Abstract
Urban areas, i.e. dense housing and reduced green spaces, can significantly impact avian health, through altering land use and increasing biotic and abiotic stress. This study assessed the association of urbanization on haemosporidian infections, vectors, immune response, and body condition in Parus major nestlings, across four classes of urbanization along an urban-to-rural gradient in Vienna, Austria. Contrary to our expectations, vector abundance remained consistent across the gradient, while an increase in leukocyte count is positively associated with total parasite intensity. We found that nestlings in more urbanized areas exhibited higher parasite intensity and altered immune response, as evidenced by variations in the heterophil to lymphocyte ratio and leukocyte counts. Culicidae female vectors were associated with nestlings’ total parasites, scaled mass index, and industrial units. Nestlings in highly developed areas had higher infection rates than those in forests, suggesting increased exposure to infections. However, there was no clear relationship between total female vectors and total parasites. The level of urbanization negatively affected nestling body condition, with a decrease in fat deposits from forested to highly urbanized areas. Our findings highlight the complex interplay between urbanization, vector-borne parasite transmission, and host immune response, emphasizing the need for comprehensive urban planning to improve wildlife health and guarantee ecosystem functioning. Understanding how urbanization affects bird immunity and parasite infections is critical for adapting urban landscapes for wildlife health and ecosystem integrity.
Similar content being viewed by others
Introduction
Urban areas, characterized by high housing densities, extensive impervious surfaces, and few green spaces e.g.,1,2 are globally increasing in extent. As of 2018, 55% of the global population and 74% of Europeans are residing in cities, and the numbers are rising3. Urban expansion alters ecosystems, leading to the rapid transformation or degradation of natural habitats into typically -for the hosts- less favorable habitats for many organisms4,5,6,7, affecting both, biotic and abiotic factors, depending on the level of urbanization. Many organisms, such as birds7 or dipteran vectors8 are affected by urbanization. Most studies have found that species composition changes from rural to urban9 and especially dipteran vectors are favored in cities8. Therefore, urbanization causes shifts in species ranges and abundances, resulting in, for example, a decline of specialized bird species and a predominance of generalists1,10,11. The latter are considered urban adapter species, including mainly insectivorous and omnivorous ground foragers, seed eaters, and aerial sweepers7, and their numerical increase can impact ecosystem functions12.
Physical changes from urban-to-rural environments, such as alterations in resource availability, or increasing impervious surfaces can influence organismal responses13,14. Species reactions to urbanization are species-specific and are mediated by factors like temperature variation, noise, light, air pollution15,16,17, or variation in predator and parasite densities18,19,20,21. For instance, urban birds tend to breed earlier than those in rural areas, possibly due to higher urban temperatures, leading to mismatches between insect prey availability and bird nesting periods13,15. This, combined with lower caterpillar biomass in urban areas, for example, can adversely affect body condition22 due to constrained food resources13, because the nestlings critically depend on caterpillars as food23. Combined, urbanization can reduce body condition, due to changes in temperature and the implied food limitations13.
Higher urban temperatures may also increase the development of avian blood parasites (i.e. haemosporidians) within vectors, however, specific upper and lower temperature-thresholds can inhibit this development20,23,24,25,26. Higher temperatures towards city centers are a direct consequence of increased impervious surfaces and reduced green spaces. Increased levels of urban pollution (e.g., heavy metals), through immune-toxic effects, can directly impair birds' immune responses, exacerbating the effects of parasites19. Thus, the effects of pollution and parasites can be costlier in urban habitats than in forests27, decreasing body condition of birds and their reproduction28,29. In general, urban habitats may impose greater reproductive and physiological costs on birds25,26. However, how breeding, immune response, and the influence of parasites along urbanization gradients interact remain poorly understood.
Habitat use of birds is likely associated by the interplay between blood parasites, vectors, and hosts18,30,31. Environmental conditions can affect haemosporidian development within both insect vectors and bird hosts24,32. Haemosporidian prevalence may therefore depend on the vectors’ abundance33,34, hosts reproductive-habitat conditions21,35, and the bird host should respond to altered environmental conditions36. For example, land-use changes can decrease immune response27,37, affect body condition13, or reproduction rates28. As land-use can negatively affect a bird's immune response27, positive effects on haemosporidian development within the birds can occur. As a consequence, the host's body condition, which is a first approximation to the birds' health status38, further decreases35. Hence, urbanization as one of the most intense land-use forms, can cause immunosuppression in birds19,39,40. Although urban habitats tend to show lower haemosporidian prevalence, the role of vectors along urbanization gradients is unclear18.
Habitat variations, such as those brought by urbanization, indirectly shape the distribution of avian blood parasites, influencing species or strains and affecting overall prevalence and intensity within the host population36,41,42. The presence of blood-feeding dipterans like Ceratopogonidae and Simuliidae, crucial for haemosporidian transmission, is often reduced in urban settings8 due to fewer water bodies for reproduction, potentially lowering vector abundance and, consequently, blood parasite pressure on bird hosts21,31. However, suitable larval habitats for some Culicidae species are typically more abundant towards city centers43,44 and we would expect an increase of culicids towards the city center and less of Ceratopogonidae and Simuliidae. Although haemosporidian prevalence in urban areas is generally lower compared with natural habitats27,45, the role of vectors as transmitting agents along urbanization gradients has not yet been assessed together with health of hosts, blood parasite prevalence, and host life history parameters46,47.
During a haemosporidian infection, hosts can experience severe symptoms, yet the severity and nature of these symptoms are dependent on the specific blood parasite, species or strain and the infected host species48. In other words, the infection intensity is typically related to the individual conditions and immune-response effects, and consequently may affect the (parental) fitness49. Survivors of an acute infection typically harbor low levels of blood parasites, resulting in a chronic infection with minimal or no pathological signs21,35, although a chronic infection may still negatively affect the birds’ reproductive success50,51,52. An acute infection activates the immune system, leading to symptoms marked by elevated levels of heterophils and leukocytes53. The heterophil to lymphocyte ratio (H/L-ratio) can be used as an indicator for stress54 and has been used as such in birds before55. Leukocytes can be used as an approximation for the immune response to parasitic infection53. However, little is known about how the H/L-ratio and leukocyte count are affected by the level of urbanization.
