Abstract
Fractional flow reserve (FFR) has become the gold standard for evaluating coronary lesion-specific ischemia. However, FFR is an invasive method that may cause possible complications in the coronary artery and requires expensive equipment, which limits its use. Promising noninvasive diagnostic methods, such as computed tomography angiography–derived FFR (CT-FFR) and the quantitative flow ratio (QFR), have been proposed. In this study, we evaluated the diagnostic performance of the QFR and CT-FFR in predicting coronary lesion-specific ischemia, with the FFR serving as the reference standard. Patients with suspected or known coronary artery disease who underwent coronary CT angiography revealing 30–90% diameter stenosis in the main coronary artery (≥ 2.0 mm reference diameter) were enrolled. The FFR was measured during invasive coronary angiography (within 15 days after coronary CT angiography). An FFR ≤ 0.8 was the reference standard for coronary lesion-specific ischemia. A total of 103 vessels from 92 consecutive patients (aged 59.8 ± 9.2 years; 60.9% were men) were evaluated. The diagnostic performance of a QFR ≤ 0.80 for predicting coronary lesion-specific ischemia demonstrated good diagnostic accuracy, sensitivity, and specificity (92.2%, 87.2%, and 96.4%, respectively), with an area under the receiver operating characteristic curve (AUC) of 0.987 (P < 0.0001). The diagnostic performance of a CT-FFR ≤ 0.80 for predicting coronary lesion-specific ischemia also demonstrated good diagnostic accuracy, sensitivity, and specificity (96.1%, 95.7%, and 96.4%, respectively), with an AUC of 0.967 (P < 0.0001). However, there was no significant difference in the AUC between a QFR ≤ 0.80 and a CT-FFR ≤ 0.80 for predicting coronary lesion-specific ischemia (P = 0.319). There was an excellent correlation between the QFR and FFR (r = 0.856, P < 0.0001). The CT-FFR and FFR also showed a good direct correlation (r = 0.816, P < 0.0001). The QFR and CT-FFR are strongly correlated with the FFR and can provide excellent clinical diagnostic performance for coronary lesion-specific ischemia detection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
The evaluation of the physiological function of coronary artery disease (CAD) is highly important for guiding the treatment of patients with ischemic heart disease. Fractional flow reserve (FFR) is the gold standard for detecting coronary lesion-specific ischemia and is strongly supported by guidelines for guiding revascularization decisions1,2. Compared with traditional invasive coronary angiography (ICA)-guided strategies, FFR-guided coronary artery revascularization strategies have been proven to reduce the number of implanted stents and improve clinical outcomes and cost-effectiveness in patients with CAD3,4. However, the wide application of the FFR is limited by the invasive nature of the procedure, prolonged procedural time, increased cost, and patient discomfort caused by the administration of adenosine5.
Machine learning has been used to analyze the correlation between coronary lesions and hemodynamics quickly and effectively6. Recently, machine learning has been introduced in the measurement of the FFR. Machine learning methods can predict FFR parameters. Some studies have reported the use of machine learning models based on cardiovascular images (such as CT) to predict FFR.
In recent years, image-based physiological function assessments of coronary arteries, such as computed tomography angiography–derived FFR (CT-FFR) and the quantitative flow ratio (QFR), have developed rapidly. Noninvasive CT-FFR can provide physiological functional evaluation of the coronary artery prior to the ICA on the basis of regular coronary computed tomography angiography (CCTA) data without changing the CCTA acquisition protocol or requiring additional imaging or hyperemic medications. Compared with anatomic imaging alone, CT-FFR is a supplement to CCTA and has greater positive predictive value (PPV) and specificity for detecting coronary lesion-specific ischemia. Several trials have shown a strong correlation between the CT-FFR and the FFR7,8. To improve the accessibility of functional lesion assessment in the ICA, the QFR was calculated on the basis of three-dimensional angiography reconstruction via two angiographic projections and a fluid dynamics algorithm without the need for a pressure wire or the administration of adenosine9. The QFR plays a mediating role between angiographic and physiological assessments of coronary artery lesions. Compared with FFR, the QFR can reduce the procedure time, risk, contrast agent, radiation exposure and cost. Previous studies have reported that the QFR has good diagnostic performance for detecting significant hemodynamic lesions10.
