Abstract
GNAO1 encodes G protein subunit alpha O1 (Gαo). Pathogenic variations in GNAO1 cause developmental delay, intractable seizures, and progressive involuntary movements from early infancy. Because the functional role of GNAO1 in the developing brain remains unclear, therapeutic strategies are still unestablished for patients presenting with GNAO1-associated encephalopathy. We herein report that siRNA-mediated depletion of Gnao1 perturbs the expression of transcripts associated with Rho GTPase signaling in Neuro2a cells. Consistently, siRNA treatment hampered neurite outgrowth and extension. Growth cone formation was markedly disrupted in monolayer neurons differentiated from iPSCs from a patient with a pathogenic variant of Gαo (p.G203R). This variant disabled neuro-spherical assembly, acquisition of the organized structure, and polarized signals of phospho-MLC2 in cortical organoids from the patient’s iPSCs. We confirmed that the Rho kinase inhibitor Y27632 restored these morphological phenotypes. Thus, Gαo determines the self-organizing process of the developing brain by regulating the Rho-associated pathway. These data suggest that Rho GTPase pathway might be an alternative target of therapy for patients with GNAO1-associated encephalopathy.
Similar content being viewed by others
Introduction
G protein subunit alpha O1 (GNAO1) encodes the heterotrimer-forming protein Gαo1,2. A growing number of reports have indicated that GNAO1 is associated with developmental and epileptic encephalopathy (DEE), a devastating neurodevelopmental disorder in infancy and childhood3,4,5,6. Affected patients present with severe developmental delay, intractable seizures, and involuntary movements5,6,7. To date, more than 100 patients with DEE have been reported to carry “pathogenic” or “likely pathogenic” variants of GNAO1. Trio-based sequencing indicates that these variations occur de novo3.
GNAO1 and its mammalian homologs are highly expressed in the central nervous system, where they play essential roles in neural circuit formation8,9. In differentiating neurons, Gαo is enriched in expression at the tip of the growth cone, a dynamic structure that determines the extension or retraction of neurites to organize functional connectivity with adjacent neurons10. Thus, Gαo is considered to organize local molecular signaling in mature and immature neurons so that they correctly guide the growth cone to external cues11. Indeed, as a GTP-binding protein, Gαo activates the downstream molecule CDC42 in cooperation with its binding partner GRIN111. These data support the notion that Gαo regulates morphogenic signaling in the developing brain.
We recently reported that Gαo interacts with another DEE-associated protein, spectrin alpha, non-erythrocytic 1 (SPTAN1), in vitro and in vivo12. A morphological analysis further suggested that Gαo is involved in the molecular pathways associated with cytoskeletal remodeling and neuronal connectivity12. Thus, pathogenic variants in GNAO1 directly and indirectly affect a broad category of molecular pathways underlying the development of the neural network13. However, the mechanism by which Gαo regulates downstream molecular events crucial for neuronal differentiation and cytoskeletal remodeling remains largely unknown.
In this study, we report that siRNA-mediated depletion of Gnao1 (Gαo) in Neuro2a cells perturbs the expression of genes associated with the Rho kinase (ROCK) pathway14,15. This study also highlights the aberrant morphology of growth cones in monolayer neurons expressing the p.G203R variant. We further determine the dominant effects of this variant on the ventricle-like structure and phosphorylation pattern of myosin light chain 2 (MLC2) in cortical organoids. Our data provide clues to help understand how Gαo regulates morphogenic signaling in differentiating neurons and in the human brain.
Results
RNA sequencing for Gαo-depleted Neuro2a cells
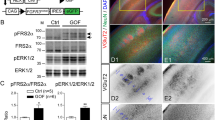
To identify the molecular pathways regulating Gnao1 (Gαo) in neuronal cells, we performed RNA sequencing of the mouse neuroblastoma cell line Neuro2a after treatment with control or Gnao1-specific siRNA (siGnao1). Quantitative polymerase chain reaction (qPCR) and Western blotting validated the successful (> 90%) knockdown of Gnao1 expression compared to that in control siRNA-treated Neuro2a cells (p = 0.100 [qPCR, n = 3] and p = 0.0286 [Western blotting, n = 4], Wilcoxon’s rank-sum test; Figs. 1a, S1a–c). In siGnao1-treated Neuro2a cells, 634 genes were upregulated (log2[fold change] > 1; p < 0.05 [− log10 > 1.301]), whereas 1257 genes were downregulated (log2[fold change] < − 1; p < 0.05) (Fig. 1b). Thus, transcriptomic analysis identified 1,891 genes that were aberrantly expressed in Neuro2a cells after treatment with siGnao1. To evaluate aberrantly expressed transcripts through alternative splicing, we applied the rMATS algorithm to the present dataset16. We found that a total of 2,948 transcripts (1,115 upregulated and 1833 downregulated) were differentially expressed in siGnao1-treated Neuro2a cells compared with control Neuro2a cells (Fig. 1c).
RNA sequencing for Gαo-depleted Neuro2a cells. (a) Box-dot plot shows the relative expression of Gnao1 mRNA to actin-β (Actb) in Neuro 2a cells after treatment with siRNA against Gnao1 (siGnao1) or control siRNA. P = 0.100 (n = 3 each group; Wilcoxon’s rank-sum test). (b) Volcano plots for genes detected by RNA sequencing. Green lines indicate the cut-off levels: log2 (Fold Change) > 1 or < − 1; p < 0.05 (− log10 [p-value] > 1.301). Among the 1891 genes with a significant increase (red) or decrease (blue) in expression, the top 10 genes with high—log10 (p-values) were annotated. (c) Heat map clustering of rMATS data for the 2948 transcripts differentially expressed in siGnao1 and control siRNA-treated Neuro2a cells. The color gradient indicates a Z score > 0 (red: high) and < 0 (blue: low) for the expression of each transcript. (d) A Reactome pathway analysis on rMATS data. Bar plots represent p-values for over-representation (blue), the number (white) and percentage (%, orange) of genes annotated in each category. Red circles indicate the categories associated with Rho GTPase signaling. (e) GSEA for RhoA and RhoB GTPase cycle pathways in the Reactome. The expression of genes in these categories did not deviate from either the upregulated or downregulated side.
A Gene Ontology (GO) analysis of rMATS data identified enrichment in “actin cytoskeleton organization” (GO: 0,030,036; p = 1.29 × 10–26, n = 42) in Biological Process (BP, Fig. S2a) and “cytoskeletal protein binding” (GO: 0,008,092; p = 3.08 × 10–8, n = 24) in Molecular Function (MF, Fig. S2b). Consistently, the Kyoto Encyclopedia of Genes and Genomes (KEGG, https://www.genome.jp/kegg/) revealed that 35 genes were overrepresented in the category of “axon guidance” (mmu04360; p = 2.68 × 10–4), 23 genes in “neurotrophin signaling pathway” (mmu04722; p = 4.64 × 10–3), and 16 genes in “ErbB signaling” (mmu04012, p = 0.0205) (Fig. S2c). These data suggest that Gnao1 is involved in cytoskeletal remodeling and the molecular pathways associated with neuronal differentiation12. We further referenced the 2948 transcripts against the Reactome database (https://reactome.org/), in which “Rho GTPase cycle” (R-MMU-9012999, p = 7.14 × 10–4, n = 74) and Rho-related pathways were recurrently annotated among the top 15 categories of enrichment (7/15, 47%; Fig. 1d, Table S2). We ensured that the 74 genes (transcripts) in “Rho GTPase cycle” category of Reactome were classified into “GTPase regulator activity” and related categories in the GO database (Fig. S3). Thus, we focused on the functional role of Gαo in the regulation of Rho-associated molecular pathways. Consistent with this finding, “chromatin organization” (GO: 0,006,325), “DNA repair” (GO: 0,006,281) and “MAPK signaling pathway” (KEGG: mmu04010) were ranked at the top of each category. These data suggest that the depletion of Gnao1 broadly affected broad molecular pathways downstream to Rho GTPase signaling in nuclear and cytoplasmic compartments of Neuro2a cells17,18,19.
