Abstract
Carbapenem-resistant Klebsiella pneumoniae (CPKP) infections seriously threaten global public health. The main objective of this study was to assess the in-vitro synergistic activity of ceftazidime–avibactam (CZA) in combination with colistin (COL), amikacin (AK), gentamicin (GEN), and fosfomycin (FOS) against CPKP isolates. The secondary goal was to determine the antibiotic susceptibility performance of BD Phoenix. OXA-48 (49.1%) was the predominant carbapenemase, followed by KPC (29.1%). We used the broth microdilution (BMD) method to determine the minimum inhibitory concentrations (MICs) of CZA, COL, AK, and GEN. Meanwhile, the MICs of FOS were determined by the agar dilution (AD) method. To examine the antibacterial activity of CZA, we conducted a checkerboard assay (CBA) with COL, AK, GEN, and FOS against CRKP isolates. We randomly selected three strains and performed synergy testing via time-kill assay (TKA). CRKP isolates were 89.1% susceptible to CZA, 16.4% to COL, 21.8% to GEN, and 29.1% to AK using BMD, 47.3% to FOS by AD. The most synergistic effects were observed in the combination of CZA-COL (78.2%) and CZA-FOS (63.6%). Given the limited therapeutic options for treating severe CRKP infections, combining CZA with COL and FOS may enhance in-vitro activity against clinical CRKP isolates.
Similar content being viewed by others
Introduction
Antimicrobial resistance in gram-negative bacteria has become a major threat to global public health in recent years. It has become increasingly challenging to find therapeutic options for multi-drug resistant (MDR) organisms and carbapenem-resistant Enterobacterales (CRE). This healthcare challenge is associated with significant morbidity and mortality, as well as the substantial cost of management1. CRE causes more than 13,000 infections and results in over 1000 deaths in the United States annually2. Klebsiella pneumoniae is the primary opportunistic pathogen of healthcare-associated CRE infections3.
Ceftazidime–avibactam (CZA) is a novel combination of ceftazidime, a broad-spectrum cephalosporin, with avibactam, a non-β-lactam β-lactamase inhibitor. CZA exhibits a broad spectrum of activity against a variety of β-lactamases expressed by Enterobacterales, including class A extended-spectrum β-lactamases (ESBLs), serine carbapenemases like KPC, class C cephalosporinases, and some class D oxacillinases. However, it is important to note that CZA does not inhibit the activity of metallo-β-lactamases (MBLs). It is worth noting that non-MBLs mediated CZA resistance in CRE is growing concern. It is important to be aware that isolates with a mutation to the blaKPC gene omega loop and those with increased expression of blaKPC in the context of reduced permeability may exhibit CZA resistance4. Although it has not been long since its approval, reports of CZA-resistant strains were published soon after its launch5.
Polymyxins, including colistin (polymyxin E) (COL), are cationic antimicrobial peptides used as the last-line therapy to treat MDR gram-negative bacterial infections. Polymyxins are bactericidal drugs that disrupt the outer cell membrane, allowing other drugs (such as CZA) to reach transpeptidase enzymes and other targets more efficiently6.
On the other hand, aminoglycosides inhibit protein synthesis by binding to the 30S ribosome. However, aminoglycoside resistance is commonly caused by mobile genes that code for aminoglycoside-modifying enzymes (AMEs). These genes can resist some aminoglycosides and are particularly common among MBLs-producing Enterobacterales. The activity of aminoglycosides may also vary against Enterobacterales. Aminoglycosides can reduce the activity of drug efflux pumps, which in turn, can reduce the resistance of other drugs. This leads to collateral sensitivity, making aminoglycosides an important tool in fighting bacterial infections7. Numerous studies have shown the combined activity of aminoglycosides and beta-lactams in treating infections caused by gram-negative organisms8.
Although fosfomycin (FOS) is an old phosphoenolpyruvate analog antibiotic, it is a potent bactericidal agent. It can be a viable alternative treatment option against carbapenem-resistant Klebsiella pneumoniae (CPKP) infections. However, when used alone, resistance to FOS develops rapidly9.
Several large-scale studies have shown no significant difference in mortality between patients who manage infections caused by CRE with CZA alone and those treated with combination regimens10. However, it is important to note that CZA resistance emergence was reported at 3.0% in the combination group and 4.1% in the monotherapy group11. Additionally, increasing rates of CZA resistance have been reported, especially in KPC-positive isolates12.
The main objective of this study was to assess the combined in vitro activity of CZA with COL, AK, GEN, and FOS against CPKP isolates. The secondary goal was to evaluate the antibiotic susceptibility performance of BD Phoenix (US) for these antibiotics.
Materials and methods
Antibiotics, media, bacterial strains, and susceptibility testing
This research was carried out at a university hospital with 900 beds, which is the largest one in the Southern Marmara region. During the period between November 2020 and November 2022, a total of 55 isolates of CRKP were arbitrarily collected from patients who were in the medicine wards and intensive care units (ICU) of our hospital. Only adult inpatients were included in the study, and each patient was represented by only one isolate. The isolates were cultured on 5% sheep blood agar plates (BD diagnostic systems, USA) and incubated at 37 °C overnight. They were identified using matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDITOF MS) (Bruker Daltonik, Bremen, Germany) (IVD v 12.0 database) before undergoing in vitro antimicrobial susceptibility tests. Only those with a score of two or higher were included in the tests.