The difference in diagnostic performance between the QFR derived from the ICA and the CT-FFR derived from noninvasive CCTA images has not been adequately studied. With the advancement of relevant novel software, the diagnostic capabilities of the QFR and CT-FFR may also be improved. This study evaluated the diagnostic performance of the QFR and CT-FFR for predicting coronary lesion-specific ischemia, with the FFR serving as the reference standard. This study further clarified the potential value of the QFR and CT-FFR for CAD patients.
Methods
Study design and study population
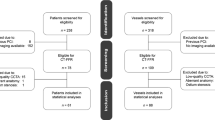
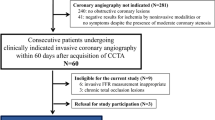
This single-center clinical trial evaluated the diagnostic performance of the QFR and CT-FFR for coronary lesion-specific ischemia, with the FFR serving as the reference standard. In this study, we continuously enrolled adults with suspected or known CAD between December 1, 2019, and June 30, 2022. The inclusion criteria were as follows: 18 years of age or older; ≥ 1 stenosis in the coronary artery with a diameter ≥ 2.0 mm and percent diameter stenosis ranging from 30 to 90%, as indicated by CCTA; ≥ 64 slice CT scanners for CCTA; and ICA within 15 days after CCTA examination. The exclusion criteria were as follows: pregnant or lactating women; women planning a short-term pregnancy; body mass index (BMI) > 35 kg/m2; iodine contrast agent allergy; inability to tolerate adenosine; artificial heart valve; previous placement of a stent, pacemaker, or defibrillator; previous coronary artery bypass grafting (CABG); left main artery lesion; hypertrophic obstructive cardiomyopathy; serum creatinine level > 178 μmol/L; within 1 month of myocardial infarction; New York Heart Association Functional Classification III or IV; poor CCTA imaging quality, severe motion artifacts, or diffuse calcification that lead to failure of coronary tree extraction or modeling; aneurysm or myocardial bridge disease; occlusive lesions; and severe bending that makes it difficult for the pressure wire to pass. This study was conducted in accordance with the Declaration of Helsinki. The Ethics Committee of Shaanxi Provincial People’s Hospital approved the study protocol (Approval No. 2019-X005). Informed consent was obtained from the enrolled subjects prior to their participation in the study.
CCTA and CT-FFR computation
CCTA was performed by using ≥ 64 slice CT scanners (uCT960+, United Imaging, Shanghai, China; GE Revolution, GE Healthcare, Milwaukee, Wisconsin; Aquilion Vision, Toshiba, Otawara, Japan; Somatom Force and Definition Flash, Siemens, Forchheim, Germany) in accordance with the CCTA guidelines11. CCTA images were interpreted by two experienced physicians. The percentage classifications of the diameter stenosis in the target lesions were as follows: 0%, 1–29%, 30–49%, 50–69%, 70–90%, or > 90%.
The CT-FFR was computed on the basis of CCTA images in a blinded fashion by well-trained technicians at the independent core laboratory with workstation-based software (Coronary Scope, version 1.0; Shenzhen Yueying Technology Co., Ltd., Shenzhen, China). This software is a package based on a computer unified device architecture with accelerated computational fluid dynamics (CFD) solved with a deep machine learning algorithm. The detailed methodology for the CT-FFR computation was recently described12.
ICA and FFR
ICA was performed by two experienced invasive cardiologists via a transfemoral or transradial approach. FFR was performed during ICA via a Radi Analyzer Xpress instrument with a Certus coronary pressure wire (St. Jude Medical, Inc., Minneapolis, MN, USA). Before the FFR was measured, nitroglycerine was administered into the coronary artery. A pressure wire was inserted distal to the coronary artery stenosis. Hyperemia was induced by intravenous administration of adenosine (140 µg/kg/min) via the femoral vein for at least 2 min. When steady-state hyperemia was reached, measurements of the mean arterial pressure measured through the coronary guiding catheter and the mean intracoronary pressure distal to the target stenosis measured by the pressure wire were recorded. During steady-state maximum hyperemia, the pressure wire was subsequently pulled back manually to the coronary artery ostium. If there is a pressure drift of |Pa- Pd|> 3 mm Hg, it is necessary to remeasure the FFR13. An FFR ≤ 0.80 was considered to indicate coronary lesion-specific ischemia.
