, there was no report of B. malayi in the annual Mf surveys from sentinel and random sites by the Lymphatic Filariasis Elimination Programme of Odisha. However, a recent study in 12 villages showed that Mf prevalence in 6230 individuals tested was 0.99%, with 5 villages having more than 1% threshold of Mf prevalence. Of the total infection, B. malayi infection was 98.4% and the remaining was W. bancrofti in Balasore district9. In addition, the data collected by ICMR-VCRC, Puducherry through Brugia Rapid Test kit and the night blood survey provides evidence of Brugian infection in the Balasore district (unpublished report). The present study reports for the first time the results of molecular xenomonitoring of filarial parasites in Culex and Mansonia mosquitoes.
Traditionally, Culex mosquitoes have been primarily associated with the transmission of W. bancrofti, while Mansonia mosquitoes to B. malayi transmission. Initially, we tried to collect mansonioides to find the B. malayi infection. However, we could not collect sufficient number of mansonioides due to low prevalence, peculiar oviposition behavior, less attractive collection method, and also less abundance of Mansonia spp. during the study period, we collected Culex spp. with assumption that Mf are ingested while feeding on human by these anthropophilic mosquitoes. We collected high density of Culex mosquitoes during the vector collection period. The mosquitoes were subjected to detect W. bancrofti and B. malayi infection in known Culex spp, as non-vector mosquitoes also play role in the transmission of filariasis. However, there was no pool of mosquitoes with B. malayi infection in the non-vector as well as vector mosquitoes. Instead, when we looked for W. bancrofti infection in its principal vector Cx. quinquefasciatus positive pools (6.7%) were observed though the villages selected for the study are reported to be endemic for B. malayi infection. Similar studies have demonstrated high percentage of positive pools for W. bancrofti infection in Cx. quinquefasciatus. McPherson et al.23 reported 18% (86/475) pools positive for W. bancrofti DNA by PCR in primary sampling units of Samoa. While, in American Samoa, 5/585 pools were positive out of 4,413 Cx. quinquefasciatus mosquitoes tested24. In Tanzania, molecular xenomonitoring was carried out to assess the infection status in mosquito vectors after the MDA. Masasi district showed 9% pools (33/365 pools) and Rufiji district showed 0.45% pools (5/1092 pools; total 5460 mosquitoes) positive in mosquito vectors tested after six and twelve rounds of MDA25,26. Srilankan studies have showed 14.7% pools (92/625) positive in coastal and 1.4% (8/583) pools positive in inland evaluation units13. In another study, filarial DNA rate in mosquitoes collected in Matotagama from February 2013 to June 2014 reported positive pools ranging from 0 to 23.4%27. Studies have also reported no positivity in pools of Cx. quinquefasciatus mosquitoes. In Gaibandha and Panchagarh districts of Bangladesh, out of 594 pools (total 10,021 Cx. quinquefasciatus mosquitoes) none of the pool was tested positive28. Similarly, Culex and Mansonia mosquitoes (1801 and 1823 mosquitoes respectively) were negative for W. bancrofti infection in four districts of Ghana29. In Indian settings, molecular xenomonitoring survey showed 11 (3.1%) of the 358 pools (8850 Cx. quinquefasciatus) positive in Cuddalore district of Tamilnadu15. Recently, molecular xenomonitoring was carried out to check the infection in vector mosquitoes during validation of mini-TAS in two non-MDA districts of Odisha. Out of 7990 Cx. quinquefasciatus mosquitoes tested, 3.3% pools (10/300 pools) found to be positive30. However, in this study we observed (6.7%) pools positive for W. bancrofti infection.