One factor used to evaluate the effect of habitat variation on the immune response on avian health is body condition, often referred to as variation in stored energy reserves38,56 of the birds in form of large levels of body mass, e.g. individual fat or muscle deposits. A robust body condition is presumed to enhance immune response capabilities, thereby increasing the likelihood of surviving a haemosporidian infection and consequently increasing fitness, reproductive success, and chance of fledgling survival57,58. While body condition as a sole metric is somewhat coarse, its utility is enhanced when combined with the evaluation of body asymmetries and scaled mass indices (SMI), providing a more comprehensive assessment of the association of habitat on bird health59,60,61,62,63.
Urban environments expose birds to chronic stressors like pollution, higher disturbance rates, and increased temperatures, which can collectively suppress the immune system, potentially easing the spread of parasite infections27. The transmission of haemosporidians is linked to vector presence31,64, thus, higher incidences and haemosporidian infection are predicted in areas with greater vector populations and vector abundance, which increase the chance of transmissions33,34. These environmental and ecological factors are expected to result in a spectrum of immune responses in birds, with those infected exhibiting a heightened immune response, especially in nestlings where a chronic stage of infection has not yet been established due to the short interval between infection and blood sampling35.
Our research assessed the variation of avian haemosporidians in Parus major nestlings along an urban-to-rural gradient concerning host health, transmission risk, and land use surrounding the nest boxes (a proxy for resource availability). We hypothesized that haemosporidian intensity in Parus major nestlings correlates positively with the level of urbanization due to reduced immune responses and lower body condition. We also expected higher transmission risk from greater vector abundance (Culdicidae) in areas with more vector reproducing habitats available. We anticipated that body condition deteriorates with increasing parasite intensity, leading to greater body asymmetries and lower scaled mass indices in highly urbanized areas. Lastly, we evaluated the relationship between life history traits, reproduction, and urbanization levels, and integrated all discussed aspects.
Methods
Study area
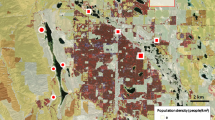
The study area covered the city of Vienna, Austria. Mean annual precipitation is about 500–800 mm and mean daily temperature per year is 9–12 °C. The level of urbanization within the city was characterized within a 2 × 2 km grid based on the variables used by Suarez-Rubio and Krenn2. Physical (percentage of land area for every land-use and land-cover class), demographic (total number of people and total number of flats) and landscape metrics variables (e.g., connectivity, number of patches) were used to assign each square to one of four urbanization categories, thus allowing the description of the urban-to-rural gradient. From high to low the urbanization levels were: Highly-developed, moderately-developed, sparsely-developed, and forest2 (Fig. 1).
In each 2 × 2 km squares, four points were formally placed within a distance of 1 km. From those, between 22 and 25 points per category were randomly selected for mounting a total of 92 nest boxes (Fig. 1). We mounted the nest boxes on trees at about 160 cm above ground and the front side was oriented towards north to north-east. We used wood-concrete nest boxes with an entry diameter of 32 mm (WoodStone "Salamanca", brown/black color, round entry).
Additionally to the four urbanization levels defining the urban-to-rural gradient, we characterized the immediate surroundings of the nest boxes at the fine scale, which constitutes a second alternative approach to characterize land use and land cover. For this, we created buffers of 94.4 m in QGIS v.3.4.4 around each nest box, which represents the circular smallest known territory of Parus major (i.e. 2.8 ha65). We then extracted out of the European Urban Atlas66 (minimum mapping unit of 0.25 ha) the area covered in m2 of the different land-use classes within each of the buffers (all land use classes are described in the Online Supporting Information Table S1). These variables were used to understand which land-use classes in the immediate surroundings of the nest boxes were associated with the hosts, the vectors and the blood parasites metrics.
Study organism and data collection
We selected the cavity-nesting Great Tit, Parus major, as model species, since they occupy a wide range of habitats, regularly accept artificial nest boxes as breeding sites, and have been used for urban vs. rural research questions29,67,68 and for research on haemosporidians. We sampled P. major nestlings, as this guarantees parasite transmission at the nesting sites and that all environmental effects can be assessed definitely from one of the four urbanization levels. Contrary to the nestlings, adult birds could obtain infections by haemosporidians elsewhere prior to breeding.
We monitored the nest boxes to relate habitat quality to the reproductive outcome along the urban-to-rural gradient. We checked nest boxes regularly once per week, starting from March 15th to June 1st, 2018. We adapted the interval of visits depending on the reproductive stages (number of eggs laid, hatchlings, nestlings). We followed chick development and on day 15 after hatching, we conducted the body measurements and drew the blood of all nestlings in the nest box.
For each individual chick, we confirmed the species and determined ectoparasite load and the birds' fat deposits and flight muscle scores. The scores were based on69 and indicate the subcutaneous fat deposits in four classes at the throat and abdomen, as well as provides a flight muscle strength estimate. We measured several eco-morphological traits: Body mass (g), bill length (mm69), and tarsometatarsus (mm) from the notch at the back of the intertarsal joint to the bend of the toe at the metatarsal joint (from now on abbreviated as "tarsus"70). We measured the right and the left tarsus each five times on each side to minimize measurement errors, and for asymmetry calculations. We used a digital caliper to the nearest of 0.01 mm for measuring the bill and tarsus length and a digital scale to determine the body mass to the nearest of 0.1 g. All nestlings were individually banded with metal bird rings for individual identification. We calculated tarsus length asymmetry with Ai = Ri/Li, where Ai is the individual asymmetry, Ri is the individual's mean right tarsus length and Li the mean left tarsus. We calculated the scaled mass index –SMI, scaled by tarsus length– as a proxy for body condition38,56.
We took blood from the brachial blood vessel with a microcapillary after puncturing with a 0.40 × 20 mm needle. Then, we prepared blood smears, air-dried, and fixed them in 100% methanol for 5 min at the sampling site. In the laboratory, we stained all blood smears with Giemsa in 1.25 g saline buffer solution of Na2HPO4 and 0.7 g of KH2PO4 for one hour.