QFR computation
The QFR was measured in a blinded fashion (AngioPlus, version 2.0, https://en.pulse-imaging.com/col.jsp?id=137; Pulse Medical Imaging Technology, Shanghai, China). The algorithms used were previously described14. Two sequences with a minimum 25° separation in projection angle were transferred to the QFR analysis system via a local network. The QFR analysis was conducted by well-trained technicians. The well-trained technicians were unaware of the FFR and CT-FFR results.
Statistical analysis
Statistical analysis was performed with MedCalc 20.0 (MedCalc Software, Ostend, Belgium). Categorical and continuous variables are expressed as the frequency and percentage and mean ± standard deviation, respectively. The diagnostic performance of a QFR ≤ 0.80 and a CT-FFR ≤ 0.80 for predicting a FFR ≤ 0.80 was assessed by the accuracy, sensitivity, specificity, PPV, negative predictive value (NPV), positive likelihood ratio (+ LR), and negative likelihood ratio (-LR). Receiver operating characteristic (ROC) curves were generated to assess the area under the curve (AUC) of the QFR and CT-FFR. The AUC was used to determine the QFR and CT-FFR as metrics of overall diagnostic performance according to the DeLong method15. Correlations between the QFR or CT-FFR and the FFR were assessed by Pearson's correlation coefficient. Agreement between the QFR, CT-FFR and FFR was determined via the Bland‒Altman plot16. A P value < 0.05 was considered to indicate statistical significance.
Results
Baseline patient characteristics
A total of 103 vessels from 92 consecutive patients were included. The mean age of the patients was 59.8 ± 9.2 years, and 56 (60.9%) were men. Forty-five (48.9%) had hypertension, 24 (26.1%) had hyperlipidemia, and 30 (32.6%) had diabetes mellitus. The baseline patient characteristics are shown in Table 1.
Vessel and lesion characteristics
Among the 103 evaluated vessels, 54 vessels (52.4%) were left anterior descending arteries, 13 vessels (12.6%) were left circumflex arteries, and 36 vessels (35.0%) were right coronary arteries. Sixteen vessels (15.5%) had 30%–49% stenosis, 59 vessels (57.3%) had 50%–69% stenosis, and 28 vessels (27.2%) had 70%–90% stenosis. An FFR ≤ 0.80 was noted for 46 vessels (44.7%). An FFR > 0.80 was noted for 57 vessels (55.3%) (Table 2).
QFR and CT-FFR for detecting coronary lesion-specific ischemia
The mean QFR was 0.786 ± 0.148, the mean CT-FFR was 0.792 ± 0.127, and the mean FFR was 0.781 ± 0.168 (Fig. 1).
The diagnostic performance of a QFR ≤ 0.80 for predicting an FFR ≤ 0.80 demonstrated good diagnostic accuracy, sensitivity, specificity, PPV, NPV, + LR, and –LR (92.2%, 87.2%, 96.4%, 95.4%, 90.0%, 24.4, and 0.13, respectively). The diagnostic performance of a CT-FFR ≤ 0.80 for predicting an FFR ≤ 0.80 also demonstrated good diagnostic accuracy, sensitivity, specificity, PPV, NPV, + LR, and –LR (96.1%, 95.7%, 96.4%, 95.7%, 96.4%, 26.8, and 0.04, respectively) (Table 3).
The AUC for a QFR ≤ 0.80 in predicting coronary lesion-specific ischemia was 0.987 (95% CI, 0.842–0.999; P < 0.0001), and the AUC for a CT-FFR ≤ 0.80 in predicting coronary lesion-specific ischemia was 0.967 (95% CI, 0.912–0.992; P < 0.0001) (Table 4; Fig. 2). However, no significant difference was found in the AUC between the QFR and CT-FFR for predicting coronary lesion-specific ischemia (P = 0.319).