In contrast, according to a Gene Set Enrichment Analysis (GSEA), genes involved in the Rho GTPase pathway did not show a deviated expression, either to the upregulated or downregulated side (Fig. 1e). Thus, enrichment of Rho GTPase-associated pathways reflected uniquely expressed splicing isoforms but not the summed expression of transcripts in siGnao1-treated Neuro2a cells. Consistent with this notion, the GSEA showed that genes associated with “RNA splicing” and “snRNP assembly” pathways were significantly downregulated in siGnao1-treated Neuro2a cells (Fig. S4).
Impaired neurite outgrowth of Gnao1-depleted Neuro2a cells
Based on the assumption that Rho-associated molecular signaling is hyper- or hypoactive in Gαo-depleted cells, we examined whether their proliferation rates differed from that of control Neuro2a cells. However, siGnao1-treated Neuro2a cells showed a growth rate similar to that of control cells (p > 0.05, Steel–Dwass’ multiple comparison; Fig. S5a). Consistently, a cell cycle analysis did not show significant differences in the proportions of G0/G1, S, G2/M, and subG1 phases between siGnao1-treated and control Neuro2a cells (p > 0.05, Steel–Dwass’ multiple comparison; Fig. S5b).
Given the versatile functions of Rho GTPases in cell cycle, epigenetic regulation, and cytoskeletal remodeling, we first tested whether the siGnao1 treated cells show disproportionate % of cells in S-phase. Actively growing cells in S-phase are also known to increase the nuclear size and the N/C ratio20,21. Thus, we determined whether siGnao1-treated cells are disproportionately classified into S-phase in comparison with that in Neuro2a cells. Morphologically, siGnao1-treated cells showed a higher nuclear-cytoplasmic (NC) ratio than those treated with control siRNA (median: 0.486 [siGnao1] vs. 0.416 [control], p = 3.23 × 10–15, Wilcoxon’s rank-sum test; Fig. S5c, d). Despite that siRNA-mediated depletion of Gnao1 did not affect either the proliferation or cell-cycle progression, siGnao1-treated Neuro2a cells showed the higher NC ratio than controls. These data suggested that Gnao1 plays more important roles in gene expression and morphological regulation than in cell-cycle progression of Neuro2a cells.
Because Rho GTPases and related pathways regulate axonal regeneration in vitro and in vivo22, we tested whether siRNA-mediated depletion of Gnao1 affects neurite outgrowth in Neuro2a cells. As previously reported12, the siGnao1-treated Neuro2a cells showed shorter and fewer neurites than those treated with control siRNA (p = 1.76 × 10–7 [length] and 3.32 × 10–5 [number], respectively, Wilcoxon’s rank-sum test; Figs. 2a, b and S6). To investigate the organization of cytoskeletal molecules in siGnao1-treated Neuro2a cells, we quantitatively analyzed the immunofluorescence signals of F-actin (phalloidin) and tubulin β3 (Tuj1) by dissecting the structure of neurites into stem and tip regions (Fig. 2c). In both regions, siGnao1-treated Neuro2a cells showed lower Gαo and TUJ1 signals than those treated with control siRNA (p < 2.2 × 10–16, Kruskal–Wallis test, n = 5 [ROIs] for each group; Fig. 2d, e). Notably, the F-actin signal was highly enriched at the tip (growth cone) of neurites in the control siRNA-treated cells. In contrast, such a gradient pattern of F-actin signals was absent in siGnao1-treated Neuro2a cells, and their neurites showed numerous flossy protrusions expressing F-actin (p < 2.2 × 10–16, Kruskal–Wallis test, n = 5; Fig. 2d, e).
Morphological analyses of Gαo-depleted Neuro2a cells. (a) An overview of control (left) and Gαo-depleted (right: siGnao1) Neuro2a cells. Merged images of F-actin (phalloidin, green), Gαo (red), Tuj1 (magenta), and DAPI (blue) are shown. The boxes indicate the regions of interest provided in Panel d. (b) Quantitative data on the length (nm) of neurites and number (counts per cell). Median values are shown as horizontal lines (light blue) on the violin plots. ***p < 0.001. (c) Schematic representation of the stem and tip portions of one neurite. The distance represents the length from the soma-neurite junction to the point of fluorescence measurement. (d) Three-channel images of the stem (left) and tip (right) regions of the neurites. (e) Quantitative measurements of F-actin, Gαo, and TUJ1 signals in panel d. Data from control (black) and siGnao1-treated Neuro2a cells (brown) are shown in box-dot plots. All p < 0.001 (siGnao1 vs. control; Kruskal–Wallis test, n = 5 [ROIs] for each group). The distance and fluorescence intensity are labelled on the X and Y axes, respectively. AU, arbitrary unit.
A dominant effect of the Gαo variant p.G203R on neuronal differentiation
To clarify the functional role of Gαo in an experimental model of the human brain, we recently established iPSCs from a healthy volunteer12. We inactivated the endogenous GNAO1 gene using the CRISPR-Cas9 system. To further investigate the pathogenic effects of a known variant of Gαo, we used iPSCs from a patient carrying a de novo heterozygous variant of p.G203R5,12. When we analyzed the growth of neurospheres during the first 15 days of differentiation, p.G203R-expressing iPSCs, but not GNAO1-knockout (KO) iPSCs, showed a significant delay in the growth of neuro-spheres (p = 8.88 × 10–14, Steel–Dwass’ multiple comparison test [factors: day and WT vs. PT; Fig. 3a, b).
Morphology of neuro-spheroids and neuronal growth cones. (a) Phase-contrast images of neuro-spheroids (arrows) from days 1 to 15. HA, iPSCs from a healthy adult; KO, Gαo-knockout iPSCs from the same individual as HA; PT, iPSCs from a patient with p.G203R variant. (b) Quantitative results of HA, KO, and PT-derived neuro-spheroids. Box-dot plots are line-connected with the mean values of spheroid size at indicated days of differentiation. ***p < 0.001 (HA vs. PT) and p = 0.484 (HA vs. KO), Steel–Dwass’ multiple comparison test (n = 6). Six independent spheroids were used for this analysis. (c) Confocal images of growth cones in monolayer neurons differentiated from HA, KO, and PT-derived iPSCs. (d) A schematic diagram of the Sholl analysis. Segmented filopodia are depicted as red dots on green protrusions, extending from a growth cone (lamellipodia). The red dots are counted as the number of filopodia at a defined radius (μm). (e) A Sholl analysis of growth cones in the HA, KO, and PT- derived neurons. Box-dot plots are line-connected with mean values of the number of filopodia at the indicated radius. P > 0.05 (HA vs. KO) and **p < 0.01 (HA vs. PT); Steel–Dwass’ test (n = 3). Three independent organoids were analyzed.