We conducted antibiotic susceptibility testing using the BD Phoenix™ M50 System per the recommendations of The European committee on antimicrobial susceptibility testing (EUCAST) guideline of 202313. In our study, we identified carbapenem-resistant strains using the BD Phoenix CPO detect (NMIC/ID-505) panel. To maintain quality control, we used the reference strain K. pneumoniae ATCC 700603. The carbapenemase content of the isolates had been previously characterized by Xpert Carba-R assay v. 2 (Cepheid, Sunnyvale, CA, USA).
Ceftazidime (Cayman Chemical Company, USA), avibactam (Pfizer, USA), colistin sulfate (Sigma, Germany), amikacin sulfate (ChemCruz, USA), gentamicin sulfate (Biobasic, Canada), fosfomycin disodium salt (Sigma, Germany), sodium carbonate (Sigma, Germany), and glucose-6-phosphate disodium salt (Biobasic, Canada) were obtained commercially.
MIC determinations
The minimum inhibitory concentrations (MIC) for CZA, COL, AK, GEN, and FOS were determined using two methods. The broth microdilution (BMD) method was used for CZA, COL, AK, and GEN on cation-adjusted Mueller Hinton Broth (CAMHB) (Oxoid, Basingstoke, United Kingdom). The agar dilution (AD) method was used for FOS. These methods were performed following the guidelines of the Clinical and Laboratory Standards Institute (CLSI)14. While the CLSI guideline was used for applying BMD and AD methods, breakpoints were determined according to EUCAST13. For FOS susceptibility testing in the AD method, cation-adjusted Mueller Hinton Agar (CAMHA) containing 25 μg/ml of glucose-6-phosphate (CAMHA-G6P) was used. The antibiotic media were prepared according to the EUCAST Document using a water bath at a temperature of 40–50 °C, and subsequent dilutions of FOS were added to the liquid agar15. The MIC ranges of individual drugs were determined according to CLSI recommendations: CZA (0.0625/4–64/4 μg/ml), COL (0.0625–64 μg/ml), AK (0.0625–64 μg/ml), GEN (0.015–16 μg/ml), and FOS (0.125–128 μg/ml), breakpoints were determined according to EUCAST.
The BMD plates (CZA, COL, AK, and GEN) were used to test each test organism. The plates were inoculated with 105 CFU/ml (1/20 McFarland 0.5) of each test organism in 100 µl CAMHB and then incubated for 18–20 h at 37 °C. One well with no antibiotic was used as a positive growth control, and another with no bacteria was used as a negative control on each plate. The plates were then read using a magnifying mirror reader for visual turbidity, and the results were recorded. The MICs were determined as the lowest drug concentration with no visible growth16. In the AD method, 107 CFU/ml (1/10 McFarland 0.5) of each test organism was prepared, and 2 µl of bacteria were inoculated onto the CAMHA-G6P with different concentrations of FOS. The plates were then incubated for 18–20 h at 37 °C. The experiments were performed at least three times on different days, and Escherichia coli ATCC 25922 was used as a quality control strain.
Analysis of the MIC results
The results of the commercial BD Phoenix M50 System were compared with those of the reference BMD and AD. The ISO 20776-2 guideline was used to calculate categorical agreement (CA), very major errors (VME), and major errors (ME). For a technique to be considered reliable, the following criteria should be met: CA ≥ 90%, VME ≤ 3%, and ME ≤ 3%17.
Checkerboard analysis of combination effects
We conducted a study to test the activity of CZA in combination with COL, AK, and GEN against CRKP isolates by using the BMD method, as well as CZA in combination with FOS using the AD method16. The MIC ranges were determined for all combinations, as shown below;
-
1. CZA+COL; using BMD, CZA (0.0625/4–64/4 μg/ml) and COL (1–64 μg/ml),
-
2. CZA+GEN; using BMD, CZA (0.0625/4–64/4 μg/ml) and GEN (0.25–16 μg/ml),
-
3. CZA+AK; using BMD, CZA (0.03/4–32/4 μg/ml) and AK (1–64 μg/ml),
-
4. CZA+FOS; using AD, CZA (0.125/4–32/4 μg/ml) and FOS (4–128 μg/ml).
We prepared the combinations using 96-well flat-bottom microtiter plates with two-fold serially diluted drug concentrations in the checkerboard assay (CBA) using the BMD method in-house. The two drugs were mixed in a 96-well plate, and then a standard bacterial suspension was added with a final concentration of 5 * 105 CFU/mL (1/20 McFarland 0.5) in CAMHB. After incubating for 18 h at 37 °C, we observed the results using a magnifying mirror reader for visual turbidity.