Correlation and agreement between the QFR, CT-FFR and FFR
The QFR and FFR had excellent correlations (r = 0.856, P < 0.0001; Fig. 3). There was good agreement between the QFR and FFR. No systematic differences were found in the Bland–Altman analysis comparing the QFR and FFR (mean difference and root mean squared deviation 0.014 ± 0.143, P = 0.326; Fig. 4).
The CT-FFR and FFR were strongly correlated (r = 0.816, P < 0.0001; Fig. 5). There was good agreement between the CT-FFR and FFR. No systematic differences were found in the Bland–Altman analysis between the CT-FFR and FFR (mean difference and root mean squared deviation 0.028 ± 0.142, P = 0.052; Fig. 6).
The QFR and CT-FFR were directly correlated (r = 0.740, P < 0.0001; Fig. 7). There was good agreement between the QFR and CT-FFR. No systematic differences were found in the Bland–Altman analysis between the QFR and CT-FFR (mean difference and root mean squared deviation -0.014 ± 0.155, P = 0.369; Fig. 8).
Discussion
The present study demonstrated that the QFR and CT-FFR are strongly correlated with the FFR. The QFR and CT-FFR can provide excellent clinical diagnostic performance for coronary lesion-specific ischemia detection. Therefore, the QFR and CT-FFR can serve as tools to guide clinical practice in determining coronary lesion-specific ischemia. This study provides key data for the clinical application of the QFR and CT-FFR in patients with CAD.
The ICA is the most widely used method for guiding PCI in patients with CAD. However, physician assessments of CAD severity cannot satisfactorily distinguish between significantly functional lesions and nonsignificant functional lesions. There are studies indicating that the relationship between the severity of quantitative stenosis on coronary angiography and the level of functional blood flow restriction is weak17,18. A simple percentage description of a narrowed coronary artery does not necessarily represent the full physiological impact on its perfused myocardium19. The FFR has the ability to measure myocardial ischemia induced by lesions via a pressure wire under maximum hyperemic conditions (adenosine administration). Compared with visual angiography, physiological measurements based on pressure wires can more accurately identify blood flow-limiting lesions in patients with CAD. The FFR is considered the gold standard for detecting coronary lesion-specific ischemia and is strongly supported in guiding revascularization decisions20. The FAME trial followed up for 1 year, and the FAME 2 trial followed up for 3 years and reported that FFR-guided PCI can significantly reduce major cardiovascular events21,22. Although there is scientific evidence suggesting that coronary artery revascularization based on functional assessment is beneficial, the application of the FFR still remains significantly underutilized23. However, according to a recent study, the use of the FFR in patients with intermediate lesions in the United States has increased slowly but steadily from 14.8 to 18.5% in recent years24.
The QFR is obtained through coronary angiography imaging and does not require pressure guide wires or hyperemic agents25. The acquisition of the QFR is very fast in the catheterization laboratory (median 266 s)26. Therefore, compared with the FFR, the QFR can reduce the procedure time, risk, contrast agent, radiation exposure and costs. The QFR can help clinical physicians make decisions on coronary artery revascularization by identifying significantly functional lesions27. The software used for QFR in this study was AngioPlus, and recent studies (such as the FAVOR II China study and the FAVOR III study) have confirmed its application in coronary lesions. The FAVOR II China study revealed that the diagnostic accuracy of the QFR was 92.7%, with a sensitivity of 94.6% and a specificity of 91.7%9. The FAVOR III study confirmed that, compared with standard angiography guidance, QFR-guided PCI can reduce the risk of 1-year major adverse cardiac events by 35%28. The QFR is easier to implement than the FFR is, which helps to incorporate the QFR into routine clinical practice.
