Abstract
The pan-immune-inflammation value (PIV), calculated as (neutrophil × platelet × monocyte)/lymphocyte count, may be useful for estimating survival in breast cancer patients. To determine the prognostic value of PIV for overall survival in breast cancer patients in Lima, Peru. A retrospective cohort study was conducted. 97 breast cancer patients diagnosed between January 2010 and December 2016 had their medical records analyzed. The primary dependent variable was overall survival, and the key independent variable was the PIV, divided into high (≥ 310) and low (< 310) groups. Patient data included demographics, treatment protocols and other clinical variables. Statistical analysis involved Kaplan–Meier survival curves and Cox proportional hazards modeling. Patients with a PIV ≥ 310 had significantly lower 5-year survival functions (p = 0.004). Similar significant differences in survival were observed for clinical stage III-IV (p = 0.015), hemoglobin levels < 12 mg/Dl (p = 0.007), histological grade (p = 0.019), and nuclear grade (p < 0.001); however, molecular classification did not show a significant survival difference (p = 0.371). The adjusted Hazard Ratios showed that PIV ≥ 310 was significantly associated with poor outcome (5.08, IC95%: 1.52–16.92). While clinical stage and hemoglobin levels were associated with survival in the unadjusted model. These factors did not maintain significance after adjustment. PIV is an independent predictor of reduced survival in Peruvian breast cancer patients.
Similar content being viewed by others
Introduction
Breast cancer continues to be the most frequently diagnosed malignant tumor in women globally, and according to the World Health Organization, it represents the highest incidence among all cancers affecting women1. In Latin America and the Caribbean, there were more than 210,000 new diagnoses of breast cancer and almost 68,000 deaths in 20201. Breast cancer in Latin America is a significant public health issue, marked by the existing barriers between patients and healthcare, evaluation by experts, and identification of strategies and priorities for improved clinical outcomes2. In Peru, breast cancer is the most common among women and the sixth deadliest, with 28 new cases and 9.2 deaths per 100,000 annually3.
Despite significant improvements in breast cancer overall survival rates due to advances in diagnosis and treatment in recent decades, heterogeneity in prognostic outcomes remains an issue1. Such variability is particularly evident in low and middle-income countries, where the mortality rates of breast cancer are steady or even increasing, unlike the continuous substantial improvements exceeding 2% annual mortality reduction in high-income countries4. This disparity accentuates the urgent need for a reliable, easily accessible prognostic tool, particularly in Latin America, where a significant portion of breast cancer diagnoses occur in late stages5.
Recent research in cancer biology indicates that immune-inflammatory responses play a pivotal role in cancer, both enhancing anti-tumor immunity and facilitating tumor growth and resistance to treatment, reflecting the diverse effects of inflammation in cancer progression6,7,8. In breast cancer research, several immune-inflammatory biomarkers (IIBs)—notably, the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and systemic immune-inflammation index (SII)—have been identified as potentially independent prognostic factors9,10,11,12,13. Despite their potential, the complex interplay between tumor dynamics and host immune responses limits the effectiveness of these individual IIBs as prognostic tools.
A novel biomarker, the pan-immune-inflammation value (PIV), encompassing neutrophil, platelet, monocyte, and lymphocyte counts, has emerged as a robust predictor of survival outcomes in cancer. PIV has been identified as a significant predictor of pathological complete response to neoadjuvant chemotherapy, overall survival, and progression-free survival in various breast cancer studies9,10,11,12,13. Its ease of obtainment from routine blood tests makes it a particularly promising tool in public health for breast cancer prognosis. This accessibility positions PIV as a cost-effective tool, particularly relevant in regions like Latin America where healthcare resources may be limited. However, its effectiveness in these diverse populations, which may have a higher genetic predisposition to more aggressive types of breast cancer as well as environmental factors and socio-cultural barriers for better management2,14, remains to be thoroughly evaluated. Implementing its use to local contexts could enhance its role in early detection and treatment strategies in breast cancer management.
Considering the growing incidence and mortality of breast cancer in Latin America, and particularly in Peru, this study aims to evaluate the practicality and effectiveness of PIV as a prognostic tool accessible to healthcare providers at all levels, including those in primary healthcare settings. This research is important for enhancing personalized treatment strategies and survival outcomes in Latin American breast cancer patients. It is especially significant as it provides a simple, yet effective tool that can be utilized even by clinicians in diverse clinical environments. The objective of this study is to determine the prognostic value of PIV for overall survival in breast cancer patients at the Central Military Hospital Coronel Arias Schreiber (HMC) in Lima, Peru.
