Abstract
We evaluated the diagnostic performance of newly developed microfluidic microplate-based fluorescent ELISA for anti-SARS-CoV-2 antibody detection: the Veri-Q opti COVID-19 IgG and IgM ELISAs (hereafter, “Opti IgG/M”; MiCo BioMed, Gyeonggi-do, Republic of Korea), in comparison with conventional ELISAs. A total of 270 serum samples were analyzed, among which 90 samples were serially obtained from 25 COVID-19 patients. Another 180 samples were collected from 180 SARS-CoV-2–negative individuals. As comparative assays, we used SCoV-2 Detect IgG/M ELISA (hereafter, “InBios IgG/M”; InBios, Seattle, WA, USA) and Veri-Q COVID-19 IgG/IgM ELISA (hereafter, “Veri-Q IgG/M”; MiCo BioMed). Compared with conventional ELISAs, the Opti IgG yielded 97.1–100.0% positive percent agreement, 95.2–98.0% negative percent agreement, 96.3–97.8% total percent agreement, and kappa values of 0.90–0.94. Between the Opti IgM and the InBios IgM, the values were 93.7%, 96.6%, 95.9%, and 0.89, respectively. For the Opti IgG, sensitivities for the samples collected from 0–7, 8–14, 15–21, and ≥ 22 days after symptom onset were 40.0, 58.3, 94.1, and 100.0%, respectively. The values for the Opti IgM were 30.0, 54.2, 88.2, and 80%, respectively. The diagnostic specificities of the Opti IgG and IgM were 99.4 and 97.2%, respectively. The microfluidic microplate-based fluorescent ELISAs showed comparable diagnostic performance to conventional ELISAs for detecting anti-SARS-CoV-2 antibodies. With the combination of high throughput, a simplified workflow, and the ability to analyze reduced volumes, this new technology has great potential for improving SARS-CoV-2 serologic testing.
Similar content being viewed by others
Introduction
Enzyme-linked immunosorbent assays (ELISAs), notable for their diagnostic accuracy, are routinely performed in research and clinical laboratories1. Among various types of ELISA, the heterogeneous ELISA is commonly used because of its higher sensitivity. However, conventional heterogeneous assays require multiple, time-consuming washing and incubation steps to separate the bound antigen from the free antigen. These steps limit the applicability of conventional heterogeneous ELISA for high-throughput applications1,2.
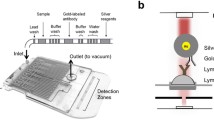
Opti96 technology (previously called the Optimiser, MiCo BioMed, Gyeonggi-do, Republic of Korea) is a microfluidic microplate-based fluorescent ELISA that uses the 96-well layout of microchannels. Compared with conventional ELISA plates, Opti96 capillary microchannels provide a 50% increase in surface area and, thus, a 50-fold increase in surface-area-to-volume ratio in conjunction with dramatically reduced diffusion distances. This results in increased surface binding and rapid assay kinetics. Additionally, the microchannels eliminate the need for traditional washing steps, which are replaced by simple flushing. The emergence of this technology has significantly reduced sample and reagent volume requirements, shortened assay times (< 70 min), and enabled high-sensitivity diagnostics3. Figure 1 depicts a schematic illustration of this technology.
Schematic illustration of the microfluidic microplate-based fluorescent ELISA. (A) The Opti96 microplate (MiCo BioMed, Gyeonggi-do, Republic of Korea). Each well contains capillary microfluidic channels where the analytic reactions occur. (B) 1. The wells in the Opti96 microfluidic platform are used for loading all reagents and samples. The assay reaction occurs as they flow through the microchannel of the microplate. 2. All assay reactions take place in the 200 × 200 μm microfluidic reaction chamber located under each loading well. The capture antigen is coated on the internal surfaces of the plate’s microchannels. Antibodies targeting SARS-CoV-2 present in the samples are specifically captured on the microchannel surface, and a reaction by the detection antibody consequently occurs. 3. Traditional wash steps are replaced by simple flushing.