CCTA is a noninvasive and widely used clinical tool for detecting suspicious CAD. However, CCTA cannot be used to evaluate the hemodynamics of coronary artery lesions29. Before performing ICA, it is necessary to evaluate the physiological function of coronary artery lesions on the basis of CCTA data. The CT-FFR is a validated noninvasive diagnostic method based on CCTA datasets that can accurately detect the hemodynamics of coronary artery lesions30. In patients without significant functional lesions, the CT-FFR can reduce the number of unnecessary coronary angiographies. A prospective study confirmed that the CT-FFR can reduce the incidence of ICA by 61%31. The advantage of CT-FFR lies not only in its high diagnostic accuracy in detecting lesion-specific ischemia but also in not changing the CCTA data collection protocol, providing additional images, or administering medication7,32. Therefore, the CT-FFR is a feasible and safe alternative to ICA. In this study, Coronary Scope software was used for the CT-FFR measurements. Coronary Scope is a novel deep machine learning-based software for calculating the CT-FFR for predicting coronary ischemia12,33,34. Recently, a multicenter study from six clinical medical centers with 324 vessels from 301 patients used the Coronary Scope software to detect the diagnostic accuracy of the CT-FFR. The results showed that on a per-vessel basis, the diagnostic sensitivity, specificity, and accuracy of the CT-FFR for detecting coronary lesion-specific ischemia were 95%, 92%, and 94%, respectively. In addition, the CT-FFR values were strongly correlated with the FFR values (Spearman’s rank correlation, r = 0.88; P < 0.001)12.
Machine learning models have been reported to be able to predict the FFR based on cardiovascular images, such as CT, angiography images, intravascular ultrasound (IVUS), and optical coherence tomography (OCT). Although CFD-based CT-FFR has good consistency with the FFR, the calculation time varies with complexity. Coenen et al. reported that machine learning-based CT-FFR closely reproduces CFD-based CT-FFR and can be executed virtually without delay. In addition, the diagnostic accuracy of machine learning-based CT-FFR can improve with increasing image quality35. Arefinia et al. designed a fast, end-to-end, automated deep learning model to estimate the FFR via angiography images. This model did not require manual annotation, with an overall accuracy of 81%36. This model can be used to evaluate physiological conditions without the need for additional data input and manual annotation, as it only requires the input of vascular images. Lee et al. reported that IVUS-based machine learning algorithms had good diagnostic accuracy for identifying lesion-specific ischemia and had good correlations with the FFR37. Although these studies have shown good FFR prediction performance, there is a trend toward better performance with respect to high-resolution images6. OCT has better resolution than CT, angiography and IVUS. Compared with wire‑based FFR, OCT-based machine learning-based FFR for left anterior descending artery lesions showed a sensitivity of 100% and a specificity of 92.9% in terms of ischemia diagnosis (FFR ≤ 0.8) and good correlation (r = 0.853) with the wire‑based FFR13. Cha et al. developed OCT-based machine learning-based FFR for all coronary artery territories. The results showed that the OCT-based machine learning-based FFR was strongly correlated with the wire-based FFR (r = 0.8782), with an overall accuracy of 91.7%6.
With the advancement of relevant novel algorithms, the diagnostic capabilities of the QFR and CT-FFR have also improved. In this study, we used the novel software Coronary Scope with a deep machine learning algorithm for the CT-FFR measurement and AngioPlus software for the QFR measurement. The results revealed that the QFR provided good diagnostic accuracy, sensitivity, and specificity (92.2%, 87.2%, and 96.4%, respectively) for predicting coronary lesion-specific ischemia, with an AUC of 0.987. The QFR and FFR exhibited an excellent direct correlation (r = 0.856). However, despite relatively good predictive values and correlations, the QFR misclassified 7.8% of the stenoses with respect to the FFR, indicating functional significance. That is, if the QFR is chosen as the basis for percutaneous coronary intervention, 8 of 103 stenoses receive inappropriate percutaneous coronary intervention or do not receive appropriate intervention. The CT-FFR also provided good diagnostic accuracy, sensitivity, and specificity (96.1%, 95.7%, and 96.4%, respectively) for predicting coronary lesion-specific ischemia, with an AUC of 0.967. These predictive values indicate that the CT-FFR misclassified 3.9% of stenoses with respect to the FFR, indicating functional significance. The CT-FFR and FFR also exhibited a good direct correlation (r = 0.816). As previously reported using Coronary Scope software for CT-FFR measurement and AngioPlus software for QFR measurement9,12, this study also revealed that the QFR and CT-FFR are strongly correlated with the FFR. In this study, the QFR and CT-FFR were more strongly correlated with the FFR than were those reported in previous studies38,39. One possible reason may be that this study used different CT-FFR and QFR software than was used in the other studies. Machine learning models were used for geometry extraction. Another possible reason is that a larger range of percent diameter stenoses identified on CCTA was included in this study. The strength of the correlation is known to be weakest for intermediate stenoses40. Hirshfeld et al. reported that the diagnostic performance of CT-FFR was weaker in the moderate stenosis subgroup (50% − 90%) evaluated by CCTA and the weakest in the subgroup with FFR values between 0.70 and 0.8540. The differences in diagnostic performance among these studies may be due to differences in the distribution of stenosis severity among different study populations. Therefore, the larger range of percent diameter stenosis identified on CCTA in this study may be a reason for the greater correlations reported here.