To identify blood cells and blood parasites, we scanned each blood smear with a Leica microscope DM 5500B, using oil immersion at 1000 × magnification. We prepared 300 images per blood smear with the Leica Application Suite (v.4.5.0). We counted the number of erythrocytes per image until we reached the necessary 10,000 erythrocytes per bird individual, and we counted the parasites per these 10,000 erythrocytes to calculate the parasite prevalence and intensity71. Since haemosporidians in nestlings are only represented by the early stages, we differentiated between parasites we surely identified as such and those which probably showed also parasitic stages but we were not absolutely sure. Consequently, we differentiated between large, certain parasitic stages and small, uncertain parasitic stages. We labelled these two procedures as progressive versus conservative parasite counts. We defined conservative parasite counts as big, clear stages of haemosporidians, elongated around the nucleus or roundish with granules. We included all parasites with clearly pale blue cytoplasm, pink or dark blue or brownish/black granules, or green-shaded globules visible. We only counted parasites as conservative if parasites were completely visible within a cell and did not overlay the cells' nucleus and had a clearly defined border. Contrastingly, we defined progressive parasite counts as small parasitic stages in pale blue/darker blue to pinkish/purple color72. We did not count small structures in a brownish/black color. In these small, often roundish stages mostly no granules were visible. We excluded bi-lobed nuclei or micro-nuclei of the erythrocytes73, which sometimes look similar. Once we detected a parasite, we assumed an infection ("prevalence"). For the statistical analysis we focused on conservative and progressive counts combined (henceforth "total parasite intensity"). In addition to the blood parasites, we screened and quantified each individual for ectoparasites and counted attached ticks, feather mites, and louse files escaping from the hatchlings' plumage. We also counted all leukocytes per 10,000 erythrocytes and distinguished between lymphocytes, heterophils, monocytes and undetermined leukocytes53. We used the differential white blood cell count, which is the sum of all leukocytes per 10,000 erythrocytes, and calculated the H/L-ratio (heterophils to lymphocytes).
To assess the potential role of vectors at the nesting sites, we set up mosquito traps (BG-Sentinel 1, Biogents AG, Regensburg, Germany) attracting with sweat-scent at each of the nest boxes. We installed the traps below the nest boxes in shady and sheltered positions and operated overnight for typically 20 h per night (only in few instances up to 22 h) between June 19th and July 13th, 2018. Despite the temporal mismatch between the blood sampling of nestlings and vector sampling, we considered that this is not a significant issue given that the sampling period of the vectors (from June 19th to July 13th) still entails a comprehensive snapshot of the local dipteran populations during the peak activity period for these vectors, ensuring that the data still reflects the potential vector transmission on the nestlings. We froze the captured insects on the same day and afterwards vectors, i.e. Culicidae, Ceratopogonidae, and Simuliidae, counted and determined to family level. We used the total female vector count and considered in a second step only the Culicidae females for further analysis, as only the female dipterans feed on blood and therefore transmit haemosporidians35.
Data analysis
From all parameters available, we a priori excluded from the datasets: male vectors since there is no direct function in transmission; the five repetitive measures of tarsus left and tarsus right—we retained the mean values on both body sides, however, tarsus left mean was further excluded in most models since correlated with tarsus right. We further tested for multicollinearity using Variance Inflator Factors (vif < 3; parameters retained for analysis are listed in Table S1) using the R package usdm74. We scaled parameters to reduce scaling issues using the R-function "scale".
We used Generalized Linear Models (GLM, family = gaussian) and Generalized Mixed Effects Models (GLMM) to evaluate how the level of urbanization, vectors, health indicators, body condition indicators and nest box surroundings affected total parasite intensity. We created models based on a combination of hypothesis-driven (i.e. models we thought of a priori) and by excluding non-significant parameters. The full and alternative models we tested are listed with the parameters in Table S2, naming also all predictors for each model.
For the GLMM we added the nest box site as random effect (i.e. '(1|plot)') since the nestlings within the same nest box were not independent and needed to be considered as nested. Following the model creation, we performed model averaging on three sets of models: (1) total parasites as response (including random effects) to model the effects of the level of urbanization, nest box surroundings by the urban land use classes and vector transmission on the parasites; (2) all female vectors and the Culicidae female vectors as response (two independent sets of models), to explain, besides the level urbanization, any of the land use effects surrounding the nest boxes on the vectors; and (3) H/L-ratio as well as leukocytes as response (two sets of independent models) to explain both the effects of the level of urbanization and land use surrounding the nest boxes on the immune-response/function. We selected the best fitting model out of the set of models based on ΔAICc and AICc values to find the most parsimonious model—while we report models within the 95% CI of the model averaging in Table S3A, we add also BIC and ΔBIC75.
We performed the GLM with 'glm' (Package: stats Version: 4.2.3) and the GLMM with 'lmer' for models including random effects (Package: lme4 Version: 1.1-33)76. Model selection followed Burnham and Anderson77 in the package AICcmodavg Version: 2.3-3. All tests and GLM have been performed with R version 4.2.378. The data are included in Table S4.
Under/Overdispersion was tested with ‘testDispersion' (package DHARMa, non-parametric dispersion test via s.d. of residuals fitted vs. simulated) and outliers with ‘testOutliers' (Package: DHARMa, Version: 0.4.6). None of the models had P values below the typical threshold for overdispersion (0.05), suggesting no significant overdispersion issues (Table S2). The P values for outliers indicated that there might be some issues with outliers for ‘Total female Vectors', ‘Female Culicidae Vectors' and ‘H/L-ratio' as responses, but the significance was marginal (Table S2). However, exclusion of outliers did not change the outcome, hence we included all outliers in all analyses.
Ethical approval
Capturing and handling of animals was performed in compliance with laws and regulations of Austrian federal and state legislation, as well as corresponding EU regulations. All permits to access protected areas, capturing/handling of species/protected species, and handling wildlife were granted by the Bundesministerium für Wissenschaft, Forschung und Wirtschaft (Gz. BMWFW-66.016/0010-WF/V/3b/2015), access to land by Magistratsabteilung MA22 (Gz. MA22-94959/2015) and Magistratsabteilung MA49 (Gz. MA49-33232/2016/3). All land owners and land users approved access to their sites prior to the study.
Results
From the 92 nest boxes placed along the urban-to-rural gradient in Vienna, 54 nest boxes were occupied by Great Tits (Parus major), five by Blue Tits (Cyanistes caerulaeus) and two by Sparrows (Passer sp.). Of all nest boxes occupied by Great Tits, most were situated in moderately-developed areas (31.48%) and fewest in highly-developed areas (12.96%; Table 1). Of those nests with eggs, the average clutch size was largest in sparsely-developed areas and smallest in moderately-developed areas. The mean number of nestlings on day 15 were highest in forest and lowest in moderately-developed areas (Table 1). The hatching date differed between urbanization categories, with later hatching dates in forests and earlier hatching dates in highly-developed areas.