Methods
Study design and setting
A retrospective cohort study was performed to leverage existing clinical data to analyze the potential prognostic significance of PIV, calculated as:
The research was undertaken at the HMC, a facility with a comprehensive archive of patient records pertinent to breast cancer treatment and research. This site was chosen due to its extensive repository of detailed patient data, including immunohistochemistry results.
The patient cohorts comprised individuals diagnosed from January 2010 to December 2016, as identified from the center's database. This period was chosen to ensure all patients had at least a 5-year follow-up by the time of data collection in 2022. Assessments of PIV were conducted at the time of initial diagnosis, with values sourced from existing pathology and medical records. Patient follow-up data were accumulated over a minimum duration of five years post-diagnosis.
Population and sample
We analyzed the medical records of 97 patients diagnosed with breast cancer at the HMC from January 2010 to December 2016. The cohort was divided based on PIV, with those exhibiting high PIV (≥ 310) designated as the exposed group and those with low PIV (< 310) as the non-exposed group. Following the application of selection criteria, 81 patients were included in the final analysis. Statistical power was calculated at a 95% confidence level to discern expected survival differences—96.2% in patients with low PIV and 39.1% in those with high PIV12; additionally, for an expected hazard ratio (HR) of 6.489. In both cases, study's statistical power exceeded 80%.
No sampling was performed as the study included all breast cancer patients at the HMC during the study period. The inclusion criteria encompassed patients with invasive ductal carcinoma who underwent immunohistochemical studies and those diagnosed with primary breast cancer. Exclusion criteria included patients without complete medical records16, those diagnosed with synchronous malignant neoplasms in other organs (0), and patients who received follow-up care at other hospitals (0).
Variables and instruments
The primary dependent variable was overall survival, measured in months from the date of diagnosis to the date of death or last known follow-up. Patients who are still alive at the end of the study or lost to follow-up were censored at their last known alive date.
Covariates included in the study were age, marital status, origin, oncological family history, molecular classification based on the immunohistochemical profile, immunohistochemical profile, histological grade, nuclear grade, clinical stage, and body mass index (BMI). All of these covariates were measured using data from patient medical records.
Procedures
Data collection was conducted using clinical records requested from the hospital's archives, following the approval of the hospital's ethics committee. Variables were collected using a data collection sheet specifically designed for this study. All patients who met the inclusion criteria were recorded. The data obtained were then transferred to a Microsoft Excel Spreadsheet for analysis.
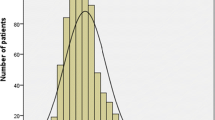
The cutoff point for PIV was 310, based on an important previous study10; this cutoff was considered for the formation of comparison groups as it allowed for an evaluation comparable to that precedent. Therefore, a ROC curve analysis (Fig. 1) was performed to determine the best cutoff point for PIV in the evaluated sample using the Youden method, resulting in ≥ 346.6.
Statistical analysis
Descriptive analysis was conducted using frequencies and percentages for categorical variables and measures of central tendency and dispersion for numerical variables, depending on their normality. Kaplan–Meier survival analysis was performed to compare overall survival functions across groups. The log-rank test was employed to evaluate the statistical significance of the differences in these overall survival functions. Additionally, Cox proportional hazards modeling was used to calculate both crude (HR) and adjusted hazard ratios (aHR) with their 95% confidence intervals (CI), providing insights into the prognostic significance of PIV in breast cancer overall survival. A Mantel–Haenszel exploratory analysis was conducted to assess if PIV acts as an effect modifier between neoadjuvant chemotherapy and worse survival.
Institutional review board statement
The study was conducted in compliance with the ethical standards established in the Helsinki Declaration, ensuring patient anonymity during data collection for maximum confidentiality. The study was approved by the Ethics Committee for Research (CEI) of Ricardo Palma University with the code PG 103—2021.