Using the AD method for the CBA, a combination of CZA and FOS agents was prepared using CAMHA-G6P with two-fold serially diluted drug concentrations in-house (Fig. 1). The two drugs were mixed in each agar (Fig. 1) and a standard bacterial suspension was created with a final concentration of 107 CFU/mL (1/10 McFarland 0.5). 2 µl of each bacteria were inoculated onto the CAMHA-G6P agar surface. After 18–20 h of incubation at 37 °C, the results were observed, and the MIC was determined as the lowest concentration of the agent that inhibits growth, as judged by the naked eye (Fig. 2)15.
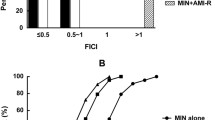
We calculated the fractional inhibitory concentrations (FIC) for each strain to determine the activity of antibiotics in combination. This was done using the formula: FIC A = MIC of antibiotic A in combination/MIC antibiotic A alone; FIC B = MIC of antibiotic B in combination/MIC antibiotic B alone. We then used the fractional inhibitory concentration index (FICI) to describe the interaction effects of the antibiotics in vitro. The FICI is the sum of the two FIC values (FIC of antibiotic A + FIC of antibiotic B) for each strain. The lowest FIC index of all the non-turbid wells along the turbidity/non-turbidity interface was used. The activity of the antimicrobial combination was categorized as synergistic when the FICI was ≤ 0.5, additive when 0.5 < FICI ≤ 1, indifferent when 1 < FICI ≤ 4, and antagonistic when FICI > 416.
Time-kill assay
Based on our PCR results, three strains were arbitrarily selected into the different classes of carbapenemase genes (class A, B, and D). CAMHB- containing 25 μg/ml of glucose-6-phosphate (CAMHB-G6P), was used for time-kill assay (TKA)16. A direct suspension from a pure overnight culture was incubated on a mechanical shaker in CAMHB at 37 °C with shaking until visibly turbid. Suspensions were adjusted to 1 McFarland standard in saline and then diluted to the last concentration of 6* 105 CFU/mL (1/5 McFarland 1). Colony counts were performed to verify inoculum densities. A sampling of the inoculum incubated with CZA, COL, AK, GEN, FOS, CZA plus COL, CZA plus AK, CZA plus GEN, and CZA plus FOS was performed at times of 0, 4, 8, and 24 h of post-incubation at 37 °C. Antibiotics were prepared in the MICs of all the strains. A growth control without any antibiotic was included in each experiment16. Synergy was defined as a ≥ 2 log10 CFU/mL decrease by the combination compared with the most active single agent at 4, 8, and 24 h. Indifference was defined as a < 2 log10 CFU/mL decrease between the combination and the most active single drug alone. Antagonism was defined as a ≥ 2 log10 CFU/mL increase decrease by the combination compared with the most active single agent18.
Ethical approval
The study adhered to the principles of the Declaration of Helsinki and received ethical approval from the Bursa Uludag University Ethics Committee (2021-18/12). Informed consent was obtained from all subjects.
Results
Bacterial isolates
Our research involved 55 CRKP strains, each belonging to a different patient. These strains were grown from clinical specimens collected between November 2020 and November 2022. The patients were of varying ages (ranging from 23–86) and genders (35 males and 20 females). The strains were collected from different parts of the body, such as blood (n:25, 45.5%), deep tracheal aspirate (n:18, 32.7%), wound pus (n:6, 10.9%), sputum (n:3, 5.5%), urine (n:2, 3.6%), and cerebrospinal fluid (n:1, 1.8%). OXA-48 production was the most common (49.1%), followed by KPC production (29.1%), co-production of KPC and OXA-48 (10.9%), NDM production (3.7%), co-production of VIM and NDM (1.8%), co-production of OXA-48 and NDM (1.8%), co-production of KPC, OXA-48, and NDM (1.8%), and the absence of any gene (1.8%).
Antimicrobial susceptibility and analysis of the MIC results
A total of 55 CRKP isolates, with no repetition, were tested for susceptibility using the automated BD Phoenix™ M50 System according to EUCAST, 2023. The results indicate that 48 (87.3%) isolates were susceptible to CZA, 14 (25.5%) to COL, 17 (30.9%) to GEN, 25 (45.5%) to AK, and 34 (61.8%) to FOS.
In reference methods, CRKP isolates showed 89.1% susceptibility to CZA (n:49), 16.4% susceptibility to COL (n:9), 21.8% susceptibility to GEN (n:12), and 29.1% susceptibility to AK (n:16) by using BMD, 47.3% susceptibility to FOS (n:26) by AD according to EUCAST, 2023.
The BD Phoenix showed unacceptable false-susceptible results (VME) rates, except for CZA results. AK and FOS showed the most inconsistent results because of their high VME and low CA rates. COL and GEN had acceptable CA results but unacceptable VME results. CZA showed the most compatible result according to the ISO 20776–2 guideline (Table 1).
We found six CZA-resistant CRKP strains. Five of them were NDM positive, and one was KPC positive.
CZA resistance rate was 10.9% (Table 2). The COL resistance rate was 83.6%, which was higher in CZA-susceptible strains (87.8%) than in CZA-resistance strains (50%). GEN resistance rate was 78.2%, higher in CZA-resistance strains (83.3%) than in CZA- susceptible strains (77.5%). AK resistance rate was 70.9%, higher in CZA-susceptible strains (73.5%) than in CZA-resistance strains (50%). FOS resistance rate was 52.7%; it was higher in CZA-susceptible strains (53.1%) than in CZA-resistance strains (50%) (Table 2).