COVID-19, the lung disease caused by SARS-CoV-2, has been a threat to individual and public health worldwide since 2019. Although the diagnostic standard for COVID-19 is molecular detection of the causative pathogen, SARS-CoV-2, serologic antibody tests have been utilized for epidemiologic research, individual risk assessments, diagnosis of COVID-19 in concert with molecular testing, and convalescent plasma therapy4. Furthermore, recovery from COVID-19 and the vaccination of millions of people against the disease have made serologic tests that identify the presence and levels of SARS-CoV-2 antibodies highly relevant. This study evaluated—in comparison with conventional ELISAs—the diagnostic performance of newly developed microfluidic ELISAs for SARS-CoV-2 IgG and IgM antibodies using Opti96 technology, Veri-Q opti COVID-19 IgG ELISA, and Veri-Q opti COVID-19 IgM ELISA (MiCo BioMed). These were developed to assess humoral immunity acquired by natural infection.
Methods
Clinical samples
A total of 270 serum samples were used to evaluate the performance of the Veri-Q opti COVID-19 IgG/IgM ELISA, among which 90 samples were serially obtained from 25 COVID-19 patients who were confirmed to be infected with SARS-CoV-2 by molecular testing of nasopharyngeal swab specimens. Among the 90 samples, 12, 29, 23, and 26 were obtained < 7, 7–14, 15–21, and ≥ 21 days after symptom onset, respectively.
Another 180 samples were collected from 180 individuals who were confirmed to be negative for SARS-CoV-2 by molecular testing. Five of these 180 samples were anti-nuclear antibody (ANA)–positive, five were hepatitis B virus surface antigen (HBsAg)–positive, five were anti-hepatitis C virus (anti-HCV)–positive, and three were convalescent sera from individuals who had been infected with coronaviruses other than SARS-CoV-2. The samples were acquired from February 2020 to January 2021, which is the pre-COVID-19 vaccination era in the Republic of Korea. All samples were stored at − 70 ℃ until their analysis.
The study protocol was approved by the institutional review board of Chung-Ang University Hospital (Seoul, Republic of Korea; IRB approval no. 2010-016-437). The protocol was performed in accordance with the Declaration of Helsinki, and obtaining informed consent from the study subjects was waived by the institutional review board.
Microfluidic microplate-based fluorescent ELISA
Veri-Q opti COVID-19 IgG and IgM ELISA (hereafter, “Opti IgG” and “Opti IgM,” respectively) are microfluidic microplate-based fluorescent ELISAs that use Opti96 technology. Opti IgG detects anti-nucleocapsid (N) SARS-CoV-2 IgG, and Opti IgM detects anti-receptor-binding domain (RBD) SARS-CoV-2 IgM, respectively. The targeted epitopes of each assay were determined following previous studies according to their binding affinity5,6.
These assays replaced the conventional microplate used in ELISA with the microfluidic microplate. Briefly, reagents and/or samples are loaded into the loading well according to the manufacturer’s instructions; a reaction occurs as they flow through the microchannel of the microplate. In detail, capture antigen (N protein for IgG and receptor-binding domain of S protein for IgM) was loaded and immobilized on the internal surfaces of the plates’ microchannels. After a 10 min flush step, 5 µL of diluted (1:20) serum samples and controls were dispensed into the wells. Then, the antibody present in the samples and controls was specifically captured on the microchannel surface. After 10 min of incubation at room temperature and another flush washing, 5 µL of horseradish peroxidase–labeled secondary antibody was added to the wells. Following two additional flush steps, 5 µL of chemifluorescent substrate was added. After 15 min, relative fluorescence unit (RFU) measurements were read at the wavelengths of Ex.530 nm and Em.590 nm using a fluorescence plate reader (Synergy HT, BIO-TEK, Winooski, VT, USA). For the wash step, 5 µL of solution after sample loading and 30 µL of solution after loading the secondary antibody were used, and the same incubation time (10 min) was used for removal through dilution of unreacted substances.
Test results were interpreted using the antibody index, which was calculated as the sample RFU divided by the blank RFU. An antibody index ≥ 12 was considered a positive result, and an index ≤ 6 was considered negative. Any other index value was categorized as a “retest” value. After retesting, index values ≥ 9 were considered positive.
Conventional ELISAs
As comparative assays, we used SCoV-2 Detect IgG/M ELISA (hereafter, “InBios IgG/M”; InBios, Seattle, WA, USA, Food and Drug Administration Emergency Use Authorized [FDA-EUA]) and Veri-Q COVID-19 IgG/IgM ELISA (hereafter, “Veri-Q IgG/M”; MiCo BioMed). In both InBios IgG and IgM, the S protein was used as the capture antigen. Veri-Q IgG detects anti-N SARS-CoV-2 IgG, and Opti IgM detects anti-RBD SARS-CoV-2 IgM, respectively, similar to Opti IgG and Opti IgM. Both assays are conventional heterogeneous sandwich-format ELISAs, and all procedures were conducted according to the manufacturer’s instructions at the same time as the microfluidic ELISAs.