To test whether the morphological findings of Neuro2a cells are reproducible in human iPSC-derived neurons, we observed neurite outgrowth in monolayer neurons differentiated from organoids on day 15 of differentiation. We observed neurite outgrowth in monolayer neurons on day 15 of differentiation. Monolayer neurons from a healthy adult’s iPSCs (HA) clearly formed lamellipodia and filopodia structures of growth cones at the end of the neurites (Fig. 3c, d). The number of filopodia per growth cone did not differ between HA and KO neurons (p = 0.085 [HA vs. KO], n = 3 at each point, Steel–Dwass’ multiple comparison test; Fig. 3e). However, the patient-derived neurons showed even more dysmorphic lamellipodia with fewer filopodia per growth cone than those in HA neurons (p = 0.00870 [HA vs. PT], Steel–Dwass’ test; Fig. 3e). These data support the notion that the p.G203R variant of Gαo has a gain-of-function and/or dominant-negative effect on neuronal differentiation from iPSCs.
Dysmorphic appearance of cortical organoids expressing the p.G203R variant of Gαo
To further characterize the structure of developing neurons, we continued the differentiation of cortical organoids until they formed the laminar structure of the ventricular zone (VZ) and cortical plate (CP) by day 60–65 of differentiation in vitro. At this stage, the HA organoids developed a VZ, which showed a ventricle-like appearance (Fig. 4a, b). In KO organoids, a more round- and ovoid-shaped VZ was more predominant in appearance than in HA organoids. The structures in PT organoids were too dysmorphic to evaluate the laminar structures of the VZ and CP. When we closely examined the VZ and CP regions, Gαo-KO organoids showed a comparable thickness of VZ (median 82.4 µm [HA] vs. 69.8 µm [KO], p = 0.063, Wilcoxon’s rank-sum test; Fig. 4c). However, KO organoids showed a thinner CP than HA organoids (median 134.6 µm [HA] vs. 68.9 µm [KO], p = 0.014, Wilcoxon’s rank-sum test; Fig. 4c). The thickness of either the VZ or CP was immeasurable in PT organoids. Thus, we evaluated the circularity of the VZ in HA, KO, and PT organoids. We confirmed that PT organoids formed a VZ with lower circularity than KO and PT organoids (p = 0.009 [HA vs. PT] and p = 2.1 × 10–5 [KO vs. PT], Kruskal–Wallis test; Fig. 4d, S7).
Morphological analyses of cortical organoids. (a) Immunofluorescence images of organoids from a healthy adult (HA), GNAO1-knockout (KO), and patient (PT, p.G203R)-derived iPSCs. Organoids on day 60 of differentiation were used in this study. HA and KO organoids clearly developed VZ structures (arrows). PT-derived organoids showed a disordered VZ structure. (b) High- magnification images of the ventricular zone (VZ) and cortical plate (CP) in HA, KO, and PT-derived organoids. The dashed lines indicate the boundaries between the VZ and CP layers. Values indicate the circularity index of each VZ. (c) Quantitative results of VZ and CP thickness. *p < 0.05 (Wilcoxon’s rank-sum test). The thicknesses of the VZ and CP were unmeasurable in the PT organoids. For C-E, three independent organoids per group were subjected to analysis. (d) A circularity analysis of the VZ in HA, KO, and PT-derived organoids. *p < 0.05 (Kruskal–Wallis test). (e) Box-dot plots for the NeuN/FoxG1 signal ratio in the VZ. Small dots represent the value in each ROI (n = 621 [HA], 406 [KO] and 628 [PT]). *p < 0.05 and ***p < 0.001 (Kruskal–Wallis test).
Expression of neuronal lineage markers in organoids
Because we did not perform the microscopic analysis at a single-cell resolution, we were unable to count the number of progenitors and terminally differentiated neurons in the VZ and CP regions. To compensate this information, we quantitatively measured the expression level of molecular markers in the respective regions. We found a contrasting expression of progenitor (FOXG1: a maker for dorsal telencephalon23,24,25,26,27,28,29,30) and mature (NeuN) neuronal markers in the VZ of HA organoids, where FOXG1 was high and NeuN was low (Figs. 4a, b and S8). Consistently, the PAX6 signal (a dorsal forebrain progenitors23,28,29,31) was higher in VZ than in CP of HA organoid. Thus, the NeuN/FOXG1 ratio was as low as 0.515 (range: 0.237–2.76) in the VZ of HA organoids. KO organoids also showed a lower NeuN/FOXG1 ratio than VZ in HA organoids (KO: median 0.503 [range: 0.233–1.28], p = 0.045 [HA vs. KO]). In contrast, PT organoids showed a higher NeuN/FoxG1 ratio (median 0.905 [range: 0.312–3.66]) than HA organoids (p < 2 × 10–16 [HA vs. PT], Kruskal–Wallis test; Fig. 4e).
Consistently, HA and KO organoids expressed another progenitor marker PAX6 at the higher level in VZ than in the CP region (Fig. S9a,b). In contrast, they expressed mature neuronal markers, Reelin and TUJ1 (tubulin-β3) in VZ at lower levels than in CP. Reelin showed the strongest signals at the outmost layer of CP (Fig. S9a), supporting the concept that the population of Reelin-expressing (Cajal-Retzius) cells are distinct from those of PAX6-expressing dorsal progenitors23,26,28,29,31. As observed in the FOXG1-NeuN immunofluorescence data, the PT organoids showed a highly disorganized pattern of VZ-CP regionalization. It was therefore suggested that p.G203R-mutant Gαο disturbed the process of neurogenesis or neuronal differentiation. These data suggest that the p.G203R variant affects the physiologically regulated process of neuronal differentiation of human iPSCs.
Aberrant phosphorylation of myosin light chain in PT organoids
To determine the relationship between Rho GTPase signaling and the dysmorphic phenotype of KO and PT organoids, we evaluated the immunofluorescence signals of phospho-myosin light chain (pMLC2), an activation marker of the Rho kinase pathway32. In HA organoids, pMLC2 signals were highest in the outer layers of the CP, where the dendrite marker MAP2 was co-expressed (HA, Fig. 5a). Similarly, higher pMLC2 and MAP2 signals were observed in the CP layer than in the VZ layer of the KO organoids (KO, Fig. 5a). However, MAP2-positive neurons were localized in the outermost layer of the CP in KO organoids. In contrast to these findings in HA and KO organoids, PT organoids showed significantly lower signals of pMLC2 in the outer layer of the CP, resulting in the loss of the “CP-high and VZ-low” gradient in pMLC signals (p = 1.47 × 10–13 [HA vs. KO], 3.34 × 10–14 [HA vs. PT], and < 2.2 × 10–16 [KO vs. PT]; n = 6 each; Steel–Dwass’ multiple comparison test; Fig. 5a,b). PT organoids also expressed lower levels of MAP2 than in KO organoids (p = 3.33 × 10–14 [HA vs. KO], 0.196 [HA vs. PT], and 2.19 × 10–12 [KO vs. PT], n = 6 each; Fig. 5a, b).