Synergy testing
The ΣFIC values were calculated using CBA for four antibiotic combinations (CZA-COL, CZA-GEN, CZA-AK, and CZA-FOS). Their synergistic, additive, and indifferent interactions are shown in Table 3. Antagonism was not detected in all of the combinations. The most synergistic results were observed in the combination of CZA-COL (78.2%) and CZA-FOS (63.6%). The synergistic result was insufficiently combined with aminoglycosides, and the indifference result was more dominant. When the results of the CZA-COL combination were examined, a synergistic effect was observed in 33.3% of the strains to which both antibiotics were resistant. In contrast, no indifferent results were observed in CZA-susceptible and COL-resistant strains, and synergistic effects were observed in 95.3%. In combinations of CZA-GEN, CZA-AK, and CZA-FOS, a synergistic effect was not observed when the strains were resistant to both antibiotics. No synergistic effect was observed in cases where isolates were susceptible to aminoglycosides but resistant to CZA. Synergistic results were found in 10.5% of CZA-susceptible and GEN-resistant strains and 19.4% of CZA-susceptible and AK-resistant strains. Synergistic results were found in 66.7% of CZA-resistant and FOS-susceptible strains and 42.3% of CZA-susceptible and FOS-resistant strains (Table 3).
We arbitrarily selected three strains that had different carbapenemase genes. Table 4 shows their antimicrobial profile and synergy results with both CBA and TKA. Only the KPC-positive strain showed synergistic effects with the combination of CZA and GEN when tested with TKA. However, all combinations with other strains resulted in indifference.
Discussion
Infections caused by CRKP are a major public health concern due to their rapid spread and limited treatment options19. CZA has been utilized as a first-line treatment for infections caused by CRKP. According to the Infectious Diseases Society of America (IDSA) 2023, the emergence of resistance is a significant concern associated with using all beta-lactams for treating CRE infections. Existing data indicates that the frequency of resistance may be most pronounced in the case of CZA. Most data on resistance emergence to CRE new agents focuses on KPC-producing isolates. Resistance to CZA commonly occurs due to mutations in the blaKPC gene, leading to changes in the KPC carbapenemase2. Since its initial discovery, FOS has been sparingly utilized in clinical settings, primarily for treating urinary tract infections20. However, it has demonstrated potent antibacterial activity against numerous MDR pathogens, including CRE. Additionally, the medication exhibits low protein binding and high tissue penetration, suggesting potential efficacy in combination with other antibiotics against MDR pathogens21. COL enhances membrane permeabilization and improves access to CZA at its target sites22. Aminoglycosides, including GEN and AK, exhibit rapid and enduring bactericidal properties, proving effective in treating CRKP bloodstream infections22. Shields et al. effectively utilized combination therapy, starting with a short course of GEN followed by de-escalation to CZA alone. This approach maximizes treatment effectiveness while limiting toxicity, primarily acute kidney injury23. Combining different medications could have a synergistic effect, which is especially beneficial for severe infections such as sepsis, septic shock, renal replacement therapy, and ventilator-associated pneumonia. Even if the bacteria are susceptible to CZA, using a combination regimen might help prevent the development of resistance to CZA22. Patients with CRKP pneumonia have worse outcomes when treated with CZA, highlighting the need for research on CZA’s activity in the lungs and the best dosage, particularly for septic patients. There is generally a lack of clinical data on the best dosing for new β-lactams and β-lactamase inhibitors (BLBLIs), and differences in extracellular volume and renal function in critically ill patients can affect the distribution of BLBLIs24. Additionally, even in the presence of MBLs, adding AK, COL, or FOS to the treatment may be beneficial, as carbapenemase enzymes do not affect these antibiotics. In vitro synergistic activities may not precisely correlate with the clinical efficacy of antibiotics’ in vivo pharmacokinetic properties. Our primary objective was to investigate the synergistic effect of a recently introduced antibiotic, CZA, and last-line antibiotics. Our study aimed to show these combinations’ in vitro activity in preventing resistance development in individuals with invasive infections. Our secondary objective was to evaluate the performance of our automated antibiotic susceptibility system.
In Turkey, CZA was approved for use in April 2021. A study conducted between 2018 and 2021 found that our country’s CZA resistance rate in CRKPs was 7.3%25. A recent multidisciplinary study in Turkey reported that 39.2% of CRKP had CZA resistance, and 21% of isolates were positive for NDM26. Nordman et al. reported that CREs had 63% CZA susceptibility, and it may contribute to optimizing the choice of first-line therapy for treating infections due to CRE27. We had six CZA-resistant CRKP strains (10.9%) in our study. Five of them were NDM-positive, and one was KPC-positive. NDM belongs to the B1 subclass of MBLs of carbapenemases, and CZA is already inactive against class B. It is rare for a KPC-positive CRKP to be resistant to CZA. Researchers have demonstrated that a single substitution mutation in the KPC gene results in resistance to CZA. The emergence of CZA resistance in KPC-positive strains presents a major challenge in the future.