InBios IgG/M results were interpreted using the immunological status ratio (ISR), which was calculated from the ratio of the optical density obtained with the test sample divided by the calculated cutoff value. An ISR ≥ 1.1 was considered a positive result, and an index ≤ 0.9 was considered negative. Any other ISR value was categorized as a “retest” value. After retesting, ISR values ≥ 1 were considered positive.
Test results of Veri-Q IgG/M were interpreted using the antibody index; an antibody index ≥ 1.1 was considered a positive result, and an index ≤ 0.7 was considered negative. Any other index value was categorized as a “retest” value. After retesting, index values ≥ 1 were considered positive.
Statistical analysis
The positive percent agreement (PPA), negative percent agreement (NPA), total percent agreement (TPA), and Cohen’s kappa between the assays for SARS-CoV-2 antibody detection were evaluated. Additionally, diagnostic sensitivity and specificity for the detection of COVID-19 patients or SARS-CoV-2 infection were calculated. All statistical analyses were performed using Microsoft Office Excel 2016 (Microsoft Corp., Redmond, WA, USA) and R version 3.6.1 (The R Foundation for Statistical Computing, Vienna, Austria) according to the Clinical and Laboratory Standards Institute guideline EP12-A27.
Results
Assay performance comparisons of the Opti IgG and IgM with the two conventional ELISAs are listed in Table 1. For IgG, compared with the conventional ELISAs, the Opti IgG yielded 97.1–100.0% PPA, 95.2–98.0% NPA, 96.3–97.8% TPA, and kappa values of 0.90–0.94 (categorized as “almost perfect” agreement). Between the Opti IgM and the InBios IgM, the values were 93.7%, 96.6%, 95.9%, and 0.89, respectively. Compared with the Veri-Q IgM, the Opti IgM yielded 100% PPA, 84.6% NPA, 86.3% TPA, and a kappa value of 0.54.
The diagnostic sensitivity and specificity of each assay for diagnosing COVID-19 using SARS-CoV-2-specific antibodies according to the number of days after symptom onset are listed in Supplementary Table 1. For the Opti IgG, the sensitivities for samples collected 0–7, 8–14, 15–21, and ≥ 22 days after symptom onset were 40.0, 58.3, 94.1, and 100.0%, respectively. The values for the Opti IgM were 30.0, 54.2, 88.2, and 80%, respectively. The diagnostic specificities of the Opti IgG and IgM were 99.4 and 97.2%, respectively. There were no false-positive results obtained from the ANA-, HBsAg-, anti-HCV-positive samples or convalescent sera positive for coronaviruses other than SARS-CoV-2.
The sensitivities of the conventional ELISA and InBios IgG/IgM for samples 0–7, 8–14, 15–21, and ≥ 22 days after symptom onset were 30.0%/30.0%, 54.2%/50.0%, 100.0%/94.1%, and 100.0%/86.7%, respectively, with specificities of 100.0%/100.0%. The sensitivities of Veri-Q IgG/IgM for each sample were 30.0%/20.0%, 41.7%/20.8%, 94.1%/52.9%, and 93.3%/46.7% respectively, with specificities of 100.0%/100.0%.
Time kinetics of SARS-CoV-2 IgG and IgM according to the signal generated by tested assays (antibody index or ISR) are illustrated in Supplementary Fig. S1. Although these are qualitative assays, they demonstrate the typical humoral immune response against SARS-CoV-2. The smoothing splines of the assays showed that IgM and IgG antibody titers rapidly increased after 7 days post-symptom onset (PSO). For IgM, the smoothing splines of both assays reached a peak at 15–21 days PSO and then gradually decreased. For IgG, the smoothing splines gradually increased and remained at similar levels or slightly decreased after 21 days PSO.
Discussion
There are several technologies for serologic detection of SARS-CoV-2, including conventional ELISA, chemiluminescent immunoassay, and lateral flow immunoassay. However, these technologies have several disadvantages, such as high costs, high sample and reagent volume requirements, low throughput, onerous procedures, and unsatisfactory diagnostic performance8. Therefore, new technologies are sought that are capable of high throughput, can yield results with low reagent and sample consumption, are less cumbersome, and can achieve high sensitivity and specificity. In this study, we evaluated the diagnostic performance of microfluidic microplate-based fluorescent ELISAs for detecting antibodies against SARS-CoV-2, in comparison with conventional ELISAs, including an FDA-approved assay.