Aberrant phosphorylation of MLC2 in patient-derived organoids. (a) Immunofluorescence signals of pMLC2 (red) and MAP2 (magenta) in healthy adult (HA), GNAO1-knockout (KO), and patient-derived (PT) organoids after 60 days of differentiation. Nuclei were counterstained with DAPI (blue). The rectangles in the left panels indicate the regions selected for higher magnification (right panels). Solid lines indicate the positions of the optical sections for the quantitative measurement of pMLC2 and MAP2 signals across the CP and VZ layers. Dashed lines denote the boundaries of the CP and VZ layers. (b) Box-dot plots for pMLC (upper) and MAP2 (lower) signals in HA, KO, and PT organoids. ***p < 0.001 (Steel–Dwass’ multiple comparison, n = 6 for each group). Six optical sections are shown with yellow lines (DAPI, panel a), from which 360–390 ROIs (single pixel) were randomly selected. AU, arbitrary unit. Three independent organoids per group were subjected to this analysis.
These results suggested that the gradient pattern of neuronal differentiation (MAP2 and NeuN signals) might reflect the regional difference in the activity of Rho GTPase signaling in HA organoids. We also wondered whether the correlation might be disturbed in KO and PT organoids. To test this possibility, we statistically analyzed the correlation between MAP2 and phosphorylated MLC2 (pMLC2). When we set 140 regions of interest (ROIs) in CP, the expression of MAP2 was correlated with pMLC2 signals in HA (p < 2.2 × 10–16, Spearman’s rank correlation, KO (p < 2.2 × 10–16), and PT organoids (p < 2.2 × 10–16; Fig. S10a, b). Notably, the correlation of pMLC2 and MAP2 signals in HA organoids was distributed over a wider range than that in the KO and PT organoids (Fig. S10b, lower). Thus, Rho kinase-associated pathways were profoundly deregulated in PT organoids and to a lesser extent in KO organoids than in PT organoids.
Causal effect of Rho signaling pathways on the dysmorphic structure of organoids
To validate the causal effect of Rho kinase activity on the anomalous development of organoids, we further investigated whether the Rho kinase inhibitor Y27632 restored the dysmorphic phenotype of PT organoids. During the first 15 days of differentiation, an increasing Y27632 concentration (50–250 µM) assisted the assembly and growth of PT neuro-spheroids (p = 5.18 × 10–9 [100 µM vs. 50 µM], 1.61 × 10–3 [250 µM vs. 50 µM], n = 6, Steel–Dwass’ multiple comparison; Fig. 6a, b). More specifically, PT spheroids treated with 100 µM Y27632 (light blue) gained significantly larger size after day 5 of differentiation compared to control (50 µM: gray) (Fig. 6a, b). When treated with a higher concentration of Y27632 (250 µM: blue), PT spheroids showed delay in aggregate formation during the first week, but they caught-up in growth by day 15 of differentiation.
Restoring the growth and differentiation of patient-derived organoids by Rho kinase inhibitor. (a) Phase-contrast images of patient (PT)-derived neuro-spheroids (arrows) after treatment with 50, 100 and 250 μM Y27632. Microscopic images were captured at the indicated time points (days 1–15). (b) Aria size of spheroids (μm2) on the indicated days of treatment. Box-dot plots are line-connected with the mean values of spheroid size at indicated days of differentiation. **p < 0.01, ***p < 0.001 (n = 6, Steel–Dwass’ multiple comparison [50 μM vs. 100 μM] and [50 μM vs. 250 μM]). Six independent spheroids were used for this analysis. (c) Immunofluorescence images of PT organoids treated with 50, 100, and 250 μM Y27632. Organoids were cryo-sectioned 15 days after treatment with Y27632. The dashed lines indicate the VZ region. Arrowheads indicate that round-shaped VZ structures were recovered after treatment with 100 μM Y27632. (d) NeuN/FoxG1 ratio in the VZ of PT organoids treated with 50, 100, and 250 μM Y27632. Small dots represent the value in each ROI (n = 1,106 [50 μM], 1,357 [100 μM] and 1438 [250 μM]) ***p < 0.001 (Kruskal–Wallis test). Three independent organoids were analyzed.
Immunofluorescence data showed that the round-shaped structure of the VZ inside the CP layer of PT organoids began to recover by day 15 of treatment with Y27632 (100 µM, Fig. 6c). Of note, treatment of PT organoids with a higher concentration (250 µM) of Y27632 failed to restore the dysmorphic structure of the VZ (Fig. 6c). However, treatment with 100 and 250 µM Y27632 redeemed the “FoxG1-high and NeuN-low” gradient pattern to VZ in PT organoids. Quantitative measurement of immunofluorescence signals revealed that the NeuN/FoxG1 ratio was lower in PT organoids treated with 100 and 250 µM Y27632 than that in organoids treated with 50 µM Y27632 (p < 2 × 10–16 [100 µM vs. 50 µM] and p < 2 × 10–16 [250 µM vs. 50 µM], p = 5.51 × 10–150, Kruskal–Wallis test; Fig. 6d).
Discussion
This study provided experimental data to rationalize the regulatory role of Gαo in Rho GTPase-associated signaling using the mouse neuroblastoma cell line Neuro2a and human brain organoids. The results of unsupervised transcriptomic analyses led us to link the distinct phenotypes of Gαo-depleted Neuro2a cells to the deregulation of Rho GTPase signaling. Morphologically, Neuro2a cells treated with siRNA against Gnao1 showed shorter and fewer neurites, which were also characterized by the loss of polarized F-actin assembly at their far-end and the presence of fluffy protrusions in the stem portion, than control Neuro2a cells. In agreement with such findings in Neuro2a, Gαo-KO cortical organoids presented an altered morphology of neuronally differentiating cells. Furthermore, the Gαo-mutant (p.G203R) organoids showed hardly any identifiable VZ and CP layers in their internal structures. These dysmorphic features were associated with aberrant patterns of pMLC2 signals in the Gαo-KO and mutant (p.G203R) organoids. The ROCK inhibitor Y27632 partly rescued the dysmorphic phenotypes of the Gαo-mutant organoids. Thus, the pathogenic variant (p.G203R) of Gαo was considered to induce hyperactivation of the Rho kinase pathway, which deregulates the proliferation and differentiation of neuronally committed progenitors. Specifically, p.G203R-expressing iPSCs might have lost the appropriate timing of differentiation from GFAP-expressing neural stem cells to FOXG1 and/or PAX6-expressing progenitors. In this regard, aberrant Rho GTPase signaling in patient (p.G203R) organoids might have driven the GFAP-expressing stem cells to exit prematurely to neuronal progenitors. Thus, the population of FOXG1-expressing progenitors might be increased in the patient organoids. Consequently, neuronal progenitors in patient (p.G203R) organoids might have missed the physiological condition of gene expression to differentiate themselves into NeuN and Reelin-expressing mature neurons.
Recent studies have shown that the p.G203R variant causes a neomorphic effect on GTPase activities of Gαo12. In other words, pathogenic variants in GNAO1, including p.G203R, exhibit opposing features of loss-of-function, gain-of-function, and dominant-negative profiles in their GTPase functions. In agreement with this concept, we found that the growth cone formation and Rho GTPase-related pathway were more profoundly affected in p.G203R-expressing neurons than in GNAO1-knockout neurons. Collectively, these data suggested that the p.G203R variant might render Gαo to exert the neomorphic functions in Rho GTPase-related molecular pathways. According to the Bgee database (https://www.bgee.org/gene/ENSG00000087258), a bulk-RNA sequencing supported evidence that GNAO1 is expressed in the fetal cortical plate at 14 weeks of post-fertilization. Thus, as recently reported33, GNAO1 is likely to regulate the process of differentiation and maturation of neuronal progenitors.