Resistance to CZA was observed in a clinical trial after treatment courses of 10, 15, and 19 days28. Using CZA as a single drug may lead to rapid resistance and treatment failure. Polymyxins are a class of bactericidal drugs that disrupt the outer cell membrane. This action enables other drugs, including CZA, to more effectively reach transpeptidase enzymes and other cellular targets6. Köle et al. reported synergy in 97.6% of the isolates when CZA was combined with COL using the CBA method29. Mataracı Kara et al. found that the best synergistic interactions were achieved with CZA+COL against OXA-48-producing Enterobacterales, when studied at 1xMIC concentrations for 24 h using the TKA method30. Shields et al. identified synergy and antagonism against 13% and 46% of the CRE isolates, respectively, when CZA+COL were combined using the TKA method31. However, Wang et al. reported that the CZA+COL combination (by TKA method) did not have a better effect than a single drug, supporting this study7. In our study, we found better synergy between CZA+COL (78.2%) using the CBA method. However, using TKA, indifference was observed in the three selected isolates. The variability in the results can be attributed to the different isolates and synergy methods used by the researchers. The studies that demonstrated the synergy between CZA and COL, including our study, were conducted in Turkey. On the other hand, the studies that showed no synergy were conducted in different countries. Rosa et al. demonstrated that KPC-2-positive K. pneumoniae complex strains displayed MDR and extensively drug-resistant profiles, emphasizing resistance to carbapenems, CZA, COL, and tigecycline, considered last-line antimicrobial treatment options32. The differences in CRKP isolates at the molecular level may have influenced the results. Although OXA-48 is dominant in our country, there are not enough studies to determine which variations of the KPC genes are dominant. The dominant KPC variant in our country and the prevalence of OXA-48 may be related to the observed synergy result.
Fosfomycin is a drug used as a last-line treatment for a broad range of infections and has activity against gram-negative bacteria. FOS serves as a time-dependent inhibitor of the MurA enzyme, which plays a pivotal role in the initial stage of peptidoglycan synthesis. Its synergistic effect with CZA is attributed to its distinct mechanisms of action in different phases of bacterial cell wall synthesis33. Various studies have reported varying results on the synergistic effect of combining CZA-FOS in CRKPs, with some showing no synergy34 and others reporting a synergistic effect3. Önal et al. found that patients infected with CRKP did not exhibit a significant difference in mortality rates when treated with combination regimens such as FOS plus one or two antibiotic combinations35. Furthermore, they did not observe any significant differences in the overall length of ICU hospitalization and the 14- and 30 day mortality rates between patients who received CZA-based and FOS/meropenem-based treatment regimens36. In our study, we found that the CZA-FOS combination showed the most synergy after the CZA-COL combination, as determined by CBA. However, TKA indicated that the selected three isolates were indifferent. CBA also showed promising synergy in approximately 42.3% of FOS-resistant CZA-susceptible isolates.
Aminoglycosides are often used in combination with CZA to treat CRKP infections. When aminoglycosides are combined with β-lactam antibiotics, which act on cell wall synthesis, this combination promotes aminoglycosides’ entry into bacteria and enhances their killing ability37. According to a study by Huang et al., synergy was more common between CZA and AK for CRKP isolates that contain aac(6ʹ)-Ib’ genes, while synergy between CZA and GEN was more common for isolates containing aac(6ʹ)-Ib genes using the TKA method38. Other researchers have also found synergy between CZA and AK against CRKP isolates3,7. However, Gaibani et al. showed no synergism was found in the CZA-GEN combination against CRKP isolates by using a gradient synergy test38. In our study, we found that the combination of aminoglycosides and CZA showed less frequent synergy than COL and FOS using the CBA method. Moreover, we found relatively less frequent synergy in aminoglycosides-resistant and CZA-susceptible isolates using the CBA method. We only observed synergy in the CZA-GEN combination for the KPC-positive CRKP isolate using the TKA method.
In our study’s combinations and methods, no antagonist results were found. This is an encouraging outcome that suggests CZA may be effective in combination therapy as well as monotherapy. The synergy rate with CBA was determined to be 95.3% in COL-resistant, CZA-susceptible isolates. In CZA-resistant FOS-sensitive isolates, the synergy rate was found to be 66.7%, while in CZA-sensitive FOS-resistant isolates, the synergy rate was 42.3%. Combination therapy in these isolates may lead to successful treatment.
Various methods are used to test for synergy but don’t always produce the same results. Determining which method is most reliable is difficult because there is no established gold-standard synergy reference method. An ideal technique would consistently predict treatment outcomes, but there have been few comparisons of in vitro synergy testing data with clinical outcomes39. For all these reasons, there may be discrepancies in the results between our CBA and TKA testing methods.