In this ELISA technique, the microfluidic microplates work by capillary action between the microchannel and absorbent pads with a passive flow regulation process. This facilitates rapid and accurate target detection. The assay principle for the novel ELISA technique was similar to that of conventional ELISAs3. Besides the structural characteristics of the assay, the most distinctive features of the reagents were the conjugate reagents. For microfluidic ELISA, 10-acetyl-3,7-dihydroxyphenoxazine was used as a conjugate; this is a more sensitive substrate than the tetramethylbenzidine-based substrates used in conventional ELISAs9. Consequently, microfluidic microplates can offer the advantage of using a highly accessible microfluidic or capillary surface and a highly sensitive substrate compared with those used in conventional ELISAs, minimizing sample and reagent volume requirements. Moreover, the novel approach eliminated the traditional washing steps and replaced them with simple and passive “flush-washing” steps. Given that traditional washing steps are onerous and highly influenced by the operator’s skill, this simple flush-washing is an attractive advantage of this assay. To date, microfluidic microplate-based fluorescent ELISA has been used for detecting the lactate dehydrogenase of Plasmodium falciparum10 and for measuring serum levels of cytokines, including human interleukin-611.
In this study, the performance of the microfluidic ELISA, Opti IgG/IgM, was comparable to those of InBios IgG/IgM, which have been approved by the FDA. Their TPA was 97.8%, and their kappa values reflected “almost perfect” agreement with the findings of the traditional ELISAs. Overall, the Opti IgG/M showed more positive results than InBios. However, some samples showed only positive results in InBios IgG/IgM, not in Opti IgG/IgM. Although the exact cause of these discrepancies is unclear, differences in target epitopes of the assays can be considered. Especially for IgG, the samples that showed Opti-negative and InBios-positive results were obtained from the same patient, suggesting that these results were caused by differences in the characteristics of the antibodies produced depending on the patient’s immune system. The effect of endogenous substances in the sample, such as rheumatoid factor, heterophil antibody, autoantibodies, cross-reactive substances, or enzyme inhibitors can also be considered.
In contrast to the InBios IgG/IgM, agreement of the novel ELISA with the conventional Veri-Q IgG/IgM ELISA was lower than that with InBios IgG/IgM. In particular, agreement between Opti IgM and Veri-Q IgM was unsatisfactory, with a TPA of 86.3% and a kappa value of only 0.54. This finding reflects the low diagnostic sensitivity of the Veri-Q IgM conventional ELISA compared with the other assays. The diagnostic sensitivity of Veri-Q IgM ranged from only 20.0%–52.9% according to the samples obtained period, but those for Opti IgM and InBios IgM were 30.0%–88.2% and 30.0–94.1%, respectively. Consequently, because Veri-Q IgM showed more negative results for the COVID-19 positive samples than other assays, the NPA value of Opti IgM to Veri-Q IgM was significantly lower (84.6%, 204/241) than to InBios IgM (96.6%, 200/207).
The diagnostic specificity values for Opti IgG and IgM (99.4 and 97.2%) were slightly lower than those associated with conventional ELISAs (100% for all), and there were more false-positive findings associated with the IgM assay than the IgG assay. Aside from the lack of benefits of using IgM testing for COVID-19 relative to IgG testing or molecular testing12, IgM false-positivity can lead to unnecessary investigations for SARS-CoV-2 infection. Thus, further improvements are needed to improve the specificity of IgM testing.
This evaluation had several limitations. First, the relatively small sample size could have affected the study outcomes. Second, antibody detection with other methods, such as chemiluminescent immunoassay or lateral flow immunoassay, was not conducted for further comparisons. Third, we did not verify the cut-off antibody index value of the assay. Additionally, the analytical limit of detection (LoD) values of the assay, although mainly used in quantitative tests, were also not evaluated. The manufacturers did not provide the LoD of the assay, but information about the LoD would be valuable. Fourth, because the symptom onset data were derived from patient recall, it is likely that these data were inaccurate by a few days. Finally, sample numbers, especially for the late period of infection, were limited, therefore, it was not possible to verify that IgG levels remain at a similar level for several months13.