The best-fitting model for total parasites (ma0; Table S3A) included the parameters total female vectors, scaled mass index (SMI), H/L-ratio, leukocytes, fat, muscle, ticks, mites, and urbanization categories, according to AICc (AICc: 781.8; Table S3A). Significant predictor in this model were leukocytes (Estimate = 0.010, S.E. = 0.002, t-value = 4.655; Table S5), indicating that an increase in leukocyte count is positively associated with total parasite intensity (Fig. 2). The second-best model (m1.5; AICc: 784.2), which included similar predictors but excluded some non-significant terms, confirmed these trends. Compared to the full model (m.full; AICc: 856.2), which included a broader range of predictors, the best-fitting model was more parsimonious and had better fit statistics (lower AICc and BIC values; Table S3).
Effect size of nested Parus major nestlings in Vienna, Austria for significant parameters in the best fitted model on total parasites and leukocytes. The blue dotted lines represent the fitted effects of each predictor, and the blue shaded areas represent the 95% confidence intervals of the fitted effects.
For total female vectors, the best model (v.lulcXy2; Table S3A) included SMI, tarsus right mean, impervious surface 50–80%, and industrial units. However, the models focusing on Culicidae female vectors only, show that the best model for Culicidae female vectors (v.culicid.xcc2; AICc: 695.8; Table S3A) included total parasites, SMI, and industrial units as predictors. Notably, SMI (Estimate = 0.253, Std. Error = 0.061, t-value = 4.135; Table S5), tarsus right mean (Estimate = 0.351, Std. Error = 0.104, t-value = 3.395), and industrial units (Estimate = 0.542, Std. Error = 0.069, t-value = 7.860) were significant, indicating these physical and environmental factors are influential in determining Culicidae female counts (Fig. 3). The second-best model (v.culicid.xcc1, AICc: 696.2; Table S3A) showed similar trends but included additional predictors like agriculture, which were not significant. The full model, which considered a wider range of predictors, had higher AICc (710.5) and BIC (788.5; Table S3) values, indicating a poorer fit.
Effect size of nested Parus major nestlings in Vienna, Austria for significant parameters in the best fitted model on Culicidae females and (A) total parasites, (B) SMI, and (C) industrial units. The blue dotted lines represent the fitted effects of each predictor, and the blue shaded areas represent the 95% confidence intervals of the fitted effects.
The best model on the H/L-ratio (hl.lulcXy AICc: 760.9; Table S3A), an approximation for stress in relation to the urban-to-rural gradient, included impervious surface 10–30% (Estimate = -0.174, Std. Error = 0.059, t-value = -2.959; Table S5) and impervious surface 30–50% (Estimate = 0.314, Std. Error = 0.0618, t-value = 5.093) in the nest box surroundings as significant predictors. This model shows that urban impervious surface density was significantly associated with the H/L-ratio, with varying effects depending on the density level (Fig. 4). The second-best model (hl.lulcXX AICc: 761.8; Table S3A) included additional non-significant predictors and had slightly higher AICc and BIC values. The full model, which included a comprehensive set of predictors, did not fit the data as well and had higher AICc (766.9; Table S3A) and BIC (805.7; Table S3B) values.
Effect size of nested Parus major nestlings in Vienna, Austria for significant parameters in the best fitted model on H/L-ratio and (A) impervious surface 10–30%, and (B) impervious surface 30–50%. The blue dotted lines represent the fitted effects of each predictor, and the blue shaded areas represent the 95% confidence intervals of the fitted effects.
For leukocytes as dependent variable, an indicator of immune functioning, the best model (leuco.lulc8 AICc: 2635.3; Table S3A), included total parasites (Estimate = 8.309, Std. Error = 1.699, t-value = 4.891; Table S5) and industrial units (Estimate = -3.390, Std. Error = 1.784, t-value = -1.901) as significant predictors. There was a positive relationship between total parasite and leukocyte count, while industrial units showed a borderline significant negative association (Fig. 5). The second-best model (leuco.lulc6 AICc: 2637.3; Table S3A) included water bodies as an additional predictor, which was not significant and slightly increased the AICc. The full model (leuco.full AICc: 2642.4, BIC: 2681.3; Table S3), which included a broader set of predictors, did not perform as well as the best model.
Effect size of nested Parus major nestlings in Vienna, Austria for significant parameters in the best fitted model on leukocytes and parasites total. The blue dotted lines represent the fitted effects of each predictor, and the blue shaded areas represent the 95% confidence intervals of the fitted effects.
Discussion
We examined the impact of urbanization on the haemosporidian infections, immune responses, and body condition of Parus major nestlings in an urban-to-rural setting. Our analysis indicates that leukocytes, SMI, and the surroundings of the nest boxes are significantly associated with parasite loads and vector populations. The significance of industrial units across multiple models highlights the impact of the nest surroundings on parasite-vector relationships. The consistent significance of leukocytes in the total parasite model highlights the role of immune response in parasite intensity.
Contrary to our initial hypotheses, we observed no large variation in total vector abundance across different urbanization categories, however we found a negative association between culicid female vector abundance and total parasites. This finding challenges our hypothesis that city centers should show higher vector presence, which in turn should correlate with higher bird infections in urbanized areas21,27,45. More specific, we have detected female culicid vectors to negatively correlating with parasite intensity. Interestingly, when including female vectors in our best-fit models, their correlation with parasitism was negative, pointing to a more complex relationship between vector abundance and haemosporidian parasites46. This complexity is further emphasized by including urbanization's impact on different haemosporidians, which could be affected by varying vector species compositions in urban versus more natural-like settings21,35,79,80. This counterintuitive correlations between parasites and vectors can origin from a short temporal mismatch between the sampling times of parasites and vectors in our study. However, factors other than vector abundance, such as urban-associated temperature variations or availability of water bodies for vector reproduction, could play a critical role in explaining lack of correlation between haemosporidians and vectors in our study, thereby influencing parasite transmission rates by vectors20,24,81.