Study limitations
We are also aware of several limitations. First, the number of subjects in this study was relatively small, and further expansion is needed. Second, this study selected only patients whose percent diameter stenosis ranged from 30 to 90%. Furthermore, this study did not observe any clinical outcomes. Finally, specific patient conditions, such as left main artery lesions and previous placement of a stent, were excluded. However, further research is needed on the diagnostic value of the QFR and CT-FFR for these patients.
Conclusions
Compared with the FFR, the QFR derived from coronary angiography is a less invasive method, and the CT-FFR derived from CCTA is a noninvasive method for evaluating coronary lesion-specific ischemia. The QFR and CT-FFR are significantly correlated with the FFR and have high diagnostic performance for predicting coronary lesion-specific ischemia.
Data availability
The dataset used during this study can be obtained from the corresponding authors upon reasonable request.
References
Lawton, J. S. et al. 2021 ACC/AHA/SCAI Guideline for coronary artery revascularization: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 79, e21–e129. https://doi.org/10.1016/j.jacc.2021.09.006 (2022).
Neumann, F. J. et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 40, 87–165. https://doi.org/10.1093/eurheartj/ehy394 (2019).
Xaplanteris, P. et al. Five-year outcomes with PCI guided by fractional flow reserve. N. Engl. J. Med. 379, 250–259. https://doi.org/10.1056/NEJMoa1803538 (2018).
Dobrić, M. et al. Current status and future perspectives of fractional flow reserve derived from invasive coronary angiography. Front. Cardiovasc. Med. 10, 1181803. https://doi.org/10.3389/fcvm.2023.1181803 (2023).
Chuang, M. J. et al. Clinical assessment of resting full-cycle ratio and fractional flow reserve for coronary artery disease in a real-world cohort. Front. Cardiovasc. Med. 9, 988820. https://doi.org/10.3389/fcvm.2022.988820 (2022).
Cha, J. J. et al. Assessment of fractional flow reserve in intermediate coronary stenosis using optical coherence tomography-based machine learning. Front. Cardiovasc. Med. 10, 1082214. https://doi.org/10.3389/fcvm.2023.1082214 (2023).
Wu, H. et al. Diagnostic performance of noninvasive coronary computed tomography angiography-derived FFR for coronary lesion-specific ischemia based on deep learning analysis. RCM https://doi.org/10.31083/j.rcm2501020 (2024).
Zhuang, B., Wang, S., Zhao, S. & Lu, M. Computed tomography angiography-derived fractional flow reserve (CT-FFR) for the detection of myocardial ischemia with invasive fractional flow reserve as reference: Systematic review and meta-analysis. Eur. Radiol. 30, 712–725. https://doi.org/10.1007/s00330-019-06470-8 (2020).
Xu, B. et al. Diagnostic accuracy of angiography-based quantitative flow ratio measurements for online assessment of coronary stenosis. J. Am. Coll. Cardiol. 70, 3077–3087. https://doi.org/10.1016/j.jacc.2017.10.035 (2017).
Westra, J. et al. Diagnostic performance of quantitative flow ratio in prospectively enrolled patients: An individual patient-data meta-analysis. Catheter. Cardiovasc. Interv. 94, 693–701. https://doi.org/10.1002/ccd.28283 (2019).
Abbara, S. et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: A report of the society of Cardiovascular Computed Tomography Guidelines Committee: Endorsed by the North American Society for Cardiovascular Imaging (NASCI). J. Cardiovasc. Comput. Tomogr. 10, 435–449. https://doi.org/10.1016/j.jcct.2016.10.002 (2016).