Results
Out of the 97 patients potentially eligible for the study, 16 were excluded due to the inability to access their complete clinical history, resulting in 81 patients for the analysis. Tables 1 and 2 provide demographic and oncological insights into these patients. Table 1 highlights that 92.6% of the patients were currently married, with 86.3% residing in Lima, and a significant proportion having comorbidities like hypertension (37.0%) and diabetes mellitus (16.0%).
The oncological characteristics in Table 2 reveal that 65.4% of patients had moderately differentiated tumors, and 50.0% presented with a moderate nuclear grade. Notably, 64.2% were estrogen receptor-positive, and a majority underwent adjuvant chemotherapy (77.8%). Further detailed percentages and patient characteristics, including blood test results and molecular classifications, are presented in the respective tables.
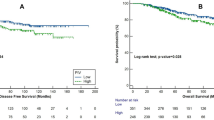
Figure 2 shows that patients with a PIV ≥ 310 had worse survival compared to those with PIV < 310 (p = 0.004). Similarly, there were significant differences in survival according to clinical stage (p = 0.015), hemoglobin level (p = 0.007), histological grade (p = 0.011), and nuclear grade (p < 0.001); but not for molecular classification (p = 0.497).
Table 3 presents the unadjusted and adjusted Hazard Ratios (HRs and aHRs) for various clinical factors affecting patient survival. In the unadjusted model, PIV ≥ 310 was significantly associated with poor outcome, with an HR of 3.41 (p = 0.006). Stage III-IV and Hemoglobin < 12 also showed significant associations with HRs of 2.85 (p = 0.02) and 3.06 (p = 0.011), respectively. However, in the adjusted model, PIV ≥ 310 remained significant with an aHR of 4.94 (p = 0.006), while Stage III-IV and Hemoglobin < 12 did not maintain their significance, with aHRs of 0.87 (p = 0.841) and 3.43 (p = 0.023). In the adjusted model, both Undifferentiated Histological Grade and High Nuclear Grade were significantly associated with increased risk, evidenced by aHRs of 4.20 (p = 0.008) and 10.96 (p = 0.001), respectively. Furthermore, the Her-2 subtype demonstrated a more favorable survival outcome, with an aHR of 0.15 (p = 0.006), compared to the Luminal A/B subtype. In contrast, the Triple Negative category did not exhibit any significant associations, although it showed worse mortality.
We conducted an exploratory evaluation of the relationship between neoadjuvant treatment and worse survival in patients with different PIV levels using a Mantel–Haenszel test to determine if PIV is an effect modifier. In the stratum with PIV < 310, the HR was 0.51 (95% CI 0.12–2.14). In the stratum with PIV ≥ 310, the HR was 0.51 (95% CI 0.17–1.56). The overall Mantel–Haenszel estimate, controlling for PIV, showed an HR of 0.51 (95% CI 0.21–1.23, p = 0.128), indicating that there is no significant evidence that PIV modifies the relationship between neoadjuvant treatment and worse survival.
Discussion
The results of our study, provide compelling evidence about the prognostic significance of PIV in breast cancer survival. Notably, patients with a PIV ≥ 310 demonstrated significantly lower overall survival rates at five years compared to those with PIV < 310, underscoring the potential of PIV as a critical biomarker in predicting patient outcomes. This finding aligns with the aHR of 5.08 for PIV ≥ 310, indicating a substantially increased risk of mortality. Furthermore, our study identified other significant clinical factors that could affect survival, such as clinical stage and hemoglobin level; however, in the adjusted model, these associations did not maintain their statistical significance, highlighting the unique predictive influence of PIV. This is further supported by a systematic review and meta-analysis15 that found patients with higher PIV levels had a significantly increased risk of death (HR: 2.00) and progression or death (HR: 1.80), reinforcing the utility of PIV as a prognostic biomarker across different cancer types and clinical settings. The implications of these findings are particularly noteworthy in the context of breast cancer treatment and management in Latin America, where accessible and efficient prognostic tools like PIV can play a crucial role in improving patient outcomes and guiding treatment strategies.
These findings are similar to those of Şahin et al.13, which demonstrated that a low PIV heralded a better survival outcomes among Turkish women. The extensive cohort of 743 patients in their research positions PIV as a potentially universal biomarker, transcending various treatment modalities. Likewise, in the study by Şahin et al., it was also observed that a low PIV led to a better response to adjuvant treatment; however, no effect modifier role of PIV was found in the relationship between said treatment and worse survival when evaluated in our study.