The higher infection rates of nestlings from clutches in highly developed areas indicates the complex interactions between host biology, vector behavior, and environmental conditions. This relationship highlights the association of temporal and biological factors on the vulnerability of avian populations to parasitic diseases within urban gradients. We discovered a notable trend where nestlings hatched later in the breeding season (forests) exhibited higher parasite intensity. This pattern aligns with the increase in vector abundance found in southern German urban forests46 through the breeding season in general terms. The data also revealed that nestlings from clutches in highly developed areas were more susceptible to haemosporidian infections, suggesting a higher likelihood of vector contact due to fewer available targets in nests with smaller clutches13. The interplay between clutch size and haemosporidian infection risk highlights the need to assess the broader ecological and evolutionary dynamics in urban environments and should include season in further research approaches.
The landscape surrounding the nest boxes presents a complex mosaic of environmental pressures that influence the transmission of haemosporidian parasites. Our results indicated higher total parasite intensities in more developed areas, with a positive correlation to impervious surface. This could be attributed to varied developmental times of haemosporidians in these environments 35. Interestingly, the immune response, particularly the H/L-ratio, was lower in forested areas compared to urban settings, contradicting our initial hypothesis but is in line with other studies that show varied immune responses across bird species and urbanization factors36,42,82. Furthermore, we observed a positive association of the leukocyte number on total parasite intensity, suggesting an intensified immune response during acute haemosporidian infections35. Our findings emphasize increased parasite intensities in nestlings residing in more urbanized areas, suggesting an interplay between urban landscape features, such as impervious surfaces, and parasite life cycles. Urban environments play an important role in influencing the epidemiology of haemosporidian infections. Despite the constant vector abundance across the urban-to-rural gradient observed by us, the increased parasite intensities in urbanized settings point to effects of non-vector factors, such as impervious surfaces.
Our study also revealed effects of urbanization on various aspects of bird physiology, like nestlings’ body condition. We noted that urbanized areas, characterized by high levels of impervious surfaces, perhaps lead to food shortages and increased stressors like pollution and disturbances. This in turn can reduce the fitness of an individual bird in form of low muscle development or reduced fat deposits due to higher energy demands for an immune response13,27,83,84. Urbanization also was associated with slower development in European Paridae nestlings22,85, however we cannot directly confirm such a link in our study. In addition, parents (adults in general) might show completely different correlations between all mentioned aspects, since e.g. they might percept their potential infection at any stage during their life cycle. Reduced fitness of the adults in turn may also affect the fitness of the nestlings for shortage of food intake or adults might serve as a reservoir of parasites such as ticks.
One significant consideration in our study is the exclusion of molecular techniques, specifically PCR/genetic detection methods, which warrants further explanation. While molecular methods offer precise identification and quantification of blood parasites, they are not without limitations86,87. Several ecological and epidemiological studies have demonstrated that the prevalence and intensity of haemosporidian infections can be reliably assessed through microscopic examination of blood smears35. Although this method is less specific in terms of identifying parasite species or strains compared to molecular techniques, it provides valuable insights into the overall health and immune response of the host53, aligning with the primary objectives of our study. A not yet fully compensated by PCR or sequencing method established benefit of microscopy allows for the assessment of various blood parameters, such as leucocyte counts and heterophil-to-lymphocyte ratios, which are crucial for understanding the physiological stress and immune status of the birds. These parameters are not directly measurable through molecular methods (yet). However, we acknowledge that the absence of molecular data limits our ability to identify specific parasite species and strains, which could offer a deeper understanding of parasite-host interactions.
Conclusion
Our findings clarify some aspects in the interplay between urbanization, haemosporidian infections, immune responses, and the overall health of Parus major nestlings. While vector abundance alone does not fully explain haemosporidian prevalence, the urban environment shapes the health and vulnerability of nestlings to parasites. Our study contributes to a deeper understanding of the host-vector-parasite triad in urban settings, offering insights that could be applicable to other similar ecological relationships, such as human malaria or the West Nile virus35,88,89. Further research into specific urbanization parameters that affect bird immune responses and can drive parasitic infections is essential for developing strategies to preserve avian health and biodiversity in urban landscapes13,90.
Data availability
The datasets used and analyzed during the current study are available from OSI Table S4.
References
Blair, R. B. Land use and avian species diversity along an urban gradient. Ecol. Appl. 6, 506–519. https://doi.org/10.2307/2269387 (1996).
Suarez-Rubio, M. & Krenn, R. Quantitative analysis of urbanization gradients: A comparative case study of two European cities. J. Urban Ecol. 4, 1–14. https://doi.org/10.1093/jue/juy027 (2018).
United Nations, D. o. E. a. S. A., Population Division. World Urbanization Prospects: The 2018 Revision, Online Edition. <https://esa.un.org/unpd/wup/Publications; accessed 27 Feb 2019.> (Department of Economic and Social Affairs, Population Division, 2018).
Marzluff, J. M. Avian Ecology and Conservation in an Urbanizing World 19–47 (Springer, 2001).
Whittaker, K. A. & Marzluff, J. M. Species-specific survival and relative habitat use in an urban landscape during the postfledging period. Auk 126, 288–299. https://doi.org/10.1525/auk.2009.07136 (2009).
Shochat, E., Warren, P. S., Faeth, S. H., McIntyre, N. E. & Hope, D. From patterns to emerging processes in mechanistic urban ecology. Trends Ecol. Evol. 21, 186–191. https://doi.org/10.1016/j.tree.2005.11.019 (2006).
McKinney, M. L. Urbanization as a major cause of biotic homogenization. Biol. Conserv. 127, 247–260. https://doi.org/10.1016/j.biocon.2005.09.005 (2006).
Perrin, A., Glaizot, O. & Christe, P. Worldwide impacts of landscape anthropization on mosquito abundance and diversity: A meta-analysis. Glob. Chang. Biol. 28, 6857–6871. https://doi.org/10.1111/gcb.16406 (2022).
Suarez-Rubio, M. et al. Bird diversity along an urban to rural gradient in large tropical cities peaks in mid-level urbanization. PeerJ https://doi.org/10.7717/peerj.16098 (2023).
Clergeau, P., Croci, S., Jokimaki, J., Kaisanlahti-Jokimaki, M. L. & Dinetti, M. Avifauna homogenisation by urbanisation: Analysis at different European latitudes. Biol. Conserv. 127, 336–344. https://doi.org/10.1016/j.biocon.2005.06.035 (2006).
McKinney, M. L. Urbanization, biodiversity, and conservation the impacts of urbanization on native species are poorly studied, but educating a highly urbanized human population about these impacts can greatly improve species conservation in all ecosystems. BioScience 52, 883–890 (2002).