Ding, Y. et al. Diagnostic accuracy of noninvasive fractional flow reserve derived from computed tomography angiography in ischemia-specific coronary artery stenosis and indeterminate lesions: results from a multicenter study in China. Front. Cardiovasc. Med. 10, 1236405. https://doi.org/10.3389/fcvm.2023.1236405 (2023).
Cha, J. J. et al. Optical coherence tomography-based machine learning for predicting fractional flow reserve in intermediate coronary stenosis: A feasibility study. Sci. Rep. 10, 20421. https://doi.org/10.1038/s41598-020-77507-y (2020).
Tu, S. et al. Diagnostic accuracy of fast computational approaches to derive fractional flow reserve from diagnostic coronary angiography: The international multicenter FAVOR pilot study. JACC Cardiovasc. Interv. 9, 2024–2035. https://doi.org/10.1016/j.jcin.2016.07.013 (2016).
DeLong, E. R., DeLong, D. M. & Clarke-Pearson, D. L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44, 837–845 (1988).
Bland, J. M. & Altman, D. G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1, 307–310 (1986).
Pijls, N. H. et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N. Engl. J. Med. 334, 1703–1708. https://doi.org/10.1056/nejm199606273342604 (1996).
Elgendy, I. Y., Denktas, A., Johnson, N. P., Jaffer, F. A. & Jneid, H. Invasive coronary physiology assessment for patients with stable coronary disease. Cardiol. Rev. 30, 263–266. https://doi.org/10.1097/crd.0000000000000396 (2022).
Cesaro, A. et al. Functional assessment of coronary stenosis: an overview of available techniques. Is quantitative flow ratio a step to the future?. Expert Rev. Cardiovasc. Ther. 16, 951–962. https://doi.org/10.1080/14779072.2018.1540303 (2018).
Mortensen, M. B. et al. Influence of intensive lipid-lowering on CT derived fractional flow reserve in patients with stable chest pain: Rationale and design of the FLOWPROMOTE study. Clin Cardiol. 45, 986–994. https://doi.org/10.1002/clc.23895 (2022).
Tonino, P. A. et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N. Engl. J. Med. 360, 213–224. https://doi.org/10.1056/NEJMoa0807611 (2009).
Fearon, W. F. et al. Clinical outcomes and cost-effectiveness of fractional flow reserve-guided percutaneous coronary intervention in patients with stable coronary artery disease: Three-year follow-up of the FAME 2 trial (fractional flow reserve versus angiography for multivessel evaluation). Circulation 137, 480–487. https://doi.org/10.1161/circulationaha.117.031907 (2018).
Elbadawi, A. et al. Contemporary use of coronary physiology in cardiology. Cardiol. Ther. 12, 589–614. https://doi.org/10.1007/s40119-023-00329-2 (2023).
Parikh, R. V. et al. Utilization and outcomes of measuring fractional flow reserve in patients with stable ischemic heart disease. J. Am. Coll. Cardiol. 75, 409–419. https://doi.org/10.1016/j.jacc.2019.10.060 (2020).
Chu, J. et al. Angiographic quantitative flow ratio in acute coronary syndrome: Beyond a tool to define ischemia-causing stenosis-a literature review. Cardiovasc. Diagn. Ther. 12, 892–907. https://doi.org/10.21037/cdt-22-334 (2022).
Yazaki, K. et al. Applicability of 3-dimensional quantitative coronary angiography-derived computed fractional flow reserve for intermediate coronary stenosis. Circ. J. 81, 988–992. https://doi.org/10.1253/circj.CJ-16-1261 (2017).
Chu, J. et al. Impact of radial wall strain on serial changes in vascular physiology in patients with intermediate coronary stenosis. RCM https://doi.org/10.31083/j.rcm2408245 (2023).
Xu, B. et al. Angiographic quantitative flow ratio-guided coronary intervention (FAVOR III China): A multicentre, randomised, sham-controlled trial. Lancet 398, 2149–2159. https://doi.org/10.1016/s0140-6736(21)02248-0 (2021).