Our diagnostic laboratory utilizes the BD Phoenix automated antibiotic susceptibility system. We found that the CA (98.2%) and VME (0%) of CZA were pleasingly within acceptable limits. Park et al. also reported similar values (CA: 97.6% and VME: 0.9%)40. However, we have observed an alarming increase in COL resistance in our hospital and country, especially in CRKP isolates. Our COL resistance rate was 83.6%, higher than the 65% reported using the BMD method by Sengel et al. in 17 CRKP isolates9. In a previous study we conducted, we found 61.1% COL resistance in Enterobacteriaceae isolates using the BMD method41. In our country, Adaleti et al.42 reported a 63% rate of COL resistance in MDR Enterobacteriaceae using the BMD method, while Kansak et al.43 reported this rate as 61%. The adhesion of polymyxins to plastics can affect in vitro susceptibility testing. Adding polysorbate 80 as a surfactant reduces COL binding to plastic, improving MIC results for Enterobacteriaceae and Pseudomonas aeruginosa44,45. The alarmingly high rate of COL resistance may be because COL binds to plastic, reducing the concentration available to interact with the instilled bacterial inoculum. Kansak et al. found the mcr-1 gene in eight MDR K. pneumoniae strains43. A comprehensive analysis of mcr genes and whether the increased COL resistance is due to the mcr gene or chromosomal origin of CRKP has not been carried out in Turkey. Koyuncu Özyurt et al. reported that the BD Phoenix system had a CA of 95% and VME of 5% for COL46. In our study, the CA was 90.9%, which is acceptable. However, the VME rate was high at 9.1%. The BD Phoenix system did not provide successful results with aminoglycosides. The acceptable range for AK was not met, with CA at 83.6% and VME at 16.4%. Although CA for GEN was within acceptable limits at 90.9%, VME at 9.1% was not. Haffler et al. reported that, the BD Phoenix system’s aminoglycoside sensitivity values for CRKP were not within acceptable limits. They reported CA as 89% for AK and 56% for GEN47. The BD Phoenix also had difficulty with FOS. The acceptable range for FOS was not met, with CA at 81.8% and VME at 16.4%. Kowalska–Krochmal et al. found that the VME was 37.84% when testing for FOS in Enterobacterales using BD Phoenix48. This indicates that when using aminoglycosides or FOS in critical patients, a confirmation test from the laboratory should be requested.
The study has limitations, including a small number of total and CZA-resistant isolates and the inability to conduct whole genome analysis on a single KPC-positive isolate among CZA-resistant ones.
Conclusion
The CA and VME of CZA were within acceptable limits in the BD Phoenix panel; however, the other antibiotics were not. This study has revealed that combining CZA with COL and FOS improves in vitro activity against CRKP clinical isolates. The findings suggest a new treatment opportunity; as limited treatment options are available for severe CRKP infections. Further clinical studies are required to comprehend the potential benefits of combination therapies fully.
Data availability
Data cannot be shared openly but are available on request from the corresponding author.
References
Thabit, A. K., Crandon, J. L. & Nicolau, D. P. Antimicrobial resistance: Impact on clinical and economic outcomes and the need for new antimicrobials. Expert Opin. Pharmacother. 16(2), 159–177 (2015).
Tamma, P. D. et al. Infectious diseases society of America 2023 guidance on the treatment of antimicrobial resistant gram-negative infections. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. https://doi.org/10.1093/cid/ciad428 (2023).
Mikhail, S. et al. Evaluation of the synergy of ceftazidime–avibactam in combination with meropenem, amikacin, aztreonam, colistin, or fosfomycin against well-characterized multidrug-resistant Klebsiella pneumoniae and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 63(8), e00779-e819 (2019).
Russo, C. & Humphries, R. Approaches to testing novel β-lactam and β-lactam combination agents in the clinical laboratory. Antibiotics 12(12), 1700 (2023).
Galani, I., Karaiskos, I. & Giamarellou, H. Multidrug-resistant Klebsiella pneumoniae: Mechanisms of resistance including updated data for novel β-lactam-β-lactamase inhibitor combinations. Expert Rev. Anti Infect. Ther. 19(11), 1457–1468 (2021).
Yin, J. et al. Mechanisms of bactericidal action and resistance of polymyxins for gram-positive bacteria. Appl. Microbiol. Biotechnol. 104(9), 3771–3780 (2020).
Wang, F., Zhou, Q., Yang, X., Bai, Y. & Cui, J. Evaluation of ceftazidime/avibactam alone and in combination with amikacin, colistin and tigecycline against Klebsiella pneumoniae carbapenemase-producing K pneumoniae by in vitro time-kill experiment. PLoS One 16(10), e0258426 (2021).
Chen, T. et al. In vitro activity of ceftazidime–avibactam alone and in combination with amikacin against colistin-resistant gram-negative pathogens. Microb. Drug Resist. 27(3), 401–409 (2021).
Erturk Sengel, B., Altinkanat Gelmez, G., Soyletir, G. & Korten, V. In vitro synergistic activity of fosfomycin in combination with meropenem, amikacin and colistin against OXA-48 and/or NDM-producing Klebsiella pneumoniae. J. Chemother. 32(5), 237–243 (2020).
Tumbarello, M. et al. Ceftazidime–avibactam use for Klebsiella pneumoniae carbapenemase-producing K pneumoniae infections: A retrospective observational multicenter study. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 73(9), 1664–1676 (2021).