This viewpoint is bolstered by Ligorio et al.12, who established PIV's prognostic value in HER2-positive advanced breast cancer patients, asserting its role as a strong survival predictor after dual anti-HER2 therapy. Furthermore, Qi et al.11 through a meta-analysis, and Lin et al.10 with a predictive scoring model, have reinforced the prognostic value of PIV, highlighting its relevance not just in a Peruvian hospital setting, but across broader demographics and clinical scenarios.
In addition to aligning with the aforementioned studies, our findings also converse with the results of Fuca et al.16, where high PIV was linked to inferior progression-free and overall survival. Moreover, the studies of Şahin et al.13 and Gulmez et al.17 echo the utility of PIV as a pre-treatment prognostic biomarker, especially in metastatic HER2-positive contexts. Notably, the study of Provenzano et al. elucidated PIV's prognostic discrimination capacity in advanced triple-negative breast cancer patients, associating it with clinical outcomes and potential for future PIV-based risk stratification.
Contrastingly, the study by Demir et al. suggested that while a higher PIV was associated with more advanced cancer stages in younger patients18, it did not translate into a statistically significant survival difference.
The results of the PIV evaluation are similar to those found with other biomarkers in breast cancer. Thus, in the studies by Tong et al.19, and Yamanouchi and Maeda20, PIV was compared with other biomarkers like the systemic immune-inflammation-index (SII), indicating its potential over other inflammation-related prognostic tools. PIV demonstrates significant advantages over other biomarkers such as SII, HPR, and NLR. It not only reflects systemic inflammation but also provides insights into local inflammation, vascular patency, and oxygenation. These characteristics are crucial, as they relate to various aspects of cancer progression, including hypoxia, vascular thrombosis, and fibrosis21. These cumulative insights underscore PIV's robustness as a prognostic tool, capable of providing a valuable perspective on patient survival prospects across different breast cancer populations and treatment approaches, while also highlighting the need for its validation in diverse clinical settings.
The significant association between PIV and survival outcomes observed in our study may be rooted in the intricate interplay between inflammation and cancer progression. PIV, as an aggregate biomarker, encompasses various components of the systemic inflammatory response, including neutrophils, monocytes, platelets, and lymphocytes. Neutrophils and monocytes, through their secretion of growth factors and proteases, along with platelets aiding in immune evasion and metastasis, contribute to tumor growth and spread. Conversely, lymphocytes, especially T cells, are key in combating tumors. The balance between these cell types, reflected in their ratios, indicates the interplay between tumor-promoting inflammation and tumor-suppressing immunity6,7. An elevated pan-immune-inflammation value (PIV) suggests an inflammatory state that fosters a tumor-friendly environment, aiding genomic instability, angiogenesis, and immune escape, while also potentially affecting chemotherapy effectiveness13,22,23. At the molecular level, PIV components are sources of cytokine and chemokine, influencing the tumor microenvironment towards cancer cell survival and proliferation. Specifically, neutrophils secrete elements like neutrophil elastase that facilitate tumor invasion, and monocytes transform into macrophages that support angiogenesis and hinder T-cell activity, often correlating with poor cancer prognoses24.
The prognostic value of PIV could, therefore, be a composite reflection of these pro-inflammatory and immunosuppressive pathways that are pivotal in cancer progression. Understanding the exact molecular mechanisms by which PIV correlates with survival may provide valuable insights into the potential for targeting the inflammatory milieu as part of comprehensive cancer therapy. These findings suggest that modulating the inflammatory response, alongside conventional treatments, could augment therapeutic efficacy and improve patient survival in breast cancer.
PIV could be a promising prognostic biomarker for cancer, particularly valuable in resource-constrained settings such as those found in many parts of Latin America. In regions where access to advanced diagnostic tools and targeted therapies may be limited25,26, PIV stands out for its reliance on routine blood parameters, making it an accessible and cost-effective option. Derived from standard blood counts, PIV aids in early risk assessment and strategic resource allocation in Latin American cancer care, crucial in settings with limited oncology resources. Its simplicity allows for broad adoption across healthcare facilities, helping prioritize treatment and monitoring, potentially enhancing survival in economically challenged areas.