Given the higher NeuN/FOXG1 ratio in the patient’s organoids than control and KO organoids, one could hypothesize that the patient’s organoids gained the phenotype of accelerated neurogenesis, but not impairment in the process of neuronal differentiation. We previously reported that the patient’s organoid showed higher signals of GFAP-expressing neurons on the surface layer than KO and control organoids12. From these data, however, we cannot still conclude that the p.G203R variant Gαο promotes neurogenesis or neuronal differentiation. To address this issue, we need to clarify the amount of proliferating GFAP-expressing stem cells with immunolabelling the BrdU incorporation. It will be also informative to characterize when GFAP-positive neurons start expressing the differentiation markers (FOXG1, PAX6, Reelin, and NeuN) during 60 days of organoid development.
The Rho family of GTPases consists of 20 subclasses in mammalian species, all of which belong to the Ras superfamily34. Among them, the three most common Rho GTPases (Cdc42, Rac1, and RhoA) have been extensively studied35. As small GTP-binding proteins (21 kDa), Rho GTPases play versatile roles in cell mobility, the cell cycle, adhesion, neurite outgrowth, and synaptic plasticity14,15,36. Specifically, local activation of Rho GTPases stimulates regional actin polymerization and cytoskeletal remodeling by stimulating numerous downstream effectors37. In excitatory synapses, RhoA and Cdc42 relay the transient CaMKII activation to synapse-specific, long-term signaling required for spinal structural plasticity14,15. Thus, the phosphorylation status of potential targets of Rho GTPase must be further investigated. For the same reason, it is worth studying the gene expression profiles of p.G203R-expressing neurons before and after treatment with Y27632 and other ROCK inhibitors.
Recent studies have focused on Rho GTPases as potential biomarkers and therapeutic targets for schizophrenia38,39,40 and epilepsy41. For example, rare copy number variations (exonic deletions and duplications) and a single nucleotide variant (p.S490P) have been recurrently identified in ARHGAP10 among Japanese patients with schizophrenia38. Animal studies also indicated that ROCK is a promising target of treatment for emotional and cognitive dysfunctions of Arhgap10-mutant mice39,40. Furthermore, pathogenic variants in Rho-related BTB domain-containing protein 2 (RHOBTB2) have been identified in patients with DEE, the most severe form of epilepsy syndrome in childhood42,43. Intriguingly, RHOBTB2 is a substrate of the ubiquitin ligase, Cullin-3 (CUL3), another DEE-associated protein44. In this study, we found that HA organoids developed MAP2-expressing neurons in association with the activity of ROCK (pMLC2 signal), one of the molecular markers for the activity of Rho GTPase signaling. In contrast, KO and PT organoids appeared to have impairments in efficiently mediating the Rho GTPase signals to the downstream pathways and conversion of ROCK-dependent signaling to neurogenesis or neuronal differentiation. Given the regulatory role of Gαo in the Rho-kinase pathway, a certain group of DEE-associated proteins may share a convergent molecular function, enabling them to cooperatively organize neural circuits in the developing brain.
Clinically, GNAO1-associated DEE is characterized by severe developmental delays, uncontrollable seizures, and various types of involuntary movements. Most patients show only minor features on brain MRI; however, a thin corpus callosum with dilated ventricles is commonly observed1,45. Hypoplastic caudate nuclei have also been identified in multiple patients1,45,46. Thus, both regional and interspherical neural connectivity are likely to be affected in patients with GNAO1-associated DEE. Our previous data also suggested that the expression of the pathogenic variant of Gαo in differentiating neurons disturbs the process of neurite extension required for neural network formation12. Consistent findings were also shown with human iPSC-derived neurons33. Progressive choreoathetosis and dystonia have been reported in patients carrying GNAO1 variants47,48,49. These findings suggest a pathogenic link between Gαo and Rho-kinase in degenerative changes in dopaminergic neurons50,51.
An MRI study identified a right frontal T2-hyperintense lesion, which was pathologically diagnosed as diffuse astrocytoma (WHO grade II), in 1 patient at 16 years old45. It might be reasonable to hypothesize that hypomorphic variants of GNAO1 cause aberrant differentiation of neurons or tumorigenic conditions as a consequence of hyperactive Rho GTPase signaling. Although no other patients have been reported to develop brain tumors, the pathogenic variant p.R209C in GNAO1 was identified as a driver of infantile-onset acute lymphoblastic leukemia52. Thus, the number of patients with low-grade astrocytoma or glioma may be further determined through long-term observation of children and adults with GNAO1-associated DEE.
In our bioinformatics analysis, Rho families and related molecular pathways were recurrently annotated in Reactome but not in the KEGG database. These dissociated results stemmed mainly from the difference in the number of “hit” genes that were successfully retrieved in these databases. Of the 2948 differentially expressed transcripts, 1280 (43.4%) genes were listed in the Reactome, whereas only 845 (28.7%) were identified in KEGG. Thus, our experimental data were evaluated in more detail by submitting the dataset to Reactome rather than to KEGG. In addition, we used the rMATS algorithm to attribute the read depth of the transcripts to their expression16. This approach detected splicing isoforms that were differentially expressed among samples at a higher resolution than that of the conventional method for measuring the expression of transcripts at the gene level. Because ROCK is known to regulate alternative splicing in the brain53, our data suggest that the loss of Gαo expression induces an unbalanced expression of splicing isoforms.
Several limitations remain to be addressed before this conclusion can be confirmed. First, we did not perform a single-cell transcriptome analysis using GNAO1-KO or patient-derived organoids expressing the p.G203R mutant of Gαo. A comparative analysis of their RNA expression with that of HA organoids will clarify the neuronal populations and the timing at which they start showing hyperactive Rho-associated kinase signaling. Thus, it also remains to be elusive whether ROCK inhibitor (Y27632) shows therapeutic effects on morphological phenotype of patient’s organoids only at the early stage (within the first week) of differentiation. For this purpose, we must test whether Y27632 might be also effective when administered at 2 weeks of differentiation or later. Second, we established only classical (cortical) organoids using a conventional method54. Directing the patient-derived iPSCs to differentiate into striatal and inhibitory neurons will further clarify the distinctive roles of Gαo in different subtypes of brain organoids and in “assembloids”55. Third, we cannot exactly characterize the molecular features of the patient-derived organoids until we establish those expressing the wild-type Gαo on the isogenic backgrounds. For this purpose, it will be worth investigating whether the neuronal phenotypes of the patient’s cortical organoids might be mitigated by the CRISPR/Cas9-based gene editing system or the knockdown of p.G203R-mutant Gαο expression. The ectopic expression of the mutant Gαο in the control iPSCs may further identify molecular pathways that might be specifically altered in neurons expressing p.G203R-mutant Gαο, but not in those expressing other mutants. Thus, the combined analysis of single-cell RNA sequencing for patient-derived organoids with the current dataset will provide further insight into the unique role of Gαo in Rho GTPase signaling.
Conclusions
We first demonstrated that GNAO1 (Gαo) regulated gene expression associated with Rho GTPase signaling in Neuro2a cells. In combination with the findings in human iPSC-derived organoids, our data suggest that Gαo acts as a molecular switch for Rho-associated kinase, which plays versatile roles in the developing brain. Further analyses of how Gαo interacts with Rho GTPases and their effector molecules will facilitate the identification of molecular targets of therapy for patients with GNAO1-associated DEE.
Methods
Reagents
Key reagents including antibodies and oligonucleotide primers are summarized in Table S1. Details in experimental proce‹dures are described in Supplementary Materials.