Yahav, D. et al. New β-lactam-β-lactamase inhibitor combinations. Clin. Microbiol. Rev. 34(1), e00115-e120 (2020).
Compain, F. & Arthur, M. Impaired inhibition by avibactam and resistance to the ceftazidime–avibactam combination due to the D179Y substitution in the KPC-2 β-lactamase. Antimicrob. Agents Chemother. 61(7), e00451-e517 (2017).
The European Committee on Antimicrobial Susceptibility Testing, EUCAST. Breakpoint tables for interpretation of MICs and zone diameters. Version 13.0. http://www.eucast.org. Accessed 17 Jul 2023. (2023).
Clinical and Laboratory Standards Institute. CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard 10th edn, M07-A11 (Clinical and Laboratory Standards Institute, 2018).
European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by agar dilution. Clin. Microbiol. Infect. https://doi.org/10.1046/j.1469-0691.2003.00790.x (2003).
Leber, A. L. Clinical Microbiology Procedures Handbook 4th edn. (ASM Press, 2016).
ISO 20776–2:2021; Clinical laboratory testing and in vitro diagnostic test systems. Susceptibility testing of infectious agents and evaluation of performance of antimicrobial susceptibility test devices—Evaluation of performance of antimicrobial susceptibility test devices against reference broth micro-dilution. https://www.iso.org/standard/79377.html. Accessed 24 Jul 2023. (2023).
Lorian, V. Antibiotics in Laboratory Medicine 5th edn. (Lippincott Williams & Wilkins, 2005).
Lee, Y. L., Ko, W. C. & Hsueh, P. R. Geographic patterns of global isolates of carbapenem-resistant Klebsiella pneumoniae and the activity of ceftazidime/avibactam, meropenem/vaborbactam, and comparators against these isolates: Results from the antimicrobial testing leadership and surveillance (ATLAS) program, 2020. Int. J. Antimicrob. Agents 60(5–6), 106679 (2022).
Avery, L. M., Sutherland, C. A. & Nicolau, D. P. In vitro investigation of synergy among fosfomycin and parenteral antimicrobials against carbapenemase-producing Enterobacteriaceae. Diagn. Microbiol. Infect. Dis. 95(2), 216–220 (2019).
Grabein, B., Graninger, W., Rodríguez Baño, J., Dinh, A. & Liesenfeld, D. B. Intravenous fosfomycin-back to the future. Systematic review and meta-analysis of the clinical literature. Clin. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 23(6), 363–372 (2017).
Meini, S., Viaggi, B. & Tascini, C. Mono vs. combo regimens with novel beta-lactam/beta-lactamase inhibitor combinations for the treatment of infections due to carbapenemase-producing Enterobacterales: Insights from the literature. Infection 49(3), 411–421 (2021).
Shields, R. K. et al. Ceftazidime–avibactam is superior to other treatment regimens against carbapenem-resistant Klebsiella pneumoniae bacteremia. Antimicrob. Agents Chemother. 61(8), e00883-e917 (2017).
Shields, R. K. et al. Pneumonia and renal replacement therapy are risk factors for ceftazidime–avibactam treatment failures and resistance among patients with carbapenem-resistant Enterobacteriaceae infections. Antimicrob. Agents Chemother. 62(5), e02497-e2517 (2018).
Hoşbul, T. et al. In vitro activity of ceftazidime–avibactam and colistin against carbapenem-resistant Klebsiella pneumoniae clinical isolates. Mikrobiyol. Bul. 56(2), 218–229 (2022).
Pınarlık, F. et al. Klebsiella pneumoniae: Surveillance of antimicrobial resistance, clinical and molecular characteristics. ECCMID 2023. poster0133. (2023).
Nordmann, P., Bouvier, M. & Poirel, L. Efficacy of ceftazidime–avibactam, meropenem–vaborbactam, and imipenem–relebactam combinations against carbapenemase-producing Enterobacterales in Switzerland. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 42(9), 1145–1152 (2023).
Shields, R. K. et al. Clinical outcomes, drug toxicity, and emergence of ceftazidime–avibactam resistance among patients treated for carbapenem-resistant Enterobacteriaceae infections. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 63(12), 1615–1618 (2016).
Köle, M., Sesli Çetin, E., Şirin, M. C. & Cicioğlu Arıdoğan, B. Evaluation of in vitro efficacy of ceftazidime–avibactam, meropenem, and colistin single and binary combinations against carbapenem resistant Klebsiella pneumoniae strains isolated from various clinical specimens. Mikrobiyol. Bul. 56(2), 230–250 (2022).
Mataraci Kara, E., Yilmaz, M., Istanbullu Tosun, A. & Özbek Çelik, B. Evaluation of the synergy of ceftazidime/avibactam in combination with colistin, doripenem, levofloxacin, tigecycline, and tobramycin against OXA-48 producing Enterobacterales. J. Chemother. 32(4), 171–178 (2020).
Shields, R. K., Nguyen, M. H., Hao, B., Kline, E. G. & Clancy, C. J. Colistin does not potentiate ceftazidime–avibactam killing of carbapenem-resistant Enterobacteriaceae in vitro or suppress emergence of ceftazidime–avibactam resistance. Antimicrob. Agents Chemother. 62(8), e01018-e1118 (2018).