Detection of filarial infection in the Culex mosquitoes offers several advantages compared to the Filariasis Test Strip (FTS) kits. Firstly, the FTS kits are expensive and have a short expiry date, which can limit their availability and practicality for large-scale studies. Furthermore, the invasive procedure of the FTS kits limits its applicability for routine surveillance and monitoring purposes. In contrast, the non-invasive collection of Culex mosquitoes offers a more practical and efficient approach through molecular xenomonitoring. It plays a crucial role in elucidating the transmission patterns of LF. By detecting parasite DNA in mosquito vectors, this technique provides valuable information on the presence and prevalence of infection in specific vector species, helping to identify high-risk areas and inform targeted control measures. Molecular xenomonitoring does not appear to be more practical as it requires laboratory and skilled technicians. However, this can be solved at field level by establishing regional laboratories for processing the mosquitoes for molecular assay and training grass root level laboratory technicians in sampling mosquitoes, identification and storing. It should be possible as the collection is seasonal and the stored mosquitoes can be processed by pooling samples from different locations. Njenga et al.31 conducted integrated epidemiological surveys using standard parasitological and entomological methods in Kenya and provided useful information on co-endemic parasitic diseases. The coexistence of B. malayi9 with W. bancrofti (current study) was evident during MDA. However, the present study showed only the presence of W. bancrofti infection in highly anthropophilic Culex quinquefasciatus mosquitoes. This suggests possible disappearance of B. malayi infection from the area as it is more susceptible to DEC. Studies have established the fact that B. malayi parasite is more susceptible to DEC, compared to W. bancrofti32. The parasite species has probably either disappeared or reduced to undetectable levels due to treatment and by the restricted distribution of host plants (Pistia striatus) due to change in the ecology and human activities. Our study has not detected any B. malayi parasite in mosquitoes in the study area, suggesting low prevalence of B. malayi infection. However, more studies with adequate sample size are required to confirm the absence of B. malayi in Mansonia mosquitoes and its transmission.
We observed that even full-fed female Culex mosquitoes do not show any evidence of B. malayi parasite infection. This shows that the same geographical area, molecular xenomonitoring provides valuable insights into the transmission dynamics of LF due to two parasites. This observation suggests that individuals in the area may be at risk of acquiring multiple filarial infections, potentially leading to more severe clinical outcomes and challenges in treatment. Understanding the prevalence and distribution of both the species of parasites is crucial for tailoring interventions and optimizing treatment approaches in areas with multiple parasite species that circulate in a community. Molecular xenomonitoring is one such tool which can be employed to understand the transmission dynamics of filarial parasites when they co-exist.
Conclusion
This study provides valuable insights to the ongoing transmission of W. bancrofti and highlights the importance of molecular xenomonitoring using Culex mosquitoes for understanding LF transmission dynamics in Balasore district of Odisha. This information is crucial for designing effective control and surveillance strategies for elimination of lymphatic filariasis in this region.
Data availability
The data supporting the conclusions of this article are included within the article. The datasets analysed during the present study are available from the corresponding author (PRA) upon reasonable request.
References
WHO. Epidemiology of Lymphatic Filariasis (World Health Organization, Geneva, 2023).
WHO. Global Programme to Eliminate Lymphatic Filariasis: Progress report on mass drug administrations in 2005. Weekly Epidemiological Record 22: 221–232. World Health Organization (2006)
Ottesen, E. A., Hooper, P. J., Bradley, M. & Biswas, G. The global programme to eliminate lymphatic filariasis: health impact after 8 years. PLoS Negl. Trop. Dis. 2(10), e317 (2008).
Fischer, P., Supali, T. & Maizels, R. M. Lymphatic filariasis and Brugia timori: prospects for elimination. Trends Parasitol. 20, 351–355 (2004).
NCVBDCP. National Center for Vector Borne Diseases Control. https://ncvbdc.mohfw.gov.in/index4.php?lang=1&level=0&linkid=458&lid=3730 (2023)
Agrawal, V. K. & Sashindran, V. K. Lymphatic filariasis in India: Problems, challenges and new initiatives. Med. J. Armed Forces India. 62(4), 359–362 (2006).
Sabesan, S. et al. Diethylcarbamazine citrate-fortified salt for lymphatic filariasis elimination in India. Indian J. Med. Res. 155(3&4), 347–355 (2022).