Wu, P. W. et al. Added value of computed tomography virtual intravascular endoscopy in the evaluation of coronary arteries with stents or plaques. Diagnostics https://doi.org/10.3390/diagnostics12020390 (2022).
Qiao, H. Y. et al. Role of quantitative plaque analysis and fractional flow reserve derived from coronary computed tomography angiography to assess plaque progression. J. Thorac. Imaging. 38, 186–193. https://doi.org/10.1097/rti.0000000000000697 (2023).
Douglas, P. S. et al. Clinical outcomes of fractional flow reserve by computed tomographic angiography-guided diagnostic strategies vs. usual care in patients with suspected coronary artery disease: the prospective longitudinal trial of FFR(CT): Outcome and resource impacts study. Eur. Heart J. 36, 3359–3367. https://doi.org/10.1093/eurheartj/ehv444 (2015).
Röther, J. et al. Comparison of invasively measured FFR with FFR derived from coronary CT angiography for detection of lesion-specific ischemia: Results from a PC-based prototype algorithm. J. Cardiovasc. Comput. Tomogr. 12, 101–107. https://doi.org/10.1016/j.jcct.2018.01.012 (2018).
Dong, M. et al. Diagnostic performance of coronary computed tomography angiography-derived fractional flow reverse in lesion-specific ischemia patients with different Gensini score levels. Ann. Transl. Med. 10, 412. https://doi.org/10.21037/atm-22-881 (2022).
Tao, Y. et al. Diagnostic performance of coronary computed tomography (CT) angiography derived fractional flow reserve (CTFFR) in patients with coronary artery calcification: Insights from multi-center experiments in China. Ann. Transl. Med. 10, 788. https://doi.org/10.21037/atm-22-3180 (2022).
Coenen, A. et al. Diagnostic accuracy of a machine-learning approach to coronary computed tomographic angiography-based fractional flow reserve: Result from the MACHINE consortium. Circ. Cardiovasc. Imaging. 11, e007217. https://doi.org/10.1161/circimaging.117.007217 (2018).
Arefinia, F. et al. Non-invasive fractional flow reserve estimation using deep learning on intermediate left anterior descending coronary artery lesion angiography images. Sci. Rep. 14, 1818. https://doi.org/10.1038/s41598-024-52360-5 (2024).
Lee, J. G. et al. Intravascular ultrasound-based machine learning for predicting fractional flow reserve in intermediate coronary artery lesions. Atherosclerosis 292, 171–177. https://doi.org/10.1016/j.atherosclerosis.2019.10.022 (2020).
Tanigaki, T. et al. QFR versus FFR derived from computed tomography for functional assessment of coronary artery stenosis. JACC Cardiovasc. Interv. 12, 2050–2059. https://doi.org/10.1016/j.jcin.2019.06.043 (2019).
Kawashima, H. et al. Diagnostic concordance and discordance between angiography-based quantitative flow ratio and fractional flow reserve derived from computed tomography in complex coronary artery disease. J. Cardiovasc. Comput. Tomogr. 16, 336–342. https://doi.org/10.1016/j.jcct.2022.02.004 (2022).
Hirshfeld, J. W. Jr. & Nathan, A. S. QFR and FFR(CT): Accurate Enough?. JACC Cardiovasc. Interv. 12, 2060–2063. https://doi.org/10.1016/j.jcin.2019.07.029 (2019).
Funding
This study was partially supported by the Science and Technology Talent Support Program of Shaanxi Provincial People's Hospital (Nos. 2021JY-24, 2022LJ-03, 2022YJY-35, and 2023YJY-57).
Author information
Authors and Affiliations
Contributions
W.H., L.L. and H.W.: study design, data analysis and interpretation, and manuscript revision. L.L., T.H., L.S., Y.L. and F.C.: data collection and manuscript revision. W.H., Z.W., Y.C. C.Z. and W.H.: data collection, data analysis and manuscript drafting. All the authors of this study read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Han, W., Liang, L., Han, T. et al. Diagnostic performance of the quantitative flow ratio and CT-FFR for coronary lesion-specific ischemia. Sci Rep 14, 16969 (2024). https://doi.org/10.1038/s41598-024-68212-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68212-1
- Springer Nature Limited