Our study reveals that HER2-positive patients showed better survival compared to Luminal A and B subtypes, likely due to effective targeted therapies like dual anti-HER2 treatment. For instance, a study analyzing breast cancer-specific survival among women diagnosed during 2010 to 2013 in the U.S. found that the best survival was observed in HR+/HER2− subtype, followed by HR+/HER2+ and HR−/HER2+ subtypes, with the worst survival in triple-negative cases27. In contrast, Triple Negative breast cancer, known for its aggressiveness and lack of specific treatments, exhibited poorer survival. However, the significance of this finding was limited by the small number of Triple Negative cases in our study.
Limitations
Our study, while informative, has limitations. The retrospective design and reliance on a single center's records may limit broader applicability and introduce information bias. Additionally, the sample size, though statistically powered, is modest and specific to a set timeframe, which may not reflect more recent medical advances or treatment approaches.
Conclusion
The study conducted at the HMC in Lima, Peru, indicates that PIV is a significant prognostic factor for overall survival in breast cancer patients. Patients with a PIV of 310 or higher were found to have a considerably increased risk of mortality. While other clinical factors such as clinical stage, hemoglobin levels, histological grade, and nuclear grade also correlate with survival outcomes, their significance was not as pronounced in the adjusted models as PIV. This suggests that PIV could serve as a robust and independent prognostic marker. The findings advocate for the integration of PIV into routine clinical assessments to aid in the prognostication and management of breast cancer, especially in resource-limited healthcare settings like those in Peru. This could potentially lead to more personalized treatment plans and improved patient management strategies. Further studies are encouraged to validate these findings and explore the utility of PIV in broader clinical applications.
Despite these limitations, our study offers a unique and valuable perspective on PIV's role in breast cancer prognosis in environments similar to those found in many parts of Latin America. This makes it a significant contribution to the limited but growing body of oncological research specific to this region.
Data availability
Data are available upon request from the corresponding author.
References
World Health Organization. Fact sheets. 2023 [citado 2 de enero de 2024]. Breast cancer. Available at: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (2023).
Ayala, N. et al. Status of breast cancer in Latin American: Results of the breast cancer revealed initiative. Crit Rev Oncol Hematol 181, 103890 (2023).
Vallejos-Sologuren, C. S. Situación del cáncer en el Perú. Diagnóstico 59(2), 77–85 (2020).
Trapani, D. et al. Global challenges and policy solutions in breast cancer control. Cancer Treat Rev. 104, 102339 (2022).
de Lemos, L. L. P. et al. Stage at diagnosis and stage-specific survival of breast cancer in Latin America and the Caribbean: A systematic review and meta-analysis. PloS One 14(10), e0224012 (2019).
Greten, F. R. & Grivennikov, S. I. Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity 51(1), 27–41 (2019).
Zhao, H. et al. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target Ther. 6(1), 263 (2021).
Lillo, S. & Saleh, M. Inflammasomes in cancer progression and anti-tumor immunity. Front. Cell Dev. Biol. 10, 839041 (2022).
Provenzano, L. et al. The pan-immune-inflammation value is associated with clinical outcomes in patients with advanced TNBC treated with first-line, platinum-based chemotherapy: An institutional retrospective analysis. Ther. Adv. Med. Oncol. 15, 17588359231165978 (2023).
Lin, F. et al. Pan-immune-inflammation value: A new prognostic index in operative breast cancer. Front. Oncol. 12, 830138 (2022).
Qi, X. et al. Clinical utility of the pan-immune-inflammation value in breast cancer patients. Front. Oncol. 13, 1223786 (2023).
Ligorio, F. et al. The pan-immune-inflammation-value predicts the survival of patients with human epidermal growth factor receptor 2 (HER2)-positive advanced breast cancer treated with first-line taxane–trastuzumab–pertuzumab. Cancers 13(8), 1964 (2021).
Şahin, A. B. et al. Low pan-immune-inflammation-value predicts better chemotherapy response and survival in breast cancer patients treated with neoadjuvant chemotherapy. Sci. Rep. 11(1), 14662 (2021).
Zavala, V. A., Serrano-Gomez, S. J., Dutil, J. & Fejerman, L. Genetic epidemiology of breast cancer in Latin America. Genes 10(2), 153 (2019).
Guven, D. C. et al. The association between the pan-immune-inflammation value and cancer prognosis: A systematic review and meta-analysis. Cancers 14(11), 2675 (2022).