Rath, R. N., Mohapatra, B. N. & Das, B. Detection of a new focus of Brugiamalayi infection in Orissa. J. Commun. Dis. 21(1), 39–40 (1989).
Somalkar, N. et al. Challenges in elimination of filariasis: New foci of B. Malayi infection in Odisha India. J. Commun. Dis. 53(1), 5–9 (2021).
Foo, P. K. et al. High prevalence of Wuchereria bancrofti infection as detected by immunochromatographic card testing in five districts of Orissa, India, previously considered to be non-endemic. Trans. R. Soc. Trop. Med. Hyg. 105(2), 109–114 (2011).
World Health Organization. Lymphatic filariasis: a handbook of practical entomology for national lymphatic filariasis elimination programmes. WHO/HTM/NTD/PCT/2013 (2013)
World Health Organization. Strategy Development and Monitoring for Parasitic Diseases and Vector Control Team. Defining the roles of vector control and xenomonitoring in the Global Programme to Eliminate Lymphatic Filariasis: report of the informal consultation (WHO/HQ, Geneva, 29–31 January 2002)
Rao, R. U., Samarasekera, S. D., Nagodavithana, K. C., Punchihewa, M. W., Dassanayaka, T. D., P K D, G., Ford, E., Ranasinghe, U. S., Henderson, R. H., & Weil, G. J. Programmatic use of molecular xenomonitoring at the level of evaluation units to assess persistence of lymphatic filariasis in Sri Lanka. PLoS Negl. Trop. Dis. 10(5), e0004722 (2016)
Rao, Ramakrishna U., Kumara C. Nagodavithana, Sandhya D. Samarasekera, Asha D. Wijegunawardana, Welmillage D. Y. Premakumara, Samudrika N. Perera, Sunil Settinayake, J. Phillip Miller, and Gary J. Weil. A comprehensive assessment of lymphatic filariasis in srilanka six years after cessation of mass drug administration. PLoS Negl. Trop. Dis. 8(11), e3281 (2014)
Subramanian, S. et al. Molecular xenomonitoring as a post-MDA surveillance tool for global programme to eliminate lymphatic filariasis: Field validation in an evaluation unit in India. PLoS Negl. Trop. Dis. 14(1), e0007862 (2020).
Subramanian, S., Jambulingam, P., Chu, B. K., Sadanandane, C., Vasuki, V., Srividya, A., MohideenAbdulKader, M. S., Krishnamoorthy, K., Raju, H. K., Laney, S. J., Williams, S. A., & Henderson, R. H. Application of a household-based molecular xenomonitoring strategy to evaluate the lymphatic filariasis elimination program in Tamil Nadu, India. PLoS Negl. Trop. Dis. 11(4) (2017)
Barraud, P.J. The fauna of British India, including Ceylon and Burma. Diptera Vol. V. Family Culicidae. Tribes Megarhinini and Culicini. Taylor and Francis, London, 463 pp (1934)
Vasuki, V., Subramanian, S., Hoti, S. L. & Jambulingam, P. Use of a simple DNA extraction method for high-throughput detection of filarial parasite Wuchereria bancrofti in the vector mosquitoes. Parasitol. Res. 111(6), 2479–2481 (2012).
Rao, R. U., Weil, G. J., Fischer, K., Supali, T. & Fischer, P. Detection of Brugia parasite DNA in human blood by real-time PCR. J. Clin. Microbiol. 44(11), 3887–3893 (2006).
Rao, R. U. et al. A real-time PCR-based assay for detection of Wuchereria bancrofti DNA in blood and mosquitoes. Am. J. Trop. Med. Hygiene. 74(5), 826–832 (2006).
Katholi, C. R., Toé, L., Merriweather, A. & Unnasch, T. R. Determining the prevalence of Onchocerca volvulus infection in vector populations by polymerase chain reaction screening of pools of black flies. J. infect. Dis. 172(5), 1414–1417 (1995).