Alberti, M. The effects of urban patterns on ecosystem function. Int. Reg. Sci. Rev. 28, 168–192. https://doi.org/10.1177/0160017605275160 (2016).
Seress, G. et al. Impact of urbanization on abundance and phenology of caterpillars and consequences for breeding in an insectivorous bird. Ecol. Appl. Publ. Ecol. Soc. Am. 28, 1143–1156. https://doi.org/10.1002/eap.1730 (2018).
Renner, S. C. et al. Food preferences of winter bird communities in different forest types. PLoS One 7, e53121. https://doi.org/10.1371/journal.pone.0053121 (2012).
Proppe, D. S., Sturdy, C. B. & St Clair, C. C. Anthropogenic noise decreases urban songbird diversity and may contribute to homogenization. Glob. Chang. Biol 19, 1075–1084. https://doi.org/10.1111/gcb.12098 (2013).
Lepczyk, C. A. et al. in Ecology and Conservation of Birds in Urban Environments (eds Enrique Murgui & M Hedblom) Ch. Chapter 2, 13–33 (Springer, 2017).
Murgui, E. & Hedblom, M. Ecology and Conservation of Birds in Urban Environments (Springer, 2017).
Ferraguti, M. et al. Does land-use and land cover affect vector-borne diseases? A systematic review and meta-analysis. Landsc. Ecol. 38, 2433–2451. https://doi.org/10.1007/s10980-023-01746-3 (2023).
Bichet, C. et al. Urbanization, trace metal pollution, and malaria prevalence in the house sparrow. PLoS One 8, e53866. https://doi.org/10.1371/journal.pone.0053866 (2013).
Buyantuyev, A. & Wu, J. Urban heat islands and landscape heterogeneity: linking spatiotemporal variations in surface temperatures to land-cover and socioeconomic patterns. Landsc. Ecol. 25, 17–33. https://doi.org/10.1007/s10980-009-9402-4 (2009).
Ferraguti, M. et al. Effects of landscape anthropization on mosquito community composition and abundance. Sci. Rep. 6, 29002. https://doi.org/10.1038/srep29002 (2016).
Biard, C. et al. Growing in cities: An urban penalty for wild birds? A study of phenotypic differences between urban and rural great tit chicks (Parus major). Front. Ecol. Evol. https://doi.org/10.3389/fevo.2017.00079 (2017).
Balen, J. H. V. A comparative sudy of the breeding ecology of the great tit parus majorin different habitats. Ardea 38–90, 1–93. https://doi.org/10.5253/arde.v61.p1 (2002).
Paaijmans, K. P. & Thomas, M. B. The influence of mosquito resting behaviour and associated microclimate for malaria risk. Malar. J. 10, 183. https://doi.org/10.1186/1475-2875-10-183 (2011).
Vanderberg, J. P. & Yoeli, M. Effects of temperature on sporogonic development of Plasmodium berghei. J. Parasitol. 52, 559–564 (1966).
LaPointe, D. A., Goff, M. L. & Atkinson, C. T. Thermal constraints to the sporogonic development and altitudinal distribution of avian malaria Plasmodium relictum in Hawai’i. J. Parasitol. 96, 318–324. https://doi.org/10.1645/GE-2290.1 (2010).
Bailly, J. et al. Negative impact of urban habitat on immunity in the great tit Parus major. Oecologia 182, 1053–1062. https://doi.org/10.1007/s00442-016-3730-2 (2016).
Chamberlain, D. E. et al. Avian productivity in urban landscapes: a review and meta-analysis. Ibis 151, 1–18. https://doi.org/10.1111/j.1474-919X.2008.00899.x (2009).
Charmantier, A., Demeyrier, V., Lambrechts, M., Perret, S. & Grégoire, A. Urbanization is associated with divergence in pace-of-life in great tits. Front. Ecol. Evol. 5, 1–13. https://doi.org/10.3389/fevo.2017.00053 (2017).
van Hoesel, W. et al. Management of ecosystems alters vector dynamics and haemosporidian infections. Sci. Rep. 9, 8779. https://doi.org/10.1038/s41598-019-45068-4 (2019).
van Hoesel, W., Santiago Alarcon, D., Marzal, A. & Renner, S. C. Effects of forest structure on the interaction between avian hosts, dipteran vectors and haemosporidian parasites. BMC Ecol. 20, 47. https://doi.org/10.1186/s12898-020-00315-5 (2020).
Paaijmans, K. P. et al. Temperature variation makes ectotherms more sensitive to climate change. Glob. Chang. Biol. 19, 2373–2380. https://doi.org/10.1111/gcb.12240 (2013).
Ferraguti, M. et al. Ecological determinants of avian malaria infections: An integrative analysis at landscape, mosquito and vertebrate community levels. J. Anim. Ecol. 87, 727–740 (2018).
Tchoumbou, M. A. et al. Effect of deforestation on prevalence of avian haemosporidian parasites and mosquito abundance in a tropical rainforest of Cameroon. Int. J. Parasitol. 50, 63–73. https://doi.org/10.1016/j.ijpara.2019.10.006 (2020).
Valkiūnas, G. Avian Malaria Parasites and Other Haemosporidia (CRC Press, 2005).
Renner, S. C. et al. Forests of opportunities and mischief: disentangling the interactions between forests, parasites and immune responses. Int. J. Parasitol. 46, 571–579. https://doi.org/10.1016/j.ijpara.2016.04.008 (2016).
Czech, B., Krausman, P. R. & Devers, P. K. Economic associations among causes of species endangerment in the United States. BioScience 50, 593–601 (2000).
Peig, J. & Green, A. J. New perspectives for estimating body condition from mass/length data: The scaled mass index as an alternative method. Oikos 118, 1883–1891. https://doi.org/10.1111/j.1600-0706.2009.17643.x (2009).
Sorace, A. & Gustin, M. Bird species of conservation concern along urban gradients in Italy. Biodivers. Conserv. 19, 205–221. https://doi.org/10.1007/s10531-009-9716-1 (2010).
Jokimäki, J. & Huhta, E. Artificial Nest Predation and Abundance of Birds along an Urban Gradient. The Condor 102, 838–847 (2000).
Chasar, A. et al. Prevalence and diversity patterns of avian blood parasites in degraded African rainforest habitats. Mol. Ecol. 18, 4121–4133. https://doi.org/10.1111/j.1365-294X.2009.04346.x (2009).