Fucà, G. et al. The pan-immune-inflammation value is a new prognostic biomarker in metastatic colorectal cancer: Results from a pooled-analysis of the Valentino and TRIBE first-line trials. Br. J. Cancer 123(3), 403–409 (2020).
Gulmez, A. & Harputluoglu, H. Pan-immune-inflammation value in metastatic HER2-positive breast cancer patients. Eurasian J. Med. Investig. 7(3), 209–214 (2023).
Demir, H. et al. A new prognostic index in young breast cancer patients. J. Coll. Phys. Surg. Pak. 32(1), 86–91 (2022).
Tong, Y. S., Tan, J., Zhou, X. L., Song, Y. Q. & Song, Y. J. Systemic immune-inflammation index predicting chemoradiation resistance and poor outcome in patients with stage III non-small cell lung cancer. J. Transl. Med. 15(1), 221 (2017).
Yamanouchi, K. & Maeda, S. The efficacy of inflammatory and immune markers for predicting the prognosis of patients with stage IV breast cancer. Acta Med. Okayama 77(1), 37–43 (2023).
Yilmaz, B., Topkan, E., Besen, A. A., Mertsoylu, H. & Selek, U. The Predictive Power of Biomarkers in Osteoradionecrosis 1–24 (Springer, 2024). https://doi.org/10.1007/16833_2024_266.
Chen, Y. et al. Pan-immune-inflammation and its dynamics: Predictors of survival and immune-related adverse events in patients with advanced NSCLC receiving immunotherapy. BMC Cancer 23(1), 944 (2023).
Pérez-Martelo, M. et al. Clinical significance of baseline pan-immune-inflammation value and its dynamics in metastatic colorectal cancer patients under first-line chemotherapy. Sci. Rep. 12(1), 6893 (2022).
Rapoport, B. L., Steel, H. C., Theron, A. J., Smit, T. & Anderson, R. Role of the neutrophil in the pathogenesis of advanced cancer and impaired responsiveness to therapy. Molecules 25(7), 1618 (2020).
Torres Vigil, I., Aday, L. A., De Lima, L. & Cleeland, C. S. What predicts the quality of advanced cancer care in Latin America? A look at five countries: Argentina, Brazil, Cuba, Mexico, and Peru. J. Pain Symptom Manag. 34(3), 315–327 (2007).
Houghton, N., Bascolo, E. & del Riego, A. Monitoring access barriers to health services in the Americas: A mapping of household surveys. Rev. Panam. Salud Públ. 44, e96 (2020).
Howlader, N., Cronin, K. A., Kurian, A. W. & Andridge, R. Differences in breast cancer survival by molecular subtypes in the United States. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cospons. Am. Soc. Prev. Oncol. 27(6), 619–626 (2018).
Acknowledgements
In this section, you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Author information
Authors and Affiliations
Contributions
Conceptualization, J.A.D.L.C.-V. and M.A.G.-P.; Methodology, J.A.D.L.C.-V. and D.M.Q.-L.; Software, J.A.D.L.C.-V.; Validation, J.A.D.L.C.-V.; Formal Analysis, D.M.Q.-L., L.A.Q.-G. and M.A.G.-P.; Investigation, I.G.P.S., L.A.Q.-G., M.L.P.-T. and R.M.-P.; Resources, J.A.D.L.C.-V. and M.L.P.-T.; Data Curation, D.M.Q.-L., L.A.Q.-G. and M.A.G.-P.; Writing—Original Draft Preparation, I.G.P.S., L.A.Q.-G., L.A.Q.-G., M.L.P.-T. and M.L.P.-T.; Writing—Review & Editing, J.A.D.L.C.-V, D.M.Q.L. and R.M.-P.; Visualization, I.G.P.S.; Supervision, J.A.D.L.C.-V.; Project Administration, J.A.D.L.C.-V.; Funding Acquisition, J.A.D.L.C.-V.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Palomino-Secca, I., Peña-Tuya, M., Quintana-García, L.A. et al. Pan-immune-inflammation value and survival in patients with breast cancer from a Peruvian reference hospital. Sci Rep 14, 17132 (2024). https://doi.org/10.1038/s41598-024-68304-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68304-y
- Springer Nature Limited