Rosa, R. D. S. et al. Genetic diversity of KPC-2-producing Klebsiella pneumoniae complex from aquatic ecosystems. World J. Microbiol. Biotechnol. 40(6), 177 (2024).
Wu, Y. et al. Effect of ceftazidime–avibactam combined with different antimicrobials against carbapenem-resistant Klebsiella pneumoniae. Microbiol. Spectr. https://doi.org/10.1128/spectrum.00107-24 (2024).
Romanelli, F. et al. In vitro activity of ceftazidime/avibactam alone and in combination with fosfomycin and carbapenems against KPC-producing Klebsiella pneumoniae. New Microbiol. 43(3), 136–138 (2020).
Önal, U. et al. Evaluation of the combination treatments with intravenous fosfomycin for carbapenem-resistant Klebsiella pneumoniae. Rev. Assoc. Med. Bras. 69(11), e20230727 (2023).
Önal, U. et al. A comparative study of ceftazidime/avibactam-based and fosfomycin plus meropenem-based regimens for managing infections caused by carbapenem-resistant Klebsiella pneumoniae in critically ill patients. J. Chemother. https://doi.org/10.1080/1120009X.2024.2349439 (2024).
Huang, Y. et al. Generating genotype-specific aminoglycoside combinations with ceftazidime/avibactam for KPC-producing Klebsiella pneumoniae. Antimicrob. Agents Chemother 65(9), e0069221 (2021).
Gaibani, P. et al. In vitro interaction of ceftazidime–avibactam in combination with different antimicrobials against KPC-producing Klebsiella pneumoniae clinical isolates. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 65, 1–3 (2017).
Brennan-Krohn, T. & Kirby, J. E. When one drug is not enough: Context, methodology, and future prospects in antibacterial synergy testing. Clin. Lab. Med. 39(3), 345–358 (2019).
Park, B. Y. et al. Performance evaluation of the newly developed BD phoenix NMIC-500 panel using clinical isolates of gram-negative bacilli. Ann. Lab. Med. 39(5), 470–477 (2019).
Tüzemen, N. Ü., Işık, Ö., Akça, B. & Özakın, C. Determination of colistin resistance in gram negative bacteria by modified broth disk elution method. Turk. Mikrobiyol. Cemiy. Derg. 52, 184–191 (2022).
Adaleti, R. et al. Evaluation of in vitro efficacy of meropenem/colistin and meropenem/fosfomycin combinations on multidrug resistant gram-negative bacilli. Mikrobiyol. Bul. 57(3), 365–377 (2023).
Kansak, N., Aksaray, S., Aslan, M., Adaleti, R. & Gönüllü, N. Detection of colistin resistance among multidrug-resistant Klebsiella pneumoniae and Escherichia coli clinical isolates in Turkey. Acta Microbiol. Immunol. Hung. 68(2), 99–106 (2021).
Sutherland, C. A. & Nicolau, D. P. To add or not to add polysorbate 80: Impact on colistin MICs for clinical strains of Enterobacteriaceae and Pseudomonas aeruginosa and quality controls. J. Clin. Microbiol. 52(10), 3810 (2014).
Sharafi, T. & Ardebili, A. Plastic binding feature of polymyxins: The effect on MIC susceptibility measurements. Infect. Drug Resist. 12, 2649–2653 (2019).
Koyuncu Özyurt, Ö. et al. Evaluation of the BD phoenix100 system and colistin broth disk elution method for antimicrobial susceptibility testing of colistin against gram-negative bacteria. Mikrobiyol. Bul. 53(3), 254–261 (2019).
Haffler, Z. J., Kulengowski, B., Ribes, J. A. & Burgess, D. S. Evaluation of the BD Phoenix automated system for determining antimicrobial susceptibility against carbapenem-resistant Enterobacteriaceae compared with broth microdilution. Int. J. Antimicrob. Agents 54(2), 249–254 (2019).
Kowalska-Krochmal, B. et al. Reliability of E-Tests and the phoenix automated method in assessing susceptibility to IV fosfomycin—Comparative studies relative to the reference method. Pathogens 12(5), 700 (2023).
Acknowledgements
We thank Pfizer Global Medical Grants for providing us with avibactam powder, with grant number 71027415.
Funding
This study was supported by the Bursa Uludag University Scientific Research Project (TGA-2022-898) and Pfizer Global Medical Grants, 71027415.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of this study. NÜT, UÖ, OM, BA, CÖ, BE, and HA were involved in material preparation, data collection, and analysis. NÜT wrote the first draft of the manuscript, and all authors provided feedback on previous versions. Writing—review, and editing were performed by CÖ and HA. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tüzemen, N.Ü., Önal, U., Merdan, O. et al. Synergistic antibacterial activity of ceftazidime–avibactam in combination with colistin, gentamicin, amikacin, and fosfomycin against carbapenem-resistant Klebsiella pneumoniae. Sci Rep 14, 17567 (2024). https://doi.org/10.1038/s41598-024-67347-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-67347-5
- Springer Nature Limited