Ludtke, B. et al. Associations of forest type, parasitism and body condition of two European passerines, Fringilla coelebs and Sylvia atricapilla. PLoS One 8, e81395. https://doi.org/10.1371/journal.pone.0081395 (2013).
Troyo, A. et al. Seasonal profiles of Aedes aegypti (Diptera: Culicidae) larval habitats in an urban area of Costa Rica with a history of mosquito control. J. Vector Ecol. 33, 76–88. https://doi.org/10.3376/1081-1710(2008)33[76:spoaad]2.0.co;2 (2008).
Medeiros-Sousa, A. R. et al. Diversity and abundance of mosquitoes (Diptera:Culicidae) in an urban park: Larval habitats and temporal variation. Acta Trop. 150, 200–209. https://doi.org/10.1016/j.actatropica.2015.08.002 (2015).
Fokidis, H. B., Greiner, E. C. & Deviche, P. Interspecific variation in avian blood parasites and haematology associated with urbanization in a desert habitat. J. Avian Biol. 39, 300–310. https://doi.org/10.1111/j.2008.0908-8857.04248.x (2008).
Santiago-Alarcon, D., Havelka, P., Pineda, E., Segelbacher, G. & Schaefer, H. M. Urban forests as hubs for novel zoonosis: blood meal analysis, seasonal variation in Culicoides (Diptera: Ceratopogonidae) vectors, and avian haemosporidians. Parasitology 140, 1799–1810. https://doi.org/10.1017/S0031182013001285 (2013).
Santiago-Alarcon, D. et al. Parasites in space and time: A case study of haemosporidian spatiotemporal prevalence in urban birds. Int. J. Parasitol. 49, 235–246. https://doi.org/10.1016/j.ijpara.2018.08.009 (2019).
Dimitrov, D. et al. Plasmodium spp.: An experimental study on vertebrate host susceptibility to avian malaria. Exp. Parasitol. 148, 1–16. https://doi.org/10.1016/j.exppara.2014.11.005 (2015).
Marzal, A., de Lope, F., Navarro, C. & Moller, A. P. Malarial parasites decrease reproductive success: An experimental study in a passerine bird. Oecologia 142, 541–545. https://doi.org/10.1007/s00442-004-1757-2 (2005).
Asghar, M., Hasselquist, D. & Bensch, S. Are chronic avian haemosporidian infections costly in wild birds?. J. Avian Biol. 42, 530–537 (2011).
Knowles, S. C. L., Palinauskas, V. & Sheldon, B. C. Chronic malaria infections increase family inequalities and reduce parental fitness: Experimental evidence from a wild bird population. J. Evol. Biol. 23, 557–569. https://doi.org/10.1111/j.1420-9101.2009.01920.x (2010).
Schoepf, I., Olson, S., Moore, I. T. & Bonier, F. Experimental reduction of haemosporidian infection affects maternal reproductive investment, parental behaviour and offspring condition. Proc. Biol. Sci. R. Soc. 289, 20221978. https://doi.org/10.1098/rspb.2022.1978 (2022).
Campbell, T. W. & Ellis, C. K. Avian and Exotic Animal Hematology and Cytology (John Wiley & Sons, 2007).
Müller, C., Jenni-Eiermann, S. & Jenni, L. Heterophils/Lymphocytes-ratio and circulating corticosterone do not indicate the same stress imposed on Eurasian kestrel nestlings. Funct. Ecol. 25, 566–576. https://doi.org/10.1111/j.1365-2435.2010.01816.x (2011).
Moreno, J., Merino, S., MartÍnez, J., Sanz, J. & Arriero, E. Heterophil/lymphocyte ratios and heat-shock protein levels are related to growth in nestling birds. Écoscience 9, 434–439. https://doi.org/10.1080/11956860.2002.11682731 (2016).
Peig, J. & Green, A. J. The paradigm of body condition: A critical reappraisal of current methods based on mass and length. Funct. Ecol. 24, 1323–1332. https://doi.org/10.1111/j.1365-2435.2010.01751.x (2010).
Gonzalez, G. et al. Immunocompetence and condition-dependent sexual advertisement in male house sparrows (Passer domesticus). J. Anim. Ecol. 68, 1225–1234. https://doi.org/10.1046/j.1365-2656.1999.00364.x (1999).
Monrós, J. S., Belda, E. J. & Barba, E. Post-fledging survival of individual great tits: The effect of hatching date and fledging mass. Oikos 99, 481–488. https://doi.org/10.1034/j.1600-0706.2002.11909.x (2002).
Brown, C. R. & Brown, M. B. Ectoparasites cause increased bilateral asymmetry of naturally selected traits in a colonial bird. J. Evol. Biol. 15, 1067–1075 (2002).
De Coster, G. et al. Fluctuating asymmetry and environmental stress: Understanding the role of trait history. PLoS One 8, e57966. https://doi.org/10.1371/journal.pone.0057966 (2013).
Helle, S., Huhta, E., Suorsa, P. & Hakkarainen, H. Fluctuating asymmetry as a biomarker of habitat fragmentation in an area-sensitive passerine, the Eurasian treecreeper (Certhia familiaris). Ecol. Indic. 11, 861–867. https://doi.org/10.1016/j.ecolind.2010.11.004 (2011).
Lens, L., Dongen, S., Kark, S. & Matthysen, E. Fluctuating asymmetry as an indicator of fitness: Can we bridge the gap between studies?. Biol. Rev. 77, 27–38 (2002).
Letters, E. Fluctuating and directional asymmetry in natural bird populations exposed to different levels of habitat disturbance , as revealed by mixture analysis. (2000).
Cator, L. J. et al. The role of vector trait variation in vector-borne disease dynamics. Front. Ecol. Evol. https://doi.org/10.3389/fevo.2020.00189 (2020).
East, M. L. & Hofer, H. The use of radio-tracking for monitoring Great Tit Parus major behaviour: A pilot study. Ibis 128, 103–114. https://doi.org/10.1111/j.1474-919X.1986.tb02097.x (1986).
European Environmental Agency. European Urban Atlas. <https://www.eea.europa.eu/ds_resolveuid/5b71306a54ad9115e93429a251315e71> (EU, 2011).
Riddington, R. & Gosler, A. G. Differences in reproductive success and parental qualities between habitats in the Great Tit Parus major. Ibis 137, 371–378. https://doi.org/10.1111/j.1474-919X.1995.tb08035.x (2008).