Real-time quantitative PCR
Total RNA was extracted using an RNeasy Mini Kit (#74,104; Qiagen, Germantown, MD, USA). Complementary DNA was synthesized using a High-Capacity RNA to cDNA Kit (#4,387,406; Thermo Fisher Scientific) according to the manufacturer's protocol. Assays were performed using the SYBR Green system (#438,561; Thermo Fisher Scientific)12. The delta-delta cycle threshold algorithm was applied for the quantitative measurement of mRNA expression. Human or murine homolog of actin beta (Actb) was used as an internal control (Table S1).
RNA sequencing
The Neuro2a control group (n = 3) was compared with the siGnao1-treated group (n = 3). Sequencing was performed on an Illumina platform (Illumina Inc., San Diego, CA, USA) with a read length of 150 bp pair-ends and 50–60 million reads per sample. The resulting FASTQ files were quality-checked using FastQC version 0.11.9 and trimmed with trimmomatic version 0.38. The quality-checked data were then mapped to the mouse reference genome (GRCm39) using the HISAT2-2.1.0 software program. Reads were counted using Bowtie2 and RSEM 1.3.0. Normalized counts per million (CPM) were then obtained using the EdgeR program56. Transcripts containing at least one sample with CPM < 0.1 in each group were filtered. The rMATS algorithm16 was further applied for an in-depth analysis of splicing isoforms that were differentially expressed in Neuro2a cells with or without Gαo deletion. Differentially expressed genes (DEGs) and transcripts were defined as those with a p-value < 0.05, false discovery rate (FDR) < 0.05, and fold change (FC) > 2 in the two-group comparison. Transcriptomic data are available at the Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/) under accession number GSE241258.
Cortical organoids and monolayer neurons
Cortical neurons were differentiated from iPSCs using the serum-free floating culture of embryoid body-like aggregates with the quick reaggregation (SFEBq) method12,31,54. On day 18, cell aggregates were transferred to a non-adhesive dish (EZSPHERE, #4020–900; IWAKI, Baibara-gun, Shizuoka, Japan) and cultured in the following organoid medium: DMEM/F12-GlutaMAX (#10,565–018; Thermo Fisher Scientific) with 1% Chemically Defined Lipid Concentrate, 1% N-2 supplement, and 1% antibiotic–antimycotic (#11,905–031, #17,502,048, and #15,240,062, respectively; Thermo Fisher Scientific) at 37˚C under 40% O2 and 5% CO2. The medium was replenished every 3–4 days. On day 35, organoids were cultured in the following neuronal maturation medium: 10% FBS, 1% Matrigel Growth Factor Reduced (#356,230; Corning, Gkendale, AZ, USA), and 5 μg/mL heparin was added to the organoid medium. The medium was replaced every 3–4 days. For the morphological analysis of single neurons and growth cones, cerebral organoids were dissociated on day 15 using the enzyme solution of a Neuron Dissociation Solution (#291–78,001; Wako) and plated on poly-D-lysine, fibronectin and iMatrix-511 coated glass-bottom dish at a density of 75,000 cells/cm2 in the neural maturation medium. The medium was refreshed every 2–3 days.
Neuronal differentiation from iPSCs
Karyotyping and the quality check of iPSCs were completed as previously reported12. iPSCs were pretreated for 1 h with 50 μM Y27632 (#036–24,023; FUJIFILM, Wako, Japan), dissociated with TrypLE Express (#12,605–010; Thermo Fisher Scientific), and quickly reaggregated using low cell-adhesion 96-well culture plates with V-bottomed conical wells (#MS-9096VZ; Sumitomo Bakelite, Tokyo, Japan) in the following differentiation medium (10,000 cells/well, 100 μL): Glasgow-MEM (#G5154; Sigma-Aldrich) containing 20% Knockout Serum Replacement (#10,828–010; Thermo Fisher Scientific), 0.1 mM MEM Non-Essential Amino Acids Solution (#11,140,050; Thermo Fisher Scientific), 1 mM pyruvate (#S866; Sigma-Aldrich), 0.1 mM 2-mercaptoethanol (#21,417–52; Nacalai Tesque, Inc., Kyoto, Japan), 5 μM SB431542 hydrate (#S4317; Sigma-Aldrich), 3 μM IWR1e (#13,659; Cayman Chemical, Ann Arbor, MI, USA), and 1% P/S at 37 °C under a normoxic, 5% CO2 condition. The medium was replenished every 3–4 days. Y27632 (50 μM) was added to the differentiation medium from day 0 to 3 unless otherwise stated.
Morphological analyses
The ImageJ software program, version 2.9.0 (https://imagej.nih.gov/ij/index.html) was used for the quantitative analysis of neurite outgrowth, morphology of growth cone, and fluorescence signals of immunolabelled proteins. Sholl analyses were performed to identify the number of intersections at radial intervals of 30 μm starting from the central point of the growth cone (lamellipodia) using the ImageJ plug-in, Sholl Analysis 4.1.1257. The circularity index was obtained with the conventional formula 4π [area size] / [perimeter]2,58.
Statistics
All statistical analyses were performed using R version 4.2 (https://R-project.org). The R package clusterProfiler version 4.6.259 and DAVID (https://david.ncifcrf.gov/summary.jsp) were used for enrichment analysis. The collected data are presented as the median (range: min–max) unless otherwise stated. P values < 0.05 (*) were considered significant (**p < 0.01; ***p < 0.001).
Ethical approval
All experimental procedures were performed in strict compliance with the institutional guidelines and protocols approved by the Institutional Review Board of Kyushu University for clinical studies and experiments with human samples (#28–88, 29–393 and 678–01). The establishment of iPSCs was based on the consent of a healthy donor and the patient’s parents. Informed consent was obtained from all subjects and/or their legal guardians of the patient.
Data availability
Clinical data are not publicly available due to privacy or ethical restrictions. RNA-seq data are available at Gene Expression Omibus (https://www.ncbi.nlm.nih.gov/geo) with accession number GSE241258.
References
Kim, S. Y. et al. Spectrum of movement disorders in GNAO1 encephalopathy: In-depth phenotyping and case-by-case analysis. Orphanet. J. Rare Dis. 15, 343. https://doi.org/10.1186/s13023-020-01594-3 (2020).
Larasati, Y. A. et al. Restoration of the GTPase activity and cellular interactions of Galpha(o) mutants by Zn(2+) in GNAO1 encephalopathy models. Sci. Adv. 8, eabn9350. https://doi.org/10.1126/sciadv.abn9350 (2022).
Nakamura, K. et al. De Novo mutations in GNAO1, encoding a Galphao subunit of heterotrimeric G proteins, cause epileptic encephalopathy. Am. J. Hum. Genet. 93, 496–505. https://doi.org/10.1016/j.ajhg.2013.07.014 (2013).
Perez-Duenas, B. et al. The genetic landscape of complex childhood-onset hyperkinetic movement disorders. Mov. Disord. 37, 2197–2209. https://doi.org/10.1002/mds.29182 (2022).
Saitsu, H. et al. Phenotypic spectrum of GNAO1 variants: Epileptic encephalopathy to involuntary movements with severe developmental delay. Eur. J. Hum. Genet. 24, 129–134. https://doi.org/10.1038/ejhg.2015.92 (2016).
Thiel, M. et al. Genotype-phenotype correlation and treatment effects in young patients with GNAO1-associated disorders. J. Neurol. Neurosurg. Psychiatry https://doi.org/10.1136/jnnp-2022-330261 (2023).