In conclusion, the novel microfluidic microplate-based fluorescent ELISA tools, Veri-Q opti COVID-19 IgG and IgM, had comparable diagnostic performance to that of conventional ELISAs. The combination of high throughput, the ability to analyze reduced volumes, and a simplified workflow that minimized steps that increase susceptibility to error bode well for the utility of these tools in SARS-CoV-2 serologic testing.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Aydin, S. A short history, principles, and types of ELISA, and our laboratory experience with peptide/protein analyses using ELISA. Peptides 72, 4–15. https://doi.org/10.1016/j.peptides.2015.04.012 (2015).
Banala, S., Arts, R., Aper, S. J. & Merkx, M. No washing, less waiting: engineering biomolecular reporters for single-step antibody detection in solution. Org. Biomol. Chem. 11, 7642–7649. https://doi.org/10.1039/c3ob41315b (2013).
Kai, J. et al. A novel microfluidic microplate as the next generation assay platform for enzyme linked immunoassays (ELISA). Lab chip 12, 4257–4262. https://doi.org/10.1039/c2lc40585g (2012).
Theel, E. S. et al. The role of antibody testing for SARS-CoV-2: Is there one?. J. Clin. Microbial. https://doi.org/10.1128/jcm.00797-20 (2020).
Ku, Z. et al. Nasal delivery of an IgM offers broad protection from SARS-CoV-2 variants. Nature 595, 718–723. https://doi.org/10.1038/s41586-021-03673-2 (2021).
Haynes, W. A. et al. High-resolution epitope mapping and characterization of SARS-CoV-2 antibodies in large cohorts of subjects with COVID-19. Commun. Biol. 4, 1317. https://doi.org/10.1038/s42003-021-02835-2 (2021).
Clinical and Laboratory Standards Institute (CLSI). User Protocol for Evaluation of Qualitative Test Performance; Approved Guideline-Second Edition. CLSI document EP12-A2 (ISBN 1-56238-654-9). Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087 USA, 2008.
Carter, L. J. et al. Assay techniques and test development for COVID-19 diagnosis. ACS Cent. Sci. 6, 591–605. https://doi.org/10.1021/acscentsci.0c00501 (2020).
Lü, J. M., Lin, P. H., Yao, Q. & Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med. 14, 840–860. https://doi.org/10.1111/j.1582-4934.2009.00897.x (2010).
Lee, W. S. et al. Simple, rapid, and accurate malaria diagnostic platform using microfluidic-based immunoassay of Plasmodium falciparum lactate dehydrogenase. Nano Converg. 7, 13. https://doi.org/10.1186/s40580-020-00223-w (2020).
Montaha, L., Junhai, K. & Nelson. S. Novel Biomarkers Detection and Identification by Microfluidic- Based MicroELISA. Translational Medicine. 01. https://doi.org/10.4172/2161-1025.S1-006 (2012).
Kweon, O. J. et al. Antibody kinetics and serologic profiles of SARS-CoV-2 infection using two serologic assays. PloS ONE 15, e0240395. https://doi.org/10.1371/journal.pone.0240395 (2020).
Ortega, N. et al. Seven-month kinetics of SARS-CoV-2 antibodies and role of pre-existing antibodies to human coronaviruses. Nat. Commun. 12, 4740. https://doi.org/10.1038/s41467-021-24979-9 (2021).
Acknowledgements
We thank MiCo BioMed Inc. for providing the Veri-Q opti COVID-19 IgG and IgM ELISA kits for this study. MiCo BioMed Inc. provided technical support only and had no role in the study design, data collection, or interpretation.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No.2020R1A5A1018052).
Author information
Authors and Affiliations
Contributions
M.-K.L. conceived the presented idea and supervised the findings of this work. O.J.K. collected literature and wrote the first draft of the manuscript. Y.K.L. performed the statistics. H.K.K. and K.W.C. designed the figures. S.M.Y. and O.J.K. performed the experiments. O.J.K. and K.W.C. revised the manuscript. M.-K.L. received funding support.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kweon, O.J., Yoon, S., Choe, K.W. et al. Performance evaluation of microfluidic microplate-based fluorescent ELISA for qualitative detection of SARS-CoV-2–specific IgG and IgM. Sci Rep 14, 18200 (2024). https://doi.org/10.1038/s41598-024-67977-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-67977-9
- Springer Nature Limited