Hargitai, R. et al. Effects of breeding habitat (woodland versus urban) and metal pollution on the egg characteristics of great tits (Parus major). Sci. Total Environ. 544, 31–38. https://doi.org/10.1016/j.scitotenv.2015.11.116 (2016).
Eck, S. et al. Measuring Birds/Vögel Vermessen (Christ Media, 2011).
Svensson, L. Identification Guide to European Passerines (British Trust for Ornithology, 1992).
Godfrey, R. D. Jr., Fedynich, A. M. & Pence, D. B. Quantification of hematozoa in blood smears. J. Wildl. Dis. 23, 558–565. https://doi.org/10.7589/0090-3558-23.4.558 (1987).
De Angeli Dutra, D. et al. Haemosporidian infections affect antioxidant defences in great tits Parus major but are not related to exposure to aerial pollutants. Parasitol. Open https://doi.org/10.1017/pao.2017.4 (2017).
Clark, N. J., Adlard, R. D. & Clegg, S. M. Molecular and morphological characterization of Haemoproteus (Parahaemoproteus) ptilotis, a parasite infecting Australian honeyeaters (Meliphagidae), with remarks on prevalence and potential cryptic speciation. Parasitol. Res. 114, 1921–1928. https://doi.org/10.1007/s00436-015-4380-8 (2015).
Naimi, B., Hamm, N. A. S., Groen, T. A., Skidmore, A. K. & Toxopeus, A. G. Where is positional uncertainty a problem for species distribution modelling?. Ecography 37, 191–203 (2014).
Arnold, T. W. Uninformative parameters and model selection using Akaike’s Information Criterion. J. Wildl. Manage. 74, 1175–1178. https://doi.org/10.2193/2009-367 (2010).
Bates, D., Maechler, M., Bolker, B. & Walker, S. lme4: Linear mixed-effects models using Eigen and S4. R package version 1 (2014).
Burnham, K. P. & Anderson, D. R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach (Springer, 2002).
R: A language and environment for statistical computing. v. 4.2.3 (R Foundation for Statistical Computing. http://www.r-project.org/, Vienna, Austria, 2023).
Santiago-Alarcon, D., Havelka, P., Schaefer, H. M. & Segelbacher, G. Bloodmeal analysis reveals avian Plasmodium infections and broad host preferences of Culicoides (Diptera: Ceratopogonidae) vectors. PLoS One 7, e31098. https://doi.org/10.1371/journal.pone.0031098 (2012).
Santiago-Alarcon, D., Palinauskas, V. & Schaefer, H. M. Diptera vectors of avian Haemosporidian parasites: Untangling parasite life cycles and their taxonomy. Biol. Rev. Camb. Philos. Soc. 87, 928–964. https://doi.org/10.1111/j.1469-185X.2012.00234.x (2012).
Arnfield, A. J. Two decades of urban climate research: A review of turbulence, exchanges of energy and water, and the urban heat island. Int. J. Climatol. 23, 1–26. https://doi.org/10.1002/joc.859 (2003).
Gross, W. B. & Siegel, H. S. Evaluation of the heterophil/lymphocyte ratio as a measure of stress in chickens. Avian Dis. https://doi.org/10.2307/1590198 (1983).
Grieco, F. Greater food availability reduces tarsus asymmetry in nestling blue tits. Condor https://doi.org/10.1093/condor/105.3.599 (2003).
Bańbura, J. et al. Body condition parameters of nestling great titsparus majorin relation to experimental food supplementation. Acta Ornithol. 46, 207–212. https://doi.org/10.3161/000164511x625991 (2011).
Corsini, M. et al. Growing in the city: Urban evolutionary ecology of avian growth rates. Evol. Appl. 14, 69–84. https://doi.org/10.1111/eva.13081 (2021).
Levin, I. I. et al. Hippoboscid-transmitted Haemoproteus parasites (Haemosporida) infect Galapagos Pelecaniform birds: evidence from molecular and morphological studies, with a description of Haemoproteus iwa. Int. J. Parasitol. 41, 1019–1027. https://doi.org/10.1016/j.ijpara.2011.03.014 (2011).
Jarvi, S. I., Schultz, J. J. & Atkinson, C. T. Pcr diagnostics underestimate the prevalence of avian malaria (Plasmodium Relictum) in experimentally-infected passerines. J. Parasitol. 88, 153–158. https://doi.org/10.1645/0022-3395(2002)088[0153:Pdutpo]2.0.Co;2 (2002).
Chaves, L. F. et al. Climatic variability and landscape heterogeneity impact urban mosquito diversity and vector abundance and infection. Ecosphere https://doi.org/10.1890/es11-00088.1 (2011).
Rappole, J. H., Derrickson, S. R. & Hubalek, Z. Migratory birds and spread of West Nile virus in the Western Hemisphere. Emerg. Infect. Dis. 6, 319–328. https://doi.org/10.3201/eid0604.000401 (2000).
Chace, J. F. & Walsh, J. J. Urban effects on native avifauna: a review. Landsc. Urban Plan. 74, 46–69. https://doi.org/10.1016/j.landurbplan.2004.08.007 (2006).
Acknowledgements
We thank A Wieshaider and M Nemeth from the Österreichische Bundesforste, S Jeitler from Stift Schotten and M Landrichter Stift St. Peter, and Wiener Umweltschutzabteilung MA 22 for collaboration and to mount nest boxes on their properties. Many helped monitoring the nest boxes: J Meininghaus, M Kogler, F Kaunitzer, Z Hostnig, I Tarmann, S Fleischmann, M Peer, E Kraus, C Klein, E Wiedenegger, T Müllner, and M Ranalter. W van Hoesel supported identification of vectors. L Vibert assisted during the scanning process, analysis of blood samples and vector counts. Last but not least we thank the editor and three anonymous reviewers for their critical comments improving our work.
Funding
The study was performed without institutional or other organizational funding. The publication fees have been provided by the NHM Wien.
Author information
Authors and Affiliations
Contributions
S.C.R., M.S.R. developed the concept; A.E.N., M.S.R., S.C.R. performed fieldwork and the analysis. All authors contributed to text writing and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Neumann, A.E., Suarez-Rubio, M. & Renner, S.C. Haemosporidian intensity and nestlings' life-history along an urban-to-rural gradient. Sci Rep 14, 17018 (2024). https://doi.org/10.1038/s41598-024-67824-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-67824-x
- Springer Nature Limited