Schirinzi, T. et al. Phenomenology and clinical course of movement disorder in GNAO1 variants: Results from an analytical review. Parkinsonism Relat. Disord. 61, 19–25. https://doi.org/10.1016/j.parkreldis.2018.11.019 (2019).
Hwangpo, A. et al. G protein-regulated inducer of neurite outgrowth (GRIN) modulates sprouty protein repression of mitogen-activated protein kinase (MAPK) activation by growth factor stimulation. J. Biol. Chem. 287, 13674–13685. https://doi.org/10.1074/jbc.M111.320705 (2012).
Ghil, S. H., Kim, B. J., Lee, Y. D. & Suh-Kim, H. Neurite outgrowth-induced by cyclic AMP can be modulated by the α subunit of go. J. Neurochem. 74, 151–158. https://doi.org/10.1046/j.1471-4159.2000.0740151.x (2000).
Furutani, Y. & Yoshihara, Y. Proteomic analysis of dendritic filopodia-rich fraction isolated by telencephalin and vitronectin interaction. Front. Synaptic Neurosci. 10, 1–12. https://doi.org/10.3389/fnsyn.2018.00027 (2018).
Nakata, H. & Kozasa, T. Functional characterization of Galphao signaling through G protein-regulated inducer of neurite outgrowth 1. Mol. Pharmacol. 67, 695–702. https://doi.org/10.1124/mol.104.003913 (2005).
Akamine, S. et al. GNAO1 organizes the cytoskeletal remodeling and firing of developing neurons. FASEB J. 34, 16601–16621. https://doi.org/10.1096/fj.202001113R (2020).
Parenti, I., Rabaneda, L. G., Schoen, H. & Novarino, G. Neurodevelopmental disorders: From genetics to functional pathways. Trends Neurosci. 43, 608–621. https://doi.org/10.1016/j.tins.2020.05.004 (2020).
Fujita, Y. & Yamashita, T. Axon growth inhibition by RhoA/ROCK in the central nervous system. Front. Neurosci. 8, 338. https://doi.org/10.3389/fnins.2014.00338 (2014).
Murakoshi, H., Wang, H. & Yasuda, R. Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature 472, 100–104. https://doi.org/10.1038/nature09823 (2011).
Shen, S. et al. rMATS: robust and flexible detection of differential alternative splicing from replicate RNA-Seq data. Proc. Natl. Acad. Sci. U S A 111, E5593-5601. https://doi.org/10.1073/pnas.1419161111 (2014).
Arildsen, N. S. et al. Involvement of chromatin remodeling genes and the Rho GTPases RhoB and CDC42 in ovarian clear cell carcinoma. Front. Oncol. 7, 109. https://doi.org/10.3389/fonc.2017.00109 (2017).
Govek, E. E., Newey, S. E. & Van Aelst, L. The role of the Rho GTPases in neuronal development. Genes Dev. 19, 1–49. https://doi.org/10.1101/gad.1256405 (2005).
Magalhaes, Y. T., Farias, J. O., Silva, L. E. & Forti, F. L. 2021 GTPases, genome, actin: A hidden story in DNA damage response and repair mechanisms. DNA repair 100, 103070. https://doi.org/10.1016/j.dnarep.2021.103070 (2021).
Balachandra, S., Sarkar, S. & Amodeo, A. A. The nuclear-to-Cytoplasmic ratio: Coupling DNA content to cell size, cell cycle, and biosynthetic capacity. Ann. Rev. Genet. 56, 165–185. https://doi.org/10.1146/annurev-genet-080320-030537 (2022).
Cantwell, H. & Nurse, P. Unravelling nuclear size control. Curr. Genet. 65, 1281–1285. https://doi.org/10.1007/s00294-019-00999-3 (2019).
Hall, A. & Lalli, G. Rho and Ras GTPases in axon growth, guidance, and branching. Cold Spring Harb. Perspect Biol. 2, a001818. https://doi.org/10.1101/cshperspect.a001818 (2010).
Bagley, J. A., Reumann, D., Bian, S., Levi-Strauss, J. & Knoblich, J. A. Fused cerebral organoids model interactions between brain regions. Nat. Methods 14, 743–751. https://doi.org/10.1038/nmeth.4304 (2017).
Hanashima, C., Fernandes, M., Hebert, J. M. & Fishell, G. The role of and dorsal midline signaling in the generation of cajal-retzius subtypes. J. Neurosci. 27, 11103–11111. https://doi.org/10.1523/Jneurosci.1066-07.2007 (2007).
Hou, P. S., HAilín, D. O., Vogel, T. & Hanashima, C. Transcription and beyond: Delineating FOXG1 function in cortical development and disorders. Front. Cell. Neurosci. 14, 35. https://doi.org/10.3389/fncel.2020.00035 (2020).
Kumamoto, T. & Hanashima, C. Evolutionary conservation and conversion of Foxg1 function in brain development. Dev. Growth. Differ 59, 258–269. https://doi.org/10.1111/dgd.12367 (2017).
Kumamoto, T. et al. Foxg1 coordinates the switch from nonradially to radially migrating glutamatergic subtypes in the neocortex through spatiotemporal repression. Cell Rep. 3, 931–945. https://doi.org/10.1016/j.celrep.2013.02.023 (2013).
Li, C. et al. Single-cell brain organoid screening identifies developmental defects in autism. Nature 621, 373–380. https://doi.org/10.1038/s41586-023-06473-y (2023).
Mariani, J. et al. FOXG1-dependent dysregulation of GABA/Glutamate neuron differentiation in autism spectrum disorders. Cell 162, 375–390. https://doi.org/10.1016/j.cell.2015.06.034 (2015).
Toma, K., Kumamoto, T. & Hanashima, C. The timing of upper-layer neurogenesis is conferred by sequential derepression and negative feedback from deep-layer neurons. J. Neurosci. 34, 13259–13276. https://doi.org/10.1523/Jneurosci.2334-14.2014 (2014).
Sakaguchi, H. et al. Self-organized synchronous calcium transients in a cultured human neural network derived from cerebral organoids. Stem Cell Rep. 13, 458–473. https://doi.org/10.1016/j.stemcr.2019.05.029 (2019).
Azzarelli, R., Kerloch, T. & Pacary, E. Regulation of cerebral cortex development by Rho GTPases: Insights from in vivo studies. Front. Cell Neurosci. 8, 445. https://doi.org/10.3389/fncel.2014.00445 (2014).
Benedetti, M. C. et al. Cortical neurons obtained from patient-derived iPSCs with GNAO1 p.G203R variant show altered differentiation and functional properties. Heliyon 10, e26656. https://doi.org/10.1016/j.heliyon.2024.e26656 (2024).
Bar-Sagi, D. & Hall, A. Ras and Rho GTPases: A family reunion. Cell 103, 227–238. https://doi.org/10.1016/s0092-8674(00)00115-x (2000).
Stankiewicz, T. R. & Linseman, D. A. Rho family GTPases: Key players in neuronal development, neuronal survival, and neurodegeneration. Front. Cell. Neurosci. 8, 314. https://doi.org/10.3389/fncel.2014.00314 (2014).
Tolias, K. F., Duman, J. G. & Um, K. Control of synapse development and plasticity by Rho GTPase regulatory proteins. Prog. Neurobiol. 94, 133–148. https://doi.org/10.1016/j.pneurobio.2011.04.011 (2011).