Babu, B. V. & Kar, S. K. Coverage, compliance and some operational issues of mass drug administration during the programme to eliminate lymphatic filariasis in Orissa India. Trop. Med. Int. Health TM & IH 9(6), 702–9 (2004).
McPherson, B. et al. Evaluating molecular xenomonitoring as a tool for lymphatic filariasis surveillance in Samoa, 2018–2019. Trop. Med. Infect. Dis. 7(8), 203 (2022).
Schmaedick, M. A. et al. Molecular xenomonitoring using mosquitoes to map lymphatic filariasis after mass drug administration in American Samoa. PLoS Negl. Trop. Dis. 8(8), e3087 (2014).
Jones, C. et al. Lymphatic filariasis transmission in Rufiji District, southeastern Tanzania: Infection status of the human population and mosquito vectors after twelve rounds of mass drug administration. Parasit. Vect. 11(1), 588 (2018).
Lupenza, E., Gasarasi, D. B. & Minzi, O. M. Lymphatic filariasis, infection status in Culex quinquefasciatus and Anopheles species after six rounds of mass drug administration in Masasi District Tanzania. Infect. Dis. Povert. 10(1), 20 (2021).
Takagi, H. et al. Surveillance of Wuchereria bancrofti infection by anti-filarial IgG4 in urine among schoolchildren and molecular xenomonitoring in Sri Lanka: a post mass drug administration study. Trop. Med. Health 47, 39 (2019).
Irish, S. R. et al. Molecular xenomonitoring for Wuchereria bancrofti in Culex quinquefasciatus in two districts in Bangladesh supports transmission assessment survey findings. PLoS Negl. Trop. Dis. 12(7), e0006574 (2018).
Pi-Bansa, S. et al. Assessing the presence of Wuchereria bancrofti infections in vectors using Xenomonitoring in lymphatic filariasis endemic districts in Ghana. Trop. Med. Infect. Dis. 4(1), 49 (2019).
Panda, B. B. et al. Mini-TAS as a confirmatory mapping tool for remapping areas with uncertain filarial endemicity to exclude/ include for mass drug administration: A report from field validation in India. PloS one 18(11), e0293641 (2023).
Njenga, S. M. et al. Integrated survey of helminthic neglected tropical diseases and comparison of two mosquito sampling methods for lymphatic filariasis molecular xenomonitoring in the River Galana area, Kilifi County, coastal Kenya. PloS one 17(12), e0278655 (2022).
Edeson, J. F. The epidemiology and treatment of infection due to Brugia malayi. Bull. World Health Organ. 27(4–5), 529–541 (1962).
Acknowledgements
The project was funded by the Indian Council of Medical Research-Vector Control Research Centre, Puducherry, through an intramural grant (IM-1901). The support extended by the NVBDCP officer and VBD consultant, Balasore district is gratefully acknowledged. We further acknowledge the technical assistance rendered by Mr. D. Gunasekanran and Mr. Shanta Kumar for collection of mosquitoes and Ms. Shabura for assisting in DNA extraction and Real-Time PCR assays.
Author information
Authors and Affiliations
Contributions
All authors contributed significantly to this study. Supervision and implementation of field work was undertaken by P.R.A. Laboratory work was performed by B.R. and P.M. Data were analyzed by B.R., P.R.A. and P.M. The first draft of the manuscript was written by PM and P.R.A. The manuscript was revised by K.K., S.L.H. and A.K. Advice and final editorial feedback prior to manuscript submission was provided by K.K., S.L.H. and A.K. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abraham, P.R., Ramalingam, B., Mohapatra, P. et al. Detection of Wuchereria bancrofti infection in mosquitoes in areas co-endemic with Brugia malayi in Balasore district, Odisha, India. Sci Rep 14, 16780 (2024). https://doi.org/10.1038/s41598-024-67188-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-67188-2
- Springer Nature Limited