Bishop, A. L. & Hall, A. Rho GTPases and their effector proteins. Biochem. J. 348(Pt 2), 241–255 (2000).
Sekiguchi, M. et al. ARHGAP10, which encodes Rho GTPase-activating protein 10, is a novel gene for schizophrenia risk. Transl. Psychiatry 10, 247. https://doi.org/10.1038/s41398-020-00917-z (2020).
Takase, S. et al. Antipsychotic-like effects of fasudil, a Rho-kinase inhibitor, in a pharmacologic animal model of schizophrenia. Eur J Pharmacol 931, 175207. https://doi.org/10.1016/j.ejphar.2022.175207 (2022).
Tanaka, R. et al. Inhibition of Rho-kinase ameliorates decreased spine density in the medial prefrontal cortex and methamphetamine-induced cognitive dysfunction in mice carrying schizophrenia-associated mutations of the Arhgap10 gene. Pharmacol. Res. 187, 106589. https://doi.org/10.1016/j.phrs.2022.106589 (2023).
Wang, Z., Ren, D. & Zheng, P. The role of Rho/ROCK in epileptic seizure-related neuronal damage. Metab. Brain Dis. 37, 881–887. https://doi.org/10.1007/s11011-022-00909-6 (2022).
Belal, H. et al. De novo variants in RHOBTB2, an atypical Rho GTPase gene, cause epileptic encephalopathy. Hum. Mutat. 39, 1070–1075. https://doi.org/10.1002/humu.23550 (2018).
Straub, J. et al. Missense variants in RHOBTB2 cause a developmental and epileptic encephalopathy in humans, and altered levels cause neurological defects in drosophila. Am. J. Hum. Genet. 102, 44–57. https://doi.org/10.1016/j.ajhg.2017.11.008 (2018).
Nakashima, M. et al. De novo variants in CUL3 are associated with global developmental delays with or without infantile spasms. J. Hum. Genet. 65, 727–734. https://doi.org/10.1038/s10038-020-0758-2 (2020).
Danti, F. R. et al. GNAO1 encephalopathy: Broadening the phenotype and evaluating treatment and outcome. Neurol. Genet. 3, e143. https://doi.org/10.1212/NXG.0000000000000143 (2017).
Kelly, M. et al. Spectrum of neurodevelopmental disease associated with the GNAO1 guanosine triphosphate-binding region. Epilepsia 60, 406–418. https://doi.org/10.1111/epi.14653 (2019).
Feng, H., Khalil, S., Neubig, R. R. & Sidiropoulos, C. A mechanistic review on GNAO1-associated movement disorder. Neurobiol. Dis. 116, 131–141. https://doi.org/10.1016/j.nbd.2018.05.005 (2018).
Feng, H. et al. Movement disorder in GNAO1 encephalopathy associated with gain-of-function mutations. Neurology 89, 762–770. https://doi.org/10.1212/WNL.0000000000004262 (2017).
Menke, L. A. et al. Recurrent GNAO1 mutations associated with developmental delay and a movement disorder. J. Child Neurol. 31, 1598–1601. https://doi.org/10.1177/0883073816666474 (2016).
Schirinzi, T. et al. Phenomenology and clinical course of movement disorder in GNAO1 variants: Results from an analytical review. Parkinsonism Relat. Disord. 61, 19–25. https://doi.org/10.1016/j.parkreldis.2018.11.019 (2019).
Vasconcellos, L. F., Soares, V. P. & de Ricchezza, L. L. Dystonic cerebral palsy phenotype due to GNAO1 variant responsive to levodopa. Tremor Other Hyperkinet. Mov. (N Y) https://doi.org/10.5334/tohm.746 (2023).
Song, L. et al. Identification of functional cooperative mutations of GNAO1 in human acute lymphoblastic leukemia. Blood 137, 1181–1191. https://doi.org/10.1182/blood.2020005622 (2021).
Rozic, G., Lupowitz, Z., Piontkewitz, Y. & Zisapel, N. Dynamic changes in neurexins’ alternative splicing: Role of Rho-associated protein kinases and relevance to memory formation. PLoS One 6, e18579. https://doi.org/10.1371/journal.pone.0018579 (2011).
Eiraku, M. et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3, 519–532. https://doi.org/10.1016/j.stem.2008.09.002 (2008).
Pasca, S. P. et al. A nomenclature consensus for nervous system organoids and assembloids. Nature 609, 907–910. https://doi.org/10.1038/s41586-022-05219-6 (2022).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140. https://doi.org/10.1093/bioinformatics/btp616 (2010).
Langhammer, C. G. et al. Automated Sholl analysis of digitized neuronal morphology at multiple scales: Whole cell Sholl analysis versus Sholl analysis of arbor subregions. Cytometry A 77, 1160–1168. https://doi.org/10.1002/cyto.a.20954 (2010).
Szabo, M. et al. The kynurenic acid analog SZR104 induces cytomorphological changes associated with the anti-inflammatory phenotype in cultured microglia. Sci Rep 13, 11328. https://doi.org/10.1038/s41598-023-38107-8 (2023).
Wu, T. et al. clusterProfiler: A universal enrichment tool for interpreting omics data. Innov. (Camb) 2, 100141. https://doi.org/10.1016/j.xinn.2021.100141 (2021).
Acknowledgements
We thank Professor Toshiro Hara (Former President, Fukuoka Children’s Hospital), Professor Shigenobu Kanba (Kyushu University), and Professor Kazuaki Nonaka (Kyushu University) for their essential support; Dr. Yuko Arioka (Nagoya University) for supervising the organoid sectioning; Dr. Tamami Tanaka and Ms. Ryoko Unose (Kyushu University) for their technical assistance; and Drs. Pin Fee Chong, Yuri Sonoda, Kenta Kajiwara and all of the lab members for their helpful discussions.
Funding
This study was supported by JSPS Kakenhi grant numbers JP22K15913 (Akamine), JP21K07865 (Okuzono), JP23K07334 (Sakai); AMED grant numbers JP20ek0109411, JP23wm0325069, a research grant for prion diseases from the Ministry of Health, Labour and Welfare of Japan (JP23FC0201 and JP21FC1005), Japan Epilepsy Research Foundation, and Kawano Masanori Memorial Public Interest Incorporated Foundation for Promotion of Pediatrics (Sakai).
Author information
Authors and Affiliations
Contributions
R.T.: Conceptualization; Formal analysis; Writing—original draft; Writing—review and editing. S.A.: Data curation; Funding acquisition; Writing—review and editing. S.O.: Investigation; Writing—review and editing. F.F.: Formal analysis; Data curation; Writing—review and editing. E.H.: Investigation. K.Y.: Investigation. R.T.: Investigation. H.K.: Investigation. K.M.: Investigation. T.A.K.: Data curation; Supervision; Methodology. R.K.: Resources; Supervision. K.T.: Resources; Supervision; Methodology. K.Y.: Formal analysis; Supervision; Methodology. N.O.: Resources; Supervision; Methodology. S.O.: Investigation; Resources; Supervision; Funding acquisition; Writing—review and editing. Y.S.: Conceptualization; Formal analysis; Supervision; Data curation; Funding acquisition; Writing—original draft; Writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Taira, R., Akamine, S., Okuzono, S. et al. Gnao1 is a molecular switch that regulates the Rho signaling pathway in differentiating neurons. Sci Rep 14, 17097 (2024). https://doi.org/10.1038/s41598-024-68062-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68062-x
- Springer Nature Limited